Abstract
Objective.
Despite the feasibility of short-term neural recordings using implantable microelectrodes, attaining reliable, chronic recordings remains a challenge. Most neural recording devices suffer from a long-term tissue response, including gliosis, at the device–tissue interface. It was hypothesized that smaller, more flexible intracortical probes would limit gliosis by providing a better mechanical match with surrounding tissue.
Approach.
This paper describes the in vivo evaluation of flexible parylene microprobes designed to improve the interface with the adjacent neural tissue to limit gliosis and thereby allow for improved recording longevity. The probes were coated with an ultrafast degrading tyrosine-derived polycarbonate (E5005(2K)) polymer that provides temporary mechanical support for device implantation, yet degrades within 2 h post-implantation. A parametric study of probes of varying dimensions and polymer coating thicknesses were implanted in rat brains. The glial tissue response and neuronal loss were assessed from 72 h to 24 weeks post-implantation via immunohistochemistry.
Main results.
Experimental results suggest that both probe and polymer coating sizes affect the extent of gliosis. When an appropriate sized coating dimension (100 μm × 100 μm) and small probe (30 μm × 5 μm) was implanted, a minimal post-implantation glial response was observed. No discernible gliosis was detected when compared to tissue where a sham control consisting of a solid degradable polymer shuttle of the same dimensions was inserted. A larger polymer coating (200 μm × 200 μm) device induced a more severe glial response at later time points, suggesting that the initial insertion trauma can affect gliosis even when the polymer shuttle degrades rapidly. A larger degree of gliosis was also observed when comparing a larger sized probe (80 μm × 5 μm) to a smaller probe (30 μm × 5 μm) using the same polymer coating size (100 μm × 100 μm). There was no significant neuronal loss around the implantation sites for most device candidates except the group with largest polymer coating and probe sizes.
Keywords: intracortical probe, glial response, flexible probe, biodegradable polymer
1. Introduction
In recent years, intracortical neural probes have received increased interest as tools to study neuroscience and understand brain functions, and for use in promising therapeutic devices for various biomedical/rehabilitative applications, including neuromodulation, brain–computer interfaces, and neural prosthetics [1–5]. Despite several decades of neural probe development, the ability to achieve consistent long-term neural recordings still remains a major challenge and has prevented the widespread clinical use of intracortical probe technology in humans [6–9]. One of the prominent causes for recording failure is disruption of the electrode-cell interface resulting from the native tissue foreign body response. Implantation of an intracortical probe causes acute, immediate tissue damage including potential disruption of the blood vessels [10, 11], an acute and chronic inflammatory response [12, 13], neural damage and degeneration [14], and ultimately the development of a glial scar [14, 15], which diminishes probe recording quality over time.
Astrocytes and microglia are the two major glial cell types involved in the neural tissue response. Microglia are activated following device implantation and initiate the wound healing response through the secretion of soluble factors, which in turn activate astrocytes, other microglia, immune cells, and cellular pathways through inflammatory cytokines [16, 17]. Activated microglia recruit macrophages which attempt to phagocytize the implanted foreign matter [12, 18, 19]. Although this acute inflammatory response may interrupt probe performance, it usually subsides within a few weeks following device implantation [12, 13]. A longer term chronic tissue response follows, which is characterized by the development of an encapsulation layer around the probe, primarily composed of astrocytes [10, 20, 21]. This glial layer isolates the implant from adjacent neural cells and increases the electrode impedance to neural signal acquisition of local field potentials [22, 23]. Such disruption can cause diminished and/or inconsistent signal quality, which eventually limits the probe’s functionality. Most conventional intracortical electrodes (e.g. microwires, Blackrock Microsystems (Utah) arrays, or NeuroNexus (Michigan) arrays) can often sustain recording performance for several months (6–12 months with good signal-to-noise ratio) before the signal quality begins to drop significantly approximately 18–24 months after device implantation [20, 24, 25] until the majority of probes ultimately become incapable of acquiring signals at all.
Various interventions have been investigated to improve the electrode-cell interface and limit the degree of gliosis around the probes. Some researchers have reported biochemical approaches to limit gliosis by modifying conventional neural probes by incorporating or coating them with substances such as anti-inflammatory drugs (e.g. Dexamethasone (DEX) [26–31], Flavopirido [32]) or neural cell adhesion molecules, such as L1 [33, 34]. Also, by coating the recording electrodes with conductive materials such as carbon nanotubes [35–37] and conductive polymers [31, 38–40], the electrode impedance can also be decreased to allow better electrical integration with neural tissue. These interventions improve probe longevity by reducing the inflammatory response induced by the electrode, enhancing neural cell attachment to the electrode for higher fidelity recording, or increasing the electrode conductivity for improved signal-to-noise ratio.
Another approach to improve the electrode-cell interface is to manipulate device design parameters such as probe size and material. Several studies have shown that subcellular sized probes can significantly reduce the degree of glial scar formation compared to conventional silicon probes and are able to sustain their recording quality for extended periods of time [41–43]. Smaller probes are expected to produce less tissue disruption during device implantation as well as less mechanical mismatch/shearing between the probe and the surrounding tissue. The stiffness of the chosen probe material also contributes to the mechanical mismatch between the neural probe and adjacent tissue layer, where a larger, stiffer probe is expected to have a greater degree of mechanical mismatch, which induces a foreign body response due to the interfacial strain. Furthermore, friction from the device micromotion at the probe-tissue interface has been shown to worsen the inflammatory response and disrupt the cellular microenvironment [44–46]. Several groups have suggested that fabrication of probes from more flexible materials (SU-8 [47, 48], polyimide [49], poly(p-xylylene) (parylene) [9, 50], shape memory polymers [51, 52], and adaptive polymer nanocomposite materials [53, 54]) that provide a better mechanical match of the device compliance with that of adjacent tissues will minimize tissue responses [55–61].
Fabricating smaller and more flexible (compliant) neural probes represents a promising approach towards attenuating chronic tissue responses. However, implantation of compliant devices can be challenging. Flexible probes are soft and pliable and therefore often buckle during penetration through the meningeal layers. They may also bend and curl easily, leading to device misplacement within the brain. Furthermore, the electronics on the flexible probe can be sensitive to the mechanical forces experienced during insertion and so they need to be protected from distortion and fracture of the electrode traces. Various techniques have been investigated to aid insertion of flexible probes. One approach is to couple a support such as a needle or silicon shank to the flexible probes as a temporary insertion shuttle, which is often removed after implantation [9, 63–65]. A recent study used a 10 μm wide carbon fiber as an insertion shuttle for a SU-8 based neural probe and showed minimal glial response [63]. Another approach, and our chosen method, is to coat or support the probe with a degradable polymer that is fully resorbed within hours so that no rigid support remains permanently. Several groups have explored coating probes with biodegradable polymers, including silk [65, 66], poly(D,L-lactide-co-glycolide) (PLGA) [67], poly(ethylene glycol) (PEG) [68] and carboxyl-methylcellulose (CMC) [7] to stiffen the devices for cortical implantation. The biodegradable polymers provide temporary support for the probe to withstand insertion forces and then degrade within the tissue, leaving the probe in place for signal acquisition. However, most reports describe a dip-coating method to coat the probes with the supportive polymers. The coating dimensions were less defined and commonly thicker than what is required for insertion (~200–500 μm) [62, 67]. Dip-coating may also introduce large variabilities in the coating dimensions, as well as increased risk of tissue damage during insertion due to an unnecessarily thick polymer coating. Complications may also be related to extended polymer degradation times and/or non-biocompatible degradation metabolites.
Our group previously reported the biocompatibility of an ultrafast degrading tyrosine-derived polycarbonate E5005(2K) polymer following insertion into rat brain showing minimal astrocyte and microglia activation compared to other degrading polymers such as PLGA and did not show any significant difference in tissue response within randomized implantation sites [69]. When the E5005(2K) polymer was coated onto microwires by dip coating, the tissue response to the microwire showed no significant differences compared to an uncoated control and electrophysiological signal recording capability was demonstrated up to one week after the polymer coating had completely degraded [70]. Building upon this preliminary work, a fabrication process for coating non-functional SU-8 probes with an ultrafast degrading tyrosine-derived polycarbonate (E5005(2K)) was developed [71]. The polymer coating procedure was controlled by molding the polymer within a microchannel structure to ensure consistent dimensions. The coating degraded within 2 h post implantation, without disruption of the SU-8 probes. We also reported the development of a finite element model (FEM) to simulate insertion of coated neural probes of varying dimensions and material properties, and identified several possible coating thicknesses which could be used on probe candidates for successful insertion into the brain tissue, and in vivo characterization [72]. The computational model predicted that, with the E5005(2K) polymer as a coating material, a minimum coating size of 75 μm × 100 μm in cross section would allow 100% of devices to be inserted without buckling or fracture. In this work, we used a more conservative 100 μm × 100 μm minimum coating size to ensure successful probe insertion. We also adapted our fabrication process to develop polymer coated microprobes made from parylene. Parylene has emerged as a promising material for fabricating neural probes due to its: (1) biocompatibility, (2) chemical inertness, and (3) conformal nature (pin-hole free deposition) [73].
The purpose of this study was to perform a parametric study to evaluate the tissue responses to different sized non-functional (lacking electrode traces) probe candidates implanted in a rat model. The effects of different design parameters (probe and polymer coating dimensions) on the acute (72 h) to chronic (up to 24 weeks) glial cell response and neuronal loss around the implantation sites were assessed by the morphological appearance of tissue slices via immunohistochemistry to identify probes sizes and coating dimensions that obviate the glial response to parylene probes. It was hypothesized that a smaller, more flexible intracortical probe would limit gliosis by providing a better mechanical match with surrounding tissue. A secondary investigation was to determine the effect of the coating dimensions on glial cell activation. Results demonstrated that an appropriate sized coating dimension and probe size induced gliosis equivalent to tissue where a blank control consisting of a solid degradable polymer shuttle of the same dimensions was inserted and that the degree of initial insertion trauma can have a direct effect on long term gliosis even when the polymer shuttle degrades rapidly. These results can be used to help guide the design and insertion strategies for flexible probes in order to limit the glial response to functional probes in order to support long term recording fidelity.
2. Materials and methods
2.1. Polymer coated parylene probe fabrication
2.1.1. Polymer preparation.
The tyrosine-derived polycarbonate co-polymer was synthesized according to a previously published procedure [74, 75]. This biodegradable polymer has previously been shown to be biologically benign with a tailorable degradation rate. The effect of resorption and degradation kinetics on surrounding brain tissue in vivo was previously investigated [70]. These polymers showed minimal astrocyte and microglia activation compared to other degrading polymers such as PLGA. The mechanical and chemical properties of this class of polymers depend on the relative molar percentages of three monomers: desaminotyrosyl-tyrosine alkyl ethyl ester (DTE), desaminotyrosyl-tyrosine (DT), and poly(ethylene glycol) (PEG). The naming convention used for the polymer is EXXYY(MW) as previously described [71], which corresponds to poly(DTE-co-XX%DT-co-YY%-(PEGMW carbonate) where XX is the mole percent of DT, YY is the mole percent of the PEG and the MW is the average molecular weight of the PEG. From the available library of polymers in this class, the specific one used for this study was E5005(2K) (E = 1.6 GPa, Tg = 57°C, Mn = 100K). The E5005(2K) exhibited optimal properties as an insertion aid, balancing a high Young’s modulus, required for device implantation, together with a rapid degradation rate, so that the shuttle degrades into natural (tyrosine) or non-toxic (PEG) metabolites which are cleared from the surrounding tissue within a few hours post-insertion [69–71] The E5005(2K) polymer solution was prepared as a 9% w/w solution in anhydrous 1, 4-dioxane (Sigma-Aldrich, St. Louis, MO, USA).
2.1.2. Device fabrication.
Parylene was chosen as the non-functional probe material. Parylene is a flexible, biocompatible USP Class VI biomaterial that has been widely used for various FDA-approved medical devices [76, 77]. To fabricate the probe, a 5 μm thick parylene layer (Labcoter 2 (PDS 2010), Specialty Coating Systems, Indianapolis, IN, USA) was first deposited onto a glass substrate (figure 1). An aluminum masking layer was then deposited using physical vapor deposition (PVD75, Kurt J. Lesker Company, Jefferson Hills, PA, USA) and patterned using a lift off process to define the probe geometry. The unmasked parylene was isotropically etched using oxygen plasma etching (PX-250, March Instruments, Nordson March Corp., Concord, CA, USA) (600 mTorr, 100 W) for 30 min, which was long enough to etch through a ~7.5 μm thick parylene layer and ensure the removal of all of the unmasked parylene layer while defining the probe geometry. The aluminum masking layer was dissolved using aqueous aluminum etchant. The parylene probe was coated with the E5005(2K) degradable polymer according to our previously published procedure [71]. Briefly, a polydimethylsiloxane (PDMS) mold defining the coating dimensions was fabricated by soft lithography and aligned with the parylene probe. The E5005(2K) polymer solution was infused from the molding inlet using the micromolding in capillaries (MIMIC) [78] process, where the polymer solution was introduced into a microchannel reservoir and filled the cavities through capillary action. The polymer solution was refilled three times to ensure a uniform coating. The polymer coated probe was placed in a vacuum oven under −15 inHg vacuum at 50 °C for an hour, and finally in full vacuum (−29.92 inHg) for at least 1 d to ensure complete solvent evaporation. Finally, the PDMS mold was peeled off from the substrate and the probe was lifted off from the substrate using tweezers.
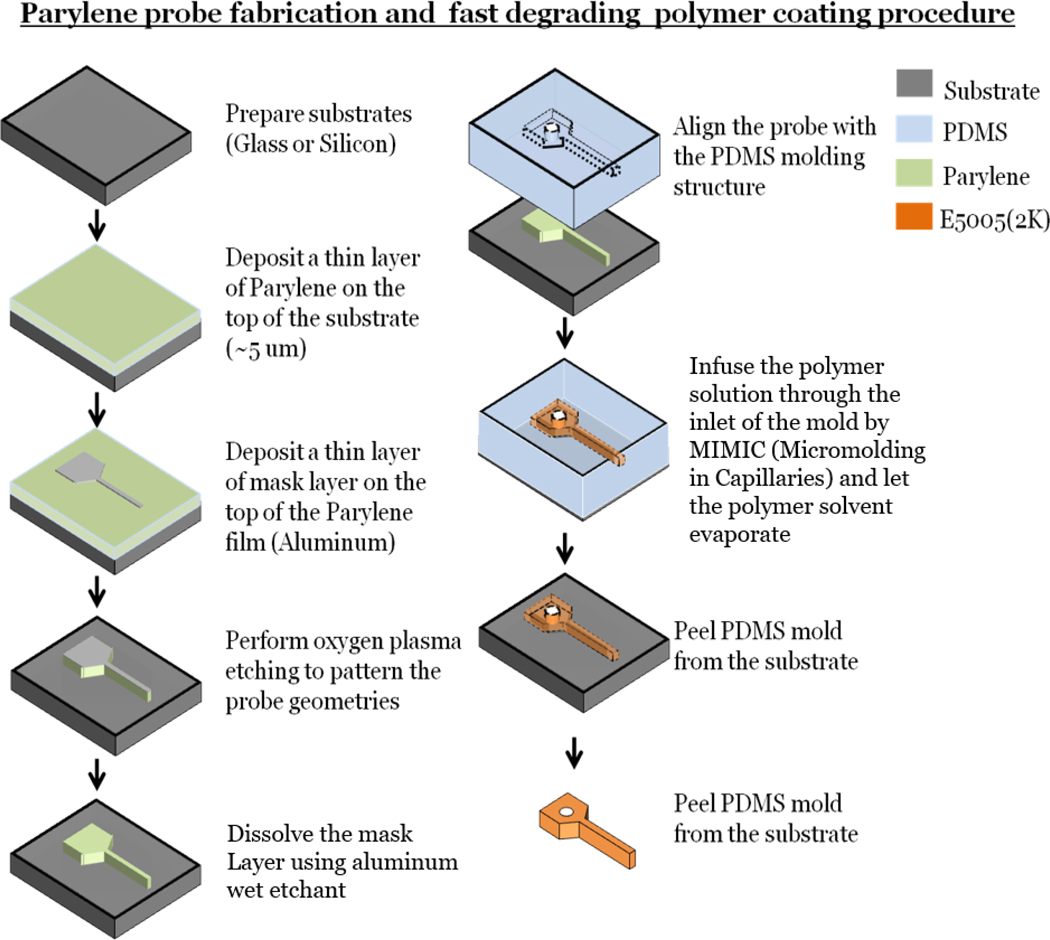
Figure 1.
Parylene probe fabrication protocol, polymer coating procedure. First, a thin layer of parylene was deposited. A thin aluminum mask layer that defined the probe geometry was deposited onto the top of the parylene layer with a lift-off method. An oxygen plasma etch was performed to pattern the probe. The mask layer was dissolved with a chemical etchant to complete probe fabrication. The probe was coated with the polymer through MIMIC, and the whole device was dried and released from the substrate [71].
2.2. In vivo characterization using animal models
2.2.1. Polymer-coated probe candidates selection and preparation.
The goal of this parametric study was to investigate how the size of the probe and of the polymer coating affects gliosis. Five different designs of varying probe and polymer coating sizes were selected for the study (table 1). Smaller (100 μm × 100 μm) versus larger (200 μm × 200 μm) sized polymer coatings were used to encapsulate the same sized probes to determine whether the initial mechanical trauma induced by the polymer coating during insertion plays a role in long term gliosis (even though the polymer coating rapidly degrades). Different sized probes (no probe, 30 μm wide, and 80 μm wide) encapsulated within the same polymer coating size (100 μm × 100 μm) were used to determine how cells reacted to the different sized probes after receiving the same insertion trauma. Finally, the probe dimensions of one group (Group 5—320 μm wide) were chosen as a positive control because they matched the width of several probes reported in the literature [9, 62, 79]. A total of ten probes (two from each group) were simultaneously implanted in each animal so that n = 6 of each probe type were tested in n = 3 animals at each timepoint. The probes were fixed to a custom 3D-printed surgical holder using epoxy glue to create a single ten-probe assembly. Probes were each spaced ~2 mm apart from each other, to prevent overlapping tissue response interference. The probe assemblies were treated with UV light (30 min per side) to minimize the risk of infection following implantation. They were then vacuum sealed and stored in a freezer at −20 °C until use. Each device was thawed under vacuum for 15 min prior to implantation to prevent moisture from condensation being absorbed by and damaging the polymer coating.
Table 1.
Polymer coated parylene probe candidates.
| Group | Parylene probe dimensions (width × thickness × length) | Polymer coating dimension (width × thickness × length) | Description |
|---|---|---|---|
| 1 | 30 μm × 5 μm × 3.5 mm | 100 μm × 100 μm × 4 mm | Small probe |
| Small coating | |||
| 2 | 80 μm × 5 μm × 3.5 mm | 100 μm × 100 μm × 4 mm | Large probe |
| Small coating | |||
| 3 | 30 μm × 5 μm × 3.5 mm | 200 μm × 200 μm × 4 mm | Small probe |
| Large coating | |||
| 4 | None | 100 μm × 100 μm × 4 mm | Negative control |
| Small coating only | |||
| 5 | 320 μm × 5 μm × 3.5 mm | 350 μm × 100 μm × 4 mm | Positive control |
| Similar dimensions to those found in literature [9, 62, 79] |
2.2.2. Surgical procedure.
All surgical procedures were performed under a protocol approved by Rutgers’ Institutional Animal Care and Use Committee (IACUC). A total of eighteen animals (male Sprague-Dawley rats; Charles River Labs, Wilmington, MA, USA) were used for the study. Each animal was first anesthetized with 5% isoflurane gas, followed by intraperitoneal ketamine (100 mg ml−1) and xylazine (10 mg ml−1). The animal’s head was shaved from between the eyes to behind the ears and then fixed in a stereotaxic station. A sagittal incision was made over the midline of the scalp. Bregma and lambda were identified as reference points. Four screws were placed (two on each side, 4 mm from the sagittal suture: the first two 8mm anterior to bregma and two 4 mm posterior to bregma) to act as anchors for the head stage. Two craniotomies (dimensions shown in figure 2(A)) were drilled 1.5 mm from the midline on each side, anterior to bregma. These implantation locations were selected due to the brain’s relative homogeneity and flatness in this region aiming to minimize any variability in the brain tissue between probe sites within each animal as much as possible.
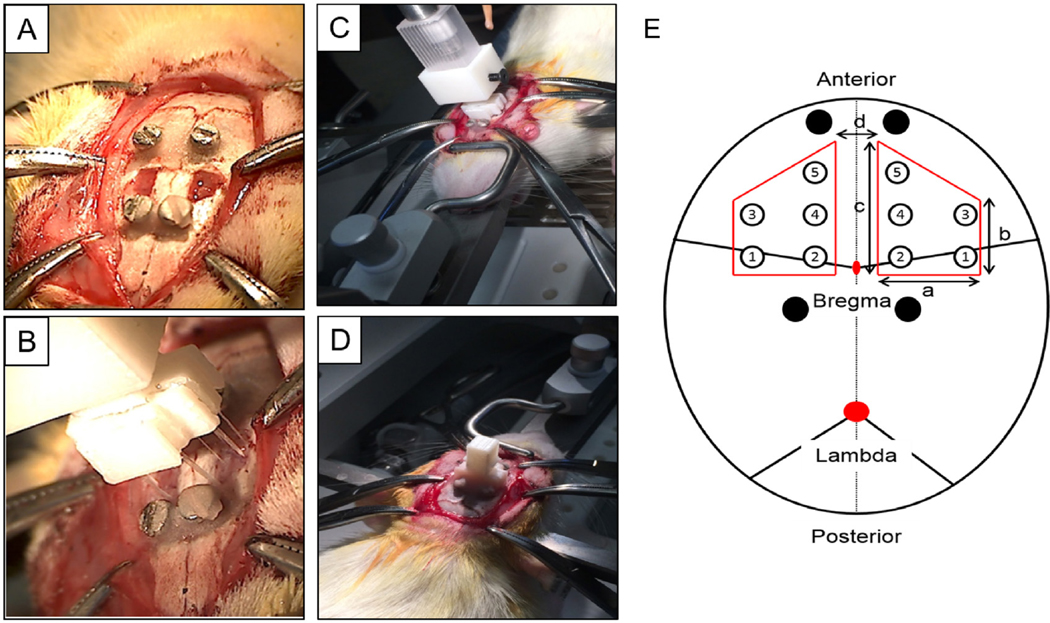
Figure 2.
Surgical procedure for the animal study. (A) Two craniotomies were performed. (B), (C) Two probes from each of the five groups were implanted simultaneously via a custom designed surgical holder. (D) Dental cement was used to fix the surgical holder, and the wound was sutured. The animal was placed on a heating pad until recovery from anesthesia. (E) Location and dimension of the two craniotomies and the ten probes (table 1 for number reference) with different dimensions. Black circle display the location of the four skull screws. A = 4 mm; b = 4 mm; c = 6 mm; d = 3 mm.
The craniotomies were kept moist with saline. To minimize exposure of the humidity-sensitive polymer-coated probes to the ambient environment, a surrogate surgical assembly with ten non-degradable polymer-coated probes was first fixed to the stereotaxic manipulator. The implantation location was then adjusted to align with the craniotomies, and the precise x-y implantation coordinates were defined (figure 2(B)). Dura was removed prior to device implantation. The surrogate surgical assembly was replaced with the definitive probe assembly, and the probes were inserted manually (at ~2 mm min−1) into the brain. Dental cement (Stoelting Co., Wood Dale, IL, USA) was applied to build a headstage, fixing the probe assembly to the calvarium and anchor screws. The scalp was sutured closed around this headstage. The animal was placed on a water-perfused heating pad until recovery from anesthesia.
2.2.3. Tissue processing and immunohistochemistry.
Animals (n = 3) were sacrificed at one of six discrete time points (72 h, 10 d, 3 weeks, 7 weeks, 12 weeks and 24 weeks). The animal was first deeply anesthetized with an overdose of pentobarbital. Once the animal reached the surgical plane of anesthesia, it was perfused transcardially with room temperature phosphate buffered saline (PBS) followed by 4% paraformaldehyde (4 °C) to fix the tissues. The surgical head stage was removed manually along with the flexible probes. The whole brain was extracted from the skull and preserved in 4% paraformaldehyde for 48 h, before transfer into a sucrosesaline cryoprotectant solution until sectioning.
Brain tissue was sectioned in the transverse plane with a cryostat into 30 μm slices. Slices were collected from 1.5 mm, 2 mm, and 2.5 mm deep to the brain’s cranial surface. At each of these levels, immunohistochemistry was performed on three consecutive slices to label various cell types. For the first slice, the primary antibodies used were specific to: (1) astrocytes: polyclonal chicken anti-glial fibrillary acidic protein (GFAP) (Cat#GFAP, dilution 1:500, Aves Lab, Tigard, OR, USA); and (2) endothelial cells: polyclonal rabbit anti-human Von Willebrand Factor, (Cat#A0082, dilution 1:200, Dako North America Inc., Carpinteria, CA, USA). The secondary antibodies used were: (1) goat anti-chicken IgY (H + L) (Alexa 647, Cat#A-21449, Life Technologies Inc, Grand Island, NY, USA); and (2) goat anti-rabbit IgY (H + L) (Alexa 488 Cat#A11008, Life Technologies Inc, Grand Island, NY, USA). Endothelial cell identification was used to assist in distinguishing probe implantation sites from blood vessels of similar size. For the second slice, a polyclonal rabbit anti-NeuN (Cat # ab104225, dilution 1:500, Abcam Inc., Cambridge, MA, USA) specific to neurons was used, followed by a goat anti-rabbit IgY (H + L) secondary antibody (Alexa 488 Cat#A11008, dilution 1:500, Life Technologies Inc, Grand Island, NY, USA). For the third slice, the primary antibody was specific to microglia: polyclonal rabbit anti-Iba-1 (Cat#CTK6675, dilution 1:200, Wako Inc., Richmond, VA). The secondary antibody was goat anti-rabbit IgY (H + L) (Alexa 568 Cat#A11011, Life Technologies Inc, Grand Island, NY, USA). Activated microglia were identified by morphology.
2.2.4. Imaging protocol.
Immunohistological images were acquired by imaging the entire brain slice using a 4× objective in epifluorescence mode (Olympus IX-81, Waltham, MA, USA) with a CCD camera (Hamamatsu Orca, Hamamatsu Photonics K.K, Hamamatsu City, Shizuoka, Japan). Due to the large overall size of the brain slice, full-brain images were obtained by acquiring multiple images across the brain region and digitally stitching these into a montage image. A grid was applied over the montage to identify individual implantation sites (figure 3). The grid consisted of ten regions of interest (ROIs), each 1 mm × 1 mm in size, located over the expected probe insertion sites according to the surgical assembly dimensions. Each ROI was then reimaged and analyzed at a higher magnification using a 10× objective to quantify cellular responses around each respective implantation site.
Figure 3.
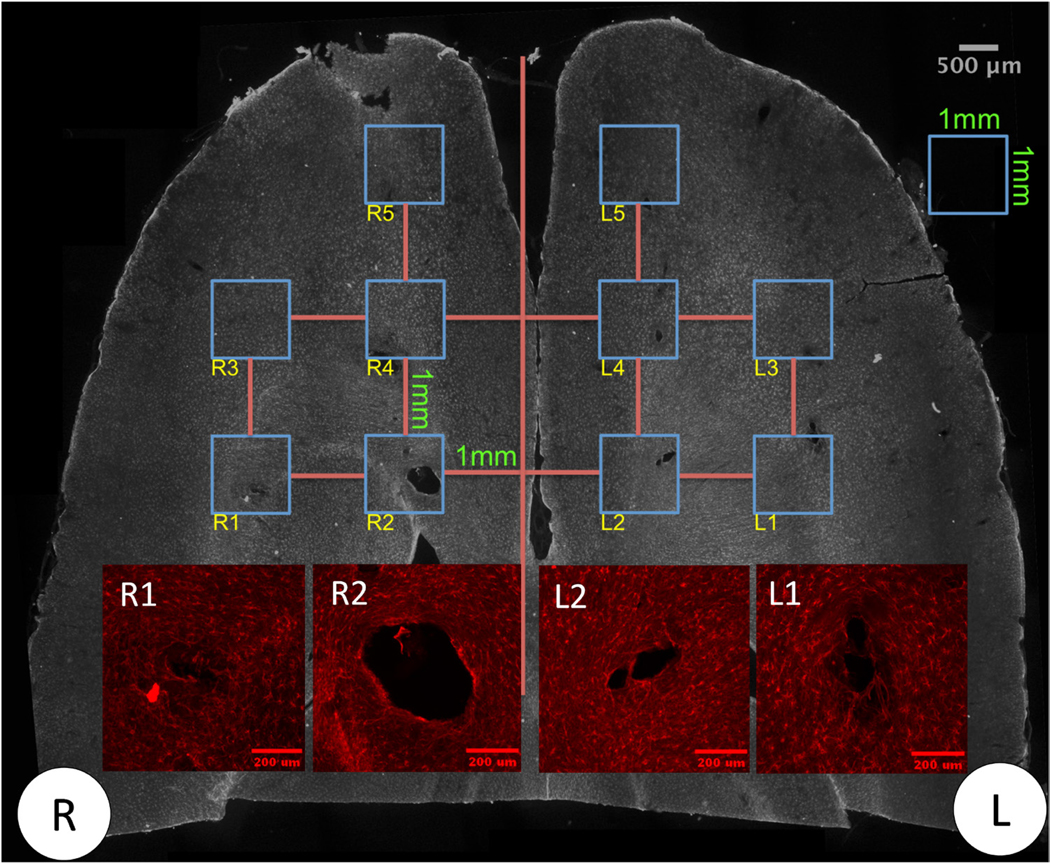
Representative image of (1) a montage of images with a grid for implantation site identification. Scale bar: 500 μm. (2) Inset: magnified images of several implantation sites for downstream image analysis process. Sample ID: 72 h control animal sectioned 2 mm deep from the surface of the brain stained with GFAP (astrocytes). R and L indicate right hemisphere and left hemisphere for the animal. Scale bar: 200 μm.
2.2.5. Image and data analysis.
A Matlab script was developed to perform a rotational intensity sweep profile analysis on individual images of sections from each implantation site (figure 4(A)). The local cell density across the image was correlated to the fluorescence intensity of the labeling antibody at each location. The image was first segmented by thresholding to identify the implantation site. The centroid of the implantation site was identified and set as the starting point for analysis. An end point was defined radially 800 μm from the starting point within an undamaged brain region. The analysis did not exclude the wound sites so that we could also quantify the wound size within each probe group. The end point was selected as a control reference intensity with respect to the implantation sites. Fluorescent label intensity values were obtained along an intensity line trace defined from the starting to the end points. The analysis was repeated in a circular fashion by rotating the line in 1° increments for a full circle sweep. The intensity values were then averaged across the different line traces and normalized to the intensity values of the undamaged area of tissue occurring 600–800 μm from the implantation center, which was normalized to a fluorescence intensity value of 1.
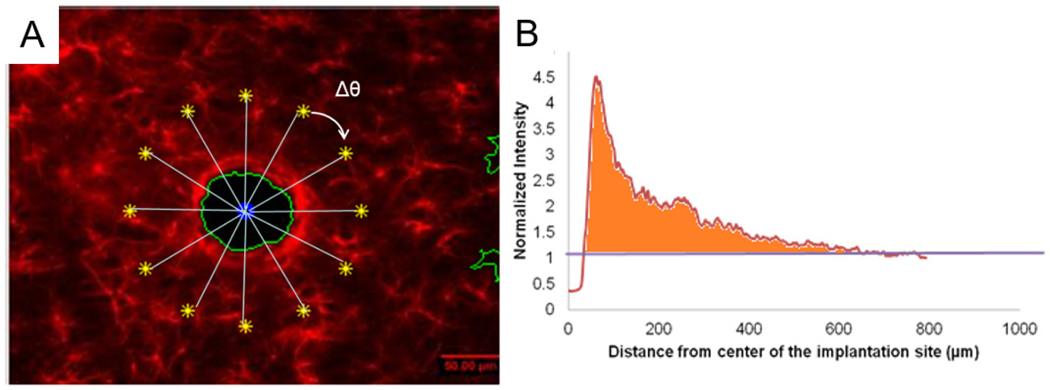
Figure 4.
(A) Schematic of the rotational intensity sweep profile anaysis. (B) Represensative data showing normalized intensity versus distance from the center of the implantation site. The intensity values were normalized by the undamaged area, which was denoted with a value of 1. Area under the curve and above 1 was obtained to indicate the relative image area where the cell density was greater compared to the undamaged area within the region of interest.
This approach allowed the assessment of cell behavior around the implantation site for various probe and coating sizes with respect to the distance from the center of the implantation site. The data were further processed to obtain a single representative intensity value for comparison among different probe groups, animals, and time points. This representative value was obtained by calculating the total area under the curve when the normalized fluorescence intensity value was above the normalized value of 1, which was indicative of the relative image area where the cell density was comparably greater compared to the undamaged area within the ROI (i.e. an indication of cellular accumulation around the implantation site) (figure 4(B)). In this manner, the implantation site normalized intensity value was always lower than 1 and excluded from the analysis. One way analysis of variance (ANOVA) was performed to evaluate if there were significant differences (P < 0.05) among probe candidates at a specific time point. For time points that were identified as significant by the omnibus ANOVA, pairwise comparisons with Tukey’s post hoc test (P < 0.05) were used to identify the devices that were significantly different from each other at that specific time point.
3. Results
3.1. Glial response—astrocyte
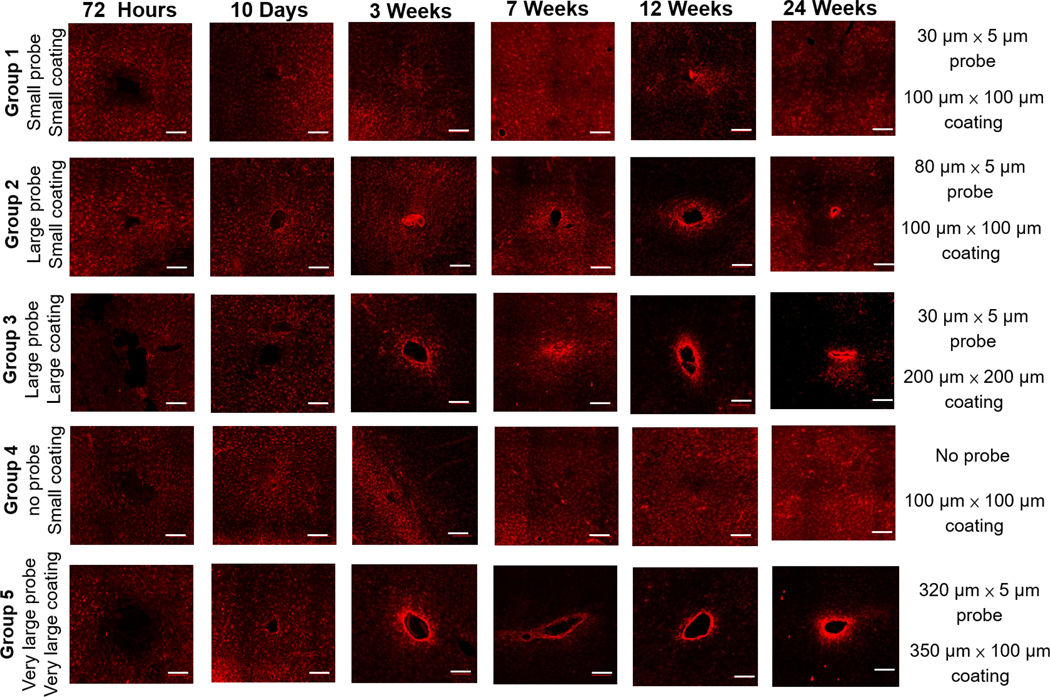
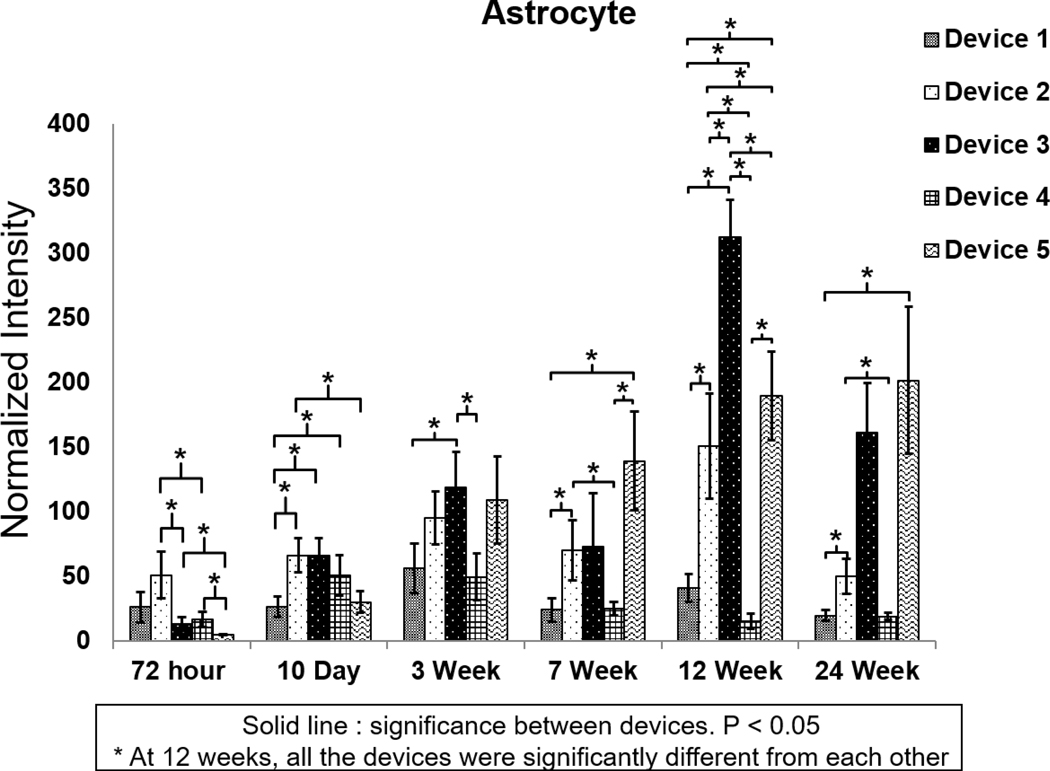
Figure 5 displays representative images of GFAP immune-fluorescence for each of the five probe candidates at each time point. Figure 6(A) shows the normalized staining intensity and statistical comparisons across different groups at various time points to examine how varying device size and coating dimensions affects glial scar formation. Figures 6(B) and (C) show the average intensity of immunolabeled astrocytes from the center of the implantation sites for all five probe groups at two different time points: 72 h and 24 weeks, respectively. At the 72 h time point, the GFAP intensity was low across all five groups, indicating minimal acute astrocyte activation. Furthermore, GFAP intensity was lower immediately adjacent to the implantation sites for all the groups compared to the undamaged area (figure 6(B)). This might result from tissue necrosis induced by mechanical trauma during insertion or the prevalence of other cell types near the implantation sites. Ten days post device implantation, the GFAP intensity increased, and the insertion wounds had begun to close for all five groups. The groups with the larger polymer coatings (Group 3— 200 μm × 200 μm coating and Group 5—350 μm × 100 μm coating) exhibited lower astrocyte density around the implantation site than the groups with the smaller polymer coatings (Groups 1, 2 and 4—100 μm × 100 μm coating). The groups with the larger polymer coatings created larger wounds upon insertion, which may have prolonged the wound healing time course compared to the groups with the smaller polymer coatings.
Figure 5.
Representative images of GFAP immunostaining for different devices at different time points. Scale bar: 200 μm.
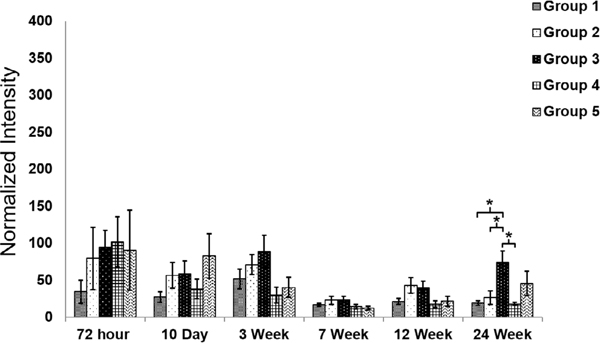
Figure 6.
(A) Normalized GFAP intensity at different time points for the five probe groups. *Significance between different groups (p < 0.05). **Significance between different groups (p < 0.01). ***Significance between different groups (p < 0.001). GFAP intensity over the distance from the center of the implantation site for different groups. (B) 72 h post device implantation. (C) 24 weeks post device implantation. The shaded area represents the standard error for each data point.
At the three-week time point, the astrocyte response started to differentiate among the different groups. A GFAP-positive astrocyte layer formed around the groups with the larger coatings (Group 3—200 × 200 μm coating and Group 5—350 μm × 100 μm coating). There was a statistically significant GFAP intensity difference between Group 1, the small probe (30 μm × 5 μm probe) with the small coating (100 μm × 100 μm coating) and Group 3, the small probe (30 μm × 5 μm probe) with the large coating (200 μm × 200 μm coating), suggesting that the larger polymer coating led to a more severe astrocyte response (figure 6(A)). Between groups with the same coating dimensions (100 μm × 100 μm coating), the larger probe (Group 2—80 μm × 5 μm probe) induced a higher GFAP intensity compared to the smaller probe (Group 1—30 μm × 5 μm probe), but the difference was not statistically significant at this time point.
By week seven, the smaller probes in Group 1 showed significantly lower GFAP staining intensity than the larger probes in Group 2 with the same coating size (figure 6(A)). There was no significant difference in GFAP intensity between Group 1 and Group 4, the sham device with no probe. The GFAP intensity remained significantly higher for the coated probes (Group 2) than the coating alone (Group 4). (figure 6(A)). These results indicate that the overall probe size plays a role in the glial scar response. As such, to limit astrocyte accumulation, probes would optimally be less than 80 μm wide.
At the 12 week time point, the astrocyte response was significantly different among all groups (figures 5 and 6(A)). The GFAP intensity was positively correlated to the probe sizes for Groups 1, 2, and 4, which have the same coating size. The insertion wounds of most devices in Group 4 (polymer coating only with no probe) were healed and could not be identified (figure 5). The GFAP intensity was also positively correlated to the polymer coating size for Groups 1 and 3, which had the same probe size but different coating dimensions. At this time point, the GFAP intensity was the highest for Group 3 (the 30 μm × 5 μm probe with the 200 μm × 200 μm coating) even compared to Group 2 (80 μm × 5 μm probe with the 100 μm × 100 μm coating) and Group 5, the largest probe (320 μm × 5 μm probe) with a large coating (350 μm × 100 μm coating) indicating that the coated device geometry aspect ratio and/or cross sectional area affected gliosis. The cross-sectional geometry for Group 5 was wider but thinner than for Group 3 and therefore may foster wound closure across the smaller dimension. The increased coating cross-sectional area in Group 3 (200 μm × 200 μm coating) may lead to more severe acute tissue damage during insertion, which ultimately translates to a more severe chronic astrocyte activation response. These results suggest that overall polymer coating size (and/or cross sectional area) may exhibit a more significant effect on astrocyte response than the overall probe size, despite the polymer coating dissolving at a much earlier time point.
At the 24 week time point (figure 6(C)), the astrocyte response stabilized and subsided compared to week 12. At this time point, the GFAP intensity for the group with smallest implanted probe (Group 1) was not significantly different than the sham, polymer alone group (Group 4). This indicates that the small, flexible probe attenuated astrocyte activation, and therefore the tissue response was minimized. However, the group with the large, 200 μm × 200 μm coating induced a more severe tissue response comparable to or even more severe than the positive control Group 5.
3.2. Glial response—microglia
Figure 7 displays representative Iba-1 stained images for the five groups at different time points while figure 8 shows the normalized intensity and average intensity traces of immunolabeled microglia from the center of the implantation sites for all five probe groups at 72 h and 24 weeks, respectively. Iba-1 immunolabels both resting and reactive microglia, and morphology was used to identify activated microglia. Quiescent microglia have a highly branched morphology. Upon activation, they begin to proliferate and exhibit an amoeboid morphology [17, 80]. For all of the groups, reactive microglia were observed at the 72 h time point, and a compact layer of microglia was observed around the implantation sites.
Figure 7.
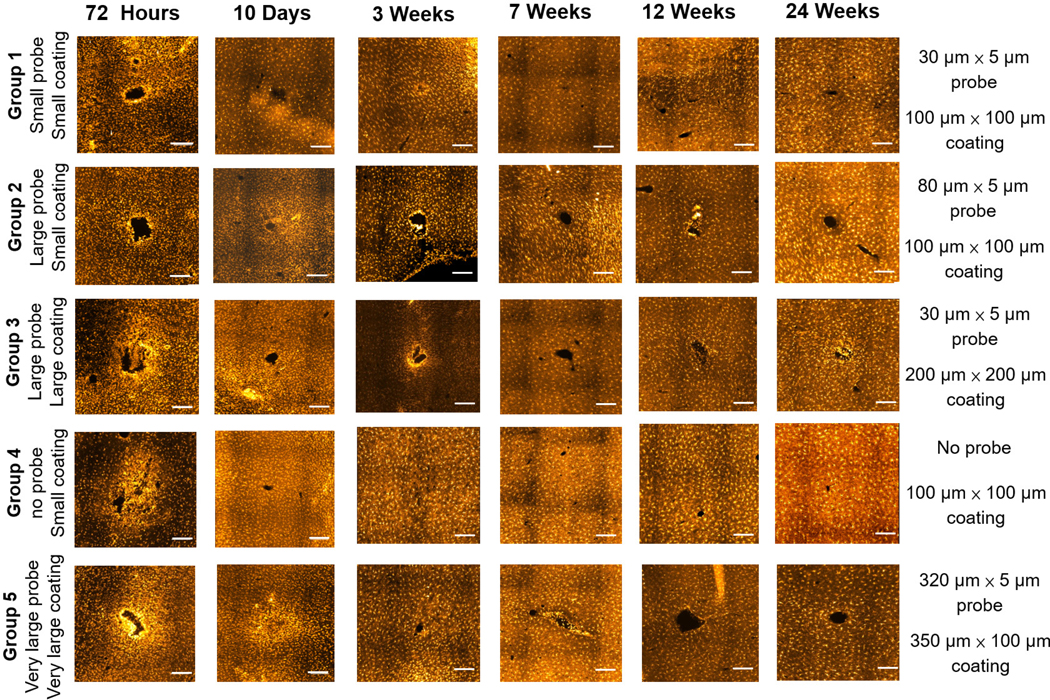
Representative images of Iba-1 immunostaining for different groups at different time points. Scale bar: 200 μm.
Figure 8.
(A) Normalized intensity of Iba-1 staining at different time points for the five probe groups. **Significance between different groups (p < 0.01), Iba-1 intensity over the distance from the center of the implantation site for different groups. (B) 72 h post device implantation. (C) 24 weeks post device implantation. The shaded area represents the standard error for each data point.
The microglial response stabilized 10 d post device implantation. Reactive microglia with an amoeboid morphology were still observed closely packed around the implantation sites, but the overall intensity decreased compared with the intensity after 72 h (figures 7 and 8(A)). At the three-week time point, the Iba-1 intensity increased for all groups except the pure polymer sham negative control (Group 4). Thus, the acute microglia response induced by insertion trauma was resolved by this three-week time point and had transitioned to a chronic microglial neuro-inflammation response observed in the groups with an encapsulated probe. The time course of microglia activation observed in this study aligns with results reported from several other studies [10, 15, 18].
At the seven and 12 week time points, the chronic response of microglia had decreased and stabilized in all five groups. At the 24 week time point, the microglial response for the groups with the 200 μm × 200 μm coating (Group 3) and the 320 μm × 5 μm probe (Group 5) were more severe when compared to the rest of the groups. These results aligned with the astrocyte response observed for the larger probe or coating sizes. Even though the glial scar response consists mainly of astrocytes, microglia have also been observed to reside within the region of gliosis.
3.3. Neuronal response
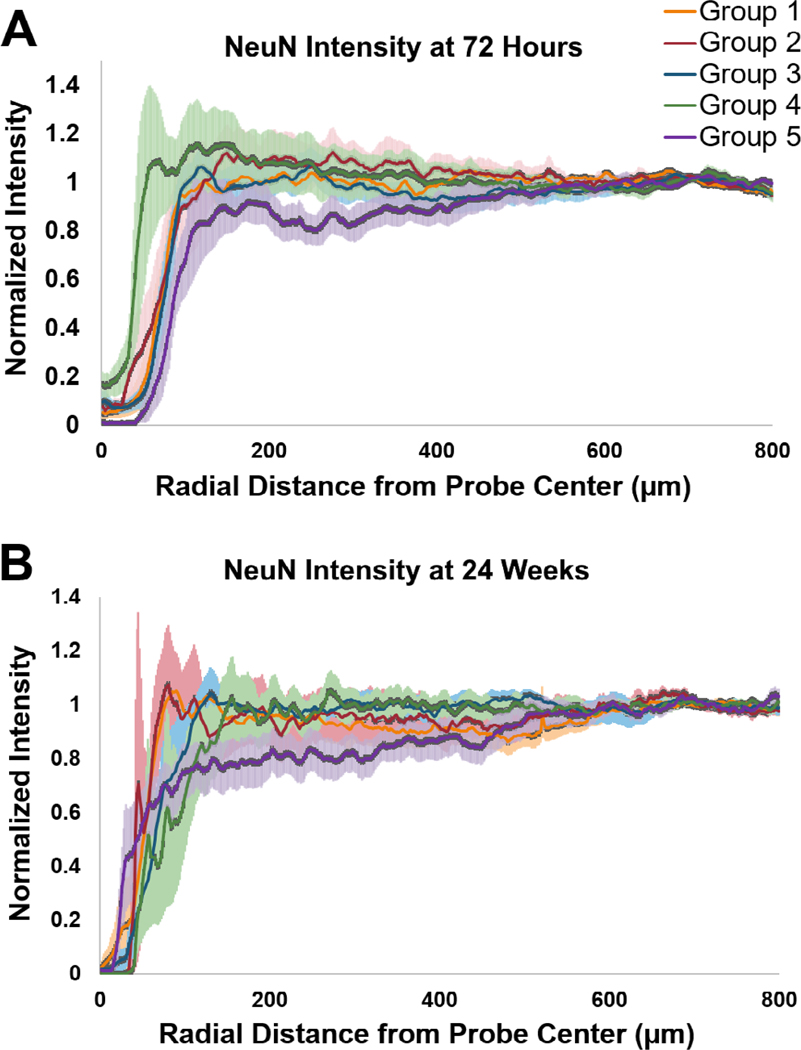
Figure 9 displays the intensity of immunolabeled neurons over the distance from the center of the implantation sites for all five different groups at two different time points: 72 h and 24 weeks. At the 72 h time point, the 320 μm × 5 μm probe (Group 5) suffered approximately 20% neuron loss up to 400 μm from the center of the implantation site. This might result from the larger wound size as well as the more severe acute glial cell activation, leaving less space for neurons close to the implantation site. The pure polymer sham negative control (Group 4) exhibited both a smaller wound size and the lowest degree of neuronal loss. Regardless, there was no statistically significant difference in terms of the percentage of neuronal loss among all five groups. At the 24 week time point, the wound size became smaller for each of the probe groups and neurons were found to be located within 50–100 μm of the center of the implantation site. The wound size for the smaller 100 μm × 100 μm coating groups (Groups 1 and 2) were smaller compared to the larger 200 μm × 200 μm and 350 μm × 100 μm coating groups (Groups 3 and 5, respectively). The largest 320 μm × 5 μm probe (Group 5) resulted in about 10–20% neuron loss up to 500 μm from the center of the implantation site. The rest of the probe groups did not exhibit significant neuronal loss (<10%) around the implantation sites.
Figure 9.
NeuN intensity over the distance from the center of the implantation site for different groups. (A) 72 h post device implantation. (B) 24 weeks post device implantation. The shaded area represents the standard error for each data point.
4. Discussion
Five device candidates of varying probe and polymer coating sizes were evaluated at six different time points ranging from acute (72 h) to chronic (24 weeks), to determine the effects on the development of the glial scar. The biocompatibility of the polymer used for this work was previously studied [70, 74, 81, 82]. In this study, the size of the polymer coating was shown to affect the glial scar, but there was no statistically significant difference in neuronal loss observed around the implantation sites for any of the probe groups.
We previously characterized the polymer coating procedure and degradation profile and demonstrated that the coating degraded within 2 h post implantation in an in vitro model [71]. Therefore, it was expected that the polymer coating would only affect the short term glial response, and that the probe size would play the primary role in chronic response. When a coating size of 100 μm × 100 μm was used, the larger probes (80 μm wide × 5 μm thick) induced a significantly more severe glial response than smaller probes (30 μm wide × 5 μm thick) at 7, 12, and 24 week time points. These data support the hypothesis that a smaller and more flexible probe limits gliosis. The larger sized probe is calculated to have ~2.67 times the bending stiffness of the smaller probe, which may produce an appreciable increase in interfacial strain due to device micromotion. These results are consistent with many other reports in the literature [44, 45, 83, 84]. Furthermore, the degree of astrocyte activation induced by the small probe was of comparable intensity to that from the smaller pure polymer shank across all time points. Based on these data, we identified the 30 μm wide × 5 μm thick parylene probe with a 100 μm wide × 100 μm thick coating as a threshold design to limit the glial scarring, which may improve a device’s signal recording consistency.
However, probes of this size and smaller can severely limit the recording potential of the probe. Most conventional neural probes have recording impedances of around 1 MΩ at a 1 kHz recording frequency [85–87]. Previous studies have shown that to achieve this impedance, a recording electrode area of 400–600 μm2 is typically required. The width of the recording window is usually about ~20 μm diameter. Therefore, a 30 μm wide probe would be the minimum size to support such a recording site. These smaller probes would also limit the number of recording electrodes that can be incorporated within the device. Fewer electrodes allow for less signal acquisition. Thus, probe design requires a balance of signal recording consistency and efficiency, and probes between the 30 and 80 μm widths may need to be investigated to optimally achieve this balance. Coating electrodes with conductive polymers such as poly(3,4-ethylenedioxythiophene) (PEDOT) and poly(pyrrole) (PPy) can also help improve the electrode-cell interface and lower electrical impedance due to their high charge injection capacity, compliant mechanical properties compared to brain tissue, and biocompatibility which may allow smaller electrodes to be used [88].
It was also noted that, for the same sized probe (30 μm × 5 μm), the larger polymer coating (200 μm × 200 μm) induced a more severe chronic astrocyte response than the smaller polymer coating (100 μm × 100 μm). This was a somewhat unexpected result since the coating dimensions are still smaller than other coating strategies such as dip coating, or other insertion strategies such as using a needle or other retractable solid support. It was expected that the coating would resorb within a few hours, and that the influence of the coating dimensions would diminish following wound closure. However, it appears that the more severe mechanical trauma produced by the larger coating let to a sustained increase in the associated acute glial reactivity. These results from the chronic time points indicate that even though the polymer coating is only a few hundred microns in size and degrades within hours, it can also affect the long-term astrocyte response. It appears that if the initial mechanical trauma is too severe, the size and shape of the insertion shuttle can have an impact on the wound healing and glial response, perhaps though disruption of blood vessels and the blood-brain barrier. The increased damage to the microvasculature may sensitize the tissue towards a foreign body insult. Additionally, a larger wound may not be able to heal and close as effectively as a smaller wound and therefore leads to a more severe long term glial response. A similar phenomenon was reported by Kozai et al in which a biodegradable carboxymethyl cellulose (CMC) was used as the insertion shuttle [7]. The wound sites for the majority of the larger CMC shuttles (300 μm × 125 μm) never closed and induced a severe astrocyte response, which aligns with our findings and demonstrates the importance of minimizing primary trauma.
We also observed that the microglia response diminished around seven weeks, and that there were no significant differences in microglia activation among the various groups. Microglia are expected to be the main glial cell type residing around implantation sites during the acute tissue response phase. The mechanical insertion trauma activates microglia to phagocytose foreign matter and release inflammatory factors to aid in injury recovery [89–91]. We hypothesize that once the polymer coating was resorbed, the microglia that had initially been activated reverted to a more quiescent phenotype (by week seven). Interestingly, a secondary microglia response was observed at the 24 week time point with Groups 3 and 5, which had larger coatings as well as a parylene probe. The astrocyte cell population is the main component of the long term glial scar layer. However, several studies have also shown that the continued presence of a probe could also induce microglia activation at longer time points [15, 14, 92]. Activated microglia that remain around the implantation site would attempt to phagocytose the foreign material completely.
The initial insertion trauma may also explain the variability in the observed glial cell responses within animals in the same group. Although we implanted the probes in the same anatomical regions, the local brain microvasculature structure will vary from animal to animal. If a probe happens to shear a larger blood vessel during insertion, it may lead to a more severe tissue reaction even for a smaller probe or implant size. Larger sized coatings are, of course, expected to disrupt more blood vessels during insertion which could induce a more severe tissue response.
We also evaluated the neuronal loss around the implantation sites for all five groups at the short term (72 h) and long term (24 week) time points. Only one group (Group 5, 320 μm × 5 μm probe with the 350 μm × 100 μm coating) demonstrated appreciable neuronal loss (~20%) at both time points. These results are noteworthy as neuronal health is believed to be one of the most essential factors for consistent long term signal acquisition. Although there is evidence that the glial scar can play a beneficial role in encouraging axon regeneration following spinal cord injury [93], there is a general consensus in the literature that, for applications with neural recordings, the glial scar could disturb the cellular environment and adversely affect neuronal health or isolate the probe from adjacent neurons through the glial sheath layer [22, 23]. The minimal neuronal loss in this work suggests that functional probes of similar materials and dimensions may still be able to acquire neuronal signals up to 24 weeks post device implantation. The current study only evaluated the glial and neuronal response of non-functional probes with the focus on the material and the sizes of the polymer coating and probes. Future work will evaluate the recording capability of functional probes to further investigate the correlation between glial scar formation and electrode performance.
There are some limitations in the study that may affect the results and thus the conclusions. The overall purpose of this work was to parametrically study probe and coating size to identify combinations that limit the glial response as a design tool for further development of functional probes. In this study, all probes were spaced a minimum of 2 mm apart to prevent any contiguous or overlapping tissue responses from adjacent probes. This can be seen in figures 6(B), (C), 8(B), (C) and 9, where the normalized staining intensity is always approximately 1 from 800 μm from the center of the implantation site outward. The probe groups were always implanted at the same location to aid in identifying the implantation sites for each group. This lack of randomization may affect the glial response due to spatial differences in tissue architecture. However, in our previously study by Lewitus et al, when the sites were randomized, no significant differences in tissue response were observed [69]. Further, as seen in figure 2(B), Group 2 (80 μm × 5 μm probe, 100 μm × 100 μm coating) and Group 3 (30 μm × 5 μm probe, 200 μm × 200 μm coating), which displayed the greatest differences in gliosis were always placed adjacent to Group 1 (30 μm × 5 μm probe, 100 μm × 100 μm coating) either medially or anteriorly. Thus, the tissue architecture was not expected to vary dramatically between adjacent probes to account for the differences in glial responses observed. Finally, the location of the 4 mm wide coronal strip of brain chosen for implantation was selected due to the relative homogeneity and flatness of the cortex in this region (just anterior to bregma). In future studies, we can decrease the number of the probes implanted in each rat and randomize the implantation sites to adjust for the biological difference within the brain.
Another complication arises from the limited number of probes implanted in each animal due to the sparse probe spacing limiting the number of probes, which could be implanted at a time to ten. Since each probe group was implanted in duplicate in each animal, we chose five experimental probe/coating groups to explore how the probe and coating dimensions affect gliosis including a pure polymer sham control and a large probe group with dimensions comparable to other published reports. Thus, there is no internal control for the 200 μm × 200 μm coating size and its influence on gliosis. However, a preliminary experiment conducted showed that the glial isolation layer around a pure 200 μm × 200 μm E5005(2K) fast-degrading polymer was minimal four weeks post device implantation (data not shown), and the previous work by Lewitus et al [69], a 180 μm cast filament was inserted into rat brain after four weeks showed a glial response comparable to a control craniotomy site (prepped but no implantation conducted). It has been suggested that the polymer degradation products may also influence the glial response. This could be tested by comparing the glial response of an implanted polymer coating of various sizes against a control ‘stab’ wound where the filament is immediately removed following insertion as was conducted by Potter et al, using silicon probes [94].
In this work, we demonstrated a method to mechanically augment flexible probes during implantation using an ultra-fast degrading tyrosine-derived polycarbonate. This polymer may be used to coat any neural probe design and does not require any modification of the probe fabrication process or design. Although the polymer coating size was significantly larger than the probe size, our results showed that a pure 100 μm × 100 μm coating exhibited minimal glial response and neural loss and therefore could still be an ideal coating size. Moreover, the tyrosine-derived polycarbonate was purely to aid in device insertion. The polymer could be further expanded to incorporate with (1) anti-inflammatory drugs proposed in the literature such as Dexamethasone to help stabilize the wound sites for better wound healing outcomes [26, 31] or neural cell adhesion molecules, such as L1 [33, 34, 95] to help better neuron attachment on the probe for consistent signal acquisition. One advantage of the tyrosine-derived polycarbonate over other polymer candidates such as silk [65, 66] or PEG [68] is its highly tunable degradation properties [82, 96]. Therefore, the release of the drugs or biological molecules can be precisely controlled as desired to maximize the effects over an extended period of time.
5. Conclusions
We have developed small and flexible neural probes, coated with an ultrafast degrading polymer as a temporary aid to insertion into the brain. The effects of different device parameters on glial and neuronal cell response progression were studied over an extended time course using non-functional probes. Our results support the idea that mechanical trauma from device implantation can affect the long-term tissue response. We also showed that the use of the rapidly degrading E5005(2K) polymer as an insertion shuttle could attenuate glial response while remaining amenable to insertion. The tyrosine-derived polycarbonate could also be an ideal carrier as a shuttle to deliver therapeutics or biological molecules to promote wound healing or improve the tissue-electrode interface with highly tunable degradation properties. Finally, we have also observed that the glial response is not well-correlated to neuronal loss around the implantation sites. Future work will investigate the recording performance of the probe and its relationship with both glial scarring and neuronal response.
Significance.
These results suggest that: (1) the degree of mechanical trauma at device implantation and mechanical mismatches at the probe-tissue interface affect long term gliosis; (2) smaller, more flexible probes may minimize the glial response to provide improved tissue biocompatibility when used for chronic neural signal recording; and (3) some degree of glial scarring did not significantly affect neuronal distribution around the probe.
Acknowledgments
This work was supported by the New Jersey Commission on Spinal Cord Research (NJCSCR) under the grant numbers CSCR12IRG001 and CSCR16IRG007. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the NJCSCR.
References
- [1].Schwartz AB, Cui XT, Weber DJ and Moran DW 2006. Brain-controlled interfaces: movement restoration with neural prosthetics Neuron 52 205–20 [DOI] [PubMed] [Google Scholar]
- [2].Daly JJ and Wolpaw JR 2008. Brain-computer interfaces in neurological rehabilitation Lancet. Neurol 7 1032–43 [DOI] [PubMed] [Google Scholar]
- [3].Kozai TDY et al. 2015. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays J. Neurosci. Methods 242 15–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fernandez E et al. 2014. Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects Front. Neuroeng 7 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gunasekera B, Saxena T, Bellamkonda R and Karumbaiah L 2015. Intracortical recording interfaces: current challenges to chronic recording function ACS Chem. Neurosci 6 68–83 [DOI] [PubMed] [Google Scholar]
- [6].Fekete Z 2015. Recent advances in silicon-based neural microelectrodes and microsystems: a review Sensors Actuators B 215 300–15 [Google Scholar]
- [7].Kozai TDY et al. 2014. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes Biomaterials 35 9255–68 [DOI] [PubMed] [Google Scholar]
- [8].Polikov VS, Tresco PA and Reichert WM 2005. Response of brain tissue to chronically implanted neural electrodes J. Neurosci. Methods 148 1–18 [DOI] [PubMed] [Google Scholar]
- [9].Kim BJ. et al. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013;10 doi: 10.1088/1741-2560/10/4/045002. 045002. [DOI] [PubMed] [Google Scholar]
- [10].Turner JN et al. 1999. Cerebral astrocyte response to micromachined silicon implants Exp. Neurol 156 33–49 [DOI] [PubMed] [Google Scholar]
- [11].Saxena T et al. 2013. The impact of chronic blood-brain barrier breach on intracortical electrode function Biomaterials 34 4703–13 [DOI] [PubMed] [Google Scholar]
- [12].Fujita T, Yoshimine T, Maruno M and Hayakawa T 1998. Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex Acta Neurochir. 140 275–9 [DOI] [PubMed] [Google Scholar]
- [13].Leskovar A, Moriarty L, Turek J, Schoenlein I and Borgens R 2000. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems J. Exp. Biol 203 1783–95 [DOI] [PubMed] [Google Scholar]
- [14].Biran R, Martin DC and Tresco PA 2005. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays Exp. Neurol 195 115–26 [DOI] [PubMed] [Google Scholar]
- [15].Szarowski DH et al. 2003. Brain responses to micro-machined silicon devices Brain Res. 983 23–35 [DOI] [PubMed] [Google Scholar]
- [16].Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC and Cui XT 2016. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo Biomaterials 87 157–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nimmerjahn A, Kirchhoff F and Helmchen F 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo Science 308 1314–8 [DOI] [PubMed] [Google Scholar]
- [18].Giordana MT et al. 1994. Reactive cell proliferation and microglia following injury to the rat brain Neuropathol. Appl. Neurobiol 20 163–74 [DOI] [PubMed] [Google Scholar]
- [19].Vetter RJ, Williams JC, Hetke JF, Nunamaker EA and Kipke DR 2004. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex IEEE Trans. Biomed. Eng 51 896–904 [DOI] [PubMed] [Google Scholar]
- [20].Rousche PJ and Normann RA 1998. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex J. Neurosci. Methods 82 1–15 [DOI] [PubMed] [Google Scholar]
- [21].Edell DJ, Toi VV, McNeil VM and Clark LD 1992. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex Biomed. Eng. IEEE Trans 39 635–43 [DOI] [PubMed] [Google Scholar]
- [22].Mercanzini A, Colin P, Bensadoun JC, Bertsch A and Renaud P 2009. In vivo electrical impedance spectroscopy of tissue reaction to microelectrode arrays IEEE Trans. Biomed. Eng 56 1909–18 [DOI] [PubMed] [Google Scholar]
- [23].Williams JC, Hippensteel JA, Dilgen J, Shain W and Kipke DR 2007. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants J. Neural Eng 4 410–23 [DOI] [PubMed] [Google Scholar]
- [24].Williams JC, Rennaker RL and Kipke DR 1999. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex Brain Res. Protoc 4 303–13 [DOI] [PubMed] [Google Scholar]
- [25].Nicolelis MAL et al. 2003. Chronic, multisite, multielectrode recordings in macaque monkeys Proc. Natl Acad. Sci. USA 100 11041–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhong Y and Bellamkonda RV 2007. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes Brain Res. 1148 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abidian MR, Kim D-H and Martin DC 2006. Conducting-polymer nanotubes for controlled drug release Adv. Mater 18 405–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spataro L et al. 2005. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex Exp. Neurol 194 289–300 [DOI] [PubMed] [Google Scholar]
- [29].Kim D-H and Martin DC 2006. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery Biomaterials 27 3031–7 [DOI] [PubMed] [Google Scholar]
- [30].Lee CD et al. 2016. Matrigel coatings for Parylene sheath neural probes J. Biomed. Mater. Res. B 104 357–68 [DOI] [PubMed] [Google Scholar]
- [31].Boehler C et al. 2017. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study Biomaterials 129 176–87 [DOI] [PubMed] [Google Scholar]
- [32].Purcell EK, Thompson DE, Ludwig KA and Kipke DR 2009. Flavopiridol reduces the impedance of neural prostheses in vivo without affecting recording quality J. Neurosci. Methods 183 149–57 [DOI] [PubMed] [Google Scholar]
- [33].Azemi E, Lagenaur CF and Cui XT 2011. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface Biomaterials 32 681–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kolarcik CL et al. 2012. In vivo effects of L1 coating on inflammation and neuronal health at the electrode–tissue interface in rat spinal cord and dorsal root ganglion Acta Biomater. 8 3561–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bareket-Keren L and Hanein Y 2013. Carbon nanotube-based multi electrode arrays for neuronal interfacing: progress and prospects Front. Neural Circuits 6 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Keefer EW, Botterman BR, Romero MI, Rossi AF and Gross GW 2008. Carbon nanotube coating improves neuronal recordings Nat. Nanotechnol 3 434–9 [DOI] [PubMed] [Google Scholar]
- [37].Fang S-P et al. 2016. A carbon nanofiber (CNF) based 3D microelectrode array for in vitro neural proliferation and signal recording 2016 IEEE 29th Int. Conf. on Micro Electro Mechanical Systems (IEEE; ) pp 423–6 ( 10.1109/MEMSYS.2016.7421651) [DOI] [Google Scholar]
- [38].Abidian MR, Corey JM, Kipke DR and Martin DC 2010. Conducting-polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes Small 6 421–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vitale F, Summerson SR, Aazhang B, Kemere C and Pasquali M 2015. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes ACS Nano 9 4465–74 [DOI] [PubMed] [Google Scholar]
- [40].Kozai T et al. 2015. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings IEEE Trans. Biomed. Eng 63 111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kang M et al. 2014. Subcellular neural probes from single-crystal gold nanowires ACS Nano 8 8182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Seymour JP and Kipke DR 2007. Neural probe design for reduced tissue encapsulation in CNS Biomaterials 28 3594–607 [DOI] [PubMed] [Google Scholar]
- [43].Kozai TDY et al. 2012. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces Nat. Mater 11 1065–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Harrison DG et al. 2006. Endothelial mechanotransduction, nitric oxide and vascular inflammation J. Intern. Med 259 351–63 [DOI] [PubMed] [Google Scholar]
- [45].Lund T et al. 2010. Shear stress regulates inflammatory and thrombogenic gene transcripts in cultured human endothelial progenitor cells Thromb. Haemost 104 582–91 [DOI] [PubMed] [Google Scholar]
- [46].Uhlig S 2002. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am. J. Physiol. Lung Cell. Mol. Physiol 282 L892–6 [DOI] [PubMed] [Google Scholar]
- [47].Altuna A et al. 2012. SU-8 based microprobes with integrated planar electrodes for enhanced neural depth recording Biosens. Bioelectron 37 1–5 [DOI] [PubMed] [Google Scholar]
- [48].Cho S-H et al. 2008. Biocompatible SU-8-based microprobes for recording neural spike signals from regenerated peripheral nerve fibers IEEE Sens. J 8 1830–6 [Google Scholar]
- [49].Fukushima M et al. 2014. An electrocorticographic electrode array for simultaneous recording from medial, lateral, and intrasulcal surface of the cortex in macaque monkeys J. Neurosci. Methods 233 155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K and Suzuki T 2005. Parylene flexible neural probes integrated with microfluidic channels Lab Chip 5 519–23 [DOI] [PubMed] [Google Scholar]
- [51].Zhao Q, Qi HJ and Xie T 2015. Recent progress in shape memory polymer: new behavior, enabling materials, and mechanistic understanding Prog. Polym. Sci ( 10.1016/j.progpolymsci.2015.04.001) [DOI] [Google Scholar]
- [52].Jorfi M et al. 2015. Mechanically adaptive materials for intracortical implants 2015 7th Int. IEEE/EMBS Conf. on Neural Engineering (IEEE; ) pp 601–2 ( 10.1109/NER.2015.7146694) [DOI] [Google Scholar]
- [53].Hess-Dunning AE et al. 2014. Microscale characterization of a mechanically adaptive polymer nanocomposite with cotton-derived cellulose nanocrystals for implantable BioMEMS J. Microelectromech. Syst 23 774–84 [Google Scholar]
- [54].Nguyen JK et al. 2016. Influence of resveratrol release on the tissue response to mechanically adaptive cortical implants Acta Biomater. 29 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stieglitz T, Beutel H, Blau C and Meyer JU 1997. Flexible multichannel microelectrodes with integrated leads for use in neuroprosthetics Biomed. Technol 42 449–50 [DOI] [PubMed] [Google Scholar]
- [56].Takeuchi S and Shimoyama I 2000. Three-dimensional shape memory alloy microelectrode with clipping structure for insect neural recording J. Microelectromech. Syst 9 24–31 [Google Scholar]
- [57].Hoogerwerf AC and Wise KD 1994. A three-dimensional microelectrode array for chronic neural recording IEEE Trans. Biomed. Eng 41 1136–46 [DOI] [PubMed] [Google Scholar]
- [58].Sridharan A, Nguyen JK, Capadona JR and Muthuswamy J 2015. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo J. Neural Eng 12 036002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Barz F, Ruther P, Takeuchi S and Paul O 2015. Flexible silicon-polymer neural probe rigidified by dissolvable insertion vehicle for high-resolution neural recording with improved duration 2015 28th IEEE Int. Conf. on Micro Electro Mechanical Systems (IEEE; ) pp 636–9 ( 10.1109/MEMSYS.2015.7051036) [DOI] [Google Scholar]
- [60].Fan B, Rechenberg R, Becker MF and Li W 2015. Fabrication of polycrystalline diamond on a flexible Parylene substrate 2015 Transducers—2015 18th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) pp 892–5 (IEEE; ) ( 10.1109/TRANSDUCERS.2015.7181067) [DOI] [Google Scholar]
- [61].Schander A et al. 2015. Design and fabrication of multi-contact flexible silicon probes for intracortical floating implantation 2015 Transducers—2015 18th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) (IEEE; ) pp 1739–42 ( 10.1109/TRANSDUCERS.2015.7181281) [DOI] [Google Scholar]
- [62].Felix SH. et al. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 2013 doi: 10.3791/50609. e50609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Luan L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1601966. e1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Köhler P. et al. Influence of probe flexibility and gelatin embedding on neuronal density and glial responses to brain implants. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119340. e0119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hwang S-W et al. 2014. High-performance biodegradable/transient electronics on biodegradable polymers Adv. Mater 26 3905–11 [DOI] [PubMed] [Google Scholar]
- [66].Kundu B et al. 2014. Silk proteins for biomedical applications: bioengineering perspectives Prog. Polym. Sci 39 251–67 [Google Scholar]
- [67].Foley C, Nishimura N, Neeves K, Schaffer C and Olbricht W 2009. Flexible microfluidic devices supported by biodegradable insertion scaffolds for convection-enhanced neural drug delivery Biomed. Microdevices 11 915–24 [DOI] [PubMed] [Google Scholar]
- [68].Chen C-H et al. 2009. Three-dimensional flexible microprobe for recording the neural signal 2009 IEEE Int. Conf. on Nano/Molecular Medicine and Engineering pp 278–81 ( 10.1109/nanomed.2009.5559071) [DOI] [Google Scholar]
- [69].Lewitus DY, Smith KL, Shain W, Bolikal D and Kohn J 2011. The fate of ultrafast degrading polymeric implants in the brain Biomaterials 32 5543–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lewitus D, Smith KL, Shain W and Kohn J 2011. Ultrafast resorbing polymers for use as carriers for cortical neural probes Acta Biomater. 7 2483–91 [DOI] [PubMed] [Google Scholar]
- [71].Lo M. et al. Coating flexible probes with an ultra fast degrading polymer to aid in tissue insertion. Biomed. Microdevices. 2015;17:34. doi: 10.1007/s10544-015-9927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Singh S. et al. Modeling the insertion mechanics of flexible neural probes coated with sacrificial polymers for optimizing probe design. Sensors. 2016;16:330. doi: 10.3390/s16030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Senkevich JJ and Wang P-I 2009. Molecular layer chemistry via parylenes Chem. Vap. Depos 15 91–4 [Google Scholar]
- [74].Schut J et al. 2007. Glass transition temperature prediction of polymers through the mass-per-flexible-bond principle Polymer 48 6115–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rojas R, Harris NK, Piotrowska K and Kohn J 2009. Evaluation of automated synthesis for chain and step-growth polymerizations: can robots replace the chemists? J. Polym. Sci. A 47 49–58 [Google Scholar]
- [76].Westedt U et al. 2006. Paclitaxel releasing films consisting of poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) and their potential as biodegradable stent coatings J. Control. Release 111 235–46 [DOI] [PubMed] [Google Scholar]
- [77].Rodger DC and Humayun MS 2008. Implantable parylene-based wireless intraocular pressure sensor 2008 IEEE 21st Int. Conf. on Micro Electro Mechanical Systems (IEEE; ) pp 58–61 ( 10.1109/MEMSYS.2008.4443592) [DOI] [Google Scholar]
- [78].Kim E, Xia Y and Whitesides GM 1996. Micromolding in capillaries: applications in materials science J. Am. Chem. Soc 118 5722–31 [Google Scholar]
- [79].Kim EGR et al. 2014. A hybrid silicon–parylene neural probe with locally flexible regions Sensors Actuators B 195 416–22 [Google Scholar]
- [80].Tremblay M-È, Lowery RL and Majewska AK 2010. Microglial interactions with synapses are modulated by visual experience PLoS Biol. 8 e1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hooper KA, Macon ND and Kohn J 1998. Comparative histological evaluation of new tyrosine-derived polymers and poly (L-lactic acid) as a function of polymer degradation J. Biomed. Mater. Res 41 443–54 [DOI] [PubMed] [Google Scholar]
- [82].Bourke SL and Kohn J 2003. Polymers derived from the amino acid l-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol) Adv. Drug Deliv. Rev 55 447–66 [DOI] [PubMed] [Google Scholar]
- [83].Waters CM 2004. Reactive oxygen species in mechanotransduction Am. J. Physiol. Lung Cell. Mol. Physiol 287 L484–5 [DOI] [PubMed] [Google Scholar]
- [84].Subbaroyan J et al. 2005. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex J. Neural Eng 2 103–13 [DOI] [PubMed] [Google Scholar]
- [85].Cogan SF 2008. Neural stimulation and recording electrodes Annu. Rev. Biomed. Eng 10 275–309 [DOI] [PubMed] [Google Scholar]
- [86].Ward MP, Rajdev P, Ellison C and Irazoqui PP 2009. Toward a comparison of microelectrodes for acute and chronic recordings Brain Res. 1282 183–200 [DOI] [PubMed] [Google Scholar]
- [87].Karp FB, Bernotski NA, Valdes TI, Bohringer KF and Ratner BD 2008. Foreign body response investigated with an implanted biosensor by in situ electrical impedance spectroscopy IEEE Sens. J 8 104–12 [Google Scholar]
- [88].Harris AR. et al. Conducting polymer coated neural recording electrodes. J. Neural Eng. 2013;10 doi: 10.1088/1741-2560/10/1/016004. 016004. [DOI] [PubMed] [Google Scholar]
- [89].Banati RB, Gehrmann J, Schubert P and Kreutzberg GW 1993. Cytotoxicity of microglia Glia 7 111–8 [DOI] [PubMed] [Google Scholar]
- [90].Woodroofe MN et al. 1991. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production J. Neuroimmunol 33 227–36 [DOI] [PubMed] [Google Scholar]
- [91].Nakajima K et al. 2001. Neurotrophin secretion from cultured microglia J. Neurosci. Res 65 322–31 [DOI] [PubMed] [Google Scholar]
- [92].Gentleman SM et al. 2004. Long-term intracerebral inflammatory response after traumatic brain injury Forensic Sci. Int 146 97–104 [DOI] [PubMed] [Google Scholar]
- [93].Anderson MA et al. 2016. Astrocyte scar formation aids central nervous system axon regeneration Nature 532 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Potter KA, Buck AC, Self WK and Capadona JR 2012. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses J. Neural Eng 9 046020 [DOI] [PubMed] [Google Scholar]
- [95].Eles JR et al. 2017. Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy Biomaterials 113 279–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Magno MHR. et al. Synthesis, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds. J. Mater. Chem. 2010;20:8885. [Google Scholar]