Abstract
Depression represents the number one cause of disability worldwide and is often fatal. Inflammatory processes have been implicated in the pathophysiology of depression. It is now well established that dysregulation of both the innate and adaptive immune systems occur in depressed patients and hinder favorable prognosis, including antidepressant responses. In this review, we describe how the immune system regulates mood and the potential causes of the dysregulated inflammatory responses in depressed patients. However, the proportion of never-treated major depressive disorder (MDD) patients who exhibit inflammation remains to be clarified, as the heterogeneity in inflammation findings may stem in part from examining MDD patients with varied interventions. Inflammation is likely a critical disease modifier, promoting susceptibility to depression. Controlling inflammation might provide an overall therapeutic benefit, regardless of whether it is secondary to early life trauma, a more acute stress response, microbiome alterations, a genetic diathesis, or a combination of these and other factors.
Major Depressive Disorder (MDD)
Mood disorders are the most common of the severe psychiatric illnesses. Episodes of major depression occur in both unipolar depression (in which mood varies between euthymia and depressed) and bipolar disorder (mood has pathological “highs,” termed hypomania and mania, as well as euthymia and depression). Major depressive episodes are defined in DSM-5 by a constellation of signs and symptoms (DSM-5, 2013). Patients with major depression exhibit alterations in a variety of critical functions including sleep, appetite, psychomotor activity, cognition, and, of course, mood.
Lifetime prevalence of major depression in the United States is 21% of women and 11%–13% of men (Belmaker and Agam, 2008; Kessler et al., 2003). It is the major cause of suicide, now in the top 10 cases of death in the United States, with almost 50,000 reported suicides per year (Mann et al., 2005). Indeed, major depression is associated with a significant reduction in lifespan, in part due to suicide and the remainder due to the marked increase in vulnerability to major medical disorders, including cardiovascular disease and stroke, autoimmune disease, diabetes, and cancer (Benros et al., 2013; Windle and Windle, 2013; Bortolato et al., 2017). Not only are depressed patients more vulnerable to these and other disorders, but their treatment outcomes for these medical disorders are poorer (Katon, 2011). The morbidity and mortality associated with major depression renders it the number one cause of disability worldwide and exerts an extraordinary economic burden on society in terms of lost productivity (Bloom et al., 2011).
Risk factors for depression include family history of depression (approximately 35% of the risk is hereditary), early life abuse and neglect, as well as female sex and recent life stressors. Medical illness also increases the risk of depression, with particularly high rates associated with metabolic (e.g., cardiovascular disease) and autoimmune disorders.
Treatment of depression includes three major modalities: (1) antidepressants and other medications that augment antidepressant action, (2) evidence-based psychotherapy such as cognitive-behavior therapy (CBT) and inter-personal psychotherapy (IPT), and (3) somatic non-pharmacological treatments including electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and vagus nerve stimulation (VNS) (Gartlehner et al., 2017). Monotherapy with either an antidepressant or evidence-based psychotherapy results in the virtual absence of any depressive symptoms and return to the premorbid state, termed remission, in approximately 50% of previously untreated depressed patients (Dunlop et al., 2017) and in 28% in a more heterogeneous mix of “real-world” patients in an effectiveness study (Trivedi et al., 2006). At the current time, there are no clinically useful predictors of response in a given individual to one antidepressant versus another (Zeier et al., 2018) in spite of claims to the contrary (Greden et al., 2019). Such biomarkers are of great interest, as ongoing depression is associated with increasing treatment resistance and increased risk for substance abuse and suicide. Depressed patients with increases in inflammatory markers may represent a relatively treatment-resistant population. In this regard, it is of interest to note that patients with autoimmune disorders have inordinately high prevalence rates of depression. This is discussed in further detail in subsequent sections.
Peripheral and Central Immunity
Mammals are protected by the immune system from infectious agents and many types of insults that cause injury. Immunity involves (1) recognition of infection or damage, (2) immune functions to contain the infection/damage, (3) regulation limiting the magnitude and duration of the immune response that can itself be damaging to tissues, and (4) memory to enhance the future response to the same infectious agent/damage if reencountered (Murphy, 2012). Inflammation or inflammatory response are the result of the activation of the immune system that often manifests as a localized reaction resulting from irritation, injury, or infections; are associated with warmth, redness, swelling and pain, and sometimes fever; and are necessary to eliminate the insult. Many types of immune cells and mechanisms are in place to maintain homeostasis, but dysregulation of their actions often contributes to diseases, with increasing evidence that this occurs in psychiatric disorders, including depression (Murphy, 2012).
The immune system is classically divided into innate and adaptive arms, though these two act cooperatively to ensure proper immune actions. The innate immune system is the first line of defense, because innate immune myeloid cells (e.g., macrophages/monocytes, dendritic cells) and lymphoid cells (e.g., natural killer [NK]) constantly patrol the circulation to provide rapid responses. Receptors on these cells are activated when they encounter damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs); DAMPs (also called alarmins) are host molecules that signal cellular damage (e.g., Heat shock proteins), whereas PAMPS are present on infectious pathogens (Gong et al., 2020). After activation, macrophages and dendritic cells produce cytokines (interleukins and/ or chemokines), which recruit other immune cells to the site of infection or insult. As part of the inflammatory activity, dendritic cells initiate the adaptive immune response by presenting antigens to cells of the adaptive immune system and are therefore also called antigen-presenting cells (APCs).
The adaptive immune system, composed of lymphocytes (T and B cells), is slower to respond, as it often requires recruitment, activation, and differentiation of the lymphocytes to exert effector functions. A key characteristic of adaptive immune cells is their capacity to clonally express a large repertoire of antigen-specific receptors, T cell receptors (TCR), and B cell receptors (BCR), which are produced by site-specific somatic recombination (Smith-Garvin et al., 2009). Each lymphocyte expresses one unique antigen receptor variant. This confers an antigen specificity to the adaptive immune system, which does not exist in the innate immune system, highlighting the specialization of the adaptive immune system in contrast to the innate immune system that respond to a wide variety of DAMPs and PAMPs. Until lymphocytes are activated by “their” antigen, they are considered naive and inactive cells. Upon antigen recognition, they are activated and undergo clonal differentiation to become fully functional effector lymphocytes. B cells clonally proliferate and differentiate into plasma cells, which produce antigen-specific antibodies. Activated T cells can become one of three broad types of effector T cells: cytotoxic, helper, and regulatory. Thus, cytotoxic T cells (CD8+ cells) kill infected cells. T helper (Th) cells influence the behavior and activity of other immune cells, and regulatory T cells (Tregs) suppress the activity of other lymphocytes that control or limit immune responses to prevent autoimmunity. Some activated B and T cells differentiate into memory cells, which can mount a rapid immune response if the same antigen is encountered again by differentiating into a large pool of specific effector cells (Murphy, 2012).
Microglia—The CNS Immune System
The brain possesses specialized immune cells called microglia that comprise 5%–10% of total brain cells and carry out macrophage-like and other specialized functions (Kim and de Vellis, 2005). Microglia are maintained by self-renewal with minimal contribution from immune cells outside of the CNS, and their main functions are to maintain CNS homeostasis and to provide rapid responses to damage or infection. Microglia exhibit a broad spectrum of activation states upon receiving various stimuli. Recent findings have shown that microglia are important for synaptic modulation (e.g., synapse pruning and neurogenesis) and are activated in many neurodegenerative and neuropsychiatric diseases, where they contribute to pathology by promoting neuroinflammation (Yirmiya et al., 2015). The heterogeneity of microglia suggests that microglia subsets have distinct roles in the brain (Masuda et al., 2019), but a more complete understanding of the complex roles of microglia is necessary to provide further insights in understanding their role in brain function and pathology.
Interfaces between CNS and Peripheral Immunity
There is a role for non-microglial cells in CNS immunity with three other types of CNS macrophages: perivascular, meningeal, and choroid plexus macrophages (for review, see Li and Barres, 2018) as well as lymphoid cells ( Beureland Lowell, 2018). These macrophages are localized at the interface of the parenchyma and blood vessels. Under physiological conditions, peripheral immune cells do not enter the brain parenchyma, though some are present in cerebrospinal fluid (CSF) and the meninges (Wilson et al., 2010). However, in certain conditions, macrophages, and T cells, can cross the blood-brain barrier (BBB) and enter the brain parenchyma, generally producing damage (Wilson et al., 2010). The BBB is composed of specialized endothelial cells linked by tight junctions, limiting the entry of immune cells, various blood constituents, and pathogens. Indeed, the BBB prevents >98% of antibodies and small molecules from entering the parenchyma, while assuring the efflux of other molecules. Various hypotheses have been proposed to explain how peripheral immune cells may cross the BBB under pathological conditions (for review, see Ousman and Kubes, 2012; Ransohoff and Engelhardt, 2012). In conditions that weaken the BBB or in regions where the BBB is more permissive, such as the circumventricular organs and choroid plexus, immune cells infiltrate the brain parenchyma via diapedesis. Because the choroid plexus has a secretory epithelium that produces CSF, it also allows the passage of lymphocytes to access and provide immune surveillance of the CSF (for review, see Ransohoff and Engelhardt, 2012). In physiological circumstances, few immune cells are present in the CSF, but a higher percentage of memory or CNS antigen experienced CD4+ T cells are found in the CSF compared to the circulation (Ransohoff and Engelhardt, 2012).
Activated T cells gain access to the brain by extravasation into the tissue, by upregulating many adhesion molecules and integrins, allowing them to roll and adhere to the vessel walls. Upregulation of very late antigen-4 (VLA-4) or lymphocyte function-associated-1 (LFA-1) on T cells promotes the binding to vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) present on endothelial cells, and infiltration to the parenchyma. Furthermore, the gradient of chemokines produced by the choroid plexus (e.g., CCL9, CCL20) also attracts T cell subsets to the brain, which has particularly been demonstrated in studies of autoimmune diseases (Oukka and Bettelli, 2018; Reboldi et al., 2009). Finally, immune cells are present in the meninges, and the role of meningeal immune cells has been mainly studied in the context of viral, bacterial, or parasitic infections (for review, see Forrester et al., 2018). The recent (re) discovery of the lymphatic system within the meninges of the brain has revealed another pathway for immune cells to reach the meninges (Sandrone et al., 2019). Indeed, the lymphatic system is critical for the drainage of immune cells and soluble factors from the CNS into the deep cervical lymph nodes (Louveau et al., 2015). It has also been proposed that the lymphatic vessels maintain anergy of CNS-reactive T cells within the meningeal spaces promoting T cell tolerance, whereas infections may trigger CNS-reactive T cells to attack the CNS. The immune response within the CNS is not always detrimental, such as after CNS injury when the immune response limits secondary degeneration (for review, see Louveau et al., 2017). Similarly, although pathogenic T cells have been associated with autoimmune diseases and neuropsychiatric and neurodegenerative disease, not all T cells are detrimental to brain function. For example, T cells support cognition under physiological conditions (Kipnis, 2016). Clearly, the immune system in the CNS functions in a unique way compared to peripheral tissues.
Cytokine Production and Regulation
Cytokines are small proteins that affect cell functions and interactions and can have either pro-inflammatory or anti-inflammatory effects. There are many families of cytokines that provide specialized functions. Cytokines are predominantly produced by immune cells, including microglia in the CNS, but other CNS cells such as neurons and astrocytes also produce cytokines. Immune activity including cytokine production is influenced by a myriad of factors, including but not limited to genetics, and previous exposures to pathogens (MacGillivray and Kollmann, 2014). The most studied cytokines in the context of psychoneuroimmunology are interleukin (IL)-6, tumor necrosis factor (TNF), IL-1b, and interferons (IFNs) on the inflammatory side and IL-10 on the resolving side. Table 1 summarizes the cytokines and related molecules studied in the context of depression and lists their main functions.
Table 1.
Cytokines and Their Peripheral Immune Functions and Blood Levels in MDD Patients Compared to Healthy Control Subjects
| Cytokines | Function | Role in MDD | References |
|---|---|---|---|
| CCL2 | attracts to site of inflammation: T cells (Th2 > Th1), monocytes, basophils, immature dendritic cells, NK cells | varies | Köhler et al., 2017; Leighton et al., 2018 |
| CCL3 | attracts to site of inflammation: T cells (Th1 > Th2), monocytes/macrophages, NK cells, basophils, immature dendritic cells, eosinophils, fibroblasts, neutrophils, astrocytes, osteoclasts |
↑ | Syed et al., 2018; Leighton et al., 2018 |
| CCL4 | targets T cells (Th1 > Th2), NK cells, monocytes/macrophages, basophils, immature dendritic cells, eosinophils, B cells | ↓ | Syed et al., 2018; Leighton et al., 2018 |
| CCL5 | targets T cell (memory cell > T cell, Th1 > Th2), NK cells, eosinophils, neutrophils, immature dendritic cells, monocytes/macrophages | ↑ | Syed et al., 2018 |
| CCL11 | recruits eosinophils, implicated in allergic response | ↑ | Leighton et al., 2018 |
| CXCL4 | released from platelets, attracts neutrophils, fibroblasts, and monocytes, arrests monocytes on the endothelium, important in wound healing and in promoting coagulation and artherogenesis | ↑ | Leighton et al., 2018 |
| CXCL7 | released from platelets, attracts neutrophilsangionenic, first chemokine to arrive at the site of injury | ↑ | Leighton et al., 2018 |
| CXCL10 | targets NKcells, B cells, activated Tcells (Th1 > Th2), endothelial cells | ↓ | Syed et al., 2018 |
| G-CSF | stimulates neutrophil development and differentiation | ↑ | Kirali et al., 2017; Syed et al., 2018 |
| GM-CSF | promotes granulocyte maturation and proliferation, monocyte development | ↑ | Kirali et al., 2017 |
| IFN-γ | induces macrophage activation, increased expression of MHC molecules and antigen processing components, Immunoglobulin class switching, suppresses Th2 cells | varies | Köhler et al., 2017 |
| IL-1β | induces fever, T cell activation, macrophage activation | varies | Köhler et al., 2017 |
| IL-1RA | antagonizes IL-1 function | varies | Köhler et al., 2017 |
| IL-2 | promotes T cell proliferation | ↑ | Köhler et al., 2017 |
| IL-4 | induces B cell activation, IgE switch, and differentiation toward Th2 cells | ↓ | Köhler et al., 2017 |
| IL-5 | promotes eosinophil growth, differentiation | ↑ | Köhler et al., 2017 |
| IL-6 | induces T and B cell growth and differentiation, acute phase production, fever | ↑ | Köhler et al., 2017 |
| IL-7 | induces growth of preB-cells and preT-cells | ↑ | Syed et al., 2018 |
| IL-8 /CXCL8 | targets neutrophils, basophils, CD8 cell subsets, endothelial cells | varies | Köhler et al., 2017; Leighton et al., 2018 |
| IL-9 | induces mast cell activity, stimulates Th cells | ↑ | Syed et al., 2018 |
| IL-10 | potent suppressant of macrophage functions, anti-inflammatory | ↑ | Köhler et al., 2017 |
| IL-12 | activates NK cells, induces CD4 T cell differentiation into Th1-like cells | ↑ | Köhler et al., 2017 |
| IL-13 | induces B cell growth and differentiation, inhibits macrophage inflammatory cytokine production and Th1 cells, induces allergy/ asthma | ↑ | Köhler et al., 2017 |
| IL-15 | IL-2 like cytokine, stimulates growth of intestinal epithelium, T cells and NK cells, enhances memory CD8 T cell survival | ↑ | Syed et al., 2018 |
| IL-17A | pro-inflammatory, induces cytokine production by epithelia, endothelia, astrocytes, and fibroblasts | ↑ | Köhler et al., 2017 |
| IL-18 | induces IFN-γ production by T cells and NK cells, promotes Th1 induction | ↑ | Köhler et al., 2017 |
| sIL-2 receptor | increased in autoimmune disease | ↑ | Köhler et al., 2017 |
| sIL-6 receptor | promotes IL-6 signal | no change | Köhler et al., 2017 |
| TGFβ1 | anti-inflammatory | no change | Köhler et al., 2017 |
| sTNFR2 | activated by TNF | ↑ | Köhler et al., 2017 |
| TNF | promotes inflammation, endothelial activation | ↑ | Köhler et al., 2017 |
In the brain, cytokines produced by microglia and other CNS cells are crucial positive modulators of several CNS functions, such as maintenance of neuroplasticity (Stellwagen and Malenka, 2006; Yirmiya and Goshen, 2011). However, excess or prolonged inflammatory cytokine activity perturbs multiple neuronal functions, including impairment of neurotransmitter signaling, disruption of the synthesis, reuptake, and release of neurotransmitters (Deverman and Patterson, 2009; Elmer and McAllister, 2012; Stephan et al., 2012). This, in turn, affects neurocircuit function, including that implicated in mood and cognition (Dantzer et al., 2008; Figure 1). The effects of cytokines on the dopaminergic system have been recently reviewed (Treadway et al., 2019; Felger and Treadway, 2017; Capuron et al., 2012). Relevant mechanisms that may increase cytokine activity in the brain to pathological levels include psychological and physical stressors. Nevertheless, it remains unclear how the same cytokine exhibit opposite effects on neuronal function depending on the context. It has been proposed that the source and the combination of cytokines dictate the effects of cytokines on brain function. The field of neuroinflammation has been focusing on central cytokines, whereas peripheral cytokines certainly contribute to behavioral effects, as suggested by findings showing that blocking peripheral cytokines is sufficient to tighten the BBB and that blocking BBB disruption is sufficient to exhibit antidepressant actions (Cheng et al., 2018; Menard et al., 2017). There are several well-documented pathways by which peripheral cytokines reach the brain, similarly to the immune cells: (1) through “leaky” regions of the BBB, such as the circumventricular organs, or through disease-induced disruptions of the BBB (Quan and Banks, 2007; Vitkovic et al., 2000), (2) through a neural route via afferent nerve fiber cytokine receptors that relay the signal to the brain parenchyma (Watkins et al., 1995) and (3) through the infiltration of immune cells that produce cytokines after being attracted by a chemokine gradient to the meninges or brain parenchyma (Lewitus et al., 2008).
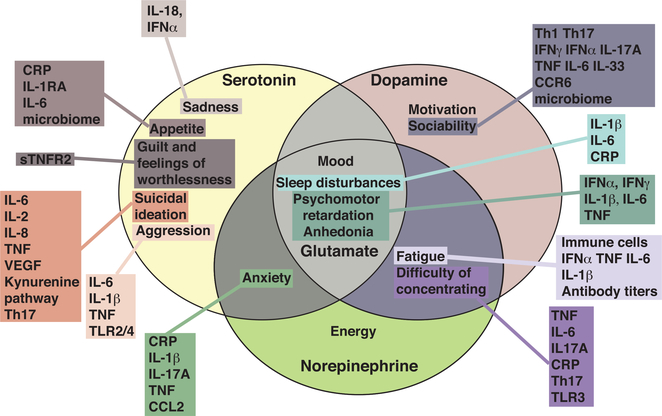
Figure 1. Symptoms of Depression Associated with Different Immunological Changes.
Monoamine neurotransmitters have been associated with various symptoms of depression as depicted in each oval. Some symptoms are dependent on multiple neurotransmitters, such as psychomotor retardation that is regulated by serotonin, dopamine, norepinephrine, and glutamate. Each box represents the different immunological changes (e.g., cytokines, immune cell, and others) associated with each symptom.
Cytokines are one of the most studied components of the immune system in depression (for review, Dantzer et al., 2008; Miller and Raison, 2016; Raison et al., 2006), but little is known about the source and the contribution of cytokines in MDD and their mechanisms of action in the brain.
Immune Findings in Depression
Over the past two decades, there has been growing evidence that MDD is associated with a systemic immune activation, comprising abnormality in inflammatory markers, immune cell numbers, and antibody titers (Gibney and Drexhage, 2013; Müller, 2014; Figure 2).
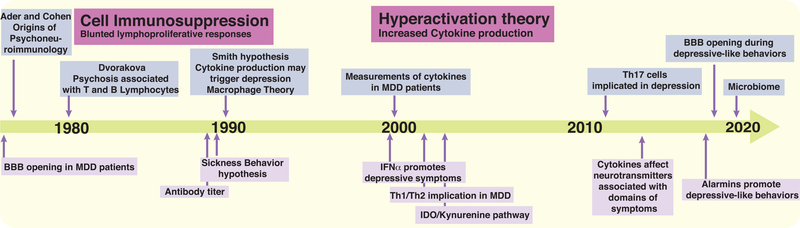
Figure 2. Timeline of Discoveries Associating Depression with Immunological and Inflammatory Changes.
This timeline depicts the origins of psychoneuroimmunology (Ader and Cohen, 1975), and several prominent inflammatory theories related to depression, starting with the cell immunosuppression theory initiated by Dvorakova et al., 1980, followed by the sickness behavior hypothesis (reviewed in Dantzer et al., 2008) and the theory of hyperactivation of the immune system (initiated by Smith, 1991, and reviewed by Raison et al., 2006) and ending with more recent findings, such as possible effects of changes in the microbiome in MDD patients.
Immune Activation
Aberrant Cytokine Production
It is now well established in multiple meta-analyses (Dowlati et al., 2010; Howren et al., 2009; Liu et al., 2012b; Kö hler et al., 2017) that proinflammatory cytokines and acute phase proteins are increased in MDD patients, with a fairly unanimous consensus of increases in IL-6, TNF, and C-reactive protein (CRP) in the blood of MDD patients compared to healthy controls (Maes et al., 2009; Miller et al., 2009; Stewart et al., 2009). With the advances in the measurement of cytokines by multiplexing (Papakostas et al., 2013), many other cytokines are now evaluated (Kiraly et al., 2017; Syed et al., 2018). A relatively recent meta-analysis of 82 studies including 3,212 MDD patients and 2,798 healthy controls reveals increased levels of IL-6, TNF, IL-10, sIL-2, C-C motif ligand (CCL)2, IL-13, IL-18, IL-12, IL-1RA, and soluble TNF receptor (sTNFR)2 in MDD patients, whereas the level of interferon-γ (IFN-γ) is reduced ( Köhler et al., 2017). Gene expression upregulation of inflammatory pathways have also been reported in peripheral blood mononuclear cells (PBMCs) of depressed patients. Similarly to the protein data, the expression of Il-1β, Il-6, tnf, macrophage migration inhibiting factor (mif), and Ifnγ genes are increased in PBMCs of MDD patients compared to healthy controls, whereas Il-4 mRNA level is decreased (Hepgul et al., 2013). Overall, there is a large heterogeneity in the data, which depends on the cytokine component studied, and is at least partly due to the absence of consideration of the clinical course and the illness duration, and the effects of potential confounding factors such as comorbidity, medications, fasting status, smoking, assay methodology, or body mass index (BMI) among others.
Are All MDD Patients Exhibiting Increased Cytokine Production?
Not all MDD patients exhibit increased inflammation. Increased inflammatory markers have been associated with atypical symptoms of depression (Lamers et al., 2018), and with suicidal MDD (Black and Miller, 2015), which contrasts with a recent study of measurement of cytokines in never-treated relatively homogeneous MDD patients, for which cytokine levels are elevated in the majority (Syed et al., 2018). This suggests that prior exposure to various regimens of antidepressants might affect the cytokine production in some MDD patients. Although most studies of cytokines in MDD include patients free of antidepressant, these patients have been previously exposed to antidepressants (Dowlati et al., 2010). Therefore, even though the study of Syed et al. reported a relatively small sample size of MDD patients, with an overrepresentation of females and high absolute levels of measured cytokines compared to many other studies, this type of study might provide benefit in understanding the impact of immune dysregulation in MDD independent of prior exposure to antidepressants. It is also plausible that different inflammatory profiles are associated with different subtypes of depression (Dunjic-Kostic et al., 2013; Kaestner et al., 2005; Karlović etal.,2012). Thus,non-melancholic patients exhibit proinflammatory states, whereas melancholic patients, in contrast, exhibit reduced proinflammatory cytokine production (for review, see Kronfol, 2002; Rothermundt et al., 2001a; Rothermundt et al., 2001b). In addition, TNF has been associated with atypical features and chronicity, while IL-6 might represent a “state indicator for acute exacerbation” in melancholic patients (Dunjic-Kostic et al., 2013). Furthermore, higher levels of IL-6 predicted over time the chronicity of depression, as well as higher severity of depression at follow-up (Lamers et al., 2019). CRP and TNF have also been associated with greater symptom severity in MDD (Haapakoski et al., 2015). This is consistent with the findings that low-grade inflammation is associated with treatment-resistant depression (Chamberlain et al., 2019; Strawbridge et al., 2015) and poor treatment response to antidepressants (Uher et al., 2014; Vogelzangs et al., 2014), which will be discussed later in this review. Although there is merit in subcategorizing MDD to fit the different cytokine profiles, the subcategorization should take into account all the disease modifiers to avoid bias in our understanding of the immune response in MDD.
Is Inflammation Unique to MDD?
It is important to note that aberrant blood levels of cytokines have been reported in other psychiatric disorders, such as bipolar disorder and schizophrenia, raising the possibility of common underlying immune pathways among MDD, schizophrenia, and bipolar disorder (Goldsmith et al., 2016). Thus, for example, IL-6, TNF, IL-1RA, and sIL2R are significantly elevated in all three of these disorders during acute illness episodes and reduced after treatment. This suggests that an acute inflammatory state might be present in acutely symptomatic psychiatric patients, which is consistent with findings that psychological stress, a prominent risk factor for depression, induces an inflammatory response, and elevation of inflammatory markers after stress in healthy volunteers, including these proinflammatory cytokines, is associated with the development of depressive symptoms (Maes et al., 1998; Miller and Raison, 2015, 2016). In addition, ∼40% of patients treated with IFN-α for hepatitis C infection or certain types of cancers develop depressive symptoms after starting treatment (Raison et al., 2005). This is reinforced by the notion that environmental factors also contribute to both depression and immune dysregulation. Thus, childhood trauma (e.g., maltreatment, sexual abuse, violence) has been shown to cause long-lasting effects on peripheral inflammation later in life (Baumeister et al., 2016; Coelho et al., 2014; Grosse et al., 2016a; Rasmussen et al., 2019) and is associated with increased risk of depression (Chapman et al., 2004). The co-occurrence of MDD and inflammation is present in individuals with a history of childhood adversity but not in those without the latter (Miller and Cole, 2012). This topic has been covered in great details in a recent review (Nemeroff, 2016). Altogether, although common causes (e.g., stress) are associated with similar outcomes (e.g., cytokine production), it is important to recognize that cytokine production is only one part of the story and a better understanding of the role of cytokines is necessary to move the field forward.
Role of Anti-inflammatory Cytokines
To contain the immune response and prevent harm to the host, there is also induction of anti-inflammatory cytokines, which presumably resolve the immune response. It is important to note that the levels of anti-inflammatory cytokines, including transforming growth factor (TGF)-β and IL-10, are also often elevated in MDD patients, raising the question of their role in depression (Dowlati et al., 2010; Howren et al., 2009; Köhler et al., 2017) and their potential impact on the cellular immune response. Defects in the anti-inflammatory response has indeed been recently associated with resistance to antidepressant treatment and the overall net effect of cytokines in MDD on the immune system seems to be anti-inflammatory (Syed et al., 2018). Therefore, although a wide variety of cytokines are upregulated in MDD patients, the role of these cytokines in depressed patients remains to be specified.
Immune Cells
PBMC Immunophenotypes
It has been speculated that, for example, elevations in IL-6 and Il-1β were associated with a possible activation of monocytes/ macrophages in MDD, whereas elevated levels of sIL-2R produced by activated T cells would serve to downregulate T cell activation. Furthermore, the number of leukocytes (Irwin et al., 1990; Kronfol and House, 1989), neutrophils (Irwin et al., 1990; Kronfol and House, 1989; Kronfol et al., 1983), and monocytes (Müller et al., 1989) are increased in depressed patients. Depressed patients also display increases in the ratio of CD4/ CD8 (T helper/T cytotoxic) cells (Darko et al., 1988; Müller et al., 1989; Tondo et al., 1988), and this increased CD4/CD8 ratio is associated with an increased percentage of CD4 cells and a decreased percentage of CD8 cells. However, evidence that “depression is accompanied by immunosuppression” also exists (Asnis and Miller, 1989; Maes, 1995) and has been exemplified by findings showing decreased lymphoproliferative responses of T cells (Kronfol et al., 1983; Schleifer et al., 1984)or NK cell activity (Irwin and Gillin, 1987; Kronfol et al., 1989; Maes et al., 1992) and decreased number of T helper cells (Schleifer et al., 1989). This raises the question as to whether these apparently discordant observations are part of a single pathological pathway within the same individual (activation of the innate immune system, but reduction of the adaptive immune system) or whether they represent two independent processes that occur either in different individuals or at different stages of the disease. Recent studies have found that levels of IL-6 are elevated (1) in bipolar patients while cytotoxic T cells are decreased (Wu et al., 2017), and (2) in MDD patients, NK cells and T helper cell maturation are deficient (Grosse et al., 2016b; Syed et al., 2018), suggesting that increased cytokine production and cell immunosuppression can occur in the same individual. It has been proposed that these observations are disease stage and age dependent, speculating that abnormality in the T cells response during aging or a depressive episode (e.g., reduced T regulatory function) would unleash the inflammatory capacity of the monocytes/macrophages to produce cytokines (Grosse et al., 2016b). Consistent with this notion, antidepressant free MDD patients exhibit a less diverse TCR repertoire expressed on T cells than matched non-depressed patients (Patas et al., 2018), resembling symptoms of chronic viral infections, in which T cell often acquire tolerance mechanisms, reducing their activity (Li et al., 2008). However, further studies are warranted to immunophenotype MDD cells and to identify which cell(s) are responsible for the cytokine production. Rather than inhibiting cytokine(s) to improve MDD, it might be more beneficial to eliminate particular immune cell(s).
PBMC Gene Networks
Some transcriptomics analyses have started to identify networks of expression of inflammatory genes in subsets of PBMCs in depressed patients. Thus, the gene expression of the ApoE receptor ApoER2 decreases in lymphocytes (Suzuki et al., 2010), whereas the gene expression of triggering receptor expressed on myeloid cells 1 (trem-1), DNAX-activation protein of 12 kDa (Dap12), and purine-rich Box-1 (pu.1) increases in monocytes of depressed patients (Weigelt et al., 2011). Furthermore, various immune-inflammatory processes, such as the nuclear factor kB (NF-κB) pathway, which is important for cytokine production as discussed later in the review, IL-1β, IL-6, and TNF signaling pathways, toll-like receptor pathway, NK cell activation pathway, IFN-α/β signaling pathway, oxidative stress pathways are affected in MDD patients’ PBMCs (Beech et al., 2010; Elovainio et al., 2015; Galecki et al., 2012; Jansen et al., 2016; Leday et al., 2018; Mostafavi et al., 2014; Yi et al., 2012), reinforcing the idea that immune pathways contribute to MDD.
Th Cell Differentiation
Cytokines are required for the differentiation of T helper (Th) subsets, suggesting that the chronic production of cytokines found in MDD patients might influence Th cell fate. There is limited information available about the roles of the T helper CD4+ cells: Th1, Th2, Th17, and Treg cells in depression, which includes the findings that depressed patients have elevated levels of Th1 and Th2 cytokines (Myint et al., 2005), and the Th1/Th2 (IFN-α/IL-4) ratio is increased in depressed patients (Maes et al., 1992). In contrast, antidepressants reduce the Th1/Th2 ratio (Kubera et al., 2001a). MDD patients also have elevated blood levels of Th17 cells (Chen et al., 2011), and the levels of Th17 cells were highest in patients with high risk of suicide (Schiweck et al., 2020). In vitro activation of CD4 cells isolated from patients with generalized anxiety disorder induces them to acquire a Th17 phenotype (Ferreira et al., 2011; Vieira et al., 2010), and patients with autoimmune diseases with elevated Th17 cells often exhibit comorbid depression (Kurd et al., 2010; Patten et al., 2017). Consistent with these findings, IL-17A was found to be elevated in some (Chen et al., 2011; Davami et al., 2016), but not all (Kim et al., 2013; Liu et al., 2012a), MDD patients, IL-17A predicts treatment response to certain antidepressants (Jha et al., 2017). Anti-IL-17A (Ixekizumab) treatment reduces depressive symptoms in 40% of psoriasis patients experiencing MDD (Griffiths et al., 2017), whereas blocking the downstream effects of IL-17A by blocking its receptor using anti-IL-17RA (Brodalumab therapy) has been associated with increased suicidality risk and psychiatric disorders in psoriasis patients (Lebwohl et al., 2018). Studies in rodents provides corroborative evidence of these detrimental links to Th17/IL-17A, such as administration of IL-17A in rodents promotes depressive-like behaviors (Nadeem et al., 2017), stress increases IL-17A levels (Cheng et al., 2018; Gu et al., 2018; Lu et al., 2017; Zhang et al., 2019), brain accumulation of Th17 cells (Beurel et al., 2013, 2018), and increased splenic Th17 cells after stress induced by social defeat (Ambrée et al., 2019). This suggests that Th1 and Th17 cells also participate to the production of proinflammatory cytokines, and targeting these cells might provide antidepressant actions.
Antibody Production
Antibodies have also been implicated in the physiopathology of depression (Denburg et al., 1988). Thus, a high titer of anti-phospholipid antibodies was found in 63 depressed patients compared to healthy controls (Gorman and Cummings, 1993; Maes et al., 1993). In addition, the presence of anti-ribosomal-P antibodies has been associated with depression and psychosis in patients with lupus erythematosus (Nojima et al., 1992; Schneebaum et al., 1991; Tzioufas et al., 2000; Watanabe et al., 1996). Furthermore, CSF anti-N-methyl-D-aspartate receptor NR2 antibodies are elevated in systemic lupus erythematosus patients with active neuropsychiatric manifestations, and these are associated with BBB damage (Hirohata et al., 2014), whereas only the serum level of anti-Sm antibodies but not serum levels of anti-NR2, anti-P, or anti-phospholipid antibodies contributes to the BBB disruption in these patients (Hirohata et al., 2018). Nevertheless, a better understanding of the role of antibodies in MDD is necessary.
Potential Causes of Immune Activation in MDD Patients
Genetic Contribution to Cytokine Production
Because of the sizeable contributions of heredity in depression vulnerability, it has been proposed that the physiology of immune function in depression may be, in part, predicted by genetic mechanisms. Over the past few years, the number of samples in genome-wide association studies (GWASs) has grown into the hundreds of thousands, with a number of gene variants contributing very small effects to depression vulnerability (Border et al., 2019; Howard et al., 2019). Among the 44 risks variants identified in MDD, 4 risk variants relate to immune responses: LACC1, OLFM4, TIAF1, and NR4A2 (Wray et al., 2018). With the advance of RNA sequencing, networks of genes with association to inflammation in depression pathogenesis have been identified. In addition, polymorphisms in the genes encoding IL-1β, IL-6, IL-10, TNF, MCP1/CCL2, CRP, and phospholipase-A2 (PLA2) have been the most replicated findings in MDD (Barnes et al., 2017). However, the contribution of these polymorphisms to MDD remains difficult to determine, as, for example, a polymorphism in the IL-1β promoter at position 511, has been associated with higher depressive symptoms severity whether the polymorphism is associated with increased IL-1β production (allele 511T) or low IL-1β production (allele 511C) (Fertuzinhos et al., 2004; Hwang et al., 2009; McCulley et al., 2004; Rosa et al., 2004; Yu et al., 2003). Similar results were found with polymorphisms in the TNF, CRP, and CCL2 promoters (Bufalino et al., 2013). This discrepancy might be due to the facts that not all depressed patients exhibit inflammation, and the environmental factors and gene-environment interactions are likely more important than pure genetic factors to account for depression. Furthermore, the same genetic variants also increase the risks for inflammation-associated metabolic diseases. Finally, genome-wide methylation profiles in whole blood showed that IL-6 methylation is decreased in depressed patients with increased levels of IL-6 and CRP (Crawford et al., 2018; Uddin et al., 2011). Altogether these findings support the notion that epigenetics profiles of inflammatory genes in MDD might provide information on the immune biology of MDD.
Is There an Infectious Contribution to Immune Alterations in MDD?
The fact that MDD is associated with a dysregulation of the immune response raises the question as to whether MDD patients are more affected by infections than the general population. A past history of MDD has been associated with an increased risk of infections (Andersson et al., 2016; Irwin et al., 2011; Seminog and Goldacre, 2013; Troidle et al., 2003). A large retrospective study of ∼50,000 US college students found increased odds of ear infection, bronchitis, sinus infection and streptococcal throat infection in students reporting depression (Adams et al., 2008). Furthermore, depression increases the risk of infections after coronary artery bypass grafting (Doering et al., 2008), for herpes zoster in older adults (Irwin et al., 2011, 2013), predicts the immune system rate decay in HIV patients (Cruess et al., 2005), and prolongs increased proinflammatory cytokines levels after influenza vaccination (Glaser et al., 2003). An increased risk of infection after the onset of depression remaining relatively stable over time, and a relationship between the risk of infections and the number of depressive episodes, with a relative risk of infections of 64% with one depressive episode increasing to 84% with ≥4 depressive episodes, were found in a Danish population-based prospective study including 976,398 individuals of whom 142,169 had an history of depression between 1995 and 2012 (Andersson et al., 2016). The interpretation of the study should be, however, taken with caution as socioeconomic status was not controlled for but could account for the differences observed. There is also evidence that various viral and bacterial infections (e.g., gastroenteritis-related virus, influenza virus, herpes virus, Epstein-Barr virus, cytomegalovirus, and Borna disease virus) are associated with depressive symptoms and are known to induce the production of cytokines (Yirmiya et al., 2015), suggesting a bidirectional communication between cytokines and mood. All these studies suggest that immune responses in MDD patients are altered in a way that MDD patients are more prone to infections, which is consistent with the observation of immunosuppression in MDD patients. This also reinforces the idea that, although there is an increased production of cytokines in MDD, other parts of the immune response such as the adaptive immune system might participate in increased susceptibility to infections that in turn might impact the long-term immune characteristics.
Autoimmune Diseases and MDD
The epidemiological link between psychiatric and autoimmune diseases has been observed for almost a century (Nissen, 1936). Thus, there is an increased risk of developing subsequent autoimmune diseases (rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, systemic lupus erythematosus) in depressed patients (Andersson et al., 2015; Dickens et al., 2002; Euesden et al., 2017; Kurina et al., 2001; Patten et al., 2017). Reciprocally, patients with autoimmune diseases have some of the highest rates of comorbid depression. Thus, for example, MDD is less common early in multiple sclerosis than in its later stages (Feinstein et al., 1992) and more prevalent in relapsing-remitting than progressive multiple sclerosis (Zabad et al., 2005) and might correlate with relapses (Mooreet al., 2012). Cytokines and T cells have been proposed to contribute to both multiple sclerosis and depression pathologies (Feinstein et al., 2014). Th17 cells in particular have attracted attention as they are pathogenic in many autoimmune diseases, and anti-IL-17A therapy induces remission of depression in 40% of psoriasis patients experiencing moderately severe depression (Griffiths et al., 2017), whereas blocking the downstream effects of IL-17A by blocking its receptor using anti-IL-17RA therapy has been associated with increased suicidality risk and psychiatric disorders in psoriasis patients (Lebwohl et al., 2018), suggesting that Th17 cells could be a potential therapeutic target in populations of MDD patients with autoimmune diseases with elevated levels of Th17 cells.
Other Co-morbidities Associated with MDD and Linked to Inflammation
It has been estimated that more than half of MDD patients have associated comorbidities, and more than a third of MDD patients exhibit drug and alcohol dependence (Hasin et al., 2018; Kessler et al., 1996), which are often associate with microglial inflammation (He and Crews, 2008). Depression also increases disease rate progression and death in cancer (Bortolato et al., 2017), cardiovascular diseases (Rudisch and Nemeroff, 2003), diabetes, renal diseases (Hedayati et al., 2009), and obesity (Hasler et al., 2004), all diseases associated with increased inflammation. In the case of diabetes, anti-diabetic drugs such as the thiazolidinediones or pioglitazone, which are peroxisome proliferators activated receptor (PPAR) agonists, increase insulin sensitivity and normalize glycemia, affecting also cytokine production (Nanjan et al., 2018), have been shown to improve depressive symptoms in diabetic patients (Moulton et al., 2018) or as add-on or monotherapy in MDD or bipolar patients (Colle et al., 2017). However, this was not confirmed in a recent study with bipolar depressed patients (Aftab et al., 2019). A large cohort study found that newly diagnosed type 2 diabetic patients treated for a year with glucagon-peptide-1 agonists and dipeptidyl peptidase-IV inhibitors therapy, which increase insulin secretion, exhibited a reduction in depression symptoms, which was correlated with reduced CRP levels, suggesting that reduction of inflammation might provide an antidepressant effect (Moulton et al., 2016). It remains, however, to be determined whether, the observed antidepressant effect resulted from improvement of the diabetic pathology, and if MDD patients without diabetes would benefit from such agents. The same precautious can be applied to other co-morbidities associated with inflammation and depression.
Peripheral versus Central Inflammation
Besides systemic inflammation, many studies are now focusing on CNS inflammation. In MDD patients, central immune dysregulation, also called neuroinflammation, has been analyzed at the level of cytokines in CSF or post-mortem tissue, and at the level of the cells involved in the immune response (e.g., microglia, astrocytes, or infiltrating immune cells) using both post-mortem tissue and positron emission tomography (PET) imaging. Indeed, expression level of the translocator protein (TSPO), analyzed by PET scans using TSPO ligands, is low in the healthy brain and is upregulated locally during neuropathological conditions, and has been therefore used to measure neuroinflammation (Rupprecht et al., 2010). It is, however, important to note that the expression of TSPO initially thought to represent microglia activation, has been recently proposed to also measure local myeloid cell proliferation, or monocytes infiltration (Owen et al., 2017). Using the [18F]FEPPATSPO ligand, elevations of TSPO volume in prefrontal cortex, insula, and anterior cingulate cortex that correlated with depression severity (Setiawan et al., 2015) and duration ( Setiawanet al., 2018) have been reported, whereas no correlation was found with other ligands (Hannestad et al., 2013). A recent study found that the serum level of products synthetized by activated microglia and actively removed from the brain (e.g., TNF and prostaglandin E2), normalized to peripheral CRP level predicts TSPO volume in depressed patients, reinforcing the role of gliosis in depression (Attwells et al., 2019). Microglial activation is also found in the hippocampus of multiple sclerosis patients and correlates with depressive symptomatology (Colasanti et al., 2014). Increased Toll-like receptor (TLR)3 and TLR4 mRNA in post-mortem tissue also correlate with increased microglial activation, as TLR3 and TLR4, which recognize DAMPs and PAMPS, are critical in the induction of cytokine production (Pandey et al., 2014). Cytokine concentrations are also elevated in post-mortem brain tissue (Shelton et al., 2011). Furthermore, a recent meta-analysis suggests increased microglial activity is associated with increased IL-6, IL-8, and TNF levels in CSF and brain parenchyma of MDD patients (Wang and Miller, 2018) and reduced astrocytes and oligodendrocytes numbers in MDD patients (Enache et al., 2019). It has been speculated that the reduction of the astrocytic population is associated with a more permeable BBB, allowing the recruitment and infiltration of monocytes to the brain parenchyma (Enache et al., 2019). It is important to note that suicidal patients exhibit increased recruitment of monocytes (Torres-Platas et al., 2014), as well as increased microglial priming and activation (Steiner et al., 2008), whether or not they exhibit psychiatric symptoms. Microglial activation has also been reported in illness-associated depression. Indeed, many of the bacterial and viral infections we discussed previously associated with depression, induce microglia activation (Rock et al., 2004). Similarly, immune challenges in humans (e.g., endotoxin [LPS] or Salmonella typhi administrations) are known to activate microglia and induce depressive symptoms; the severity of the symptoms directly correlates with high blood levels of proinflammatory cytokines (Grigoleit et al., 2011; Harrison et al., 2009; Reichenberg et al., 2001). Consistent with this, neuroinflammation induced by microglia has been thought to be responsible for the high prevalence of depression in HIV-infected patients (Del Guerra et al., 2013; Kaul et al., 2001). However, data also suggest that microglia are suppressed during depression, as, for example, results of PET studies demonstrating reductions in glial cells, but not neurons, in the subgenual anterior cingulate (Cotter et al., 2001a, 2001b; Ongür et al., 1998) or in many brain regions (Hannestad et al., 2013) of depressed patients compared to healthy controls. This might explain some of the discordant results found with NSAIDs on depressive symptoms (discussed later in the review). Indeed, cyclooxygenase-1 (COX-1) inhibitors, are associated with increased depressive symptoms (as opposed to COX-2 inhibitors that are antidepressant), and COX-1 is predominantly active in microglia, whereas COX-2 is active in neurons and astrocytes (Choi et al., 2009) reinforcing the notion that suppression of certain microglial activity is associated with depressive symptoms. With the recent findings on the various phenotypes of microglia in healthy brain, it is plausible that certain populations of microglia have beneficial roles, whereas others, in contrast, are detrimental in depression. Loss of beneficial microglia or enrichment of detrimental microglia may enhance depression, but such a hypothesis will need further testing experimentally.
Effects of Antidepressants on Inflammation
The role of inflammation in treatment response is of paramount importance. There are two major questions that have been addressed. The first is whether successful treatment of depression is associated with a reduction in inflammation. The second is whether anti-inflammatory treatments are effective antidepressants, especially in depressed patients with evidence of increased inflammation. Although no currently approved treatments for depression were developed with the intent of modulating the immune response, there is evidence that conventional antidepressants have an anti-inflammatory effect and that response may depend partially on immune phenotype. The largest meta-analysis of 45 studies representing 1,517 MDD patients revealed that antidepressant treatment significantly decreases peripheral levels of IL-6, TNF, IL-10, and CCL2, but these are not associated with treatment response (Köhler et al., 2018). Reduction of IL-6 by antidepressants has been reported in various meta-analyses (Hannestad et al., 2011; Hiles et al., 2012; Strawbridge et al., 2015; Wang et al., 2019; Wie dłocha et al., 2018), although they are very heterogeneous. Sources of heterogeneity include baseline inflammation, BMI, smoking, methodology/standardization, type of depression (melancholic versus atypical depression), and class of antidepressants. These factors were not always taken into account due to lack of data availability. For example, the selective serotonin reuptake inhibitors (SSRIs) reduce IL-1β, IL-6, and TNF (Wang et al., 2019). CBT also exhibits anti-inflammatory actions in responders (Syed et al., 2018). In other studies, in contrast, antidepressants such as serotonin and norepinephrine reuptake inhibitors (SNRIs) induce IL-6 and TNF production (Hannestad et al., 2011; Warner-Schmidt et al., 2011; Piletz et al., 2009). Treatment with ECT also induces transient elevation of plasma proinflammatory cytokine levels (Hestad et al., 2003; Lehtimäki et al., 2008), and increases the numbers of monocytes, NK cells and granulocytes. In rodents, ECT has been associated with microglial activation (Wennström et al., 2006). Altogether, these findings suggest that the effects of antidepressants on cytokines remain unclear, though it is generally thought that antidepressants shift the balance toward an anti-inflammatory response (Kubera et al., 2001b; Lanquillon et al., 2000; Maes et al., 1999; Sluzewska et al., 1997). Conversely, proinflammatory cytokine levels, especially TNF, have been shown to be elevated in treatment-resistant depressed patients, suggesting a negative correlation between treatment response and proinflammatory cytokine levels (Kubera et al., 2001b; Lanquillon et al., 2000). In contrast, elevated levels of IL-17A at baseline is associated with greater reduction of depression severity after treatment with two antidepressants in combination therapy, buproprion-SSRI, (Jha et al., 2017), whereas higher CRP levels at baseline were reported to predict better treatment outcomes with either an SSRI or SNRI (Uher et al., 2014).
Impact of Anti-inflammatory Approaches on MDD Symptoms
There is a comparatively larger body of literature examining depression response following treatment that modulates the immune system. Patients with inflammatory illnesses, especially autoimmune diseases, treated with immune suppressive drugs often experience improvement in depression symptoms. Trials in this area have focused on two drug classes, non-steroidal anti-inflammatory drugs (NSAIDs), and cytokine inhibitors. Most of the studies have used NSAIDs as add-ons to conventional antidepressants, though data also exist on monotherapy with NSAIDs. A recent meta-analysis comprised of 36 RCTs including data from 10,000 patients found that both monotherapy and add-on NSAID therapy, cytokine-inhibitor monotherapy, statin add-on therapy, glucocorticoid add-on therapy, and minocycline (microglia inhibitor) add-on and monotherapy possess antidepressant efficacy (Köhler-Forsberg et al., 2019). However, previous studies concluded that the efficacy of NSAIDs on depressive symptoms is negligible (Eyre et al., 2015), possibly due to the inclusion of studies using aspirin that has no effect on depression. Cytokine inhibitor monotherapies are promising as 4 out of 6 anti-inflammatory drugs ameliorate depression (Kappelmann et al., 2018; Köhler-Forsberg et al., 2019). However, it is important to note that these studies were conducted in patients with comorbid inflammatory diseases (e.g., psoriasis, rheumatoid arthritis), which may have a distinct pathophysiology. Furthermore, TNF inhibitors such as etanercept (Tyring et al., 2013; Tyring et al., 2006), adalimumab (Loftus et al., 2008; Menter et al., 2010), IL-4Ra antagonists (Simpson et al., 2015) or IL-12/IL-23 antagonists (Langley et al., 2010), anti-IL-17A antibody (Ixekizumab [Griffiths et al., 2017]) or anti-IL-6 antibody (Sirukumab [Sun et al., 2017]) have all been shown to be more efficacious than placebo in the treatment of MDD symptoms. In non-randomized and/or non-placebo controlled trials that targeted TNF or IL-6, similar effects have been observed (Kappelmann et al., 2018), indicating an improvement of depressive symptoms with cytokine inhibitor treatments. Infliximab, a TNF-neutralizing antibody, only benefits a sub-population of treatment-resistant MDD patients with elevated levels of inflammation (CRP >5 mg/ L) (Miller and Raison, 2015; Raison et al., 2013) or patients with a history of childhood trauma (McIntyre et al., 2019). In a recent multisite study, infliximab did not significantly reduce depressive-symptoms in bipolar depressed patients (McIntyre et al., 2019). All these anti-inflammatory treatments show relatively goodsafety profiles, without major side effects noted, but caution should be taken as the trials were of short duration (Köhler-Forsberg et al., 2019). Overall, these findings suggest that cytokine inhibitor approaches provide benefit in depressed patients with prominent inflammation, but it remains to be determined whether the improvement is due, at least in part, to their effects on somatic diseases. Although all these drugs aim at reducing inflammation, they all target different mechanisms involved in the inflammatory process. NSAIDs inhibit COX-2, which is involved in the induction of inflammation. Cytokine inhibitors selectively inhibit cytokines. Glucocorticoids act upon a myriad of targets. Statins decrease CRP levels and inhibit lymphocytes. In contrast, drugs targeting circulating monocytes to prevent their infiltration in the brain, such as the C-C chemokine receptor (CCR)2 inhibitor (pioglitazone) have no effect on depressive symptoms (Dean et al., 2017; Rasgon et al., 2016; Sepanjnia et al., 2012). Anti-inflammatory drug adjunctive treatment of antidepressants seems also to improve efficacy of the antidepressant, and treatment-resistant depressed patients may also benefit from anti-inflammatory drugs (Raison et al., 2013). Mesenchymal stem cell therapy, which has been studied fairly extensively in rodent models of a variety of neuroinflammatory conditions (Regmi et al., 2019) and in humans, produces a pan-inhibition of inflammation after intravenous administration characterized by long-lasting (>6 months) reductions in TNF and CRP (Tompkins et al., 2017). There is currently an ongoing NIH-funded clinical trial in patients with comorbid MDD and alcohol-use disorder.
Overall, there is a large body of evidence that immune responses are dysregulated in MDD patients (Figure 3). Most of these findings have been replicated and expanded in rodent and non-human primate models to identify the mechanisms of action and the cause of the dysregulated immune responses in order to develop new treatments targeting the immune system that may benefit MDD patients.
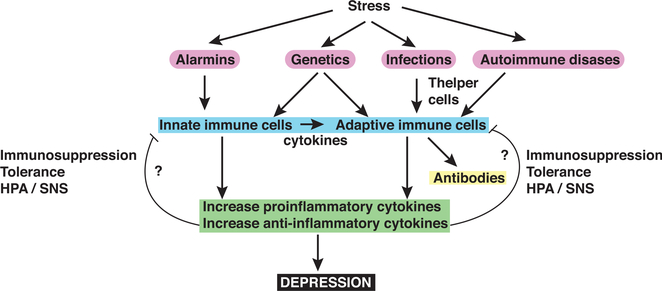
Figure 3. Diagram of Potential Immune Response Dysregulations in MDD Patients.
Stress induces the production of alarmins, molecules that trigger the production of proinflammatory cytokines by innate immune cells that promotes depressive-like behaviors in rodents. Genetically mediated susceptibility, a history of infection, and autoimmune diseases also can prime the immune system’s response to stress, through both the innate and adaptive immune systems. Infection and autoimmune diseases, for example, may act on T helper cells and affect cytokine production and antibody production. The proinflammatory cytokines are thought to promote depression, whereas the role of anti-inflammatory cytokines is less understood, and it remains to be determined whether anti-inflammatory cytokines induce cell immunosuppression or tolerance, and whether the hypothalamic-pituitary-adrenal axis (HPA) and/or the sympathetic nervous system (SNS) axis are implicated in this phenomena.
Possible Molecular Basis of Inflammation in MDD
As discussed in the previous section, there are likely several “sources” of immune dysfunction that contribute to the pathogenesis of depression: infection, microbiome alterations, medical illness, stress, and other factors (Figure 4).
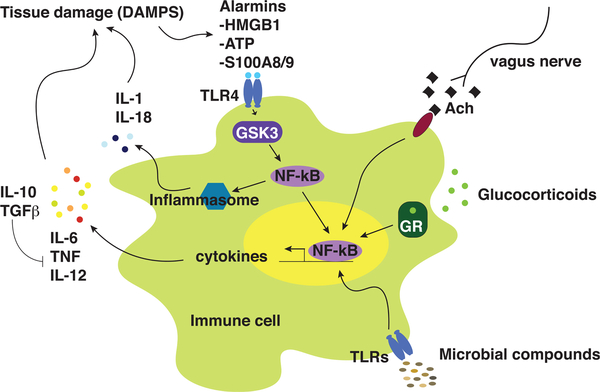
Figure 4. Multiple Mechanisms May Contribute to the Dysregulation of Cytokines in MDD.
Immune cell production of cytokines is regulated by multiple mechanisms, including anti-inflammatory actions by glucocorticoids, and vagus nerve pathways, and proinflammatory actions influenced by the microbial composition and stress-induced DAMPs involving both the inflammasome and the NF-κB pathways. Ach, acetylcholine; ATP, adenosine triphosphate; DAMP, danger-associated molecular pattern; GR, glucocorticoid receptor; GSK3, glycogen synthase kinase-3; HMGB1, high-mobility group binding protein-1; IL, interleukin; NF-κB, nuclear factor-kappa B; TLR, Toll-like receptor; TGF, transforming growth factor; TNF, tumor necrosis factor
HPA Axis and SNS
From Stress to Inflammation
The HPA axis and the sympathetic nervous system (SNS) are activated in response to various types of stress and are known to be immunoregulatory (for review, see Sternberg, 2006). The HPA axis consists of hypothalamic corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which release adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, which, in turn, releases cortisol (corticosterone in rodents) from the adrenal cortex, whereas the SNS promotes the secretion of catecholamines, norepinephrine, and epinephrine, from the adrenal medulla and sympathetic nerve endings. Both cortisol and catecholamines regulate inflammation, acting as immunosuppressants, inhibiting leukocyte trafficking and activation, as well as inflammatory cytokine production (for review, see Dhabhar, 2009). Some subsets of T cells even undergo apoptosis upon receiving a glucocorticoid signal (Pariante and Lightman, 2008).
These relationships are also bidirectional in that inflammatory cytokines also activate the HPA axis and the SNS, similarly to what occurs in infection and injury (for review, see Kenney and Ganta, 2014). Depression is often associated with hypercortisolemia and glucocorticoid resistance (Raison and Miller, 2003). Stress, particularly in early life, including maternal stress during the intrauterine period, affects glucocorticoid sensitivity via epigenetic mechanisms, turning down the sensitivity of the immune system to cortisol (for review, see Wadhwa et al., 2011). These changes in the communication between the HPA axis and immune system lead to increased rates of inflammatory and metabolic diseases in survivors of childhood abuse and neglect, as well as increased depression (Heim et al., 2008).
The autonomic nervous system is also altered in depression, with increased sympathetic activity (Murphy, 1991) and lower parasympathetic tone. The parasympathetic nervous system has also been implicated in immune function. Sickness behavior, a physiological and behavioral response associated with increased immune response activity, is, in part, mediated by the vagus nerve, through immune cells (e.g., macrophages and dendritic cells) present in the perineural sheath (Dantzer, 2009) that relay the signals from proinflammatory cytokines (IL-6, TNF, IL-1β) to the brain ( Dantzeret al., 2008). Stimulation of the vagus nerve through cholinergic signaling, in contrast, exerts anti-inflammatory properties, reducing proinflammatory cytokine production.
TLR4-Mediated Inflammation
It has become clear that immune activity in the brain itself is important. Thus, brain-induced immune activation has been partially illuminated by the discovery of alarmins produced in the brain in response to stress that trigger toll-like receptor pathway-dependent cytokine production. Toll-like receptors are a major class of receptors that detect DAMPs and PAMPs and are critical for the innate immune response. Although the prototypic pathway involving lipopolysaccharide (LPS)-induced sickness behavior has pointed toward the role of TLR4 in regulating cytokine-dependent induction of depressive-like behavior, the finding that TLR4 knockout mice are resistant to depressive-like behavior (Cheng et al., 2016) confirmed its importance. Upon ligand recognition, TLR4 activates glycogen synthase kinase-3 (GSK3) that activates NF-κB to promote proinflammatory cytokine production (Martin et al., 2005). However, only recently have some additional ligands responsible for the activation of TLR4 in depressive-like behaviors been discovered. These include the alarmins: high-mobility group box 1 protein (HMGB1), adenosine triphosphate (ATP), or Myeloid-related protein 8/14 (Mrp8/14, also called S100A8/9) (Cao et al., 2013; Cheng et al., 2016; Gong et al., 2018; Wang et al., 2018; Wu et al., 2015). Psychological stressors increase TLR4-induced inflammation (Jope et al., 2017). TLR4 activation promotes upregulation of its own expression, and TLR4 mRNA and protein have been found elevated in both the periphery and CNS of MDD patients (Hung et al., 2014). Furthermore, TLR4 levels are restored after successful treatment of MDD, confirming a role for TLR4 in MDD patients (Raison and Miller, 2017).
Inflammasome
Activation of the TLR4 pathway is also associated with activation of the inflammasome pathway (Fleshner et al., 2017), though it is not required. The inflammasome pathway is part of the innate immune response and is responsible for the production of IL-1b and IL-18 (for review, see Guo et al., 2015). Nod-like receptor (NLR), caspase-1, and apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain (ASC)-1 comprise the inflammasome complex. Once activated, pro-IL-1b and pro-IL-18 are cleaved by caspase-1 to produce active IL-1b and IL-18. NLRP3 induces CNS inflammation and increases susceptibility to depressive-like behaviors, and NLRP3- and caspase-1-deficient mice are resilient to depressive-like behaviors (Alcocer-Gómez et al., 2014; Iwata et al., 2016; Wong et al., 2016). In contrast, inflammasome activation is prevented by antidepressants (Alcocer-Gómez et al., 2017). In addition, expression of NLRP3 and caspase-1 in circulating immune cells of MDD patients is increased, suggesting that MDD patients have an activated NLRP3 inflammasome, which correlated with increased blood IL-1β and IL-18 (Alcocer-Gó mez et al., 2014; Syed et al., 2018). Interestingly, caspase-1 cleavage of glucocorticoid receptors induces glucocorticoid resistance in leukemia cells (Paugh et al., 2015), and glucocorticoid resistance has been associated with MDD (Raison and Miller, 2003), suggesting a potential pathway whereby glucocorticoid resistance might originate.
IDO/Kynurenine Pathway
Stress has also been shown to induce the indoleamine 2,3-dioxygenase (IDO)/kynurenine pathway through cytokine production. IDO is responsible for the first step of tryptophan catabolism. It reduces tryptophan levels so less tryptophan is available for the synthesis of serotonin, which is important because serotonin depletion has been hypothesized to promote depression. IDO is activated in macrophages, dendritic cells, endothelial cells, and brain glial cells comprising microglia (Dantzer, 2009) by signaling from proinflammatory cytokines, such as IL-1β, TNF, and IFN-γ, as well as psychological stress or glucocorticoids (Kiank et al., 2010) and IDO is inhibited by anti-inflammatory cytokines (Cervenka et al., 2017). IFN-α-induced depression development and severity in hepatitic C patients is directly associated with an increase in CSF and peripheral tryptophan metabolism through the kynurenine pathway (Capuron et al., 2002, 2003; Raison et al., 2010). When tryptophan is catabolized, intermediates collectively known as kynurenines are produced (Cervenka et al., 2017). Consistent with this, in rodents, administration of L-kynurenine induced depressive-like behaviors, whereas LPS-induced depressive-like behaviors are blocked by an IDO competitive inhibitor (O’Connor et al., 2009a, 2009b).
BBB Disruption
It is only recently that researchers are starting to tease apart the contribution of peripheral and central inflammation in depression with the discovery of the disruption of the BBB in depressive-like behaviors allowing peripheral signals to reach the brain, reinforcing the importance of the findings in MDD patients of a dysregulated peripheral immune response. A compromised BBB was described 40 years ago in MDD patients (Niklasson and Agren, 1984) but only recently in mice exhibiting depressive-like behaviors, independently of the stressor (Cheng et al., 2018; Menard et al., 2017). Both IL-6 and TNF have been shown to increase BBB permeability, and blocking IL-6 or TNF actions decreases stress-induced BBB opening (Cheng et al., 2018; Menard et al., 2017). Furthermore, closing of the BBB, using the sphingosine-1 phosphate receptor inhibitor, fingolimod, is sufficient to rescue learned helplessness in mice (Cheng et al., 2018). One question remaining regarding the opening of the BBB after stress is the biological consequence for the brain, and whether immune cells infiltrating the brain take advantage of this mechanism. It has been shown that both T cells and monocytes infiltrate the brain after stress. Thus, Th17 cells are able to accumulate in the hippocampus and prefrontal cortex of mice exhibiting depressive-like behavior and Th17 cells are sufficient to promote depressive-like behaviors (Beurel et al., 2013; Beurel et al., 2018). Whether these brain Th17 cells are required to induce depressive-like behavior remains to be determined. Similarly, peripheral monocytes infiltrate the brain and promote anxiety-like behaviors (McKim et al., 2018; Wohleb et al., 2013, 2014). These findings provide new avenues to identify potential relevant peripheral biomarkers associated with MDD and selective target(s) to induce antidepressant effects.
Microbiome
The dysregulated peripheral immune response in MDD patients might also result from changes at the microbiome level. The microbiome has increasingly been implicated in shaping the immune response and brain functions (gut-brain axis) (for review, see Foster et al., 2017). Recent evidence indicates the presence of microbiome alterations in depressed patients (Rogers et al., 2016), which therefore might contribute to dysregulated inflammatory responses. MDD patients exhibit significant changes in the relative abundance of Firmicutes, Actinobacteria, and Bacteroidetes compared to healthy individuals (Zheng et al., 2016; for review, see Cheung et al., 2019). A recent study with two large cohorts of Europeans reported that patients with depression are deficient in several species of gut bacteria (Coprococcus and Dialister) (Valles-Colomer et al., 2019). Coprococcus in particular has been associated with activity of the dopamine pathway, which is affected in depressed patients, and also leads to the production of butyrate, an anti-inflammatory signal, yet, depressed patients are inflamed. In addition, Coprococcus is positively associated with measures of quality of life (Valles-Colomer et al., 2019). A recent meta-analysis of 10 studies reported that the findings were inconsistent at the phylum level, whereas at the family level, Veillonellaceae, Prevotellaceae, and Sutterellaceae were less abundant and Actinomycetaceae more abundant in MDD patients than healthy controls (Sanada et al., 2020). At the genus level, Coprococcus, Faecalibacterium, Ruminococcus, Bifidobacterium, and Escherichia were reduced in MDD patients compared to healthy controls (Sanada et al., 2020). Nevertheless, it remains to be determined how microbial compounds produced in the gut influence mood. In mice, the use of germ-free mice has allowed the study of the role of the microbiome in cognition and mood (Cruz-Pereira et al., 2020). Similarly, antibiotic treatments alter multiple behaviors of mice, suggesting that bacteria influence neurobehavioral outcomes (Desbonnet et al., 2015; Hao et al., 2013; Hoban et al., 2016; Majidi et al., 2016; O’Mahony et al., 2014; Wang et al., 2017). Also, the development of fecal transfer approaches has opened new pathways to understand microbiome alteration effects on behaviors (Zheng et al., 2016). In addition, there is evidence for the role of probiotics in regulating behaviors, although the efficacy of probiotics in humans remains questionable (Suez et al., 2019). Nonetheless, meta-analysis of 6 studies using probiotics in MDD patients shows a positive effect of the probiotics in combination with antidepressant treatments (Sanada et al., 2020). Probiotics act by a variety of mechanisms of action, which include (1) increasing the biosynthesis of GABA, which may be reduced in MDD patients (Dhakal et al., 2012), (2) downregulating the HPA axis, which is often overactive in MDD patients (Ait-Belgnaoui et al., 2014), and (3) upregulating the production of tryptophan and therefore serotonin availability (Desbonnet et al., 2008). As for the studies of the role of inflammatory markers in depression, there are limitations in the studies of the microbiome in MDD patients, comprising the small size of most studies, the fact that the different populations in the studies have different ages, and age affect the microbiota composition (Chen et al., 2020), the absence of consideration of the diet, or of the effects of the antidepressant treatment, the regional variations (most studies are from Asia), and the methodology to sequence the microbiome, which might affect the results. In a more controlled environment, where a large population of psychiatric inpatients (comprising 74% MDD patients with or without other comorbidities) remain in the hospital for an 50 days, where diet was controlled for as patients received the same meals, remission was associated with an increased richness of the microbiome (Madan et al., 2020). Altogether, this suggests that the microbiota remains an interesting avenue to understand the dysregulation of the immune system in depression.
Although these mechanisms appear disjointed, they have the common theme of regulating the cytokine production, which seems central to MDD symptomatology.
Future Directions
Although substantial progress has been made in understanding immune system dysregulation in depression, many questions remain (Table 2). Thus, clinical studies have provided mixed results concerning the potential efficacy of anti-inflammatory agents in depression. Whether this is the result of a poor understanding of the immune system defect, the presence of comorbidity that complicates the clinical picture or a narrow focus on targeting a single cytokine to improve mood symptoms remains to be determined. We also lack a clear understanding of how signals from the environment (such as childhood maltreatment or stress in adulthood) initiate changes in neuroinflammation, or peripheral inflammation, and whether one precedes the other. For example, although rodent studies suggest DAMP production is important in initiating the immune response to stress, leading to the production of cytokines, we do not yet understand how stress causes DAMP production and where the production is initiated or how it is regulated. Similarly, factors that may determine the magnitude of the immune response, whether it includes downstream effector pathways such as kynurenine metabolism and/or excess of neuroinflammation are still unanswered and will have a major impact on the field.
Table 2.
Open Research Questions
| Questions |
|---|
| 1. Does dysregulation of the immune system contribute to MDD pathology? |
| If so: |
| What are the important immune system components that contribute to MDD? |
| Do these act independently or in synergy to promote MDD? |
| Is central or peripheral immune system dysregulation mediating the effects? |
| What are the CNS systems that are altered by the immune system dysregulation that promote MDD? |
| Are there different immune system alterations that promote MDD in different individuals, or are specific changes common to many MDD patients? |
| 2. What causes immune system dysregulation linked to MDD? |
| To what extent do genetic influences determine these immune system characteristics? |
| Does stress (and is it acute or chronic) contribute to immune dysregulation linked to MDD? |
| How does the environment modulate the immune system dysregulation linked to MDD? |
| Do repeated episodes of depression cause long-lasting changes in immune characteristics? |
| Does the microbiome contribute to immune system dysregulation in MDD? |
| 3. Can treating immune system dysregulation facilitate recovery from MDD and/or promote resilience to MDD onset? |
| What are the immune system targets that are effective interventions for MDD? |
| Can controlling the stress response normalize immune system characteristics? |
| Is controlling peripheral immune system characteristics sufficient to counteract MDD or does the central immune system need to be targeted? |
| Do non-invasive interventions (therapy, nutrition, exercise) normalize immune system alterations associated with MDD? |
| Are microbiome interventions that alter the immune system effective in MDD? |
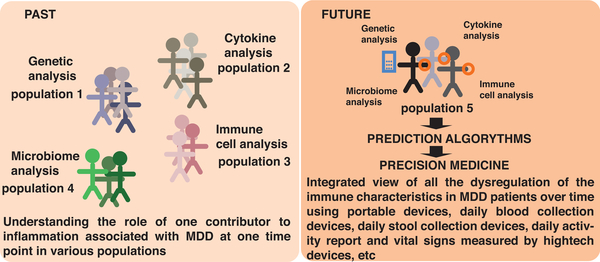
From a clinical perspective, it is also poorly understood whether the role of the immune system in depression is of clinical importance in most patients or only in a subset of cases (Figure 5). For example, an immune index analogous to the polygenic risk factor score in genomics, may more effectively classify the immune state in depressed patients compared with the use of a single marker such as CRP. Such an index or signature might help define composite criteria for clinical trials addressing the contribution of the immune system to MDD pathogenesis. Such criteria may also include imaging approaches to assess inflammation both peripherally and centrally, which are currently lacking, and/or algorithms taking into account multiple cytokines, immune cell subset prevalence or function, and microbiota species to predict the probability of developing MDD, or responding to particular treatment modalities or drugs (Figure 5).
Figure 5. Where the Field Is Going.
Historically many studies of immunological factors that may affect MDD focused on a single component, such as genetic variables, cytokines, immune cell types or actions, and the microbiome composition. With technological advances that have recently become available, or are under development, we envision that researchers will be able to apply a more integrated approach to analyze multiple parameters in each individual subject to obtain a more complete and integrated picture of genetic, microbial, and immunological factors that influence the onset, course, and treatment response of MDD patients.
New paths have been taken to target inflammation to obtain antidepressant effects, such as mesenchymal stem cell therapy, which has been proposed to induce a global anti-inflammatory response and putative antidepressant effects, which are currently being tested in clinical trials. In addition, treatment of microbiota alterations might also help improve responses to antidepressant therapy similarly to exercise, providing among other, anti-inflammatory actions. There continues to be much interest in future treatment approaches using existing or novel pharmacotherapies to control inflammation and promote or potentiate antidepressant responses. A successful immunotherapy must improve MDD symptoms without excess immunosuppression, which can occur with multiple cytokine inhibition therapy. Thus, disease modifier therapy such as modulation of the gut microbiota via diet or probiotics regime might help control the unwanted immune responses of MDD in a more physiological, and safe manner. However, the contribution of the microbiota to MDD will have to be clarified first, as well as the action of probiotics, for which a great deal of debate has been generated (Suez et al., 2019). Similarly, exercise, which provides anti-inflammatory effects, besides its other known beneficial effects, is also an option to enhance healthy diet habits and antidepressant effects in MDD patients. In addition, initiating studies to determine whether non-pharmacological treatments for depression, including evidence-based psychotherapies, transcranial magnetic stimulation, or electroconvulsive therapies modulate inflammation and immune function might provide valuable new insights. Due to the major contribution of early life trauma in the development and maintenance of chronic inflammation in adults, early intervention after trauma, especially in childhood, might prevent inflammation-associated depression. Consistent with this idea, determining whether methods to reverse the consequences of trauma, for example, via epigenetic mechanisms, prevent the inflammatory response and associated depression, might also open new therapeutic avenues.
Finally, and of considerable importance, is an important fundamental philosophical difference that has plagued this field. We are referring to some who simply believe that peripheral measures of immune dysfunction or inflammation are, at best, epiphenomena and are in no way related to the pathophysiology of depression. They dismiss the evidence of increased inflammatory markers in depressed patients and of CNS effects of induced inflammation as interesting, but certainly not causal. This in spite of overwhelming evidence that increases in peripheral inflammation produces in humans and laboratory animals CNS changes as assessed by brain imaging, neurochemistry, and behavioral changes, and moreover they minimize the now well-documented pathways reviewed above by which inflammatory cytokines can indeed act upon the CNS. Believing that the only evidence worth considering are measures of increased inflammation in the brain, which, although documented in some CNS studies and PET studies, are relatively meager at the current time might be reductionist and lead to missed therapeutic opportunity. In spite of a myriad of examples of peripheral mechanisms affecting psychiatric state, e.g., hypothyroidism and hypoglycaemia, to name only two, the argument continues to plague the field. As more research is conducted, the role of inflammation and cognate immune function dysregulation in depression will become clear.
ACKNOWLEDGMENTS
Work in the author’s labs is supported by the NIH (MH104656, MH110415, MH117293, MH115326, AA024933).
Footnotes
DECLARATION OF INTERESTS
C.B.N.’s financial disclosures are as follows: consulting for the last three years for Xhale, Takeda, Taisho Pharmaceutical Inc., Signant Health, Sunovion Pharmaceuticals Inc., Janssen Research & Development LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., Sunovion, TC MSO, Inc., Intra-Cellular Therapies, Inc., EMA Wellness, Gerson Lehrman Group (GLG), and Acadia Pharmaceuticals; a stockholder in Xhale, Celgene, Seattle Genetics, Abbvie, OPKO Health, Inc., Antares, BI Gen Holdings, Inc., Corcept Therapeutics Pharmaceuticals Company, TC MSO, Inc., Trends in Pharma Development, LLC, and EMA Wellness; on the scientific advisory boards of the American Foundation for Suicide Prevention (AFSP), Brain and Behavior Research Foundation (BBRF), Xhale, Anxiety Disorders Association of America (ADAA), Skyland Trail, Signant Health, Laureate Institute for Brain Research (LIBR), Inc.; and a member of the Board of Directors of AFSP, Gratitude America, ADAA, and Xhale Smart, Inc. C.B.N. also reports income sources or equity of $10,000 or more in American Psychiatric Publishing, Xhale, Signant Health, CME Outfitters, Intra-Cellular Therapies, Inc., Magstim, and EMA Wellness; patents for method and devices for transdermal delivery of lithium (US 6,375,990B1); method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2); and compounds, compositions, methods of synthesis, and methods of treatment (CRF Receptor Binding Ligand) (US 8,551, 996 B2).
REFERENCES
- Adams TB, Wharton CM, Quilter L, and Hirsch T (2008). The association between mental health and acute infectious illness among a national sample of 18- to 24-year-old college students. J. Am. Coll. Health 56, 657–663. [DOI] [PubMed] [Google Scholar]
- Ader R, and Cohen N (1975). Behaviorally conditioned immunosuppression. Psychosom. Med. 37, 333–340. [DOI] [PubMed] [Google Scholar]
- Aftab A, Kemp DE, Ganocy SJ, Schinagle M, Conroy C, Brownrigg B, D’Arcangelo N, Goto T, Woods N, Serrano MB, et al. (2019). Double-blind, placebo-controlled trial of pioglitazone for bipolar depression. J. Affect. Disord. 245, 957–964. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, and Tompkins T (2014). Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 26, 510–520. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, and Cordero MD (2014). NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 36, 111–117. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E, Casas-Barquero N, Williams MR, Romero-Guillena SL, Cañadas-Lozano D, Bullón P, Sánchez-Alcazar JA, Navarro-Pando JM, and Cordero MD (2017). Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 121, 114–121. [DOI] [PubMed] [Google Scholar]
- Ambrée O, Ruland C, Zwanzger P, Klotz L, Baune BT, Arolt V, Scheu S, and Alferink J (2019). Social Defeat Modulates T Helper Cell Percentages in Stress Susceptible and Resilient Mice. Int. J. Mol. Sci 20, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jørgensen P, and Goodwin RD (2015). Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol. Med. 45, 3559–3569. [DOI] [PubMed] [Google Scholar]
- Andersson NW, Goodwin RD, Okkels N, Gustafsson LN, Taha F, Cole SW, and Munk-Jørgensen P (2016). Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int. J. Epidemiol. 45, 131–139. [DOI] [PubMed] [Google Scholar]
- Asnis GM, and Miller AH (1989). Phenomenology and biology of depression: potential mechanisms for neuromodulation of immunity. Depressive Disorders and Immunity (American Psychiatric Press; ), pp. 51–64. [Google Scholar]
- Attwells S, Setiawan E, Wilson AA, Rusjan PM, Miler L, Xu C, Hutton C, Husain MI, Kish S, Vasdev N, et al. (2019). Replicating predictive serum correlates of greater translocator protein distribution volume in brain. Neuropsychopharmacology 45, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Mondelli V, and Pariante CM (2017). Genetic Contributions of Inflammation to Depression. Neuropsychopharmacology 42, 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, and Mondelli V (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-a. Mol. Psychiatry 21, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech RD, Lowthert L, Leffert JJ, Mason PN, Taylor MM, Umlauf S, Lin A, Lee JY, Maloney K, Muralidharan A, et al. (2010). Increased peripheral blood expression of electron transport chain genes in bipolar depression. Bipolar Disord. 12, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, and Agam G (2008). Major depressive disorder. N. Engl. J. Med. 358, 55–68. [DOI] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, and Mortensen PB (2013). Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70, 812–820. [DOI] [PubMed] [Google Scholar]
- Beurel E, and Lowell JA (2018). Th17 cells in depression. Brain Behav. Immun. 69, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, and Jope RS (2013). Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol. Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA, Jope RS (2018). Distinct characteristics of hippocampal pathogenic TH 17 cells in a mouse model of depression. Brain Behav. Immun. 73, 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, and Miller BJ (2015). Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry 78, 28–37. [DOI] [PubMed] [Google Scholar]
- Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein A, and Weinstein C (2011). The Global Economic Burden of Noncommunicable Diseases (World Economic Forum).
- Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, and Keller MC (2019). No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. Am. J. Psychiatry 176, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Köhler CA, Fernandes BS, Stubbs B, et al. (2017). Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 52, 58–70. [DOI] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, and Pariante CM (2013). The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav. Immun. 31, 31–47. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, et al. (2013). Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, and Dantzer R (2002). Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry 7, 468–473. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, and Miller AH (2003). Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 54, 906–914. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, and Miller AH (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, and Ruas JL (2017). Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357, 357. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, Cowen PJ, Harrison NA, Pointon L, Pariante CM, and Bullmore ET (2019). Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry 214, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, and Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 82, 217–225. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, and Sun Y (2011). Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res. 188, 224–230. [DOI] [PubMed] [Google Scholar]
- Chen JJ, He S, Fang L, Wang B, Bai SJ, Xie J, Zhou CJ, Wang W, and Xie P (2020). Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany N.Y.) 12, 2764–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, and Beurel E (2016). Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav. Immun. 53, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, and Beurel E (2018). TNFa disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 69, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, and Sublette ME (2019). Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 10, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, and Bosetti F (2009). The distinct roles of cyclooxygenase-1 and −2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 30, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, and Grassi-Oliveira R (2014). Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr. Scand. 129, 180–192. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Guo Q, Muhlert N, Giannetti P, Onega M, Newbould RD, Ciccarelli O, Rison S, Thomas C, Nicholas R, et al. (2014). In Vivo Assessment of Brain White Matter Inflammation in Multiple Sclerosis with (18)F-PBR111 PET. J. Nucl. Med. 55, 1112–1118. [DOI] [PubMed] [Google Scholar]
- Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Fève B, and Corruble E (2017). Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr. Dis. Treat. 13, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, and Everall I (2001a). Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 58, 545–553. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, and Everall IP (2001b). Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res. Bull. 55, 585–595. [DOI] [PubMed] [Google Scholar]
- Crawford B, Craig Z, Mansell G, White I, Smith A, Spaull S, Imm J, Hannon E, Wood A, Yaghootkar H, et al. ; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2018). DNA methylation and inflammation marker profiles associated with a history of depression. Hum. Mol. Genet. 27, 2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess DG, Douglas SD, Petitto JM, Have TT, Gettes D, Dubé B, Cary M, and Evans DL (2005). Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am. J. Psychiatry 162, 2125–2130. [DOI] [PubMed] [Google Scholar]
- Cruz-Pereira JS, Rea K, Nolan YM, O’Leary OF, Dinan TG, and Cryan JF (2020). Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol 71, 49–78. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2009). Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am 29, 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darko DF, Lucas AH, Gillin JC, Risch SC, Golshan S, Hamburger RN, Silverman MB, and Janowsky DS (1988). Cellular immunity and the hypothalamic-pituitary axis in major affective disorder: a preliminary study. Psychiatry Res. 25, 1–9. [DOI] [PubMed] [Google Scholar]
- Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, and Atashzar MR (2016). Elevated IL-17 and TGF-b Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. 7, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean OM, Kanchanatawan B, Ashton M, Mohebbi M, Ng CH, Maes M, Berk L, Sughondhabirom A, Tangwongchai S, Singh AB, et al. (2017). Adjunctive minocycline treatment for major depressive disorder: A proof of concept trial. Aust. N. Z. J. Psychiatry 51, 829–840. [DOI] [PubMed] [Google Scholar]
- Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, and Konkiewitz EC (2013). Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J. Neurovirol. 19, 314–327. [DOI] [PubMed] [Google Scholar]
- Denburg SD, Carbotte RM, Long AA, and Denburg JA (1988). Neuropsychological correlates of serum lymphocytotoxic antibodies in systemic lupus erythematosus. Brain Behav. Immun. 2, 222–234. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, and Dinan TG (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, and Cryan JF (2015). Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 48, 165–173. [DOI] [PubMed] [Google Scholar]
- Deverman BE, and Patterson PH (2009). Cytokines and CNS development. Neuron 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2009). A hassle a day may keep the pathogens away: The fight-or-flight stress response and the augmentation of immune function. Integr. Comp. Biol. 49, 215–236. [DOI] [PubMed] [Google Scholar]
- Dhakal R, Bajpai VK, and Baek KH (2012). Production of gaba (γ - Aminobutyric acid) by microorganisms: a review. Braz. J. Microbiol. 43, 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, and Creed F (2002). Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom. Med. 64, 52–60. [DOI] [PubMed] [Google Scholar]
- Doering LV, Martínez-Maza O, Vredevoe DL, and Cowan MJ (2008). Relation of depression, natural killer cell function, and infections after coronary artery bypass in women. Eur. J. Cardiovasc. Nurs 7, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, and Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, Poznanovic ST, Jovanovic A, Nikolic T, and Jasovic-Gasic M (2013). Melancholic and atypical major depression–connection between cytokines, psychopathology and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 43, 1–6. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Kelley ME, Aponte-Rivera V, Mletzko-Crowe T, Kinkead B, Ritchie JC, Nemeroff CB, Craighead WE, and Mayberg HS; PReDICT Team (2017). Effects of Patient Preferences on Outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) Study. Am. J. Psychiatry 174, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova, et al. (1980). T and B lymphocytes in psychotic patients. Psychopharmacology (Berl.) 67, 245–248.6770403 [Google Scholar]
- Elmer BM, and McAllister AK (2012). Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovainio M, Taipale T, Seppalälä I, Mononen N, Raitoharju E, Jokela M, Pulkki-Råback L, Illig T, Waldenberger M, Hakulinen C, et al. (2015). Activated immune-inflammatory pathways are associated with long-standing depressive symptoms: Evidence from gene-set enrichment analyses in the Young Finns Study. J. Psychiatr. Res. 71, 120–125. [DOI] [PubMed] [Google Scholar]
- Enache D, Pariante CM, and Mondelli V (2019). Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun 81, 24–40. [DOI] [PubMed] [Google Scholar]
- Euesden J, Danese A, Lewis CM, and Maughan B (2017). A bidirectional relationship between depression and the autoimmune disorders - New perspectives from the National Child Development Study. PLoS ONE 12, e0173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre HA, Air T, Proctor S, Rositano S, and Baune BT (2015). A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 57, 11–16. [DOI] [PubMed] [Google Scholar]
- Feinstein A, du Boulay G, and Ron MA (1992). Psychotic illness in multiple sclerosis. A clinical and magnetic resonance imaging study. Br. J. Psychiatry 161, 680–685. [DOI] [PubMed] [Google Scholar]
- Feinstein A, Magalhaes S, Richard JF, Audet B, and Moore C (2014). The link between multiple sclerosis and depression. Nat. Rev. Neurol. 10, 507–517. [DOI] [PubMed] [Google Scholar]
- Felger JC, and Treadway MT (2017). Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TB, Kasahara TM, Barros PO, Vieira MM, Bittencourt VC, Hygino J, Andrade RM, Linhares UC, Andrade AF, and Bento CA (2011). Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J. Neuroimmunol. 238, 58–66. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos SM, Oliveira JR, Nishimura AL, Pontual D, Carvalho DR, Sougey EB, Otto PA, and Zatz M (2004). Analysis of IL-1alpha, IL-1beta, and IL-1RA [correction of IL-RA] polymorphisms in dysthymia. J. Mol. Neurosci. 22, 251–256. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Frank M, and Maier SF (2017). Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 42, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester JV, McMenamin PG, and Dando SJ (2018). CNS infection and immune privilege. Nat. Rev. Neurosci. 19, 655–671. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rinaman L, and Cryan JF (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 7, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki P, Gałecka E, Maes M, Chamielec M, Orzechowska A, Bobinska K, Lewinski A,andSzemraj J(2012).The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depres7 sive disorder. J. Affect. Disord. 138, 360–366. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Wagner G, Matyas N, Titscher V, Greimel J, Lux L, Gaynes BN, Viswanathan M, Patel S, and Lohr KN (2017). Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open 7, e014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney SM, and Drexhage HA (2013). Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 8, 900–920. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, and Kiecolt-Glaser JK (2003). Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch. Gen. Psychiatry 60, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, and Miller BJ (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Su WJ, Cao ZY, Lian YJ, Peng W, Liu YZ, Zhang Y, Liu LL, Wu R, Wang B, et al. (2018). Hippocampal Mrp8/14 signaling plays a critical role in the manifestation of depressive-like behaviors in mice. J. Neuroinflammation 15, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Liu L, Jiang W, and Zhou R (2020). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112. [DOI] [PubMed] [Google Scholar]
- Gorman DG, and Cummings JL (1993). Neurobehavioral presentations of the antiphospholipid antibody syndrome. J. Neuropsychiatry Clin. Neurosci. 5, 37–42. [DOI] [PubMed] [Google Scholar]
- Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, Conway CR, Forester BP, Mondimore FM, Shelton RC, et al. (2019). Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled trial. J. Psychiatr. Res. 111, 59–67. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, Acharya N, and Rapaport MH (2017). Impact of Ixekizumab Treatment on Depressive Symptoms and Systemic Inflammation in Patients with Moderate-to-Severe Psoriasis: An Integrated Analysis of Three Phase 3 Clinical Studies. Psychother. Psychosom 86, 260–267. [DOI] [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, and Schedlowski M (2011). Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE 6, e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Ambrée O, Jörgens S, Jawahar MC, Singhal G, Stacey D, Arolt V, and Baune BT (2016a). Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology 73, 24–31. [DOI] [PubMed] [Google Scholar]
- Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, and Bergink V (2016b). Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl.) 233, 1679–1688. [DOI] [PubMed] [Google Scholar]
- Gu M, Li Y, Tang H, Zhang C, Li W, Zhang Y, Li Y, Zhao Y, and Song C (2018). Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients 10, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Callaway JB, and Ting JPY (2015). Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, and Kivimäki M (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, and Bloch M (2011). The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36, 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee JY, O’Connor KC, Pelletier D, and Carson RE (2013). The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C]PBR28 PET study. Brain Behav. Immun. 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao K, Qi Q, Hao H, Wang G, Chen Y, Liang Y, and Xie L (2013). The pharmacokinetic-pharmacodynamic model of azithromycin for lipopolysaccharide-induced depressive-like behavior in mice. PLoS ONE 8, e54981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, and Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, and Grant BF (2018). Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 75, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D, Rössler W, and Angst J (2004). The associations between psychopathology and being overweight: a 20-year prospective study. Psychol. Med. 34, 1047–1057. [DOI] [PubMed] [Google Scholar]
- He J, and Crews FT (2008). Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, and Rush AJ (2009). Prevalence of major depressive episode in CKD. Am. J. Kidney Dis. 54, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, and Nemeroff CB (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33, 693–710. [DOI] [PubMed] [Google Scholar]
- Hepgul N, Cattaneo A, Zunszain PA, and Pariante CM (2013). Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med 11,28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Tønseth S, Støen CD, Ueland T, and Aukrust P (2003). Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J. ECT 19, 183–188. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, and Attia J (2012). Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol. Med. 42, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Arinuma Y, Yanagida T, and Yoshio T (2014). Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res. Ther 16, R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata S, Sakuma Y, Matsueda Y, Arinuma Y, and Yanagida T (2018). Role of serum autoantibodies in blood brain barrier damages in neuropsychiatric systemic lupus erythematosus. Clin. Exp. Rheumatol. 36, 1003–1007. [PubMed] [Google Scholar]
- Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O’Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, and Cryan JF (2016). Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 339, 463–477. [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, et al. ; 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, and Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Hung YY, Kang HY, Huang KW, and Huang TL (2014). Association between toll-like receptors expression and major depressive disorder. Psychiatry Res. 220, 283–286. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Hsu CD, and Liou YJ (2009). Interleukin-1 beta −511C/T genetic polymorphism is associated with age of onset of geriatric depression. Neuromolecular Med. 11, 322–327. [DOI] [PubMed] [Google Scholar]
- Irwin M, and Gillin JC (1987). Impaired natural killer cell activity among depressed patients. Psychiatry Res. 20, 181–182. [DOI] [PubMed] [Google Scholar]
- Irwin M, Caldwell C, Smith TL, Brown S, Schuckit MA, and Gillin JC (1990). Major depressive disorder, alcoholism, and reduced natural killer cell cytotoxicity. Role of severity of depressive symptoms and alcohol consumption. Arch. Gen. Psychiatry 47, 713–719. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, Caulfield MJ, Weinberg A, Chan IS, Clair J, et al. (2011). Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav. Immun. 25, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, Carrillo C, Stanley HA, Caulfield MJ, Weinberg A, et al. (2013). Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin. Infect. Dis. 56, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, et al. (2016). Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 80, 12–22. [DOI] [PubMed] [Google Scholar]
- Jansen R, Penninx BW, Madar V, Xia K, Milaneschi Y, Hottenga JJ, Hammerschlag AR, Beekman A, van der Wee N, Smit JH, et al. (2016). Gene expression in major depressive disorder. Mol. Psychiatry 21, 339–347. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, and Trivedi MH (2017). Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav. Immun. 66, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Cheng Y, Lowell JA, Worthen RJ, Sitbon YH, and Beurel E (2017). Stressed and Inflamed, Can GSK3 Be Blamed? Trends Biochem. Sci. 42, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, Arolt V, Cassens U, and Rothermundt M (2005). Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J. Affect. Disord. 87, 305–311. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, and Khandaker GM (2018). Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlović D,Serretti A, Vrkić N,Martinac M,and Marčinko D(2012). Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 198, 74–80. [DOI] [PubMed] [Google Scholar]
- Katon WJ (2011). Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin. Neurosci. 13, 7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, and Lipton SA (2001). Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, and Ganta CK (2014). Autonomic nervous system and immune system interactions. Compr. Physiol. 4, 1177–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, and Blazer DG (1996). Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br. J. Psychiatry Suppl. June, 17–30. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, and Wang PS; National Comorbidity Survey Replication (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105. [DOI] [PubMed] [Google Scholar]
- Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, and Schuett C (2010). Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE 5, e11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SU, and de Vellis J (2005). Microglia in health and disease. J. Neurosci. Res. 81, 302–313. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, Lee HJ, Ham BJ, and Lee HS (2013). Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig 10, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J (2016). Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, Patel M, Hodes GE, Russo SJ, Merad M, et al. (2017). Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl. Psychiatry 7, e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, et al. (2017). Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, et al. (2018). Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 55, 4195–4206. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O, N Lydholm C, Hjorthøj C, Nordentoft M, Mors O, and Benros ME (2019). Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr. Scand. 139, 404–419. [DOI] [PubMed] [Google Scholar]
- Kronfol Z (2002). Immune dysregulation in major depression: a critical review of existing evidence. Int. J. Neuropsychopharmacol. 5, 333–343. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, and House JD (1989). Lymphocyte mitogenesis, immunoglobulin and complement levels in depressed patients and normal controls. Acta Psychiatr. Scand. 80, 142–147. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Silva J Jr., Greden J, Dembinski S, Gardner R, and Carroll B (1983). Impaired lymphocyte function in depressive illness. Life Sci. 33, 241–247. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Nair M, Goodson J, Goel K, Haskett R, and Schwartz S (1989). Natural killer cell activity in depressive illness: preliminary report. Biol. Psychiatry 26, 753–756. [DOI] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, and Maes M (2001a). Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J. Clin. Psychopharmacol. 21, 199–206. [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Holan V, Basta-Kaim A, Roman A, and Shani J (2001b). Prolonged desipramine treatment increases the production of interleukin-10,ananti-inflammatorycytokine,inC57BL/6micesubjectedtothe chronicmildstressmodelofdepression.J.Affect. Disord. 63,171–178. [DOI] [PubMed] [Google Scholar]
- Kurd SK,Troxel AB,Crits-Christoph P,andGelfand JM(2010). Therisk ofdepression,anxiety,andsuicidalityinpatientswithpsoriasis: apopulation-basedcohortstudy.Arch.Dermatol.146,891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurina LM, Goldacre MJ,Yeates D,and Gill LE(2001).Depressionand anxiety inpeoplewithinflammatorybowel disease.J.Epidemiol.Community Health 55,716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y,deJonge P,Giltay EJ,and Penninx BWJH (2018). Metabolicandinflammatorymarkers:associationswithindividual depressive-symptoms.Psychol.Med 48, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y,Smit JH,Schoevers RA,Wittenberg G,and Penninx BWJH(2019). Longitudinal Association Between Depression and Inflammatory Markers:Results From the Netherlands Study of Depression and Anxiety.Biol.Psychiatry 85, 829–837. [DOI] [PubMed] [Google Scholar]
- Langley RG,Feldman SR,Han C, Schenkel B,Szapary P,Hsu M-C, Ortonne J-P,Gordon KB,andKimball AB(2010).Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J. Am. Acad. Dermatol. 63, 457–465. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, and Vedder H (2000). Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 370–379. [DOI] [PubMed] [Google Scholar]
- Lebwohl MG, Papp KA, Marangell LB, Koo J, Blauvelt A, Gooderham M, Wu JJ, Rastogi S, Harris S, Pillai R, and Israel RJ (2018). Psychiatric adverse events during treatment with brodalumab: Analysis of psoriasis clinical trials. J. Am. Acad. Dermatol. 78, 81–89.e5. [DOI] [PubMed] [Google Scholar]
- Leday GGR, Vértes PE, Richardson S, Greene JR, Regan T, Khan S, Henderson R, Freeman TC, Pariante CM, Harrison NA, et al. ; MRC Immunopsychiatry Consortium (2018). Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol. Psychiatry 83, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki K, Keränen T, Huuhka M, Palmio J, Hurme M, Leinonen E, and Peltola J (2008). Increase in plasma proinflammatory cytokines after electroconvulsive therapy in patients with depressive disorder. J. ECT 24, 88–91. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, and Schwartz M (2008). Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 22, 1108–1114. [DOI] [PubMed] [Google Scholar]
- Li Q, and Barres BA (2018). Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Li S, Gowans EJ, Chougnet C, Plebanski M, and Dittmer U (2008). Natural regulatory T cells and persistent viral infection. J. Virol. 82, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ho RC, and Mak A (2012a). The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int. J. Rheum. Dis. 15, 183–187. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC-M, and Mak A (2012b). Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J. Affect. Disord. 139, 230–239. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Feagan BG, Colombel J-F, Rubin DT, Wu EQ, Yu AP, Pollack PF, Chao J, and Mulani P (2008). Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am. J. Gastroenterol. 103, 3132–3141. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, and Kipnis J (2017). Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, and Ho RC (2017). Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 12, e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray DM, and Kollmann TR (2014). The role of environmental factors in modulating immune responses in early life. Front. Immunol. 5, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A, Thompson D, Fowler JC, Ajami NJ, Salas R, Frueh BC, Bradshaw MR, Weinstein BL, Oldham JM, and Petrosino JF (2020). The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 264, 98–106. [DOI] [PubMed] [Google Scholar]
- Maes M (1995). Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 19, 11–38. [DOI] [PubMed] [Google Scholar]
- Maes M, Stevens W, Peeters D, DeClerck L, Scharpe S, Bridts C, Schotte C, and Cosyns P (1992). A study on the blunted natural killer cell activity in severely depressed patients. Life Sci. 50, 505–513. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer H, Jacobs J, Suy E, Calabrese J, Minner B, and Raus J (1993). Autoimmunity in depression: increased antiphospholipid autoantibodies. Acta Psychiatr. Scand. 87, 160–166. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, et al. (1998). The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10, 313–318. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A-H, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, and Scharpé S (1999). Negative immunoregulatory effects of antidepressants: inhibition of interferon-g and stimulation of interleukin-10 secretion. Neuropsychopharmacology 20, 370–379. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, and Maj M (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 24, 27–53. [DOI] [PubMed] [Google Scholar]
- Majidi J, Kosari-Nasab M, and Salari AA (2016). Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety-anddepression-likebehaviors,hippocampalinflammation,andHPA axisactivity in adult mice. Brain Res. Bull. 120, 1–13. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, Hegerl U, Lonnqvist J, Malone K, Marusic A, et al. (2005). Suicide prevention strategies: a systematic review. JAMA 294, 2064–2074. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, and Michalek SM (2005). Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar C. Scheiwe, Nessler S, Kunz P, van Loo G, et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. [DOI] [PubMed] [Google Scholar]
- McCulley MC, Day IN, and Holmes C (2004). Association between interleukin 1-beta promoter (-511) polymorphism and depressive symptoms in Alzheimer’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet 124B, 50–53. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, Rosenblat JD, Brietzke E, Soczynska JK, Cosgrove VE, et al. (2019). Efficacy of Adjunctive Infliximab vs Placebo in the Treatment of Adults With Bipolar I/II Depression: A Randomized Clinical Trial. JAMA Psychiatry 76, 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, et al. (2018). Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, et al. (2017). Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, Bao Y, and Mulani P (2010). The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J. Am. Acad. Dermatol. 62, 812–818. [DOI] [PubMed] [Google Scholar]
- Miller GE, and Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry 72, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, and Raison CL (2015). Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA Psychiatry 72, 527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, and Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, and Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, and Robertson NP (2012). Multiple sclerosis relapses and depression. J. Psychosom. Res. 73, 272–276. [DOI] [PubMed] [Google Scholar]
- Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, Beckman K, Haudenschild C, McCormick C, Mei R, et al. (2014). Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol. Psychiatry 19, 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Pickup JC, Amiel SA, Winkley K, and Ismail K (2016). Investigating incretin-based therapies as a novel treatment for depression in type 2 diabetes: Findings from the South London Diabetes (SOUL-D) Study. Prim. Care Diabetes 10, 156–159. [DOI] [PubMed] [Google Scholar]
- Moulton CD, Hopkins CWP, Ismail K, and Stahl D (2018). Repositioning of diabetes treatments for depressive symptoms: A systematic review and meta-analysis of clinical trials. Psychoneuroendocrinology 94, 91–103. [DOI] [PubMed] [Google Scholar]
- Müller N (2014). Immunology of major depression. Neuroimmunomodulation 21, 123–130. [DOI] [PubMed] [Google Scholar]
- Müller S, Chang HC, and Köhler H (1989). Perturbation of the idiotypic network. I. Induction with multiple alloantigen stimulation. Cell. Immunol. 119, 353–372. [DOI] [PubMed] [Google Scholar]
- Murphy MM (1991). Can menopause cause autonomic dysreflexia? SCI Nurs. 8, 83. [PubMed] [Google Scholar]
- Murphy K (2012). Janeway’s Immunobiology, Eighth Edition (Garland Science). [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, and Kim YK (2005). Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 88, 167–173. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, and Attia SM (2017). IL-17A causes depression-like symptoms via NF-κB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 97, 14–24. [DOI] [PubMed] [Google Scholar]
- Nanjan MJ, Mohammed M, Prashantha Kumar BR, and Chandrasekar MJN (2018). Thiazolidinediones as antidiabetic agents: A critical review. Bio-org. Chem. 77, 548–567. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB (2016). Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 89, 892–909. [DOI] [PubMed] [Google Scholar]
- Niklasson F, and Agren H (1984). Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol. Psychiatry 19, 1183–1206. [PubMed] [Google Scholar]
- Nissen HSK (1936). The Psychogenic Problem (Endocrinal and Metabolic) in Chronic Arthritis. N. Engl. J. Med. 214, 576–581. [Google Scholar]
- Nojima Y, Minota S, Yamada A, Takaku F, Aotsuka S, and Yokohari R (1992). Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 51, 1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, and Kelley KW (2009a). Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 182, 3202–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, and Dantzer R (2009b). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, et al. (2014). Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 277, 885–901. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, and Price JL (1998). Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 95, 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukka M, and Bettelli E (2018). Regulation of lymphocyte trafficking in central nervous system autoimmunity. Curr. Opin. Immunol. 55, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, and Kubes P (2012). Immune surveillance in the central nervous system. Nat. Neurosci. 15, 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, Galloway D, Williams JB, Lehr J, Mandhair H, et al. (2017). Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J. Cereb. Blood Flow Metab. 37, 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, and Dwivedi Y (2014). Toll-like receptors in the depressed and suicide brain. J. Psychiatr. Res. 53, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI Sr., Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, Pi B, Thurmond L, and Bilello JA (2013). Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol. Psychiatry 18, 332–339. [DOI] [PubMed] [Google Scholar]
- Pariante CM, and Lightman SL (2008). The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468. [DOI] [PubMed] [Google Scholar]
- Patas K, Willing A, Demiralay C, Engler JB, Lupu A, Ramien C, Schäfer T, Gach C, Stumm L, Chan K, et al. (2018). T Cell Phenotype and T Cell Receptor Repertoire in Patients with Major Depressive Disorder. Front. Immunol 9, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Marrie RA, and Carta MG (2017). Depression in multiple sclerosis. Int. Rev. Psychiatry 29, 463–472. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Bonten EJ, and Evans WE (2015). Inflammasome-mediated glucocorticoid resistance: The receptor rheostat. Mol. Cell. Oncol. 3, e1065947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, and Devane CL (2009). Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J. Biol. Psychiatry 10, 313–323. [DOI] [PubMed] [Google Scholar]
- Quan N, and Banks WA (2007). Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735. [DOI] [PubMed] [Google Scholar]
- Raison CL, and Miller AH (2003). When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 160, 1554–1565. [DOI] [PubMed] [Google Scholar]
- Raison CL, and Miller AH (2017). Pathogen-Host Defense in the Evolution of Depression: Insights into Epidemiology, Genetics, Bioregional Differences and Female Preponderance. Neuropsychopharmacology 42, 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, and Miller AH (2005). Depressive symptoms and viral clearance in patients receiving interferonalpha and ribavirin for hepatitis C. Brain Behav. Immun. 19, 23–27. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, and Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, and Miller AH (2010). CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, and Miller AH (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, and Engelhardt B (2012). The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Lin KW, Lin J, Epel E, and Blackburn E (2016). Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: a preliminary study. Transl. Psychiatry 6, e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LJH, Moffitt TE, Arseneault L, Danese A, Eugen-Olsen J, Fisher HL, Harrington H, Houts R, Matthews T, Sugden K, et al. (2019). Association of Adverse Experiences and Exposure to Violence in Childhood and Adolescence With Inflammatory Burden in Young People. JAMA Pediatr. Published online November 3, 2019 10.1001/jamapediatrics.2019.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, and Sallusto F (2009). C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523. [DOI] [PubMed] [Google Scholar]
- Regmi S, Pathak S, Kim JO, Yong CS, and Jeong JH (2019). Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 98, 151041. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, and Pollmächer T (2001). Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445–452. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, and Peterson PK (2004). Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17, 942–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, and Wesselingh S (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 21, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Papiol S, Cuesta MJ, Serrano F, Martínez-Larrea A, and Fañanás L (2004). Interleukin-1beta (IL-1beta) gene and increased risk for the depressive symptom-dimension in schizophrenia spectrum disorders. Am. J. Med. Genet. B. Neuropsychiatr. Genet 124B, 10–14. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Fenker J, Gutbrodt H, Peters M, and Kirchner H (2001a). Different immune patterns in melancholic and non-melancholic major depression. Eur. Arch. Psychiatry Clin. Neurosci. 251, 90–97. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Peters M, Gutbrodt H, Fenker J, Kersting A, and Kirchner H (2001b). Inflammatory markers in major depression and melancholia. J. Affect. Disord. 63, 93–102. [DOI] [PubMed] [Google Scholar]
- Rudisch B, and Nemeroff CB (2003). Epidemiology of comorbid coronary artery disease and depression. Biol. Psychiatry 54, 227–240. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, and Schumacher M (2010). Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, et al. (2020). Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 266, 1–13. [DOI] [PubMed] [Google Scholar]
- Sandrone S, Moreno-Zambrano D, Kipnis J, and van Gijn J (2019). A (delayed) history of the brain lymphatic system. Nat. Med. 25, 538–540. [DOI] [PubMed] [Google Scholar]
- Schiweck C, Valles-Colomer M, Arolt V, Müller N, Raes J, Wijkhuijs A, Claes S, Drexhage H, and Vrieze E (2020). Depression and suicidality: A link to premature T helper cell aging and increased Th17 cells. Brain Behav. Immun. Published online February 14, 2020 10.1016/j.bbi.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, and Stein M (1984). Lymphocyte function in major depressive disorder. Arch. Gen. Psychiatry 41, 484–486. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Bond RN, Cohen J, and Stein M (1989). Major depressive disorder and immunity. Role of age, sex, severity, and hospitalization. Arch. Gen. Psychiatry 46, 81–87. [DOI] [PubMed] [Google Scholar]
- Schneebaum AB, Singleton JD, West SG, Blodgett JK, Allen LG, Cheronis JC, and Kotzin BL (1991). Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am. J. Med. 90, 54–62. [DOI] [PubMed] [Google Scholar]
- Seminog OO, and Goldacre MJ (2013). Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax 68, 171–176. [DOI] [PubMed] [Google Scholar]
- Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, and Akhondzadeh S (2012). Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology 37, 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, and Meyer JH (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, and Meyer JH (2018). Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 5, 339–347. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, and Mirnics K (2011). Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 16, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Worm M, Soong W, Blauvelt A, Eckert L, Wu R, Ardeleanu M, Graham N, Pirozzi G, and Sutherland ER (2015). Dupilumab improves patient-reported outcomes (PROs) in a Phase 2 study in adults with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 135, AB167. [Google Scholar]
- Sluzewska A, Sobieska M, and Rybakowski JK (1997). Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology 35, 123–127. [DOI] [PubMed] [Google Scholar]
- Smith RS (1991). The macrophage theory of depression. Med. Hypotheses 35, 298–306. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, and Jordan MS (2009). T cell activation. Annu. Rev. Immunol. 27, 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, and Bogerts B (2008). Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, and Stevens B (2012). The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 35, 369–389. [DOI] [PubMed] [Google Scholar]
- Sternberg EM (2006). Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, and Kamarck TW (2009). A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 23, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, and Cleare AJ (2015). Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Suez J, Zmora N, Segal E, and Elinav E (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, Vermeulen J, Kent JM, Singh J, Drevets WC, et al. (2017). The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav. Immun. 66, 156–164. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Iwata Y, Matsuzaki H, Anitha A, Suda S, Iwata K, Shinmura C, Kameno Y, Tsuchiya KJ, Nakamura K, et al. (2010). Reduced expression of apolipoprotein E receptor type 2 in peripheral blood lymphocytes from patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1007–1010. [DOI] [PubMed] [Google Scholar]
- Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, Mayberg HS, Dhabhar F, Dietrich WD, Keane RW, et al. (2018). Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 99, 914–924.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV, Heldman AW, Miki R, Goldschmidt-Clermont PJ, Goldstein BJ, et al. (2017). Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci 72, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo L, Burrai C, Scamonatti L, Weissenburger J, and Rush J (1988). Comparison between clinician-rated and self-reported depressive symptoms in Italian psychiatric patients. Neuropsychobiology 19, 1–5. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, and Mechawar N (2014). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Cooper JA, and Miller AH (2019). Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends Cogn. Sci 23, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, et al. ; STAR*D Study Team (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, and Finkelstein FO (2003). Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am. J. Kidney Dis. 42, 350–354. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, et al. (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35. [DOI] [PubMed] [Google Scholar]
- Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EHZ, Gandra SR, Shi Y, and Kricorian G (2013). Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from a randomized, double-blind, placebo-controlled study of etanercept. J. Eur. Acad. Dermatol. Venereol. 27, 125–128. [DOI] [PubMed] [Google Scholar]
- Tzioufas AG, Tzortzakis NG, Panou-Pomonis E, Boki KA, Sakarellos-Daitsiotis M, Sakarellos C, and Moutsopoulos HM (2000). The clinical relevance of antibodies to ribosomal-P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann. Rheum. Dis. 59, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Koenen KC, Aiello AE, Wildman DE, de los Santos R, and Galea S (2011). Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychol. Med. 41, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, and McGuffin P (2014). An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 171, 1278–1286. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araújo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, et al. (2010). Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J. Neuroimmunol. 229, 212–218. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, and Jacque C (2000). Cytokine signals propagate through the brain. Mol. Psychiatry 5, 604–615. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, van Reedt Dortland AK, Schoevers RA, Giltay EJ, de Jonge P, and Penninx BW (2014). Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 39, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, and Lu MC (2011). The contribution of maternal stress to preterm birth: issues and considerations. Clin. Perinatol. 38, 351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AK, and Miller BJ (2018). Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 44, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Huang FL, Hu ZL, Zhang WJ, Qiao XQ, Huang YQ, Dai RP, Li F, and Li CQ (2017). Early-Life Social Isolation-Induced Depressive-Like Behavior in Rats Results in Microglial Activation and Neuronal Histone Methylation that Are Mitigated by Minocycline. Neurotox. Res. 31, 505–520. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu J, Liu Y, Li Z, and Li X (2018). TLR4-NF-κB Signal Involved in Depressive-Like Behaviors and Cytokine Expression of Frontal Cortex and Hippocampus in Stressed C57BL/6 and ob/ob Mice. Neural Plast 2018, 7254016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang R, Liu L, Qiao D, Baldwin DS, and Hou R (2019). Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav. Immun. 79, 24–38. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, and Greengard P (2011). Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Natl. Acad. Sci. USA 108, 9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sato T, Uchiumi T, and Arakawa M (1996). Neuropsychiatric manifestations in patients with systemic lupus erythematosus: diagnostic and predictive value of longitudinal examination of anti-ribosomal P antibody. Lupus 5, 178–183. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, and Goehler LE (1995). Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 57, 1011–1026. [DOI] [PubMed] [Google Scholar]
- Weigelt K, Carvalho LA, Drexhage RC, Wijkhuijs A, de Wit H, van Beveren NJ, Birkenhäger TK, Bergink V, and Drexhage HA (2011). TREM-1 and DAP12 expression in monocytes of patients with severe psychiatric disorders. EGR3, ATF3 and PU.1 as important transcription factors. Brain Behav. Immun. 25, 1162–1169. [DOI] [PubMed] [Google Scholar]
- Wennström M,Hellsten J,Ekstrand J,Lindgren H,andTingström A (2006). Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol. Psychiatry 59, 178–186. [DOI] [PubMed] [Google Scholar]
- Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M,Janus M, Dębowska W, Mosiołek A, Waszkiewicz N, and Szulc A (2018). Effect of antidepressant treatment on peripheral inflammation markers - A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 80, 217–226. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, and Hunter CA (2010). Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, and Windle RC (2013). Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. J. Affect. Disord. 150, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, and Sheridan JF (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, and Godbout JP (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh SL, et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MK, Huang TL, Huang KW, Huang YL, and Hung YY (2015). Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr. Dis. Treat 11, 1853–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zheng YL, Tian LP, Lai JB, Hu CC, Zhang P, Chen JK, Hu JB, Huang ML, Wei N, et al. (2017). Circulating T lymphocyte subsets, cytokines, and immune checkpoint inhibitors in patients with bipolar II or major depression: a preliminary study. Sci. Rep. 7, 40530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z, Cui J, Shi T, and Fang Y (2012). Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS ONE 7, e31283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, and Goshen I (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, and Reshef R (2015). Depression as a microglial disease. Trends Neurosci. 38, 637–658. [DOI] [PubMed] [Google Scholar]
- Yu YW, Chen TJ, Wang YC, Liou YJ, Hong CJ, and Tsai SJ (2003). Association analysis for neuronal nitric oxide synthase gene polymorphism with major depression and fluoxetine response. Neuropsychobiology 47, 137–140. [DOI] [PubMed] [Google Scholar]
- Zabad RK, Patten SB, and Metz LM (2005). The association of depression with disease course in multiple sclerosis. Neurology 64, 359–360. [DOI] [PubMed] [Google Scholar]
- Zeier Z, Carpenter LL, Kalin NH, Rodriguez CI, McDonald WM, Widge AS, and Nemeroff CB (2018). Clinical Implementation of Pharmacogenetic Decision Support Tools for Antidepressant Drug Prescribing. Am. J. Psychiatry 175, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, Zhao S, and Song C (2019). Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav. Brain Res. 356, 348–357. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. [DOI] [PubMed] [Google Scholar]