Abstract
Background
Mobile apps provide an accessible way to test new health-related methodologies. Tobacco is still the primary preventable cause of death in industrialized countries, constituting an important public health issue. New technologies provide novel opportunities that are effective in the cessation of smoking tobacco.
Objective
This paper aims to evaluate the efficacy and usage of a mobile app for assisting adult smokers to quit smoking.
Methods
We conducted a cluster randomized clinical trial. We included smokers older than 18 years who were motivated to stop smoking and used a mobile phone compatible with our mobile app. We carried out follow-up visits at 15, 30, and 45 days, and at 2, 3, 6, and 12 months. Participants of the intervention group had access to the Tobbstop mobile app designed by the research team. The primary outcomes were continuous smoking abstinence at 3 and 12 months.
Results
A total of 773 participants were included in the trial, of which 602 (77.9%) began the study on their D-Day. Of participants in the intervention group, 34.15% (97/284) did not use the app. The continuous abstention level was significantly larger in the intervention group participants who used the app than in those who did not use the app at both 3 months (72/187, 38.5% vs 13/97, 13.4%; P<.001) and 12 months (39/187, 20.9% vs 8/97, 8.25%; P=.01). Participants in the intervention group who used the app regularly and correctly had a higher probability of not being smokers at 12 months (OR 7.20, 95% CI 2.14-24.20; P=.001) than the participants of the CG.
Conclusions
Regular use of an app for smoking cessation is effective in comparison with standard clinical practice.
Trial Registration
Clinicaltrials.gov NCT01734421; https://clinicaltrials.gov/ct2/show/NCT01734421
Keywords: tobacco smoking, tobacco use cessation, mobile application, primary public health, clinical trial, mobile phone
Introduction
The number of health interventions involving mobile-based technologies has been increasing in recent years, given the potential of reaching larger audiences who own and use smartphones on a daily basis. In the case of Spain, between 80% and 90% of the population have at least one smartphone [1], which places the country at the top of European mobile phone usage, with 23 million people owning smartphones [2]. Mobile apps provide an accessible way to test new health-related methodologies, which also address user concerns around the availability and confidentiality of their personal data [3]. In Spain, users download around 4 million apps every day and, more importantly, two-thirds of teenagers (and young users) downloaded and used a mobile health app in the last year.
Tobacco consumption levels around the world vary significantly; while in some low-income countries there has been an increase in the prevalence of tobacco, in industrialized countries the observed decrease in consumption in recent years seems to have stopped. Nonetheless, tobacco is still the primary preventable cause of death in industrialized countries, constituting an important public health issue despite all the medical advances and resources invested to reduce tobacco-related death and diseases [4-7].
There are many available mobile apps designed to support smokers in the process of tobacco cessation. However, most of these apps lack scientific evidence that prove their effectiveness [8]. In a recent systematic review [9] focused on analyzing available apps that support the process of smoking cessation, the authors found that only 6 out of 158 apps are supported by low-quality scientific evidence, 3 of which are currently available on smartphone markets, and only 2 of which are in the top 50 most popular apps for smoking cessation [9]. This creates two important issues: the limited number of scientifically validated apps and the unavailability of such apps for the general population. In order to increase the quality and the availability of apps, experts suggest that it is necessary to develop an innovative framework capable of scientifically evaluating different properties of the apps, and then improve distribution channels to make those certified apps easier to find for the consumer. Moreover, certified apps will also be easier to use in health care environments, given that they should comply with a set of standards and regulations [10]. Official institutions also suggest the use of simple language to widen the dissemination of health-related information.
Our research team has been working since 2013 to evaluate the efficacy of a gamified mobile app with the goal of increasing the success rate of smoking cessation interventions in adults (individuals older than 18 years) that are already motivated to quit smoking.
Methods
Study Design
The protocol of the study was previously published [11] and was executed in 2 phases. First, an interdisciplinary team composed of doctors, nurses, educators, designers, and computer engineers designed and implemented the Tobbstop mobile app, which combines gamification principles with the latest mobile technologies to create a novel experience designed to follow a tobacco withdrawal guide. Second, a cluster randomized clinical trial was conducted in order to evaluate the efficacy of the mobile app and the features included within.
Setting
We included participants from the primary health care regions of Tarragona and Terres de l’Ebre (Catalonia, Spain).
Recruitment of Primary Health Care Professionals
The participation of the primary health care professionals was voluntary. Health professionals—doctors and nurses—were informed of the goals and scope of the study, provided with the study protocol [11] and related documentation, and trained by the members of the research team to inform them of the details of the study. Individuals that participated in the study were recruited by the health professionals, provided that they met our inclusion criteria.
Participant Eligibility Criteria
Our inclusion criteria were (1) current smokers aged 18 years or older who smoked at least 10 cigarettes per day, (2) owners of an Android or iOS (Apple Inc) mobile phone compatible with our mobile app, and (c) smokers with a moderate-high motivation to quit smoking (Richmond test score ≥5) [12].
The exclusion criteria were (1) patients addicted to psychoactive substances other than tobacco, (2) patients with a psychotic disorder, (3) patients without a smartphone with the minimum hardware requirements necessary to run the app, and (4) participants who had a low motivation to quit smoking (Richmond test score <5).
If a participant did not meet the motivation criterion, health professionals provided information and measures to increase motivation and arranged a second session to try to recruit the participant into our study.
Random Allocation
The unit of randomization was the primary health care centers that participated in the recruiting. Each center enrolled was assigned randomly to one of the 2 groups (control or intervention). Centers were stratified according to rural or urban locations and the number of health professionals available in each of them. We ensured comparability between the 2 groups. For the randomization, we used the software EpiData (version 3.0, EpiData Association).
Blinding
Given the nature of the intervention we could not prevent the participants and health professionals from knowing to which of the 2 groups they were assigned. However, in the data analysis phase, we blinded the data so no identification process could be carried out.
Intervention
During the recruitment process (June 2014-May 2016) professionals invited active smokers older than 18 years. The participants that met the different selection criteria and were willing to participate were asked to sign a written consent form.
In the first visit (defined as visit 0), we collected demographic data, asked the participant to fill in the study questionnaire, and fixed the D-Day upon which they would initiate the process of smoking cessation. A few days before the D-Day, each participant had a second visit, where the smoker and the health professional discussed the plan to be followed throughout the process, in accordance with the Clinical Practice Guidelines of the Institut Català de la Salut—Catalan Health Institute [13]—and planned all the remaining monitoring visits.
Both groups received information on the standard guidelines of clinical practice. In the event that a participant did not attend a follow-up meeting, professionals called their phone to reintroduce them into the study. If they could not be reached, they were considered as relapsed as of that point.
Description of the Mobile App
The participants assigned to the intervention group (IG) received a numeric code to activate their access to the Tobbstop app and a detailed explanation of its basic features. This app is included in the Serious Games category, which are apps that include components from games designed to facilitate the achievement of the goals of the app, increasing user engagement and improving user experience. Tobbstop was designed with the goal of engaging the participants to use the app for at least the 3-month period that they were in the clinical study, and it included features to motivate them to use the app every day during this period. We also included features that covered Bartle’s taxonomy of player types (killers, socializers, achievers, and explorers) and that adapted to the different stages of the tobacco withdrawal process: start, euphoria, grief, normalization, and consolidation. All this work was performed with an interdisciplinary team of experts that included experts on tobacco withdrawal (in smokers and ex-smokers), computer engineers, graphic designers, game designers, educators, and health professionals (doctors and nurses). For more details about the app features, see Multimedia Appendix 1.
The clinical trial was designed to measure success 12 months after the start of the study, but the app was only designed to cover the first 3 months. Once participants completed the path through the island in the app, they could continue using the app and all the features provided without limitation.
Control Group
Participants assigned to the control group (CG) only followed the recommendations of the health professionals included in the study [13].
Data Collection
During the first visit, we collected all sociodemographic and anthropometric data, such as date of birth, gender, level of education, civil status, weight (kg), height (cm), and blood pressure (mmHg), as well as data on the presence of other pathologies, such as high blood pressure, chronic obstructive pulmonary disease, diabetes mellitus, stroke, neoplasia, acute myocardial infarction, dyslipidemia, angina pectoris, and intermittent claudication. We also collected data on tobacco consumption, including number of cigarettes smoked per day, usage of electronic cigarettes, age at which the individual started smoking, number of previous attempts to quit smoking, longest period without smoking, and presence of other smokers in the family. Nicotine dependence level was measured using the Fagerström test [14] and their motivation was measured using the Richmond test [12].
In each of the follow-up visits we measured weight and blood pressure and asked about tobacco consumption and the existence of abstinence syndrome. Tobacco abstinence was confirmed by the level of carbon monoxide (CO) in exhaled breath. We recorded any other treatments used for tobacco cessation (ie, pharmacological treatment).
If participants did not attend the follow-up interviews, we attempted to call them by phone. In some cases, when the participant was unable to attend the visit at the health center, we conducted the follow-up interview via phone in order to ensure the participant’s continuance with the study, but in such cases we were unable to collect all the measures (weight, height, CO-oximetry).
The follow-up period had the following end-points: (1) when the participant did not attend one of the visits and we were unable to contact them thereafter, (2) when the participant decided to quit the study, (3) when the participant started to smoke again, or (4) after 12 months without smoking.
Computed Variables
We computed the body mass index using height and weight and mean arterial pressure according to the formula [(diastolic arterial pressure x 2) + systolic arterial pressure] / 3.
Sample Size
To compute the sample size, which was randomized by the primary health care centers that participated, we multiplied the number of individuals required by the design effect. We accepted an α risk of 5% and a β risk of 20% in a bilateral contrast. We counted 222 participants in each group, detecting a difference of less than 5%, with measurements based on Epidat (version 3.1; Xunta de Galicia). In order to compute the design effect, we estimated an intracluster correlation coefficient in randomized clinical trials, which is usually lower than 0.05. The average size was 20 with a design effect of 1.36. Using all these values, we set the sample size to 604 participants, with 302 in each group.
Statistical Analysis
We performed intention-to-treat analysis to evaluate the comparability of the 2 groups.
Quantitative measures are described with mean and standard deviation if they have a normal distribution, or median and interquartile range if they do not. Qualitative variables are described with percentages and 95% confidence intervals. Baseline quantitative measures are compared using Student’s t test, while qualitative measures are compared using Pearson’s chi-square test.
The primary outcomes were continuous abstinence at 3 and 12 months.
We evaluated the app efficacy using a protocol analysis, comparing the CG participants with the IG participants who used the app. We computed the crude and adjusted hazard ratios using a multilevel Cox regression with 2 effects (fixed and random). The fixed component included group assignment and, in the adjusted models, sociodemographic variables. The random component included assignment to a primary health care center. Data analysis was performed using R version 3.4.3 [15].
Ethical Aspects of the Study
The study, in its revised and updated version, was carried out following the Declaration of Helsinki principles and the Spanish Clinical Practice Guidelines. The study protocol was approved by the Clinical Research Ethics Committee at the Institut Universitari d'Investigació en Atenció Primària Jordi Gol. Data confidentiality was guaranteed by the Spanish law that regulated the protection of personal data at the time of the study, the Ley Orgánica de Protección de Datos de Carácter Personal (15/1999, December 13).
Results
Participant Characteristics
We recruited 773 participants for the study, of which 602 (77.9%) started the study on their D-Day. In Figure 1 we detail the flow of the participants within the study [16].
Figure 1.

Flowchart of the participants included in the analysis.
The participants who set their D-Day belonged to one of the 22 health care centers assigned to the IG, and they were treated by 67 health professionals (nurses and doctors). There were no significant differences in the basic characteristics of the participants prior to the intervention if we compare the centers in which they were recruited or the professionals who recruited them. The basic characteristics of the participants are detailed in Table 1. Even though there were not large differences between the 2 groups, we observed that the IG participants were younger than those in the CG (42.2 years vs 48.8 years; P<.001), and they had a higher education level (P=.001), lived alone (P=.03), had a lower CO level (P=.001), had lower dependence (P=.02), had more family members who smoke (P=.03), and had lower blood pressure (P=.003) than participants in the CG. The CG used more pharmacological treatment for tobacco withdrawal (P<.001).
Table 1.
Basic characteristics of the Tobbstop study participants based on their assigned group.
| Characteristics | Control group (n=318) | Intervention group (n=284) | P value | Total (n=602) | |||||
| Mean age, years (SD) | 48.8 (11.0) | 42.2 (10.2) | <.001 | 45.7 (11.1) | |||||
| Men (%) | 155 (48.7) | 120 (42.3) | .13 | 275 (45.7) | |||||
| Education, n (%) |
|
|
.001 |
|
|||||
|
|
No studies or primary | 126 (39.6) | 70 (24.7) |
|
196 (32.6) | ||||
|
|
Secondary | 129 (40.6) | 143 (50.5) |
|
272 (45.3) | ||||
|
|
University or higher | 63 (19.8) | 70 (24.7) |
|
133 (22.1) | ||||
| Marital status, n (%) |
|
|
.03 |
|
|||||
|
|
Single | 55 (17.3) | 72 (25.4) |
|
127 (21.1) | ||||
|
|
With a partner | 205 (64.5) | 171 (60.2) |
|
376 (62.5) | ||||
|
|
Widowed | 15 (4.7) | 5 (1.8) |
|
20 (3.3) | ||||
|
|
Divorced | 43 (13.5) | 36 (12.7) |
|
79 (13.1) | ||||
| Body mass indexa (kg/m2), mean (SD) | 27.4 (8.9) | 26.9 (8.5) | .47 | 27.2 (8.7) | |||||
| Mean arterial pressurea (mm/Hg), mean (SD) | 91.4 (10.6) | 60.5 (10.7) | .30 | 91 (10.7) | |||||
| COb-oximetry (ppm), mean (IQRc) | 16.8 (8.00-21.00) | 13.6 (6.00-20.00) | .001 | 15.2 (6.75-20.00) | |||||
| Electronic cigarette users, n (%) | 49 (15.4) | 54 (19) | .29 | 103 (17.1) | |||||
| Cigarettes per day, n (%) |
|
|
.12 |
|
|||||
|
|
0-10 | 60 (18.9) | 73 (25.7) |
|
133 (22.1) | ||||
|
|
11-20 | 183 (57.5) | 154 (54.2) |
|
337 (56.0) | ||||
|
|
21-30 | 60 (18.9) | 38 (13.4) |
|
98 (16.3) | ||||
|
|
31-40 | 12 (3.7) | 15 (5.3) |
|
27 (4.5) | ||||
|
|
>40 | 3 (0.9) | 4 (1.4) |
|
7 (1.2) | ||||
| Fagerström test (dependence), n (%) |
|
|
.02 |
|
|||||
|
|
High | 37 (11.6) | 35 (12.3) |
|
72 (12.0) | ||||
|
|
Moderate | 217 (68.2) | 165 (58.1) |
|
382 (63.5) | ||||
|
|
Low | 64 (20.1) | 84 (29.6) |
|
148 (24.6) | ||||
| Number of previous attempts, mean (IQR) | 1.90 (1.00-3.00) | 2.61 (1.00-3.00) | .08 | 2.24 (1.00-3.00) | |||||
| Maximum number of withdrawal months, mean (SD) | 13.8 (31.1) | 10.4 (20.9) | .12 | 12.2 (26.8) | |||||
| Age of starting smoking (years), mean (SD) | 17.0 (4.1) | 16.7 (3.4) | .33 | 16.8 (3.8) | |||||
| No smokers in the family, n (%) | 154 (48.4) | 111 (39.1) | .03 | 265 (44.0) | |||||
| Use of a pharmacological treatment for tobacco cessation, n (%) | 244 (76.7) | 148 (52.1) | <.001 | 392 (65.1) | |||||
| Pathologies, n (%) |
|
|
|
|
|||||
|
|
Hypertension | 72 (22.6) | 37 (13.0) | .003 | 109 (18.1) | ||||
|
|
Chronic obstructive pulmonary disease | 24 (7.5) | 11 (3.9) | .08 | 35 (5.8) | ||||
|
|
Type 2 diabetes mellitus | 23 (7.2) | 16 (5.6) | .53 | 39 (6.5) | ||||
|
|
Stroke | 3 (0.9) | 5 (1.8) | .49 | 8 (1.3) | ||||
|
|
Neoplasia | 5 (1.6) | 6 (2.11) | .85 | 11 (1.8) | ||||
|
|
Dyslipidemia | 83 (26.1) | 55 (19.4) | .06 | 138 (22.9) | ||||
|
|
Coronary heart disease |
16 (5) | 6 (2.1) | .09 | 22 (3.6) | ||||
| 3-month withdrawal, n (%) | 101 (31.8) | 85 (29.9) | .69 | 186 (30.9) | |||||
| 12-month withdrawal, n (%) | 60 (18.9) | 47 (16.5) | .53 | 107 (17.8) | |||||
aAt baseline.
bCO: carbon monoxide.
cIQR: interquartile range.
When looking at the efficacy of the study by participant group, we observed that there were no significant statistical differences between the 2 groups, with slightly better results observed in the CG.
App Usage
Out of the 284 participants enrolled in the IG, 97 (34.1%) did not use the app, never used the code to activate the app, or did not send any data on their usage from their mobile phone to our server.
We observed significant statistical differences between the participants who used the app and those who did not. Those who did not use the app had lower CO levels (P=.03) and smoked more cigarettes per day (P=.04). The IG participants who did not use the app indicated less pharmacological treatment than those who used the app (41/97, 42.3% vs 107/187, 57.2%; P=.02).
Finally, we observed that abstinence was significantly larger in the IG participants who used the app than in those who did not use the app at both 3 months (72/187, 38.5% vs 13/97, 13.4%; P<.001) and 12 months (39/187, 20.9% vs 8/97, 8.2%; P=.01; Figures 2 and 3).
Figure 2.
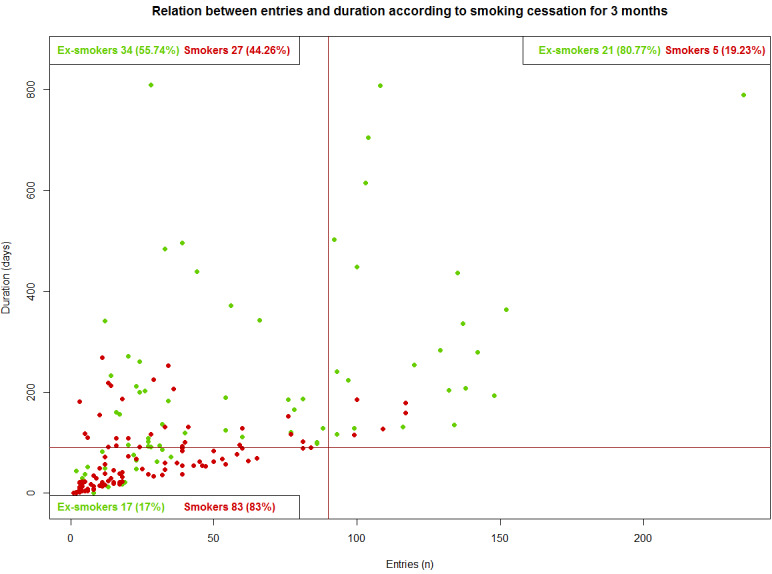
Relation between entries and duration according to smoking cessation for 3 months.
Figure 3.
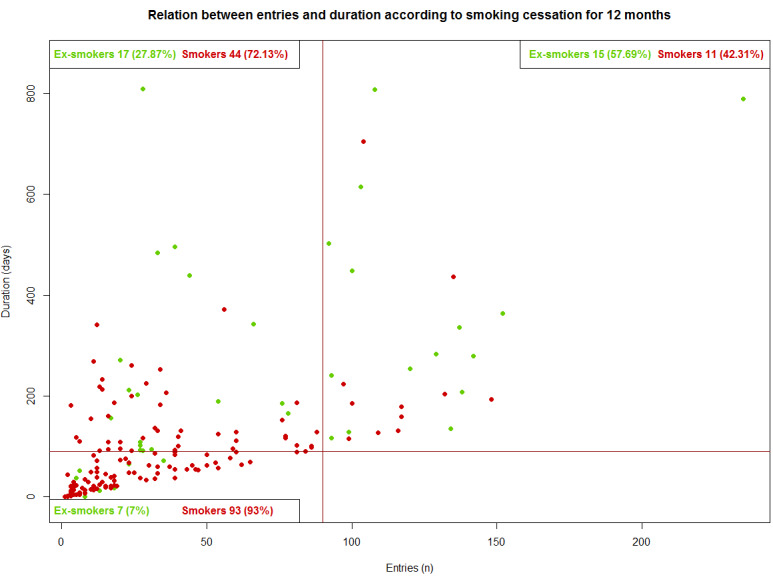
Relation between entries and duration accoding to smoking cessation for 12 months.
Results According to the App Features Used
We consider the good users of the app (based on our design goals) to be those who used the app for at least 90 days and accessed the app at least 90 times. In accordance with these 2 conditions, we consider 26 of the 187 participants who used the app at least once to be good users. Among the main differences, we observed that the participants of the good users group smoked fewer cigarettes per day, had smaller dependencies according to the Fagerström test, and used less pharmacological treatment than those in the CG. The 3-month abstinence of the participants included in the good users group was 80.8% (21/26) compared with 31.7% (51/161) in the other users (P<.001). The 12-month abstinence rate for the good users was 57.7% (15/26) compared with 14.9% (24/161) for the other users of the app (P<.001; Figure 4).
Figure 4.

Kapplan-Meier curve GC (control group) vs GUG (good users group).
We evaluated which app features were used the most by the IG. Those who succeeded were more active users of the app and logged in more than twice as much as the other participants. With the exception of the chat feature, which was used independently of whether the participant relapsed, those who did not relapse used the app features more than those who relapsed, as seen in Table 2. Out of the 187 participants who used the app, 38.5% (15/39) of the ex-smokers were considered good users, while only 7.4% (11/148) of those who relapsed were good users (P<.001).
Table 2.
List of a set of app metrics, classified according to the success rate at the end of the study (12 months from the day at which they stopped smoking) for participants of the intervention group who used the app (n=187).
| Characteristics | Relapse (n=148) | Abstinence at 12 months (n=39) | P value | ||||
| Pharmacological treatment, n (%) | 91 (61.5) | 16 (41.0) | .03 | ||||
| App metrics |
|
|
|
||||
|
|
Duration within the app (days), mean (SD) | 85.2 (94.8) | 262 (220.0) | <.001 | |||
|
|
Number of different days connected, mean (SD) | 31.7 (33.0) | 67.4 (53.5) | <.001 | |||
|
|
Chat usage, n (%) | 78 (52.7) | 23 (59.0) | .60 | |||
|
|
Trivial maximum level, mean (SD) | 12.7 (17.1) | 18.3 (16.7) | .07 | |||
|
|
Trivial highest score, mean (SD) | 612 (1011.0) | 956 (989.0) | .06 | |||
|
|
Fruit game maximum level, mean (SD) | 1.67 (1.3) | 2.15 (1.3) | .04 | |||
|
|
Fruit game highest score, mean (SD) | 1174 (2258.0) | 2020 (2451.0) | .06 | |||
|
|
Number of challenges completed, mean (SD) | 1.42 (3.1) | 2.95 (4.5) | .05 | |||
|
|
Island sections completed, mean (SD) | 6.26 (10.5) | 12.5 (12.4) | .006 | |||
|
|
Number of times they consulted the information section, mean (SD) | 3.61 (4.5) | 6.44 (7.8) | .04 | |||
Protocol Analysis of App Efficacy
Observing that 34.1% (97/284) of the IG did not use the app, we evaluated app usage using a protocol analysis. When we selected the IG participants who had at least one activity record stored in the server and compared them with the CG participants, we did not find significant differences between the groups in terms of tobacco cessation at 3 months and 12 months (P=.26 and P=.94, respectively), as seen in Table 3.
Table 3.
Adjusted association in smoking cessation at 3 months and 12 months between control group and app users.a
| Clinical outcomes | Controlb, n (%) | App usersc, n (%) | ICCd | ORe (95% CI) | P value |
| Abstinent at 3 months | 101 (31.8) | 72 (38.5) | 0.012 | 1.31 (0.82-2.09) | .26 |
| Abstinent at 12 months | 60 (18.9) | 39 (20.9) | 0.019 | 1.02 (0.58-1.79) | .94 |
aAdjusted by clinic group, age, gender, body mass index, education, Fagerström test assessment, number of previous attempts to quit, smokers in the family, use of electronic cigarettes, and use of a pharmacological treatment for tobacco cessation.
bn=318.
cn=187.
dICC: intracluster correlation coefficient.
eOR: odds ratio.
We repeated the same analysis with the participants of the IG that we considered good users, taking into account that the size of the sample was small (n=26). We observed that those belonging to this group had a higher probability of being abstinent at 12 months (OR 7.20, 95% CI 2.14-24.20; P=.001) than the participants of the CG, as seen in Table 4.
Table 4.
Adjusted association in smoking cessation at 3 months and 12 months between control group and good users.a
| Clinical outcomes | Controlb, n (%) | Good usersc, n (%) | ICCd | ORe (95% CI) | P value |
| Abstinent at 3 months | 101 (31.8) | 21 (80.8) | 0.000 | 9.88 (3.37-28.91) | <.001 |
| Abstinent at 12 months | 60 (18.9) | 15 (57.7) | 0.053 | 7.20 (2.14-24.20) | .001 |
aAdjusted by clinic group, age, gender, body mass index, education, Fagerström test assessment, number of previous attempts to quit, smokers in the family, use of electronic cigarettes, and use of a pharmacological treatment for tobacco cessation.
bn=318.
cn=26.
dICC: intracluster correlation coefficient.
eOR: odds ratio.
Discussion
Principal Findings
Our intervention trial, based on the use of a mobile app for smoking cessation in people older than 18 years who were motivated to quit smoking, included 773 participants who were monitored during their abstinence period for up to 12 months. The study demonstrated success in quitting smoking at 3 and 12 months among regular mobile app users compared with participants in the CG. Our study contributes to the literature on the design and evaluation of mobile health apps designed to help patients in the process of tobacco cessation [8-10,17], since most of the current research is based on the use of text messages to mobile phones [18].
Previous studies have shown that mobile phone app–based interventions may be useful tools for lifestyle interventions, such as weight loss [19], increased physical activity [20], or long-term condition management [21]. Moreover, a recent meta-analysis that included 5 studies that assess the effectiveness of smoking cessation using mobile apps alone to compare lower-intensity smoking cessations support found no evidence of a favorable effect of mobile apps in comparison with other types of interventions [22]. This meta-analysis also included one study that compared mobile app plus text messaging with a web-based intervention, which found evidence of a benefit of the app plus text messaging intervention [22]. Compared with the studies included in the meta-analysis, our study presents the longest follow-up (12 months) and the highest sample size.
We know that most attempts to quit smoking are not successful; according to the Centers for Disease Control and Prevention, only 12.2% of those who try to stop smoking remain abstinent [3]. For this reason, interventions that help young people and the general population stop smoking are needed. In our study, young people (aged 18 to 44 years) represent 57.7% (108/187) of those that used the app and 42.3% (11/26) of the good users. Mobile app usage seems more aligned with the lifestyle of the younger population, so this type of app has a double benefit in young people, since it reduces both smoking prevalence and comorbidity as patients grow older. Our mobile app includes some intervention components that have been successful in promoting smoking cessation, such as social compromise [23,24] and strategies to cope with abstinence syndrome and moments of craving [24,25].
Studies based on apps with this type of design should make sure that participants have a minimum level of digital skills with which to use the apps and that they are motivated to use them. As other researchers point out, it is important to study the usage patterns of the users in order to identify the features that are most helpful in the process of quitting smoking [26]. Researchers need to continue working on designs capable of identifying the key elements that help participants, as well as redesigning features (including new features) that can increase the efficacy of the app. Researchers should also consider how to personalize the intervention (and the available features within the app) to each of the population subgroups in order to maximize the engagement of the participants and, therefore, the probability of the success of the intervention. It is important to note that recent interventions that use an app for tobacco cessation are well received and viewed favorably by the participants [27], and it is vital that future apps take the needs of the users into account [28]. Mobile apps that use services hosted on online servers have an extra layer of complexity in complying with the technical and legal requirements of working with personal data.
In this initial Tobbstop study, users of the app appeared to exhibit patterns of participation and follow-up over time and demonstrated encouraging rates of tobacco cessation. Future research is warranted in order to evaluate the efficacy of Tobbstop in larger sample sizes.
We are currently working on adapting the app to other specific situations in which new features can improve its efficacy. In particular, we are designing a large-scale study of tobacco cessation among pregnant women. We have already performed a pilot study with 42 participants and have seen high success rates, even when the app was not designed for this specific group [29].
In our study, 34.1% (97/284) of the IG users never used the app, so we have to take this into account when making our analysis. This prevalence is similar to those obtained in previous studies [30]. Engagement and user retention are common and critical problems in mobile health. Previous studies have shown that more than two-thirds of people who downloaded a mobile phone app used it once and then stopped using it [31]. As a result, we have performed a sensitivity analysis between users and good users to obtain more accurate information about the efficacy of long-term usage of mobile phone apps in achieving smoking cessation. Our results have confirmed the hypothesis that long-term use of mobile apps improves the continuance of tobacco abstinence [32].
Despite the fact that the number of participant relapses were considered high, with 64.6% (183/284) of the IG and 73.3% (233/318) of the CG relapsing, our results have higher success rates than other studies that are also based on apps and have similar population samples [24,32]. In our study, we only enrolled participants with a high motivation to quit smoking. This parameter is unknown in other studies.
Conclusions
A mobile app to help the process of quitting smoking presents higher success rates than standard interventions, indicating the viability of conducting a randomized community trial based on smartphone technologies.
Acknowledgments
The authors would like to thank all who participated in the Tobbstop study, including all the health professionals; the Catalan Health Institute for publicizing our study; and the Institut Universitari d'Investigació en Atenció Primària Jordi Gol for all of the support we received.
The project received a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain). It was awarded by the 2012 call under Health Strategy Action 2013-2016 within the National Research Program oriented to Societal Challenges of the Technical, Scientific, and Innovation Research National Plan 2013-2016, with reference PI12/01977, cofunded by the European Regional Development Fund (European Union).
JD was funded by the Ministry of Economy and Competitiveness project (FIS2016-78904-C3-1-P), the Generalitat de Catalunya project (2017SGR-896), and the Universitat Rovira i Virgili project (2018PFR-B2-41).
Abbreviations
- CG
control group
- CO
carbon monoxide
- IG
intervention group
Appendix
Main features and information about the Tobbstop application.
CONSORT checklist.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Informe 2018: Usuarios Internet, Social Media y Móvil en España y en el Mundo. [2019-02-01]. Luces y sombras de las marcas Internet https://fatimamartinez.es/2018/01/30/informe-2018-usuarios-internet-social-media-y-movil-en-espana-y-en-el-mundo/
- 2.Ditrendia Ditrendia. 2018. [2020-05-31]. Informe ditrendia: Mobile en España y en el Mundo 2018 https://mktefa.ditrendia.es/informe-mobile-2018?hsCtaTracking=ee534c46-775d-4920-8b36-90aaa48576bb%7Cd20196ea-869c-4c41-a9b1-0ea9f6150ea5.
- 3.Robinson C, Seaman E, Grenen E, Montgomery L, Yockey R, Coa K, Prutzman Yvonne, Augustson Erik. A content analysis of smartphone apps for adolescent smoking cessation. Transl Behav Med. 2020 Feb 03;10(1):302–309. doi: 10.1093/tbm/iby113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal A, King B, Neff L, Whitmill J, Babb S, Graffunder C. Centers for Disease Control and Prevention. 2016. [2019-03-08]. Current Cigarette Smoking Among Adults — United States, 2005–2015 https://www.cdc.gov/mmwr/volumes/65/wr/pdfs/mm6544a2.pdf.
- 5.World Health Organization World Health Organization. 2017. [2020-05-26]. WHO Report on the Global Tobacco Epidemic, 2017: monitoring tobacco use and prevention policies https://apps.who.int/iris/bitstream/handle/10665/258503/WHO-NMH-PND-17.4-eng.pdf?sequence=1.
- 6.Bilano V, Gilmour S, Moffiet T, d'Espaignet Edouard Tursan, Stevens G, Commar A, Tuyl Frank, Hudson Irene, Shibuya Kenji. Global trends and projections for tobacco use, 1990-2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015 Mar 14;385(9972):966–76. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 7.Drope J, Drope J, Schluger N, Cahn Z, Drope J, Hamill S. The Tobacco Atlas. American Cancer Society Inc; Vital Strategies; 2018. [2019-03-20]. Prevalence https://tobaccoatlas.org/topic/prevalence/ [Google Scholar]
- 8.Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017 Jun;7(2):292–299. doi: 10.1007/s13142-017-0492-2. http://europepmc.org/abstract/MED/28527027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton L, Quinn C, Birrell L, Guillaumier A, Shaw B, Forbes E, Deady M, Kay-Lambkin F. Free smoking cessation mobile apps available in Australia: a quality review and content analysis. Aust N Z J Public Health. 2017 Dec;41(6):625–630. doi: 10.1111/1753-6405.12688. [DOI] [PubMed] [Google Scholar]
- 10.Ghorai K, Akter S, Khatun F, Ray P. mHealth for Smoking Cessation Programs: A Systematic Review. J Pers Med. 2014 Jul 18;4(3):412–23. doi: 10.3390/jpm4030412. https://www.mdpi.com/resolver?pii=jpm4030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdivieso-López Empar, Flores-Mateo G, Molina-Gómez Juan-Domingo, Rey-Reñones Cristina, Barrera Uriarte María-Luisa, Duch J, Valverde A. Efficacy of a mobile application for smoking cessation in young people: study protocol for a clustered, randomized trial. BMC Public Health. 2013 Aug 01;13:704. doi: 10.1186/1471-2458-13-704. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond R L, Kehoe L, Webster I. Multivariate models for predicting abstention following intervention to stop smoking by general practitioners. Addiction. 1993 Aug;88(8):1127–35. doi: 10.1111/j.1360-0443.1993.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 13.Institut Català de la Salut . Guies de pràctica clínica. Barcelona: Departament de Salut; 2009. Apr 1, [2020-05-31]. Detecció i tractament del consum del tabac http://ics.gencat.cat/ca/assistencia/coneixement-assistencial/guies-de-practica-clinica/ [Google Scholar]
- 14.Becoña E, Vázquez F L. The Fagerström Test for Nicotine Dependence in a Spanish sample. Psychol Rep. 1998 Dec;83(3 Pt 2):1455–8. doi: 10.2466/pr0.1998.83.3f.1455. [DOI] [PubMed] [Google Scholar]
- 15.Therneau T. R package version 2. [2020-05-31]. Cox me: Mixed Effects Cox Models https://cran.r-project.org/package=coxme.
- 16.CONSORT. [2020-05-31]. Welcome to the CONSORT Website http://www.consort-statement.org/
- 17.Ubhi HK, Michie S, Kotz D, Wong WC, West R. A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. J Med Internet Res. 2015 Jan 16;17(1):e17. doi: 10.2196/jmir.3479. https://www.jmir.org/2015/1/e17/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker R, McRobbie H, Bullen C, Borland Ron, Rodgers Anthony, Gu Yulong. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012 Nov 14;11:CD006611. doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Flores Mateo G, Granado-Font E, Ferré-Grau Carme, Montaña-Carreras Xavier. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J Med Internet Res. 2015 Nov 10;17(11):e253. doi: 10.2196/jmir.4836. https://www.jmir.org/2015/11/e253/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romeo A, Edney S, Plotnikoff R, Curtis R, Ryan J, Sanders I, Crozier A, Maher C. Can Smartphone Apps Increase Physical Activity? Systematic Review and Meta-Analysis. J Med Internet Res. 2019 Mar 19;21(3):e12053. doi: 10.2196/12053. https://www.jmir.org/2019/3/e12053/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead L, Seaton P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res. 2016 May 16;18(5):e97. doi: 10.2196/jmir.4883. https://www.jmir.org/2016/5/e97/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019 Oct 22;10:CD006611. doi: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore S, Teixeira A, Stewart S. Effect of network social capital on the chances of smoking relapse: a two-year follow-up study of urban-dwelling adults. Am J Public Health Internet. 2014 Dec;:e72–e76. doi: 10.2105/AJPH.2014.302239. http://ajph.aphapublications.org/doi/10.2105/AJPH.2014.302239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacoviello BM, Steinerman JR, Klein DB, Silver TL, Berger AG, Luo SX, Schork NJ. Clickotine, A Personalized Smartphone App for Smoking Cessation: Initial Evaluation. JMIR Mhealth Uhealth. 2017 Apr 25;5(4):e56. doi: 10.2196/mhealth.7226. https://mhealth.jmir.org/2017/4/e56/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploderer B, Smith W, Pearce J, Borland R. A mobile app offering distractions and tips to cope with cigarette craving: a qualitative study. JMIR Mhealth Uhealth. 2014;2(2):e23. doi: 10.2196/mhealth.3209. http://mhealth.jmir.org/2014/2/e23/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver JA, Hallyburton MB, Pacek LR, Mitchell JT, Vilardaga R, Fuemmeler BF. What Do Smokers Want in A Smartphone-Based Cessation Application? Nicotine Tob Res Internet. Nicotine Tob Res Internet. 2018 Nov 15; doi: 10.1093/ntr/ntx171. https://academic.oup.com/ntr/article/20/12/1507/4061438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cupertino AP, Cartujano-Barrera F, Ramírez M, Rodríguez-Bolaños R, Thrasher JF, Pérez-Rubio G, Falfán-Valencia R, Ellerbeck EF, Reynales-Shigematsu LM. A Mobile Smoking Cessation Intervention for Mexico (Vive sin Tabaco... ¡Decídete!): Single-Arm Pilot Study. JMIR Mhealth Uhealth. 2019 Apr 25;7(4):e12482. doi: 10.2196/12482. https://mhealth.jmir.org/2019/4/e12482/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struik LL, Bottorff JL, Baskerville NB, Oliffe J, Crichton S. Comparison of Developers' and End-Users' Perspectives About Smoking Cessation Support Through the Crush the Crave App. JMIR Mhealth Uhealth. 2019 Mar 07;7(3):e10750. doi: 10.2196/10750. https://mhealth.jmir.org/2019/3/e10750/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin-Gomez F, Garcia-Moreno Marchán Rocio, Mayos-Fernandez A, Flores-Mateo G, Granado-Font E, Barrera Uriarte Ml, Duch J, Rey-Reñones Cristina. Exploring Efficacy of a Serious Game (Tobbstop) for Smoking Cessation During Pregnancy: Randomized Controlled Trial. JMIR Serious Games. 2019 Mar 27;7(1):e12835. doi: 10.2196/12835. https://games.jmir.org/2019/1/e12835/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll JK, Moorhead A, Bond R, LeBlanc WG, Petrella RJ, Fiscella K. Who Uses Mobile Phone Health Apps and Does Use Matter? A Secondary Data Analytics Approach. J Med Internet Res. 2017 Apr 19;19(4):e125. doi: 10.2196/jmir.5604. https://www.jmir.org/2017/4/e125/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K, Kwon H, Lee B, Lee G, Lee JH, Park YR, Shin S. Effect of self-monitoring on long-term patient engagement with mobile health applications. PLoS One. 2018;13(7):e0201166. doi: 10.1371/journal.pone.0201166. http://dx.plos.org/10.1371/journal.pone.0201166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BinDhim NF, McGeechan K, Trevena L. Smartphone Smoking Cessation Application (SSC App) trial: a multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid 'app'. BMJ Open. 2018 Jan 21;8(1):e017105. doi: 10.1136/bmjopen-2017-017105. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=29358418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main features and information about the Tobbstop application.
CONSORT checklist.


