Abstract
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is devastative to the humankind for which neither vaccines nor precise therapeutic molecules for treatment are identified. The search for new drugs and repurposing of existing drugs are being performed; however, at the same time, research on plants to identify novel therapeutic compounds or testing the existing ones is progressing at a slower phase. In this context, genomics and biotechnology offer various tools and strategies to manipulate plants for producing those complex biopharmaceutical products. This review enumerates the scope for research on plant-based molecules for their potential application in treating SARS-CoV-2 infection. Strategies to edit gene and genome, overexpression and silencing approaches, and molecular breeding for producing target biomolecules in the plant system are discussed in detail. Altogether, the present review provides a roadmap for expediting research on using plants as a novel source of active biomolecules having therapeutic applications.
Keywords: SARS-CoV-2, Coronavirus, Genomics, Genetic manipulation, Therapeutics, Plant-based drugs
Highlights
-
•
Plants are a source of biomolecules that have application in treatment of SARS-CoV-2.
-
•
Identifying active biomolecules for therapeutic purposes is not adequately performed.
-
•
High-throughput strategies promote large-scale screening of plant-based drugs.
-
•
Plant biotechnology facilitates the production of complex biopharmaceutical products.
1. Introduction
Advancement of civilization at a rapid pace faces the spread of complex diseases and infections that threatens the human race. Among the diseases, several pandemics have wiped a predominant human population throughout history. To note a few, flu, plague, pox, yellow fever, malaria, leprosy, tuberculosis, measles, dengue, HIV/AIDS, H1N1, SARS, Ebola, and MERS were disastrous. Among these, the present-day COVID-19 (caused by Severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) is potentially contagious and lethal that has forced almost all the countries to practice a strict lockdown for minimizing the spread [69]. The virulence of this virus, along with the evolution of different strains, has urged the scientific community to identify vaccines, therapeutic drugs, and diagnostic methods for rapid detection, treatment, and immunization of the human population. Though progress has been made in the development of biological- and chemical-based strategies to diagnose the disease, there is no drug or vaccine invented so far to treat the infected patients effectually [55]. The appearance of disease symptoms at a late stage and the issue of asymptomatic infections have constrained the diagnosis procedures; however, testing the entire population for the virus infection holds the key to identify the infected ones, isolate and treat them in a suitable way (WHO, 2020). In case of treatment of infected patients, the use of existing antiviral drugs is prevalent, and also, repurposing of drugs is suggested for testing and application. Currently, antiviral drugs such as Lopinavir/Ritonavir and Remdesivir are predominantly used in the treatment. These commercially synthesized molecules inhibit the virus replication either by regulating the ion channel transport or inhibiting the serine protease activity [16,97]. However, the search for novel compounds that could interfere with viral replication, assembly, and spread within the human system is in progress. Devising new molecules through computational and integrative approaches that target one or the other process of virus infection and spread could be an amenable strategy. Still, the chemical synthesis of such a molecule is time-consuming and labor-intensive. Screening of existing molecules for repurposing is already being carried out, but at the same time, studying the plants for novel phytochemicals that possess potential antiviral activity is largely ignored.
Large-scale screening of plants for active antiviral compounds dates to 1952, where around 288 plant extracts were tested for their activity against influenza A virus [11]. Later, Debiaggi et al. [19] had shown the antiviral effect of Chamaecyparis lawsoniana on herpes simplex virus type 2. Similarly, Serkedjieva [78] identified the antiviral effect of Geranium sanguineum extract against influenza A virus, and Asres and Bucar [3] showed anti-HIV activity of Combretum mole. An aqueous extract of Agrimonia eupatoria and root extract of Boehmeria nivea were reported to inhibit hepatitis B virus [32,47]. Kotwal et al. [43] had demonstrated that an acidic extract of Trifolium species could show a broad-spectrum antiviral activity, including anti-SARS activity. Thus, examining natural antiviral compounds from plants gains momentum in the present scenario. These plant-based therapeutic drugs have several advantages as compared to synthetic molecules. Naturally available biomolecules are safe, economical, and have minimal side effects. Further, if a plant-based compound is found to regulate the COVID-19 infection, it can facilitate an immediate use in the treatment regime after obtaining necessary ethical clearances. However, these compounds might not be stable or produced at minimal levels since they might play limited roles in planta. A holistic approach is required to identify such potential compounds, study the biosynthesis pathway, analyze the genes underlying those pathways, and finetuning them using genomics and biotechnological interventions could promote the use of plants as a source and bio-manufacturer of antiviral compounds in large-scale. While mainstreaming plant-based research to identify biomolecules having anti-SARS-CoV-2 activity itself is in a nascent stage, exploiting the potential plants as biofactories is a long way to go. However, the advent of tools and techniques in plant molecular biology and biotechnology could expedite this process provided a roadmap need to be established towards achieving these targets. Given this, the present review enumerates the plant-based compounds that have been identified and characterized so far for treating viral infections with emphasis on coronavirus strains. The strategies for the identification of additional compounds, implementing omics tools for the over-production of those compounds, customization of biomolecules, and scaling-up of the process for large-scale production have been elaborated. Altogether, the review provides a roadmap for extrapolating the biomolecule repertoire of plants and use genomics and biotechnological approaches for their production and effective use in SARS-CoV-2 treatments.
2. Plant-based biomolecules with antiviral activities
Presently, traditional medicines from different parts of the world have been studied for their therapeutic effect against SARS-CoV-2. Traditional medicines-based defense methodology has been used against different human viruses, including SARS-CoV, Ebola, and Zika viruses [101]. Recently, Sehailia and Chemat [77] had compared the mechanism of infection of SARS-CoV-2 and malaria plasmodium and found that both the pathogens infect the lungs to cause the crystallization of carbon dioxide. Based on this information, artemisinin molecule (sesquiterpene lactone), isolated from Artemisia annua, has been proposed to be used in the treatment of SARS-CoV-2 patients. Artemisinin is popularly used in treating malaria; however, it has also been tested for its activity against different viral diseases, including MERS-CoV and SARS-CoV [18,27]. Currently, WHO has backed the clinical trials on Artemisia annua for its use in treating COVID-19, and collaborative research in this direction is being performed at Max-Planck research center, Germany and Mateon Therapeutics, California. The use of medicinal plants for treating infectious diseases is found to be associated with the tradition and custom of several populations around the globe. For example, Traditional Chinese Medicine (TCM) is one of the well-studied and documented practices followed in China that has been reported to cure many diseases or infections, including SARS-CoV [51,89,104]. Approximately 85% of COVID-19 patients were treated with TCM that might act due to a close homology between SARS-CoV-2 and SARS-CoV [101]. These plants could possess active biomolecules that regulate virus accumulation by restricting their multiplication. For example, flavonoids (herbacetin, isobavaschalcone, rhoifolin, pectolinarin, quercetin 3-β-D-glucoside, and epigallocatechin gallate) were found inhibit the enzymatic activity of one of the viral proteases; MERS-CoV 3CL protease and myricetin and scutellarein have the potential to regulate the helices activity of nsP13 (SARS-CoV helicase protein). Yang et al. [101] had reported that at least fifteen clinical trials are underway to test the efficacy of TCM in treating SARS-CoV-2 infection.
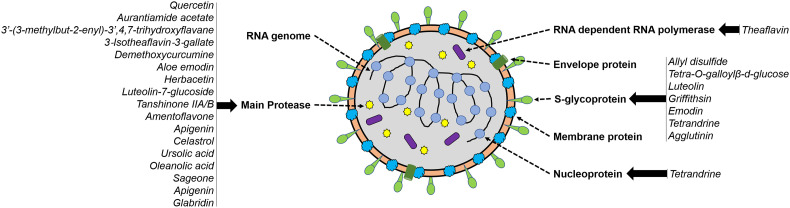
Countries other than China focus very less on their traditional medicinal plants that could be a potential source of active antiviral molecules. For example, India has a long record of using plants in treating virus-induced respiratory diseases; however, further research towards pinpointing the molecules having roles in antiviral activity is limited. A few Indian collections of medicinal herbs were reported to demonstrate anti-inflammatory and antioxidant properties and can be used for the treatment of COVID-19 [92]. For instance, Vitex trifolia and Sphaeranthus indicus, the medicinal plants from Southern India, have been reported to target cytokines, thus reducing the inflammation during respiratory diseases, including SARS-CoV at a concentration of 400 ng/mL [86]. Similarly, several species used in the daily diet contain metabolites such as curcumin and quercetin, which were found to interact with proteases of SARS-CoV-2 [76]. Further, Unani medicine, having an antiviral effect against measles and similar virus disease, has been proposed to provide an inhibitory effect on SARS-CoV-2 [62]. The antiviral compounds reported so far in plant species are summarized in Table 1 , and their target sites of action on SARS-CoV-2 are shown in Fig. 1 . In addition to antiviral activity, plant extracts also possess antioxidant, antipyretic, anti-asthmatic, bronchodilator, expectorant, analgesic, and anti-inflammatory activities that have application in the treatment regime of respiratory diseases (Table 2 ). Several herbs have been reported to possess immune-boosting properties, and this includes Ocimum tenuiflorum, Zingiber officinale, Trigonella foenum-graecum, Allium sativum, and Curcuma longa. However, there is no comprehensive study performed to identify the active biomolecules that interact with the immune system of the human body to enhance the resistance to diseases. The prevalence of COVID-19 as a pandemic has mandated research and development for identifying vaccines, therapeutic and diagnostic molecules; however, such searching for those biomolecules in plants is not being performed at the right pace. In case of plant-based vaccines, research is appropriately being carried out to express novel virus-like particles (VLP) in plants that could serve as potential antigens for eliciting immune responses. The prime advantages of plant-based vaccines are their purity (free from human pathogens), low cost for production, transportation, and storage [70]. In case of diagnostic reagents, plants serve as a suitable host for expressing viral proteins to develop assay kits for effective detection and diagnosis of SARS-CoV-2 (reviewed in [10]). Given this, the review explicitly covers the identification and isolation of plant-based therapeutic molecules and the role of genomics and biotechnology in reaping the maximum benefit out of plants for effective treatment of potential diseases.
Table 1.
Plant-based compounds reported to possess antiviral activity.
| Plant | Compound | Target | Mode of action | Reference |
|---|---|---|---|---|
| Allium sativum | Allyl disulfide | S protein | ACE2 receptor inhibitor | [87]. |
| Anethum graveolens | Quercetin | main protease (Mpro) | Virus replication | [37] |
| Artemisia annua | Aurantiamide acetate | Inhibition of CoV protease | Virus replication | [63] |
| Broussonetia papyrifera | 3′-(3-methylbut-2-enyl)-3′,4,7-trihydroxyflavane | Inhibition of CoV protease | Virus replication | [66] |
| Camellia sinensis | 3-Isotheaflavin-3-gallate | main protease (Mpro) | Virus replication | [12] |
| Theaflavin | RNA-dependent RNA polymerase | Virus replication | [54] | |
| Cinnamomi sp. | Procyanidin A2 | – | Inhibition of virus entry | [108] |
| Curcuma longa | Demethoxycurcumine | main protease (Mpro) | Virus replication | [37] |
| Dioscoreae Rhizoma | – | – | Inhibition of virus growth | [99] |
| Galla chinensis | Tetra-O-galloylβ-d-glucose | S protein | ACE2 receptor inhibitor | [102] |
| Luteolin | Spike protein | Virus entry | ||
| Griffithsia sp. | Griffithsin | Spike protein | Inhibition of virus entry | [59] |
| Isatis indigotica | Aloe emodin | main protease (Mpro) | Virus replication | [49] |
| Linum usitatissimum | Herbacetin | main protease (Mpro) | Virus replication | [36] |
| Lycoris radiata | Lycorine | – | Inhibited cell divison | -[79] |
| Malus domestica | Apigenin | main protease (Mpro) | Inhibited virus replication | [76] |
|
Olea europaea L Averrhoa belimbi Capsicum annum Allium fistulosum |
Luteolin-7-glucoside | main protease (Mpro) | Virus replication | [37] |
| Rheum officinale | Emodin | Inhibited binding of S protein to ACE2 | Virus entry | [88] |
| Salvia miltiorrhiza | Tanshinone IIA/B | Inhibition of CoV protease | Virus replication | [65] |
| Salvia officinalis | Safficinolide | main protease (Mpro) | Inhibited virus replication | [76] |
| Stephania tetrandra | Tetrandrine | S and N protein | Virus replication | [40] |
| Torreya nucifera | Amentoflavone, Apigenin | main protease (Mpro) | Virus replication | [109] |
| Tripterygium regelii | Celastrol | Inhibition of CoV protease | Virus replication | [74] |
| Urtica dioica | agglutinin | Spike protein | Inhibition of virus entry | [45] |
‘-‘No information available.
Fig. 1.
Structural proteins of SARS-CoV-2 and the potential plant-based biomolecules interacting with these proteins. The mode of action of these biomolecules are given in Table 1, Table 2.
Table 2.
Potential plant-based biomolecules useful for therapeutic applications to treat SARS-CoV-2.
| Compound | Source | Class | Mode of action | Reference |
|---|---|---|---|---|
| Anti-asthmatic compounds | ||||
| 1,8-Cineol | Eucalyptus globulus | Monoterpene | NF-κB p65 translocation to nucleus is inhibited thus, hampering NF-κB mediated transcription. | [24] |
| 3-Methoxy-catalposide | Pseudolysimachion rotundum | Iridoid glycoside | Inhibitory effect on lipopolysaccharide stimulated RAW264.7 macrophages. | [73] |
| 7-Glucuronic acid-5,6-dihydroxyflavone | Scutellaria baicalensis | Flavonoid | Inhibitor of phosphodiesterase 4A and 4B. Downregulates the expression of TNF-α. | [67] |
| 5,7-dihydroxyflavon | Passiflora caerulea | Flavonoid | Suppression of mast cell mediated release of pro-inflammatory cytokines. | [6] |
| Crocetin | Crocus sativus | Carotenoid | Asthma mitigation by activation of Foxp3 through TIPE2 in asthma associated Treg cells. | [20] |
| Curcumin | Curcuma longa | Polyphenol | Inhibition of Notch1–GATA3 signalling pathway preventing the development of allergic airway inflammation. | [15] |
| Diallyl-disulfide | Zingiber officinale | Organosulphur | Suppression of airway inflammation by activation of Nrf-2/HO-1 pathway and downregulation of NF-kappaB. | [82] |
| D-α-tocopheryl acetate | Glycine max | Vitamin | Modulates atopic asthma and inhibits oxidative stress. | [31] |
| Ellagic acid | Rubus fruticosus | Polyphenol | Inhibition of NF-κB mediated transcription | [106] |
| 1,3,8-Trihydroxy-6-methylanthraquinone | Rheum palmatum | Anthraquinone | Inhibition of NF-κB signalling pathway | [83] |
| Homoegonol | Styrax japonica | Lignan | Reduction in the number of inflammatory cells and Th2 cytokines | [81] |
| Kaempferol-O-rhamnoside | Camellia sinensis | Flavonoid | Reduction in the number of inflammatory cells and Th2 cytokines | [17] |
| L-Theanine | Camellia sinensis | Amino acid | NF-κB and MMP-9 levels are reduced leading to anti-inflammatory activity | [33] |
| Luteolin | Perilla frutescens | Flavonoid | Airway mucus accumulation is inhibited by inhibition of GABAergic system | [80] |
| Mangosteen | Garcinia mangostana | Xanthone | Inhibition of cytokine production and histamine release | [35] |
| Naringin | Citrus paradisi | Flavone | Induction of calcium signalling leading to bronchoconstriction inhibition | [98] |
| Oxymatrine | Sophora flavescens | Alkaloid | Anti-asthmatic effect by regulation of CD-40 signalling | [105] |
| Piperine | Piper nigrum | Alkaloid | Inhibition of Th2 and mast cell hyperactivity | [9] |
| Antipyretic compounds | ||||
| Acacetin | Potentilla evestita | Flavone | Probably inhibits activity of prostaglandins | [72] |
| Viscosine | Dodonaea viscosa | Flavonoid | Inhibition of cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1) | [61] |
| 16-hydroxy betulinic acid | Mikania cordata | Triterpenoid | Unknown | [84] |
| 6-(3-carboxybut-2-enyl)-7-hydroxycoumarin | Peucedanum ostruthium | Flavonoid | Inhibitor of cyclooxygenase and 5-lipoxygenase activity involved in prostaglandin synthesis | [29] |
| Linarin | Buddleia cordata | Flavonoid | Unknown | [58] |
| 6-methoxy-7-hydroxy-α-dunnione | Sinningia canescens | Napthaquinone | Reduction in lipopolysaccharide-induced fever | [53] |
| Mangiferin | Mangifera indica | Xanthone | Synthesis of TNF-α is inhibited which acts as a pyrogen | [85] |
| 2-hydroxy-1,4-naphthaquinone | Lawsonia inermis | Napthaquinone | Unknown | [2] |
| Usnic Acid and Diffractaic Acid | Usnea diffracta | Dibenzofuran and | Unknown | [64] |
| Artemisinin | Artemisia annua | Sesquiterpene | Unknown | [63] |
| Neochlorogenic Acid | Hibiscus sabdariffa | Polyphenol | Inhibition of lipopolysaccharide-induced fever in BV2 microglial cells | [41] |
| Phenacetin | Bursera grandifolia | Acetamide | Unknown | [91] |
| Analgesic compounds | ||||
| Aspirin | Spiraea ulmaria | Acetylsalicyclic acid | Inhibitor of cyclooxygenase 1 and cyclooxygenase 2 | [90] |
| Morphine | Papaver somniferum | Opiate alkaloid | Analgesic effect by binding to mu opoid receptors present in the central and peripheral nervous system cells | [48] |
| Codeine | Papaver somniferum | Opiate alkaloid | Similar mode of action to morphine. Codeine is metabolised to morphine in animal body. | [8] |
| Thebaine | Papaver somniferum | Opiate alkaloid | Similar mode of action to morphine. Codeine is metabolised to morphine in animal body. | [42] |
| Salvinorin A | Salvia divinorum | Diterpenoid | Binds to kappa opoid receptors present in central and peripheral nervous system leading to analgesic effect | [68] |
| Menthol | Mentha spicata | Terpene | Binds to kappa opoid receptors present in central and peripheral nervous system leading to analgesic effect | [23] |
| Pawhuskin A | Dalea purpurea | Stillbene | Acts as opoid antagonist and binds selectively to kappa receptors | [28] |
| Cocaine | Erythroxylum coca | Tropane alkaloid | Acts supraspinally in a dopamine mediated and non-opiate manner to produce analgesic effect | [50] |
| Tetrahydrocannabinol | Cannabis sativa | Cannabinoid | Analgesia by inhibition of release of neurotransmitters and neuropeptides from nerve endings. | [93] |
| Bronchodilation compounds | ||||
| Emodin | Folium Sennae | Anthraquinone | Inhibition of acetylcholine mediated contraction of airway smooth muscle | [71] |
| Curcumin | Curcuma longa | Polyphenol | Relaxation of tracheal smooth muscles contraction mediated by KCl. Regulation involves calcium channel blocking and potassium channel opening | [22] |
| Berberine | Berberis aristata | Alkaloid | Inhibition of histamine receptors, cyclooxygenase pathway and nitric oxide which are involved in bronchoconstriction | [75] |
| 9-octadecenamide | Anacardium occidentale | Amide derivative of fatty acid | Inhibition of histamine mediated bronchoconstriction | [5] |
| Cyclomicrobuxine and its derivatives | Buxus papillosa | Steroidal alkaloids | Inhibition of calcium channels involved in bronchoconstriction | [38] |
| Citral | Zingiber officinale | Terpenoid | Bronchodialation by regulation of β-adrenergic receptor | [57] |
| Expectorant compounds | ||||
| chlorogenic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid | Tussilago farfara | Caffeoylquinic acids | Unknown | [100] |
| Liquiritin apioside, liquiritigenin and liquiritin | Glycyrrhiza uralensis | Flavonoid | Unknown | [44] |
| Caffeoylquinic acids, astersaponins, and aster peptides | Aster tataricus | Caffeoylquinic acid, saponin and peptide | Unknown | [103] |
| Imperialine, imperialine-β-N-oxide, isoverticine, and isoverticine-β-N-oxide | Fritillaria wabuensis | Alkaloids | Unknown | [94] |
| imperialine, chuanbeinone, verticinone and verticine | Fritillariae cirrhosae | Alkaloids | Unknown | [95] |
| Vasicine, deoxyvasicine and vasicinone | Peganum harmala | Quinazoline alkaloids | Unknown | [52] |
3. Identification of novel therapeutic biomolecules in plants
Plants could serve as a reservoir of potential drugs and therapeutic molecules, and an integrated approach is required to identify and characterize those molecules. Proteomic and metabolomic profiling using high-throughput platforms are a boon for large-scale analysis of plant extracts to identify the biomolecular composition of those extracts. A comprehensive review of literature that exists in use within the community or population can help to identify the potential plants that can be targeted for metabolic screening. Predominantly, metabolites possess therapeutic properties than the simple protein molecules which are reported to be involved in cellular and biological processes of plants. Sampling of plants at different stages of development, preparation of tissue-specific extracts (using different solvents), and analysis of those extracts using Gas and/or Liquid Chromatography-Mass spectrometry can identify metabolites and other volatile compounds present in the extract. High-Performance Thin-Layer Chromatography can assist in fingerprinting of secondary metabolites and separating those metabolites at high-resolution. Computational tools coupled with these platforms, aid in the identification of the metabolites, whose chemical structure can be resolved at high-resolution using several available in silico approaches. For screening the efficacy of these biomolecules and studying the potent inhibitors against coronavirus, computer-aided drug design (CADD) serves as a reliable approach [96,107] as it facilitates a multidimensional study of molecular interactions between putative anti-COVID-19 compounds and target proteins. This approach has suggested the interaction of SARS-CoV-2 encoded proteins with different phytochemicals. 3C-like protease (3CLpro), also known as Main protease (Mpro) is an attractive target for anti-CoV drug design. It is a cysteine protease and is responsible for cleaving the replicase polypeptide into various functional proteins and essential for virus multiplication. Phenolic compounds such as coumarin, flavones [39], baicalin, cyanidin 3-glucoside, and α-ketoamide-11r [34] have structural similarity with the protease and might be the potential and safer inhibitors against the SARS-CoV-2. Apart from this, the Moroccan Medicinal plants containing Crocin, Digitoxigenin, and β-Eudesmol were projected as COVID-19 inhibitors based on the computational investigation with a benefit of oral intake [1]. Phytochemicals such as Belachinal, Macaflavanone E, and Vibsanol B have been found to restrict the formation of ion channels by oligomerization of ‘SARS-CoV2 E', thus inhibiting the virus pathogenesis [25].
The interaction between Spike (S) Glycoprotein of SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2) receptor is crucial for the entry of SARS-CoV-2 into the human alveolar epithelial cells. Different plant-derived lectins such as Griffithsin and Urtica dioica agglutinin lectin interact with S protein and inhibit the binding onto the host cell [45,59]. Simultaneously, phytoestrogens, especially Diadiazin, Genistein, Formontein, and Biochanin A were found to have a high affinity towards the substrate-binding domain β (SBDβ) of Heat Shock Protein A5 (HSPA5). It is the host-cell receptor that interacts with S protein of COVID-19 and leads to the entry of the pathogen into the host cell. These phytoestrogens have been reported to act as competitive inhibitors against Spike protein by binding onto the active site of HSPA5 [21]. Recently, Withania somnifera compound, Withanone (Wi-N) was found to weaken the binding between ACE2-RBD complex and was thus proposed to control the virus entry into the host cell [7]. Kumar et al. [46] had also shown that Wi-N and caffeic acid phenethyl ester of W. somnifera interact with the highly conserved protease, Mpro of SARS-CoV-2. These reports justify that plant-based anti-COVID drugs could save time and cost for designing/developing new therapeutic molecules; however, large-scale screening of plants for identifying such potential biomolecules is imperative. Such compounds once identified will be processed to the further steps for laboratory trials and clinical trials followed by large-scale production, purification, and application at the end-user level.
4. Genetics and genomics approaches for the production of therapeutic biomolecules
4.1. Forward and reverse genetics approaches
Plant biotechnology offers a broad spectrum of production strategies at different levels, including whole plant, tissue, and cell. Hairy roots and cell suspension cultures facilitate the synthesis of biomolecules at large-scale [26]. In addition, advances in genetics and genomics had also facilitated the manipulation of genes and pathways underlying the biosynthesis of therapeutic molecules. Numerous approaches for genetic manipulation of genes and genomes are available in plants, including stable transformation (transgenics and transplastomics), transient and inducible expression systems, gene silencing approaches (knockdown and knockout), and genome editing methods. A transient expression for vaccine production is beneficial over stable transgenics as it saves time and expedites the large-scale manufacturing in pandemic situations. Fig. 2 illustrates how genomics and biotechnology could intervene in producing plant-based drugs. Next-generation sequencing provides comprehensive information about the genes, non-coding regions, and regulatory elements present in the genome, which enables rapid identification of the genes involved in the biosynthesis of therapeutic molecules. Forward genetics approach of estimating the biomolecule content (or large-scale metabolite profiling) in a given population followed by genotyping (genotyping-by-sequencing, double digest restriction-site associated DNA sequencing, whole-genome resequencing, etc.) will identify the genomic regions regulating the biosynthesis of individual molecules. These genomic regions (genes/alleles/QTLs) can be effectually used in genomics-assisted breeding for developing elite lines producing higher levels of target molecules. On the other hand, gene cloning enables the isolation and molecular characterization of target genes encoding for active biomolecules that can be transformed into a different plant system (Nicotiana, for example, is a widely used system for plant-based drugs) for the ease of expression, optimization of production and purification. For instance, N. benthamiana is now being used as an efficient system for expressing VLP of SARS-CoV-2 to produce a plant-based vaccine. Similarly, these heterologous systems can be used to produce the desired biomolecules that could be isolated and purified for further downstream approaches. A few metabolites could be tissue- or development-specific, and therefore, finetuning their expression throughout the life cycle of the respective plant is imperative to ensure a maximum harvest. For example, β–Eudesmol has been found to have antibacterial and antiviral properties; however, its usage as an antiviral drug is limited due to its low level (2.39%) of production in Lauris nobilis [4]. Promoter cloning or manipulation of cis-regulatory elements in the promoter regions could provide an amenable solution to this issue. CRISPR/Cas9 approach can be deployed to edit the cis-elements present upstream to the gene(s) for ensuring its expression throughout the life cycle of the target plant. Genetic elements regulating post-translational events acting on the therapeutic biomolecule of interest should also be studied.
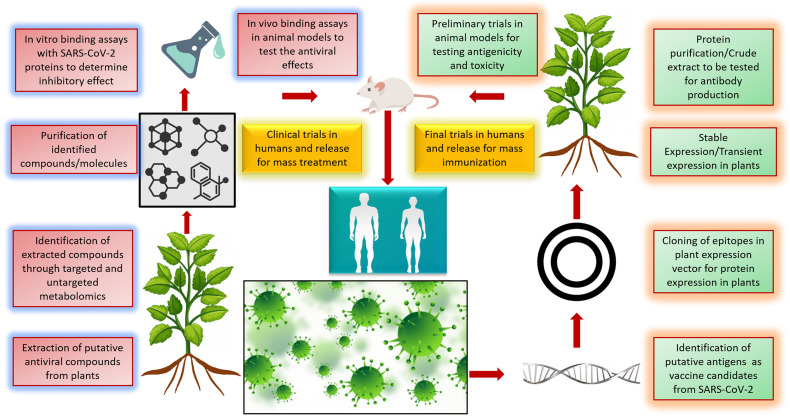
Fig. 2.
Different approaches for using plant-based biomolecules against SARS-CoV-2. Plants are a store house of active metabolites, and identification of these metabolites through targeted and untargeted metabolomics is important. These metabolites can be tested for their antiviral activity and released for treating viral diseases. On the other hand, plant based edible vaccines can be synthesized by expressing viral epitopes in plants. These vaccines are easy to store and propagate. (Image created using freepik.com).
4.2. Genome editing and overexpression systems
CRISPR/Cas9 method enables precise editing of genes, particularly this applies to knock out the enzymes that utilize the target biomolecule as a precursor for further processing and to facilitate rate-limiting processes for over-production of desired metabolites. Transient approaches, including RNA interference or virus-induced gene silencing, could also assist in finetuning the biosynthetic machinery to achieve a higher level of production. Stable transformation of genes and overexpression in the plant system could be achieved through several approaches existing for genetic transformation. Agrobacterium-mediated transformation is one such reliable approach where the gene of interest is cloned into the Ti-plasmid and transferred to a plant for integration into the genome [56]. This approach has been widely used in crop improvement for stress tolerance and agronomic traits. Given the broader application, this could be easily adapted for producing target biomolecules in diverse plant systems, where further downstream processing and purification is easy and straightforward. For instance, extracts of Chinese medicinal plants, Panax ginseng and Magnolia officinalis have anti-inflammatory properties, but since they are endangered species, their use in therapeutics is restricted. In such a case, studying the biosynthetic pathways and engineering them into a model plant species such as maize, tomato, rice, and tobacco reduces the pressure on parent medicinal plants [60] and provides the key to achieve production of beneficial compounds. Some natural compounds may require chemical modifications to increase their potency so that they can be used as a therapeutic drug. For example, a naturally occurring saponin, glycyrrhizin, was found to inhibit the replication of coronavirus, but modifying its glycoside chain enhances the antiviral activity by ten folds [30]. Similarly, increased antiviral activity was observed for tomentins and quercetin-7-rhamnoside as compared to their non-modified precursors [13,14]. These reports accentuate that biotechnology can also bridge the gap between the identification of naturally derived compound(s) and their usage as the therapeutic drug with a reduced timeline and increased efficacy. Also, the administration of plant-based drugs either through topical application or oral intake reduces the stringencies associated with ultra-high purification and storage issues. This will simultaneously reduce manufacturing as well as downstream processing costs.
5. Conclusions and future perspectives
Plants are primarily under-studied for their use in therapeutic purposes for treating infectious diseases; however, the traditional medicines have taken complete advantage of the entire plant kingdom. The gap between conventional treatment using herbs and extracts and the knowledge on the bioactive compounds present in those plant extracts, as well as their mode of action leading to disease recovery, needs to be bridged. Advances in science and advent of next-generation scientific research and discovery had delivered several tools, techniques, approaches, and strategies that can be effectually used in dissecting the metabolomic profile of plant species to identify the potential compounds that could possess anti-SARS-CoV-2 activities. Computational methods, including molecular modeling, docking, structure analysis, etc. can assist in performing experiments and validation of results virtually before undertaking further in vitro and in vivo studies. The intervention of genomics and biotechnological approaches to modulate the genes underlying biosynthesis and accumulation of these therapeutic biomolecules gains importance in the current scenario (Fig. 3 ). The identification of unique compounds present in plants may be laborious, but once identified, screening the compounds for therapeutic applications in treating COVID-19 infected patients, devising appropriate strategies for optimized production and purification of such compounds, and pursuing further validation studies in the laboratory and clinical trials may be expedited keeping in view the eco-friendliness and safety aspects of plant-based drugs. Also, the availability of a repository of plant-based therapeutic biomolecules will play an important role in confronting future health emergencies.
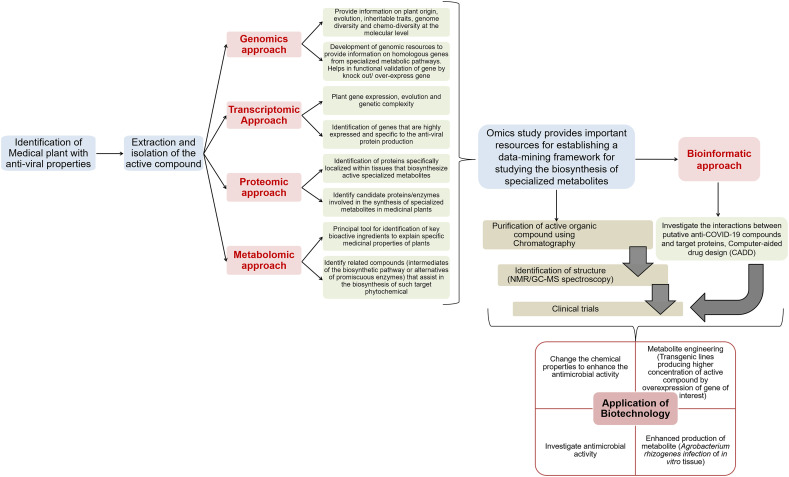
Fig. 3.
Schematic representation of biotechnology and omics-based strategies for profiling and application of plant-based antiviral phytochemicals against COVID-19. It includes identification of the plant encoding the active molecule, purification and evaluation for its role against SARS-CoV2. The Omics-based approach helps in recognition of different biological components associated with the pathway of phytochemical synthesis. Initially, Genomic approach is used to detect the gene coding for the active molecule. This information can be used to identify the homologous genes from other plant species and for functional characterization of the gene. The transcriptomic and proteomic approach depicts the active pathways in the plant associated with the molecule production. The metabolomic approach is the primary tool for detecting the main bioactive compound and the biosynthetic pathway regulating its synthesis in the plant. The metabolite or protein identified by integration of these approaches is studied for its role against SARS-CoV2. This is achieved either by computational/bioinformatics-based docking between the active compound and virus encoded proteins followed by clinical trials or by purification of the phytochemical through chromatography followed by its clinical trial. Further, by applying biotechnology the concentration of these metabolites can be increased significantly. It includes developing stable transformations in plants or by expressing the synthesis machinery into a heterologous system or using transformed root cultures or through chemical modifications in the active molecule to increase its potency as an antiviral compound.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors' work in this area is supported by J.C. Bose National Fellowship Grant of Department of Science and Technology, Government of India (File No.: JCB/2018/000001). N.S. acknowledges the SERB Women Excellence Award from Science and Engineering Research Board, Govt. of India (File No. WEA/2020/000004). A.P. acknowledges the Council for Scientific and Industrial Research, Govt. of India for research fellowship. Authors are thankful to DBT-eLibrary Consortium (DeLCON) for providing access to e-resources.
References
- 1.Aanouz I., Belhassan A., El-Khatabi K., Lakhlifi T., El-Ldrissi M., Bouachrine M. Moroccan medicinal plants as inhibitors against SARS-CoV-2 main protease: computational investigations. J. Biomol. Struct. Dyn. 2020;2020:1–9. doi: 10.1080/07391102.2020.1758790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali B.H., Bashir A.K., Tanira M.O.M. Anti-inflammatory, antipyretic, and analgesic effects of Lawsonia inermis L. (henna) in rats. Pharmacology. 1995;51:356–363. doi: 10.1159/000139347. [DOI] [PubMed] [Google Scholar]
- 3.Asres K., Bucar F. Anti-HIV activity against immunodeficiency virus type 1 (HIV-I) and type II (HIV-II) of compounds isolated from the stem bark of Combretum molle. Ethiop. Med. J. 2005;43:15–20. [PubMed] [Google Scholar]
- 4.Astani A., Reichling J., Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. 2011;2011:1–8. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awakan O.J., Malomo S.O., Adejare A.A., Igunnu A., Atolani O., Adebayo A.H., Owoyele B.V. Anti-inflammatory and bronchodilatory constituents of leaf extracts of Anacardium occidentale L. in animal models. J. Integr. Med. 2018;16:62–70. doi: 10.1016/j.joim.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Bae Y., Lee S., Kim S.H. Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol. 2011;254:56–64. doi: 10.1016/j.taap.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Balkrishna A., Pokhrel S., Singh J., Varshney A. Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Res. Sq. 2020 doi: 10.21203/rs.3.rs-17806/v1. [DOI] [Google Scholar]
- 8.Bhandari M., Bhandari A., Bhandari A. Recent updates on codeine. Pharm. Methods. 2011;2:3–8. doi: 10.4103/2229-4708.81082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bui T.T., Piao C.H., Song C.H., Shin H.S., Shon D.H., Chai O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell. Immunol. 2017;322:64–73. doi: 10.1016/j.cellimm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Capell T., Twyman R.M., Armario-Najera V., Ma J.K., Schillberg S., Christou P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020 doi: 10.1016/j.tplants.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantrill B.H., Coulthard C.E., Dickinson L., Inkley G.W., Morris W., Pyle A.H. The action of plant extracts on a bacteriophage of Pseudomonas pyocyanea and on influenza a virus. J. Gen. Microbiol. 1952;6:74–84. doi: 10.1099/00221287-6-1-2-74. [DOI] [PubMed] [Google Scholar]
- 12.Chen C.N., Lin C.P., Huang K.K., Chen W.C., Hsieh H.P., Liang P.H., Hsu J.T. Inhibition of SARS-CoV 3C-like protease activity by Theaflavin-3,3′-digallate (TF3) Evid. Based Complement. Altern. Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., Park K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 2013;21:3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H.J., Kim J.H., Lee C.H., Ahn Y.J., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir. Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong L., Zhang W., Nie Y., Yu G., Liu L., Lin L., Wen S., Zhu L., Li C. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1–GATA3 signaling pathway. Inflammation. 2014;37:1476–1485. doi: 10.1007/s10753-014-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.H., Huang X., Peiris M. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. (104786–104786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung M.J., Pandey R.P., Choi J.W., Sohng J.K., Choi D.J., Park Y. Il. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. Int. Immunopharmacol. 2015;25:302–310. doi: 10.1016/j.intimp.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 18.D’Alessandro S., Scaccabarozzi D., Signorini L., Perego F., Ilboudo D.P., Ferrante P., Delbue S. The use of antimalarial drugs against viral infection. Microorganisms. 2020;8:85. doi: 10.3390/microorganisms8010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debiaggi M., Pagani L., Cereda P.M., Landini P., Romero E. Antiviral activity of Chamaecyparis lawsoniana extract: study with herpes simplex virus type 2. Microbiologica. 1988;11:55–61. [PubMed] [Google Scholar]
- 20.Ding J., Su J., Zhang L., Ma J. Crocetin activates Foxp3 through TIPE2 in asthma-associated treg cells. Cell. Physiol. Biochem. 2015;37:2425–2433. doi: 10.1159/000438595. [DOI] [PubMed] [Google Scholar]
- 21.Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emami B., Shakeri F., Gholamnezhad Z., Saadat S., Boskabady M., Azmounfar V., Sadatfaraji H., Boskabady M.H. Calcium and potassium channels are involved in curcumin relaxant effect on tracheal smooth muscles. Pharm. Biol. 2020;58:257–264. doi: 10.1080/13880209.2020.1723647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galeotti N., Di Cesare Mannelli L., Mazzanti G., Bartolini A., Ghelardini C. Menthol: a natural analgesic compound. Neurosci. Lett. 2002;322:145–148. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 24.Greiner J.F.W., Müller J., Zeuner M.T., Hauser S., Seidel T., Klenke C., Grunwald L.M., Schomann T., Widera D., Sudhoff H., Kaltschmidt B., Kaltschmidt C. 1,8-cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. Biophys. Acta, Mol. Cell Res. 2013;1833:2866–2878. doi: 10.1016/j.bbamcr.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Gupta M.K., Vemula S., Donde R., Gouda G., Behera L., Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1751300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez-Valdes N., Häkkinen S.T., Lemasson C., Guillet M., Oksman-Caldentey K.M., Ritala A., Cardon F. Hairy root cultures-A versatile tool with multiple applications. Front. Plant Sci. 2020;3(11):33. doi: 10.3389/fpls.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn F., Fröhlich T., Frank T., Bertzbach L.D., Kohrt S., Kaufer B.B., Stamminger T., Tsogoeva S.B., Marschall M. Artesunate-derived monomeric, dimeric and trimeric experimental drugs - their unique mechanistic basis and pronounced antiherpesviral activity. Antivir. Res. 2018;152:104–110. doi: 10.1016/j.antiviral.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Hartung A.M., Beutler J.A., Navarro H.A., Wiemer D.F., Neighbors J.D. Stilbenes as κ-selective, non-nitrogenous opioid receptor antagonists. J. Nat. Prod. 2014;77:311–319. doi: 10.1021/np4009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiermann A., Schantl D. Antiphlogistic and antipyretic activity of Peucedanum ostruthium. Planta Med. 1998;64:400–403. doi: 10.1055/s-2006-957468. [DOI] [PubMed] [Google Scholar]
- 30.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Doerr H.W., Cinatl J. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 31.Hoskins A., Roberts J.L., Milne G., Choi L., Dworski Y. Natural source d-α-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67:676–682. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang K.L., Lai Y.K., Lin C.C., Chang J.M. Inhibition of hepatitis B virus production by Boehmeria nivea root extract in HepG2 2.2.15 cells. World J. Gastroenterol. 2006;12 doi: 10.3748/wjg.v12.i35.5721. (5721–5721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang Y.P., Jin S.W., Choi J.H., Choi C.Y., Kim H.G., Kim S.J., Kim Y., Lee K.J., Chung Y.C., Jeong H.G. Inhibitory effects of L-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017;99:162–169. doi: 10.1016/j.fct.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Islam R., Parves R., Paul A.S., Uddin N., Rahman M.S., Mamun A.A., Hossain M.N., Ali M.A., Halim M.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1761883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang H.Y., Kwon O.K., Oh S.R., Lee H.K., Ahn K.S., Chin Y.W. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem. Toxicol. 2012;50:4042–4050. doi: 10.1016/j.fct.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. 2020. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. [DOI] [Google Scholar]
- 38.Khan A. Ullah, Ali S., Gilani A.H., Ahmed M., Choudhary M.I. Antispasmodic, bronchodilator, vasorelaxant and cardiosuppressant effects of Buxus papillosa. BMC Complement. Altern. Med. 2017;17:54. doi: 10.1186/s12906-017-1558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 40.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Park C.M., Song J.H., Kim H.R., Kim S., Jin Y.H., Kwon S. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human Lung cells. Biomolecules. 2019;9:696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M., Choi S.Y., Lee P., Hur J. Neochlorogenic acid inhibits lipopolysaccharide-induced activation and pro-inflammatory responses in BV2 microglial cells. Neurochem. Res. 2015;40:1792–1798. doi: 10.1007/s11064-015-1659-1. [DOI] [PubMed] [Google Scholar]
- 42.Kodaira H., Spector S. Transformation of thebaine to oripavine, codeine, and morphine by rat liver, kidney, and brain microsomes. Proc. Natl. Acad. Sci. U. S. A. 1988;85:1267–1271. doi: 10.1073/pnas.85.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotwal G.J., Kaczmarek J.N., Leivers S., Ghebremariam Y.T., Kulkarni A.P., Bauer G., De Beer C., Preiser W., Mohamed A.R. Anti-HIV, anti-poxvirus, and anti-SARS activity of a nontoxic, acidic plant extract from the Trifollium species Secomet-V/anti-vac suggests that it contains a novel broad-spectrum antiviral. Ann. N. Y. Acad. Sci. 2005;1056:293–302. doi: 10.1196/annals.1352.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuang Y., Li B., Fan J., Qiao X., Ye M. Bioorganic & Medicinal Chemistry Antitussive and expectorant activities of licorice and its major compounds. Bioorg. Med. Chem. 2018;26:278–284. doi: 10.1016/j.bmc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.K.K., Chan P.K.S., Smee D.F. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antivir. Res. 2011;90:22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar V., Dhanjal J.K., Kaul S.C., Wadhwa R., Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon D.H., Kwon H.Y., Kim H.J., Chang E.J., Kim M.B., Yoon S.K., Song E.Y., Yoon D.Y., Lee Y.H., Choi I.S., Choi Y.K. Inhibition of hepatitis B virus by an aqueous extract of Agrimonia eupatoria L. Phytother. Res. 2005;19:355–358. doi: 10.1002/ptr.1689. [DOI] [PubMed] [Google Scholar]
- 48.Leite Junior J.B., de Mello Bastos J.M., Samuels R.I., Carey R.J., Carrera M.P. Reversal of morphine conditioned behavior by an anti-dopaminergic post-trial drug treatment during re-consolidation. Behav. Brain Res. 2019;359:771–782. doi: 10.1016/j.bbr.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y., Morrow T.J., Roy J.A.K., Terry L.C., Casey K.L. Cocaine: evidence for supraspinal, dopamine-mediated, non-opiate analgesia. Evidence-based Complement. Altern. Med. 1989;479:306–312. doi: 10.1155/2020/1021258. [DOI] [PubMed] [Google Scholar]
- 51.Liu X., Zhang M., He L., Li Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst. Rev. 2012;10 doi: 10.1002/14651858.CD004882.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W., Wang Y., He D., Li S., Zhu Y., Jiang B., Cheng X., Wang Z., Wang C. Phytomedicine Antitussive, expectorant, and bronchodilating effects of quinazoline parts of Peganum harmala L. Phytomedicine. 2015;22:1088–1095. doi: 10.1016/j.phymed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Lomba L.A., Vogt P.H., Souza V.E.P., Leite-Avalca M.C.G., Verdan M.H., Stefanello M.E.A., Zampronio A.R. A naphthoquinone from Sinningia canescens inhibits inflammation and fever in mice. Inflammation. 2017;40:1051–1061. doi: 10.1007/s10753-017-0548-y. [DOI] [PubMed] [Google Scholar]
- 54.Lung J., Lin Y.S., Yang Y.H., Chou Y.L., Shu L.H., Cheng Y.C., Liu H.T., Wu C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020;92:693–697. doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 56.Ma R., Yu Z., Cai Q., Li H., Dong Y., Oksman-Caldentey K.M., Rischer H. Agrobacterium-mediated genetic transformation of the medicinal plant Veratrum dahuricum. Plants. 2020;9:191. doi: 10.3390/plants9020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangprayool T., Kupittayanant S., Chudapongse N. Participation of citral in the bronchodilatory effect of ginger oil and possible mechanism of action. Fitoterapia. 2013;89:68–73. doi: 10.1016/j.fitote.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Vázquez M., Ramírez Apan T.O., Aguilar M.H., Bye R. Analgesic and antipyretic activities of an aqueous extract and of the flavone linarin of buddleia cordata. Planta Med. 1996;62:137–140. doi: 10.1055/s-2006-957836. [DOI] [PubMed] [Google Scholar]
- 59.Millet J.K., Séron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon K.B., Park J.S., Park Y.I., Song I.J., Lee H.J., Cho H.S., Jeon J.H., Kim H.S. Development of Systems for the Production of plant-derived biopharmaceuticals. Plants (Basel) 2019;24(9):30. doi: 10.3390/plants9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muhammad A., Khan B., Iqbal Z., Khan A.Z., Khan I., Khan Kashif, Alamzeb M., Ahmad N., Khan Khalid, Lal Badshah S., Ullah A., Muhammad S., Jan M.T., Nadeem S., Kabir N. Viscosine as a potent and safe antipyretic agent evaluated by yeast-induced pyrexia model and molecular docking studies. ACS Omega. 2019;4:14188–14192. doi: 10.1021/acsomega.9b01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikhat S., Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci. Total Environ. 2020;728:138859. doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okebe J., Bojang K., D’Alessandro U. Use of artemisinin and its derivatives for the treatment of malaria in children. Pediatr. Infect. Dis. J. 2014;33:522–524. doi: 10.1097/INF.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 64.Okuyama E., Umeyama K., Yamazaki M., Kinoshita Y., Yamamoto Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995;61:113–115. doi: 10.1055/s-2006-958027. [DOI] [PubMed] [Google Scholar]
- 65.Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., Kwon H.J., Park S.J., Lee W.S., Ryu Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017;2:504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park K., Lee J.S., Choi J.S., Nam Y.J., Han J.H., Byun H.D., Song M.J., Oh J.S., Kim S.G., Choi Y. Identification and characterization of baicalin as a phosphodiesterase 4 inhibitor. Phyther. Res. 2016;30:144–151. doi: 10.1002/ptr.5515. [DOI] [PubMed] [Google Scholar]
- 68.Paton K.F., Kumar N., Crowley R.S., Harper J.L., Prisinzano T.E., Kivell B.M., 1School The analgesic and anti-inflammatory effects of Salvinorin A analogue β-tetrahydropyran Salvinorin B in mice. Eur. J. Pain. 2017;21:1039–1050. doi: 10.1002/ejp.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prasad A., Prasad M. SARS-CoV-2: the emergence of a viral pathogen causing havoc on human existence. J. Genet. 2020;99:37. doi: 10.1007/s12041-020-01205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prasad A., Muthamilarasan M., Prasad M. Synergistic antiviral effects against SARS-CoV-2 by plant-based molecules. Plant Cell Rep. 2020 doi: 10.1007/s00299-020-02560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu J., Ma L., Liu B., Zhang W., Liu M., Wang G. Folium Sennae and emodin reverse airway smooth muscle contraction. Cell Biol. Int. 2020 doi: 10.1002/cbin.11393. [DOI] [PubMed] [Google Scholar]
- 72.Rauf A., Khan R., Khan H., Ullah B., Pervez S. Antipyretic and antinociceptive potential of extract/fractions of Potentilla evestita and its isolated compound, acacetin. BMC Complement. Altern. Med. 2014;14:448. doi: 10.1186/1472-6882-14-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryu H.W., Lee S.U., Lee S., Song H.H., Son T.H., Kim Y.U., Yuk H.J., Ro H., Lee C.K., Hong S.T., Oh S.R. 3-Methoxy-catalposide inhibits inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Cytokine. 2017;91:57–64. doi: 10.1016/j.cyto.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saadat S., Naghdi F., Ghorani V., Rakhshandeh H., Boskabady M.H. Histamine (H1) receptors, cyclooxygenase pathway and nitric oxide formation involved in rat tracheal smooth muscle relaxant effect of berberine. Iran. J. Allergy Asthma Immunol. 2019;18:320–331. doi: 10.18502/ijaai.v18i3.1125. [DOI] [PubMed] [Google Scholar]
- 76.Sampangi-Ramaiah M.H., Vishwakarma R., Shaanker U. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020;118:1087–1092. [Google Scholar]
- 77.Sehailia M., Chemat S. In-silico studies of antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12098652.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serkedjieva J. Influenza virus variants with reduced susceptibility to inhibition by a polyphenol extract from Geranium sanguineum L. Pharmazie. 2003;58:53–57. [PubMed] [Google Scholar]
- 79.Shen L., Niu J., Wang C., Huang B., Wang W., Zhu N., Deng Y., Wang H., Ye F., Cen S., Tan W. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019;93 doi: 10.1128/JVI.00023-19. (e00023–19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen M.L., Wang C.H., Lin C.H., Zhou N., Te Kao S., Wu D.C. Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Sci. Rep. 2016;6:32756. doi: 10.1038/srep32756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin I.S., Ahn K.S., Shin N.R., Jeon C.M., Kwon O.K., Chin Y.W., Lee K., Oh S.R. Homoegonol attenuates the asthmatic responses induced by ovalbumin challenge. Arch. Pharm. Res. 2014;37:1201–1210. doi: 10.1007/s12272-013-0327-8. [DOI] [PubMed] [Google Scholar]
- 82.Shin I.S., Hong J., Jeon C.M., Shin N.R., Kwon O.K., Kim H.S., Kim J.C., Oh S.R., Ahn K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. 2013;62:506–513. doi: 10.1016/j.fct.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Shrimali D., Shanmugam M.K., Kumar A.P., Zhang J., Tan B.K.H., Ahn K.S., Sethi G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341:139–149. doi: 10.1016/j.canlet.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 84.Siddiqui S.A., Rahman A., Rahman M.O., Akbar M.A., Ali M.A., Al-Hemaid F.M.A., Elshikh M.S., Farah M.A. A novel triterpenoid 16-hydroxy betulinic acid isolated from Mikania cordata attributes multi-faced pharmacological activities. Saudi J. Biol. Sci. 2019;26:554–562. doi: 10.1016/j.sjbs.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh K.S., Sinha S.K., Prasad S.K., Kumar R., Bithu B.S., Sadish Kumar S., Singh P. Synthesis and evaluation of novel analogues of mangiferin as potent antipyretic. Asian Pac J Trop Med. 2011;4:866–869. doi: 10.1016/S1995-7645(11)60210-1. [DOI] [PubMed] [Google Scholar]
- 86.Srivastava R.A.K., Mistry S., Sharma S. A novel anti-inflammatory natural product from Sphaeranthus indicus inhibits expression of VCAM1 and ICAM1, and slows atherosclerosis progression independent of lipid changes. Nutr. Metab. 2015;12:20. doi: 10.1186/s12986-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Thi Phuong Loan H., Triet N.T., Anh T.T.V., Quy P.T., Tat P.V., Hue N.V. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toney J.H., Navas-Martín S., Weiss S.R., Koeller A. Sabadinine: a potential non-peptide anti-severe acute-respiratory-syndrome agent identified using structure-aided design. J. Med. Chem. 2004;47:1079–1080. doi: 10.1021/jm034137m. [DOI] [PubMed] [Google Scholar]
- 89.Tong X., Li A., Zhang Z., Duan J., Chen X., Hua C., Zhao D., Xu Y., Shi X., Li P., Tian X., Lin F., Cao Y., Jin L., Chang M., Wang Y. TCM treatment of infectious atypical pneumonia--a report of 16 cases. J. Tradit. Chin. Med. 2004;24:266–269. [PubMed] [Google Scholar]
- 90.Vane J.R., Botting R.M. The mechanism of action of aspirin. Thromb. Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 91.Velázquez F., Manríquez R., Maya L., Barrientos L., López-Dellamary F. Phenacetin isolated from Bursera grandifolia, a herbal remedy with antipyretic properties. Nat. Prod. Commun. 2009;4:1575–1576. [PubMed] [Google Scholar]
- 92.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;4:725. doi: 10.1016/j.scitotenv.2020.138277. (138277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vučkovic S., Srebro D., Vujovic K.S., Vučetic Č., Prostran M. Cannabinoids and pain: new insights from old molecules. Front. Pharmacol. 2018;9:1–19. doi: 10.3389/fphar.2018.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D., Wang S., Chen X., Xu X., Zhu J., Nie L., Long X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012;139:189–193. doi: 10.1016/j.jep.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 95.Wang D., Zhu J., Wang S., Wang X., Ou Y., Wei D., Li X. Fitoterapia Antitussive, expectorant and anti-in fl ammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia. 2011;82:1290–1294. doi: 10.1016/j.fitote.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Wang J. Fast identification of possible drug treatment of coronavirus Disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020 doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Lu Y., Luo M., Shi X., Pan Y., Zeng H., Deng L. Evaluation of pharmacological relaxation effect of the natural product naringin on in vitro cultured airway smooth muscle cells and in vivo ovalbumin-induced asthma Balb/c mice. Biomed. Reports. 2016;5:715–722. doi: 10.3892/br.2016.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wen C.C., Shyur L.F., Jan J.T., Liang P.H., Kuo C.J., Arulselvan P., Wu J.B., Kuo S.C., Yang N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011;1:41–50. doi: 10.1016/S2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Q., Zhao D., Xiang J., Zhang M., Zhang C. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2016;54:1117–1124. doi: 10.3109/13880209.2015.1075048. [DOI] [PubMed] [Google Scholar]
- 101.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu P., Cheng S., Xiang J., Yu B., Zhang M., Zhang C., Xu X. Expectorant, antitussive, anti-in fl ammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015;164:328–333. doi: 10.1016/j.jep.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M.M., Liu X.M., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J. Gastroenterol. 2004;10:3500–3505. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang T.Z., Fu Q., Chen T., Ma S.P. Anti-asthmatic effects of oxymatrine in a mouse model of allergic asthma through regulating CD40 signaling. Chin. J. Nat. Med. 2015;13:368–374. doi: 10.1016/S1875-5364(15)30028-5. [DOI] [PubMed] [Google Scholar]
- 106.Zhou E., Fu Y., Wei Z., Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014;5:2106–2112. doi: 10.1039/c4fo00384e. [DOI] [PubMed] [Google Scholar]
- 107.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhuang M., Jiang H., Suzuki Y., Li X., Xiao P., Tanaka T., Ling H., Yang B., Saitoh H., Zhang L., Qin C. Procyanidins and butanol extract of Cinnamomi cortex inhibit SARS-CoV infection. Antivir. Res. 2009;82:73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryu YB, Jeong HJ, Kim JH, Nguyen TT, Park SJ, Chang JS, Park KH, Rho MC, Lee WS. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]