Abstract
Objective
To evaluate the feasibility of an electronic symptom-tracking platform for patients recovering from ambulatory surgery.
Method
We assessed user response to an electronic system designed to self-report symptoms. Endpoints included compliance, postoperative symptoms, patient satisfaction. An 8-item symptom inventory (pain, nausea, vomiting, shortness of breath, fever, swelling, discharge, redness) was developed and made available on postoperative days (POD) 2–6. Responses exceeding defined thresholds of severity triggered alerts to healthcare providers. Symptoms, alerts, actions taken, urgent care center (UCC) visits, hospital admissions were tracked until POD 30. Patient satisfaction was evaluated on POD 7. A patient was defined as “responder” if at least 5/8 items on at least 3 PODs were completed. The assessment method was deemed successful if 64/100 patients responded.
Results
97/102 patients were evaluable; 65 met “responder” criteria (67% responder rate; 95% CI 57–76%). 321 surveys were completed (median 4/patient), 248 (77%) in ≤2 min. Involving caregivers and allowing additional symptom-reporting improved the responder rate to 72% (95% CI 58–84%). Most commonly-reported moderate, severe, very severe symptoms were pain, nausea, swelling; 71% reported moderate to very severe pain on POD 2. Phone calls and adjustment of medications adequately addressed most symptoms. Two patients (2%) presented at UCC before, 6 (6%) after, POD 6; 1 (1%) was admitted. Most agreed or strongly agreed that electronic symptom-tracking was helpful, easy to use, and would recommend it to others.
Conclusion
Electronic symptom-tracking is feasible for patients undergoing ambulatory gynecologic cancer surgery. Symptom burden is high in the early postoperative period. Addressing patient-reported symptoms in a timely, automated manner may prevent severe downstream adverse events, reduce UCC visits and admission rates, and improve outcomes.
Keywords: Patient-reported outcomes, Electronic patient-reported symptom monitoring, Minimally invasive surgery
Highlights
-
•
This study assessed user response of an electronic system designed to self-report symptoms.
-
•
Electronic postoperative symptom-tracking is feasible for patients undergoing ambulatory gynecologic cancer surgery.
-
•
Symptom burden is high in the early postoperative period.
-
•
Electronic patient-reported symptom-tracking reduces adverse events and urgent care/readmission rates and improves outcomes.
1. Introduction
Cancer and its treatment are highly morbid, and assessment of patient symptoms is a key part of oncology care. It has been widely recommended that patient-reported outcomes (PROs) should be integrated into patient management. Indeed, randomized trials have shown that use of PROs for chemotherapy patients decreases morbidity and improves survival, compared with routine care [1]; a similar randomized trial showed benefits of symptom management in patients after lung resection [2]. While the value of PROs has been demonstrated in randomized controlled trials, work is needed to refine their implementation into clinical practice.
Surgery is associated with multiple symptoms and potentially serious complications in the postoperative period. Traditional manual systems for following up with patients after discharge and managing postoperative concerns are error-prone due to delays and loss of important information [3,4]. The process can be administratively inefficient, incomplete, and may not reflect the patient's actual experience. There is growing evidence that physicians tend to underestimate patient symptoms, and that patients are often reluctant or forget to report important aspects of their health status on routine postoperative assessments [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14]].
As the length of hospital stay continues to decrease, a greater proportion of patients' recovery takes place at home, which makes comprehensive follow-up of symptoms logistically challenging. Addressing patient-reported symptoms following surgery in a timely and automated manner is known to prevent adverse downstream events, reduce unnecessary urgent care visits and readmission rates, and ultimately improve outcomes and reduce health care costs [2,[15], [16], [17]]. We have previously evaluated the use of a weekly web-based model of symptom tracking in patients recovering from major gynecologic surgery [18]. The system was feasible. However weekly recall of symptoms was not described as actionable or helpful by healthcare providers. The purpose of our study was to evaluate the feasibility of tracking early postoperative and daily patient self-reported symptoms in the ambulatory surgical population, with secondary specific aims being: (1) refine the system based on patient and provider feedback through an interim evaluation; (2) describe postoperative patient-reported symptoms in the ambulatory population undergoing minimally invasive surgery; (3) evaluate the impact of this tool on patient-provider communication, urgent care center (UCC) visits and hospital re-admissions.
2. Methods
2.1. The electronic symptom inventory tool
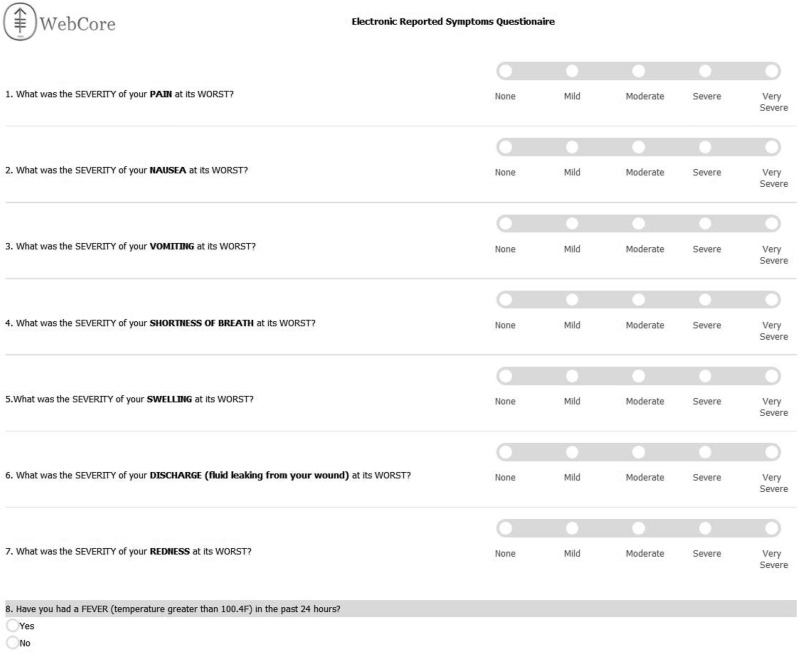
This is an investigator-initiated single-arm study tracking PROs in cancer patients undergoing ambulatory minimally invasive surgery. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) and registered at ClinicalTrials.gov (NCT02700256). Potentially eligible patients were identified from the outpatient Gynecologic Oncology practices and informed consent was obtained. The tool used was adopted from the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE). An 8-item symptom inventory was developed (Fig. 1 ). Seven items were taken from the patient language adaption of the NCI CTCAE (PRO-CTCAE) [12]: pain, nausea, vomiting, shortness-of-breath, swelling, wound discharge and redness, with five response items ranging from none to very severe. A yes / no question on fever was added. The survey was administered in Web Survey Core, an institutional resource. Web Survey Core is a stand-alone site that creates secure electronic surveys for use in research. Patients received daily emails reminders to complete the survey. After completing the survey, the patients received one of three feedback prompts. If all symptoms grades were none or mild, patients were told their responses did not suggest there was anything to worry about, and if they had any concerns to please call the doctor's office. If at least one symptoms grade was moderate, an email alert was sent to the doctor and nurse team. Patients were told a nurse would be contacting them within one business day. If at least one symptoms grade was severe or very severe, an email alert was sent to the doctor and nurse team and patients were told to call the doctor's office immediately, or an answering service if it was after business hours. A brief 4-question satisfaction survey was sent to patients on POD 7 adapted from the system usability scale (SUS) and the Net Promoter Score (NPS).
Fig. 1.
Symptom questionnaire interface with grading.
2.2. The study population
Procedures targeted in this population included minimally invasive, laparoscopic or robotic-assisted surgery planned as ambulatory procedures on the Gynecology Service at MSKCC. Patient eligibility criteria included: patients were scheduled to undergo ambulatory surgery; patients had to be English-speaking, had to have a phone number or email address, and had to express willingness to self-report postoperative symptoms over an automated phone or online platform. Patients had the choice to choose between the automated phone call or the online web-based questionnaire.
2.3. Scheme of the pilot including interim analysis
On postoperative days (POD) 2 through 6, all patients were asked to complete the symptom inventory (online or via automated phone calls). If responses exceeded defined thresholds of severity, email alerts were triggered to the healthcare providers. Postoperative symptoms, alerts, actions taken, UCC visits and hospital admissions were tracked (Supplementary Fig. S1). Patient satisfaction was evaluated on POD 7. Separate semi-structured qualitative interviews were performed at their respective postoperative visits. UCC visits and readmission were tracked until POD 30.
A patient was defined as “responder” if she completed at least 5 of the 8 symptom items on at least 3 of 6 days postoperatively. In keeping with standards for the assessment of feasibility of online platforms the symptom assessment method was deemed successful if 64 of the 100 patients responded [18]. With this decision rule, we had 86% power and a type I error rate of 3.2% to detect a promising response rate of 70% versus an unpromising response rate of 50%. After defining feasibility and responder rate, we planned to enroll 100 patients on this study, with a pre-defined interim analysis after 50 patients.
In the pre-planned interim analysis, patient and healthcare provider feedback were used to make adjustments to the electronic symptom monitoring. Due to under-use of the automated phone system in the initial phase (only 2 patients chose the automated phone call system), in the second phase only the web-based platform was continued. Based on semi-structured interviews and patient and provider feedback after the first phase, patient caregivers were included in the system, and opportunity for additional patient-reported symptoms (free text) and grading was incorporated. In addition, after the interim analysis patients had the option to choose if they wished to be contacted by a healthcare provider if a symptom was moderate (grade 3), and discharge paper instructions were enhanced to remind the patient about the electronic symptom tracking.
Semi-structured patient interviews were conducted during follow-up appointments and over the phone. A total of 71 (26 before and 45 after the interim analysis) were interviewed. Interviews were also conducted with 4 nurses. Patient and nurse responses were manually recorded and later transcribed to an Access database. The responses were categorized into sub-themes and then into larger themes by two different coders.
3. Results
3.1. Patient characteristics
In total, 102 patients were consented and enrolled in the study preoperatively. Five were excluded from the study, due to cancellation of surgery in 3 and conversion to laparotomy and admission in 2 patients. Patient characteristics are shown in Table 1 . Patients ranged in age from 25 to 83, with a median of 53 (Q1-Q3: 42–63). Forty (41%) patients enrolled in the study were diagnosed with endometrial hyperplasia or uterine cancer. Other common indications for surgery were risk-reducing surgery for hereditary breast and ovarian cancer syndrome (HBOC) or Lynch syndrome (10%), or endocrine ablation for patients with hormone receptor positive breast cancer (10%). The most commonly performed procedures included laparoscopic or robotic-assisted hysterectomy, with or without salpingo-oophorectomy, with or without sentinel lymph node mapping/sampling (61%); followed by laparoscopic salpingo-oophorectomy (36%). In total, 8 patients underwent simultaneous breast reconstruction surgery at the time of the gynecologic procedure.
Table 1.
Patient demographic and procedural data.
| Characteristics |
Median (interquartile range) |
|---|---|
| Age at diagnosis (years) | 53 (Q1-Q3: 42–63) |
| n (%) | |
| Race | |
| Asian | 9 (9.3) |
| Black | 4 (4.1) |
| Native American | 1 (1.0) |
| White | 76 (78.4) |
| Refused to report | 7 (7.2) |
| Primary diagnosis | 97 (100) |
| Uterine Cancer | 32 (33.0) |
| Endometrial Hyperplasia | 8 (8.2) |
| HBOC*, Lynch Syndrome | 10 (10.3) |
| Benign ovarian mass | 13 (13.4) |
| Leiomyoma/adenomyosis | 10 (10.3) |
| Ovarian Cancer | 5 (5.2) |
| Breast Cancer | 10 (10.3) |
| Cervical dysplasia/cancer | 2 (2.0) |
| Other | 7 (7.2) |
| Procedures | |
| Hysterectomya | 59 (60.8) |
| Laparoscopic USOb/BSO | 35 (36.1) |
| Other laparoscopy | 3 (3.1) |
| Median (interquartile range) | |
| Operative time (minutes) | 117 (Q1-Q3: 77–155) |
| Estimated blood loss (mL) | 25 (Q1-Q3: 20–50) |
Hysterectomy could have been performed with or without either bilateral salpingo-oophorectomy (BSO) or lymph node sampling.
Unilateral Salingo oophorectomy.
3.2. Electronic symptom monitoring usage
Eighty-seven (90%) of 97 evaluable patients completed at least one postoperative survey, and 65 completed at least 5 of 8 symptom items on at least 3 days postoperatively, for a responder rate of 67% (95% CI: 57–76%), meeting our criterion for success (Table 2 ). Before the interim analysis there were a total of 47 evaluable patients. Of these, 29 were considered “responders”, for a responder percentage of 62% (95% CI: 47–74%). After the interim analysis, and after making adjustments to the system, there were a total of 50 evaluable patients. Of these, 36 were considered “responders”, for a responder percentage of 72% (95% CI: 57–84%). The difference in responder percentage before and after interim analysis was not statistically significant (p = .4). A significantly higher percentage of patients in the latter phase (96%) completed at least one postoperative survey compared to patients in the initial phase (83%) (p = .047). A total of 321 surveys were completed (mean = 3.7 surveys per patients). Of these, 248 (77%) were completed in 2 min or less.
Table 2.
Survey completion rates, urgent care visits, hospital admissions.
| Number of surveys completed | n (%) |
|---|---|
| 0 | 10 (10%) |
| 1 | 13 (13%) |
| 2 | 9 (9%) |
| 3 | 11 (11%) |
| 4 | 13 (13%) |
| 5 | 41 (42%) |
| Caregiver assistance used (n = 50) | 33 (65%) |
| Urgent care visit | 8 (8%) |
| Hospital admission | 1 (1%) |
Survey completion rates increased over the postoperative period. Fifty-three (51%) of 97 evaluable patients completed the survey on POD 2 compared with 72 (74%) who completed the survey on POD 6. The difference in responder percentage was not statistically significant (p = .4). Common reasons for not using the electronic symptom monitoring survey included being too tired, not remembering, or having technical difficulties with the computer.
After the interim analysis patients were given the option to report additional symptoms via free text. Of 48 patients who completed at least one survey after the interim analysis, 20 (42%) reported additional symptoms and 12 (25%) reported additional symptoms on multiple surveys. Most patients reported 1 additional symptom; however, 7 patients reported 2 or more additional symptoms.
3.3. Patient-reported symptoms
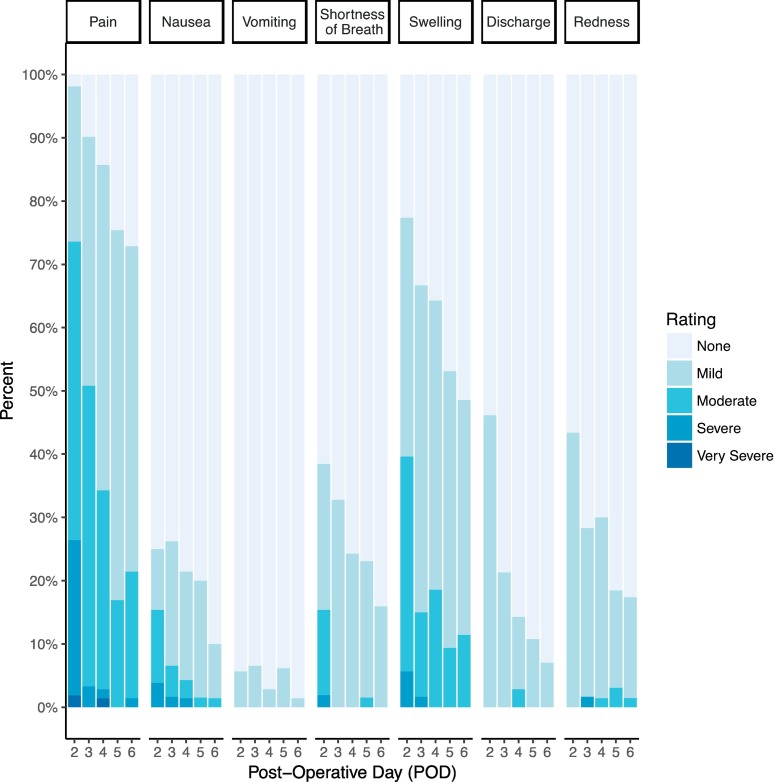
Of the 87 patients who completed at least one survey, 75 (86%, 95% CI: 77–93%) reported at least 1 moderate or higher-grade symptom and 28 (32%; 95% CI: 23–43%) reported at least 1 severe or higher-grade symptom. In total 2226 symptoms were reported; of those, 219 (9.8%) were moderate to very severe symptoms (Fig. 2 ). Symptom severity was highest on POD 2, with patients reporting an average of 18% of the maximum possible symptom severity (Table 3 .). The score decreased consistently over time to 7% on POD 6. The most commonly reported postoperative symptoms included pain, nausea, and swelling. On POD 2, 25 (29%) of 87 patients reported moderate pain, 13 (15%) reported severe pain and 1 (1%) reported very severe pain. Severity of pain decreased on subsequent days. Only 5 patients reported severe or very severe pain after POD 2.
Fig. 2.
Patient-reported symptoms after minimally invasive ambulatory surgery.
Table 3.
The symptom total summary score.
| POD | n | Mean score (SD) |
|---|---|---|
| 2 | 53 | 17.7 (9.1) |
| 3 | 61 | 11.8 (6.6) |
| 4 | 70 | 10.3 (7.1) |
| 5 | 65 | 8.0 (6.1) |
| 6 | 71 | 6.9 (4.9) |
The Symptom Total Summary Score was calculated for each patient by summing the symptom scores for a given POD, and then transforming the score so that it ranges from 0 (no symptoms) to 100 (worst possible rating for every symptom). This type of score is sometimes called a “POMP” score (Percent Of Maximum Possible), and it is a convenient way to quantify a patient-reported outcome into a meaningful metric with an intuitive interpretation.
For example, in the table, the POD 2 mean of 17.7 which indicates that patients reported an average of 17.7% of the maximum possible symptomatology on the survey. This consistently decreased over time to a low of 6.9% at POD 6.
In total, 53 symptoms triggered phone calls to patients, addressing moderate to very severe symptoms during the survey period. In a majority (51 symptoms) no action beyond the phone call was necessary. Phone calls with review of symptoms and reinforcement of discharge instructions were sufficient to address most symptoms adequately. One patient was prescribed additional medication as a result of a phone call, and 1 patient was advised to go to the UCC for further evaluation. After the interim analysis patients were given the option to request contact through their healthcare provider for moderate symptoms. Sixteen percent requested contact for moderate symptoms and 84% did not want to be contacted for their moderate symptoms.
After the interim analysis patients had the opportunity to enter additional symptoms and grade their symptoms. Twenty of 50 patients reported at least 1 additional symptom and 12 patients reported more than 1 additional symptom. Fourteen reported symptoms were mild, 18 were moderate, and 7 were severe. Older patients (>50 years) were twice as likely to report additional symptoms. Most additional symptoms fell under the categories of pain, incision, gastrointestinal function, dizziness and fatigue.
3.4. Urgent care center visits and readmissions
Of 97 evaluable patients, 8 (8.2%) presented at the UCC and 1 (1%) was admitted to the hospital within 30 days after surgery. The median time from surgery to UCC visit was 13 days (range: 2–27) postoperatively, with a majority of patients (n = 6) presenting after completion of the electronic symptom monitoring survey (POD 6).
Two patients presented at the UCC during the survey. Of these, 1 patient who had undergone a laparoscopic bilateral salpingo-oophorectomy for endocrine ablation presented on POD 3 with fever-like symptoms; she was diagnosed with an upper respiratory infection and discharged home with antibiotics. This patient did not use the survey until POD 4. The other patient, with a history of breast cancer and BRCA mutation who underwent a robotic total laparoscopic hysterectomy and bilateral salpingo-oophorectomy in combination with a breast expander exchange, presented to UCC for reported severe pain and moderate shortness of breath with fever-like symptoms on POD 2; as a result of the survey, she was contacted and advised to come to UCC. A CT scan was performed and did not reveal any signs of abdominal infection or pulmonary embolus. The patient was sent home and advised to use incentive spirometry.
Of the 6 patients who presented to UCC after the survey period but <30 days postoperatively, 2 presented with fever (1 on POD 20, the other on POD 11), 1 presented with vaginal bleeding (POD 19), 1 presented with abdominal pain (POD 27) and 1 presented with jaw pain (POD 7). One patient called with low appetite on POD 14, a swelling at the surgical incision and nausea on PODs 16 and 18 and was prompted to come to UCC; she was found to have a hematoma requiring admission, drainage and antibiotic treatment. Symptom severity was not a significant predictor of UCC visits (p = .8).
3.5. Qualitative patient feedback
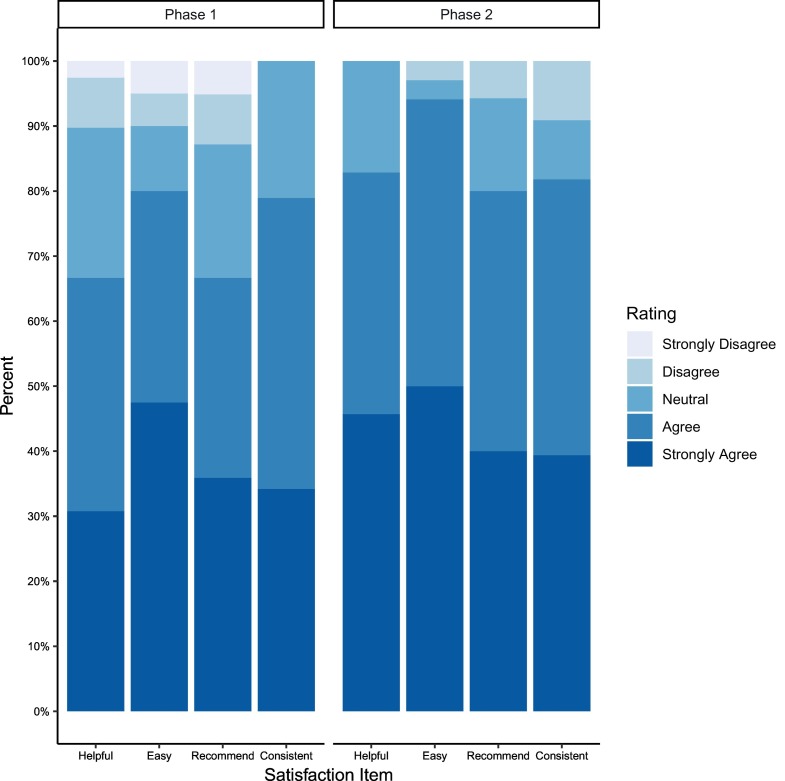
On POD 7 patients were asked to fill out a patient satisfaction survey. Responses were received from 74 patients. Most agreed or strongly agreed that the electronic survey was helpful (n = 5, 74%), consistent (n = 57, 80%), easy to use (n = 64, 86%), and would recommend it to others (n = 54, 73%) (Fig. 3 ). Semi-structured patient interviews were conducted during follow-up appointments and over the phone. Patients reported that the surveys made them feel that they had not been forgotten. “It made me feel like I wasn't alone.” (Patient 28) In addition to feeling cared for, patients found that the survey provided context as to whether their symptoms were normal. “[The survey] was helpful to pinpoint how I'm feeling, and it was good to know I'm okay.” (Patient 39) Knowing they would be contacted if they were experiencing something abnormal was very comforting for patients. “I didn't have to worry that anything was wrong because if I had a complication I would have been contacted.” (Patient 5).
Fig. 3.
Satisfaction questionnaire (postoperative day 7).
Patients reported faster communication through use of the survey. Patients often delay contacting their clinician due to uncertainty over whether their symptoms warrant a call, or guilt about bothering a clinician. “People feel bad about calling a doctor, this [survey] removes that burden.” (Patient 25). This allowed the clinicians to respond in real time. “[The] doctor's office was awesome, they responded within minutes of reporting a symptom.” (Patient 20).
Patients reported that the 8 symptom items were “too limiting” and wanted to enter their own symptoms. After the interim analysis, patients were given the ability to enter up to 3 free-text symptoms. Patients reported the free-text options to be a useful way to engage with their care team. “I commented on swelling I was experiencing. I had surgery on an ovary and had a heavier than expected period. I found it helpful to enter my own symptoms.” (Patient 13).
The feedback on caregivers revealed that caregivers played many different roles in patient adherence. For some patients, a caregiver acted as a facilitator of adherence. “[He] played a big role, he would bring the computer upstairs and we would do it together.” (Patient 42) Other patients were completely self-sufficient and did not require a caregiver's aid. Some caregivers actively chose not to remind the patient so as to not overburden them during their early recovery.
Patients found that adding reminders about the survey to the discharge papers were unhelpful. Patients felt overwhelmed by the amount of paper information that was given to them: “I couldn't find them, wasn't helpful at all. I was given too much stuff at discharge, it was overwhelming.” (Patient 31) In some cases, the papers ended up in the hands of a family member and never made it into the hands of a patient. One patient, when shown an example of what discharge papers looked like, said “I never saw them, maybe my sister got them.” (Patient 10).
4. Discussion
The goal of this pilot study was to test an electronic postoperative symptom-tracking platform and determine its clinical usefulness in the first week after surgery for patients undergoing minimally invasive ambulatory surgery. We developed an 8-item symptom inventory using items from the patient language adaption of the NCI CTCAE (PRO-CTCAE) including pain, nausea, vomiting, shortness-of-breath, swelling, discharge and redness, with the addition of fever. In total, 65 patients completed at least 3 of the 8 symptom items after discharge on at least 3 days postoperatively, meeting our primary feasibility endpoint.
In this study, patient-reported symptom severity was high in the early postoperative period after minimally invasive surgery, with over two-thirds reporting moderate to very severe symptoms on POD 2. The most commonly reported moderate, severe and very severe symptoms included pain, nausea, and swelling, with 71% of patients reporting moderate to very severe pain on POD 2. Phone calls with review or adjustment of supportive medications were sufficient to address most symptoms adequately.
This information can be used to educate patients preoperatively and prepare them more realistically when planning minimally invasive gynecologic surgery. In addition, this information can be used to give patients normative feedback when they report a symptom after surgery resulting in reassurance, fewer phone calls and fewer unnecessary UCC visits, and at the same time identify severe complications early. In contrast to our previous study [18], we believe that the early postoperative daily recall of symptoms in the current study is more accurate and clinically more useful and actionable than a weekly recall of symptoms.
This study gives us the opportunity to plan future clinical trials addressing postoperative symptoms. While we have demonstrated feasibility of an early and daily postoperative symptom tracking system after minimally invasive surgery, several limitations need to be discussed. Only 8 patients experienced symptoms prompting an UCC visit. Most of the UCC visits were after the 6-day symptom-tracking was completed. Severity of symptoms reported during the symptom-tracking period was not associated with UCC visits. Longer symptom-tracking and more patients and events may improve prediction of unplanned UCC visits in future studies [19].
Another limitation is that the PRO-CTCEA was mainly designed and studied to address chemotherapy-associated toxicity. Its usefulness in patients recovering from surgery is less established and may not have appropriate discretion as an instrument for surgical pain. While there are broad categories of PROs (domains of well-being include physical, functional, emotional and social), disease-specific symptoms after specific surgical procedures may be better defined with qualitative feedback from the patient. Additionally, specific tools to address different domains must be investigated and tailored to a population. For example, pain after minimally invasive surgery may be related to incisions but also to CO2 gas insufflation, with referred pain to the upper abdomen, shoulders, neck and chest. These symptoms may not be adequately captured with the symptom inventory used in this study, and patient feedback will help us develop more accurate descriptions of specific postoperative symptoms in the future. In addition, postoperative symptoms are dependent on the nature of surgery and individual patient characteristics. For example, the removal of both ovaries can result in menopausal symptoms in younger premenopausal patients, in addition to surgery-related symptoms. Hot flashes can be interpreted as chills and fever (which happened in this study) and may result in unnecessary interventions. For that reason, we have included patient feedback and given patients the opportunity to describe and grade their symptoms in their own way. This has improved response rates and was generally well received. Although there is commonality among all postoperative patients, patient demographics, disease- and treatment-specific toxicities, and duration of treatment impact PROs. This study did not apply a rigorous methodology for qualitative interviews as this was not our primary goal. Future studies may analyze patient-reported symptoms specific to procedures and patient characteristics and integrate these into clinical trials as endpoints [20,21].
More data will result in a more accurate representation of symptoms after surgery among specific patient populations. This will inform patients preoperatively and when they recover from surgery. Normative feedback may reassure patients during recovery from surgery, but also identify outliers who are not experiencing the same symptoms with the same severity as the majority. The study was performed in an urban setting, and generalizability to other parts of the country or the world may be difficult due to differences in access to the internet. However, the Coronavirus 2019 pandemic has seen an unprecedented accelerated expansion of telehealth across the globe [22] and access to web-based communication tools is expanding worldwide.
In conclusion, daily postoperative electronic postoperative symptom-tracking is feasible for patients undergoing ambulatory gynecologic cancer surgery. Symptom burden is high in the early postoperative period. Addressing patient-reported symptoms following surgery in a timely and automated manner may improve patient-provider communication and prevent severe downstream adverse events, reduce urgent care visits and admission rates, and ultimately improve outcomes. The next steps will be to integrate daily self-reporting into our routine care, refine the instruments used specifically relating to procedures performed, and incorporate healthcare provider feedback. The future web-based electronic symptom monitoring after minimally invasive surgery should cover a longer period than 6 days after surgery and introduce the possibility of entering and grading patient-generated symptoms, with the goals of reducing unscheduled UCC visits and readmissions and improving quality of life. In addition, the role of caregivers in this setting can be explored further to assess how best to engage them.
The following is the supplementary data related to this article.
Flowchart of symptom management.
Funding
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Disclosures
Dr. Straubhar reports patent (W02019195097A1) for perineal heating device, outside the submitted work.
Dr. Carter reports grants from Fidia (Grant PI; Fidia provided product and financial support for research study assistant and biostatistics support to MSK); grants from Sprout (Grant Co-PI; Sprout provided product and research funds to MSK), outside the submitted work.
Dr. Jewell is a consultant for Intuitive Surgical Inc., and reports personal fees from Covidien/Medtronic, outside the submitted work.
Dr. Chi reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc. (now Apyx Medical Corp.), personal fees from C Surgeries, other from Intuitive Surgical Inc., other from TransEnterix Inc., personal fees from Biom ‘Up, outside the submitted work.
Dr. Abu-Rustum reports grants from Stryker/Novadaq, grants from Olympus, grants from GRAIL, outside the submitted work.
Dr. Leitao is a consultant for Intuitive Surgical Inc., outside the submitted work.
Declaration of Competing Interest
None declared.
References
- 1.Basch E., Deal A.M., Dueck A.C. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine Cancer treatment. JAMA. 2017;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleeland C.S., Wang X.S., Shi Q. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J. Clin. Oncol. 2011;29(8):994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotti A., Colevas A.D., Setser A. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen S.M., Dörr W., Anscher M.S. Normal tissue effects: reporting and analysis. Semin. Radiat. Oncol. 2003;13(3):189–202. doi: 10.1016/S1053-4296(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 5.Basch E., Iasonos A., McDonough T. Patient versus clinician symptom reporting using the National Cancer Institute common terminology criteria for adverse events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland C.S. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J. Natl. Cancer Inst. Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland C.S., Mendoza T.R., Wang X.S. Assessing symptom distress in cancer patients: the M.D. Anderson symptom inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland C.S., Reyes-Gibby C.C. When is it justified to treat symptoms? Measuring symptom burden. Oncology (Williston Park) 2002;16(9 Suppl 10):64–70. [PubMed] [Google Scholar]
- 9.Cleeland C.S. A computerized telephone monitoring and alert system to reduce postoperative symptoms: a randomized trial. ASCO Ann Mtg Proc. 2008;2008 [Google Scholar]
- 10.Basch E., Artz D., Dulko D. Patient online self-reporting of toxicity symptoms during chemotherapy. J. Clin. Oncol. 2005;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 11.Basch E., Artz D., Iasonos A. Evaluation of an online platform for cancer patient self-reporting of chemotherapy toxicities. J. Am. Med. Inform. Assoc. 2007;14(3):264–268. doi: 10.1197/jamia.M2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E., Iasonos A., Barz A. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J. Clin. Oncol. 2007;25(34):5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- 13.Basch E., Ulbricht C., Basch S. An evidence-based systemic review Echinacea E. angustifolia DC, E. pallida, E. purpurea by the natural standard research collaboration. J. Herb. Pharmacother. 2005;5(2):57–88. [PubMed] [Google Scholar]
- 14.Basch E.M., Somerfield S.R., Beer T.M. American Society of Clinical Oncology endorsement of the Cancer Care Ontario practice guideline on nonhormonal therapy for men with metastatic hormone-refractory (castration-resistant) prostate cancer. J. Clin. Oncol. 2007;25(33):5313–5318. doi: 10.1200/JCO.2007.13.4536. [DOI] [PubMed] [Google Scholar]
- 15.Nipp R.D., El-Jawahri A., Ruddy M. Pilot randomized trial of an electronic symptom monitoring intervention for hospitalized patients with cancer. Ann. Oncol. 2019;30(2):274–280. doi: 10.1093/annonc/mdy488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lizée T., Basch E., Trémolières P. Cost-effectiveness of web-based patient-reported outcome surveillance in patients with lung cancer. J. Thorac. Oncol. 2019;14(6):1012–1020. doi: 10.1016/j.jtho.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Denis F., Lethrosne C., Pourel N. Randomized trial comparing a web-Mediated follow-up with routine surveillance in lung cancer patients. J. Natl. Cancer Inst. 2017;109(9) doi: 10.1093/jnci/djx029. [DOI] [PubMed] [Google Scholar]
- 18.Cowan R.A., Suidan R.S., Andikyan V. Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: a prospective study measuring symptoms and health-related quality of life. Gynecol. Oncol. 2016;143(2):362–366. doi: 10.1016/j.ygyno.2016.08.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer D.K., Travers D., Wyss A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J. Clin. Oncol. 2011;29(19):2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E., Deal A.M., Kris M.G. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stabile C., Temple L.K., Ancker J.S. Ambulatory cancer care electronic symptom self-reporting (ACCESS) for surgical patients: a randomised controlled trial protocol. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2019-030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shachar C., Engel J., Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020 May 18 doi: 10.1001/jama.2020.7943. [Online ahead of print.] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of symptom management.