Abstract
Background:
There is a lack of predictive markers informing on the risk of colitis in patients treated with immune checkpoint inhibitors (ICIs). The aim of this study was to identify potential factors associated with development of ICI colitis.
Methods:
We performed a retrospective analysis of melanoma patients at Dana-Farber Cancer Institute who received PD-1, CTLA-4, or combination ICIs between May 2011 to October 2017. Clinical and laboratory characteristics associated with pathologically confirmed ICI colitis were evaluated using multivariate logistic regression analyses. External validation was performed on an independent cohort from Massachusetts General Hospital.
Results:
The discovery cohort included 213 patients of whom 37 developed ICI colitis (17%). Vitamin D use was recorded in 66/213 patients (31%) before starting ICIs. In multivariable regression analysis, vitamin D use conferred significantly reduced odds of developing ICI colitis (OR 0.35, 95% CI 0.1–0.9). These results were confirmed in the validation cohort (OR 0.46, 95% CI 0.2–0.9) of 169 patients of whom 49 developed ICI colitis (29%). Pre-treatment neutrophil/lymphocyte ratio (NLR) ≥5 predicted reduced odds of colitis (OR 0.34, 95% CI 0.1–0.9) only in the discovery cohort.
Conclusions:
This is the first study to report that among patients treated with ICIs, vitamin D intake is associated with reduced risk for ICI colitis. This finding is consistent with prior reports of prophylactic use of vitamin D in ulcerative colitis and graft-versus-host-disease. This observation should be validated prospectively in future studies.
Keywords: Immunotherapy, irAE, colitis, vitamin D, toxicity, melanoma
Precis:
In a retrospective cohort study of melanoma patients receiving checkpoint inhibitors, supplementation with vitamin D was associated with decreased risk of developing colitis. This finding was externally validated through an independent cohort.
Background
Immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and the programmed cell death-1 (PD-1)/ PD-ligand 1 (PD-L1) pathway have improved the prognosis for many malignancies.1,2 The anti-tumor activity of these agents results from augmentation of the T-cell immune response by blocking two immune checkpoints, CTLA-4 and PD-1/PD-L1 that protect against detrimental inflammation and autoimmunity.3,4 This augmented immune response can also lead to a range of systemic and organ specific immune-related adverse effects (irAE).5 The burden of irAEs hinders the clinical benefit of ICIs, with over 50% of patients on combination immunotherapy developing grade 3–4 toxicities.6,7 In particular, immune-related colitis is among the most frequent and severe irAEs often leading to treatment interruption, immunosuppression that may negatively impact tumor immune response, and ICI discontinuation.8–11 The incidence of diarrhea and colitis is higher using CTLA-4-blocking agents compared to PD1/PD-L1 blocking agents,6,12 and highest with a combination of anti-CTLA-4 and anti-PD-1 antibodies.11,13
ICI-induced colitis shares similar phenotypical, histological, and serological characteristics with inflammatory bowel disease (Crohn’s disease [CD] and ulcerative colitis [UC]), albeit with a more aggressive disease course involving acute rather than chronic inflammation.11,14,15 Several individual (e.g., male gender) and environmental (e.g., smoking, vitamin D deficiency, NSAID use, PPI use) risk factors have been associated with the risk of IBD or its complications.2,16,17,18,19,20,21 High vitamin D levels are a known protective factor for both CD and UC.18 A range of comorbidities, likely due to a common pathogenesis of immune dysregulation, have also been shown to correlate with IBD, including history of autoimmune disease22,23 and eosinophilia.24,25 There are comparatively few predictive markers for determining ICI-induced colitis risk.
In this retrospective cohort study, we reviewed potential factors that correlate with grade 3–4 irAEs in melanoma patients who received ICIs at Dana-Farber Cancer Institute (DFCI). We found an association between lower levels of albumin prior to treatment with ICIs and the development of high-grade irAEs.26 Subsequently, we performed a secondary analysis to identify factors that are associated with specific irAEs. Here we report findings pertaining to ICI-induced colitis in our DFCI cohort. These results were independently validated in a cohort of melanoma patients treated with ICIs at Massachusetts General Hospital (MGH).
Methods
Discovery Cohort Patient Population and Characteristics
246 patients included in the discovery cohort of this study were identified from DFCI’s melanoma bio-specimen banking protocol (DFCI protocol 05–042). Patients in this cohort received at least one course of pembrolizumab, nivolumab, or ipilimumab alone, or the combination of ipilimumab and nivolumab between May 2011 to October 2017. Patient characteristics and concomitant medications were collected from the electronic medical record (EMR) at the time of the first ICI treatment initiation. Pre-treatment use of vitamin D was evaluated based on reports of vitamin D intake at the time the first ICI treatment was initiated, including therapeutic vitamin D (ergocalciferol and cholecalciferol) and multivitamin use containing at least 400 IU of vitamin D. Vitamin D intake was divided into 3 dosage categories: no use, ≤ 1000 IU, and > 1000 IU per day.
Other laboratory values (albumin, lactate dehydrogenase [LDH], neutrophil-to-lymphocyte ratio [NLR], and eosinophils) were collected at the time of the treatment initiation for a given line of therapy. Peri-treatment vaccination history with influenza and pneumococcal pneumonia vaccines was determined based on documentation of immunization within 3 months before or after initiating ICI therapy. Infections (pneumonia, bacteremia, UTI, or sepsis) within 6 months of initiating ICIs were noted. Comorbidities including autoimmune disease, asthma, or seasonal allergies were recorded. Tumor characteristics, including prior therapy within 6 months of the ICIs initiation, common tumor mutations (c-KIT, BRAF, NRAS), and number of metastatic sites were collected. The protocol was approved by the DFCI Institutional Review Board.
Outcomes
The primary outcome was the development of immune-related colitis as confirmed by histopathological biopsies of the colon. irAE grading was determined by review of the EMR. If specific grades were not documented in the chart by clinicians, then a single reviewer graded the irAEs using the NIH Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Data abstraction on the DFCI cohort was concluded by October 2017. irAEs were reviewed by a second reviewer to confirm the events.
Validation Cohort
External validation was performed on an independent cohort of 169 melanoma patients treated with PD-1, CTLA-4 inhibitors or the combination at MGH from December 2010 until July 2018 under IRB approved protocol (2017P002740/PHS). All diagnoses of colitis in this cohort were histopathologically confirmed and graded by an independent reviewer.
Statistical Methods
Univariate analysis was used to evaluate the relationships between the development of ICI-induced colitis and patient and clinical covariates of interest. Fisher’s exact tests were used for comparisons of categorical variables and Wilcoxon rank-sum tests for continuous variables. A multivariable prediction model for ICI-induced colitis was constructed using logistic regression. Since patients could have received more than one checkpoint therapy, ICI treatment and irAE data relevant to that ICI treatment were correlated within each patient. To address this, a multivariable, generalized linear model was fit to the data, allowing for multiple occurrences of irAEs on different ICI therapies. The binary outcome variable was modeled using a logit link function with a binomial distribution. A multivariable regression model of immune-related colitis was fit using characteristics that had a nominal univariate Fisher’s exact P-value of 0.30 or less as candidate predictors. Forward, backward, and stepwise elimination were used to assure model consistency. Predictors with a P-value of 0.05 or less were selected for the final model. For the validation, ICI class, NLR divided at 5, and vitamin D use were the only independent predictors in the logistic regression. Results are reported as odds ratios (OR) with 95% Wald confidence intervals. All reported P-values are 2-sided. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Discovery cohort characteristics and incidence of immune-related adverse events
We assessed the incidence of irAEs in 246 patients with stage III or IV melanoma treated with ICIs at DFCI from May 2011 to October 2017 (Figure 1). We limited the dataset to standard-of-care ICIs that were FDA approved at the time of the analysis, which further reduced the dataset to 213 patients. Of these 213 patients included in the analysis, 49 patients received more than one ICI regimen during their course of treatment, resulting in 267 individual ICI treatment regimens. 164 patients received a single regimen, 44 had two, and 5 had three regimens (two PD-1 and one CTLA-4 inhibitor). Of the 267 ICI regimens, 66 were ipilimumab, 17 nivolumab, 91 pembrolizumab, and 93 combined ipilimumab and nivolumab (Table 1). The most common toxicities were dermatitis and colitis (17.8% each), followed by rheumatologic (12.7%), endocrine (10.3%), hepatitis (7.5%), and pneumonitis (7.5%, Supplemental Table 1).
Figure 1.
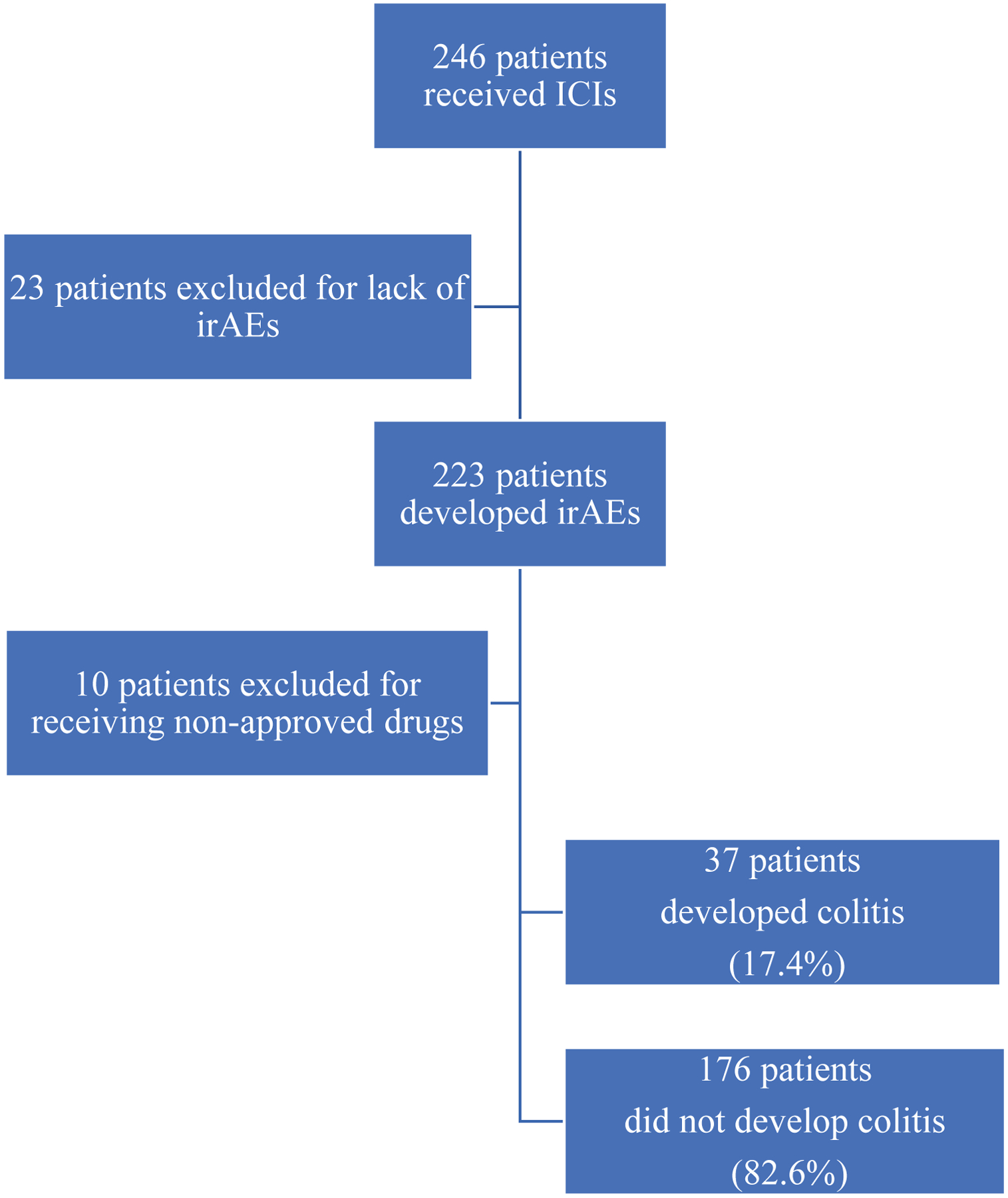
Study Schema for Patients Included in Discovery Cohort Analysis
Table 1.
Immune-Checkpoint Inhibitor Regimen at Time of Highest Grade irAE in the Discovery Cohort
| Maximum irAE grade | ||
|---|---|---|
| 1–2 | 3–4 | |
| N | N | |
| Ipilimumab and Nivolumab | 30 | 63 |
| Ipilimumab | 33 | 33 |
| Nivolumab | 12 | 5 |
| Pembrolizumab | 59 | 32 |
This table categorizes the commercially available immunotherapy treatment that patients in the discovery cohort were taking at the time of their highest grade irAE, delineated by grades 1–2 vs. grades 3–4.
Among the 213 individual patients in the discovery cohort, there were 38 reports of pathologically confirmed ICI-induced colitis in 37 patients (17.4%, Table 2); 8 presentations were grade 2, 27 were grade 3, and 3 were grade 4 colitis. The majority of colitis cases (32/38, 84%) occurred during therapy with ipilimumab monotherapy (17/38, 45%) or during combined therapy of nivolumab and ipilimumab (15/38, 39%); the rest occurred during nivolumab or pembrolizumab therapy (6/38, 16%, Table 2).
Table 2.
Incidence of Colitis Based on Immune Checkpoint Inhibitor Regimen in Discovery and Validation Cohorts
| Colitis in Discovery Cohort | Colitis in Validation Cohort | |||
|---|---|---|---|---|
| No (N=229) | Yes (N=38) | No (N=196) | Yes (N=50) | |
| N (%) | N (%) | N (%) | N (%) | |
| Checkpoint class | ||||
| Combination (Ipilimumab + Nivolumab) | 78 (34.1) | 15 (39.5) | 22 (11.2) | 11 (22.0) |
| Ipilimumab | 49 (21.4) | 17 (44.7) | 61 (31.2) | 23 (46.0) |
| Nivolumab | 16 (7.0) | 1 (2.6) | 14 (7.1) | 3 (6.0) |
| Pembrolizumab | 86 (37.6) | 5 (13.2) | 99 (50.5) | 13 (26.0) |
This table summarizes the incidence of immune-related colitis based on ICI regimen, in the discovery cohort of 213 patients receiving a total of 267 regimens, and the validation cohort of 169 patients receiving 246 regimens.
Association of clinical characteristics with ICI colitis
We performed bivariable analysis to evaluate the association of development of ICI colitis and clinical characteristics (demographics, comorbidities, peri-treatment immunization, pre-treatment laboratory values, tumor characteristics, and concomitant medications, Table 3). Vitamin D use was recorded in 66/213 patients (31%) before starting ICIs and showed a statistically significant association with decreased incidence of ICI colitis by univariate analysis (34.1% of patients without colitis reported vitamin D intake vs. 16.2% of patients with colitis, P = 0.03). Of the 66 patients taking vitamin D, 30 reported dosages of 1000 IU or less, and 36 had dosages greater than 1000 IU.
Table 3.
Patient Characteristics, Comorbid Disease History, Pre-treatment Laboratory Values, Disease and Tumor Characteristics, and Concomitant Medications in the Discovery Cohort
| Colitis | Fisher’s exact P-value | ||
|---|---|---|---|
| No (N=176) | Yes (N=37) | ||
| N (%) | N (%) | ||
| Patient Characteristics | |||
| Male | 102 (58.0) | 22 (59.5) | 0.99 |
| Age < 70 | 117 (66.5) | 21 (56.8) | 0.26* |
| Obese (BMI ≥ 30) | 43 (24.4) | 7 (18.9) | 0.53 |
| Pre-treatment ECOG PS ≥ 1 | 34 (19.3) | 5 (13.5) | 0.49 |
| History of smoking | 80 (45.5) | 18 (48.6) | 0.72 |
| History of alcohol use | 118 (67.0) | 24 (64.9) | 0.85 |
| Influenza immunization within 3 months of treatment | 34 (19.3) | 12 (32.4) | 0.08* |
| Pneumococcal pneumonia immunization within 3 months of treatment | 35 (19.9) | 13 (35.1) | 0.05* |
| Influenza OR pneumonia immunization within 3 months of treatment | 50 (28.4) | 17 (45.9) | 0.05* |
| Comorbid Diseases | |||
| Infection in previous 6 months | 13 (7.4) | 3 (8.1) | 0.99 |
| Autoimmune disease | 12 (6.8) | 2 (5.4) | 0.99 |
| Asthma | 17 (9.7) | 2 (5.4) | 0.54 |
| Seasonal allergies | 20 (11.4) | 4 (10.8) | 0.99 |
| Asthma OR seasonal allergies | 32 (18.2) | 6 (16.2) | 0.99 |
| Pre-treatment Laboratory Values | |||
| Albumin ≤ 4.2 | 96 (54.5) | 22 (59.5) | 0.72 |
| Normal LDH (110 – 220 U/L) | 110 (62.5) | 26 (70.3) | 0.45 |
| Neutrophil-to-lymphocyte ratio (NLR) ≥ 5 | 55 (31.3)a | 6 (16.2) | 0.16* |
| NLR ≥ 3 | 107 (60.8) | 18 (48.6) | 0.34 |
| Absolute eosinophil count > 400 /uL | 29 (16.5)b | 5 (13.5) | 0.81 |
| Cancer - Disease and Treatment Characteristics | |||
| Prior chemotherapy | 34 (19.3) | 7 (18.9) | 0.99 |
| Prior targeted therapy | 37 (21.0) | 5 (13.5) | 0.37 |
| Prior chemotherapy or targeted therapy within previous 6 months | 10 (5.7) | 0 (0) | 0.22* |
| Prior radiation therapy in the previous 6 months | 44 (25.0) | 12 (32.4) | 0.41 |
| c-KIT mutation | 5 (2.8) | 1 (2.7) | 0.99 |
| BRAF mutation | 49 (27.8) | 9 (24.3) | 0.84 |
| NRAS mutation | 26 (14.8) | 4 (10.8) | 0.79 |
| c-KIT, BRAF, or NRAS mutation | 77 (43.8) | 14 (37.8) | 0.59 |
| Number of metastatic sites >1 | 174 (98.9) | 37 (100) | 0.99 |
| Number of metastatic sites >3 | 85 (48.3) | 21 (56.8) | 0.37 |
| Concomitant Medications | |||
| Angiotensin-converting enzyme inhibitor (ACEi) | 29 (16.5) | 7 (18.9) | 0.81 |
| ACEi OR Angiotensin II receptor blocker (ARB) | 41 (23.3) | 10 (27.0) | 0.67 |
| Aspirin | 29 (16.5) | 8 (21.6) | 0.48 |
| Aspirin OR nonsteroidal anti inflammatory (NSAID) | 38 (21.6) | 11 (29.7) | 0.29* |
| Proton-pump inhibitor (PPI) | 42 (23.9) | 6 (16.2) | 0.39 |
| Statin | 39 (22.2) | 10 (27.0) | 0.52 |
| Vitamin D supplementation | 60 (34.1) | 6 (16.2) | 0.03* |
This table summarizes the characteristics of 213 patients based upon those who developed ICI colitis vs. those who did not develop colitis.
P < 0.30, which met inclusion for consideration in the multivariable model
Missing NLR values from 1 colitis and 7 non-colitis patients
Missing eosinophil count from 1 colitis and 3 non-colitis patients
Age divided at 70, peri-treatment pneumococcal or influenza immunization, NLR ≥ 5, prior chemotherapy and/or targeted therapy, and concomitant aspirin or NSAIDs met the univariate P-value threshold of 0.30 for potential association with increased risk of ICI colitis and were thus included in the multivariable regression model as candidate predictors.
Certain factors that were reviewed, including demographics, comorbidities, pre-treatment laboratory values, tumor characteristics, prior treatment, and concomitant medications, were not found to demonstrate statistically significant association with the development of ICI colitis (Table 3).
Multivariable Model of Predictors for ICI Colitis
A multivariable regression model of immune-related colitis was fit using the candidate predictors that met the univariate P-value threshold of 0.30. Multivariable regression revealed three independent predictors of immune-related colitis: checkpoint inhibitor class, baseline NLR ≥ 5, and vitamin D use (Table 4).
Table 4:
Multivariable Logistic Regression Analysis of Risk Factors for Developing Colitis
| Discovery Cohort (N = 213) | Validation Cohort (N = 169) | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P value | Odds Ratio | 95% CI | P value | |
| Immune Checkpoint Inhibitor Class | ||||||
| Ipi + Nivo vs. Pembrolizumab | 3.34 | 1.1 – 9.8 | 0.001 | 3.37 | 1.4 – 8.1 | 0.009 |
| Nivolumab vs. Pembrolizumab | 1.04 | 0.1 – 9.3 | 1.25 | 0.3 – 5.3 | ||
| Ipilimumab vs. Pembrolizumab | 7.48 | 2.6 – 21.8 | 2.68 | 1.5 – 4.9 | ||
| Ipi + Nivo vs. Ipilimumab | 0.45 | 0.2 – 1.0 | 1.26 | 0.5 – 2.9 | ||
| Neutrophil-to-Lymphocyte Ratio | ||||||
| ≥ 5 vs. < 5 | 0.34 | 0.1 – 0.9 | 0.046 | 0.61 | 0.3 – 1.3 | 0.38 |
| Vitamin D Intake | ||||||
| Yes vs. No | 0.35 | 0.1 – 0.9 | 0.01 | 0.46 | 0.2 – 0.9 | 0.03 |
Characteristics with a univariate Fisher’s exact P-value of 0.30 or less were incorporated into a multivariable logistic regression. The model demonstrated higher odds of developing colitis in patients treated with combination (ipilimumab + nivolumab) or ipilimumab monotherapy compared to those treated with pembrolizumab monotherapy. Furthermore, NLR ≥ 5 and pre-treatment vitamin D supplementation were associated with decreased odds of colitis. The validation cohort confirmed the relationship between colitis and vitamin D intake.
Treatment with combination therapy (ipilimumab and nivolumab) was associated with significantly increased odds of developing ICI colitis compared to treatment with pembrolizumab monotherapy (OR 3.34, 95% CI 1.1–9.8). In addition, treatment with ipilimumab alone conferred significantly increased odds of colitis compared to pembrolizumab monotherapy (OR 7.48, 95% CI 2.6–21.8). There was no statistically significant difference in the risk of developing ICI colitis between patients treated with nivolumab monotherapy compared to pembrolizumab monotherapy (OR 1.04, 95% CI 0.1–9.3).
Notably, patients taking vitamin D at the time their first ICI treatment was initiated had significantly reduced odds of developing colitis compared with patients who were not on pre-treatment vitamin D (OR 0.35, 95% CI 0.1–0.9, P = 0.01). Furthermore, pre-treatment NLR ≥ 5 was also associated with reduced odds of colitis (OR 0.34, 95% CI 0.1–0.9, P = 0.046).
Given the association of vitamin D with decreased risk of colitis, we compared different dosages of vitamin D intake and their association with ICI colitis. In the refit multivariable regression model, there was no statistically significant difference in the risk of developing ICI colitis between patients taking > 1000 IU of vitamin D compared to patients taking ≤ 1000 IU.
Validation Cohort Analysis
External validation of our multivariable model was performed on an independent cohort from MGH, which included 169 melanoma patients treated with 246 regimens of ipilimumab, nivolumab, or pembrolizumab alone, or the combination of ipilimumab and nivolumab. In the validation cohort, there were 50 reports of immune-related colitis in 49 of the 169 patients (29.9%); 22 presentations were grade 2, 15 cases were grade 3, and none were grade 4 colitis. Thirty-four of the 50 cases of colitis occurred during ipilimumab monotherapy or during combined therapy of ipilimumab with nivolumab (Table 2). Vitamin D use at the time of first ICI treatment initiation was reported in 103 of the 169 patients (60.9%), or 137 of the 246 ICI treatment regimens.
Patients in the validation cohort treated with combination therapy had increased odds of developing colitis compared to patients on pembrolizumab alone (OR 3.37, 95% CI 1.4–8.1, Table 4). Similarly, patients in the validation cohort treated with ipilimumab monotherapy were more likely to develop colitis compared to patients treated with pembrolizumab alone (OR 2.68, 95% CI 1.5–4.9). We did not find a statistically significant difference in the risk of developing ICI colitis between patients treated with nivolumab monotherapy compared to pembrolizumab monotherapy (OR 1.25, 95% CI 0.3–5.3).
Furthermore, multivariable regression analysis of the validation cohort confirmed our finding of an association between vitamin D intake and decreased odds of ICI colitis (OR 0.46, 95% CI 0.2–0.9, P = 0.03). However, the validation cohort did not confirm an association between NLR ≥ 5 and decreased risk for ICI colitis (OR 0.61, 95% CI 0.3–1.3, P = 0.38).
Discussion
Our study analyzed patient demographic characteristics, medical comorbidities, tumor characteristics, prior therapy, pre-treatment laboratory values, and concomitant medications to identify factors associated with the development of immune-related colitis. We found that pre-treatment vitamin D intake significantly lowered the odds of developing ICI colitis in advanced melanoma patients and validated these results in an independent cohort of melanoma patients. To our knowledge, this is the first study that identifies vitamin D as a protective factor against the development of ICI colitis.
Prior studies in patients with checkpoint inhibitor colitis have suggested NSAID use,15 pre-existing IBD,27–30 baseline microbiota (enriched in Firmicutes and poor in Bacteroidetes)31 as putative risk factors for the development of ICI colitis. Tumor histology has also been proposed as a risk factor for ICI colitis (melanoma as compared to non-small cell lung carcinoma and renal cell carcinoma) although these findings may be confounded by the disproportionate use of ipilimumab and awareness of colitis risk in melanoma patients.32,33 Various predictive biomarkers have also been studied, with higher serum interleukin 17 (IL-17) levels at baseline shown to correlate with development of grade 3 colitis,34 and increase in absolute eosinophil count by week 4 of therapy correlated with any irAE.35 However, the role of vitamin D in immune checkpoint inhibitor-induced colitis has not been previously reported.
There are compelling data to suggest that vitamin D plays an important role in the risk of autoimmune disorder exacerbation36–38 and more specifically IBD.39,40 Vitamin D has immunomodulatory properties by decreasing the pro-inflammatory Th1 response and increasing anti-inflammatory Th2 cells.41 In mouse models, vitamin D prevents autoimmune disorders by suppression of IL-17A and Th17 cells.42,43 In addition, vitamin D may also promote self-tolerance by inhibiting the differentiation and maturation of dendritic cells and increasing T regulatory cells.44,45 Studies also suggest that vitamin D has a role in Toll-like receptor activation of macrophages, which triggers antimicrobial activity against intracellular bacteria, and gut vitamin D receptor (VDR) signaling, which inhibits colitis by protecting the epithelial mucosal barrier.46,47 Polymorphisms in the VDR gene are also associated with susceptibility to inflammatory bowel disease.48
In the multivariate regression model of our discovery cohort, we also found that elevated baseline NLR ≥ 5 was negatively correlated with risk of developing ICI colitis. This finding is similar to that of a recent study in South Korea reporting lower risk of irAEs in patients with baseline NLR ≥ 3 who were treated with pembrolizumab, although this study did not examine colitis specifically.49 Neutrophil-to-lymphocyte ratio is an increasingly used biomarker of systemic inflammation, but there are conflicting reports around its prognostic value in IBD. While some studies have shown that high NLR predicts increased severity of active UC and CD,50,51 low NLR has also been found to be strongly associated with risk of UC relapse in patients receiving tacrolimus therapy.52 In our study, NLR evaluated at the threshold of 3 did not yield statistically significant associations with ICI colitis. In addition, our initial finding of decreased risk of ICI colitis in patients with NLR ≥ 5 was not confirmed in the validation cohort. Further studies on NLR as a predictive biomarker for irAEs and immune-related colitis are warranted using a larger sample size.
We acknowledge that our study has key limitations, including its retrospective nature, the fact that it only included melanoma patients, and that we evaluated vitamin D intake before the initiation of the first ICI rather than each ICI. The heterogeneity in study populations is both a weakness and strength. Patients without irAEs were not included in the discovery cohort; however, those without any irAEs were a small percentage of the patients. It should be noted that the validation cohort from MGH included a higher percentage of patients with ICI colitis, as well as a higher proportion of patients without any irAEs. The differences between these cohorts may reflect heterogeneity of the patient population or cross-institutional variability in irAE grading. We were also limited by sample size for some of the patient characteristics evaluated (medical comorbidities, tumor mutation status, prior infection, and concomitant medication use). The limited number of exposed patients may prevent meaningful analysis of these factors. In particular, the subgroup analysis comparing different vitamin D dosages and association with ICI colitis is limited due to the small sample size of each dosage category. We also were not able to analyze baseline serum levels of vitamin D in patients due to limited serum samples, which may be a more meaningful measure of physiologic vitamin D levels compared to vitamin D intake as a proxy. Patients reporting high dosages of vitamin D intake (> 1000 IU) may represent a confounding factor due to being prescribed for underlying disease processes (vitamin D deficiency, osteoporosis, etc.). Future studies should seek to investigate a relationship between specific dosage levels of vitamin D and decreased risk for ICI colitis, as well as confirming results through baseline vitamin D serum levels.
In conclusion, here we report that patients taking vitamin D at the time their first ICI treatment was initiated had significantly reduced odds of developing ICI colitis over the course of their immunotherapy regimen. These results may suggest benefit in prophylactic use of vitamin D supplementation to prevent ICI colitis, as is currently recommended for ulcerative colitis53–56 and graft-versus-host disease.57–59 The specific mechanism by which vitamin D may prevent immune-related colitis should be further explored through future correlative studies, including cytokine analyses and immune profiling at baseline and at the time of colitis. The results from this retrospective study should be validated prospectively in larger cohorts, which may lead to a better understanding of factors that drive and prevent the development of immune checkpoint inhibitor-induced colitis.
Supplementary Material
Funding:
The authors thank the Parker Institute of Immunotherapy for their support of this project (5812101). MD is supported by an American Gastroenterological Association Research Scholars Award and an NIH/NIDDK Mentored Clinical Scientist Training Grant (1K08 DK114563-01). SB is supported by the Massachusetts General Hospital T32 (2T32CA071345-21A1).
Footnotes
Competing Interests:
The authors report the following competing interests:
SG is employed as Senior Physician Editor, Gastroenterology for UpToDate, a Wolters Kluwer company.
MD has grant support from Novartis, is a member of the scientific advisory board for Neoleukin Therapeutics, and has been a consultant for Tillotts Pharma, Genentech, and Partner Therapeutics.
SB reports consulting with Two River Consulting and Third Rock Ventures as well as equity in Kronos Bio and Allogene Therapeutics.
RH has research grant support from Novartis and BMS as well as a consulting arrangement with Tango Therapeutics.
EB has served on advisory boards for Array Biopahrma, Bristol-Myers Squibb (BMS), Trieza Therapeutics and Novartis, and she also receives clinical trial support from Eli Lilly, Novartis, BMS, Genentech and BVD.
JI reports consulting for Two River, Trammel Therapeutics, and Tango Therapeutics, and owns equity in Jounce Therapeutics and Kronos Therapeutics.
PO reports the following: advisory roles for Alexion, Array, BMS, Celldex, CytomX, Genentech, Merck, Neon Therapeutics, Novartis, Pfizer, and TRM Oncology; institutional grants from Armo Biosciences, AstraZeneca/MedImmune, BMS, Celldex, CytomX, Genentech, Merck, Neon Therapeutics, Novartis, and Pfizer; and a speaking engagement from Medscape.
SH reports the following: grants from BMS and Novartis; personal fees from BMS, Merck, Serono, Novartis, Takeda, Surface Pharmaceuticals, Genentech/Roche, Compass Therapeutics, Apricity, Bayer, Aduro, Partners Therapeutics, Sanofi, Pfizer, Pionyr Immunotherapeutics, 7 Hills Pharma, Verastem Oncology, Rheos Medicines, and Kairos Therapeutics; equity in Torque Therapeutics; and patents #20100111973 and #7250291 issued as well as #20170248603, #20160340407, #20160046716, #20140004112, #20170022275, #20170008962, and “Methods of Using Pembrolizumab and Trebananib” pending.
OR has research support from Merck; educational grants from BMS and Merck; consulting agreements with Merck, Celgene, Five Prime Therapeutics, GSK, GFK, Defined Health INC, Roche/Genentech, Puretech, Imvax, Leerink and PRMA Consulting; and a patent “Methods of Using Pembrolizumab and Trebananib” pending.
Ethics Approval: Patients evaluated in this study were identified from the Dana-Farber Cancer Institute melanoma bio-specimen banking protocol (DFCI IRB approved protocol 05–042) and Massachusetts General Hospital (MGH IRB approved protocol 2017P002740/PHS).
Availability of Data and Material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Couzin-Frankel J Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. [DOI] [PubMed] [Google Scholar]
- 2.Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8(5):583–600. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. [DOI] [PubMed] [Google Scholar]
- 4.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 6.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. [DOI] [PubMed] [Google Scholar]
- 7.Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019;40(6):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67(11):2056–2067. [DOI] [PubMed] [Google Scholar]
- 9.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–3714. [DOI] [PubMed] [Google Scholar]
- 10.Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J Immunother. 2018;41(3):101–108. [DOI] [PubMed] [Google Scholar]
- 11.Dougan M Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front Immunol. 2017;8:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(7):785–792. [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertha M, Bellaguara E, Kuzel T, Hanauer S. Checkpoint Inhibitor-Induced Colitis: A New Type of Inflammatory Bowel Disease? ACG Case Rep J. 2017;4:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marthey L, Mateus C, Mussini C, et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(4):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology. 2018;155(4):1079–1089 e1073. [DOI] [PubMed] [Google Scholar]
- 17.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. [DOI] [PubMed] [Google Scholar]
- 18.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019;157(3):647–659 e644. [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric Acid Suppression Is Associated with an Increased Risk of Adverse Outcomes in Inflammatory Bowel Disease. Digestion. 2017;95(3):188–193. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156(5):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan SS, Luben R, Bergmann MM, et al. Aspirin in the aetiology of Crohn’s disease and ulcerative colitis: a European prospective cohort study. Aliment Pharmacol Ther. 2011;34(6):649–655. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129(3):827–836. [DOI] [PubMed] [Google Scholar]
- 23.Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23(33):6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgenstern S, Brook E, Rinawi F, Shamir R, Assa A. Tissue and peripheral eosinophilia as predictors for disease outcome in children with ulcerative colitis. Dig Liver Dis. 2017;49(2):170–174. [DOI] [PubMed] [Google Scholar]
- 25.Azad S, Sood N, Sood A. Biological and histological parameters as predictors of relapse in ulcerative colitis: a prospective study. Saudi J Gastroenterol. 2011;17(3):194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum S, Ishizuka J, Qazi T, et al. Hypoalbuminemia as a predictor factor for immune related adverse events (irAEs) in advanced melanoma patients treated with immune checkpoint inhibitors (ICIs) Presented at Society for Immunotherapy of Cancer (SITC); November 7–11, 2018, Washington D.C. [Google Scholar]
- 27.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016;2(2):234–240. [DOI] [PubMed] [Google Scholar]
- 28.Kahler KC, Eigentler TK, Gesierich A, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother. 2018;67(5):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Sbeih H, Faleck DM, Ricciuti B, et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. Journal of Clinical Oncology (in press) JCO.19.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. [DOI] [PubMed] [Google Scholar]
- 32.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. [DOI] [PubMed] [Google Scholar]
- 33.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6(10):e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindler K, Harmankaya K, Kuk D, et al. Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab. Journal of Clinical Oncology. 2014;32(15_suppl):9096–9096. [Google Scholar]
- 36.Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front Immunol. 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4(8):404–412. [DOI] [PubMed] [Google Scholar]
- 38.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2017;15(2):240–246 e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142(3):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stucci S, Palmirotta R, Passarelli A, et al. Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol Lett. 2017;14(5):5671–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi S, Pantalena LC, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7(4):3011–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol. 2009(188):251–273. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123(9):3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. [DOI] [PubMed] [Google Scholar]
- 48.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47(2):211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eun Y, Kim IY, Sun JM, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep. 2019;9(1):14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celikbilek M, Dogan S, Ozbakir O, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27(1):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acarturk G, Acay A, Demir K, Ulu MS, Ahsen A, Yuksel S. Neutrophil-to-lymphocyte ratio in inflammatory bowel disease - as a new predictor of disease severity. Bratisl Lek Listy. 2015;116(4):213–217. [DOI] [PubMed] [Google Scholar]
- 52.Nishida Y, Hosomi S, Yamagami H, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction. PLoS One. 2019;14(3):e0213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S, Yoon S, Zhang YG, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karimi S, Tabataba-Vakili S, Yari Z, et al. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr J. 2019;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahamed ZR, Dutta U, Sharma V, et al. Oral Nano Vitamin D Supplementation Reduces Disease Activity in Ulcerative Colitis: A Double-Blind Randomized Parallel Group Placebo-controlled Trial. J Clin Gastroenterol. 2019;53(10):e409–e415. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Chen N, Wang D, Zhang J, Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta-analysis. Medicine (Baltimore). 2018;97(46):e12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Bahr L, Blennow O, Alm J, et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50(9):1217–1223. [DOI] [PubMed] [Google Scholar]
- 58.Carrillo-Cruz E, Garcia-Lozano JR, Marquez-Malaver FJ, et al. Vitamin D Modifies the Incidence of Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation Depending on the Vitamin D Receptor (VDR) Polymorphisms. Clin Cancer Res. 2019;25(15):4616–4623. [DOI] [PubMed] [Google Scholar]
- 59.Caballero-Velazquez T, Montero I, Sanchez-Guijo F, et al. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin Cancer Res. 2016;22(23):5673–5681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


