Abstract
Introduction
Obstructive sleep apnoea (OSA) and type 2 diabetes mellitus (T2DM) often occur concurrently, and untreated OSA may potentially amplify the high risk of cardiovascular disease in T2DM. Compliance with continuous positive airway pressure (CPAP), the conventional treatment for OSA, can be poor and considering weight loss is the most effective treatment for OSA. This trial examines whether the glucagon-like peptide-1 receptor agonist liraglutide, a glucose-lowering therapy associated with significant weight loss used in T2DM, can improve the severity and symptoms of OSA.
Methods and analysis
This is an outpatient, single-centred, open-labelled, prospective, phase IV randomised controlled trial in a two-by-two factorial design. One hundred and thirty-two patients with newly diagnosed OSA (apnoea–hypopnoea index (AHI) ≥15 events/hour), and existing obesity and T2DM (glycated haemoglobin (HbA1c) ≥47 mmol/mol), will be recruited from diabetes and sleep medicine outpatient clinics in primary and secondary care settings across Liverpool. Patients will be allocated equally, using computer-generated random, permuted blocks of unequal sizes, to each of the four treatment arms for 26 weeks: (i) liraglutide (1.8 mg once per day) alone, (ii) liraglutide 1.8 mg once per day with CPAP, (iii) CPAP alone (conventional care) or (iv) no treatment (control). The primary outcome measure is change in OSA severity, determined by AHI. Secondary outcome measures include effects on glycaemic control (glycated haemoglobin (HbA1c)), body weight and quality of life measures. Exploratory measures include measures of physical activity, MRI-derived measures of regional body composition including fat mass (abdominal subcutaneous, visceral, neck and liver fat) and skeletal muscle mass (cross-sectional analysis of thigh), indices of cardiac function (using transthoracic echocardiography) and endothelial function.
Ethical approval
The study has been approved by the North West Liverpool Central Research Ethics Committee (14/NW/1019) and it is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Trial registration numbers
ISRCTN16250774. EUDRACT No. 2014-000988-41. UTN U1111-1139-0677.
Keywords: sleep medicine, diabetes & endocrinology, protocols & guidelines
Strengths and limitations of this study.
This is the first study to address the treatment of type 2 diabetes mellitus and obstructive sleep apnoea concomitantly using an glucagon-like peptide-1 receptor agonist (GLP-1RA) (liraglutide) in combination with continuous positive airway pressure to target long-term weight loss and immediate symptomatic relief.
This study is designed to examine the impact of weight-loss-induced reductions in apnoea–hypopnoea index (AHI).
Further assessment of changes in glycaemic control and body composition provides metabolic, mechanical and physiological correlates.
The sample size is relatively small, though it provides sufficient statistical power to address the primary research question.
Higher doses of liraglutide 3.0 mg once per day and newer GLP-1RAs (semaglutide) would produce a greater magnitude of weight loss (and thus potentially a greater reduction in AHI); although recently licenced these were not available at study initiation, nor are widely available currently.
Introduction
Obstructive sleep apnoea (OSA) is characterised by repeated closure of the upper airway during sleep and it has been associated with significant cardiovascular morbidity including hypertension, myocardial infarction, atrial fibrillation, congestive heart failure and stroke. The obstruction causes breathing to be interrupted for up to 60s (with hypopnoea or complete apnoea), resulting in recurrent oxyhaemoglobin desaturations and arousals.1
There is a particularly high prevalence of OSA in patients with obesity and type 2 diabetes mellitus (T2DM) (23%–86%).2–4 Recently it has been shown that this relationship is bidirectional with insulin-treated diabetes associated with a higher risk of OSA, particularly in women.5 If effective treatment is not administered, OSA is associated with significant long-term health risks including impaired quality of life,6 irritability and depression, decreased performance in work and potentially road traffic accidents,7 hypertension, increased risk of microvascular complications8 and increased risk of stroke and cardiovascular disease.9 The standard care option for OSA patients is continuous positive airway pressure (CPAP)10 which facilitates normal breathing patterns during sleep by splinting open the upper airway. Other treatment options include diet-induced weight loss,11 intensive lifestyle intervention12 and metabolic (bariatric) surgery.13 Therefore, the beneficial effects of treatment may be derived from mechanical (CPAP) and/or metabolic interventions (diet and exercise); however, compliance with these current treatment pathways is poor. Weight loss is particularly difficult to achieve and the use of CPAP is usually associated with slight weight gain14 which may further exacerbate the associated metabolic complications.15 Thus, the optimal treatment strategy for a T2DM patient with OSA would involve concomitantly targeting both weight loss and glycaemic control (metabolic interventions) in addition to offering CPAP (mechanical intervention).16
This research study assesses the impact of pharmacological treatment with liraglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), a subcutaneously injected agent licenced for glucose lowering in T2DM at doses up to 1.8 mg.17 This therapy has also been licenced at higher dose (3 mg) for treatment of obesity18 19; however, it is important to note that this study commenced before approval of 3 mg dose of liraglutide. There have been limited studies examining the impact of liraglutide in patients with OSA.20 21 There is however very recent unpublished data, released by NovoNordisk, from the first completed phase 3a trial in the STEP programme, the STEP4 study (Semaglutide Treatment Effect in People with obesity), demonstrating the magnitude of weight loss observed with semaglutide, a once-weekly GLP-1RA. Over a 68-week period, weight loss of up to ~18% of total body weight was observed. These results demonstrate greater weight loss than that previously observed with pharmacotherapy in individuals with obesity. It will be interesting to examine the impact of semaglutide, and the associated greater weight loss, in people with obesity complicated by OSA.
We aim to determine whether liraglutide, at the 1.8 mg dose approved for T2DM treatment, can provide a useful therapeutic adjunctive effect in patients with obesity, T2DM and OSA, either as a stand-alone treatment or as an adjunct to CPAP. The coexistence of obesity and insulin resistance in T2DM and OSA provides a strong rationale for the therapeutic administration of liraglutide to obese individuals with T2DM and OSA. We assess the effects of liraglutide on OSA symptoms and severity on glycaemic control in obese individuals with T2DM and OSA, either as a monotherapy (without CPAP) or in combination with CPAP. The data collected will help determine optimal treatment strategies for this challenging and increasingly common clinical disorder.
Methods and analysis
One hundred and thirty-two obese individuals (body mass index (BMI) ≥30 kg/m2) with a clinical diagnosis of T2DM and OSA will be recruited (with the aim of 128 participants completing the study, n=32 per study arm) from across the Liverpool area from both primary and secondary care settings (diabetes and sleep medicine outpatient clinics and community care).
Primary objective
To determine whether 26 weeks of liraglutide treatment (up to 1.8 mg once per day) can provide a useful treatment for patients with obesity, T2DM and OSA, either as a stand-alone treatment or as an adjunct to CPAP. The primary outcome measure of interest is change in apnoea–hypopnoea index (AHI) (the principal measure of OSA severity) from baseline.
Secondary objectives
The key secondary outcome measures are change in HbA1c (the principal measure of glycaemic control) from baseline and change in total body weight (kg). Additionally, the trial will provide useful measures of changes in daytime sleepiness (Epworth score) and quality of life and assess treatment compliance as well as the rate of adverse events.
Further exploratory outcome measures include changes in (i) physical activity (measured by a multisensor array), (ii) fat volume and distribution (abdominal visceral adipose tissue, abdominal subcutaneous adipose tissue, neck fat, submental fat, tongue fat and liver fat) and skeletal muscle mass using MRI-based techniques, (iii) cardiac function using transthoracic echocardiography and (iv) arterial structure (carotid intima-media thickness (cIMT)) and function (flow-mediated dilatation (FMD)) using duplex ultrasonography.
Trial design
This is an outpatient, single-centred, open-labelled, prospective, phase IV randomised controlled trial in a two-by-two factorial design. The trial is designed to provide evidence of benefit of liraglutide on the severity of OSA, body composition and cardiometabolic complications of OSA, when used alone or in combination with CPAP over a period of 26 weeks. Patient–public involvement was not incorporated into the design of this trial.
Methods: participants, interventions and outcomes
This protocol document reflects version 7. Various aspects of the protocol were updated based on early trial monitoring and evaluation by the research team and Trial Steering Committee (TSC).
Study setting
Patients will be recruited from T2DM and OSA clinics at University Hospital Aintree, Liverpool, UK commencing September 2015. Further patients will be sourced from approved patient identification centres consisting of community clinics across the Liverpool and Knowsley areas. Study sites are controlled and monitored by the Liverpool Cancer Trials Unit (LCTU), University of Liverpool. At study outset, we anticipated that five participants would be recruited per month based on clinic numbers. Initially, we projected study completion be 1 October 2017; however early study monitoring revealed a slower recruitment rate, which was evaluated by the TSC, and recruitment was extended accordingly until March 2018. Study amendments were also submitted to maximise patient recruitment opportunities.
Eligibility criteria
Population
T2DM patients with OSA will be initially identified based on BMI >30 kg/m2, by screening with the STOP-BANG questionnaire, or based on clinical suspicion from the medical history in patients with T2DM. Patients managed by diet alone or any combination of metformin and sulfonylureas can be included. Patients receiving Dipeptidyl peptidase (DPP-IV) inhibitors may be included if treatment ceases before baseline tests. Patients with current CPAP usage or whose diabetes is treated at recruitment with pioglitazone, sodium-glucose co-transporter-2 (SGLT2) inhibitors, GLP-1RAs or insulin or any history of pancreatitis identified in medical history will be excluded. Patients with excessive sleepiness (Epworth Sleepiness Score (ESS) >14) will be discussed with a sleep consultant and excluded if they drive a heavy goods vehicle or have any other occupational risk if not treated.
Potentially eligible patients must perform a screening visit 2–21 days before randomisation to assess eligibility to participate as determined by the detailed inclusion/exclusion criteria. This will include medical history and concomitant medications, physical examination, height, weight, waist and neck measurements, blood tests and an overnight home sleep study. Patients can only be randomised if the overnight sleep study confirms moderate–severe OSA as assessed by polysomnographic criteria and HbA1c ≥47 mmol/mol. Detailed inclusion/exclusion criteria are summarised as follows.
Detailed inclusion criteria
Men or women, age 18–75 years.
A clinical diagnosis of T2DM.
HbA1c ≥47 mmol/mol.
BMI ≥30 kg/m2.
Currently treated with either diet or any combination of metformin and sulfonylureas (excluding patients treated with DPP-IV inhibitors*, pioglitazone, SGLT2 inhibitors, GLP-1RAs or insulin).
No current use of liraglutide treatment.
Patients with moderate–severe OSA as assessed by polysomnographic criteria, either by:
AHI ≥15 events/hour with overnight domiciliary multichannel sleep study device (Nox T3).
Overnight desaturation index (ODI; pulse oximetry): ODI≥10 (4% dip in oxygen saturation more than 10 events/hour).
Currently symptomatic for OSA, with daytime sleepiness.
*Patients who are currently treated with DPP-IV inhibitors can be included providing the treatment is discontinued before baseline tests.
Detailed exclusion criteria
Medical history and concurrent diseases
Women of childbearing potential (WOCBP) who are not using adequate contraceptive methods or who are planning a pregnancy in the next 6 months.
Treatment with SGLT2 inhibitors, pioglitazone, subcutaneous insulin injections or with any antiobesity medication (eg, orlistat). We wished to avoid other glucose-lowering drugs that would either promote weight loss (SGLT2 inhibitors) or weight gain (pioglitazone or insulin). DPP-IV inhibitors were contraindicated as they cannot be used in patients who take GLP1-RA.
Patients in whom there may be occupational implications to a diagnosis of OSA, for example, professional drivers or machinery operators.
Type 1 diabetes mellitus.
Congestive heart failure class III–IV.
Renal impairment: estimated glomerular filtration rate < 30 mL/min/1.73 m2.
Previous history of acute pancreatitis.
Hyperthyroidism.
Hypothyroidism (participants with a normal circulating thyroid stimulating hormone (TSH) and free thyroxin (T4) concentrations, and on a stable dose of thyroxine for at least 3 months may be included).
Uncontrolled hypertension (blood pressure ≥170/120 mm Hg).
Recent (<6 months) myocardial infarction.
Previous stroke (with residual neurological deficit).
Significant cardiac dysrhythmias (including pacemaker or implantable cardioverter defribrillator (ICD)).
Presence of any other medical condition that would, in the opinion of the investigator or their clinician, preclude safe participation in the study. This decision should be informed by liraglutide precautions for use statements which will be provided to all clinicians and the research team.
Alcohol consumption in excess of daily recommended limits (21 units/week women, 28 units/week men). Alcohol consumption was determined using simple recall.
History of seizures or unexplained syncope.
Severe sleepiness*.
* If a patient scores >14 on the Epworth Sleepiness Scale, the sleep apnoea specialist will be consulted to assess and that confirm inclusion/exclusion criteria are met, especially regarding driving, but will not be an automatic exclusion.
Allergies and adverse drug reactions
Participants with a history of any serious hypersensitivity reaction to GLP-1RA.
Sex and reproductive status
WOCBP who are unwilling or unable to use an acceptable method to avoid pregnancy for the study duration plus 8 weeks.
Women who are pregnant or breastfeeding.
Prohibited treatments and/or therapies
Diabetes treated with pioglitazone, GLP-1RA analogues or insulin.
Use of other weight loss medication or any drug that might affect body weight or appetite (including antidepressants, antipsychotics and corticosteroids).
Other exclusion criteria
Prisoners or participants who are involuntarily incarcerated.
Participants who are compulsorily detained for treatment of either a psychiatric or physical (eg, infectious) illness.
Additional exclusion criteria for MRI scanning
Any history of internal metal, pacemakers, ferromagnetic metallic implants, intraocular foreign bodies or cerebral aneurysm clips.
Weight >250 kg (due to limitations of MRI scanner).
Outcome measures
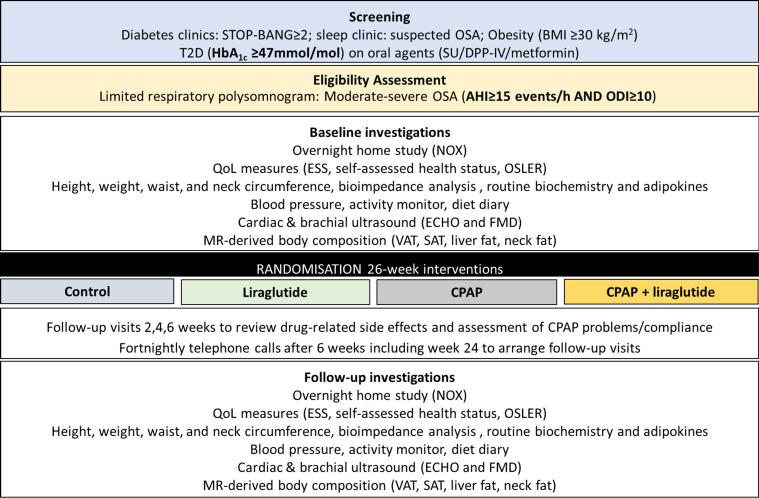
Over the course of three visits (figure 1), the following outcomes will be assessed in all patients at baseline and following the 26-week intervention period. For those patients treated with CPAP, we will perform an additional overnight sleep study at the end of the study period, while receiving CPAP and after 4–7 days of CPAP withdrawal.
Figure 1.
Schematic of protocol. AHI, apnoea–hypopnoea index; BMI, body mass index; CPAP, continuous positive airway pressure; ECHO, echocardiogram; ESS, Epworth Sleepiness Score; FMD, flow-mediated dilatation; HbA1c, glycated haemoglobin; NOX, sleep testing device; ODI, overnight desaturation index; OSA, obstructive sleep apnoea; QoL, quality of life; SAT, subcutaneous adipose tissue; SU, sulphonylureas; T2DM, type 2 diabetes mellitus; VAT, visceral adipose tissue.
Primary outcomes
The primary outcome variable is AHI22 derived via an overnight multichannel sleep study (NOX T3 PSG Recorder, Nox Medical, Reykjavik, Iceland). Although a number of studies using CPAP have used the ESS as the primary outcome measure, for the present study analysis of the ESS may lead to misleading conclusions as excessive daytime sleepiness is very common in obese and T2DM participants and is not restricted to those with OSA, particularly in patients with poor glycaemic control.23
Secondary outcomes
Anthropometric measurements
Weight, height and waist and hip circumference will be measured by a single research technician. Participants will then be rested for 5 min before blood pressure will be determined from an average of three measures.
Questionnaires
A series of self-assessed health and sleep questionnaires will be administered including: (i) STOP-BANG questionnaire (sleep apnoea questionnaire), (ii) BERLIN questionnaire (risk of sleep-disordered breathing), (iii) Epworth Sleepiness Scale (index of excessive daytime somnolence), (iv) SF-36 (self-administered questionnaire to determine quality of life) and (v) SAQLI (sleep apnoea quality of life index), a sleep apnoea-specific quality of life questionnaire.
Oxford sleep resistance test (OSLER)
The OSLER test, a modified measure of the ‘maintenance of wakefulness’ test that gives a validated objective measure of sleepiness, will be performed.23 The test uses a small LED that lights up every 3 s, which the patient is required to cancel by tapping a sensor while sitting on a standard chair in a quiet dark room. The test will be terminated if the patient misses a number of lights in succession, at which point the patient is considered to have fallen asleep. Time to sleep onset and/or the number of misses over a 40 min period will be recorded. The test will be carried out once during the day and will be repeated post-intervention.
Biochemical analysis
All patients will have a routine 15 mL blood sample taken for glucose, insulin, lipid profile and liver function tests. Insulin sensitivity will be measured by homeostatic model assessment of insulin resistance (HOMA-IR).24
CPAP and liraglutide compliance
We shall examine CPAP usage within the first 3 months of the study, and CPAP usage >4 hours per night will be taken as adherent with total hours and average usage also reported. Similarly, for liraglutide we shall look at patient records and prescriptions returned from pharmacy where this information is available/recorded.
Exploratory analyses and substudies
Physical activity monitoring
Physical activity will be tracked throughout using a SenseWear mini armband (BodyMedia, Pittsburgh, Pennsylvania) for a 4-day period. Patients will be instructed to wear at all possible times. Data collected from the armband include: daily average step count, total energy expenditure, active energy expenditure and time spent in domains of physical activity including sleep, lying, sedentary (<1.5 metabolic equivalents (METS)), light (1.5–3 METS), moderate (3–6 METS), vigorous (6–9 METS) and very vigorous (>9 METS) and are analysed using SenseWear Professional software (V.8.0).
Food diaries
Patients will be asked to complete a food diary detailing exactly what they eat and drink over the same 4-day period. Total energy consumption, carbohydrate, protein and fat content will be determined from dietary records using Nutritics (Nutrition Analysis Software for Professionals).
Cardiorespiratory fitness
A V̇O2peak cardiopulmonary exercise test (CPET) will be performed on a treadmill (Model 77OCE, RAM Medisoft Group, Manchester, UK) in a temperature-controlled room. The CPET provided breath-by-breath monitoring and analysis of expiratory gases and ventilation as well as continuous ECG monitoring (Love Medical Cardiopulmonary Diagnostics, Manchester, UK). The modified Bruce protocol will be employed, after an initial 2 min warm-up at 2.2 km/h on a flat gradient, stepwise increments in speed and gradient were employed each minute. V̇O2peak was determined by any of the following: respiratory exchange ratio >1.15, heart rate >90% predicted maximum, plateau in V̇O2 or exhaustion.
Body composition, neck anatomy and liver fat
MRI will be carried out at the Liverpool Magnetic Resonance Imaging Centre (LiMRIC), University of Liverpool using a Siemens Symphony 1.5T MR scanner. For body composition, transverse whole-body MRI data will be acquired using a T1-weighted turbo spin-echo (TSE) sequence with 1 cm slice thickness and gap.
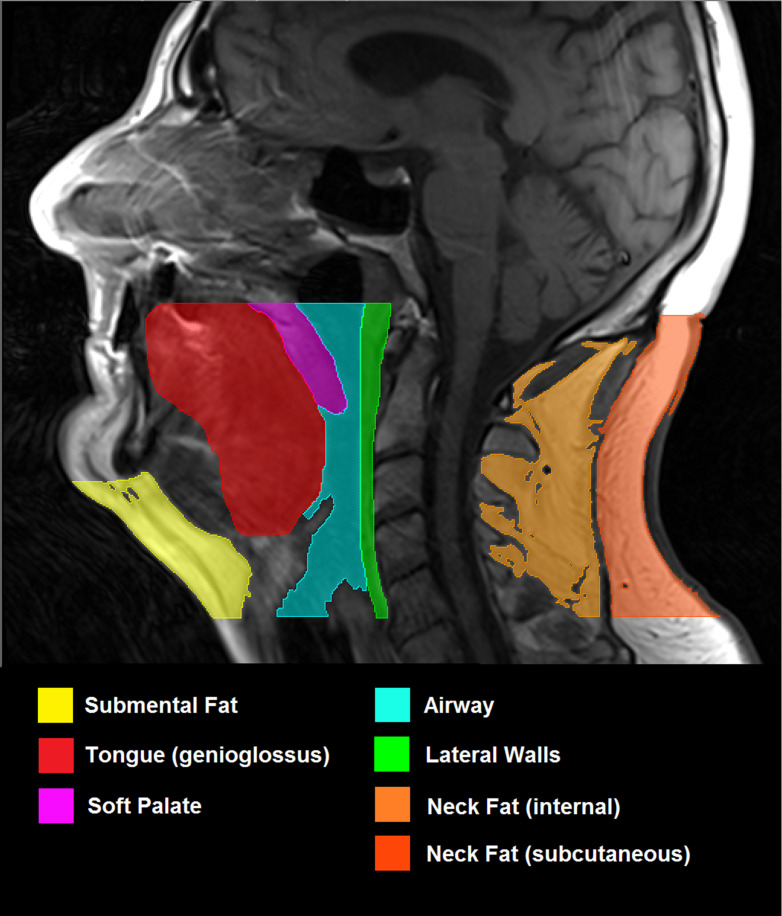
From these scans, subcutaneous and visceral fat content will be determined using serial sections of the trunk and skeletal muscle mass from serial sections of the thigh. Liver proton magnetic resonance spectroscopy (1H MRS) will be performed at three standardised sites using the integral body coil with TR 1500 ms TE 135 ms, free breathing. 1H MRS data will be analysed to determine intrahepatic triglyceride concentration as marker of non-alcoholic fatty liver disease. To assess the neck anatomy, T1-weighted TSE axial contiguous 4 mm slices will be acquired from the hard palate to the vocal chords, using a cervical spine coil with participants lying supine and breathing quietly through their nose.25 Within the region of interest, volumetric measurements of submental fat, neck fat, tongue, soft palate, lateral pharyngeal walls and airway will be obtained (figure 2).
Figure 2.
MRI (T1-weighted, spin echo, 4 mm slice thickness) showing the mid-sagittal slice of head and neck. Region of interest includes all tissues inferior to hard palate and superior to vocal cords. The following structures to be included in analysis have been highlighted: submental fat, defined as all fat anterior to hyoid and inferior to mandible; tongue (genioglossus); soft palate; airway; lateral parapharyngeal walls; internal neck fat; subcutaneous neck fat.
Endothelial function
A 10 MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (Siemens Medical Solutions, Malvern, Pennsylvania) will be used to image the brachial artery in the distal third of the upper right arm. Once an optimal image is acquired, the probe is held stable and the ultrasound parameters set to optimise longitudinal, B-mode images of the lumen–arterial wall interface. Continuous Doppler velocity assessment will also be used. Nitric oxide-mediated endothelial function will be assessed by measuring FMD in response to a 5 min ischaemic stimulus, induced by forearm cuff inflation.26 Baseline images will be recorded using specialised recording software (Camtasia; TechSmith, Okemos, Michigan). A rapid inflation and deflation pneumatic device (D.E. Hokanson, Bellevue, Washington) will be used with an inflation cuff placed immediately distal to the olecranon process of the imaged arm to provide a stimulus for forearm ischaemia. A baseline recording lasting 1 min will be acquired before the forearm cuff is inflated (È220 mm Hg) for 5 min. Artery diameter and blood flow velocity recordings resumed 30 s before cuff deflation and continued for 3 min thereafter.26 Peak brachial artery diameter and blood flow velocity, and the time taken to reach these peaks after cuff release, will be recorded.
Vascular structure
An image of the carotid artery will be recorded using the same technology and measurement of cIMT, or artery wall thickness, will be calculated using custom-designed software.27 In brief, the anterolateral, posterolateral and mediolateral planes are to be acquired. Patients will be instructed to lay supine with a slight hyperextension of the neck and a 45° lateral flexion away from the side being scanned (right). An R-wave triggered optimal recording of the far wall, 1 cm proximal to the carotid bulb, will be stored as a digital DICOM file on the PC for analysis of cIMT and common carotid arterial diameter.
Cardiac structure and function
Indices of cardiac function and subclinical indices of myocardial systolic and diastolic function (strain and strain rate) will be determined using tissue Doppler echocardiography.28 All echocardiograms will be performed using a GE Vivid 7 or E9 machine with a 2.5 MHz phased array transducer and the patient in the left lateral position on a reclining couch. A combination of 2D, M-mode, pulsed wave and continuous wave Doppler and tissue Doppler is to be used. Conventional echocardiographic views will be obtained (parasternal long axis, parasternal short axis, apical four chamber, apical long axis, apical two chamber and subcostal).
Left ventricular (LV) diameter and wall thicknesses will be measured in the parasternal long axis view using two-dimensional or M-mode measurements. LV mass will be calculated using the Devereux formula and indexed to body surface area. Modified Simpson’s biplane method will be used to determine LV ejection fraction. Mitral inflow velocities and deceleration times are to be measured using pulsed wave Doppler in the apical four-chamber view. Isovolumetric relaxation time will also be calculated using continuous wave Doppler, with the cursor midway between LV outflow and mitral inflow. For tissue Doppler imaging, colour tissue Doppler loops will be recorded using a frame rate >100 frames/s. Myocardial longitudinal function will be assessed from three consecutive cycles of tissue Doppler imaging in the apical four-chamber, apical two-chamber and apical long-axis views.
Echocardiographic data has to be analysed using Echopac V.9.01 (GE, Horten, Norway). Peak systolic and early and late diastolic myocardial tissue velocities will be obtained from the basal segment of all six LV walls. Myocardial deformation curves will be obtained from the basal segment of all six LV walls. Wall motion will be manually tracked throughout the cardiac cycle to maintain continuity of the sampling area. Data will be excluded if a smooth curve was unobtainable, or if the angle between the ventricular wall and the scan line was >200. From these curves, peak systolic strain, systolic and early and late diastolic strain rates will be obtained. Using data from each of the three cardiac cycles, the values from each wall can be averaged to give a mean value.
Follow-up Investigation Repeat procedures identical to the baseline visits will be performed at the end of the 26-week intervention period. Importantly, the time of day of these assessments will remain consistent to control for circadian variation.
Interventions
Patients will be randomised in equal allocation to one of the four arms:
Arm A: Control arm, comprising conventional care for existing patients with T2DM with no intervention for OSA.
Arm B: Conventional care, plus liraglutide (up to 1.8 mg once per day).
Arm C: Conventional care, plus CPAP.
Arm D: Conventional care, plus liraglutide (up to 1.8 mg once per day) and CPAP.
Randomisation
Sequence generation
Patients will be allocated equally to each of the four treatment arms using computer-generated random, permuted blocks of unequal sizes. This will be created by the trial statistician in accordance with the LCTU’s standard operating procedure. The allocation sequence will be held centrally at the LCTU with access confined to the trial statistician, the LCTU trial coordinator and the data managers assigned to the study.
Control (no intervention)
This group will not use placebo medication. Patients will be asked to continue with their usual antidiabetic medications and if titration of any glucose-lowering therapy is necessary, due to worsening glycaemic control, this will be recorded. This diabetes therapy titration may include initiation of subcutaneous insulin therapy if glycaemic control significantly deteriorates. Patients will not be given CPAP for this period for their OSA.
Liraglutide
Liraglutide is administered as a once per day subcutaneous injection in the abdomen, thigh or upper arm. It will be commenced at a starting dose of 0.6 mg once per day, increasing after 1 week to 1.2 mg once per day and after a further week to 1.8 mg once per day. Those patients who cannot tolerate the increased dose after the first week will be asked to remain at 0.6 mg/day and will be rechallenged with 1.2 mg dose accordingly. Should drug intolerance persist, patients will be asked to remain on the lowest, tolerated dose. This will be managed by the research team on an individual basis. The trial-specific prescription will allow the prescriber to specify individual doses and quantities for those patients who do not tolerate the intended dose. Due to the dose-escalation nature of the trial design, dose modifications are not appropriate.
Continuous positive airway pressure
The CPAP device that will be used will be the ResMed AirSense 10 Elite in a fixed pressure delivery mode, with the therapeutic pressure defined in accordance with standard clinical protocols.29
Preparation, dosage and administration of study treatment
Existing clinical staff within the relevant diabetes and sleep clinics at University Hospital Aintree will initiate the appropriate treatment pathway in patients enrolled onto the study according to their randomisation. It is important to note that individuals who enrol onto the study will be fast-tracked through the referral pathways and will commence their allocated treatment immediately following baseline assessment.
Additional visits
Additional visit for patients randomised to arms B, C and D to collect the CPAP device, liraglutide prescription and instructions for use. This is optional for arm A patients who should be given the choice of a visit or telephone consultation.
Female patients randomised to liraglutide will require an additional pregnancy test (within 0–72 hours before the first dose of study drug).
Assessment of compliance with study treatment
All patients randomised to arms B or D will be instructed to return used (empty or part full) liraglutide pens at each prescribing visit. These are assessed by pharmacy staff and volumes recorded and compliance can be calculated accordingly as percentage used per protocol and per individual prescription (if maximum dose is not tolerated).
The CPAP device will be interrogated to determine the duration of usage (minutes per night) with the usage recorded on the case report form (CRF). The initial 3 months of CPAP data will be used for study analysis as per remote monitoring with Airview (Resmed) or by downloading the CPAP machine directly. Compliance calculations will be described in greater detail in the statistical analysis plan but patients returning their machine will still remain in their allocated study arm. Patients will also be issued with treatment diaries to facilitate discussion at each study visit and the data will also be recorded. Patients will be routinely counselled on the importance of using their study intervention as prescribed.
Monitoring/dispensing visits
Follow-up visits or telephone calls for patients randomised to arms B, C and D will take place at weeks 2, 4 and 6 of treatment intervention to review drug-related side effects and assessment of any CPAP-related issues and compliance with regimen. Patients in arm A will be reviewed by telephone conversation.
After 6 weeks, specialist T2DM and OSA nurses will provide fortnightly telephone support to all patients and where necessary face-to-face reviews to monitor patient compliance to treatment pathways and manage their drug escalation and deal with any problems identified with the CPAP device (liraglutide and liraglutide +CPAP arms).
For patients randomised to arms A and B, these are repeat procedures identical to visits 2, 3 and 4
For patients randomised to arms C and D who have been issued with a CPAP device, an additional sleep study will be performed at visit 10 following a period of 4–7 days without CPAP.
Study withdrawal
If a patient wishes to withdraw from trial treatment, the importance of remaining on trial follow-up, or failing this, of allowing routine follow-up data to be used for trial purposes, will be explained. Generally, follow-up will continue unless the patient explicitly also withdraws consent for follow-up.
Patients who withdraw from the trial for other reasons have previously consented to follow-up in the trial. Data up to this time can be included in the trial if anonymised. They may need to reaffirm that they consent to follow-up through usual NHS mechanisms. If the patient explicitly states their wish not to contribute further data to the study, an End of Study CRF should be completed documenting the reason for withdrawal. It must be noted that any safety data collected up to the point of withdrawal cannot be removed from the trial analysis.
Pharmacovigilance
All adverse events and assignment of the severity/grading (mild, moderate, severe, life-threatening, death) made by the investigator responsible for the care of the participant will be reported. The assignment of causality will be made by the chief investigator. All non-serious adverse events (SAEs), whether expected or not, will be recorded and updated at each study visit. All new SAEs will be reported from the point of consent until 70 days after discontinuation of the investigational medical product; this includes those thought to be associated with protocol-specified procedures. Investigators will report SAEs, serious adverse reactions (SARs) and sudden unexpected SARs (SUSARs) to LCTU within 24 hours of the local site becoming aware of the event. LCTU will notify the Medicines and Healthcare products Regulatory Agency (MHRA) and main Research Ethic Committee (REC) of all SUSARs occurring during the study: fatal and life-threatening SUSARs within 7 days of notification and non-life-threatening SUSARs within 15 days. All adverse events will be followed until satisfactory resolution or until the investigator responsible for the care of the participant deems the event to be chronic or the patient to be stable.
Statistical analysis plan
Sample size calculation
We assume a clinically minimum relevant difference in AHI between control and intervention groups of ~10 units, approximating the difference between the midpoints of mild and moderate AHI scores. This assumption is reasonable based on a weight loss of ~5 kg being associated with a reduction in AHI of 14 units in patients with similar baseline characteristics to those proposed in the current study.30
Treating the two-by-two factorial design as two unrelated comparisons, using the method for the unpaired t-test and based on a SD of 15 units,31 90% power and a 1% significance level, we require a total sample size of 128 patients (64 in the marginal total for each group or 32 in each arm). Considering a small dropout rate, this will entail screening approximately 132 patients. Given the non-invasive nature of the interventions and the short follow-up planned, patient retention is not envisaged to be an issue. Furthermore, early study withdrawals formed part of the evaluation by the joint data monitoring committee (DMC)/TSC oversight committee so that this assumption could be evaluated during the course of the study.
This sample size does not consider the possibility of an interaction effect, but without prior knowledge of any such interaction we believe that this assumption is reasonable. Furthermore, we recognise that the power to detect an effect on HbA1c is low.
Statistical methods
Categorical variables will be summarised as frequencies and percentages. Continuous variables will be summarised as mean (95% SD). The analysis of the factorial design will use a general linear model with main effect terms for treatment together with baseline value of the response variable (for each of the response variables). Full details of the planned analyses will be given in a separate statistical analysis plan, to be completed and signed off before data lock. Data access is granted to assigned trial statisticians.
Subgroup analyses
A test for interaction will be performed. No others are specified at the time of writing.
Significance levels
For analysis of the primary outcome, statistical significance will be declared if a two-sided p value of <0.05 is obtained in favour of liraglutide or CPAP. For the primary end point, the mean difference from baseline (adjusted for baseline) will be presented with a corresponding 95% two-sided CI. Secondary end points will also be presented with 95% CIs and 5% two-sided significance levels.
Analysis populations
The main analysis for primary and secondary end points will use the full analysis set, consisting of all randomised patients, with participants analysed according to the group to which they were originally allocated and with outcomes included irrespective of protocol adherence, to follow the intention-to-treat principle.
The per-protocol population will consist of those patients in the full-analysis set without any major deviations in treatment or assessment that could affect the outcome. This population will be used in a sensitivity analysis for the primary end point. The safety population, consisting of all patients who actually receive a trial intervention, according to the treatment received, will be used for analysis of toxicity and adverse events.
Missing data
Missing data will be handled by multiple imputation (MI), performing separate MIs by treatment arm, using the method of chained equations as implemented in Stata V.12 or higher. Imputation is planned only for cases of missing follow-up outcome data which is anticipated to be small, and therefore the scope for any bias due to the MI routine is limited. MI using chained equations will be applied to each treatment arm individually. Imputation methods will include baseline outcome data and other key prognostic information (eg, sex and age).
Safety analysis
Information related to adverse events (eg, hypoglycaemia) will be tabulated and summarised descriptively.
Trial oversight
Management structure
The trial will be overseen by a TSC and operated on a day-to day basis by a trial management group (TMG). The trial coordinator (TC) will produce monthly recruitment reports, to allow the TSC and TMG to regularly review the trial across sites. The TSC will comprise of experienced medical experts and trialists. Meetings will be held at regular intervals dependent on need, but no less than once a year. The responsibilities will include as follows:
Report to the TSC.
Maintain the Trial Master File.
Confirm all approvals are in place before release of the trial treatment and the start of the trial at a site.
Provide training about the trial.
Provide study materials.
Data management centre.
Give collaborators regular information about the progress of the study.
Respond to any questions (eg, from collaborators) about the trial.
Ensure data security and quality and observe data protection laws.
Safety reporting.
Ensure trial is conducted in accordance with the International Conference on Harmonisation Good Clinical Practice (ICH GCP).
Statistical analysis.
Publication of trial results.
The role of the TSC is to provide overall supervision of the trial. In particular, the TSC will concentrate on the progress of the trial, adherence to the protocol, patient safety and consideration of new information. The TSC must be in agreement with the final protocol and, throughout the trial, will take responsibility for:
Major decisions such as need to change the protocol for any reason.
Monitoring and supervising the progress of the trial.
Reviewing relevant information from other sources.
Considering recommendations from the DMC.
Informing and advising the TMG on all aspects of the trial.
Patient and public involvement
No patient or public involvement.
Ethics and dissemination
This study is being conducted in accordance with GCP, as defined by the ICH and in compliance with the European Union Directive 2001/20/EC transposed into UK law as statutory instrument 2004 No. 1031: Medicines for Human Use (Clinical Trials) Regulations 2004 and all subsequent amendments and the United States Code of Federal Regulations, Title 21, Part 50 (21CFR50). The trial protocol has received the favourable opinion of the NRES North West—Liverpool Central Research Ethics Committee (14/NW/1019; protocol number UoL000977). An appropriate patient information sheet and consent forms (online supplementary files) describing in detail the trial interventions/products, trial procedures and risks were approved by the ethical committee. The investigator will then explain the study to the patient and answer any questions posed. A contact point where further information about the trial may be obtained will be provided. After being given adequate time to consider the information, the patient will be asked to sign the informed consent document by a member of the study team. A copy of the informed consent document will be given to the patient for their records and a copy placed in the medical records, with the original retained in the investigator site file. The patient may withdraw from the trial at any time by revoking their informed consent. The rights and welfare of the patients will be protected by emphasising to them that the quality of medical care will not be adversely affected if they decline participation.
bmjopen-2020-038856supp002.pdf (184.7KB, pdf)
bmjopen-2020-038856supp001.pdf (183.3KB, pdf)
The results will be analysed together and published as soon as possible. The Uniform Requirements for Manuscripts Submitted to Biomedical Journals (http://www.icmje.org/) will be respected. The ISRCTN allocated to this trial would be attached to any publications resulting from this trial.
Dissemination plan (publications, data deposition and curation)
It is our intention to present our research findings to all our research participants in a written lay summary and hold an open feedback session where the results will be presented in a lay-friendly manner. We plan to present the scientific findings as oral communications and abstracts at regional, national and international scientific meetings related to obesity, T2DM, respiratory and sleep medicine. We also intend to publish our findings in peer-reviewed journals in the subspecialties described earlier.
Supplementary Material
Acknowledgments
We would like to acknowledge the Liverpool Clinical Trials Unit, particularly Emma Clark, Kate Culshaw and Julie Perry along with trial statisticians, particularly James Dodd, for their contributions to the study.
Footnotes
Twitter: @torisprung
Contributors: VSS, JPHW, SEC and DJC wrote the study protocol. DJC is the principal investigator for this study. VSS is the postdoctoral research fellow responsible for the running of the clinical trial and is a co-investigator. VSS and DJC drafted the protocol in the journal format. GJK, AW, VA, KM and RJS developed the imaging methodology for the protocol. SE, MT, AM and SEC developed the respiratory assessment and analysis included in the trial. MB developed the cardiac outcome measures. AJN developed the statistical plan. All authors are co-investigators for the study and have contributed to the revision of the manuscript.
Funding: Funding support for the project was an investigator-initiated research project by Novo Nordisk with all intellectual content, data collection and analysis and writing of manuscripts performed independently.
Competing interests: DJC has competing interests with AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals and Lilly & Novo Nordisk. JW has acted as a consultant, received institutional grants and given lectures on behalf of pharmaceutical companies developing or marketing medicines used for the treatment of diabetes, specifically AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Novo Nordisk and Sanofi & Takeda.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This trial has been registered with the MHRA and has been granted a Clinical Trial Authorisation (CTA).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 2.Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 2004;25:735–41. 10.1016/j.ehj.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 3.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017–9. 10.2337/dc08-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006;61:945–50. 10.1136/thx.2005.057745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang T, Lin BM, Stampfer MJ, et al. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care 2018;41:2111–9. 10.2337/dc18-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyer CA, Sonnad SS, Garetz SL, et al. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med 2001;2:477–91. 10.1016/S1389-9457(01)00072-7 [DOI] [PubMed] [Google Scholar]
- 7.Young T, Blustein J, Finn L, et al. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997;20:608–13. 10.1093/sleep/20.8.608 [DOI] [PubMed] [Google Scholar]
- 8.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy. Am J Respir Crit Care Med 2012;186:434–41. 10.1164/rccm.201112-2135OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–41. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 10.Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006:CD001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the sleep ahead study. Arch Intern Med 2009;169:1619–26. 10.1001/archinternmed.2009.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ 2009;339:b4609. 10.1136/bmj.b4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax 2012;67:442–9. 10.1136/thx.2010.151225 [DOI] [PubMed] [Google Scholar]
- 14.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the mosaic randomised controlled trial. Thorax 2012;67:1090–6. 10.1136/thoraxjnl-2012-202178 [DOI] [PubMed] [Google Scholar]
- 15.Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 2015;70:258–64. 10.1136/thoraxjnl-2014-205361 [DOI] [PubMed] [Google Scholar]
- 16.Chirinos JA, Gurubhagavatula I, Teff K, et al. Cpap, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014;370:2265–75. 10.1056/NEJMoa1306187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 Mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 19.Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract 2017;3:3–14. 10.1002/osp4.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes 2016;40:1310–9. 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Peralta F, Abreu C, Castro JC, et al. An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes. BMC Endocr Disord 2015;15:78. 10.1186/s12902-015-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions Task force of the American Academy of sleep medicine. J Clin Sleep Med 2012;8:597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res 1997;6:142–5. 10.1046/j.1365-2869.1997.00039.x [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 25.Turnbull CD, Wang SH, Manuel AR, et al. Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath 2018;22:673–81. 10.1007/s11325-017-1599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijssen DHJ, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2–12. 10.1152/ajpheart.00471.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor LH, Green DJ, Jones TW, et al. Endothelial function and carotid intima-medial thickness in adolescents with type 2 diabetes mellitus. J Pediatr 2011;159:971–4. 10.1016/j.jpeds.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 28.Dobson R, Burgess MI, Sprung VS, et al. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes 2016;40:153–61. 10.1038/ijo.2015.151 [DOI] [PubMed] [Google Scholar]
- 29.Stradling JR, Hardinge M, Paxton J, et al. Relative accuracy of algorithm-based prescription of nasal CPAP in OSA. Respir Med 2004;98:152–4. 10.1016/j.rmed.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland K, Lee RWW, Phillips CL, et al. Effect of weight loss on upper airway size and facial fat in men with obstructive sleep apnoea. Thorax 2011;66:797–803. 10.1136/thx.2010.151613 [DOI] [PubMed] [Google Scholar]
- 31.Pepperell JCT, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002;359:204–10. 10.1016/S0140-6736(02)07445-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038856supp002.pdf (184.7KB, pdf)
bmjopen-2020-038856supp001.pdf (183.3KB, pdf)