Abstract
Purpose:
To compare the effect of different echo times (TE) on the detection of prostate cancer (PCa) on T2-weighted (T2W) MR images.
Materials and Methods:
This study recruited patients (n=38) with histologically confirmed PCa who underwent preoperative 3T MRI. Three radiologists independently marked region on interests (ROIs) on suspected PCa lesions on T2W images at different TEs: 90, 150 and 180 ms obtained with Turbo Spin Echo imaging protocol with multiple echoes. The ROIs were assigned a value 1-5 indicating the reviewer’s confidence in accurately detecting PCa. These ROIs were compared to histologically confirmed PCa (n=95) on whole mount prostatectomy sections to calculate sensitivity, positive predictive value (PPV), and confidence score.
Results:
Two radiologists (R1, R2) showed significantly increased sensitivity for PCa detection at 180ms TE compared to 90ms (R1: 43.2, 50.5, 50.5%, R2: 45.3, 44.2, 53.7% at TE of 90, 150, 180ms respectively) (p=0.048, 0.033 for R1 and R2). Sensitivity was similar for radiologist 3 (45.3-46.3%) at different TE values (p=0.953). No significant difference in the PPV (R1: 64.1-70.6%, R2: 46.7-56.0%, R3: 70.5-81.5%) and the confidence score assigned (R1: 4.6-4.8, R2: 4.6-4.8 R3: 4.3-4.4) was found for either of the radiologists.
Conclusion:
Our results suggest improved detection of PCa with similar PPV and confidence scores when higher TE values are utilized for T2-weighted image acquisition.
Keywords: T2-weighted, echo time, prostate cancer, confidence score, sensitivity
Introduction
Prostate cancer is the second leading cause of cancer related death among men in the United States (1). Multiparametric MRI (mpMRI) is increasingly being used for prostate cancer diagnosis and biopsy guidance, and has excellent diagnostic performance for detecting prostate cancer (2-4). Prostate Imaging Reporting and Data System version 2 (PI-RADS v2 and v2.1) guidelines (5, 6) recommend T1 and T2-weighted images, diffusion-weighted imaging and dynamic contrast enhanced MRI as part of routine mpMR protocol. T2-weighted (T2W) imaging remains an integral part of prostate mpMRI, as it provides high resolution anatomical images and has been shown to be highly effective in diagnosing prostate cancer. As part of T2-weighted imaging, PI-RADS recommends 3 mm slice thickness with no gaps, a 12-20 cm field-of-view encompassing the whole prostate and seminal vesicles, and an in-plane resolution of ≤0.7 mm (phase) x ≤0.4 mm (frequency). While PI-RADS recommends an echo time (TE) ≤90 ms be utilized for diffusion weighted images, no recommendation is given for T2W images by PI-RADS v2 as well as updated guidelines in PI-RADS v2.1 (6). Studies have shown that varying mpMRI parameters, such as diffusion time and b-value in diffusion-weighted imaging and temporal resolution in DCE-MRI can affect quantitative MR values as well as imaging performance (7-14).
While the choice of TE in diffusion weighted imaging has been studied and shown to alter measurements and diagnostic performance (15), there is little available data regarding how different TEs affects the performance of T2-weighted images for the detection of prostate cancer. For instance, there are no standard clinical imaging parameters for echo time (TE) for T2W imaging, and the reported TEs used at several major institutions vary from 90 to 120 ms (16-23). This variability makes meta-data analysis and comparing images and radiologist performance across institutions difficult, and it is unclear whether some TEs are superior to others. Therefore, determining the optimal TE for detecting prostate cancer on T2W images may be useful for improving prostate cancer diagnosis and to standardize protocols across institutions. This study aims to compare the effect of different echo times (TE) on the detection of prostate cancer on T2-weighted MR images.
Materials and Methods
Study patients:
This study involved retrospective analysis of prospectively acquired data which was conducted after institutional review board approval. Informed patient consent was previously obtained and the study was compliant with the Health Insurance Portability and Accountability Act. A total of 84 patients with biopsy-proven prostate cancer underwent mpMRI between March 2014 and March 2016 at our research center followed by subsequent radical prostatectomy. Inclusion criteria involved patients undergoing multi-echo T2-weighted with the standard clinical Turbo Spin Echo (TSE) imaging protocol. Exclusion criteria included any prior radiation or hormonal replacement therapy. Exclusion of cases in this study, included 11 cases where no multi-echo T2W images were acquired and 35 cases where multi-echo T2W imaging was performed using research k-t-T2 research sequence (21, 24) that used different imaging parameters instead of TSE. The final set included 38 patients. The mean of the patients was 58.3 years (range 40-76 years) and mean PSA was 7.9 ng/mL (range 0.81-66.05 ng/mL) prior to MR imaging.
MR imaging:
Patients underwent preoperative MRI with a 3T Philips Achieva MR system with a 6-channel cardiac phased array coil placed around the pelvis, and with an endorectal coil (Medrad eCoil, Bayer Healthcare). To limit peristalsis of the rectal wall 1mg dose of glucagon (Glucagon, Eli Lilly & Co., Indianapolis) was injected prior to imaging. T2W images and multi echo T2W images were acquired, and only patients who underwent T2 mapping were included in the study. The echo times used were 90, 150, and 180 ms. The field-of-view was 160 mm x 160 mm and the matrix size was 212 x 212 with an in-plane resolution of 0.75 x 0.75 mm. The repetition time (TR) was 7.5 ms.
MR image analysis:
The multi-echo T2W images for all 38 patients were loaded on a custom module, PCampReview in 3D Slicer (10). Three sets of images were created with images at one of the three TEs assigned randomly for every patient. Three expert radiologists (R1, WW with 15 years of experience, R2: XX, 10 years of experience, and R3: YY, 5 years of experience, all fellowship trained in abdominal imaging) working independently and blinded to the histology results and echo time (TE) evaluated the images and marked regions of interest (ROIs) that were suspicious for PCa lesions on T2W images. The radiologists were blinded to the TE and patient information and were given a two-week gap (memory washout period to forget the MR images) between reviewing each of the three case sets. Each ROI for suspected cancer lesion was assigned a score of 1-5 based on the confidence in accurately detecting cancer, with 5 being the highest confidence.
After radical prostatectomy, the whole prostates of the patients were fixed in formalin and serially sectioned approximately in approximately the same plane as the axial MR images. Whole mount tissue sections were embedded in paraffin and stained with hematoxylin and eosin. The cancers were marked on the histologic slides by an expert pathologist (ZZ, 10 years’ experience). The sites of known malignancy on the specimens were then compared to the preoperative MR imaging; only lesions with at least one dimension 5 mm or greater were included in the analysis. The histologically confirmed PCa on whole mount sections were matched with ROIs drawn on T2W images by the radiologists. In cases for which the radiologist marked multiple ROIs for the same cancer lesion, only the highest confidence score was selected.
Statistical analysis:
Statistical analysis was performed using SPSS (IBM Corporation, Armonk, NY). Sensitivity, precision or positive predictive value (PPV) and radiologist’s mean confidence score for PCa detection using T2-weighted imaging at different echo times were calculated. The analysis was performed for all cancers, cancer in the peripheral and transition zones and for all clinically significant cancers (≥ Gleason 3+4). The difference in sensitivity and confidence scores was analyzed using Friedman test with post hoc Wilcoxon signed rank test. The difference in PPV was assessed using z-test (hypothesis testing). Cronbach alpha (α) was calculated for measuring inter-observer agreement between the three radiologists at each TE. Cronbach alpha ≥ 0.7 is considered acceptable inter-observer agreement or consistency between the three radiologists. While α ≥ 0.8 and < 0.9 is considered good and α ≥ 0.9 considered excellent.
Results
A total of 95 histologically confirmed PCa lesions with dimensions greater than 5 mm were found on whole mount sections of prostates from 38 patients. 35 lesions were Gleason score 3+3, 48 lesions were Gleason 3+4, 11 lesions were Gleason 4+3, and 1 lesion was Gleason 4+5. The mean tumor size was 1.5 cm × 0.8 cm (range 0.5-5.3 cm× 0.2-2.2 cm). The clinical information for patients and cancer lesions can be found in Table 1. Representative examples of marked T2W images at different TEs and corresponding histologic sections are shown in Figures 1-3. The detailed statistics sensitivity, PPV, and confidence score for PCa detection using T2W images at different TEs each radiologist is provided in Tables 2-4.
Table 1:
Clinical information for patients and cancer lesions
| Mean Age | 58 years (range 40 - 76 years) |
| Mean PSA | 7.92 ng/ml (range 0.81 - 66.05 ng/ml) |
| Cancer lesions | 95 |
| Peripheral zone | 71 (75%) |
| Transition zone | 24 (25%) |
| Mean Tumor size | 1.5 cm × 0.8 cm (range 0.5 - 5.3 cm× 0.2 - 2.2 cm) |
| Gleason Score | |
| Gleason score 3+3 | 35 |
| Gleason score 3+4 | 48 |
| Gleason score 4+3 | 11 |
| Gleason score 4+5 | 1 |
Figure 1:
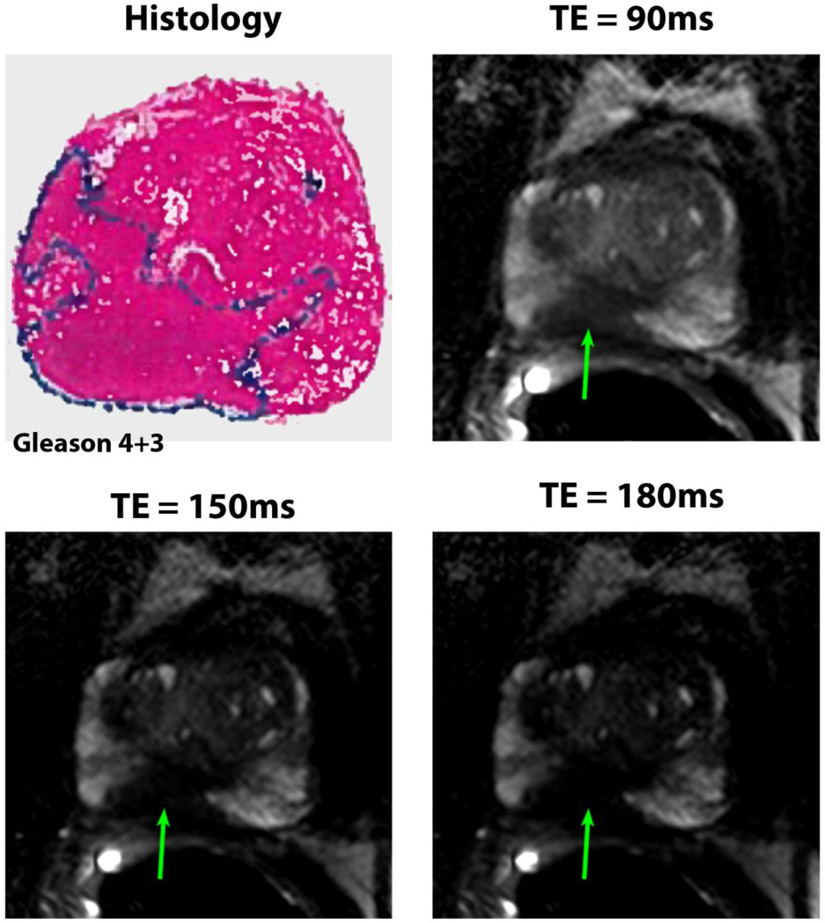
50 years old patient with PSA of 4.8 ng/mL. The Gleason 4+3 cancer (4.0 cm × 1.7 cm primarily in the right peripheral zone in mid prostate shown by arrows) was detected and very well visualized by radiologists at the 3 different echo times with confidence score of 5 for the 3 radiologists.
Figure 3:
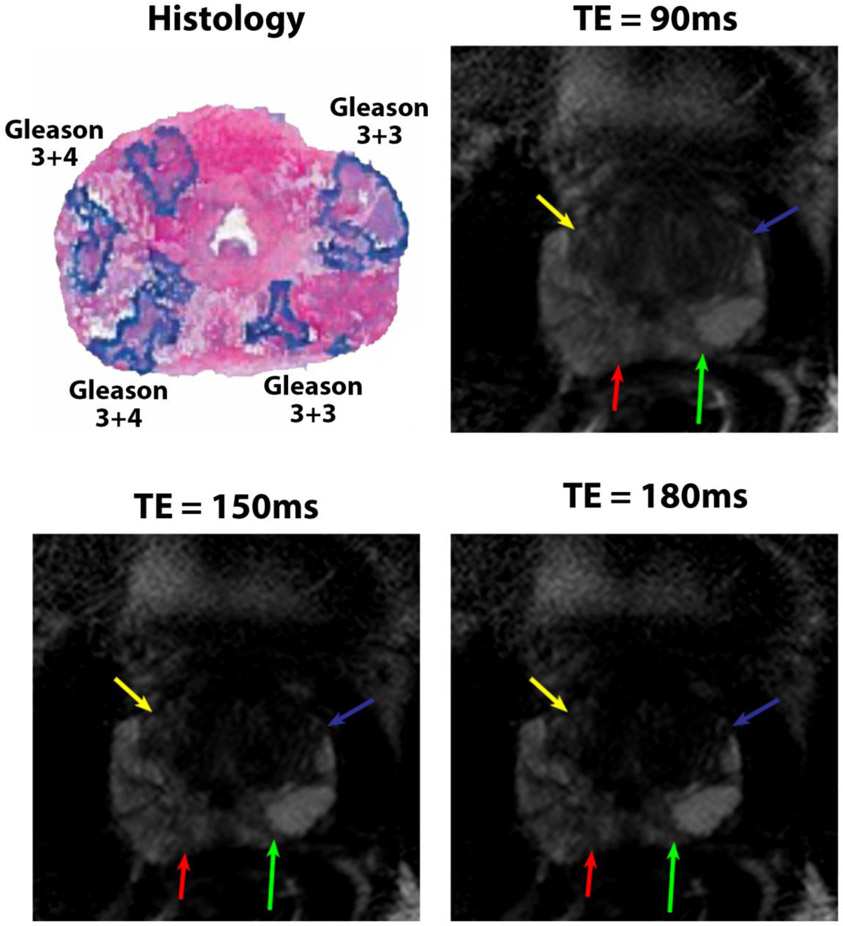
49 years old patient with PSA of 2.8 ng/mL. The Gleason 3+4 cancer (1.7 cm × 1.2 cm in the right anterior peripheral zone shown by yellow arrows) was only detected at TE = 180 ms by R1 and R2. The Gleason 3+4 cancer (1.5 cm × 0.6 cm in the right posterior peripheral zone shown by red arrows) was detected at TE = 90 and 180ms by R1 and at all TE’s by R3 but not by R2. The Gleason 3+3 cancer (2.0 cm × 1.4 cm in the left anterior peripheral zone shown by blue arrows) was detected at TE = 150 ms by R1 and at all TEs by R2, but not by R3. The Gleason 3+3 cancer (0.7 cm × 0.5 cm in the left posterior peripheral zone shown by green arrows) was detected at TE = 150 and 180 ms by R1 and at all TE’s by R3, but not by R2.
Table 2:
Radiologist Performance for all cancers (n=95)
| Radiologist 1 | |||
|---|---|---|---|
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 41 | 48 | 48 |
| False Positive | 23 | 20 | 24 |
| Sensitivity (%) | 43.2 | 50.5 | 50.5 |
| PPV (%) | 64.1 | 70.6 | 66.7 |
| Confidence Score | 4.8±0.4 | 4.7±0.6 | 4.6±0.7 |
| Radiologist 2 | |||
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 43 | 42 | 51 |
| False Positive | 43 | 48 | 40 |
| Sensitivity (%) | 45.3 | 44.2 | 53.7 |
| PPV (%) | 50.5 | 46.7 | 56.0 |
| Confidence Score | 4.8±0.4 | 4.7±0.6 | 4.6±0.7 |
| Radiologist 3 | |||
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 44 | 44 | 43 |
| False Positive | 10 | 12 | 18 |
| Sensitivity (%) | 46.3 | 46.3 | 45.3 |
| PPV (%) | 81.5 | 78.6 | 70.5 |
| Confidence Score | 4.3±0.5 | 4.3±0.6 | 4.4±0.5 |
Table 4:
Radiologist Performance for different prostatic zone
| Peripheral zone (n=71) | Transition zone (n=24) | |||||
|---|---|---|---|---|---|---|
| Radiologist 1 | ||||||
| Echo Time (ms) | Echo Time (ms) | |||||
| 90 | 150 | 180 | 90 | 150 | 180 | |
| True Positive | 36 | 41 | 43 | 5 | 7 | 5 |
| False Positives | 17 | 13 | 19 | 6 | 7 | 5 |
| Sensitivity (%) | 50.7 | 57.7 | 60.6 | 20.8 | 29.2 | 20.8 |
| PPV (%) | 67.9 | 75.9 | 69.3 | 45.4 | 50.0 | 50.0 |
| Confidence Score | 4.8 ± 0.4 | 4.7 ± 0.6 | 4.6 ± 0.7 | 4.8 ± 0.4 | 4.7 ± 0.5 | 4.8 ± 0.4 |
| Radiologist 2 | ||||||
| Echo Time (ms) | Echo Time (ms) | |||||
| 90 | 150 | 180 | 90 | 150 | 180 | |
| True Positive | 40 | 39 | 46 | 3 | 3 | 5 |
| False Positives | 35 | 38 | 33 | 8 | 10 | 7 |
| Sensitivity (%) | 56.3 | 54.9 | 64.8 | 12.5 | 12.5 | 20.8 |
| PPV (%) | 53.3 | 50.6 | 58.2 | 27.3 | 23.1 | 41.7 |
| Confidence Score | 4.1 ± 1.1 | 4.3 ± 0.9 | 3.9 ± 1.1 | 3.3 ± 0.6 | 3.3 ± 1.2 | 3.2 ± 0.8 |
| Radiologist 3 | ||||||
| Echo Time (ms) | Echo Time (ms) | |||||
| 90 | 150 | 180 | 90 | 150 | 180 | |
| True Positive | 39 | 38 | 38 | 5 | 6 | 5 |
| False Positives | 8 | 8 | 13 | 2 | 4 | 5 |
| Sensitivity (%) | 54.9 | 53.5 | 53.5 | 20.8 | 25.0 | 20.8 |
| PPV (%) | 82.9 | 82.6 | 74.5 | 71.4 | 60.0 | 50.0 |
| Confidence Score | 4.3 ± 0.5 | 4.3 ± 0.7 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.2 ± 0.4 | 4.6 ± 0.5 |
Sensitivity:
Radiologist 1 showed significantly different (χ2 = 6.091, p = 0.048) sensitivities at different TEs for PCa detection with sensitivity of 43.2% at 90 ms, 50.5% at 150 ms and 50.5% at 180 ms. Post-hoc test showed that the sensitivity for detecting PCa for this radiologist improved at higher TE: 180 ms compared to 90 ms (p = 0.035). However, no significant difference was found between 90 and 150 ms (p = 0.052) and between 150 and 180 ms (p = 1.000).
Radiologist 2 showed significantly different (χ2 = 8.111, p = 0.017) sensitivities at different TEs for PCa detection with sensitivity of 45.3% at 90 ms, 44.2% at 150 ms and 53.7% at 180 ms. Post-hoc test showed that the sensitivity for detecting PCa for this radiologist improved at higher TE of 180 ms compared to 90 ms (p = 0.033) and 150 ms (p = 0.020). However, no significant difference was found between 90 and 150 ms (p = 0.705).
Radiologist 3 showed no significantly difference (χ2 = 0.095, p = 0.953) in sensitivities between different TEs for PCa detection with sensitivity of 46.3% at 90 ms, 46.3% at 150 ms and 45.3% at 180 ms.
For clinically significant cancers only (Table 3), the sensitivity was nominally higher at either TEs 150 and 180 ms for all 3 radiologists compared to TE of 90 ms. Radiologist had highest sensitivity at TE of 150 ms (61.7%), while radiologists 2 and 3 performed using T2W images with TE of 180 ms with sensitivity of 65.0% and 60.0% respectively. Statistical significance was not achieved due to small size unlike the subset for all cancer that is statistically powered to determine statistical significance.
Table 3:
Radiologist Performance for clinically significant cancers (n=60)
| Radiologist 1 | |||
|---|---|---|---|
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 34 | 37 | 36 |
| Sensitivity (%) | 56.7 | 61.7 | 60.0 |
| Confidence Score | 4.9±0.3 | 4.8±0.5 | 4.8±0.5 |
| Radiologist 2 | |||
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 34 | 34 | 39 |
| Sensitivity (%) | 56.7 | 56.7 | 65.0 |
| Confidence Score | 4.1±1.0 | 4.3±0.9 | 4.0±1.1 |
| Radiologist 3 | |||
| Echo Time (ms) | |||
| 90 | 150 | 180 | |
| True Positive | 33 | 35 | 36 |
| Sensitivity (%) | 55.0 | 58.3 | 60.0 |
| Confidence Score | 4.4±0.6 | 4.4±0.6 | 4.5±0.6 |
Of these lesions 71 lesions (75%) were in the peripheral zone and 24 lesions (25%) were in the transition zone. The mean size of the peripheral (1.5 cm × 0.9 cm) and transition (1.6 cm × 0.8 cm) zone lesions were similar (p = 0.4). The sensitivity of detecting cancers at higher TEs was nominally higher for radiologists 1 and 2 in the peripheral zone. Radiologist 1 showed higher sensitivity at 150 ms (41/71 = 57.7%) and 180ms (43/71 = 60.6%) compared to 90 ms (36/71 = 50.7%). Radiologist 2 showed higher sensitivity at 180ms (46/71 = 64.8%) compared to 90 ms (40/71 = 56.3%) and 150 ms (39/71 = 54.9%). Radiologists 3 showed no difference in sensitivity for detecting peripheral zone cancer at different TEs (39/71 = 54.9% at 90ms and 38/71 = 53.5% at 150 and 180ms). However, no significant difference in sensitivity for detecting transition zone cancers was found (R1: 21-29%, R2: 13-21%, R3: 21-25%), possibly due to small sample of lesions in the transition zone. However, with this limited cohort, the sensitivity for detecting TZ cancers was best at TE=150ms for 2 radiologists (R1, R3) and at TE=180ms for one radiologist (R2). Detailed statistics can be found in Table 4.
Positive predictive value:
The PPV for radiologist 1 was 64.1%, 70.6% and 66.7% at 90, 150 and 180 ms TE respectively. There were no significant differences in the PPV calculated between the different TEs (90 and 150ms: p = 0.424, 90 and 180ms: p = 0.749, 150 and 180ms: p = 0.617). Similarly, there were no significant differences in the PPV calculated between the different TEs (90 and 150ms: p = 0.656, 90 and 180ms: p = 0.418, 150 and 180ms: p = 0.201) for radiologist 2 with PPV of 50.5%, 46.7% and 56.0% at 90, 150 and 180 ms TE respectively. The PPV for radiologist 3 was 46.3%, 46.3% and 45.3% at 90, 150 and 180 ms TE respectively, with no significant differences between the different TEs (90 and 150ms: p = 0.704, 90 and 180ms: p = 0.171, 150 and 180ms: p = 0.317). Similar analysis involving cancer in the peripheral and transition zones (Table 4) and for all clinically significant cancers (Table 3) found no significant differences in PPV at different TEs. PPV was not calculated for clinically significant cancer as the radiologists were tasked with detecting all cancers, so the number of false positives are same as those for all cancers.
Confidence score:
The confidence scores can be found in Tables 2-4. No significant difference in confidence score assigned by radiologist 1 (χ2 = 2.146, p = 0.342), radiologist 2 (χ2 = 4.867, p = 0.088) or radiologist 3 (χ2 = 0.875, p = 0.646) was found for all cancers. Similar analysis involving cancer in the peripheral and transition zones and for all clinically significant cancers found no significant differences in confidence score assigned by radiologists at different TEs.
Inter-observer agreement:
There was excellent overall inter-observer agreement in the assigned confidence score between the 3 radiologists (α = 0.912), with similarly good agreement at each TE: 90 ms (α = 0.845), 150 ms (α = 0.828) and 180 ms (α = 0.829).
Discussion
T2 weighted imaging is an integral component of prostate cancer imaging in MRI as per PI-RADS guidelines (5, 6). However, PI-RADS consensus guidelines does not specifically address which TE values should be used for T2 weighted images, and there is variability in the TE values used by different institutions. This study investigated the effect of different TE on the detection of prostate cancer on T2W MR images. Our results suggest that TE has an effect on the contrast of cancerous lesion with respect to surrounding benign prostatic tissue. Our observer study found that there is a higher sensitivity for prostate cancer detection when higher TE values are utilized for T2-weighted image acquisition lesions compared to the lower TE values typically utilized routinely in clinical MRI for 2 out of the 3 radiologists in analysis for all cancer, clinically significant cancer and cancer in the peripheral zone. Furthermore, this higher sensitivity was associated with similar PPV and confidence scores based on radiologist assigned lesion conspicuity index for detected. These results have an important clinical relevance as using optimal TE to acquire may lead to improved detection of prostate cancer as compared to TEs (90-120ms) typically used for prostate MRI.
Our result showing that higher TE values may provide better contrast between cancerous lesions and normal soft tissue can be explained by effect of TE on “T2-weighting” on 2 tissue types, where if TE is short, the echo occurs without much time for T2-decay to have taken place and hence the tissues are not well differentiated. While if TE is long, the relative differences in signal decay or contrast between the two tissues types become more visible. There is some evidence to support the finding that higher TE values may provide better contrast between cancerous lesions and normal soft tissue. Benign prostate tissue has been shown to have a higher fractional volume of water in lumen as compared to cancerous tissue, resulting in high signal intensity on T2W images (25-27). Lumen has been shown to have higher T2 and therefore it should have significant signal at higher TEs in benign tissue but not in prostate cancer (25, 28, 29). While, one study demonstrated that benign prostatic tissue demonstrates long-lived signal intensity on T2W images at higher TEs, with significant signal intensity remaining at very high TEs of up to 800 ms (28), we however did not utilize such high TEs and this may be of interest for future studies. However, the signal-to-noise ratio is expected to be much lower at very long TE and this may affect the radiologists’ performance. Therefore, determining the optimal TE for detecting prostate cancer on T2W images may be useful for improving prostate cancer diagnosis and to standardize protocols across institutions.
The overall sensitivity and PPV in detecting prostate cancers using T2W images in this study are similar to values published literature on T2W imaging of the prostate (2, 21, 30-33). While, our study suggests the use of longer TE for T2W imaging of the prostate, the readers should be careful in ascertaining the optimal TE. A larger sample size and more readers in a multi-center study may be needed to ascertain an optimal TE to be recommended by future version of PI-RADS. Also, we did not investigate whether the PI-RADS score was altered by the use of different TEs, especially for the transition zone lesions where T2W is the dominant sequence.
The diagnostic performance for detecting prostate cancers from the different prostatic zones are different. Cancers in the peripheral zone (the most common site for prostate cancers) are better detected at higher TEs, while the investigated TEs had no effect on diagnostic performance of cancer detection in the transition zone. The sensitivity in the peripheral zone was significantly higher than the transition zone, as there are big differences in SNR in the different zones, especially since the endorectal coil is used with large SNR drop-off in the anterior prostate which leads to reduced sensitivity as shown by previous work (34). The combination of higher SNR in the peripheral zone due to use of endorectal coil, and considering the fact that peripheral zone has more lumen volume (prostate luminal fluid in the lumen has very long T2 relaxation times) than benign transition zone (25, 27), produces good contrast. The contrast between cancer and benign peripheral zone tissue is better especially at higher TE, where the signal from cancer is minimal while surrounding benign tissue has high signal from lumen, creating good contrast for cancer detection. In addition, there may be different amount of lumen in people from different ages (especially in older men), which may affect the SNR and therefore choice of optimal TE for T2W imaging.
Our study found very low sensitivity (13-29%) for detecting transition zone cancers. This is lower than literature values (25, 34). This could be due to the SNR drop-off in the anterior prostate when endorectal coil was used, due to presence of benign prostatic hyperplasia which can mimic cancers and affect diagnostic accuracy of prostate MRI (35), as well as use of slightly lower resolution T2W images used in this study compared to standard clinical protocol. This nonetheless, highlights the problem with detecting cancers in the transition zone, where PI-RADS (5, 6) recommends T2W as the dominant sequence.
The radiologist assigned confidence scores for different TEs exhibit similar excellent overall inter-observer agreement between the 3 radiologists. It suggests that use of different TEs in the clinic should ideally not require additional training and we can expect that it won’t add to the already large inter-observer variation in mpMRI interpretation (36). The windowing of the T2W images is an important step that our radiologists noted and is also something others radiologists should keep in mind to get the best contrast.
The increased use of biparametric protocol (T2W and DWI) for PCa imaging in recent literature (37, 38), and the focus on T2W images in the PI-RADS guidelines, as seen in v2.1, the optimization of imaging protocols such as echo time (TE) can be very important as shown by this study. Since our results suggest that the degree of T2–weighting is important, it is possible that the exact parameters used for the fast spin echo sequence are important as well – e.g. number of echoes – type of refocusing pulse – TR etc., as well as coherence spoiling pulses, phase cycling etc. All of these details could significantly affect contrast. It would be desirable in the future to look at these parameters and compare fast spin echo with the conventional single echo T2W sequence, which yields much purer T2 weighting.
The study has a few limitations. A multi-echo TSE imaging technique was used in this study to cover a large range of TEs, and therefore the spatial resolution was lower than the PI-RADS recommended. In the future, it would be desirable to use imaging protocol with high resolution typically used (0.4 mm × 0.4 mm) for T2W imaging. As this study was limited to a single scanner, its applicability to machines from different vendors and/or with different field strengths needs to be investigated. A lesion based analysis was used and therefore specificity and negative predictive value could not be determined in this study. This limitation is not unique, as previous observer studies have this inherent described this limitation (21, 32, 39). Future studies may also consider a quantitative approach to determining the optimal TE that provides the best contrast.
In conclusion, our study shows that the performance of T2-weighted imaging for the detection of all prostate cancers is improved at higher echo times (150 and 180 ms) compared to the range used most commonly in the clinics worldwide (90-120 ms). However, a large cohort, specifically with more transition zone cancer and more readers in a multi-center study may be needed to ascertain an optimal TE.
Figure 2:
49 years old patient with PSA of 2.8 ng/mL. The Gleason 3+4 cancer (1.9 cm × 1.2 cm in the right peripheral zone in mid prostate shown by arrows) was detected by all three radiologists at the 3 different echo times. However, the confidence score assigned by the radiologists 2 and 3 were lower at TE = 90ms (R1 = 5, R2 = 4, R3 = 3) than at 150ms (R1 = 5, R2 = 5, R3 = 4) and 180ms (R1 = 5, R2 = 5, R3 = 3).
Acknowledgement:
We would like to thank Dr Teodora Szasz from the University of Chicago Research Computing Center for developing the framework for image annotation for the observer study.
Funding sponsors: Philips Healthcare and National Institutes of Health (NIH R01 CA172801 and NIH 1S10OD018448-01)
Disclosures: Dr Aytekin Oto has the following disclosures - Research Grant, Koninklijke Philips NV Research Grant, Guerbet SA Research Grant, Profound Medical Inc. Medical Advisory Board, Profound Medical Inc Speaker, Bracco Group
Abbreviations
- DCE
Dynamic Contrast Enhanced
- mpMRI
multiparametric MRI
- MRI
Magnetic Resonance Imaging
- PCa
Prostate Cancer
- PI-RADS
Prostate Imaging – Reporting and Data System
- PPV
Positive Predictive Value
- ROI
Region of Interest
- T2W
T2 weighted
- TE
Echo Time
- TR
Repetition Time
- TSE
Turbo Spin Echo
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflict of interests.
IRB statement: The study was conducted after institutional review board approval, informed patient consent and was compliant with Health Insurance Portability and Accountability Act.
Ethics approval: The study was conducted after institutional review board approval and was compliant with Health Insurance Portability and Accountability Act.
Informed consent: Informed patient consent was obtained for recruiting patients in this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018; 68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Baur AD, Maxeiner A, Franiel T, et al. Evaluation of the prostate imaging reporting and data system for the detection of prostate cancer by the results of targeted biopsy of the prostate. Invest Radiol. 2014; 49(6):411–20. [DOI] [PubMed] [Google Scholar]
- 3.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. New England Journal of Medicine. 2018; 378(19):1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet. 2017; 389(10071):815–22. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, Version 2. European Urology. 2016; 69(1): 16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. European Urology. 2019; 76(3):340–51. [DOI] [PubMed] [Google Scholar]
- 7.Lemberskiy G, Rosenkrantz AB, Veraart J, Taneja SS, Novikov DS, Fieremans E. Time-Dependent Diffusion in Prostate Cancer. Investigative Radiology. 2017; 52(7):405–11. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y, Jiang Y, Antic T, et al. Apparent Diffusion Coefficient for Prostate Cancer Imaging: Impact of b Values. American Journal of Roentgenology. 2014; 202(3):W247–W53. [DOI] [PubMed] [Google Scholar]
- 9.Othman AE, Falkner F, Weiss J, et al. Effect of Temporal Resolution on Diagnostic Performance of Dynamic Contrast-Enhanced Magnetic Resonance Imaging of the Prostate. Invest Radiol. 2016; 51(5):290–6. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee A, He D, Fan X, et al. Performance of ultrafast DCE-MRI for diagnosis of prostate cancer. Academic Radiology. 2018; 25(3):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurrell SL, McGarry SD, Kaczmarowski A, et al. Optimized b-value selection for the discrimination of prostate cancer grades, including the cribriform pattern, using diffusion weighted imaging. Journal of medical imaging (Bellingham, Wash). 2018; 5(1):011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning P, Shi D, Sonn GA, et al. The impact of computed high b-value images on the diagnostic accuracy of DWI for prostate cancer: A receiver operating characteristics analysis. Sci Rep. 2018; 8(1):018–21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Trani MG, Nezzo M, Caporale AS, et al. Performance of Diffusion Kurtosis Imaging Versus Diffusion Tensor Imaging in Discriminating Between Benign Tissue, Low and High Gleason Grade Prostate Cancer. Acad Radiol. 2019; 26(10):1328–37. [DOI] [PubMed] [Google Scholar]
- 14.Nezzo M, Di Trani MG, Caporale A, et al. Mean diffusivity discriminates between prostate cancer with grade group 1&2 and grade groups equal to or greater than 3. Eur J Radiol. 2016; 85(10):1794–801. [DOI] [PubMed] [Google Scholar]
- 15.Feng Z, Min X, Wang L, et al. Effects of Echo Time on IVIM Quantification of the Normal Prostate. Scientific Reports. 2018; 8(1):2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. Journal of Magnetic Resonance Imaging. 2013; 37(5): 1035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dregely I, Margolis DAJ, Sung K, et al. Rapid quantitative T2 mapping of the prostate using three-dimensional dual echo steady state MRI at 3T. Magnetic Resonance in Medicine. 2016; 76(6):1720–9. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkrantz AB, Meng X, Ream JM, et al. Likert score 3 prostate lesions: Association between whole-lesion ADC metrics and pathologic findings at MRI/ultrasound fusion targeted biopsy. Journal of Magnetic Resonance Imaging. 2016; 43(2):325–32. [DOI] [PubMed] [Google Scholar]
- 19.Turkbey B, Mani H, Aras O, et al. Prostate Cancer: Can Multiparametric MR Imaging Help Identify Patients Who Are Candidates for Active Surveillance? Radiology. 2013; 268(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litjens GJS, Barentsz JO, Karssemeijer N, Huisman HJ. Clinical evaluation of a computer-aided diagnosis system for determining cancer aggressiveness in prostate MRI. European Radiology. 2015; 25(11):3187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee A, Devaraj A, Matthew M, et al. Performance of T2 maps in the detection of prostate cancer. Academic Radiology. 2019; 26(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahiem EI, Mohsen T, Nabeeh AM, Osman Y, Hekal IA, Abou El-Ghar M. DWI-MRI: Single, Informative, and Noninvasive Technique for Prostate Cancer Diagnosis. The Scientific World Journal. 2012; 2012:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkel DJ, Heye TJ, Benz MR, et al. Compressed Sensing Radial Sampling MRI of Prostate Perfusion: Utility for Detection of Prostate Cancer. Radiology. 2019; 290(3):702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sénégas J, Liu W, Dahnke H, Song H, Jordan EK, Frank JA. Fast T2 relaxometry with an accelerated multi-echo spin-echo sequence. NMR in Biomedicine. 2010; 23(8):958–67. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee A, Bourne RM, Wang S, et al. Diagnosis of Prostate Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology. 2018; 287(3):864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer DL, van der Kwast TH, Evans AJ, et al. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology. 2010; 255(2):485–94. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee A, Watson G, Myint E, Sved P, McEntee M, Bourne R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology. 2015; 277(3):751–62. [DOI] [PubMed] [Google Scholar]
- 28.Storås TH, Gjesdal K-I, Gadmar ØB, Geitung JT, Kløw N-E. Prostate magnetic resonance imaging: Multiexponential T2 decay in prostate tissue. Journal of Magnetic Resonance Imaging. 2008; 28(5): 1166–72. [DOI] [PubMed] [Google Scholar]
- 29.Sabouri S, Chang SD, Savdie R, et al. Luminal Water Imaging: A New MR Imaging T2 Mapping Technique for Prostate Cancer Diagnosis. Radiology. 2017; 284(2):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilanova JC, Barceló-Vidal C, Comet J, et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-total prostate-specific antigen ratio in the detection of prostate cancer. AJR American journal of roentgenology. 2011; 196(6):W715–W22. [DOI] [PubMed] [Google Scholar]
- 31.Turkbey B, McKinney YL, Trivedi H, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. The Journal of urology. 2011; 186(5): 1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkrantz AB, Deng F-M, Kim S, et al. Prostate Cancer: Multiparametric MRI for Index Lesion Localization—A Multiple-Reader Study. American Journal of Roentgenology. 2012; 199(4):830–7. [DOI] [PubMed] [Google Scholar]
- 33.Greer MD, Brown AM, Shih JH, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. Journal of Magnetic Resonance Imaging. 2017; 45(2):579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammadian Bajgiran A, Afshari Mirak S, Shakeri S, et al. Characteristics of missed prostate cancer lesions on 3T multiparametric-MRI in 518 patients: based on PI-RADSv2 and using whole-mount histopathology reference. Abdom Radiol. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee A, Gallan AJ, He D, et al. Revisiting quantitative multi-parametric MRI of benign prostatic hyperplasia and its differentiation from transition zone cancer. Abdom Radiol. 2019; 44(6):2233–43. [DOI] [PubMed] [Google Scholar]
- 36.Niaf E, Lartizien C, Bratan F, et al. Prostate Focal Peripheral Zone Lesions: Characterization at Multiparametric MR Imaging—Influence of a Computer-aided Diagnosis System. Radiology. 2014; 271(3):761–9. [DOI] [PubMed] [Google Scholar]
- 37.Scialpi M, Prosperi E, D'Andrea A, et al. Biparametric versus Multiparametric MRI with Non-endorectal Coil at 3T in the Detection and Localization of Prostate Cancer. Anticancer Res. 2017; 37(3):1263–71. [DOI] [PubMed] [Google Scholar]
- 38.Niu X-k, Chen X-h, Chen Z-f, Chen L, Li J, Peng T. Diagnostic Performance of Biparametric MRI for Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. American Journal of Roentgenology. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 39.Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: Correlation with whole-mount histopathology. Journal of Magnetic Resonance Imaging. 2014; 39(6):1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]