Abstract
Two recent Lancet and Lancet Oncology papers report that cancer patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have higher mortality rates. Common independent factors associated with increased risk of death were older age, history of smoking status, number of comorbidities, more advanced performance status, and active cancer.
Two recent Lancet and Lancet Oncology papers report that cancer patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have higher mortality rates. Common independent factors associated with increased risk of death were older age, history of smoking status, number of comorbidities, more advanced performance status, and active cancer.
Main Text
The disease course of cancer patients infected by SARS-CoV-2, which causes the illness designated as COVID-19, is phenotypically heterogeneous. Some patients suffer only mild or no symptoms, but on the other hand, some individuals develop very severe symptoms and can follow a severe clinical phenotype with the development of respiratory failure, cytokine release syndrome, and multi-organ failure (Liang et al., 2020; Miyashita et al., 2020; Anil et al., 2020). Subgroups of COVID-19 patients have been identified who appear to be at increased risk of extreme morbidity and mortality, including patients of advancing age, male gender (versus female), and those with co-morbidities such as hypertension, chronic lung disease, diabetes, and active cancer while receiving chemotherapy (Kuderer et al., 2020; Garassino et al., 2020). Since COVID-19 disease started to spread across the globe in early 2020, patients with a diagnosis of cancer were designated as a particularly vulnerable subgroup of the population. Cancer patients have been reported to be not only at increased risk of contracting SARS-CoV-2 infection but also of running a more severe disease course, with a higher proportion requiring higher levels of intensive care, having a more rapidly evolving disease, and with increased risk of death. As a consequence of an assumption of “COVID-19 vulnerable” patients, cancer patients (of any age, gender, or tumor subtype and stage) have been labeled as high risk from COVID-19 disease, and this has led to sweeping changes in cancer management for all cancer types over the last few months, including abbreviation of radiotherapy, switching from intravenous to oral chemotherapy regimens, and development of specific recommendations (ESMO). Are all cancer patients more vulnerable in case of SARS CoV-2 infection, and should we assess the individualised risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender? Do studies from the COVID-19 and Cancer Consortium (CCC19) and from the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) (Kuderer et al., 2020; Garassino et al., 2020) support sufficient evidence to perform an optimal risk assessment for any cancer patients? CCC-19 and TERAVOLT are large real-world datasets investigating risk for hospitalization and death in cancer patients. The CCC-19 population included patients with several types of malignancies (breast cancer, 21%; prostate cancer, 16%; gastrointestinal, 12%; thoracic cancers, 10%; hematological malignancies, 22%; and others, 19%); 45% of the patients had cancer in remission, 32% stable, and only 11% progressing. The overall mortality rate in the CCC-19 was 13% (121 out of 928 eligible patients). The TERAVOLT trial included 200 patients with thoracic cancers, 74% with stage IV disease, and the reported mortality rate was 33% (66 out of 152 hospitalized patients). In both studies, independent factors associated with increased mortality are older age, history of smoking status, number of comorbidities, more advanced performance status, and active cancer.
Although a global effort has been performed, there are many limitations in both studies. They are both cross-sectional studies. In a cross-sectional study, the investigator measures the outcome and the exposures in the study participants at the same time. These studies are also prone to certain biases, and we have to be careful about interpreting the associations and direction of associations from a cross-sectional survey. These studies can usually be conducted relatively faster and are inexpensive, but they are limited by multiple biases, including selection bias, recall bias, confounding by indication, and changes in practice and/or disease evolution. There is no propensity score adjustment, no control with non-cancer patients, and no stratification for stage and lines of previous therapies and other covariate adjustment.
Which are the open questions and the research priorities? First, in patients who died from Covid-19-associated respiratory failure, the histologic pattern in the peripheral lung is diffuse alveolar damage with perivascular T cell infiltration. The lungs from patients with Covid-19 showed distinctive vascular features, consisting of severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes. Histologic analysis of pulmonary vessels in patients with Covid-19 showed widespread thrombosis with microangiopathy (Ciceri et al., 2020; Varga et al., 2020; Ackermann et al., 2020) (Figure 1 ). In cancer patients, this process can also be sustained by cancer-induced thrombophilia. Second, cancer care prioritization and cancer care intensity should be adapted to the pandemic scenario (from 1 to 4 according to European Centre for Disease Prevention and Control [ECDC]), to local R0 index, and to health facilities resources. R0 is the measure used to track how many people, on average, will be infected for every person who has the disease and is the most internationally used index. R0 may also be locally used to shape prioritization of treatments and safety measures at local level. Possible R0 cut off levels are <0.5, 0.5–1, >1–2, >2–3, and >3. Important factors are the status of the viral outbreak, in terms of availability of hospital technical and human resources, as well as of on-site ICU ventilation capacities (Chowdhury et al., 2020). This may explain why in the TERAVOLT trial there is also higher mortality rate related to lower admission to ICU of patients with stage IV disease, who may be excluded at triage. In this context, should all cancer patients or patients who we consider at risk be tested for SARS-CoV-2 before hospital admittance for any surgical or medical treatment? It is imperative, in my opinion, that all cancer patients requiring inpatient admission with fever and upper respiratory symptoms or chest radiographical findings with suspected COVID-19 (new peripheral opacities) should be segregated and isolated and have a COVID-19 swab performed (unless done within the past 48 h). Third, longitudinal serological studies are urgently needed to determine the extent and duration of immunity to SARS-CoV-2 in cancer patients. We need to develop an epidemiological model to estimate the cumulative incidence of COVID-19 within a specific time frame and pandemic scenario for multiple solid tumors according to data we have. We need to identify viral, environmental, and immunologic factors that in combination will determine the dynamics of SARS-CoV-2 in cancer patients. Finally, we need to determine the COVID-19 morbidity and mortality according to treatment (chemotherapy, targeted therapy, immune-checkpoint blockade). Overall, the most important message is to allocate specific health facilities not only for treatment of suspected and confirmed cases of an epidemic disease but also for treatment of cancer patients. Cancer treatment prioritization should balance interventions based on the magnitude of benefit and clinical setting and stage of disease. For cancer patients in the curative setting, regimens and doses of adjuvant and neoadjuvant systemic therapies should be followed, surgery, if curative, should not be delayed, and in a multidisciplinary discussion, risk/benefit analysis and discussion with the patient should be done.
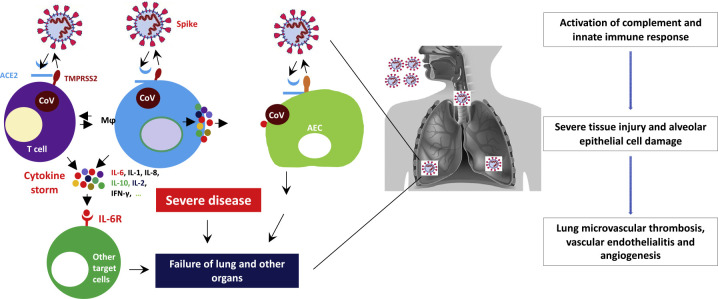
Figure 1.
Microvascular COVID-19 Lung Vessels Obstructive Thromboinflammatory Syndrome (MicroCLOTS) as Potential Mechanism of Death in Cancer Patients
SARS-CoV-2 enters target cells through the cell surface receptor angiotensin-converting enzyme 2 (ACE2) or through transmembrane protease, serine 2 (TMPRSS2), which is expressed on the surface of lung epithelial cells. Viral particles may elicit innate immune responses of the host through different mechanisms, such as the activation of alveolar macrophages (MƟ) and of the complement cascade. The activation of complement cascade not only directly causes endothelial damage but further recruits leucocytes for a massive local release of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, and interferon-γ. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS) is one of the most important mechanisms of death for cancer patients, who have increased risk of developing venous thromboembolism (VTE). This progressive endothelial thromboinflammatory syndrome may also involve the microvascular bed of the brain and other vital organs, leading to multiple organ failure and death.
ACE2, angiotensin-converting enzyme 2; IL-6, interleukin 6; IL-1, interleukin 1; IL-8, interleukin 8; IL-10, interleukin 10; IL-2, interleukin 2; IFN-γ, interferon gamma; IL6 R, interleukin 6 receptor; TMPRSS2, transmembrane protease, serine 2; AEC, alveolar epithelial cell; CoV, coronavirus.
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil I., Arnold R., Benkwitz-Beford S., Branford S., Campton N., Cazier J.-B., Cheng V., Curley H., D’Costa J., Edmondson A., UK Coronavirus Cancer Monitoring Project team The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020;21:622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Heng K., Shawon M.S.R., Goh G., Okonofua D., Ochoa-Rosales C., Gonzalez-Jaramillo V., Bhuiya A., Reidpath D., Prathapan S., Global Dynamic Interventions Strategies for COVID-19 Collaborative Group Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur. J. Epidemiol. 2020;35:389–399. doi: 10.1007/s10654-020-00649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESMO Cancer Patient Management During the COVID-19 Pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
- Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D’Angelo A., De Cobelli F., Rovere-Querini P. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. Published online April 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C., TERAVOLT investigators COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., de Lima Lopes G. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet. 2020;395:P1907–P1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D., Cruz C. Do Patients with Cancer Have a Poorer Prognosis of COVID-19? An Experience in New York City. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.006. Published online April 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]