Abstract
BACKGROUND
Platelet (Plt)-derived extracellular vesicles (Plt-EVs) have hemostatic properties similar to Plts. In addition to hemostasis, Plts also function to stabilize the vasculature and maintain endothelial cell (EC) barrier integrity. We hypothesized that Plt-EVs would inhibit vascular EC permeability, similar to fresh Plts. To investigate this hypothesis, we used in vitro and in vivo models of vascular endothelial compromise and bleeding.
METHODS
In the vitro model, Plt-EVs were isolated by ultracentrfugation and characterized for Plt markers and particle size distribution. Effects of Plts and Plt-EVs on endothelial barrier function were assessed by transendothelial electrical resistance measurements and histological analysis of endothelial junction proteins. Hemostatic potential of Plt-EVs and Plts was assessed by multiple electrode Plt aggregometry. Using an in vivo model, the effects of Plts and Plt-EVs on vascular permeability and bleeding were assessed in non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice by an established Miles assay of vascular permeability and a tail snip bleeding assay.
RESULTS
In the in vitro model, Plt-EVs displayed exosomal size distribution and expressed Plt-specific surface markers. Platelets and Plt-EVs decreased EC permeability and restored EC junctions after thrombin challenge. Multiplate aggregometry revealed that Plt-EVs enhanced thrombin receptor–activating peptide-mediated aggregation of whole blood, whereas Plts enhanced thrombin receptor–activating peptide–, arachidonic acid–, collagen-, and adenosine diphosphate–mediated aggregation. In the in vivo model, Plt-EVs are equivalent to Plts in attenuating vascular endothelial growth factor (VEGF)-A–inducedvascular permeability and uncontrolledblood loss in a tail snip hemorrhage model.
CONCLUSION
Our study is the first to report that Plt-EVs might provide a feasible product for transfusion in trauma patients to attenuate bleeding, inhibit vascular permeability, and mitigate the endotheliopathy of trauma.
Keywords: Vascular instability, trauma, barrier disruption, hemostasis
Traumatic injury is the leading cause of death worldwide in individuals between the ages of 1 and 44 years.1–5 Hemorrhage is responsible for the majority of preventable trauma-related deaths, 80% in the military, and 40% under the age of 65 years in the civilian population.3–6 Goals for resuscitation and blood product transfusion in bleeding patients have been defined by landmark retrospective and prospective studies in which balanced ratios of blood products in a 1:1:1 ratio of red blood cells-plasma-platelets (Plts) are shown to improve survival and outcomes.6,7 Findings from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial also revealed that early Plt administration is associated with improved hemostasis and reduced mortality in severely injured bleeding patients.8 Logistically, in austere settings or settings that require prolonged field care of bleeding patients, there are challenges in obtaining, storing, and administering blood products. Platelets in particular generate a greater logistical challenge than red blood cells and plasma since current blood banking practice in the United States allows Plts to be stored for a total of only 5 day at 22°C, which leads to increased risk of infections, decreased or wasted inventory of Plts, and a demonstrable storage lesion or decline in function of the Plts with storage at 22°C.9–11
There are currently a number of preclinical and clinical endeavors to evaluate alternative Plt-derived hemostatic agents such as cold-stored Plts and freeze-dried Plts.12,13 Platelet-derived hemostatic products can circumvent some of the logistical and practical challenges of Plt transfusion and with fewer infectious risks associated with standard apheresis or whole blood–derived Plt units.14 Platelet-derived extracellular vesicles (EVs) are particles secreted from Plts that express surface receptors and contain multiple types of RNA, proteins, lipids, and DNA. Extracellular vesicles range in size from 10 nm to 1,000 nm in diameter. Extracellular vesicles in the range of 50 mm to 100 nm in size are nanoparticles called exosomes, and particles in the size range of 100 nm to 1,000 nm are called microvesicles (MVs).15–22
Extracellular vesicles mediate cell-cell communication by transferring their cargo from one cell to another.18,23 Extracellular vesicles can bind and fuse with the plasma membrane of the target cell, or alternatively, are engulfed by the target cell, through which they can alter the biological function of the recipient cell.18 These nanometer sized particles are shown to circulate systemically in the blood, in both health and disease, and are derived from a number of cellular sources including monocytes, macrophages, endothelial cells (ECs), and neutrophils.19,23,24 Over the past decade, the role and biological functions of EVs have been the focus of scientific research in multiples fields, with demonstrated diagnostic and therapeutic potential of EVs, in cancer, hemostasis, autoimmune disease, and angiogenesis.19 Platelet-derived EVs are procoagulant and can enhance induced hemostasis through EV surface receptors, which include GPIIb/IIIa, tissue factor, and phosphatidylserine.16 Phosphatidylserine can activate coagulation factors II and X, hence triggering the coagulation cascade.25 Aside from surface receptors, there are only a few studies that have aimed to characterize the proteomic content of Plt-EVs. These reports have demonstrated that, regardless of the methods of stimulation of the Plts, there are a number of proteins packaged consistently within the EVs, including thrombospondin-1, fibrinogen, integrin IIb, and platelet factor 4, to name a few.26–28
We have previously demonstrated the ability of standard apheresis Plts to attenuate vascular endothelial permeability in both in vitro and in vivo models in mice.29,30 In this article, we sought to determine if Plt-EVs could mediate hemostasis similar to apheresis Plts and also modulate vascular endothelial protection in our defined murine model of endothelial barrier dysfunction and permeability. We hypothesized that Plt-EVs would have similar therapeutic potential to Plts in vitro and in vivo and may provide an alternative hemostatic and vasculoprotective option to bleeding patients when access to Plts or blood products are limited.
MATERIALS AND METHODS
Isolation of Plt-EVs From Apheresis Plts
Leukoreduced apheresis Plts stored in plasma were obtained from Bonfils Blood Bank, Denver, Colorado. All Plts were tested for bacterial infection by the blood bank (Blood Centers of the Pacific) and found to be negative. To generate EVs from Plts, we first stored the Plts at 22°C for 3 days with gentle rocking. The Plts were spun at 1,000g for 30 minutes to obtain a cell-free supernatant. This supernatant was subsequently spun at 13,000g at 4°C for 15 minutes, from which the resulting supernatant was collected, and spun at 100,000g (Beckman Coulter Optima LE-80 K ultracentrifuge and a SW-28 T rotor) at 4°C for 1 hour. The resulting pellet was resuspended in a small volume of phosphate buffered saline (PBS) (approximately 200 μL), and the amount of protein was quantified on NanoDrop 2000 (Thermo Scientific, Waltham, MA) and then subsequently aliquoted and stored at −80°C.
Flow Cytometry Characterization of Evs
Extracellular vesicles collected from apheresis platelets were characterized for the expression of Plt-specific markers such as CD31, CD41, and CD63 using fluorescently conjugated antibodies (BioLegend, San Diego, CA). Extracellular vesicles were further tested for the expression of tetraspanin markers using CD9 and CD81 antibodies (BioLegend, San Diego, CA).
Before staining the EVs, antibodies were filtered using the Ultrafree-MC/Durapore-PVDF centrifugal filter tubes (Millipore, Hayward, CA). After 30 minutes of staining at 4°C, EVs were spun in the Ultrafree-MC/Durapore-PVDF centrifugal filter tubes. Extracellular vesicles were collected by flushing the filter membrane with 200 μL of PBS. Samples were analyzed by running on an LSR II benchtop flow cytometer (BD Biosciences, San Jose, CA). For the size determination of EVs, 0.16, 0.2, 0.24, and 0.5 μm Biocytex Megamix Plus-SSC reference beads were used (Thermo Fisher Scientific, Waltham, MA). Data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR), as described previously.21
Characterization of Plt-EVs by NanoSight
Particle size distribution of Plt-EVs was determined by nanoparticle tracking analysis using a NanoSight NS300 system (Malvern Panalytical, Westborough, MA) as previously described.22 Samples were diluted 1:400 in particle-free PBS according to the manufacturer’s recommendations. Data were analyzed using NTA 3.2 Dev Build 3.2.16 software (Malvern Panalytical, Westborough, MA).
Effects of Plt-EVs on EC Permeability
Human pulmonary microvascular endothelial cells (PECs) were obtained from Promocell (Germany) and maintained using Growth Medium MVS (Promocell, Germany). The integrity of PEC monolayers was measured using an electric cell-substrate impedance sensing system (ECIS 1600, Applied BioPhysics, Troy, NY). An increase or decline in transendothelial electrical resistance across the cell monolayers indicated accordingly decreased or increased endothelial paracellular permeability. Pulmonary microvascular endothelial cells were grown to confluence on L-cysteine reduced, 96-well plates containing electrodes in each well. Cells were treated with Plts (10 × 106/mL, 25 × 106/mL, and 50 × 10/mL) or Plt-EVs (15 μg/mL, 30 μg/mL, and 60 μg/mL) and challenged after 30 minutes with Thrombin (Sigma, St. Louis, MO) at a concentration of 0.2 U/mL. Monolayer resistance at 4/16/64 kHz was analyzed in 8-minute intervals. Data were normalized to the mean resistance of cell monolayers before the treatments.
Effect of Plt-EVs on Cytoskeletal, Tight, and Adherens Junction Protein Expression on PECs
Pulmonary microvascular endothelial cells were grown to confluence on collagen-coated cover slips before treatment. Platelets (50 × 106/mL) or Plt-EVs (30 μg/mL) were added to the PEC monolayer for 30 minutes, followed by a 0.2 U/mL of thrombin challenge for 5 minutes at 37°C. Cells were then fixed with 4% paraformaldehyde and stained with antibodies against VE-cadherin (Cell Signaling), zonula occludens-1 (ZO-1) (Invitrogen), and phalloidin (Cell Signaling) and then imaged sat 20 times magnification using the Revolve microscopy system (Echo) or Nikon Eclipse 80i microscope with RT-scmos camera.
To quantify junctional proteins, five nonoverlapping images per treatment condition were captured at same exposure settings at 20 times magnification on Nikon Eclipse 80i microscope with RT-scmos camera. Images were exported into MetaMorph software (Molecular Devices, Inc.) and thresholded at similar settings between all images and treatment groups. The number of DAPI-positive nuclei was manually counted, and integrated intensity measurements of junctional proteins and F-actin that included both cytoplasmic and membrane bound expression were measured using Measure Colocalization module. On an average, 70 cells were counted per image.
Multiple Electrode Plt Aggregometry of Plt-EVs
Blood samples were obtained from healthy volunteers as approved by the University of California Committee on Human Research as part of longitudinal study examining perturbations in coagulation and inflammation after trauma (IRB number 10–04417). Standard laboratory vacuum-sealed tubes containing 3.2% (0.109 M) sodium citrate were used for all draws. Using multiple electrolyte Plt aggregometry (Multiplate) (Roche, Basel, Switzerland), we examined Plt aggregation of whole blood treated with EVs, in response to stimulation by the agonists adenosine diphosphate (ADP), collagen, thrombin receptor–activating peptide-6 (TRAP-6), and arachidonic acid (ASPI). Briefly, 0.3 mL of whole blood was diluted in warmed normal saline containing 3 mM of CaCl2 and incubated for 3 minutes at 37°C with continuous stirring in a Multiplate test cell. Each test cell contains two sets of 3 mm silver-coated copper wires, across which electrical resistance is measured at 0.57-second intervals. Platelet activation was induced by ADP (final concentration, 6.5 μM; via P2 receptors), TRAP-6 (final concentration, 32 μM; via PAR receptors), ASPI (final concentration, 0.5 mM; via the cyclooxygenase pathway), and collagen (final concentration, 3.2 μg/mL; via GpIa/IIa and GpVI receptors). Platelet adhesion to the electrodes was detected as increasing electrical impedance, measured by duplicate sets of sensor wires in each test cell. Whole blood samples were treated with apheresis Plts or EVs for 5 minutes before agonist stimulation, and the resulting aggregation units were normalized to untreated whole blood samples.
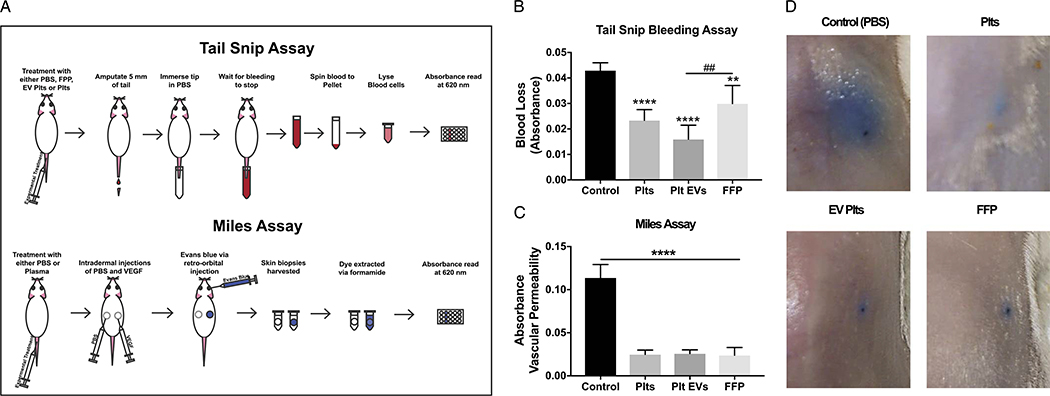
Tail Snip Bleeding Model in Mice
All animal protocols were performed with approval of the Institutional Animal Care and use Committee at PMI Preclinical (San Carlos, CA). The tail snip bleeding model was performed in 8- to 10-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratories, Sacramento, CA). Isoflurane-anesthetized mice were injected with vehicle (PBS), Plts (3 × 108), Plt-EVs (35 μg), or fresh frozen plasma (FfP) (200 μL), through the tail vein. After 30 minutes, the distal portion of the tails (~5 mm segment) were amputated and immediately immersed in 10 mL of 37°C PBS. Once bleeding ceased, the resulting blood samples were centrifuged at 500g for 10 minutes to collect a pellet. The pellet was then resuspended in 300 μL of red blood lysis buffer (Sigma, St. Louis, MO) and incubated at 22°C for 10 minutes. To quantify blood loss, the absorbance of the sample was measured using the SoftMax Pro 5 Microplate Reader at 550 nm.
Miles Assay in Mice
The modified Miles assay (Miles AA, 1952) was performed in 8- to 10-week old NSG mice (Jackson Laboratories, Sacramento, CA). Isoflurane-anesthetized mice were injected with vehicle (PBS), Plts (2 × 108) Plt-EVs (35 μg), or FFP (200 μL) via their tail veins. To stimulate permeability, 50 μL of vascular endothelial growth factor (VEGF)-A (2 ng/μL) and an equal volume of PBS were administered 30 minutes later intradermally to opposite sides of the dorsal skin. One hundred microliters of a 0.5% Evans blue dye (Sigma, St. Louis, MO) was administered in the retro-orbital sinus. After 2 hours posttreatment, mice were photographed (× 200, 1 NIKKOR, Nikon, Melville, NY) and euthanized. Barrier permeability was analyzed by removing the stimulated area via biopsy punch and extracting the Evans blue dye with formamide at 37°C for 48 hours, and the amount of leakage was quantitated by measuring absorbance at 620 nm on a SoftMax Pro 5 Microplate Reader.
Statistical Analysis
Measures of transendothelial electrical resistance (TEER), junctional protein expression, vascular permeability, and blood loss were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc tests (GraphPad Prism 7 software, GraphPad Software, La Jolla, CA). Impedance aggregometry underwent multiple group comparisons that were analyzed by two-way ANOVA and Tukey’s multiple comparisons post hoc test to perform between group comparisons. Differences between groups were considered significant with p ≤ 0.05.
RESULTS
EVs Express Intrinsic Plt-Specific and EV-Specific Cell Surface Markers
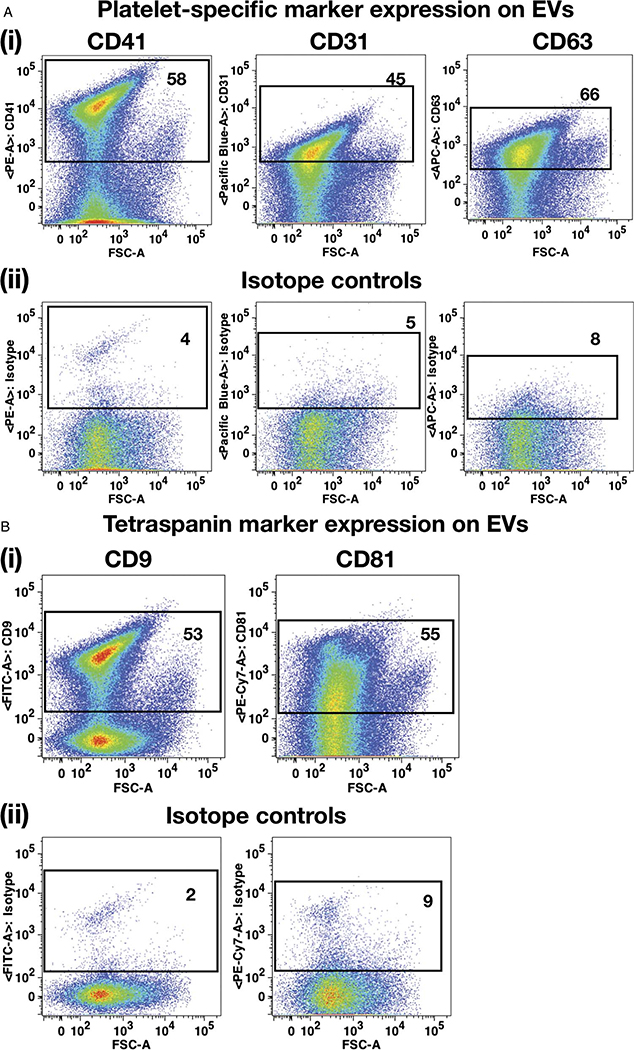
Extracellular vesicles were isolated from the stored Plts using differential ultracentrifugation procedure as described previously.31 This method can result in isolation of EVs from neutrophils, macrophages, or ECs. To confirm that the EVs are predominantly of Plt origin, they were analyzed for the Pltand EV-specific cell surface marker expression (Fig. 1). Fluorescently labeled Megamix beads containing a mixture of various reference size beads (Figs. S1 (1) and (2), http://links.lww.com/TA/B301) were used to determine the relative particle size of Plt-EVs (Fig. S1 (3), http://links.lww.com/TA/B301). It was observed that the Plt-EVs expressed high levels of Plt-specific markers such as CD31 (45%) and CD41 (58%) (Fig. 1A (1)). Expression of tetraspanin protein CD63, also known as the lysosome-associated membrane protein-3 (lamp3), has been reported to be present on the activated Plts as well as Plt-derived exosomes.32,33 Our results also revealed that CD63 was highly expressed (66%) on the Plt-EVs (Fig. 1A (1)). The representative isotype controls have been shown in Figure 1A (2). In addition, other tetraspanin proteins, such as CD9 and CD81, that are known to be present on exosomes, were also found to be expressed on Plt-EVs34 (Fig. 1B).
Figure 1.
Phenotypic characterization of EVs. (A) (1) and (2) Flow plots demonstrating Plt-specific markers (CD41, CD31, and CD63) and corresponding isotype controls expressions, respectively, on the Plt-EVs. (B) (1) and (2) Flow plots showing expression of tetraspanin markers (CD9 and CD81) and corresponding isotype controls, respectively, on Plt-EVs.
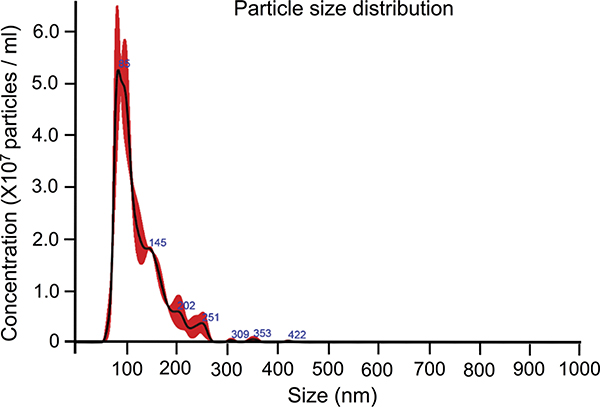
The NanoSight system was used to determine the particle size distribution of the isolate. This assay revealed the mean particle size to be 124 nm, with all particles falling between the range of 50 nm and 422 nm (Fig. 2). The final particle count allowed us to extrapolate the number of particles used in our studies, with 30 μg/mL containing approximately 3.39 × 1013 particles per milliliter. Altogether, these assays confirm that the EVs are of Plt-derived origin and primarily exosomal in size.
Figure 2.
Platelet EVs display size comparable with exosomes. Nanoparticle tracking analysis of Plts-EVs. Graph represents distribution of the various particle sizes at a 1:400 dilution.
Effect of Plt-EVs on Endothelial Barrier Function
Using the ECIS system to measure the TEER of the endothelial barrier junctions, we compared the effects of apheresis Plts and Plt-EVs on the integrity of PEC monolayers. In agreement with previously reported data, Plt treatment of unchallenged PECs induced an increase in endothelial barrier resistance, in a dose dependent manner. A dose of 10 × 106/mL of Plts resulted in an increased resistance of 3.8%, while the doses of 25 × 106/mL and 50 × 106/mL induced an increase of 15%and 17%, respectively (p <0.0001)(Fig. 3A). In contrast, Plt-EVs did not appear to increase barrier stability. Moreover, treatment of unchallenged/unstimulated PECs with Plt-EVs resulted in an initial decrease in resistance or barrier function for all three doses of Plt-EVs. Although all doses (15 μg/mL, 30 μg/mL, 60 μg/mL) of Plt-EVs initially decreased endothelial resistance initially by 17%, 14%, and 15% (p <0.01 as compared with untreated control), barrier resistance was increased by Plt-EVs after challenge/stimulation with thrombin (Fig. 3B).
Figure 3.
Treatment with Plts and Plt-EVs protects the endothelial barrier in vitro. (A) TheTEER ECIS tracing of human PECs pretreated with Plts and subsequently challenged with thrombin. Area under the curve boxplots represent AUC quantitation of changes in barrier resistance. (B) TheTEER ECIS tracing of PECs pretreated with Plt-EVs and subsequently challenged with thrombin. Area under the curve boxplots represent AUC quantitation of changes in barrier resistance. (C) The TEER ECIS tracing of PEC comparing Plt and Plt-EV pretreatment effects against thrombin challenge. Area under the curve boxplots represent AUC quantitation of barrier resistance. The TEER line graphs represent mean ± SEM, and AUC boxplots represent mean ± SD of 4 wells/condition. *Indicates significant difference from control. #indicates a significant difference between dose groups. In thrombin challenged graphs, * indicates significant difference from thrombin, while # indicates a significant difference from control as determined by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Indeed, when the PEC monolayers were challenged/stimulated with thrombin, a known inducer of vascular permeability, both Plts and Plt-EVs attenuated the resulting barrier compromise equivalently. As shown by the ECIS tracings and quantitated by area under the curve (AUC), thrombin challenge decreased the endothelial barrier resistance by 12% as compared with unchallenged controls (p < 0.0001) (Fig. 3A). Monolayers pretreated with Plts attenuated thrombin-induced loss of EC barrier resistance in a dose dependent manner (p < 0.05,10 × 106/mL vs. 50 × 106/mL) (Fig. 3A). The treatment dose 10 × 106/mL resulted in a 6% decrease after thrombin challenge (p < 0.01), while 25 × 106/mL and 50 × 106/mL attenuated the thrombin-induced barrier dysfunction to a 4% decrease in resistance (p < 0.001) and 2.5% decrease in resistance (p < 0.0001), respectively, as compared with the thrombin treated group alone (12% loss) (Fig. 3A). Similarly, pretreatment with Plt-EVs mitigated thrombin-induced increase in barrier permeability, in a dose dependent manner. While the dose of 15 μg/mL of Plt-EVs did not significantly increase barrier integrity or resistance, the doses of 30 μg/mL and 60 μg/mL attenuated the effect of thrombin on barrier integrity to 2% decrease in resistance (p < 0.001 vs. thrombin treatment) and 5% decrease in resistance (p < 0.01 vs. thrombin treatment), respectively (Fig. 3B). Furthermore, the 30 μg/mL and 60 μg/mL doses maintained the barrier resistance at levels statistically indistinguishable from control (untreated cells), demonstrating full protection against the thrombin challenge and equivalence to Plt effects on EC permeability with thrombin challenge (Fig. 3C). Overall, these results suggest that both Plts and Plt-EVs are capable of providing EC barrier protection against a relevant insult such as thrombin.
Plt and Plt-EVs Attenuate Thrombin-Induced Compromise of Adherens and Tight Junction Proteins
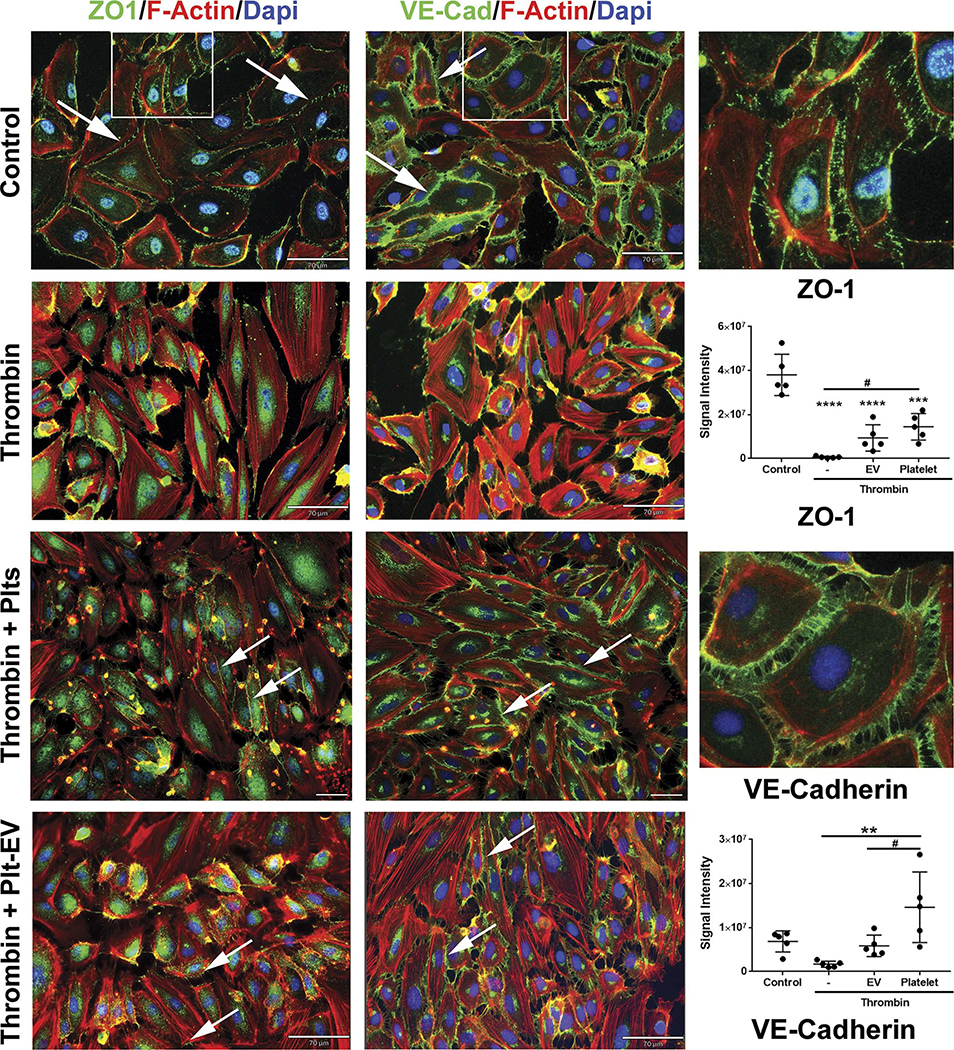
To understand the effects of Plts and Plt-EVs on endothelial junction proteins, we visualized PEC monolayers and stained for the expression of the adherens junction protein VE-cadherin, the tight junction protein ZO-1, and the structural protein Factin. The representative control group images show the undisturbed membrane junctions of VE-cadherin (green) and ZO-1 (green), as well as a quiescent level of F-actin, which is not mobilized to the periphery of the cytoplasm (red) (Fig. 4). Following thrombin challenge, a majority of the cells contract, initiating a loss of both VE-cadherin and ZO-1 expression and an increase in F-actin expression levels, which is mobilized to the periphery of the cell, ultimately resulting in the formation of large gaps throughout the endothelial monolayer (Fig. 4). Platelet pretreatment of the PECs greatly attenuated the loss of this thrombinmediated gap formation, evidenced by the preservation of VE-cadherin and ZO-1 expression at the cell membrane, and the muted level of F-actin staining (Fig. 4). Similarly, pretreatment with Plt-EVs also maintained these junctional proteins following thrombin challenge, resulting in an attenuation of gap formation in the endothelial monolayer (Fig. 4). We quantitated the amount of VE-cadherin and ZO-1 expression in response to challenge and treatment. As shown in Figure 4, the amount of ZO-1 expression decreased in response to thrombin challenge, and treatment with Plts significantly attenuated this loss, while treatment with Plt-EVs, on an average, also resulted in an increase in mean value of ZO-1 as compared with the thrombin treated group. Similar results were observed with the expression of VE-cadherin.
Figure 4.
Treatment with Plt and Plt-EV attenuates loss of endothelial junction proteins in response to thrombin challenges. Representative images of PECs stained for ZO-1, VE-cadherin, and F-actin after pretreatment with Plts or Plt-EVs and subsequent challenge with thrombin. Arrows indicate preserved junctional and adherens proteins; squares in the control panel indicate region of image shown in high magnification. Scale bars in control, thrombin and thrombin-Plt-EV, 70 μM; scale bar in thrombin-Plt, 100 μm. Graphs represent thresholded signal intensity for each of the proteins. Values are mean ± SD, with individual data sets.
Effect of Plt-EVs on Plt Aggregation
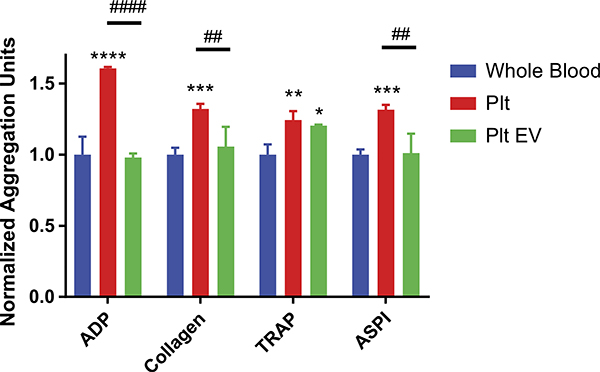
To examine possible hemostatic properties of Plt-EVs, we studied their capability to enhance Plt aggregation response to agonist activation using a multiple electrode Plt aggregometer. As expected, addition of Plts to whole blood samples increased the Plt aggregation response when individually stimulated by agonists. With Plt treatment, ADP-induced aggregation demonstrated an increase of 60% over untreated whole blood (p = <0.001), Plt treatment of collagen-induced aggregation increased by 32% over untreated whole blood (p = 0.009), Plt treatment of TRAP-induced aggregation increased by 23% over untreated whole blood (p = 0.0082), and Plt treatment of ASPI-induced aggregation increased by 31% over whole blood (p = 0.0010). In comparison, treatment with Plt-EVs only increased TRAP-induced aggregation by 20% over untreated whole blood (p = 0.0266) and did not significantly change the aggregation response to ADP, collagen, or ASPI agonists (Fig. 5).
Figure 5.
Platelet EVs and Plts promote aggregation of whole blood. Whole blood samples pretreated with Plts or Plt-EVs were stimulated by the agonists ADP, collagen, TRAP, and ASPI. Quantitation of resulting aggregation normalized to untreated whole blood aggregation. Bars represent mean + SEM. *Indicates a significant difference from untreated whole blood. #Indicates a significant difference between Plt and Plt-EV groups, as determined by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Plt-EVs Provide Hemostasis in a Bleeding Model
Using a tail snip bleeding model with NSG immunodeficient mice (Fig. 6A), we examined the hemostatic effect of Plt-EVs in comparison with the effects of Plts and FFP. These mice are immunocompromised so that they can receive human tissue xenotransplants. Measurement by absorbance level of the blood collected in saline following tail snips revealed a reduction of bleeding by Plts infusion. Fresh frozen plasma treatment resulted in a 30% decrease in blood loss compared with vehicle control (p = 0.0054), while Plt treatment resulted in a 45% reduction in bleeding compared with control p < 0.001). Importantly, treatment with Plt-EVs in a 62% reduction in bleeding compared with control (p < 0.001). Taken together, this model suggests that Plt-EVs can provide hemostatic support similar to that seen with Plts or FFP (Plt-EV significantly higher than FFP, p = 0.0029) (Fig. 6B).
Figure 6.
Platelets and Plt-EVs promote hemostasis and reduce vascular permeability in in vivo mouse models. (A) Schematic outlining procedures for mouse tail snip assay and Miles assay. (B) Quantitation of blood collected from mice treated with Plts, Plt-derived EVs, and FFP. (C) Absorbance quantitation of Evans blue dye extravagated from challenged regions of the mouse. Bars in both graphs represent mean + SEM. *Indicates a significant difference from control. #Indicates a difference between treatment groups, as determined by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Plt-EVs Attenuate VEGF-A-Induced Vascular Permeability in Vivo
To further examine the effects of Plt-EVs on vascular permeability in an in vivo setting, we used a modified Miles assay in NSG mice (Fig. 6A). As shown by the representative images, control untreated mice displayed large blue patches of Evans blue dye leakage following VEGF-A administration, a known inducer of vascular permeability (Fig. 6D). In agreement with previously reported data,23 intravenous treatment with Plts and FFP resulted in a dramatic attenuation of VEGF-A–induced leak. Indeed, quantification of the dye extravasation indicated a 79% reduction in permeability for both treatment groups. Comparatively, Plt-EVs also attenuated leakage by 77% (Fig. 6C). Similar to in vitro studies of EC barrier integrity, Plt-EVs appear to be comparable with Plts in the attenuation of vascular endothelial permeability in vivo (Fig. 6C and D).
DISCUSSION
In this study, using in vitro and in vivo models, we demonstrate that Plt-EVs have potent therapeutic effects on vascular permeability and hemostasis. These particles, derived from apheresis Plts, were confirmed to be the size of EVs,31 and expressed marker proteins were known to be associated with Plts and Plt-EV’s (Figs. 1 and 2). In addition, they demonstrated an ability, in vitro, to maintain endothelial junction protein expression and functionally protect the endothelium from barrier disruption (Figs. 3 and 4). It is of interest to note that the potency and protective effects of Plt-EVs in vitro on endothelial barrier function are diminished when unchallenged by thrombin, indicating the necessity of Plts in the protection of an unstimulated endothelium (compare Fig. 3B top and bottom panels). However, after thrombin challenge, the Plt-EVs are equally protective of barrier integrity compared with Plts (Fig. 3C). Consistent with these in vitro results with challenge, the in vivo data in the Miles assay and tail snip bleeding model suggest that Plt-EVs are equivalent to Plts in attenuating vascular permeability and promoting hemostasis. These results may suggest that some form of activation of the Plt-EVs, possibly by a stimulated endothelium, is required to initiate their EC barrier protective effects. This may be due to the release of Plt trophogens within the Plt-EVs that are known to regulate barrier stability.35
The Multiplate Plt aggregometry assays showed that Plt-EVs potentiate TRAP-induced Plt aggregation in healthy human whole blood but did not potentiate ASPI-, ADP-, or collagen-induced Plt aggregation. However, not surprisingly, the addition of Plts to healthy human whole blood potentiated all tested inducers of Plt aggregation. This observation may suggest that Plt-EVs are indeed capable of providing a hemostatic function but may not be to the extent found with Plts. It is known that the Plt response to thrombin activation potentiates further expression of surface thrombin receptors36 and that thrombin is a very potent Plt activator.28 It may be that the threshold for activation is much lower for thrombin than other agonists, and this mechanism could be a potential explanation for the potentiation of thrombin response over the other agonists with the tested dosages of Plt-EVs. Further investigations with increasing dosages of Plt-EVs may elucidate whether there is a dose-dependent response that potentiates ASPI-, ADP-, or collagen-induced Plt aggregation.
Furthermore, Plt-EVs are known to have heterogeneous and highly diverse cargo, and studies have identified the presence of both prothrombin and thrombospondin, a dominant procoagulant a granule protein, in Plt-EVs.28,37,38 Given this, it may be that the Plt-EVs potentiation of the Plt aggregation response to thrombin can be explained by differential contents of thrombin pathway-specific procoagulant proteins compared with the other Plt aggregation pathways tested in this study. In addition, proteomic studies have demonstrated differences in the content of Plt MVs by size distribution, with smaller MVs enriched for increased procoagulant content than larger MVs. This association should also be considered as potential mechanistic support that there may be a variation in procoagulant contents in the even smaller Plt-EVs, differentially potentiating the Plt response to thrombin-induced aggregation over others by contents beyond prothrombin and thrombospondin. In the future, it will be critical to identify the array of contents within these Plt-EVs.
Limitations of this study are that Plt-EVs were tested from a single donor. In our past studies, we have demonstrated that there is considerable donor dependent variability in Plts and their effects on endothelial integrity.29,30 It has been shown that EVs, primarily of exosomal origin from a particular cell type, consistently have about 50% of their “cargo” in common,17–19,39,40 hence indicating that there is a cellular program mediated by the particular cell type that packages specific proteins and others such as RNA into EVs. Plasma from trauma patients lacking Plt-EVs demonstrates a decreased response to Lipopolysaccharide (LBS),41 and trauma patients with acute traumatic coagulopathy have reduced concentration of Plt-EVs.24 Taken together, these studies on Plt-EVs indicate that they play a role in trauma-induced inflammation and coagulation; hence, the delicate balance and interaction between the patient and the effects of Plt-EVs on inflammation, coagulation, and vascular function will likely determine outcomes. It is possible that Plt-EVs as a therapeutic could result in different clinical outcomes depending on the patient, timing of delivery, and dose given in trauma. Indeed, the freeze-thaw nature of Plt-EV dose storage could very likely affect the outcomes of their therapeutic effects. Examination of any changes to the Plt-EV morphology, content, and function by long-term storage is warranted. Altogether, the role and therapeutic potential of Plt-EVs in trauma could potentially be mixed and requires further investigation.31 Future studies into donor dependent variability in EVs are warranted as well.
Another limitation of this study is that the Plt-EVs were tested in vivo by pretreating animals, which is not clinically relevant to the treatment of bleeding patients. The Plt-EVs were also not tested in models of traumatic injury for their therapeutic potential, for example, in models of hemorrhagic shock and hemorrhage-induced lung vascular permeability.42,43 This will be the focus of future studies where Plt-EVs will be administered after traumatic injury in rodent models. It would be of interest as well in future studies to distinguish between the contribution of Plts and Plt-EVs in the regulation of hemostasis and vascular stability. One potential method to investigate this question would be to study Plts and the lack of Plt-EVs in a Rab27a and Rab27b double-knockout (Rab27DKO) mice that are deficient in exosome secretion.44 One can hypothesize that the effects of Plts on both endpoints would be attenuated.
Treating uncontrolled hemorrhage is challenging in austere environments such as remote rural areas of the United States and military Role 2 facilities or during prehospital transport.1 In the military, apheresis Plts can only be stored for up to 5 days at room temperature (22°C), which makes transportation from the United States to the battlefield generally impossible. The military has moved to using cold-stored Plts or collecting Plts and Plt-containing products such as low-titer whole blood in the battlefield, but these are more difficult to use under challenging conditions.1 Platelet EVs are a potential novel hemostatic agent that could possibly be stored for extended periods at room temperature or dried, warranting further investigation. The availability of a dried hemostatic agent (i.e., dried Plt-EVs) that can bridge bleeding patients and attenuate the endotheliopathy of trauma, until patients can receive blood products, would provide several therapeutic advantages in both civilian and military settings.
Supplementary Material
Biography
DISCUSSION
SUSAN EVANS, M.D. (Charlotte, North Carolina): Thank you, Dr. Schreiber, Dr. Henry, members and guests. Platelets are well recognized as crucial to controlling hemorrhage. However, the availability of platelets remains limited due to insufficient supply and short storage life.
Dr. Pati and colleagues have attempted to address this crucial problem with a unique utilization of bloodstream constituents which, until now, have generally been considered dysfunctional byproducts of physiologic stress.
Extracellular vesicles, sometimes called microparticles, are cell fragments released by exocytosis, often following cell activation, and occur in the bloodstream in substantially increased concentration following injury or stress.
Because they carry only portions of cells, their signaling is altered from their parent cell and they have been shown to contribute to inflammation. However, Dr. Pati and her colleagues have identified a molecular response to extracellular vesicles which could be beneficial rather than detrimental.
They evaluated the impact of platelet extracellular vesicles on limiting vascular permeability and hemostasis.
In a very elegantly designed study they demonstrated that extracellular vesicles have a generally similar efficacy to platelets in preventing vascular permeability.
At the very least, these findings help our understanding of clotting and hemostasis to pursue further investigation into the mechanisms of both platelets and extracellular vesicles.
At best, they provide an intervention which can improve hemorrhage control, despite our limited supply of platelets.
Dr. Pati, I have four questions.
Number 1. At this World Trauma Conference we should recognize the contributions of our Chinese colleagues who demonstrate an upregulation of CRP and IL6 in platelet extracellular vesicles.
Furthermore, non-platelet-derived extracellular vesicles, which are most certainly included in your concentrates, have been demonstrated to enhance inflammation, as well.
How could you exclude non-platelet-derived extracellular vesicles or mitigate the inflammatory effects of the platelet-derived extracellular vesicles?
Number 2. Our Canadian colleagues have identified that only a fraction of platelet extracellular vesicles actually contain the phosphatidylserine from the lipid bilayer integral to platelet aggregation.
Additionally, you demonstrate that platelet extracellular vesicles actually decrease endothelial barrier resistance without the thrombin activation.
This is not equivalent to the platelet response, as you suggest in your title. Given these findings, could platelet extracellular vesicles lead to increased bleeding, perhaps?
Number 3. On the contrary, understanding that without the entire cell for regulation is it possible these vesicles could create upregulated thrombosis?
And, finally, coming from one of the labs that has attempted to extend access to stored platelets I appreciate your efforts to spare this precious resource.
However, your technique to harvest these fragments still requires platelet concentrates. Doesn’t that defeat the purpose of providing a treatment which, ideally, spares our limited platelet supply?
Thank you to the AAST for the opportunity to review this important manuscript. Dr. Pati, I look forward to your responses. Thank you.
HASAN B. ALAM, M.D. (Ann Arbor, Michigan):Very nicely presented work. And every time I listen to one of your talks I learn something, and this was no exception. Excellent job.
So, I have two quick questions. The first one is about these vesicles from the platelets. Are these specific to the platelets, or would you see the same effect if you have non-platelet vesicles from a variety of different sources? Is it possible that other cellular products, such as exosomes, may be at play? As you know, you can drive exosomes from multiple cellular sources. You can also scale up the production, and generate exosomes in very large quantities, which has obvious commercial implications. There is a lot of research going on showing that exosomes can stabilize the endothelium, and promote healing. So how much of the effect that you have observed is specific to platelet-derived vesicles, versus a generalized response to various cellular products that are shed in response to different stimuli?
And the second question is about finding the right balance. We know that a lot of these cellular particles act as DAMPs and can create an exaggerated inflammatory response. And here you are showing data that they are beneficial. So, how do you sort out the good versus the bad?
MICHAEL GOODMAN, M.D. (Cincinnati, Ohio): More of a technical question for you. What are your EVs resuspended in? And is this a dose-dependent response with the EVs, one, depending on the amount of EVs you use; and two, the size of the EVs that are applied?
SHIBANI PATI, M.D. Ph.D. (San Francisco, California): Thank you very much, Dr. Evans, for your comments and questions.
So I wanted to address one of the questions you had about the issue ofthrombosis and also this addresses Dr. Alam’s question of whether or not there are potentially deleterious and also beneficial effects of these platelet EVs.
So I think that this is very context dependent. And if we are aiming as using this as a therapeutic we have to be mindful of the fact that they do have pro-thrombotic effects.
They have phosphatidylserine on the surface. They activate Factors II and X. They have tissue factor, also, on the surface, GB2B and 3A. All of these taken together do, indeed, indicate that they have the capacity to be thrombotic.
In cancer research it has been shown really clearly that they are indicative of thrombotic events within cancer patients with increased platelet EVs in their circulation.
So with that in mind I think that this is where – this is the next step in our endeavors to understand this as a therapeutic in, for example, in ARDS or hemorrhagic shock-induced ARDS. Do we see microthrombi in the lung?
So to date, I think, I bet you, I’m sure that there is some issue with context and dose. And so that’s really what this requires is to understand therapeutic window, dose, and timing of delivery to really tease out whether there are deleterious effects so we should be mindful of that.
Another question that was brought up by Dr. Evans related to the variability in extracellular vesicles and also the ones that are not platelet-derived. Dr. Alam, you also alluded to this, too.
So in plasma, if you look at plasma it really actually depends on which donor you analyze the EV content from. Some donors have very high amounts, numbers of endothelial-derived microvesicles. Some donors have actually low EVs from endothelial cells and much higher from platelets.
Generally speaking, we’ve analyzed about ten donors across the board, what we’ve found is about 80 percent of the microvesicles are EVs in plasma are derived from platelets.
And so you know that’s actually a thought is can we actually start to sort out specific types of EVs. As you know, there are other EVs, for example from mesenchymal stem cells, that also circulate and these have very potent effects.
So, no, I actually do not think that the effects we are seeing are completely just derived from platelet EVs, Dr. Alam. But I actually think that there are other cell types that are in there. And that would be really a great interest in our future studies.
Another question that was asked was about what do we resuspend the EVs in, we spin them down and we re-suspend them in buffer, basically a phosphate buffered saline. We don’t put them into plasma. And then they’re directly transfused or infused intravascular.
I think that – yes. So I think that basically sums up. I hope I answered all the questions that were asked.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Byron Miyazawa, Department of Laboratory Medicine, University of California.
Alpa Trivedi, Department of Laboratory Medicine, University of California.
Padma Priya Togarrati, Blood Systems Research Institute, San Francisco, California.
Daniel Potter, Department of Laboratory Medicine, University of California.
Gyulnar Baimukanova, Blood Systems Research Institute, San Francisco, California.
Lindsay Vivona, Department of Laboratory Medicine, University of California.
Maximillian Lin, Department of Laboratory Medicine, University of California.
Ernesto Lopez, Department of Surgery, University of Texas Health Science Center at Houston.
Rachael Callcut, Department of Surgery, University of California San Francisco, San Francisco, California.
Amit K. Srivastava, Department of Pediatric Surgery, McGovern Medical School, University of Texas Health Science Center at Houston, Houston, Texas.
Lucy Z. Kornblith, Department of Surgery, University of California San Francisco, San Francisco, California.
Alexander T. Fields, Department of Surgery, University of California San Francisco, San Francisco, California.
Martin A. Schreiber, Department of Surgery, Oregon Health Science and University, Portland, Oregon..
Charles E. Wade, Department of Surgery, University of Texas Health Science Center at Houston.
John B. Holcomb, Department of Pediatric Surgery, McGovern Medical School, University of Texas Health Science Center at Houston, Houston, Texas.
Shibani Pati, Department of Laboratory Medicine, University of California.
REFERENCES
- 1.Hess JR, Lelkens CC, Holcomb JB, Scalea TM. Advances in military, field, and austere transfusion medicine in the last decade. Transfus Apher Sci. 2013;49:380–386. [DOI] [PubMed] [Google Scholar]
- 2.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368:1723–1730. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431–S437. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Fox EE, Wade CE. Mortality and ratio of blood products used in patients with severe trauma—reply. JAMA. 2015;313:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, et al. Injury severity and causes of death from operation iraqi freedom and operation enduring freedom: 2003–2004 versus 2006. J Trauma. 2008;64:S21–S26; discussion S26–27. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: The surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardenas JC, Zhang X, Fox EE, Cotton BA, Hess JR, Schreiber MA, Wade CE, Holcomb JB; PROPPR Study Group. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krailadsiri P, Seghatchian J, Williamson LM. Platelet storage lesion of WBC-reduced, pooled, buffy coat-derived platelet concentrates prepared in three in-process filter/storage bag combinations. Transfusion. 2001;41: 243–250. [DOI] [PubMed] [Google Scholar]
- 10.Holme S, Heaton WA, Courtright M. Platelet storage lesion in second-generation containers: correlation with platelet ATP levels. Vox Sang. 1987;53: 214–220. [DOI] [PubMed] [Google Scholar]
- 11.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30: 475–487. [DOI] [PubMed] [Google Scholar]
- 12.Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4°C and 22°C. Shock. 2014;41(Suppl 1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick GM, Cliff R, Tandon N. Thrombosomes: a platelet-derived hemostatic agent for control of noncompressible hemorrhage. Transfusion. 2013;53(Suppl 1):100S–106S. [DOI] [PubMed] [Google Scholar]
- 14.Bode AP, Fischer TH. Lyophilized platelets: fifty years in the making. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:125–133. [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006; Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 16.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34. [PubMed] [Google Scholar]
- 17.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6: 267–283. [DOI] [PubMed] [Google Scholar]
- 18.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. [DOI] [PubMed] [Google Scholar]
- 19.Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, et al. Applying extracellular vesicles based therapeutics in clinical trials — an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromato-graphically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglis H, Norris P, Danesh A. Techniques for the analysis of extracellular vesicles using flow cytometry. J Vis Exp. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013;2:e19671. doi: 10.3402/jev.v2i0.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matijevic N, Wang YW, Holcomb JB, Kozar R, Cardenas JC, Wade CE. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. J Extracell Vesicles. 2015;4: 29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens AP 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preusser C, Hung LH, Schneider T, Schreiner S, Hardt M, Moebus A, Santoso S, Bindereif A. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Pare A, Rousseau M, Naika GS, Levesque T, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3:24692. doi: 10.3402/jev.v3.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baimukanova G, Miyazawa B, Potter DR, Gibb SL, Keating S, Danesh A, Beyer A, Dayter Y, Bruhn R, Muench MO, et al. The effects of 22°C and 4°C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56(Suppl 1):S52–S64. [DOI] [PubMed] [Google Scholar]
- 30.Baimukanova G, Miyazawa B, Potter DR, Muench MO, Bruhn R, Gibb SL, Spinella PC, Cap AP, Cohen MJ, Pati S. Platelets regulate vascular endothelial stability: assessing the storage lesion and donor variability of apheresis platelets. Transfusion. 2016;56(Suppl 1):S65–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez E, Srivastava AK, Pati S, Holcomb JB, Wade CE. Platelet-derived microvesicles: a potential therapy for trauma-induced coagulopathy. Shock. 2018;49:243–248. [DOI] [PubMed] [Google Scholar]
- 32.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 33.Kannan K, Divers SG, Lurie AA, Chervenak R, Fukuda M, Holcombe RF. Cell surface expression oflysosome-associated membrane protein-2 (lamp2) and CD63 as markers of in vivo platelet activation in malignancy. Eur J Haematol. 1995;55:145–151. [DOI] [PubMed] [Google Scholar]
- 34.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molino M, Bainton DF, Hoxie JA, Coughlin SR, Brass LF. Thrombin receptors on human platelets. Initial localization and subsequent redistribution during platelet activation. J Biol Chem. 1997;272:6011–6017. [DOI] [PubMed] [Google Scholar]
- 37.Tao SC, Guo SC, Zhang CQ. Platelet-derived extracellular vesicles: an emerging therapeutic approach. Int J Biol Sci. 2017;13:828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuszynski GP, Rothman VL, Murphy A, Siegler K, Knudsen KA. Thrombospondin promotes platelet aggregation. Blood. 1988;72:109–115. [PubMed] [Google Scholar]
- 39.Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. [DOI] [PubMed] [Google Scholar]
- 41.Balvers K, Curry N, Kleinveld DJ, Boing AN, Nieuwland R, Goslings JC, Juffermans NP. Endogenous microparticles drive the proinflammatory host immune response in severely injured trauma patients. Shock. 2015;43: 317–321. [DOI] [PubMed] [Google Scholar]
- 42.Pati S, Potter DR, Baikamunova G, Farrell DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: factor concentrate vs. fTesh frozen plasma. J Trauma Acute Care Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 43.Potter DR, Baimukanova G, Keating SM, Deng X, Chu JA, Gibb SL, Peng Z, Muench MO, Fomin ME, Spinella PC, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg. 2015;78: S7–S17. [DOI] [PubMed] [Google Scholar]
- 44.Alexander M, Ramstead AG, Bauer KM, Lee SH, Runtsch MC, Wallace J, Huffaker TB, Larsen DK, Tolmachova T, Seabra MC, et al. Rab27-dependent exosome production inhibits chronic inflammation and enables acute responses to inflammatory stimuli. J Immunol. 2017;199:3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.