Abstract
Purpose
This study aimed to develop robust normal-tissue complication probability (NTCP) models for patients with hepatocellular carcinoma treated with radiation therapy (RT) using Child-Pugh (CP) score and albumin-bilirubin (ALBI) grade increase as endpoints for hepatic toxicity.
Methods and Materials
Data from 108 patients with hepatocellular carcinoma treated with RT between 2008 and 2017 were evaluated, of which 47 patients (44%) were treated with proton RT. Of these patients, 29 received stereotactic body RT and 79 moderately hypofractionated RT to median physical tumor doses of 43 Gy in 5 fractions and 59 Gy in 15 fractions, respectively. A generalized Lyman-Kutcher-Berman (LKB) model was used to model the NTCP using 2 clinical endpoints, both evaluated at 3 months after RT: CP score increase of ≥2 and ALBI grade increase of ≥1 from the pre-RT baseline. Confidence intervals on LKB fit parameters were determined using bootstrap resampling.
Results
Compared with previous NTCP models, this study found a stronger correlation between normal liver volume receiving low doses of radiation (5–10 Gy) and a CP score or ALBI grade increase. A CP score increase exhibited a stronger correlation to normal liver volumes irradiated than an ALBI grade increase. LKB models for CP increase found values for the volume-effect parameter of a = 0.06 for all patients, and a = 0.02/0.09 when fit to photon/proton patients separately. Subset analyses for patients with superior initial liver functions showed consistent dose—volume effects (a = 0.1) and consistent dose-response relationships.
Conclusions
This study presents an update of liver NTCP models in the era of modern RT techniques using relevant endpoints of hepatic toxicity, CP score and ALBI grade increase. The results show a stronger influence of low-dose bath on hepatic toxicity than those found in previous studies, indicating that RT techniques that minimize the low-dose bath may be beneficial for patients.
Introduction
In 2018, hepatocellular carcinoma (HCC) was the 7th most common cancer worldwide and the 3rd leading cause of cancer deaths.1 Liver transplantation remains the most effective treatment option, but is not available to many patients with HCC owing to donor-liver shortages or medical comorbidities. For these patients, multidisciplinary therapeutic approaches, including surgical resection, chemotherapy, radiation therapy (RT), and combination therapy, have been proposed.2,3 RT is gaining popularity as a viable treatment option to bridge the time to transplantation and has been shown to provide a safe and efficient alternative to conventional bridging therapies, such as transarterial chemoembolization or radiofrequency ablation.4
Early experiences with RT had limited success owing to radiation-induced liver disease (RILD),5,6 a dose-limiting complication associated with a high mortality rate in patients with HCC. Awareness of RILD and recent technological advances in the planning and delivery of RT have improved the safety and efficacy of RT for HCC treatment.7,8 With the increasing use of hypofractionated dose regimens, multiple series have demonstrated local control rates >90%.8–11 However, increased local control rates have refocused attention on normal-tissue complication probability (NTCP) models because patients without disease progression often die of liver failure.12,13 The current constraints for RT doses to the liver are based on NTCP models developed in the 1990s and early 2000s,14–16 before the increased use of hypofractionated dose regimens. These models were developed using RILD as the toxicity endpoint. RILD is rarely observed today in modern RT techniques,9,10 and the focus has shifted to using biomarkers of liver function to evaluate the effects of RT. The Child-Pugh (CP) scoring system combines 5 clinical measures of liver function (ie, ascites, encephalopathy, albumin, bilirubin, and prothrombin time) to assess prognosis in chronic liver disease.17 The albumin-bilirubin (ALBI) grading system uses only the laboratory measures albumin and bilirubin and has been used to assess prognosis in HCC.18
With the conformal techniques previously used for liver RT, mean liver dose (MLD) was found to correlate with RILD. A limitation of liver NTCP models is that they predict an increased risk of RILD in situations where a small volume of the liver is irradiated to a high dose.14,15 Recent studies for liver stereotactic body RT (SBRT) indicate that treatment parameters related to the volume of the liver receiving low doses may be more strongly associated with increases in CP score than MLD.19,20 At some centers, practice has changed to ensure that at least 700 mL of the liver receives less than a specified dose.21,22 However, these insights about the dose—volume response of liver with modern RT techniques have not been incorporated into liver NTCP models.
The purpose of this study was to investigate the dosimetric predictors of CP score and ALBI grade increases in patients with primary HCC treated with RT. Dosimetric parameters and clinical outcome data were used to parameterize NTCP models for these outcomes to determine the observed volume effect.
Methods and Materials
Patient cohort
The study population was retrospectively collected on an institutional review board—approved protocol from patients with unresectable HCC treated with ablative RT between 2008 and 2017 and is an extension of an existing cohort.13 The cohort consisted of 140 patients for which CP scores acquired before RT were available. Patients receiving prior liver-directed external RT were excluded owing to extrahepatic disease at the time of diagnosis, as were patients receiving another course of liver-directed therapy <6 months after completion of the index treatment. A total of 32 patients receiving protons were enrolled in study NCT00976898.23 Patients for whom insufficient data were available to determine either 3-month posttreatment CP score or ALBI grade increase were excluded, which reduced the study cohort to 108 patients.
Of these, 29 patients (27%) received SBRT with a median prescribed tumor dose of 43 Gy in 5 fractions. The remaining patients received moderate hypofractionated RT with a median prescribed tumor dose of 59 Gy in 15 fractions. Patients were treated with either proton therapy (43%) using a relative biologic effectiveness of 1.1 or intensity modulated photon RT. A summary of patient characteristics is shown in Table 1 and Table E1. All dose values were converted into 2-Gy equivalent dose (EQD2) using a linear-quadratic-linear model with α/β = 2.5 Gy24, although sensitivity analyses were performed using α/β = 2 and 3 Gy. After removing patients with a baseline CP score in class C or ALBI grade 3 and those with locoregional failure within 3 months of treatment, 92 patients were assessable for CP score endpoint analysis and 90 patients for ALBI grade endpoint analysis.
Table 1.
Patient characteristics
| Characteristic |
SBRT (n = 29) |
Hypofractionated (n = 79) |
Total (n = 108) |
|---|---|---|---|
| Age (y) Median (range) | 69 (36–88) | 67 (20–91) | 68 (20–91) |
| Sex | |||
| Female | 8 (28%) | 17 (22%) | 25 (23%) |
| Male | 21 (72%) | 62 (78%) | 83 (77%) |
| Underlying cirrhosis | 23 (79%) | 70 (89%) | 93 (86%) |
| Hepatitis carrier | 9 (31%) | 41 (52%) | 50 (46%) |
| Hepatitis B | 1 (3%) | 9 (11%) | 10 (9%) |
| Hepatitis C | 8 (28%) | 32 (41%) | 40 (37%) |
| Child-Pugh score | |||
| Baseline median (range) | 6 (5–8) | 6 (5–9) | 6 (5–9) |
| 3-month post median (range) | 6 (5–9) | 6 (5–13) | 6 (5–13) |
| Score change median (range) | 0 (−2 to 3) | 0 (−2 to 7) | 0 (−2 to 7) |
| Baseline class A | 22 (76%) | 56 (71%) | 78 (72%) |
| Albumin-bilirubin grade | |||
| Baseline median (range) | −2.3 (−3.4 to −1.5) | −2.6 (−3.8 to −0.9) | −2.6 (−3.8 to −0.9) |
| 3−month after median (range) | −2.0 (−3.4 to −0.9) | −2.2 (−3.6 to 0.1) | −2.2 (−3.6 to 0.1) |
| Grade change median (range) | 0.2 (−0.3 to 1.3) | 0.3 (−1.1 to 1.6) | −0.1 (−1.1 to 1.6) |
| Baseline grade 1 | 9 (31%) | 40 (51%) | 49 (45%) |
| Received photon radiation | 20 (69%) | 41 (52%) | 61 (56%) |
| Prior therapy | |||
| Any* | 14 (48%) | 32 (41%) | 46 (43%) |
| Ablation or resection | 9 (31%) | 13 (16%) | 22 (20%) |
| Chemoembolization | 6 (21%) | 14 (18%) | 20 (19%) |
| SIRT | 0 (0%) | 6 (8%) | 6 (6%) |
| Liver-GTV volume (cm3) | |||
| Median (range) | 1646 (781–2406) | 1777 (1007–2988) | 1742 (781–2988) |
| Liver-GTV EQD2 mean dose (Gy) | |||
| Median (range) | 19.7 (1.1–39.0) | 22.1 (8.5–37.6) | 21.6 (1.1–39.0) |
Abbreviations: EQD2 = 2-Gy equivalent dose; GTV = gross tumor volume; liver-GTV = total liver excluding the gross tumor volume assessed on the average intensity project of a 4-dimensional computed tomography scan; SBRT = stereotactic body radiation therapy; SIRT = selective internal radiation therapy (radioembolization).
Sum of individual therapies may exceed total number of patients receiving prior therapy because some patients received multiple forms of prior therapy
Treatment delivery
All patients underwent 4-dimensional computed tomography simulation with intravenous contrast, with and without abdominal compression. Gross tumor and clinical target volumes were determined by the treating physician, and the planning target volume expansion ranged from 0.5 to 1 cm depending on the treatment technique. If target motion exceeded 10 mm, respiratory gating was used for treatment. Proton RT was delivered using passive scattered protons and 1 to 3 beam directions. Photon treatments were delivered with intensity modulated or volumetric arc RT using 6 MV photons. Daily imaging for target localization was used for all treatment techniques.
Toxicity criteria
CP scores and ALBI grade were calculated from laboratory-acquired results 1 to 3 weeks before RT and again at 3 months after RT. The clinical endpoint used for this study was an increase in baseline CP score by ≥2 points (CP2+) or ALBI grade increase by ≥1 (ALBI1+) at 3 months after treatment.
NTCP modeling
NTCP modeling was based on a Lyman-Kutcher-Berman (LKB) model, describing the sigmoidal dose-response curve of normal tissues with the following probit form:
| (1) |
where Φ(x) is a probit function, and
| (2) |
with x = (gEUD – D50)/(m • D50) as in Equation 1. The generalized equivalent uniform dose (gEUD) represents the dose that, if delivered uniformly to the entire organ, would produce the same radiobiologic effect as the given heterogeneous dose distribution, as specified by the dose—volume histogram (DVH). The gEUD is calculated from the differential DVH pairs {Di, vi} as:
| (3) |
The LKB model with gEUD has 3 parameters: a is the volume effect parameter to account for differences in tissue architecture, with a = 1 representing mean dose and a > 1 or a < 1 increasing the weight of the high- or low-dose regions, respectively; m is the slope of the dose-response curve; and D50 is the dose at which there is a 50% complication probability rate.
NTCP modeling was also performed using a normal-liver volumetric dose parameter (ie, volume of liver receiving more than 5 Gy [V5]) in lieu of the gEUD to provide practical guidance. This model simplifies to 2 parameters: m is the slope of the dose-response curve and V50 the volume at which there is a 50% complication probability rate.
Maximum likelihood estimation
Normal-liver (liver-GTV) DVHs in EQD2 values and clinical endpoint at 3 months after treatment were used as the input data to determine the NTCP model parameters, using a maximum likelihood estimation. For a binomial outcome (clinical endpoint status, Ri) the form of the negative log likelihood (NLL) function is
| (4) |
The NLL was minimized using the Nelder-Mead method implemented in R, version 3.5.1, and the interior-point algorithm in MATLAB R2017b. A nonnegative constraint was set for the a parameter given its representation of a normal tissue volume effect.
In the presentation of the data, the observed NTCP values were binned for display purposes and to allow for a calculation of χ2 between the data and the model. For the 18 events of CP2+, the highest and lowest dose bins each contained 3 events, and the 3 middle bins each contained 4 events. For the 31 events of ALBI1+, there are 6 events in each dose bin except the middle bin, which has 7 events. The error bars were estimated by Agresti-Coull binomial proportion confidence intervals (CIs).
CIs for fit parameters were calculated using the bootstrap method,25 which consists of creating a large number of random samples with a replacement from the list of patient samples. Each bootstrap sample had the size of the patient cohort and the fitting process was repeated on each bootstrap sample. The 95% CIs were calculated from the distribution of fit parameters from 1000 bootstrap samples using MATLAB. Goodness of fit was estimated by calculating χ2 between the observed data and the model predictions, with values close to 0 indicating the model was consistent with the data. A Brier score function was computed to compare each patient outcome with the model’s probabilistic prediction. Scores closer to 0 indicate better predictions.26
Results
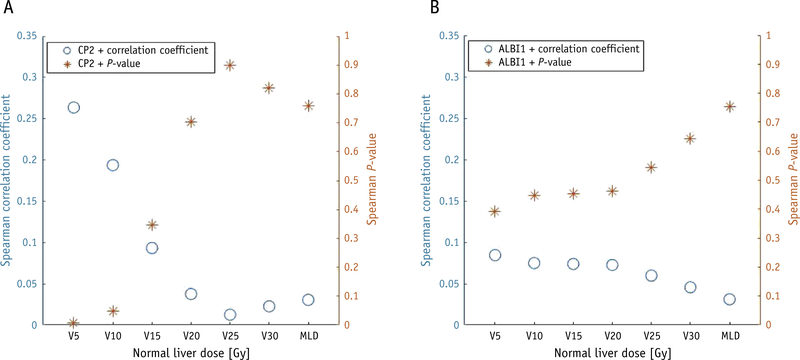
A total of 92 patients were assessable for CP score endpoint analysis and 90 patients for ALBI grade endpoint analysis. Of this cohort, 18 patients showed an increase in baseline CP score of ≥2 (CP2+) by 3 months after treatment and 31 patients showed an increase in baseline ALBI grade ≥1 (ALBI1+). As an initial exploratory analysis, Figure 1 shows Spearman correlation test results of the dosimetric parameters to CP2+ and ALBI1+ for normal liver volumetric dose parameters and the MLD. The only significant correlations were between CP2+ and normal liver V5 and V10 (volume of the normal liver receiving >5 Gy and >10 Gy, respectively), with P-values of .007 and .049, respectively. MLD did not show a significant correlation with either endpoint. The correlation of CP2+ and ALBI1+ is examined in Table E2.
Fig. 1.
Spearman correlation test of outcomes with normal liver volumetric dose parameters, with endpoints of (A) Child-Pugh score increase of ≥2, and (B) albumin-bilirubin grade increase of ≥1. Coefficients and P-values are plotted on separate axes. MLD, mean normal liver dose; V5, volume of normal liver receiving >5 Gy.
Generalized equivalent uniform dose fit results
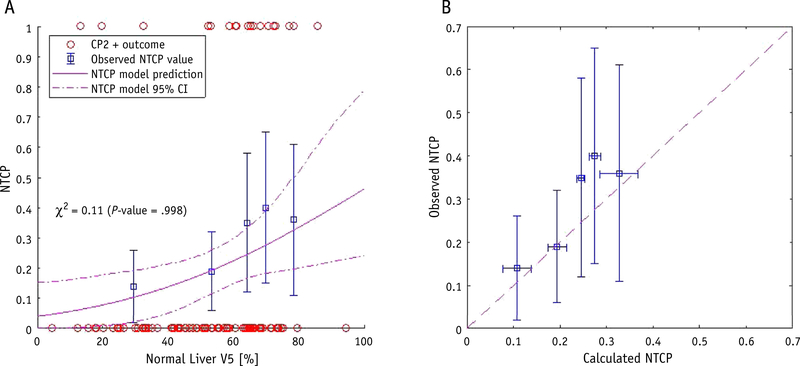
Using the maximum likelihood estimation method, an estimation of the LKB model parameters based on the DVHs and CP outcome of 92 patients was performed, and an optimal solution was found for a = 0.06, m =0 .8, and D50 = 19 Gy (Table 2). In Figure 2A, the observed patient outcome is shown versus the gEUD as open circles, with the observed NTCP values shown as open squares. The χ2 between the binned outcome and model prediction was 0.13 (P = .998) and the Brier score was 0.15. The best-fit gEUD model is shown as a solid line with 95% CI bands. In Figure 2B, the calculated NTCP probability for each bin is shown versus the observed outcome. Horizontal error bars are estimated from the standard deviation of calculated NTCP values in that bin. Qualitatively, the observed data points agree with the model within the range of uncertainty for this patient cohort.
Table 2.
Lyman-Kucher-Berman normal-tissue complication probability model fit results
| Model | Volume effect parameter, a | Slope of dose-response curve, m | Dose of 50% complication rate, D50 (Gy) or volume V50 (%) | Negative log likelihood value |
|---|---|---|---|---|
| CP2 + endpoint | ||||
| Class A + B (92 patients) | 0.06 (0.01–0.20) | 0.8 (0.6–1.0) | 19 (12–37) | 41.9 |
| 6 mo. no LRF (78 patients) | 0.09 (0.01–0.23) | 0.8 (0.5–1.1) | 19.5 (12–39) | 34.9 |
| Class A only (67 patients) | 0.10 (0.01–0.30) | 0.7 (0.4–1.0) | 20 (12–51) | 27.6 |
| Photon only (51 patients) | 0.02 (0.01–0.36) | 1.2 (0.6–2.4) | 24 (12–100) | 30.4 |
| Proton only (41 patients) | 0.09 (0.01–0.23) | 0.7 (0.2–0.9) | 7.5 (1–42) | 10.7 |
| Volumetric V5 model | ||||
| Class A + B (92 patients) | NA | 0.6 (0.3–1.0) | 106 (78–300) | 43.4 |
| Photon-treated only (51 patients) | NA | 0.6 (0.3–1.3) | 89 (70–216) | 29.6 |
| ALBI1 + endpoint | ||||
| grade 1 + 2 (90 patients) | 0.5 (0.01–1.5) | 1.5 (0.7–3) | 32 (12–100) | 57.5 |
| 6 mo. no LRF (78 patients) | 0.7 (0.03–2.0) | 1.2 (0.5–2.4) | 39 (14–100) | 48.5 |
| grade 1 only (45 patients) | 0.3 (0.01–1.6) | 1.2 (0.5–2.8) | 14 (5–48) | 28.1 |
Abbreviations: 6 mo. no LRF = exclusion of patients with locoregional failure or intrahepatic progression within 6 months after treatment rather than 3 months; ALBI1+ = increase of ≥1 in albumin-bilirubin grade 3 months after treatment; CP2+ = increase of ≥2 in Child-Pugh score 3 months after treatment; NA = not applicable; volumetric V5, normal liver volume of liver receiving >5 Gy instead of generalized equivalent uniform dose.
The 95% confidence interval is given in parentheses for each parameter.
Fig. 2.
Normal-tissue complication probability (NTCP) model with a Child-Pugh score increase of 2+ as the endpoint, with (A) normal liver generalized equivalent uniform dose versus NTCP, with patient outcomes shown as open circles, binned observed complication rates shown as open squares, and the best-fit model with a = 0.06, m = 0.8, and D50 = 19 Gy shown as a solid line with 95% confidence interval bands, and (B) calibration plot of calculated NTCP versus observed complication rates, with the dashed line representing perfect agreement.
The best-fit LKB model parameters were slightly different with ALBI1+ as the clinical endpoint, with the minimum found for a = 0.5, m = 1.5, and D50 = 32 Gy (Table 2). The χ2 between the binned outcome and model prediction was 0.04 (P = .999) and the Brier score was 0.22. Qualitatively, the observed data points also agree with this model within the range of uncertainty for this patient cohort (Fig. E2). As indicated by the exploratory Spearman analysis and the large value for the slope parameter, m, ALBI grade change is not as strongly correlated to dosimetric parameters as CP2+.
Liver volumetric dose parameter fit results
As demonstrated in Figure 1, the liver volumetric dose parameter that correlates most strongly with CP2+ outcome and the best-fit gEUD is the V5 (volume of liver receiving >5 Gy). The modified LKB model, with liver V5 in lieu of gEUD, was used to model the CP outcome of 92 patients with an optimal solution found for m = 0.6 and V50 = 106% (Table 2). The χ2 between the binned outcome and model prediction was 0.11 (P = .998) and the Brier score was 0.15. In Figure 3, the observed patient outcome is shown versus the liver V5 with the best-fit V5 NTCP model. Qualitatively, the observed data points also agree with this model within the range of uncertainty.
Fig. 3.
Liver volumetric normal-tissue complication probability (NTCP) model with a Child-Pugh score increase of 2+ as the endpoint. (A) Normal liver V5 (percentage of healthy liver receiving ≥5 Gy) versus NTCP, with patient outcomes shown as open circles, binned observed complication rates shown as open squares, and best-fit model with m = 0.6 and V50 = 106% shown as a solid line with 95% confidence interval bands. (B) Calibration plot of calculated NTCP versus observed complication rates, with the dashed line representing perfect agreement.
Model robustness results
This cohort includes all patients with HCC treated consecutively at a single cancer center, which leads to greater heterogeneity in baseline clinical characteristics and treatment modalities than in most modeling studies. To ensure the robustness of the model against these heterogeneities, additional analyses were performed for specific subpopulations (Table 2 and Table E3. To investigate the impact of underlying disease progression or preexisting liver damage, the LKB estimation was performed excluding patients with locoregional failure within 6 months after treatment and for the subset of patients with initial CP class A. Subset analyses were also performed for patients with hypofractionated treatment and patients without prior radioembolization. These subset analyses are shown in Table E3. All subset analyses were consistent with the full patient cohort model.
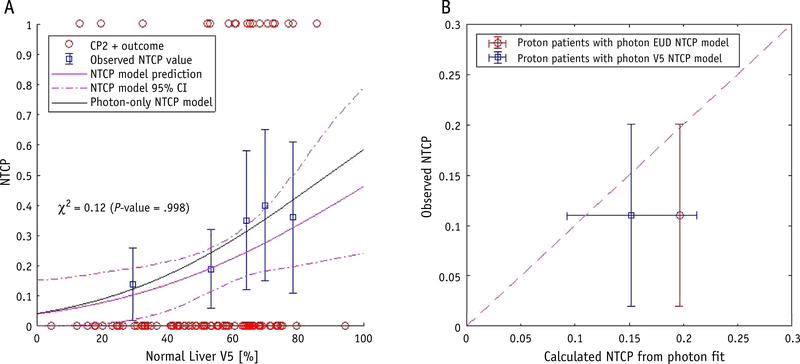
To ensure that the observed effect is not an artefact of an underlying difference between the proton and photon cohorts, we performed several additional robustness studies for these subsets. From the 92-patient cohort with 18 CP2+ events, 51 patients were treated with photons, of which 15 patients had CP2+ outcome. Table 2 shows the best-fit model parameters for the photon and proton subsets. The V5 NTCP model could not be fitted to the proton cohort owing to the low signal (3 events observed). The gEUD fit parameters for both subsets agreed with those from the fit to all patients, although the CIs are larger for the photon subset (Table 2). Figure 4A shows that the photon V5 NTCP model is well contained within the 95% CI of the model for all patients. The photon model is validated on the proton subset in Figure 4B. The calculated NTCP values for proton-treated patients from the photon models are consistent with the observed outcomes.
Fig. 4.
Robustness evaluation for photon- and proton-treated patient subsets. (A) Normal liver V5 (percentage of healthy liver receiving ≥5 Gy) versus NTCP, with patient outcomes shown as open circles, binned observed complication rates shown as open squares, and best-fit model for all patients shown as a solid line with 95% confidence interval bands. The best-fit model for photon-treated patients alone is shown as a solid black line. (B) Calibration plot of calculated NTCP versus observed complication rates for proton-treated patients alone, with the calculated NTCP from the photon-treated patient best-fit generalized equivalent uniform dose (open circle, red) and V5 (open square, blue) models. (A color version of this figure is available at https://doi.org/10.1016/jijrobp.2020.04.027 ).
Discussion
This study presents the first refitting of NTCP models in the era of modern SBRT to relevant endpoints of hepatic toxicity, CP score and ALBI grade increase while accounting for varying volume effects. As a first step, we evaluated normal-liver DVH parameters in a univariate analysis to investigate the differential association between various dose—volume ranges and hepatic toxicity. Previous liver NTCP models are based on the MLD (14–16); however, we observed higher correlations for the low-dose volume parameters (V5 and V10, or volume of normal liver receiving 5 Gy and 10 Gy respectively), which suggests that the low-dose bath may contribute significantly to hepatic toxicity.
This was corroborated by our NTCP model fit to the same data, which found a best-fit value of the LKB volume effect parameter a that was far smaller than 1, indicating that MLD alone is not a good predictor of NTCP and that lower dose—volume metrics play a significant role. Based on the NTCP model using CP2+ as the clinical endpoint, to keep the normal-liver NTCP <20%, requires an RT plan with normal-liver gEUD <8 Gy EQD2. The gEUD does not directly correspond to any one point on the DVH, but Figure E3 shows an analysis of the normal liver V5 demonstrating that a similar NTCP may be achieved by a dose constraint metric of normal liver V5 < 60% in addition to standard metrics, such as MLD. The NTCP model predicts a certain baseline rate of CP score increase even at 0 Gy, which is due to the natural progression of underlying liver disease in these patients.27,28
This analysis may be more sensitive to the low-dose bath than prior studies owing to changes in practice for RT delivery, namely the increasing use of stereotactic and proton RT.29 Proton RT can substantially reduce the low-dose bath in the liver, and including these patients in this analysis provided greater diversity in the dosimetric parameters to which the model was fit. The use of hypofractionation could have increased the importance of this low-dose bath, because now large parts of the normal liver are irradiated to nonnegligible radiation doses every fraction. The subset analysis of patients treated only with photons had much larger CIs than the fit to all patients, although the derived NTCP models agree with the fit to the overall cohort (Fig. 4).
The importance of the low-dose volume for hepatic toxicity has been seen in several clinical observations from recent years. One of the first observations was a phase 1 clinical trial of SBRT at the University of Colorado showing that the volume spared (ie, volume of normal liver receiving <15 Gy) is critical, similar in extent to surgical sparing approaches to maintain liver function after resection. Similar observations noting the importance of sparing 600 to 900 cc of uninvolved liver below a dose threshold of 15 to 25 Gy were made in several other series studying the dosimetric predictors of CP score increase.30–32
The results of our NTCP model parameter estimation agree with these clinical observations and formalize them in a quantitative fashion within the constraints of an EUD-based LKB approach. The approach presented herein is continuous in dose and effects with no critical volume component. We think this approach is justified due to the continuous nature of the relationship between decrease in liver function and dose, as demonstrated in a recent study imaging the spatial variance of liver function using Tc-99m Mebrofenin single-photon emission computed tomography and showing a continuous decrease in liver function with an increasing dose.33
Multiple analyses have shown the importance of initial liver function, as quantified by pretreatment CP score, to develop toxicity.19,32 Unfortunately, the number of patients in this study was too low to separate cohorts based on initial CP score or ALBI grade. The subset analyses performed on patients with CP score A and ALBI grade 1 showed model parameters consistent with the fit to all patients. Because this was not a stratified patient cohort, heterogeneity exists in the patient populations (Tables 1 and E1), most notably in the baseline ALBI grade, underlying cirrhosis and hepatitis B/C status, and receiving other forms of liver-directed therapy before RT.
Subset analyses were performed where possible with the limited cohort size and showed consistent results (Table 2, Table E3, Fig. 4), indicating the analysis was robust against many of these heterogeneities. However, due to the underlying heterogeneity, these results may not be applicable to all HCC populations. Future studies should either stratify the patient population or integrate the initial liver function into a predictive model together with the radiation dose similar to existing approaches.34 An additional confounding factor is that some patients with HCC will experience decreasing liver function owing to underlying liver disease or HCC recurrence, and post-RT changes on imaging may be difficult to interpret.35
Interestingly, our results showed clearer dose-response relationships for CP2+ than for ALBI1+. The correlation between the 2 is explored further in Figure E1. CP score was not designed as a biomarker for RT liver toxicity, but has historically been the main prognostic factor for survival after RT. Its increase by 2 points has been shown to correlate with outcome in multiple series.9,22,30,36 ALBI grade increase in this cohort was quite common, even among the lowest dose level (Fig. E2). Of note, ALBI is a purely quantitative subset of variables included in the CP score and was designed as a pretreatment prognostic marker specifically for outcomes after RT.18 ALBI grade increase has not be demonstrated to be a good prognostic marker of liver damage, and the thresholds between ALBI grades may be ill-suited to quantify a change in liver function after RT. This must be evaluated and correlated to observed outcomes in larger series. However, because ALBI is purely quantitative, the score is simpler to acquire for each patient than CP, which also uses partly subjective criteria, such as ascites and encephalopathy. The ease of tracking outcome data with ALBI may outweigh the benefit of additional sensitivity from using CP score.
Other popular endpoints include liver enzyme changes and general gastrointestinal toxicity, which were not investigated in this study. However, studies using these endpoints often report critical volume criteria being significantly correlated with toxicity.37 In addition, the integration of functional imaging and other biologic markers into dosimetric NTCP modeling has been shown to improve dose-response relationships and can be used to personalize model predictions compared with approaches only relying on dose.34
The slope of the dose response curve (m) for the best fit NTCP model in Figure 2A is shallow and has large CIs in some of the subset analyses in Table 2. This may be due to several factors, including uncertainty in the endpoint used for clinical outcome and the heterogeneity of the patient cohort, particularly heterogeneity in baseline liver status. A model based on a more homogenous cohort may be able to offer more precise NTCP predictions, although only for patients similar to those in the cohort used for modeling. Additionally, this study used the planned dose distributions to estimate the impact of RT on hepatic function. Steeper dose-response relationships can be obtained by estimating the actual delivered dose using daily volumetric imaging,38,39 although the results may be subject to registration errors. Volumetric imaging is not available in our proton treatment rooms and has only recently been performed on a daily basis for photon-treated patients. Future studies should be designed to use estimates of actual delivered dose.40
Conclusions
The results of this study indicate that the low-dose bath to the liver should be considered along with MLD when designing treatment plans using modern RT techniques for HCC. Most treatment planning systems do not currently allow for optimizing RT dose with biologic models of normal-tissue response, so NTCP models are typically used outside of the planning system to compare the complication risk of several different plan options. The models derived from this study may be used in that fashion. More generally, the low value of the LKB volume effect parameter in these models (a = 0.06 for CP2+ endpoint and 0.5 for ALBI1+ endpoint) indicates that the toxicity risk will be reduced when the low-dose end of the DVH is lowered, even if the tradeoff is more volume receiving a higher dose. This reduced low-dose distribution is easily achieved with proton RT. Reducing the low-dose distribution with photon RT while achieving similar MLDs is also possible by limiting the gantry angles used for treatment and choosing appropriate optimization constraints.
Supplementary Material
Summary.
Radiation therapy for hepatocellular carcinoma is limited by its impact on liver function. This work uses a cohort of patients treated with modern radiation therapy techniques to develop new normal tissue complication probability models for liver irradiation. These models show a stronger influence of the low-dose bath on hepatic toxicity than found in previous models.
Acknowledgments
The present research was supported in part by National Cancer Institute U19 CA21239 (C.G., T.S.H.), R21 CA241918 (C.G.) and C06 CA059267 (B.Y.Y.), National Institute of Health R37-CA222215 and R01-CA233487 (I.E.N.), and National Institute of Health R01-CA221971 (K.K.B.).
Footnotes
Disclosures: none.
Data sharing: Research data are not available at this time. Please contact the corresponding author for specific requests.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.04.027.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20: 4115–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villaneuva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: A critical view of the evidence. Nat Rev Gastroenterol Hepatol 2013;10:34–42. [DOI] [PubMed] [Google Scholar]
- 4.Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma: An intention-to-treat analysis. J Hepatol 2017;67:92–99. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31:1237–1248. [DOI] [PubMed] [Google Scholar]
- 6.Khozouz RF, Huq SZ, Perry MC. Radiation-induced liver disease. J Clin Oncol 2008;26:4844–4845. [DOI] [PubMed] [Google Scholar]
- 7.Guha C, Kavanagh BD. Hepatic radiation toxicity: Avoidance and amelioration. Semin Radiat Oncol 2011;21:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane FK, Hong TS. Role and future directions of external beam radiotherapy for primary liver cancer. Cancer Control 2017;24:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631–1639. [DOI] [PubMed] [Google Scholar]
- 10.Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huertas A, Baumann AS, Saunier-Kubs F, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol 2015;115:211–216. [DOI] [PubMed] [Google Scholar]
- 12.Mizumoto M, Okumura T, Hashimoto T, et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:e529–e535. [DOI] [PubMed] [Google Scholar]
- 13.Sanford NN, Pursley J, Noe B, et al. Protons vs photons for unresectable hepatocellular carcinoma: liver decompensation and overall survival proton radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2019;105:64–72. [DOI] [PubMed] [Google Scholar]
- 14.Jackson A, Ten Haken RK, Robertson JM, Kessler ML, Kutcher GJ, Lawrence TS. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys 1995;31:883–891. [DOI] [PubMed] [Google Scholar]
- 15.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810–821. [DOI] [PubMed] [Google Scholar]
- 16.Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncologica 2009;45:856–864. [DOI] [PubMed] [Google Scholar]
- 17.Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach — the ALBI grade. J Clin Oncol 2015;33:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son SH, Kay CS, Song JH, et al. Dosimetric parameter predicting the deterioration of hepatic function after helical tomotherapy in patients with unresectable locally advanced hepatocellular carcinoma. Radiat Oncol 2013;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu JIl, Park HC, Lim DH, Park WY. Predictive factors for Child-Pugh score elevation in hepatocellular carcinoma patients treated with conformai radiation therapy: Dose-volume histogram analysis. Tumori 2013;99:164–171. [DOI] [PubMed] [Google Scholar]
- 21.Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209–214. [DOI] [PubMed] [Google Scholar]
- 22.Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012;118: 5424–5431. [DOI] [PubMed] [Google Scholar]
- 23.Hong TS, Wo JY, Yeah BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astrahan M Some implications of the linear-quadratic-linear radiation dose-response with regard to hypofractionation. Med Phys 2008;35: 4161–4172. [DOI] [PubMed] [Google Scholar]
- 25.Tucker SL, Liu HH, Wang S, et al. Dose-volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;66:754–761. [DOI] [PubMed] [Google Scholar]
- 26.Brier GW. Verification of forecasts expressed in terms of probability. Monthly Weather Rev 1950;78:1–3. [Google Scholar]
- 27.Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 2003;38: 307–314. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Liu R, Long XR, et al. Postsurgical treatment with adjuvant transarterial chemoembolization in patients with hepatitis B-related hepatocellular carcinoma. Medicine 2016;95:e5517–e5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadha AS, Gunther JR, Hsieh CE, et al. Proton beam therapy outcomes for localized unresectable hepatocellular carcinoma. Radiother Oncol 2019;133:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son SH, Choi BO, Ryu MR, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: Dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys 2010;78:1073–1080. [DOI] [PubMed] [Google Scholar]
- 31.Su TS, Luo R, Liang P, Cheng T, Zhou Y, Huang Y. A prospective cohort study of hepatic toxicity after stereotactic body radiation therapy for hepatocellular carcinoma. Radiother Oncol 2018;129:136–142. [DOI] [PubMed] [Google Scholar]
- 32.Velec M, Haddad CR, Craig T, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2017;97:939–946. [DOI] [PubMed] [Google Scholar]
- 33.Hardcastle N, Haddad C, Schembri G, et al. The Liver INSPECTR Trial: Towards improved understanding of liver function following radiotherapy. J Phys 2019;1154:012009. [Google Scholar]
- 34.El Naqa I, Johansson A, Owen D, et al. Modeling of normal tissue complications using imaging and biomarkers after radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2018;100: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanuki-Fujimoto N, Takeda A, Ohashi T, et al. CT evaluations of focal liver reactions following stereotactic body radiotherapy for small hepatocellular carcinoma with cirrhosis: relationship between imaging appearance and baseline liver function. Br J Radiol 2010;83:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447–e453. [DOI] [PubMed] [Google Scholar]
- 37.Miften M, Vinogradskiy Y, Moiseenko V, et al. Radiation dose-volume effects for liver SBRT. Int J Radiat Oncol Biol Phys 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCulloch MM, Muenz DG, Schipper MJ, et al. A simulation study to assess the potential impact of developing normal tissue complication probability models with accumulated dose. Advances in Rad Oncol 2018;3:662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polan DF, Feng M, Lawrence TS, Ten Haken RK, Brock KK. Implementing radiation dose-volume liver response in biomechanical deformable image registration. Int J Radiat Oncol Biol Phys 2017;99: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swaminath A, Massey C, Brierley JD, et al. Accumulated delivered dose response of stereotactic body radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys 2015;93:639–648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.