Abstract
Nox2 is responsible for artery dysfunction via production of reactive oxidant species. RNA viruses may activate Nox2, but it is unknown if this occurs in coronavirus 2019(Covid-19). Nox2 activation by soluble Nox2-derived peptide(sNox2-dp) was measured in patients hospitalized for Covid-19 (n = 182) and controls (n = 91). sNox2-dp values were higher in Covid-19 patients versus controls and in severe versus non severe Covid-19. Patients with thrombotic events(n = 35,19%) had higher sNox2-dp than thrombotic event-free ones. A logistic regression analysis showed that sNox2 and coronary heart disease predicted thrombotic events. Oxidative stress by Nox2 activation is associated severe disease and thrombotic events in Covid-19 patients.
Keywords: Covid-19, Nox-2, NADPH oxidase, Thrombosis
Highlights
-
•
Nox2 is responsible for artery dysfunction via production of reactive oxidant species.
-
•
sNox2-dp values, markers of Nox2 activation, were high in Covid-19 patients and higher in those with severe disease.
-
•
A logistic regression analysis showed that sNox2 predicted thrombotic events.
-
•
Oxidative stress by Nox2 activation is associated severe disease and thrombotic events in Covid-19 patients.
1. Introduction
There is a growing body of evidence that Coronavirus 2019(Covid-19) poor outcome is related not only to pulmonary pneumonia but also to ischemic episodes occurring in the artery and venous circulation [1].
Among the putative mechanisms accounting for vascular disease, Nox2, one the most important cellular producers of reactive oxidant species (ROS), could play a role. Nox2 encompass, indeed, pro-inflammatory and vasoconstriction properties which may favor vascular occlusion [2,3]. These properties have been deduced by experiments in patients with chronic granulomatous disease, who are characterized by hereditary deficiency of Nox2 and display enhanced artery dilatation and reduced atherosclerotic burden [4]. Furthermore Nox2 is implicated in thrombosis as it elicits platelet aggregation via overproduction of hydrogen peroxide, isoprostane or inactivation of nitric oxide [2]. Experimental studies in Nox2 knock-out models showed impaired thrombus formation in small but not in large arteries [5,6].
As a previous study from our group reported that Nox2 is overactivated in patients with community-acquired pneumonia [7], we speculated that a similar behavior could be detected in patients hospitalized for Covid-19, which are essentially complicated by several pneumonia read-outs [8]. Thus, the aim of the present study was to assess the behavior of Nox2 in Covid-19 patients with different degrees of disease severity and its relationship with thrombosis complications.
2. Methods
Covid-19 cohort was prospectively recruited and followed-up by 3 divisions at University hospital located in Rome and Latina, (Italy).
We included in the study adult (≥18 years) patients with laboratory-confirmed Covid-19 and severe acute respiratory syndrome coronavirus-2(SARS-CoV2)-related pneumonia, consecutively hospitalized in March 2020. Covid-19 was diagnosed on the basis of the WHO interim guidance [9]. Severe disease was defined as the need of intensive care unit (ICU) admission. Patients matched for demographic and clinical characteristics but without acute infections were used as controls.
Nox2 activation was measured as soluble Nox2-derived peptide (sNox2-dp) with an ELISA method as previously reported [10].
The clinical course of the disease and its evolution were monitored during hospitalization. The appearance of new ischemic/embolic events was diagnosed as follows: 1) pulmonary thrombo-embolism by lung CT scan [11]; 2) myocardial infarction by EKG changes associated with enhanced markers of cell necrosis [12]; 3) acute brain ischemia by onset of new focal neurological signs and symptoms and confirmed, whenever possible, by NMR or CT imaging [13]; 4) acute limb ischemia diagnosed according to AHA guidelines [14].
3. Results
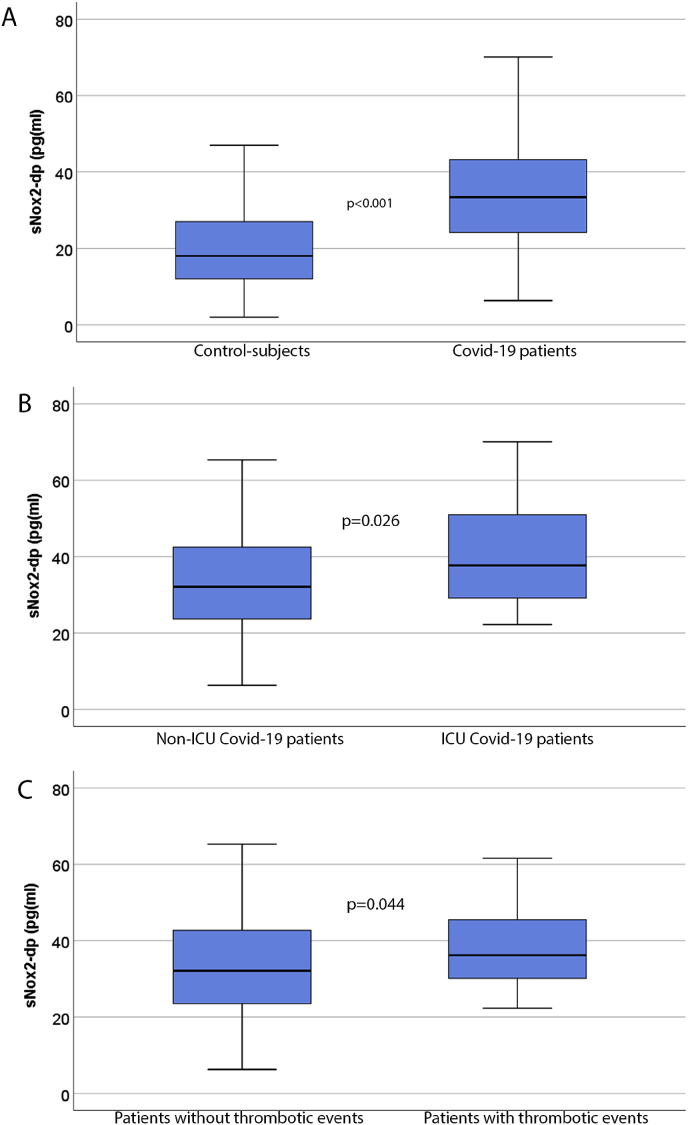
Clinical characteristics of Covid-19 patients and controls are reported in Table 1. No significant differences were present for age, sex, body mass index, smoking habit and for the prevalence of arterial hypertension, diabetes, peripheral artery disease (PAD) and atrial fibrillation between patients and controls; coronary heart disease (CAD), heart failure and chronic obstructive pulmonary disease(COPD) were more prevalent among Covid-19 patients. Serum sNox2-dp was higher in Covid-19 patients than in control subjects (Fig. 1A). Among 182 Covid-19 patients, 32 (18%) had needed intensive care unit (ICU) treatment. Clinical and laboratory characteristics of ICU and non-ICU patients are reported in Table 2. Covid-19 patients admitted to ICU showed higher levels of serum sNox2-dp than non-ICU Covid-19 ones (38 [29-51] vs. 32 [24-42] pg/ml; p = 0.026) (Fig. 1B).
Table 1.
Clinical characteristics of Covid-19 patients and control subjects.
| Control subjects | COVID-19 patients | p | |
|---|---|---|---|
| N. | 91 | 182 | |
| Age (years) | 61.7 ± 10.3 | 63.9 ± 14.4 | 0.142 |
| BMI (kg/m2) | 26.1 ± 3.5 | 27.5 ± 9.0 | 0.389 |
| Male sex | 65% | 60% | 0.511 |
| Arterial hypertension | 53% | 49% | 0.608 |
| Smokers | 16% | 15% | 0.861 |
| COPD | 4% | 12% | 0.049 |
| Diabetes | 15% | 21% | 0.328 |
| CAD | 8% | 21% | 0.005 |
| PAD | 5% | 11% | 0.129 |
| Heart failure | 3% | 13% | 0.015 |
| Atrial fibrillation | 6% | 9% | 0.471 |
| ACE-inhibitors/ARBs | 40% | 35% | 0.427 |
| sNox2-dp (pg/ml) | 18 [12-27] | 33 [24-43] | <0.001 |
Legend: ACE: angiotensin converting enzyme, ARBs: angiotensin receptor blockers, BMI: body mass index, CAD: coronary heart disease, COPD: chronic obstructive pulmonary disease, sNox2-dp: serum Nox2-derived peptide. Differences between percentages were assessed by Fisher exact tests. All continuous variables were tested for normality with the Shapiro-Wilk test. Student unpaired t-test was used for normally distributed continuous variables (expressed as mean ± SD). Mann-Whitney U test was used for not-normally distributed continuous variables (expressed as median[interquartile range]).
Fig. 1.
Serum sNox2-dp in Covid-19 patients and controls (panel A), in Covid-19 patients admitted or not in intensive care unit (ICU) (panel B) and in Covid-19 patients who experienced or not thrombotic events (panel C). * Mann-Whitney U test.
Table 2.
Clinical characteristics of Covid-19 patients according to ICU admission and thrombotic events.
| Non-ICU patients | ICU patients | p | Patients without thrombotic events | Patients with thrombotic events | p | |
|---|---|---|---|---|---|---|
| N. | 150 | 32 | 147 | 35 | ||
| Age (years) | 63.6 ± 14.9 | 65.5 ± 12.3 | 0.501 | 62.5 ± 14.3 | 69.9 ± 13.7 | 0.006 |
| BMI (kg/m2) | 25.4 ± 4.0 | 29.1 ± 6.1 | 0.041 | 26.0 ± 4.2 | 27.9 ± 6.3 | 0.285 |
| Male sex | 55% | 84% | 0.002 | 60% | 60% | 1.000 |
| Arterial hypertension | 48% | 53% | 0.698 | 48% | 54% | 0.573 |
| Smokers | 15% | 19% | 0.804 | 15% | 18% | 0.800 |
| COPD | 10% | 22% | 0.075 | 12% | 14% | 0.773 |
| Diabetes | 19% | 28% | 0.337 | 20% | 26% | 0.488 |
| CAD | 17% | 37% | 0.016 | 16% | 40% | 0.004 |
| PAD | 11% | 16% | 0.540 | 8% | 26% | 0.007 |
| Heart failure | 9% | 31% | 0.002 | 10% | 23% | 0.052 |
| Atrial fibrillation | 8% | 12% | 0.489 | 8% | 11% | 0.515 |
| ACE-inhibitors/ARBs | 34% | 37% | 0.688 | 35% | 34% | 1.000 |
| SpO2 (%) | 96 [93-97] | 91 [85-97] | 0.034 | 96 [93-97] | 94 [88-97] | 0.143 |
| P/F ratio | 352 [293-421] | 171 [130-257] | <0.001 | 352 [281-414] | 217 [158-387] | 0.032 |
| hs-CRP (mg/L) | 45 [14-98] | 150 [70-263] | <0.001 | 45 [16-108] | 127 [48-203] | 0.004 |
| D-dimer (ng/ml) | 1146 [537–2026] | 4610 [1628–4800] | < 0.001 | 1090 [555–1974] | 4474 [1657–4800] | < 0.001 |
| sNox2 (pg/ml) | 32 [24-42] | 38 [29-51] | 0.026 | 32 [23-43] | 36 [30-48] | 0.044 |
Legend: ACE: angiotensin converting enzyme, ARBs: angiotensin receptor blockers, BMI: body mass index, CAD: coronary heart disease, COPD: chronic obstructive pulmonary disease, hs-CRP: high sensitivity C reactive protein, P/F: PaO2/Fi02 ratio, sNox2-dp: serum Nox2-derived peptide, SpO [2]: oxygen saturation. Differences between percentages were assessed by Fisher exact tests. All continuous variables were tested for normality with the Shapiro-Wilk test. Student unpaired t-test was used for normally distributed continuous variables (expressed as mean ± SD). Mann-Whitney U test was used for not-normally distributed continuous variables (expressed as median [interquartile range]).
A logistic regression analysis showed that sNox2-dp (OR: 1.035; 95% CI: 1.007-1.064; p = 0.015), heart failure (OR: 5.386; 95%CI: 1.900-15.271; p = 0.002) and male sex (OR: 5.344; 95%CI: 1.780-16.046; p = 0.003) predicted ICU admission, after adjusting for age, CAD, PAD and COPD.
Among 182 Covid-19 patients included in the study 35 patients (19%) experienced thrombotic events in the arterial (n = 15) and venous circulation (n = 20) during a median follow-up of 18 days (IQR: 11-27 days). Venous thrombosis included 7 superficial thrombophlebitis, 7 deep venous thrombosis and 6 pulmonary embolisms. Arterial thrombosis included 8 acute limb ischemia, 4 myocardial infarctions and 3 strokes. Patients experiencing thrombotic events were older, had a higher prevalence of CAD, PAD, and were more frequently admitted to ICU than thrombotic event -free ones (43 vs 12%). Patients with thrombotic events showed higher levels of sNox2-dp(Table 2; Fig. 1C) and D-dimer(Table 2) than thrombosis-free ones; no correlation was found between sNOX2-dp and D-dimer levels (Spearman's rho = 0.083; p = 0.336).
A logistic regression analysis showed that sNox2-dp (OR: 1.028; 95% CI: 1.001-1.055; p = 0.041) and CAD (OR: 3.481; 95%CI: 1.524-7.048; p = 0.003) predicted thrombotic events, after adjusting for age and PAD.
4. Discussion
The study provides evidence that, compared to controls, Covid-19 patients displayed overactivation of Nox2, which was more marked in patients admitted to ICU. Covid-19 patients with thrombotic complications had higher Nox2 activation compared to event-free patients suggesting a role for Nox2 as mechanism favoring thrombotic-related ischemic events.
Covid-19 binding and entry into human cells occurs via converting enzyme 2 (ACE2) and may enhance angiotensin II levels and eventually Nox2 activation [15]. Thus, a link between angiotensin II and Nox2 may occur in Covid-19 patients as the entry of Covid-19 into the cells occurs via interaction of viral S-protein with extracellular domains of the transmembrane ACE2 protein; this results in surface ACE2 expression downregulation and loss of function and is accompanied by angiotensin II up-regulation as ACE2 has a role in angiotensin II degradation [16]; in fact, angiotensin II levels have been found elevated in Covid-19 patients and linearly correlated with viral load [17,18]. The increase of angiotensin II in Covid-19 has potentially deleterious effect as angiotensin II is implicated in artery dysfunction, an effect that may be mediated by Nox2 activation via production of ROS and eventually endothelial damage [15]. Experimental study demonstrated, in fact, that angiotensin II has a negative impact on vascular disease as it promotes proinflammatory changes of artery endothelium, vascular infiltration of monocytes and ultimately vascular dysfunction via Nox2-mediated oxidative stress [15]. Our hypothesis is supported by Nox2 overactivation in Covid-19 patients with >40% increase compared to controls.
Covid-19 patients with thrombotic complications had higher Nox2 activation compared to event-free ones, independently upon confounders such as coexistent atherosclerotic burden including coronary artery disease and peripheral artery disease, that are associated to Nox2 activation [19,20] and age. We must acknowledge, however, the overlap of Nox2 activation in the Covid-19 population with and without vascular disease, which may entail that Nox2 activation is not the only factor concurring to ischemic complications. We have recently demonstrated, for instance, that serum albumin, which an important blood antioxidant, is reduced in Covid-19 and associated with a higher rate of thrombotic complications [1]; this would suggest that more than one component of redox balance may intervene in favoring thrombosis. Furthermore, Covid-19 related ischemic events could not be fully related to clotting/platelet activation as indirectly suggested by the lack of association between Nox2 and D-dimer elevation in patients with vascular complications.
Another finding of the present report is the higher levels of Nox2 activation in patients with severe disease, i.e, those needed ICU admission, which could imply a role for Nox2 as factor favoring Covid-19 infection worsening. Nox2 may be activated by Covid-19 also as consequence of up-regulation of Toll-like receptor 7 elicited by RNA viruses; thus, a previous report demonstrated that such effect has a negative impact in the defense mechanism against viruses as they used Nox2 activation to propagate the infection and hamper the immunological response [21].
The study has implications and limitations. Analysis of Nox2 in serum reflects essentially the enzyme activation by leucocytes and platelets [10], thereby inference regarding Nox2 activation at level of vascular cells, where Nox2 is also expressed [22], cannot be drawn. We did not investigate if angiotensin II, Toll-like 7 or both are trigger of Nox2 activation. Our data provide a rationale to assess if downregulation of Nox2 improves Covid-19 morbidity and mortality.
In conclusion, we report for the first time that Covid-19 is associated with Nox2-derived oxidative stress so providing a novel insight in the pathophysiology of Covid-19 and a clue to explore future therapeutic approach to fight SARS-CoV-2.
Founding information
This work was supported in part by a grant from Sapienza University of Rome (grant. n. RM118164366B89BD) to Prof. F. Violi.
Declaration of competing interest
The short communication you find enclosed is submitted to the journal for publication.
The authors involved in this study declared that they do not possess any conflict of interest concerning the study and the issue evaluated in it.
read and approved the last version of the manuscript, that has not been submitted for publication elsewhere.RM118164366B89BD
References
- 1.Violi F., Ceccarelli G., Cangemi R., Alessandri F., Gabriella d’Ettorre G., Oliva A., Pastori D., Loffredo L., Pignatelli P., Ruberto F., Venditti M., Pugliese F., Hypoalbuminemia Mastroianni CM. Coagulopathy and vascular disease in covid-19. Circ. Res. 2020;127:400–401. doi: 10.1161/CIRCRESAHA.120.317173. [DOI] [PubMed] [Google Scholar]
- 2.Violi F., Loffredo L., Carnevale R., Pignatelli P., Pastori D. Atherothrombosis and oxidative stress: mechanisms and management in elderly. Antioxidants Redox Signal. 2017;27:1083–1124. doi: 10.1089/ars.2016.6963. [DOI] [PubMed] [Google Scholar]
- 3.Sirker A., Zhang M., Shah A.M. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violi F., Carnevale R., Loffredo L., Pignatelli P., Gallin J.I. NADPH oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. Arterioscler. Thromb. Vasc. Biol. 2017;37:218–225. doi: 10.1161/ATVBAHA.116.308351. [DOI] [PubMed] [Google Scholar]
- 5.Sonkar V.K., Kumar R., Jensen M., Wagner B.A., Sharathkumar A.A., Miller F.J., Jr., Fasano M., Lentz S.R., Buettner G.R., Dayal S. Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice. Blood Adv. 2019;3:1272–1284. doi: 10.1182/bloodadvances.2018025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaney M.K., Kim K., Estevez B., Xu Z., Stojanovic-Terpo A., Shen B., Ushio-Fukai M., Cho J., Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:846–854. doi: 10.1161/ATVBAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violi F., Carnevale R., Calvieri C., Nocella C., Falcone M., Farcomeni A., Taliani G., Cangemi R., group Ss Nox2 up-regulation is associated with an enhanced risk of atrial fibrillation in patients with pneumonia. Thorax. 2015;70:961–966. doi: 10.1136/thoraxjnl-2015-207178. [DOI] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . March 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease Is Suspected: Interim Guidance V 1.2. 13. available at: https://apps.who.int/iris/rest/bitstreams/1272156. [Google Scholar]
- 10.Pignatelli P., Carnevale R., Di Santo S., Bartimoccia S., Sanguigni V., Lenti L., Finocchi A., Mendolicchio L., Soresina A.R., Plebani A., Violi F. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011;31:423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., Huisman M.V., Humbert M., Jennings C.S., Jimenez D., Kucher N., Lang I.M., Lankeit M., Lorusso R., Mazzolai L., Meneveau N., Ainle F.N., Prandoni P., Pruszczyk P., Righini M., Torbicki A., Van Belle E., Zamorano J.L. The Task Force for the d and management of acute pulmonary embolism of the European Society of C. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur. Respir. J. 2019;54 doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. White HD and executive group on behalf of the joint European society of cardiology/American college of cardiology/American heart association/world heart federation task force for the universal definition of myocardial I. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 13.Kernan W.N., Ovbiagele B., Black H.R., Bravata D.M., Chimowitz M.I., Ezekowitz M.D., Fang M.C., Fisher M., Furie K.L., Heck D.V., Johnston S.C., Kasner S.E., Kittner S.J., Mitchell P.H., Rich M.W., Richardson D., Schwamm L.H., Wilson J.A. American heart association stroke council CoC, stroke nursing CoCC and council on peripheral vascular D. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 14.Bonaca M.P., Gutierrez J.A., Creager M.A., Scirica B.M., Olin J., Murphy S.A., Braunwald E., Morrow D.A. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis-thrombolysis in myocardial infarction 50 (TRA2 degrees P-TIMI 50) Circulation. 2016;133:997–1005. doi: 10.1161/CIRCULATIONAHA.115.019355. [DOI] [PubMed] [Google Scholar]
- 15.Molitor M., Rudi W.S., Garlapati V., Finger S., Schuler R., Kossmann S., Lagrange J., Nguyen T.S., Wild J., Knopp T., Karbach S.H., Knorr M., Ruf W., Munzel T., Wenzel P. Nox2+ Myeloid cells drive vascular inflammation and endothelial dysfunction in heart failure after myocardial infarction via angiotensin II receptor type 1. Cardiovasc. Res. 2020;cvaa042 doi: 10.1093/cvr/cvaa042. [DOI] [PubMed] [Google Scholar]
- 16.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loffredo L., Carnevale R., Cangemi R., Angelico F., Augelletti T., Di Santo S., Calabrese C.M., Della Volpe L., Pignatelli P., Perri L., Basili S., Violi F. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int. J. Cardiol. 2013;165:184–192. doi: 10.1016/j.ijcard.2012.01.069. [DOI] [PubMed] [Google Scholar]
- 20.Pignatelli P., Pastori D., Carnevale R., Farcomeni A., Cangemi R., Nocella C., Bartimoccia S., Vicario T., Saliola M., Lip G.Y., Violi F. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb. Haemostasis. 2015;113:617–624. doi: 10.1160/TH14-07-0571. [DOI] [PubMed] [Google Scholar]
- 21.To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T., Chan C., Drummond G.R., Sobey C.G., Broughton B.R.S., Starkey M.R., van der Sluis R., Lewin S.R., Bozinovski S., O'Neill L.A.J., Quach T., Porter C.J.H., Brooks D.A., O'Leary J.J., Selemidis S. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat. Commun. 2017;8:69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violi F., Pignatelli P. Clinical application of NOX activity and other oxidative biomarkers in cardiovascular disease: a critical review. Antioxidants Redox Signal. 2015;23:514–532. doi: 10.1089/ars.2013.5790. [DOI] [PubMed] [Google Scholar]