Abstract
Introduction:
Although patent ductus arteriosus (PDA) has been implicated to play a role in the development of cerebral ischemia and intraventricular hemorrhage (IVH) through a cerebral steal phenomenon, there is conflicting data on the impact of PDA size on cerebral blood flow (CBF). Cerebral autoregulation is the brain’s innate protective mechanism to maintain constant CBF despite changes in blood pressure, and it is unclear if it is influenced by PDA hemodynamics.
Objective:
To delineate the relationship between PDA size and cerebral blood flow velocity (CBFv) in premature infants.
Methods:
113 premature infants born at 23–29 weeks’ gestation had echocardiograms performed during the first week after birth to evaluate for PDA. Infants were divided into three groups by PDA size: none-to-small, moderate, or large. All infants had continuous recordings of umbilical artery blood pressure (ABP) and CBFv during the first week after birth. Critical closing pressure (CrCP) was calculated from ABP and CBFv tracings. Diastolic closing margin (DCM), defined as diastolic BP minus CrCP, was calculated as a marker for the risk of developing IVH.
Results:
Infants with large PDAs (n=16) had the lowest ABP across all phases of the cardiac cycle (systole [p=0.003], mean [p=0.005], and diastole [p=0.012]) compared to infants with both moderate PDA (n=19) and none-to-small PDA (n=78). Despite BP being different, systolic, mean, and diastolic CBFv were not different among the PDA groups. Cerebral autoregulation as measured during systole was intact regardless of the PDA size. CrCP and DCM were also not different among the PDA groups.
Conclusions:
In this cohort, CBFv and cerebral autoregulation during systole were not influenced by PDA size. Intact cerebral autoregulation may play a role in maintaining CBFv regardless of PDA size and differences in ABP.
Keywords: Premature infant, Cerebral blood flow velocity, Patent ductus arteriosus, Cerebral autoregulation
Introduction
Patent ductus arteriosus (PDA) is common in premature infants and spontaneously closes in 34% of extremely low birth weight infants during the first week [1]. A persistent PDA can impact end organ perfusion, depending on PDA size and velocity of the left-to-right shunt [2–5]. In addition, an increasing left-to-right shunt through the PDA is believed to alter cerebral hemodynamics and increase the risk of developing intraventricular hemorrhage (IVH).
The hemodynamic effects of PDA on CBF are controversial. Abnormal CBF has been reported in premature infants with large PDAs [6]. In one study, high pulsatile index (PI) was reported in premature infants with large PDAs [3] that further supported the impact of the hemodynamically significant PDA. Also, absent diastolic CBFv was observed in 93% of premature infants with a PDA [7]. However, in contrast to these studies, one study did not find any relationship between PDA and CBFv during diastole [2].
In many clinical scenarios, PDA is associated with hypotension, due to low diastolic and mean arterial blood pressure (ABP) [8]. However, hypotension is not always associated with decreased CBFv when there is intact cerebral autoregulatory capacity [9, 10]. Another unique aspect of premature infants’ cerebral hemodynamics is the role of the brain’s critical closing pressure (CrCP). CrCP is the ABP at which blood flow ceases to the brain and often the mean ABP is close to or below the CrCP [11]. When this occurs, CBF is present only during systole [11, 12]. Additionally, we previously showed that elevated diastolic closing margin (DCM) is associated with severe IVH in premature infants [13].
The purpose of this study is twofold: to delineate the relationship between PDA size and CBFv and to determine the influence of PDA size on cerebral autoregulatory capacity. We hypothesized that large PDAs are associated with lower diastolic CBFv and more impaired cerebral autoregulatory capacity.
Methods
This is a re-analysis of previously published, prospectively collected data from infants born January 2003 to April 2008 at 23–29 weeks’ gestation who had echocardiograms during the first week after birth [9, 14–17]. Approval was obtained by the University of Arkansas for Medical Sciences Institutional Review Board, and parental informed consent was obtained. Very low birth weight (<1,500 grams) infants (without major congenital anomalies or chromosomal abnormalities) who were receiving mechanical ventilation and had an umbilical arterial catheter in place were included in the study.
An echocardiogram was performed during the first week after birth for clinical indications and results were confirmed by attending cardiologists. The PDA was measured at the pulmonic end of the ductus arteriosus with size and direction of the shunt noted. All infants were included for this secondary analysis even if there was no PDA present. Infants were divided into three groups by PDA size: no-to-small, moderate, or large. Moderate PDA was defined as size 1.5–2 mm. Large PDA was defined as size >2 mm with either left heart dilatation or left atrial to aorta ratio >1.4. Shortening fraction (SF) was assessed as a marker for cardiac function. During the study period, prophylactic indomethacin therapy was not used.
All infants had ABP measurement with an umbilical artery catheter. CBFv in the middle cerebral artery was continuously monitored (for 1-hour periods) using transcranial Doppler ultrasound (Nicolet Vascular/Natus Medical, San Carlos, CA). All infants underwent two 1-hour daily recording sessions on days 1–3 and then once daily 1-hour sessions on days 4–7. Analog data from ABP monitors and Doppler ultrasounds were simultaneously collected using a PowerLab 8 Channel data acquisition system (AD Instruments, Mountain View, CA).
Digitalized files were analyzed using ICM+ software (ICM+, Cambridge Enterprise, Cambridge, United Kingdom). Artifacts were removed using valid values range filters and by visual inspection of each individual recording.
Autoregulatory capacity was quantified by a moving correlation coefficient between: 1) systolic ABP and systolic CBFv (Sx); 2) mean ABP and mean CBFv (Mx); and 3) diastolic ABP and diastolic CBFv (Dx), as our previous study described [12]. Intact autoregulatory capacity is detected as a negative or near-zero correlation. Sx has previously been shown to best discriminate autoregulatory capacity in premature infants [12]. Mx has been extensively studied in adult populations, for whom values >0.3 have been associated with poor outcomes [18].
CrCP was calculated from ABP and CBFv tracings using equations derived from a model of the cerebral vasculature with resistance and compliance in a parallel circuit. The full derivation of the method has been described in detail and validated previously [19]. For each session, DCM, diastolic ABP minus CrCP, was calculated as a marker for the risk of developing IVH [13].
Clinical characteristics and hemodynamic values were included for analysis. Data was collapsed to one averaged session per subject since multiple sessions would create confusion because the number of sessions was inconsistent from subject to subject, and there was only a small degree of variability between individual subject sessions. To get uniform data across the studied infants, first week hemodynamic data except echocardiograms were condensed for analysis. Comparison across PDA groups was done with ANOVA and post hoc analysis by Bonferroni correction. SPSS 22.0 (IBM Corp., Armonk, NY) was used for all statistical analyses.
Results
Among 184 infants at 23–29 weeks’ gestation during the study period, 116 infants had an echocardiogram performed during the first week after birth for clinical indications. Three infants were excluded, two infants had right-to-left PDA shunts, and one infant had heart failure with a pericardial effusion. Thus, a total of 113 infants were included for the analysis. The gestational age was 25.7 ± 1.7 weeks’ (range: 23–29 weeks’) and birth weight was 769 ± 216 grams (range: 375–1498 grams) in the cohort. Of the study infants, 72 (64%) were born by cesarean section, 28 (25%) were from multiple gestation pregnancies, 99 (88%) were exposed to antenatal steroids, and 44 (39%) received inotropes.
The clinical characteristics according to PDA size are shown in Table 1. Although there was a trend towards lower gestational age in the moderate and the large PDA groups, this difference was not significant. Importantly and not unexpectedly, indomethacin treatment or PDA ligation was significantly more common in the moderate and the large PDA groups.
Table 1.
Clinical Characteristics by PDA Size
| No/Small PDA (n=78) | Moderate PDA (n = 19) | Large PDA (n = 16) | p value | |
|---|---|---|---|---|
| Gestational age (weeks) | 26.0 ± 1.7 | 25.0 ± 1.3 | 25.3 ± 1.3 | 0.041 |
| 0.064a, 0.445b, 1.000c | ||||
| Birth weight (grams) | 800 ± 221 | 715 ± 168 | 687 ± 220 | 0.077 |
| Male (n, %) | 38 (49%) | 9 (47%) | 10 (62%) | 0.585 |
| Race | 30 : 44 : 4 | 5 : 10 : 4 | 4 : 9 : 3 | 0.133 |
| (Black : White : Others) | ||||
| 1‵ Apgar score | 3 (2–5) | 4 (1–5) | 3 (1–5) | 0.678 |
| 5‵ Apgar score | 6 (5–7) | 6 (4–7) | 6 (4–7) | 0.627 |
| Inotropes (n, %) | 29 (37%) | 8 (42%) | 7 (44%) | 0.848 |
| Indomethacin treatment | 6 (8%) | 8 (42%) | 7 (44%) | 0.000 |
| (n, %) | 0.001a, 0.001b, 1.000c | |||
| PDA ligation | 0 (0%) | 2 (11 %) | 7 (44%) | 0.000 |
| (n, %) | 0.222a, 0.000b, 0.000c | |||
| Mortality within 1 week | 1 (1%) | 2 (11 %) | 2 (13%) | 0.051 |
| (n, %) |
Gestational age and birth weight are expressed as mean ± SD. Apgar scores are expressed as median (interquartile range).
Significant p values by ANOVA were evaluated by post hoc analysis with Bonferroni correction:
p value between no/small vs moderate PDA;
p value between no/small vs large PDA;
p value between moderate vs large PDA.
Arterial Blood Pressure and Cerebral Blood Flow Velocity
ABP was significantly lower with increasing PDA size across the cardiac cycle (Fig. 1). Especially, the large PDA group had significantly lower ABP compared to the no/small PDA group across all phases of cardiac cycle (p=0.003 in systole, p=0.008 in mean, and p=0.011 in diastole). Systolic ABP was 46.1 ± 6.5, 44.4 ± 5.3, and 41.1 ± 5.1 mmHg in the no/small, moderate, and large PDA groups, respectively. Mean ABP was 36.8 ± 4.6, 35.1 ± 3.4, and 32.7 ± 4.1 mmHg in the no/small, moderate, and large PDA groups, respectively. Diastolic ABP was 29.1 ± 4.3, 27.2 ± 3.1, and 25.6 ± 4.3 mmHg in the no/small, moderate, and large PDA groups, respectively.
Fig. 1. Arterial Blood Pressure across the Cardiac Cycle.
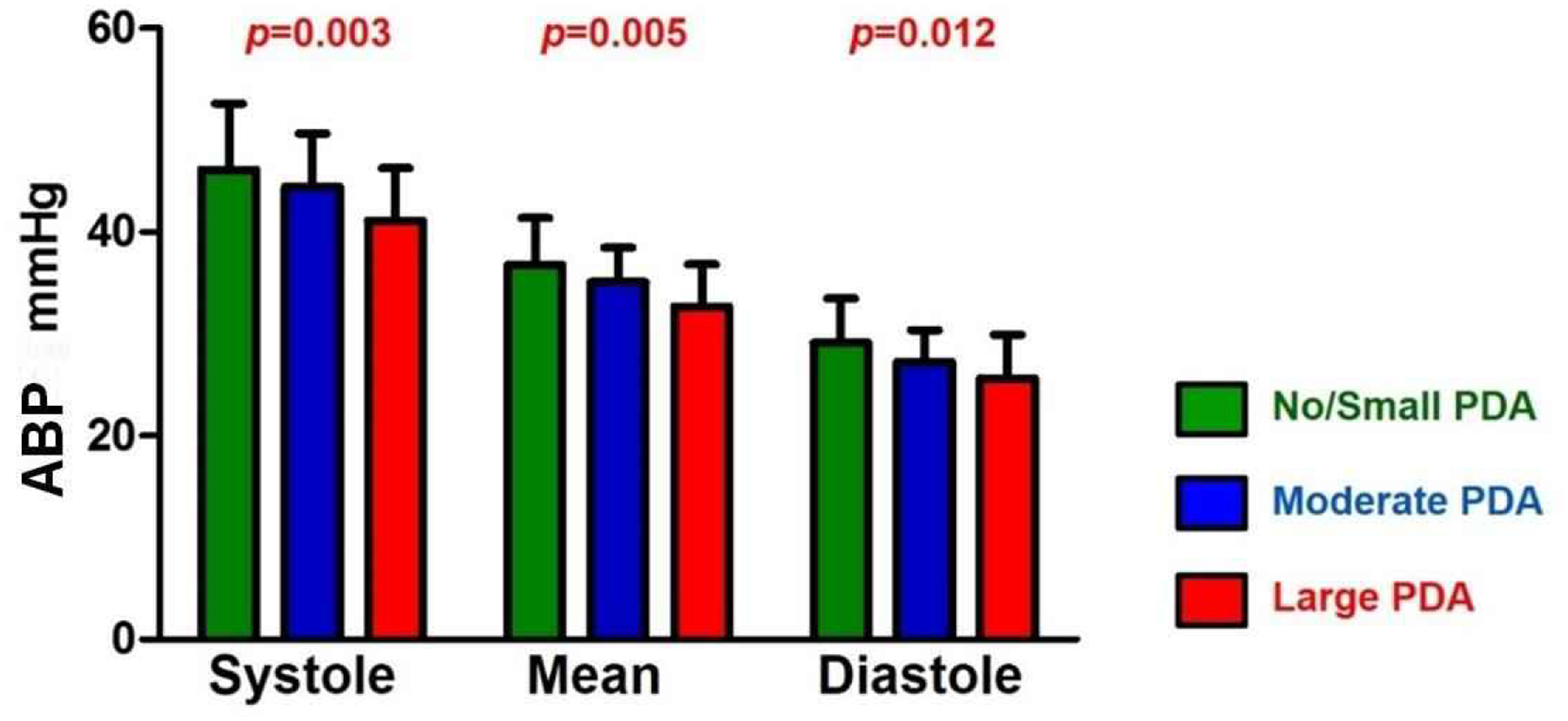
The arterial blood pressure (ABP) showed a significantly decreasing trend with increasing PDA size across the cardiac cycle (depicted p values were from ANOVA between different PDA groups).
CBFv was not different according to PDA size across the cardiac cycle (Fig. 2). Systolic CBFv was 26.2 ± 5.2, 26.9 ± 5.3, 25.9 ± 6.8 cm/sec in the no/small, moderate, and large PDA groups, respectively. Mean CBFv was 16.8 ± 3.2, 17.3 ± 3.5, and 16.7 ± 4.3 cm/sec in the no/small, moderate, and large PDA groups, respectively. Diastolic CBFv was 8.9 ± 1.5, 9.1 ± 1.9, and 8.4 ± 1.8 cm/sec in the no/small, moderate, and large PDA groups, respectively. The large PDA group had lower average CBFv at all phases of the cardiac cycle, but this was not statistically significant.
Fig. 2. Cerebral Blood Flow Velocity across the Cardiac Cycle.
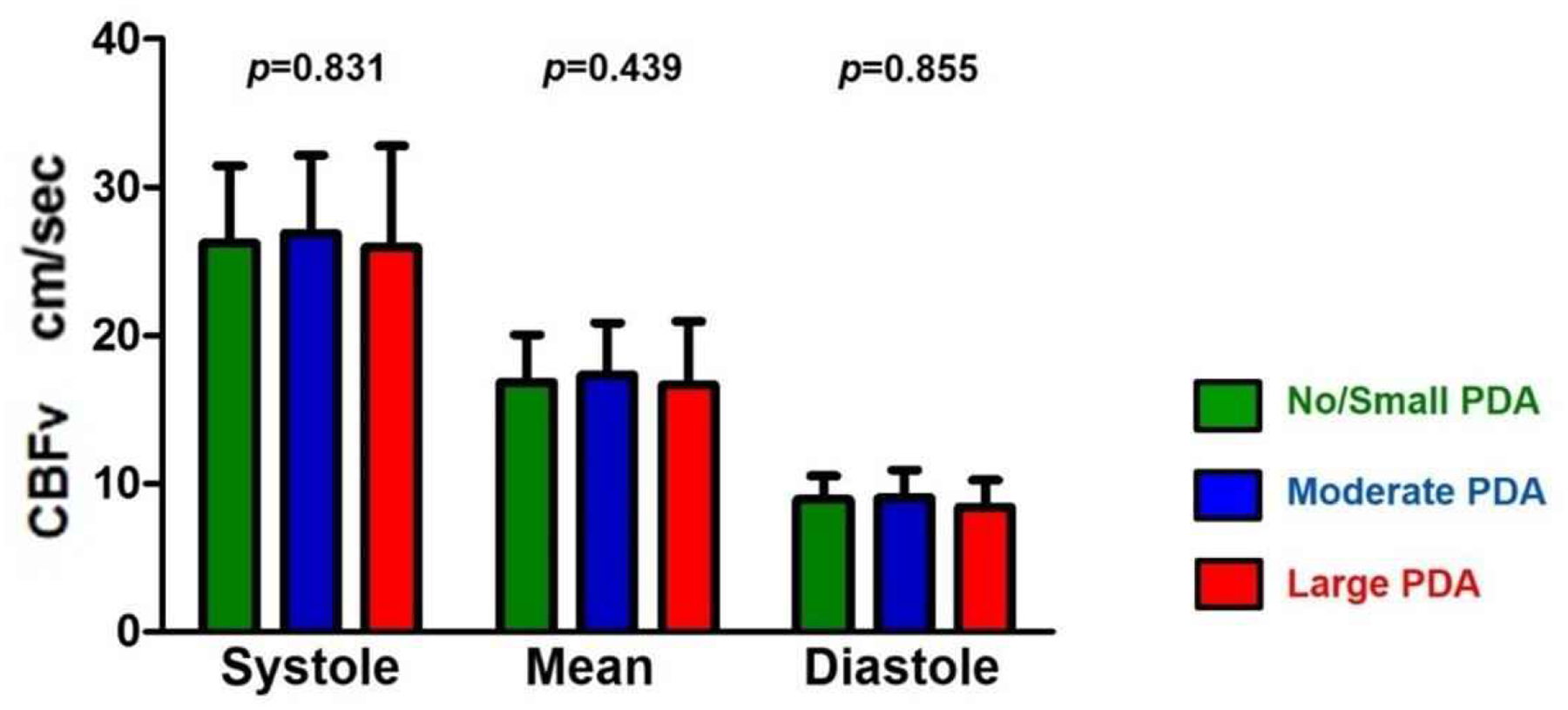
Cerebral blood flow velocity (CBFv) was not different according to PDA size across the cardiac cycle (depicted p values were from ANOVA between different PDA groups).
Quantification of Cerebral Autoregulatory Capacity and Cerebral Hemodynamics
To quantify cerebral autoregulatory capacity, the index of cerebral autoregulation was calculated across all phases of the cardiac cycle (Fig. 3). Cerebral autoregulatory capacity as measured by Sx was intact regardless of the PDA size. While cerebral autoregulatory capacity is often absent during diastole, the large PDA group showed improved autoregulatory capacity during diastole (p=0.021 by ANOVA). CrCP and DCM were not different between the PDA groups (Fig. 4).
Fig. 3. Cerebral Autoregulation across the Cardiac Cycle.
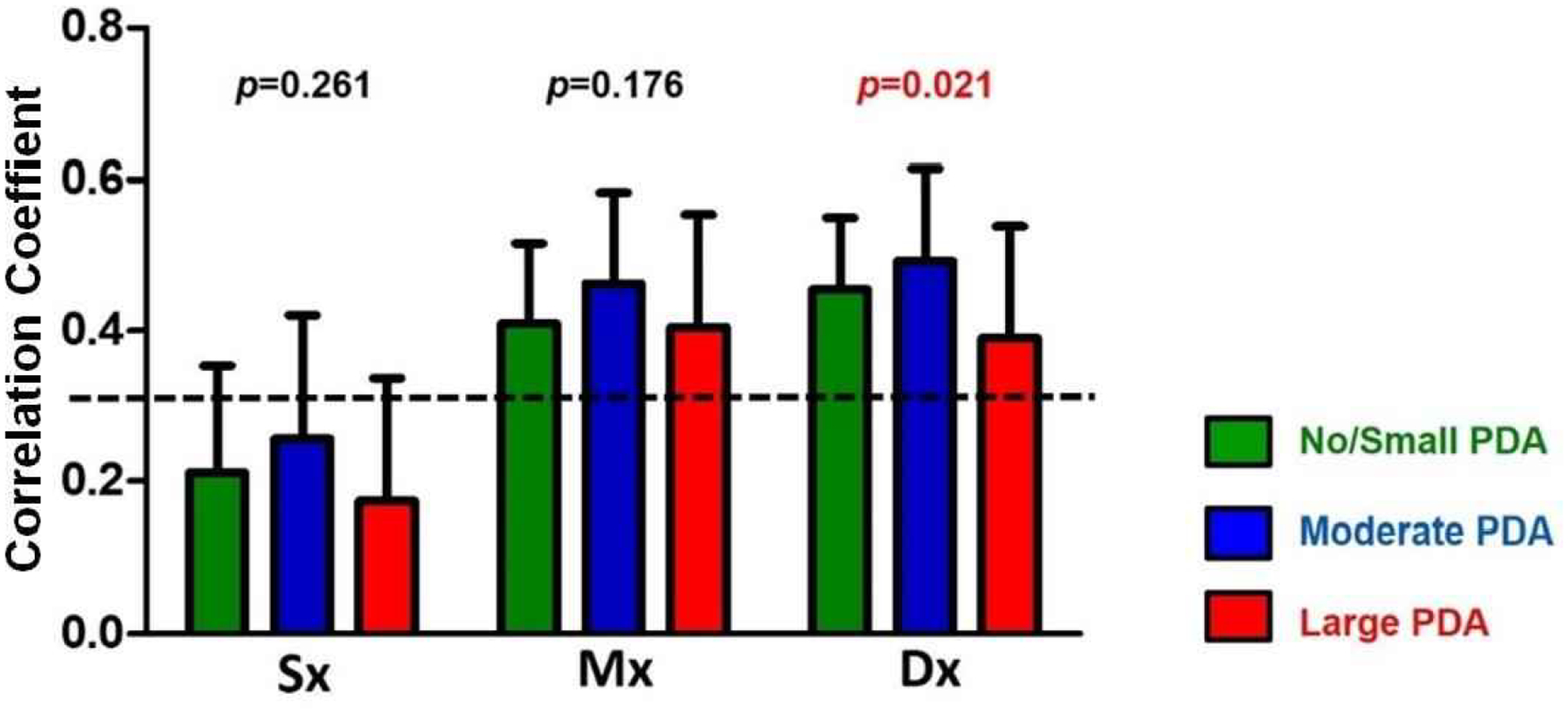
Sx denotes the correlation between systolic ABP and systolic CBFv, Mx denotes the correlation between mean ABP and mean CBFv, and Dx denotes the correlation between diastolic ABP and diastolic CBFv. Sx, as an index for cerebral autoregulation, remained intact regardless of PDA size during systole.
Fig. 4. Critical Closing Pressure and Diastolic Closing Margin by PDA Size.
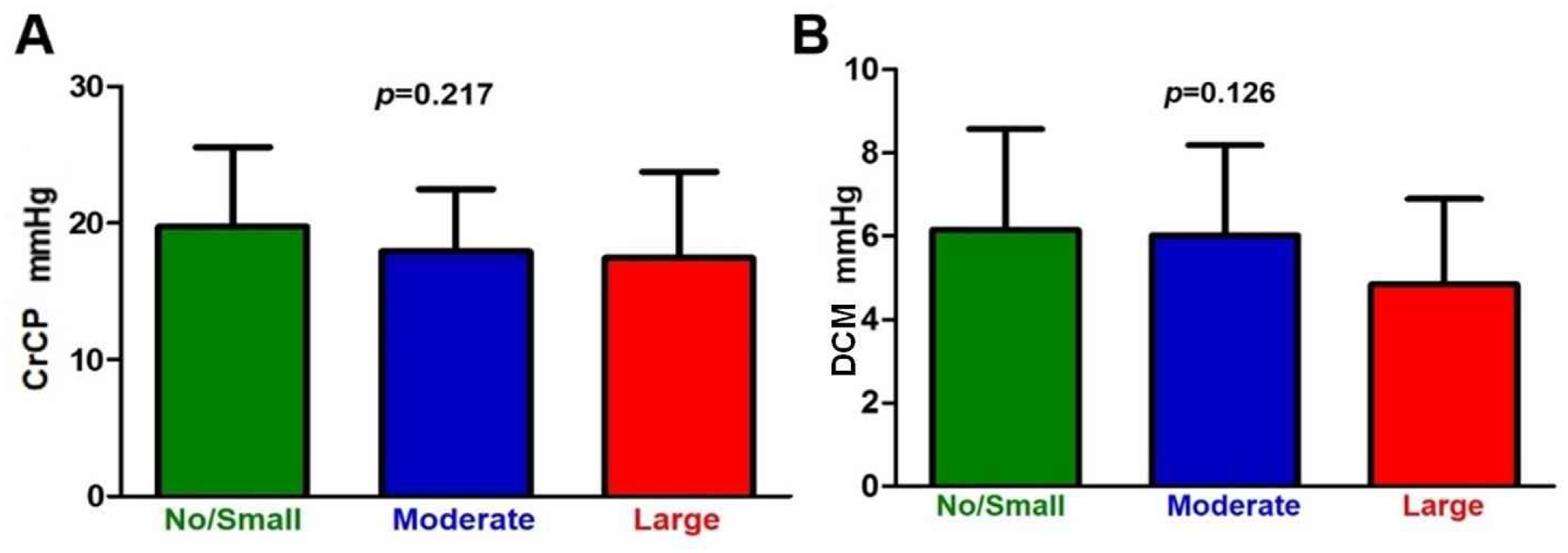
a. Critical closing pressure (CrCP) was not different according to PDA size. CrCP was 19.7 ± 5.8, 17.9 ± 4.6, and 17.4 ± 6.3 mmHg in the no/small, moderate, and large PDA groups, respectively. b. Diastolic closing margin (DCM) was not different according to PDA size. However, the large PDA group showed a trend of decreased DCM, although not significant. DCM was 6.2 ± 2.4, 6.0 ± 2.2, and 4.8 ± 2.1 mmHg in the no/small, moderate, and large PDA groups, respectively.
Quantification of Shortening Fraction
SF was not correlated with CBFv (systole, mean, and diastole CBFv, respectively, all p >0.05) or cerebral autoregulation index (Sx, Mx, and Dx, respectively, all p >0.05). SF was also not different between PDA groups (35.0 ± 6.6, 33.8 ± 7.4, and 37.9 ± 7.7 in no-to-small, moderate, and large PDA groups, respectively, p=0.217).
Discussion
This study delineates the relationship between PDA size and CBFv using transcranial Doppler ultrasound in premature infants during the first week after birth. In our study, CBFv across the cardiac cycle was not influenced by the size of the PDA. This may be because infants in this study cohort had intact cerebral autoregulatory capacity (at least during systole).
Transcranial Doppler ultrasound has been used to study the relationship between PDA and CBFv; abnormal diastolic CBFv in the setting of PDAs in premature infants is often observed [2, 3, 6]. Multiple studies have shown that infants with a hemodynamically significant PDA have an increased PI or resistive index, likely attributable to diastolic steal [3, 6, 7, 20] and low diastolic CBFv. This may represent increased resistance to blood flow and higher risk for end-organ injury. Another study by Pawar et al. measured middle cerebral artery CBFv during the first day after birth in premature infants with variable sizes of PDA and did not find any correlation between the PDA size and CBFv including end diastolic velocity and PI, consistent with our results [21].
Near-infrared spectroscopy (NIRS) has been used to evaluate the relationship between PDA and cerebral perfusion status. While transcranial Doppler ultrasound measures CBFv in cerebral arteries, NIRS measures ‘venous weighted’ cerebral oxygenation in the cerebral cortex. Cerebral oxygenation is not a measure of CBF, however, it does reflect the amount and consumption of oxygen in the brain tissue. Lemmers et al. observed decreased cerebral oxygenation and increased cerebral oxygen extraction in premature infants with a hemodynamically significant PDA. In this study, 75% of infants with a significant PDA received inotropes which may have influenced cerebral hemodynamics [22]. Another study by Van der Laan et al. reported that cerebral oxygenation was not different between infants with and without a hemodynamically significant PDA [23]. Chock et al. reported that infants with a significant PDA showed relative preservation of cerebral oxygenation [24]. Finally, Petrova et al. reported no difference in cerebral oxygenation between infants with moderate and large PDAs [25].
It is often believed that a PDA is associated with more impaired cerebral autoregulatory capacity. In this study cohort, cerebral autoregulatory capacity was present during systole, likely resulting in unchanged CBFv regardless of PDA size. Interestingly, autoregulatory capacity in diastole, as measured by the Dx, was present in the large PDA group compared to both the moderate and no-to-small PDA groups. Altered hemodynamics may play a role in the development of IVH. Our metrics of brain perfusion showed stability in the brain despite the size of the PDA. It will be important to further investigate the behaviors of these metrics and ultimately use them to help reduce the burden of IVH.
CrCP and DCM have been used in premature infants to assess cerebral hemodynamics and may have advantages compared to solely using ABP as a proxy for cerebral perfusion. This is likely due to the fact that in this population, ABP is often near or below the CrCP compared to term infants and adults [11, 26, 27]. CrCP was lower in the moderate and large PDA groups compared to the no-to-small PDA group, although differences were not statistically significant. This was likely due to the influence of lower gestational age in these groups. Additionally, DCM was not significantly different between the groups indicating relative stability of brain perfusion in the setting of PDAs.
Many factors can influence cerebral hemodynamics. In this study, partial pressure of carbon dioxide, use of inotropes, frequency of steroid use, and 5-minute Apgar score were not different between PDA groups. Other hemodynamic and cardiac-specific factors such as superior vena cava flow or measures of cardiac function may also influence cerebral hemodynamics. In our study, SF as a marker for cardiac function, did not show any significant correlation with CBFv and cerebral autoregulation index across during the entire cardiac cycle and was not significantly different between the PDA groups. A more comprehensive evaluation of cardiac assessment may be helpful to understand this relationship.
There are some limitations of our study. First, this is a post hoc analysis of prospectively collected data [9, 14–17]. While cerebral monitoring was prospectively designed, echocardiographic evaluation was done at the direction of the attending neonatologist when hemodynamically significant PDA was suspected. As such, the study was only able to include those echocardiograms done within the timing of the cerebral monitoring sessions, perhaps resulting in some data dropout. Second, echocardiogram data did not include more detailed PDA data such as PDA to left pulmonary artery ratio, presence of retrograde flow in descending aorta during diastole, or superior vena cava flow. Third, Doppler measurements of middle cerebral artery flow velocity may not be reliable proxies for CBF due to varying changes in vessel diameter, however, other modalities such as near-infrared spectroscopy and magnetic resonance angiography show good correlation between CBFv and cerebral hemodynamics [28–31]. Fourth, dampening of blood pressure waveforms could cause minimal changes to the ABP, but by visualization in this study, this was minimal. Finally, assessing the intestinal bed with NIRS or another measure of tissue bed oximetry or flow may have been added further richness to the data set, however, at the time of the recordings, no other monitoring technique was used. This will be important to include in future studies. However, when comparing the clinical characteristics of each group, the data represented the expected treatment according to PDA size.
There are also many strengths. First, the data was all collected prospectively during the first week after birth in the original cohort. Second, the study recruited a large number of infants to examine the role of PDA and cerebral hemodynamics. Finally, there was a large number of physiologic recordings of the CBFv that contributed to the data set allowing for greater understanding of the behavior of the cerebral physiology during the first week after birth.
In conclusion, cerebral hemodynamics were stable regardless of the size of the PDA in this cohort of premature infants. While cerebral autoregulatory capacity was more commonly dysregulated across the mean and diastolic phase of the cardiac cycle compared to the systolic phase, all indices of autoregulatory capacity were not different between the PDA groups. Monitoring cerebral and cardiac hemodynamics concurrently will continue to be crucial for protecting premature infant’s vital organs from injury as management of the hemodynamic PDA changes over time.
Acknowledgement
Dr. Kaiser was supported by the National Institutes of Health (1K23NS43185, RR20146 and 1R01NS060674) and the University of Arkansas for Medical Sciences Translational Research Institute (1UL1RR029884). The technical assistance of Natalie C. Sikes and Melanie J. Mason and the support of the University of Arkansas for Medical Sciences NICU are gratefully appreciated.
Funding Sources
C.J.R. is supported by the National Institute of Health (1K23NS091382). D.R.R. is supported by the National Institute of Health (1K23HLI130522).
Footnotes
Statement of Ethics
In the original trial, parents’ approval and informed consent was obtained before enrollment. The original trial was approved by the University of Arkansas for Medical Sciences Institutional Review Board.
Disclosure Statement
The authors declare no conflicts of interest
References
- 1.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–21. [DOI] [PubMed] [Google Scholar]
- 2.Shortland DB, Gibson NA, Levene MI, Archer LN, Evans DH, Shaw DE. Patent ductus arteriosus and cerebral circulation in preterm infants. Dev Med Child Neurol. 1990;32:386–93. [DOI] [PubMed] [Google Scholar]
- 3.Martin CG, Snider AR, Katz SM, Peabody JL, Brady JP. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J Pediatr. 1982;101:587–93. [DOI] [PubMed] [Google Scholar]
- 4.Wong SN, Lo RN, Hui PW. Abnormal renal and splanchnic arterial Doppler pattern in premature babies with symptomatic patent ductus arteriosus. J Ultrasound Med. 1990;9:125–30. [DOI] [PubMed] [Google Scholar]
- 5.Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Abnormal gut blood flow velocities in neonates at risk of necrotising enterocolitis. J Pediatr Gastroenterol Nutr. 1992;15:13–9. [DOI] [PubMed] [Google Scholar]
- 6.Lipman B, Serwer GA, Brazy JE. Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatrics. 1982;69:778–81. [PubMed] [Google Scholar]
- 7.Julkunen M, Parviainen T, Janas M, Tammela O. End-diastolic block in cerebral circulation may predict intraventricular hemorrhage in hypotensive extremely-low-birth-weight infants. Ultrasound Med Biol. 2008;34:538–45. [DOI] [PubMed] [Google Scholar]
- 8.Kluckow M Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum Dev. 2005;81:429–37. [DOI] [PubMed] [Google Scholar]
- 9.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154:824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison JM, Girling KJ, Mahajan RP. Effects of propofol and nitrous oxide on middle cerebral artery flow velocity and cerebral autoregulation. Anaesthesia. 2002;57:27–32. [DOI] [PubMed] [Google Scholar]
- 11.Rhee CJ, Fraser CD 3rd, Kibler K, Easley RB, Andropoulos DB, Czosnyka M, et al. Ontogeny of cerebrovascular critical closing pressure. Pediatr Res. 2015;78:71–5. [DOI] [PubMed] [Google Scholar]
- 12.Rhee CJ, Fraser CD 3rd, Kibler K, Easley RB, Andropoulos DB, Czosnyka M, et al. The ontogeny of cerebrovascular pressure autoregulation in premature infants. J Perinatol. 2014;34:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee CJ, Kaiser JR, Rios DR, Kibler KK, Easley RB, Andropoulos DB, et al. Elevated diastolic closing margin is associated with intraventricular hemorrhage in premature infants. J Pediatr. 2016;174:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr. 2004;144:809–14. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58:931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser JR, Gauss CH, Williams DK. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol. 2008;28:34–41. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser JR, Gauss CH, Williams DK. The effects of closed tracheal suctioning plus volume guarantee on cerebral hemodynamics. J Perinatol. 2011;31:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czosnyka M, Smielewski P, Lavinio A, Pickard JD, Panerai R. An assessment of dynamic autoregulation from spontaneous fluctuations of cerebral blood flow velocity: a comparison of two models, index of autoregulation and mean flow index. Anesth Analg. 2008;106:234–9. [DOI] [PubMed] [Google Scholar]
- 19.Varsos GV, Richards H, Kasprowicz M, Budohoski KP, Brady KM, Reinhard M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab. 2013;33:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlman JM, Hill A, Volpe JJ. The effect of patent ductus arteriosus on flow velocity in the anterior cerebral arteries: ductal steal in the premature newborn infant. J Pediatr. 1981;99:767–71. [DOI] [PubMed] [Google Scholar]
- 21.Pawar S, Sharma D, Murki S, Subramaniam S, Kandraju H. Construction of ductal diameter centiles in the first 24 h of life and their relation to cerebral blood flow in neonates weighing less than 1250 g in the first 24 h of life. J Trop Pediatr. 2017. 10.1093/tropej/fmx016. [DOI] [PubMed] [Google Scholar]
- 22.Lemmers PM, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–7. [DOI] [PubMed] [Google Scholar]
- 23.van der Laan ME, Roofthooft MT, Fries MW, Berger RM, Schat TE, van Zoonen AG, et al. A hemodynamically significant patent ductus arteriosus does not affect cerebral or renal tissue oxygenation in preterm infants. Neonatology. 2016;110:141–7. [DOI] [PubMed] [Google Scholar]
- 24.Chock VY, Rose LA, Mante JV, Punn R. Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. Pediatr Res. 2016;80:675–80. [DOI] [PubMed] [Google Scholar]
- 25.Petrova A, Bhatt M, Mehta R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J Perinatol. 2011;31:460–4. [DOI] [PubMed] [Google Scholar]
- 26.Panerai RB, Coughtrey H, Rennie JM, Evans DH. A model of the instantaneous pressure-velocity relationships of the neonatal cerebral circulation. Physiol Meas. 1993;14:411–8. [DOI] [PubMed] [Google Scholar]
- 27.Panerai RB, Kelsall AW, Rennie JM, Evans DH. Estimation of critical closing pressure in the cerebral circulation of newborns. Neuropediatrics. 1995;26:168–73. [DOI] [PubMed] [Google Scholar]
- 28.Bassan H, Gauvreau K, Newburger JW, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res 2005;57:35–41. [DOI] [PubMed] [Google Scholar]
- 29.Pellicer A, Valverde E, Gay F, Quero J, Caba.as F. Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol 2001;24:103–9. [DOI] [PubMed] [Google Scholar]
- 30.Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr 1997;131:549–54. [DOI] [PubMed] [Google Scholar]
- 31.Benders MJ, Hendrikse J, de Vries L, Groenendaal F, van Bel F. Doppler assessed cerebral blood flow velocity in the neonate as estimator of global cerebral blood volume flow measured using phase-contrast magnetic resonance angiography. Neonatology 2013;103:21–6. [DOI] [PubMed] [Google Scholar]


