Graphical abstract

Keywords: Cyclodextrins, Coronavirus, SARS-CoV-2, COVID-19, Rings
Abstract
A handful of singular structures and laws can be observed in nature. They are not always evident but, once discovered, it seems obvious how to take advantage of them. In chemistry, the discovery of reproducible patterns stimulates the imagination to develop new functional materials and technological or medical applications. Two clear examples are helical structures at different levels in biological polymers as well as ring and spherical structures of different size and composition. Rings are intuitively observed as holes able to thread elongated structures. A large number of real and fictional stories have rings as inanimate protagonists. The design, development or just discovering of a special ring has often been taken as a symbol of power or success. Several examples are the Piscatory Ring wore by the Pope of the Catholic Church, the NBA Championship ring and the One Ring created by the Dark Lord Sauron in the epic story The Lord of the Rings. In this work, we reveal the power of another extremely powerful kind of rings to fight against the pandemic which is currently affecting the whole world. These rings are as small as ~1 nm of diameter and so versatile that they are able to participate in the attack of viruses, and specifically SARS-CoV-2, in a large range of different ways. This includes the encapsulation and transport of specific drugs, as adjuvants to stabilize proteins, vaccines or other molecules involved in the infection, as cholesterol trappers to destabilize the virus envelope, as carriers for RNA therapies, as direct antiviral drugs and even to rescue blood coagulation upon heparin treatment.
“One ring to rule them all. One ring to find them. One ring to bring them all and in the darkness bind them.” J. R. R. Tolkien.
1. Introduction
Cyclodextrins (CDs) are within the most powerful and versatile ring structures ever known. They could be considered as the molecular level equivalent of the mythical One Ring created in Middle-earth. In this analogy, Villiers played the role of the Dark Lord Sauron, by isolating these cyclic oligosaccharides from the reaction of the enzyme cyclodextrin glycosyltransferase with starch (Crini, 2014, Szente et al., 2016, Villiers, 1891). Later on, Schardinger, pioneer of CD chemistry, gave a detailed description on their preparation and separation (French, 1957). More recently, Kurkov and Loftsson (Kurkov and Loftsson, 2013) made relevant contributions to the Ring Community. Despite having been known for more than 120 years, CDs only really took off in the 1980s, when the industrial scale production led to their first applications in the pharmaceutical and food industries. Since then, these rings are amongst the most versatile/multi-functional molecules used in molecular research and chemical applications, playing a relevant role in different fields such as pharmacy, medicine, chemistry, materials design, food or agricultural science (Ain et al., 2015, Braga, 2019, Martin et al., 2018).
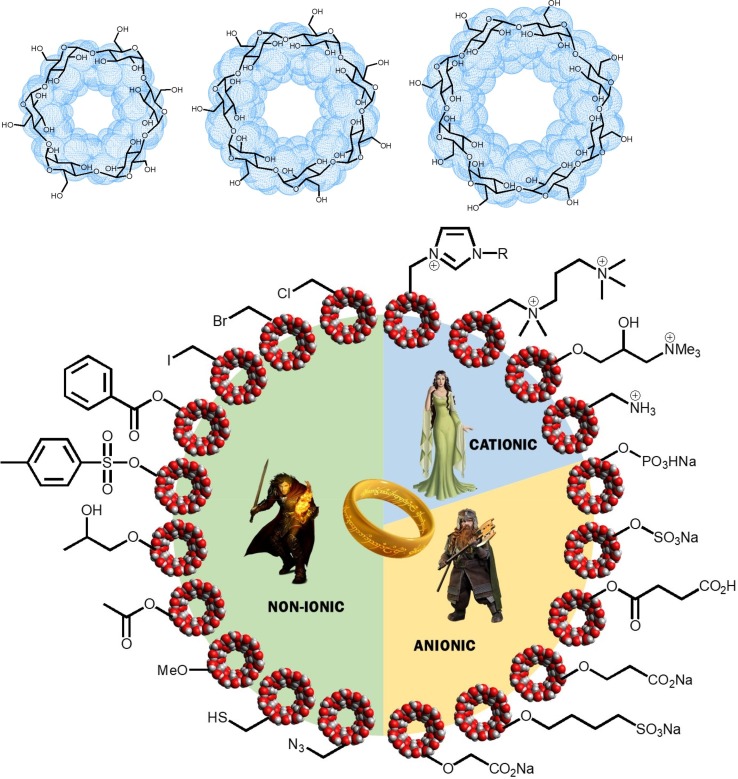
CD molecules are known to crystallize as truncated cones (Khan and Durakshan, 2013, Saokham and Loftsson, 2017), whose cavity size is determined by the number of glucopyranoside groups (GPUs): 6 (α-CD), 7 (β-CD) or 8 (γ-CD) (Fig. 1 , top). In this conformation, all the secondary hydroxyl groups (attached to the C2 and C3 carbon atoms of the glucose units) are oriented toward the wide edge of the cavity (known as the head of the CD structure), whereas the primary hydroxyls form the narrow entry of the cavity. Such distribution of polar groups determines the specific physicochemical properties of these molecules, including their solubility and their ability to encapsulate hydrophobic groups in their cavity (Koźbiał and Gierycz, 2013). As a result, CDs are widely used to increase the solubility, to protect or to reduce the toxicity of a large variety of different molecules including drugs, dyes and surfactant agents. In order to optimize the applications of CDs, their hydroxyl groups are commonly substituted, leading to synthetic derivatives that can be divided in three main groups (Fig. 1, bottom): non-ionic, such as 2-hydroxypropyl-β-CD (HP-β-CD) or randomly methylated-β-cyclodextrin (RM-β-CD), cationic, such as permethylated propylenediamine-β-CD (PEMPDA-β-CD) and ionic, such as sulfobutylether-β-CD (SBE-β-CD) (Davis and Brewster, 2004).
Fig. 1.
Top: 2D and 3D structures of native α-, β- and γ-CDs. Bottom: General classification of the most common CD derivatives and a visual comparative with the three rings of power for the Elven-kings, the seven for the Dwarf-lords and the nine rings for Mortal Men, from Tolkien’s novel.
CDs are biocompatible, they do not generate immune response and they also have low toxicity. Their successful use in inclusion complexes with bioactive compounds has allowed the development of commercially available formulations for oral, parenteral, nasal, pulmonary, and skin delivery of drugs (Adeoye and Cabral-Marques, 2017, Conceição et al., 2018, Lakkakula and Maçedo Krause, 2014, Loftsson, 2002, Loftsson and Brewster, 1996, Zhang and Ma, 2013). These features reveal the power of these versatile ring structures, and enable them, like the Hobbit Frodo Baggins' neck pendant, to be used as very powerful weapons in the crudest of battles, where viruses become enemies.
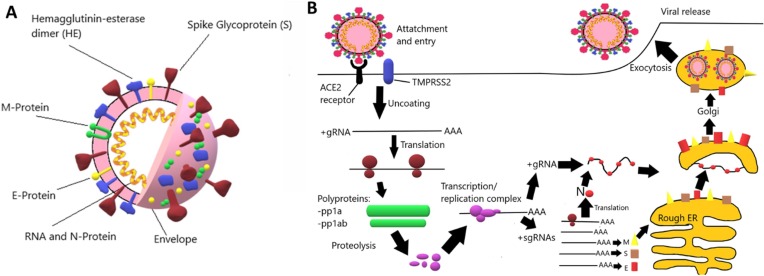
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that emerged towards the end of 2019 in Wuhan, Hubei Province (People's Republic of China) and is responsible for the coronavirus disease 2019 (COVID-19) global pandemic (Coelho Paraguassu et al., 2020). Coronaviruses are spherical-like capsules with a diameter of 80–120 nm enclosed by a lipid membrane with encrusted proteins –including the receptors employed to anchor target cells – that contain basically a single-stranded RNA chain (Fig. 2 ). They have been classified in 4 different lineages that are associated to different species: α-coronavirus, β-coronavirus, δ-coronavirus and γ-coronavirus (Chan et al., 2013). Prior to SARS-CoV-2, six coronaviruses were known to cause diseases in humans, including SARS-CoV and MERS-CoV (Zhu et al., 2020). Like them, SARS-CoV-2 is a β-coronavirus.
Fig. 2.
A. Representative structure of SARS-COV-2. B. Proposed mode of action of SARS-COV-2 towards human cells.
Coronaviruses recognize specific receptors in target cells through S proteins on their surface. In the case of SARS-CoV-2 and SARS-CoV the natural receptor is human angiotension-converting enzyme 2 (ACE2). Once the virus enters the cell, an acute infection takes place (Fig. 2). It has been reported, however, that some attack routes include host cells where the expression level of ACE2 is very low under normal conditions, like for example in the central nervous system (CNS), where they could induce neuronal injury (Baig et al., 2020, Li et al., 2020). This has been recently connected with the possible ability of the virus to use the nicotinic acetylcholine receptor (nAChR) as an alternative anchor port to infect cells (Changeux et al., 2020). The most common symptoms of COVID-19 are similar to other coronavirus infections: fever (87.9%), cough (67.7%), fatigue (38.1%) and, to a less extent, diarrhea (3.7%) and vomiting (5.0%) (Guan et al., 2020). To date, no effective and specific therapeutic method can be used to treat patients suffering from SARS-CoV-2 infection. However, due to the high rate of infection and to the global reach of this pandemic, a number of new treatments arising from intensive research in many labs and companies along the world has emerged (Liu et al., 2020, Sanders et al., 2020).
Although it might appear, a priori, that in a war of such magnitude against highly-lethal-to-humans viral enemies, small cyclic and sugary molecules could be of little use, they are revealed as extremely powerful and versatile. In this review, we will analyze the different approaches that have been carried out using CDs till the end of May 2020, both in treatments for COVID-19 and in its containment and prevention. Since hundreds of research labs over the world are focused in this problem it is difficult not to miss some significant contributions, although we hope to include at least some of the most important. Additionally, it is expected that more applications of CDs related to this pandemic will arise at the short term.
1.1. The encapsulating Army: Cyclodextrins as excipients for antiviral drugs
Antivirals are drugs used to treat viral infections. Most antivirals are focused on the inhibition of the viral replication mechanism inside the organism, so they need to be both safe and effective to avoid damaging the host and get rid of the infection. While some antivirals are effective against many different viruses –the so called broad-spectrum antivirals– others only target specific viruses. Overall, antiviral administration is conditioned by their physicochemical properties, such as solubility, stability and permeability. The distribution and bioavailability of the drug can be optimized by using excipients, which can also be employed to reduce the dose of the antiviral needed to achieve an effective therapy. In this scenario, CDs can act as Trojan horses by hiding, protecting and slowly releasing pharmaceutical active principles in a hostile environment. Their cavity allows the encapsulation of hydrophobic groups forming inclusion complexes, without modifying the structure and the chemical properties of the guest. Alternative interaction mechanisms such as the formation of non-inclusion complexes or the solubilization through aggregates can also be present in CD solutions. The use of CDs as a drug-delivery platform for antivirals has been studied by many groups. In the present section, we will summarize the role of native and modified CDs in formulations of heterogeneous antiviral treatments and then how they are used specifically in treatments of SARS-CoV-2.
1.1.1. Native Cyclodextrins in general antiviral formulations
Nicolazzi et al. reported the formation of inclusion complexes between Ganciclovir (GCV), an antiviral used specifically against human cytomegalovirus (HCMV), and β-CD (Fig. 3 ). In this case, the CD is employed to increase the poor solubility of the drug, as well as to facilitate its penetration in the organism. The comparison between CDs with rings of different diameter revealed that the middle-size structure (β-CD) is more appropriate for the encapsulation of this drug than the narrower α-CD and the wider γ-CD (Nicolazzi et al., 2001). GCV:β-CD inclusion complex provides an enhanced antiviral effect in different HCMV strains, even in those that developed a GCV resistance (Nicolazzi et al., 2002). The enhanced bioavailability of these inclusion complexes allows a reduction on the dose of GCV and, consequently, a reduction in the drug’s toxicity. Another example is Ribavirin (RBV) (Fig. 3), an antiviral used in the treatment of human respiratory syncytial virus, also employed as an oral drug against hepatitis C, hemorrhagic fever virus infections or measles virus (MEV). The use of this drug is limited by its toxicity (Grancher et al., 2004), but its complexation with α-CD or β-CD lead to a five-fold or a two-fold decrease, respectively, in the 50% inhibitory concentration (IC50) against both MEV strains. The antiviral activity of the complex RBV:α-CD was also demonstrated to be stronger than free ribavirin, in an in vivo model of measles virus (MV) encephalitis in mice, finding also a benefit of α-CD for drug delivery to the brain (Jeulin et al., 2009). Even when the action mechanism still needs to be elucidated, this animal model revealed the virological and pharmacological potential of α-CD as a drug carrier for central nervous system disorders. RBV has been tested as a therapeutic treatment for SARS-CoV and MERS-CoV patients, since it has been reported to inhibit the viral 3-chymotrypsin like protease, but no significant effect was observed (Zumla et al., 2016). CDs have occasionally been used to mask the bitter taste of some antivirals, such as Oseltamivir phosphate (an active ingredient of Tamiflu®) (Fig. 3), which is a neuraminidase inhibitor approved for the treatment of influenza virus (Sevukarajan et al., 2010). In vitro activity of this drug against SARS-CoV-2 has not been documented.
Fig. 3.
2D-structures of some antivirals that have been used in treatments for virus infections administrated with CDs as encapsulation agents. The molecules marked in the square have been tested in the treatment of COVID-19.
1.1.2. Modified Cyclodextrins in general antiviral formulations
Not only native CDs but also CD derivates have been studied as potential drug-delivery platforms to treat several viral diseases. For instance, HP-β-CD has been proved to be an effective excipient in the intravenous administration of Letermovir, an antiviral developed to deal with cytomegalovirus (CMV) in immunocompromised patients, such as transplant recipients or seropositive individuals (Fig. 3) (Erb-Zohar et al., 2017). The effectiveness of this formulation in phase III essays, without significant adverse events (Marty et al., 2017), lead to the approval of the treatment, now available as PrevymisTM Injection. HP-β-CD has also been reported as an effective platform for Acyclovir (ACV) oral delivery, a broad-spectrum antiviral used, for example, in herpes simplex (HSV) or varicella infections (Fig. 3). Nair et al. reported the formation of ACV:HP-β-CD inclusion complexes that increased the solubility of the antiviral, ensuring the dissolution of the drug in the aqueous media and its later absorption by the mucosal surface (Nair et al., 2014). Other CD-derivate platforms have been reported to enhance the solubility and bioavailability of ACV: Piperno et al. designed a platform based on β-CD/multiwalled carbon nanotubes (β-CD-MWCNT) that showed a sustained delivery of ACV and good results interfering with herpes simplex virus 1 (HSV-1) replication, higher than with the free drug (Iannazzo et al., 2014, Mazzaglia et al., 2018). Cavalli and her team characterized β-CD/poly(amidoamine) copolymers (Bencini et al., 2008), βCD-poly(4-acryloylmorpholine) nanoparticles (Cavalli et al., 2009) and carboxylated nanosponges (Lembo et al., 2013), as well as the effect of their use as ACV excipients. In vitro results were promising due to the absence of toxicity of both ACV and the CD-derivatives and to the enhanced antiviral effect shown. CD-based nanosponges have been also developed for the delivery of Ripilvirine (RPV), an antiretroviral drug used on the treatment of HIV (Fig. 3) (Rao et al., 2018). Nanosponges provided an enhanced bioavailability of RPV in vitro and in vivo, compared with the administration of free antiviral and RPV:β-CD and RPV:HP-β-CD complexes. Besides that, nanosponges’ high zeta potential prevents the aggregation of the nanoparticles in aqueous solution, allowing the formation of stable dispersions in the media. CD-based nanosponges are a promising drug-delivery platform because of their ability to encapsulate both hydrophilic and lipophilic drugs. On the other hand, Notario-Pérez et al. (Notario-Pérez et al., 2020) synthesized mucoadhesive vaginal discs for the controlled release of HIV antiretroviral drugs, Tenofovir and Dapivirine (Fig. 3). The presence of CDs in freeze-dried hydroxypropylmethyl cellulose gels, allowed the controlled release of the drugs and also modified the mechanical properties of the formulation by improving its resistance as well as the adherence to the vaginal mucosa during enough time to the complete liberation of the antiviral drug.
1.1.3. Cyclodextrins in formulations against SARS-CoV-2
CDs have also been used to encapsulate possible antivirals attacking at different stages on viral lifecycle in the battle against COVID-19. Nitazoxanide (Fig. 3), traditionally an antiparasitic drug, has also demonstrated in vitro antiviral activity against MERS and SARS-CoV-2 (Padmanabhan, 2020). Its interaction with β-CD has been studied by different physicochemical methods, showing that it forms inclusion complexes with 1:1 stoichiometry (Radi et al., 2014). Camostat mesylate (Fig. 3), a pancreatitis agent approved in Japan, has shown to prevent nCoV cell entry in vitro through inhibition of the host serine protease (Hoffmann et al., 2020). Conflicting in vitro data are not able to determine if this drug has a detrimental or protective effect in the treatment for COVID-19. Supramolecular interactions studies with α-, β-, and γ-CD revealed a 1:1 stoichiometry for all the complexes formed (Kwon et al., 2009). The results also suggested that the cavity size of γ-CD, rather than those of a α- or β-CD, is required to accommodate the guanidine group. This study also showed a multimodal molecular encapsulation with this larger CD. The combination of Lopinavir (LPV)/Ritonavir (RTV), approved for treating HIV, demonstrated in vitro activity against other novel coronaviruses via inhibition of 3-chymotrypsin-like protease (Fig. 3) (Sanders et al., 2020). The combination of CDs with these compounds is expected to handle their adverse effects. Based on phase solubility diagrams, Goyal and Vavia (Goyal and Vavia, 2012) found that γ-CD and HP-β-CD form 1:1 complexes with these drugs, so both CDs are expected to be well suited to work as excipients for them. This study also suggested that the presence of non-inclusion complexes could contribute to the considerable solubilisation enhancing of LPV. Recently, γ-CD with a high degree of 2-hydroxypropyl substitutions (HP17-γ-CD) proved to considerably increase the solubility of LPV (Adeoye et al., 2020). The preparation method of the complex has also been discussed, showing higher solubility improvement by supercritical assisted spray drying (SASD) compared with co-evaporation (CoEva). Very recently, a third active compound, interferon β-1b, was added to this cocktail with promising results (Hung et al., 2020). Remdesivir (GS-5734) (Fig. 3) has been brought up as a hopeful antiviral for treating Covid-19 (Wang et al., 2020a, Wang et al., 2020b). This compound is relatively insoluble and chemically unstable in aqueous media. In order to increase its solubility, Gilead Inc. patented last 2019 a method for comprising Remdesivir, jointly dispensed with CDs (Larson, 2019). Remdesivir was first described to specifically treat the Ebola Virus Disease, but simultaneously presented as a broad spectrum antiviral (with potential to act in vitro against other pathogenic RNA viruses, including filoviruses, arenaviruses, and coronaviruses) (Warren et al., 2016). In this first study, the vehicle used for carrying the drug consisted already in a solution of 12% SBE-CD. Other clinical trials for SARS-CoV and MERS-CoV virus have explicitly mentioned the usage of CDs as vehicle for Remdesivir (at the same percentage) (de Wit et al., 2020, Sheahan et al., 2020, Sheahan et al., 2017). They found that indeed this CD derivate is useful for an intravenous solution and showed how to prepare it. In this study, it was assumed a 1:1 complex, in agreement with the experimental observation that the drug solubility and the amount of CD are linearly dependent. It should also be remarked that upon addition of NaOH, the complex did not break, which was surprising, in their own words, as increasing the pH can result in weakened or broken complexation. SBE-β-CD can be used for parenteral administration of the drug, but it has been reported to exhibit physiological adverse effects on kidney. The antimalarial drug, chloroquine (Fig. 3) and its derivate, hydroxychloroquine, have been also been tested in the treatment of patients infected by SARS-CoV-2 (Gao et al., 2020, Touret and de Lamballerie, 2020). The enantioseparation of this racemic drug has been studied by several groups using CDs as chiral selectors, suggesting that the presence of carboxyl or sulphate functional groups on CD derivatives is advantageous for the resolution of chloroquine enantiomers (Németh et al., 2011). Despite being a hydrophilic drug, it was shown that both solubility and bioavailability of chloroquine diphosphate could be enhanced and some of its side effects reduced after encapsulation with CDs, in a 1:1 stoichiometry (ASHP, 2020, Roy et al., 2020). However, recent studies have suggested a limited efficacy of both drugs against COVID-19 (Geleris et al., 2020, Vinetz, 2020).
1.2. Collaborative weapons: Cyclodextrins as stabilizers or co-drugs
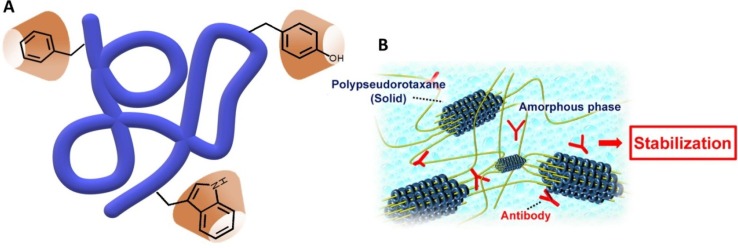
As it was shown with the Last Alliance of Elves and Men in response to the threat of conquest by the Dark Lord Sauron, unity makes strength, and that should be considered in any battle. Synergy between different combined compounds often results to be much more powerful than additive effect of different separate compounds. It has been shown that CDs can strengthen the effect of other molecules, including monoclonal antibodies as well as other proteins, peptides or even nucleotides, to enhance their activity in the combat against pathogenic agents (Fig. 4 A). Therapeutic proteins are often combined with CDs in pharmaceutical formulations to stabilize their structure against denaturation and aggregation during storage and shipment (Castellanos et al., 2006, Fernández et al., 2004, Serno et al., 2011, Serno et al., 2010, Tavornvipas et al., 2004). Monoclonal antibodies directed against key inflammatory cytokines or other aspects of the immune response represent another potential class of adjunctive therapies for COVID-19. The rationale for their use is that the underlying pathophysiology of significant organ damage in the lungs and other organs is caused by an amplified immune response and cytokine release, or “cytokine storm”. (Mehta et al., 2020). More than 130 monoclonal antibodies have already been approved for clinical use. Moreover, a broad range of therapeutic antibodies are also undergoing clinical trials (Lu et al., 2020, Marichal-Gallardo and Álvarez, 2012). The main part of these formulations is administered parenterally. However, pulmonary route is a potential alternative for local and/or systemic delivery of proteins/peptides (Bodier-Montagutelli et al., 2018, Kane et al., 2013). There are several obstacles in the formulation of inhalable proteins or peptides including intrinsic instability and drug permeation across the nasal membrane (Bajracharya et al., 2019, Schüle et al., 2008). It has been demonstrated that HP-β-CD and β-CD have a marked effect in the stability and particle properties of spray-dried IgG. The type of excipients as well as its concentration in antibody formulation influenced yield, particle size, aerodynamic behavior and antibody stability. In particular, HP-β-CD not only enhanced aerosol performance of the powder, but also contributed to preserve the secondary structure of the protein and inhibited its aggregation during spray drying. Optimal particle property and antibody stability was obtained with proper combination of CDs and simple sugars, such as trehalose (Ramezani et al., 2017). Recent findings have also suggested that polypseudorotaxane hydrogels consisting of α- or γ-CD and PEG (mw 20,000 Da) markedly improved stabilities of human IgG against heating and shaking (Higashi et al., 2015), suggesting them as stabilizers for antibody drugs without changing their pharmacokinetics (Fig. 4B) (Higashi, 2019).
Fig. 4.
A. Representation of CDs interacting with a protein; the classical inclusion involves aromatic aminoacids. B. Stabilization of Antibodies by Polypseudorotaxane Hydrogels (reproduced with permission from Yakugaku Zasshi, 139, 175–183. Copyright 2019. The Pharmaceutical Society of Japan) (Higashi et al., 2019).
Based on early case series from China, it has been reported that interleukin 6 (IL-6) could be a key driver in the dysregulated inflammation caused by COVID-19 (Zhou et al., 2020). Thus, monoclonal antibodies against IL-6 could theoretically dampen this process and improve clinical outcomes. Some of the monoclonal antibody IL-6 receptor antagonists employed in COVID-19 treatments are Tocilizumab (Lauder et al., 2013, Xu et al., 2020) and Sarilumab (Sanofi, 2020). Other monoclonal antibody or immunomodulatory agents in clinical trials in China or available for expanded access in the US include Bevacizumab (anti–vascular endothelial growth factor medication; NCT04275414), Eculizumab (antibody inhibiting terminal complement; NCT04288713) and Fingolimod (immunomodulator approved for multiple sclerosis; NCT04280588). It was found that low amounts of Fingolimod in a free form, as a salt, or as a phosphate derivative could be stabilized for long periods of time, using CDs, for oral administration. The use of the CD in the formulation process permits the blending of the different ingredients (active substance and excipients) in such a way that a mixture of uniform particle size is obtained and thus an even distribution of the drug content in the final composition is ensured (Rane, 2017). Several companies such as Regeneron, Wuxi Biologics, CytoDyn and Vir Biotechnology are trying to speed up the development of neutralizing antibodies against coronavirus (Bloomberg, 2020, CytoDyn, 2020, Pharma Advancement, 2020, Regeneron, 2020), based on previous successful treatments that include monoclonal antibodies to specifically target other viruses. An advantage of this strategy is the associated long-term effects of the resulting treatment.
On the other hand, interferons (IFNs) are a group of signaling proteins (De Andrea et al., 2002) made and released by host cells in response to the presence of different viruses. In a typical scenario, a virus-infected cell releases interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens (Parkin and Cohen, 2001). Their name comes from their ability to “interfere” with viral replication by protecting cells from virus infections. IFN-α and IFN-β have been studied for nCoVs. IFN-β demonstrated significant activity against MERS (Morra et al., 2018, Stockman et al., 2006). β-CD (Miertus et al., 1999) and HP-β-CD (Del Curto, 2010) has been suggested to play a role in the stabilization of this protein. The eventual protection of IFNs, based on patients of COVID-19 taking them for other indications (Totura and Bavari, 2019), is not clear and some conflicts between in vitro and in vivo with animal data were found. As it was mentioned in the previous section, very recently, the combination of IFN-β with Lopinavir (LPV) and Ritonavir (RTV) showed promising results (Hung et al., 2020).
CDs are also proving to be extremely valuable in prevention against viral attacks on the mucous membranes in the mouth, throat and nose, which are the major source of SARS-CoV-2 exchange through expectorations and inhalation of particles from air. Some new generation mouth rinses could contribute to lower the viral load, thus preventing oral transmission. In particular, CDs have been combined with Citrox and then tested as therapeutic oral and/or nasal rinses with the aim to reduce viral load of saliva and nasopharyngeal microbiota, including potential SARS-CoV-2 carriage. Taking advantage of the vulnerability of the virus to oxidation, the use of oxidative amphiphilic CDs has also been proposed as a complementary strategy to reduce viral load in mucous membranes (Carrouel et al., 2020a, Carrouel et al., 2020b).
Recently, Sun et al. (2020) discussed the mechanism and production of CD-soluble angiotensin-converting enzyme 2 (CD-sACE2) inclusion compounds in the treatment of SARS-CoV-2 infections by blocking S-proteins. sACE2, the extracellular region of ACE2 retains the enzyme activity of ACE2 and can bind to the S-protein of SARS-CoV, being able to inhibit SARS-CoV infected cells. Since the infection mechanism of SARS-CoV and SARS-CoV-2 is basically the same, sACE2 could also inhibit the infection of SARS-CoV-2. The formation of a complex of CD and sACE2 could effectively improve the water solubility of sACE2 so it meets the requirements for drug atomization inhalation. The inclusion conjugates release sACE2 after entering the body via atomization or other drug delivery means, and the released sACE2 would combine with SARS-CoV-2 S-proteins to block the virus's ability to infect and destroy human cells.
1.3. Using the ring as a direct weapon: Modified cyclodextrins as antiviral drugs
Like the Ring coveted by the deranged bipolar Gollum, each CD is unique and has properties that make it different from the others. A given CD sequence, i.e. the type of substitution in every glucopyranoside unit and the number of such units, endows the entire structure with specific properties. Actually, the versatility of CDs relies on the possibility to introduce different chemical substitutions in the primary or secondary hydroxyl groups, as well as to control the ratio of substituted groups. It is possible to take advantage of this feature to transform CDs in powerful weapons against a variety of targets. It has been demonstrated that modified CDs can directly act as effective broad-spectrum antivirals. Thus, they can be potent weapons to fight multiple viral infections. S. T. Jones et al, synthesized very recently (Jones et al., 2020) several biocompatible sulfonated CDs that proved to be active against a large number of herpes simplex HS-dependent viruses (Fig. 5 ). These CDs are broad-spectrum virucidal, i.e., they definitely destroy the virus, in contrast to virustatic agents that just block some of their action mechanisms. Additionally, they present a high barrier to viral resistance, they are biocompatible at μM concentrations and not cytotoxic. Several linkers were tested to join the sulfonic unit to the CD (Fig. 5) showing that its length and flexibility is key to provide virucidal activity. The known ability of CDs to extract cholesterol from membranes has also been proposed as an alternative viricidal action mechanism (vide infra). However, essays in a cholesterol rich medium highlighted that β-CD with a sulfonic group linked by an aliphatic chain of 11 carbon atoms (CD1) is antiviral in a cholesterol-independent manner. Molecular Dynamics simulations were carried out in order to determine the action mechanism of these modified CDs in the presence of glycoprotein B (gB) from HSV-2, suggesting that the CDs interacts with the binding loop of gB, producing a conformational change in the protein and thus rendering it unable to attack the host cell. The molecule has been patented and a spin-out company is being set up to continue pushing this new antiviral towards real-world use, but as far as we know it has not been tested towards SARS-CoV-2.
Fig. 5.
Structures of modified CDs and relative effective concentrations of inhibition of HSV-2 growth. Representative snapshots of the modified CDs interacting with HSV-2 gB proteins after 50 ns of Molecular Dynamics simulations. Reproduced (with corrections over the original) with permission from Science Advances, 6, 5, eaax9318. © The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/ (Jones et al., 2020).
Another study, published prior to the COVID-19 pandemic, had already suggested different modified CDs as good antiviral candidates (Pan et al., 2015). Adenovirus was chosen as a model system due to its known resistance to a broad spectrum of antiviral drugs. Here, novel polycationic star polymers with polyhexamethylene guanidine hydrochloride (PHMG) have been demonstrated to possess excellent antimicrobial and virucidal activity, which can be modulated by modifications in the topological structure (i.e., the arm number and the monomer ratio) of the composing copolymers (Fig. 6 A). Plaque assay demonstrated that the star polymer inhibits the binding of adenovirus to its cellular receptor, then blocking the cell entry, with a virucidal efficiency of 96.3% at 50 ppm. Other action mechanisms, such as cholesterol depletion, were discarded since adenovirus lacks a lipid envelope. Cytotoxicity of these structures was assessed and found to be negligible under 50 ppm concentration. Other CD derivatives with antiviral activity include conjugate compounds of γ-CD with fullerenes –showing significant antiviral activity for influenza and twice as much for OSV (Zhu et al., 2018) or terpene derivatives (Tian et al., 2017, Xiao et al., 2016) On the side of the terpene derivatives, Sulong Xiao et al. studied a series of multivalent pentacyclic triterpene-CD conjugates displaying strong anti-influenza virus activity comparable and even higher than that of Oseltamivir with a low cytotoxicity (Fig. 6B) (Xiao et al., 2016). Multiple pentacyclic triterpenes were grafted to the primary face of α-, β- and γ-CD scaffolds. They show high affinity to influenza HA protein, disrupting the interaction of hemagglutinin (a protein of the virus surface) with the sialic acid receptor and thus the attachment of viruses to host cells. A strong correlation between both the nature of the CD and the terpene and the virucidal properties was found. Too large or too small CD ring cavities were found to cause an important decrease on activity, playing the number of ligands also an important role. The structures that proved to be the most effective against influenza virus were those based on β-CD with seven pentacyclic terpenes tethered to it, with a promising IC50 of 1.60 μM, whereas virucidal activty of the isolated ligand was only detected at concentrations above 100 μM. Cytotoxic assays showed that the range of concentrations for virucidal activity is far below the concentration that must be reached before any harmful effects start to take place in the cells (>100 μM). This group also essayed a similar strategy but using per-O-methylated β-CD (Fig. 6B) (Tian et al., 2017) and, more recently, they also investigated β-CD-GA conjugates, in which a major constituent of the herb Glycyrrhiza glabra (Glycyrrhetinic acid), was covalently coupled to the primary face of β-CD using 1,2,3-triazole moiety along with varying lengths of linker. These structures provided promising results to be used as potential anti-influenza virus agents (Liang et al., 2019). All these new CDs are non-toxic against host cells and they show significant antiviral activity, but the data is still limited to in vitro studies and more research on these molecules is required to fully understand their practical utility.
Fig. 6.
A. Novel star-like polymers used for deactivating bacteria and viruses. Adapted from (Pan et al., 2015). B. Top: Multivalent pentacyclic triterpene-CD conjugates (using per-O-methylated β-CD) displaying strong anti-influenza virus activity. Reproduced with permission from European Journal of Medicinal Chemistry, 134, 133-139. Copyright 2017. Elsevier. (Tian et al., 2017). Down: Proposed mechanism for multivalent pentacyclic triterpene-functionalized per-O-methylated CD conjugates mediated blocking of influenza virus entry, wherein the multivalent pentacyclic-triterpenes tightly bind to viral homotrimeric HA protein, thus disrupting the attachment of viruses to host cells. Reproduced with permission from European Journal of Medicinal Chemistry, 134, 133-139. Copyright 2017. Elsevier. (Tian et al., 2017).
Recently, we discuss the mechanism and production of cyclodextrin-soluble angiotensin-converting enzyme 2 (CD-sACE2) inclusion compounds in the treatment of SARS-CoV-2 infections by blocking S-proteins. On the basis of the current research evidence, we believe that CD-sACE2 inclusion compounds have the potential to treat COVID-19.
1.4. Sentinels to prevent invasions: Cyclodextrins in antiviral vaccines
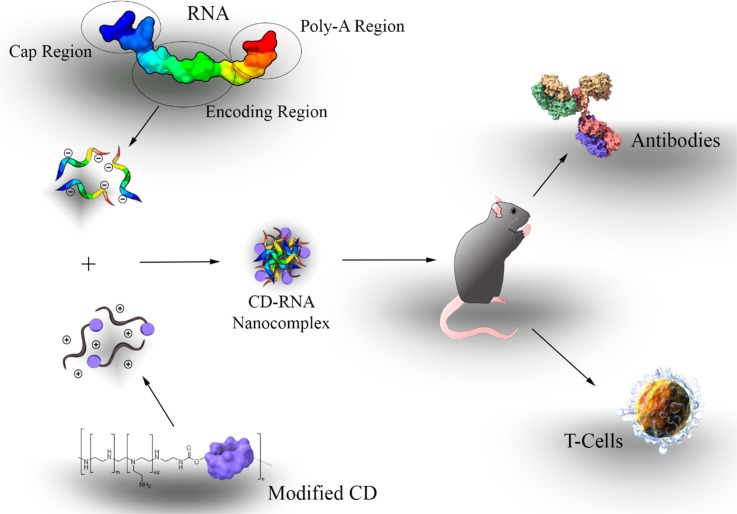
The production of stable vaccines for COVID-19 is a global urgency, with a large number of initiatives in progress since the release of the 2019-nCoV genetic sequence in early January 2020. Most projects are based on non-living subunits and mRNA technologies, which require efficient, stable and safe adjuvant components to stimulate immunity (Fig. 7 ). Intranasal vaccination with inactivated viral antigens is an attractive and valid alternative to currently available influenza (flu) vaccines. T. Kusakabe et al. examined whether HP-β-CD could act as a mucosal adjuvant for this treatment (Kusakabe et al., 2016). HP-β-CD seems to prevent the production of active immunoglobulins E (IgE), which can lead to autoimmunity; in contrast to classical adjuvants such as alum, which is well known to cause this problem. Thus, the use of HP-β-CD reduces the risk of inducing allergic IgE responses. Nevertheless, injection of HP-β-CD can also provoke some acute inflammation in lymphatic nodes but no systemic cytokine response was observed after injection in mice (Onishi et al., 2015). HP-β-CD also induced dendritic cells maturation and its subsequent activation of the T cells. It augmented the antigen-specific IgG in the blood. HP-β-CD predominantly induced IgG1 but not IgG2, indicating preferential enhancement of Th2 responses rather than Th1 responses. Type 2 T-helper (Th2) cell response, enhances antigen (vaccine)-specific antibody titers, maintains a longer immune response and induces the maturation of dendritic cells vital for a long lasting effective immune response (Kim et al., 2016, Onishi et al., 2015). HP-β-CD was selected because it has proved to be highly biocompatible. A common influenza vaccine containing 30% of HP-β-CD as adjuvant increased in the production of antibodies and produced a 100% survival rate in mice challenged with a lethal dose of influenza virus. The response produced by the vaccine takes place in every part of the body and does not remain localised at the administration site (Kim et al., 2016). It was also found that intranasal or subcutaneous administration of HP-β-CD increased the immunogenicity of influenza vaccines. HP-β-CD seems to be appropriate as a mucosal adjuvant because it induces antigen-specific IgA and IgG in the airway mucosal tissues as well as in the blood.
Fig. 7.
Scheme of a CD-based mRNA vaccine platform, with an optimized mRNA structure and administrated in a most appropriate route. Adapted from (Tan et al., 2020).
Vaccine compositions comprising inactivated poliovirus in combination with CDs, both natural and derivatized, wherein the CDs thereof protects and preserves the antigenicity and/or immunogenicity of inactivated poliovirus, especially in presence of thiomersal has also been patented (Chacornac et al., 2016). The authors have unexpectedly found that CDs are capable of minimizing the degradation of IPV (Inactivated Polio Vaccine or Virus) in presence of thiomersal, in particular in the situations described above. Whereas CDs have a wide range of applications in different areas of drug delivery and pharmaceutical industry, they have never been reported as protectant of IPV antigenicity and/or immunogenicity. More particularly, the inventors observed that CDs are capable of minimizing the loss of D-antigen titer of IPV blended to a composition comprising thiomersal and alum. The composition comprises at least one type of CD, preferably γ-CD or β-CD and hydroxypropyl, hydroxyethyl, glucosyl, maltosyl or maltotriosyl CD derivatives.
Based on this precedents, some researchers suggest the potential application of HP-β-CD as an adjuvant in developing successful vaccines for 2019-nCoV prevention (Roquette, 2020). Daiichi Sankyo is already conducting a Phase I clinical trial in Japan for their HP-β-CD adjuvanted influenza split vaccine Cyclodextrin News, 2018), which started in October 2017 and it can be the first CD-adjuvated vaccine.
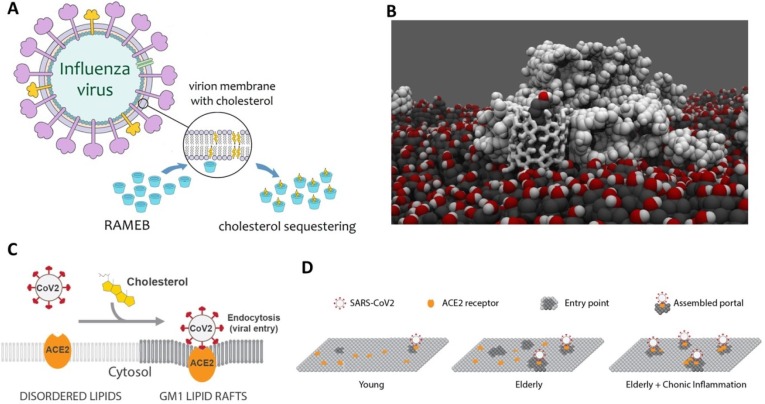
1.5. Trying to break the enemy’s borders: Cyclodextrins as cholesterol trappers to damage the viral capsid membrane
Lipid membranes are key structural part of many viruses, including SARS-CoV-2. Their main functions are to encapsulate the genetic material and also to interact with the environment through a number of partially-embedded partially-protruding proteins. The disruption of this membrane definitely destroys the virus. The presence of lipid rafts in such membranes is also though to play an important role in the virus life cycle (Li et al., 2007, Lorizate and Kräusslich, 2011, Mazzon and Mercer, 2014, Nayak and Hui, 2004, Ono and Freed, 2005, Schmitt and Lamb, 2005). Cholesterol, which is known to participate in the integrity and fluidity of bilayers, is one of the main components of these lipids rafts (Krause and Regen, 2014). Several diseases, such as Alzheimer, cancer, and atherosclerosis, have been related to the presence of cholesterol and other sterol derivatives in biological membranes (Anderson et al., 2020, Taylor and Hooper, 2007). The fluidity of membranes is a key point in the interaction between viruses and cells (Yang et al., 2016). Zawada et al. found that Influenza virions are enriched in cholesterol with respect to healthy cell membranes (Zawada et al., 2016). Additionally, they proposed that whereas this process is sensitive to concentration, it is not dependent on sterol identity for any of the sterols that they tested. Other studies revealed the requirement for cholesterol in liposomes for the fusion of Influenza virus (Nussbaum et al., 1992), as well as the clear effect of such molecule in the kinetics of this process (Domanska et al., 2013).
CDs have shown to be able to effectively extract and encapsulate cholesterol from lipid membranes (Crini, 2014, Leclercq, 2016, Mahammad and Parmryd, 2015, Nishijo et al., 2003, Zidovetzki and Levitan, 2007). A large evidence and quantitative analysis of cholesterol trapping by different CD derivatives has been carried out during the last two decades by different experimental and theoretical methods. It was concluded that β-CDs are more prone to encapsulate sterol molecules than α-CD and γ-CD (Ohtani et al., 1989, Ohvo and Slotte, 1996). Although this selectivity has been attributed to the differences in the cavity size, it is important to note that the inner cavity of a single β-CD is too narrow (diameter of ~8 Å) for totally host a cholesterol molecule (~18 Å wide). This fact, together with a quantitative thermodynamic analysis, suggests that two stacked β-CDs are required to encapsulate this molecule (Tsamaloukas et al., 2005). Another important parameter to take into account for an effective trapping is the water solubility of the CD itself (Davis and Brewster, 2004). The solubility of native β-CD is relatively low (about one order of magnitude lower than that of α-CD and β-CD). In order to increase it, a number of β-CD derivatives have been synthesized introducing different chemical modifications in the hydroxyl groups at positions 2, 3 and 6 or the glucopyranoside units. HP-β-CD and Me-β-CDs are probably the most used in cell biology studies (Uekama, 2004). Me-β-CDs has shown to be more efficient than HP and tetradecasulfated-β-CDs to encapsulate cholesterol (Atger et al., 1997, Christian et al., 1999, Christian et al., 1997, Kilsdonk et al., 1995). The extraction of cholesterol from lipid bilayers has also been simulated by Coarse-Grained Molecular Dynamics simulations using a constrained Me-β-CD dimer (Lopez et al., 2013, López et al., 2011) (Fig. 8 B).
Fig. 8.
A. Schematic representation of the mode of action proposed for the RAMEB against influenza viral particles. RAMEB sequesters membrane cholesterol, resulting in damage to the integrity of the viral envelope. Reproduced from (Braga, 2019) under Creative Common CC BY license. B. Proposed model for CD-mediated cholesterol extraction from lipid model membranes, based on Molecular Dynamics simulations: Reproduced from (López et al., 2011) under Creative Commons Attribution License. C. Proposed model for infectivity in SARS-CoV-2 in presence of cholesterol (lipid rafts) and with age and chronic disease (D). When cholesterol is low there are very few entry points and their diameter is small (similar to children). With age, average cholesterol levels in the tissue increase, thereby increasing the number and size of viral entry points (similar to adults). Reproduced from (Wang et al., 2020a), under CC-BY-NC-ND 4.0 International license.
Taking advantage of such cholesterol trapping capabilities and considering the excess of cholesterol in viral envelopes with respect to healthy cells, the use of β-CD derivatives to treat viral infections has been suggested (Braga, 2019). It has been recently demonstrated that randomly methylated β-CDs (RAMEB) are able to reduce the infectivity of viral particles of influenza A (H1N1 strain) by cholesterol depletion (Sun and Whittaker, 2003, Verma et al., 2018). The proposed mechanism (Fig. 8A) is that the extraction of cholesterol by CDs from the lipid rafts lead to the structural deformation of the viral membrane, being even possible to form holes (Barman and Nayak, 2007).
Me-β-CDs have also been used to inhibit the attachment of coronaviruses to host cells, again via cholesterol depletion (Guo et al., 2017). Moreover, Choi et al. have shown that this CD provides good activity in the treatment of murine coronavirus (Choi et al., 2005). In this case, the use of the CD does not affect the virus binding, but it is able to reduce the virus entry and the cell–cell fusion by reducing the amount of cholesterol present in the membrane. The efficiency of Me-β-CDs against coronavirus, influenza A, and canine coronavirus infections have been also probed in Vero E6 cell lines following the same mechanism (Lorizate and Kräusslich, 2011, Lu et al., 2008). Furthermore, Tang et al. have highlighted the role of the cholesterol in the Parainfluenza Virus Type 3, observing a markedly reduction in the infectivity of this virus again via the elimination of cholesterol by Me-β-CDs (Tang et al., 2019).
The importance of the cholesterol in the initial steps of SARS-CoV infection has already been proved (Glende et al., 2008, Meher et al., 2019). Additionally, very recently it has been found an abnormally low cholesterol concentration in serum among patients with COVID-19 infection, claiming again that it is playing a significant role (Hu et al., 2020). The use of CDs for the treatment of viral infections, trying to use their cholesterol depletion capabilities in order to inhibit the virus contagion, is becoming increasingly popular (Baglivo et al., 2020). HIV and hepatitis C have been successfully treated in this way already for a long time, reaching almost complete abolishment of infectivity by HIV-1 virions (Graham et al., 2003, Guyader et al., 2002, Sagan et al., 2006, Tang et al., 2012). The ability of these CDs to reduce viral infectivity has also been demonstrated against human metapneumovirus (HMPV) (Chen et al., 2019), herpes simplex virus 1 (HSV-1) (Wudiri et al., 2017, Wudiri and Nicola, 2017), varicella-zooster virus (VZV) (Hambleton et al., 2007) and a less common one, the Newcastle disease virus (NDV) (Cantín et al., 2007, Martín et al., 2012).
Other formulations have also been proposed, as the one developed recently by ASDERA (Advanced Statistics for Drug and Diet Exploration, Repurposing, and Approval). They suggest the use of an intestinally absorbed oral α-CDs against SARS-CoV-2, reducing the serum phospholipids by “intermittent fasting mimetic” (ASDERA, 2020).
In summary, given the significant influence of cholesterol in virus infections, the use of molecules able to specifically target it represent promising weapons against pandemic diseases like COVID-19. Under this panorama, CDs have been confirmed as well suited candidates for this challenge.
1.6. Intercepting messages of the enemy: Cyclodextrins in RNA therapies
RNA interference (RNAi) was proposed in the late 1990s as a method to silence pathogenic gene targets, including viral RNA. This technique consists in using small complementary RNA duplexes to target and neutralize specific mRNA molecules with identical sequence to specifically inhibit gene expression or genetic translation. Preliminary studies with SARS-CoV demonstrated the feasibility of developing siRNAs as effective anti-SARS drugs (Chang and Hu, 2006, Shi et al., 2005, Wang et al., 2004, Zhang et al., 2004). More than thirty patents describing the use of RNAi for therapeutic treatment of SARS can be found in the CAS content collection, including siRNA molecules, antisense oligonucleotides, RNA aptamers, microRNA inhibitors and even ribozyme (Liu et al., 2020). Some of these patents propose siRNAs Targeting Coronavirus Proteins M, N, or E, or the Replicase and RNA Polymerase Region.
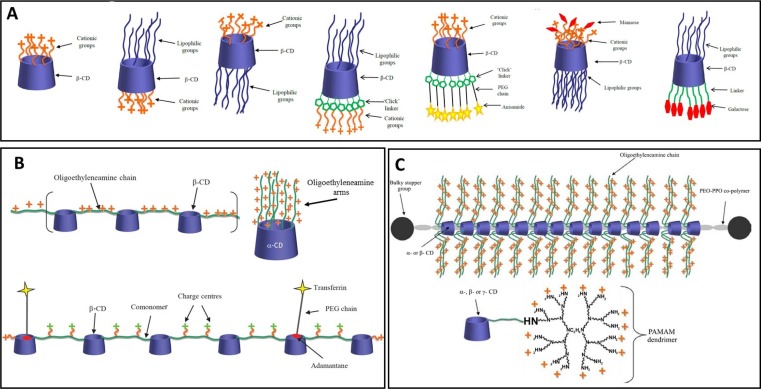
The most important limitation in the use of this technology is the delivery of the RNA-drug to reach the cytoplasm of the target cell maintaining its integrity, since siRNA is highly unstable (half-life < 1 h in human plasma). CDs are well suited to help in this task due to their very low toxicity and versatility to include different chemical modifications (Chaturvedi et al., 2011, Evenou et al., 2018; O’Mahony et al., 2013). CDs have been specifically grafted to self-assemble in the presence of nucleotides thus encapsulating and so protecting them from degradation (Mellet et al., 2011, Sallas and Darcy, 2008). For this aim, cationic modifications in CDs showed to be well suited since DNA and siRNA are negatively charged, so the predominant interaction is through complementary electrostatic charges (Guo et al., 2010). (Fig. 9 A) The additional introduction of hydrophobic groups lead to liquid-crystalline structures which can also be used as gene delivery vectors (Cryan et al., 2004). CDs can also be part of the delivery system to modify its properties. For instance, they have been incorporated to the backbone of polymers (Davis, 2009), appended to pre-existing polymeric or dendrimeric vectors (Fig. 9B) (Arima and Motoyama, 2009, Ping et al., 2011), or in polyrotaxane systems (Fig. 9C) (Li et al., 2006, Yang et al., 2009).
Fig. 9.
A. Examples of functionalised CD gene and siRNA delivery vectors. B. Schematic representations of various CD-containing polymers. C. Cationic polyrotaxanes consisting of oligoethylenimine-conjugated CDs threaded onto co-polymers of poly(propylene oxide) (PPO) and poly(ethylene oxide) (PEO). CDs conjugated to PAMAM dendrimers. Figures reproduced with permission from Pharmaceutical Nanotechnology, 1, 6–14. Copyright 2013. Bentham Science Publishers Ltd. (M. O’Mahony et al., 2013).
Antisense oligonucleotides are promising agents to treat viral infections due to their high specificity against particular species or strains (Agrawal, 1996). However, their therapeutic use is limited by their susceptibility to be degraded by nucleases before reaching their target, although this can be partially avoided by modifying their saccharide backbone. By incorporating β-CD as encapsulating agent in the formulation, a system containing a phosphodiester oligo directed toward the initiation region of the mRNA coding for the spike protein and containing the intergenic consensus sequence of an enteric coronavirus was designed (Abdou et al., 1997). CDs proved to increase the stability of the oligo, by protecting it from degradation. Antisense oligonucleotides have been developed not only for treatment and/or prevention of SARS virus but also for diagnosis purposes. Hybrid DNA/RNA antisense oligonucleotides have been designed to disrupt the pseudoknot in the frameshift site of the SARS coronavirus RNA (Crooke et al., 2008). Other antisense oligonucleotides have been proposed to target proteins involved in inflammatory processes caused by viruses (Liu et al., 2020).
1.7. Blood on the battlefield: Cyclodextrins as anticoagulant agents
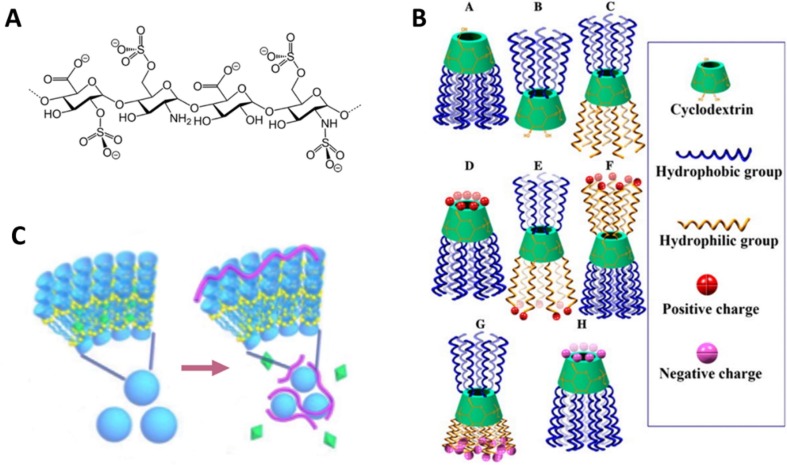
A large range of symptoms have been reported for COVID-19. Coagulopathy and disseminated intravascular coagulation were reported to appear in most of deaths (Tang et al., 2020). Anticoagulant or blood thinner factors such as heparin, have been recommended for critically ill patients, although their efficacy has not been validated yet (Song et al., 2020). Low molecular weight heparins (LMWHs) (Fig. 10 ) are considered to be as effective as unfractionated heparin (UFH) to treat deep vein thrombosis and pulmonary embolism, and it is safer since they provoke a lower immune sensitization (Shulman, 2000). In the same line as other antiviral drugs and vaccines, nasal delivery of these anticoagulant factors has been proposed. In general, this method proved to act quicker than standard subcutaneous administration but in the case of LMWHs, their structural and physicochemical properties (still relatively high molecular weight of ~5000 Da, hydrophilicity and strong negative surface charge) require the assistance of an absorption promoter (Arnold et al., 2002). As stated above, CDs are well suited for this end. In particular, dimethyl-beta-cyclodextrin (DM-β–CD) proved to be highly efficient to enhance absorption of LMWHs both in vitro and in vivo. The action mechanism seems to be the opening of the tight junctions between cells (Yang et al., 2004).
Fig. 10.
A. Heparin 2D structure. B. Examples of various amphiphilic CDs. Neutral (A,B,C), positively (D,E,F) and negatively (G,H) charged, reproduced with permission from Advanced Drug Delivery Reviews, 65, 1215–1233. Copyright 2013. Elsevier (Zhang and Ma, 2013). C. Representation of a co-assembly of amphiphilic multi-charged CDs and vitamin K (represented as green octahedrons). After de arrow, heparin (pink wires) capture and vitamin K releasing process, reproduced with permission from Chemical Communications, 55, 11790–11793. Copyright 2019. Royal Society of Chemistry (Li et al., 2019).
Controversially, CDs have been proposed and tested as heparin antidotes in a recent patent (Meijers et al., 2019). The employed CD includes at least one -S-(Cn-alkylene)-R substituent and it is intended to reverse the effect of anticoagulant agents. These CDs have also been proposed as sensors to detect heparin levels or as heparin traps to reverse surgical procedures, in a similar way Sugammadex (a modified CD that includes eight carboxyl thio ether substituted groups in a γ-CD scaffold) is widely employed to reverse neuromuscular blockade induced by several drugs in general anesthesia (Cyclolab, n.d.). Alveron Pharma is currently running a clinical study with modified CDs that promote blood coagulation by reversing the effect of blood thinners. The advantage of these CDs is that they restore coagulation independently of the type of anticoagulant used, so they are considered as universal coagulants (Thuja Capital, 2019).
New amphiphilic multi-charged CDs (AMCD) have recently been developed to work as anti-heparin coagulants (Fig. 10). Their action mechanism seems to be the assembly around UFH or LMWHs to neutralize their blood-thinner effect. A formulation consisting of AMCD and vitamin K (VK) has also been proposed to simultaneously correct VK deficiency in hemodialysis patients at the same time that heparin is neutralized by the CDs (Li et al., 2019).
In summary, several modified CDs are well suited as highly efficient coagulation restoring factors. A number of proposals have already been developed to this aim and more research is in progress in this field.
2. Conclusions
Making a comparison between CDs and the unique Ring of the legendary novel of The Lord of the Rings, J. R. R. Tolkien, the presence of these molecules in different treatments of COVID-19 have been reviewed. Their applications as encapsulating agents for antiviral drugs, as adjuvants to stabilize proteins or other molecules involved in the infection, adjuvants in vaccines, as cholesterol trappers to destabilize the virus capsid, as carriers for RNA therapies, as antivirals themselves, and even useful in anticoagulant therapies, highlight the great power of this sweet molecules. More than ever, we are in the situation to subscribe the words a thousand times pronounced by the dual character Gollum-Sméagol: “My precious”.
CRediT authorship contribution statement
Pablo F. Garrido: Writing - review & editing. Martín Calvelo: Writing - review & editing. Alexandre Blanco-González: Writing - review & editing. Uxía Veleiro: Writing - review & editing. Fabián Suárez: Writing - review & editing. Daniel Conde: Writing - review & editing. Alfonso Cabezón: Writing - review & editing. Ángel Piñeiro: Conceptualization, Writing - review & editing, Supervision. Rebeca Garcia-Fandino: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Spanish Agencia Estatal de Investigación (AEI) and the ERDF (CTQ2016-78423-R and RTI2018-098795-A-I00), and by the Xunta de Galicia and the ERDF (ED431G 2019/03 and Centro singular de investigación de Galicia accreditation 2016-2019, ED431G/09). R.G.-F. thanks to Ministerio de Ciencia, Innovación y Universidades for a “Ramón y Cajal” contract (RYC-2016-20335). M.C. thanks to Xunta de Galicia for a predoctoral fellowship (ED481A-2017/068). P. F. G. thanks the Spanish Ministry of Economy and Competitiveness and the European Social Fund for his predoctoral research grant, reference BES-2016-076761.
References
- Abdou S., Collomb J., Sallas F., Marsura A., Finance C. Beta-cyclodextrin derivatives as carriers to enhance the antiviral activity of an antisense oligonucleotide directed toward a coronavirus intergenic consensus sequence. Arch. Virol. 1997;142:1585–1602. doi: 10.1007/s007050050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeoye O., Cabral-Marques H. Cyclodextrin nanosystems in oral drug delivery: a mini review. Int. J. Pharm. 2017;531:521–531. doi: 10.1016/j.ijpharm.2017.04.050. [DOI] [PubMed] [Google Scholar]
- Adeoye O., Conceição J., Serra P.A., Bento da Silva A., Duarte N., Guedes R.C., Corvo M.C., Aguiar-Ricardo A., Jicsinszky L., Casimiro T., Cabral-Marques H. Cyclodextrin solubilization and complexation of antiretroviral drug lopinavir: in silico prediction; Effects of derivatization, molar ratio and preparation method. Carbohydr. Polym. 2020;227 doi: 10.1016/j.carbpol.2019.115287. [DOI] [PubMed] [Google Scholar]
- Agrawal S. Antisense oligonucleotides: towards clinical trials. Trends Biotechnol. 1996;14:376–387. doi: 10.1016/0167-7799(96)10053-6. [DOI] [PubMed] [Google Scholar]
- Ain S., Kumar B., Pathak K. Cyclodextrins: versatile carrier in drug formulations and delivery systems. Int. J. Pharm. Chem. Biol. Sci. 2015:5. [Google Scholar]
- Anderson A., Campo A., Fulton E., Corwin A., Jerome W.G., O’Connor M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima H., Motoyama K. Recent findings concerning PAMAM dendrimer conjugates with cyclodextrins as carriers of DNA and RNA. Sensors. 2009;9:6346–6361. doi: 10.3390/s90806346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J., Ahsan F., Meezan E., Pillion D.J. Nasal administration of low molecular weight heparin. J. Pharm. Sci. 2002;91:1707–1714. doi: 10.1002/jps.10171. [DOI] [PubMed] [Google Scholar]
- ASDERA, 2020. ASDERA alpha-cyclodextrin against coronavirus [WWW Document]. URL <http://www.asdera.com/sars-nutrition.html> (accessed 5.20.20).
- ASHP, 2020. Assessment of Evidence for COVID-19-Related Treatments [WWW Document]. URL <https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx> (accessed 5.18.20).
- Atger V.M., De La Llera Moya M., Stoudt G.W., Rodrigueza W.V., Phillips M.C., Rothblat G.H. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 1997;99:773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglivo M., Baronio M., Natalini G., Beccari T., Chiurazzi P., Fulcheri E., Petralia P., Michelini S., Fiorentini G., Miggiano G.A., Morresi A., Tonini G., Bertelli M. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020;91:161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus Interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bajracharya R., Song J.G., Back S.Y., Han H.-K. Recent advancements in non-invasive formulations for protein drug delivery. Comput. Struct. Biotechnol. J. 2019;17:1290–1308. doi: 10.1016/j.csbj.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman S., Nayak D.P. Lipid raft disruption by cholesterol depletion enhances influenza a virus budding from MDCK cells. J. Virol. 2007;81:12169–12178. doi: 10.1128/jvi.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencini M., Ranucci E., Ferruti P., Trotta F., Donalisio M., Cornaglia M., Lembo D., Cavalli R. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a β-cyclodextrin/poly(amidoamine) copolymer. J. Control. Release. 2008;126:17–25. doi: 10.1016/j.jconrel.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Bloomberg, 2020. Vir Biotechnology CEO on Finding a Coronavirus Antibody – Bloomberg [WWW Document]. URL <https://www.bloomberg.com/news/videos/2020-01-29/vir-biotechnology-ceo-confident-coronavirus-vaccine-will-be-found-video> (accessed 5.18.20).
- Bodier-Montagutelli E., Mayor A., Vecellio L., Respaud R., Heuzé-Vourc’h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expert Opin. Drug Deliv. 2018;15:729–736. doi: 10.1080/17425247.2018.1503251. [DOI] [PubMed] [Google Scholar]
- Braga S.S. Cyclodextrins: emerging medicines of the new millennium. Biomolecules. 2019;9:801. doi: 10.3390/biom9120801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantín C., Holguera J., Ferrerira L., Villar E., Muñoz-Barroso I. Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J. Gen. Virol. 2007;88:559–569. doi: 10.1099/vir.0.82150-0. [DOI] [PubMed] [Google Scholar]
- Carrouel F., Conte M.P., Fisher J., Gonçalves L.S., Dussart C., Llodra J.C., Bourgeois D. COVID-19: a recommendation to examine the effect of mouthrinses with β-cyclodextrin combined with citrox in preventing infection and progression. J. Clin. Med. 2020;9:1126. doi: 10.3390/jcm9041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Viennot S., Ottolenghi L., Gaillard C., Bourgeois D. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: a review of the current situation. Nanomaterials. 2020;10:140. doi: 10.3390/nano10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos I.J., Flores G., Griebenow K. Effect of cyclodextrins on α-chymotrypsin stability and loading in PLGA microspheres upon s/o/w encapsulation. J. Pharm. Sci. 2006;95:849–858. doi: 10.1002/jps.20512. [DOI] [PubMed] [Google Scholar]
- Cavalli R., Donalisio M., Civra A., Ferruti P., Ranucci E., Trotta F., Lembo D. Enhanced antiviral activity of Acyclovir loaded into β-cyclodextrin-poly(4-acryloylmorpholine) conjugate nanoparticles. J. Control. Release. 2009;137:116–122. doi: 10.1016/j.jconrel.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Chacornac, I., Francon, A., Vacus, P., 2016. Vaccine composition comprising ipv and cyclodextrins. WO2016012385A1.
- Chan J.F.W., To K.K.W., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z., Hu J. RNAi therapeutics: can siRNAs conquer SARS? Gene Ther. 2006;13:871–872. doi: 10.1038/sj.gt.3302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J.-P., Amoura Z., Rey F., Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Qeios. 2020 doi: 10.5802/crbiol.8. (Preprint) [DOI] [PubMed] [Google Scholar]
- Chaturvedi K., Ganguly K., Kulkarni A.R., Kulkarni V.H., Nadagouda M.N., Rudzinski W.E., Aminabhavi T.M. Cyclodextrin-based siRNA delivery nanocarriers: a state-of-the-art review. Expert Opin. Drug Deliv. 2011;8:1455–1468. doi: 10.1517/17425247.2011.610790. [DOI] [PubMed] [Google Scholar]
- Chen S., He H., Yang H., Tan B., Liu E., Zhao X., Zhao Y. The role of lipid rafts in cell entry of human metapneumovirus. J. Med. Virol. 2019;91:949–957. doi: 10.1002/jmv.25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Aizaki H., Lai M.M.C. Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release. J. Virol. 2005;79:9862–9871. doi: 10.1128/jvi.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian A.E., Haynes M.P., Phillips M.C., Rothblat G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- Christian A.E., Byun H.S., Zhong N., Wanunu M., Marti T., Fürer A., Diederich F., Bittman R., Rothblat G.H. Comparison of the capacity of β-cyclodextrin derivatives and cyclophanes to shuttle cholesterol between cells and serum lipoproteins. J. Lipid Res. 1999;40:1475–1482. [PubMed] [Google Scholar]
- Coelho Paraguassu E., Chen H., Zhou F., Xu Z., Wang M. Coronavirus and COVID-19: the latest news and views from the scientific community about the new coronavirus and COVID-19. Braz. J. Implantol. Health Sci. 2020;2:96–109. doi: 10.36557/2674-8169.2020v2n3p96-109. [DOI] [Google Scholar]
- Conceição J., Adeoye O., Cabral-Marques H.M., Lobo J.M.S. Cyclodextrins as excipients in tablet formulations. Drug Discov. Today. 2018;23:1274–1284. doi: 10.1016/j.drudis.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Crini G. Review: a history of cyclodextrins. Chem. Rev. 2014;114:10940–10975. doi: 10.1021/cr500081p. [DOI] [PubMed] [Google Scholar]
- Crooke, S.T., Ecker, D.J., Sampath, R., Freier, S.M., Massire, C., Hofstadler, S.A., Lowery, K.S., Swayze, E.E., Baker, B.F., Bennett, C.F., 2008. Compositions and methods for the treatment of severe acute respiratory syndrome (SARS). WO2005023083A2.
- Cryan S.A., Donohue R., Ravoo B.J., Darcy R., O’Driscoll C.M. Cationic cyclodextrin amphiphi]es as gene delivery vectors. J. Drug Deliv. Sci. Technol. 2004;14:57–62. doi: 10.1016/s1773-2247(04)50006-0. [DOI] [Google Scholar]
- Cyclodextrin News, 2018. Cyclodextrin News [WWW Document]. URL <https://cyclodextrinnews.com/2018/09/25/a-clinical-phase1-study-of-hydroxypropyl-beta-cyclodextrin-hpbcd-adjuvanted-influenza-split-vaccine/> (accessed 5.18.20).
- Cyclolab, n.d. Cyclodextrins as APIs [WWW Document]. URL <https://cyclolab.hu/userfiles/LMWH antidote.pdf> (accessed 5.18.20).
- CytoDyn, 2020. Leronlimab Under Evaluation for Potential Treatment of Coronavirus :: CytoDyn Inc. (CYDY) [WWW Document]. URL <https://www.cytodyn.com/newsroom/press-releases/detail/379/leronlimab-under-evaluation-for-potential-treatment-of> (accessed 5.18.20).
- Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- De Andrea M., Ravera R., Gioia D., Gariglio M., Landolfo S. The interferon system: an overview. Eur. J. Paediatr. Neurol. 2002;6:A41–A46. doi: 10.1053/ejpn.2002.0573. [DOI] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Curto, M.D., 2010. Stabilized interferon liquid formulations. WO2005058346A1.
- Domanska M.K., Wrona D., Kasson P.M. Multiphasic effects of cholesterol on influenza fusion kinetics reflect multiple mechanistic roles. Biophys. J. 2013;105:1383–1387. doi: 10.1016/j.bpj.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb-Zohar K., Kropeit D., Scheuenpflug J., Stobernack H., Hulskotte E., van Schanke A., Zimmermann H., Rübsamen-Schaeff H. Intravenous hydroxypropyl β-cyclodextrin formulation of letermovir: a phase I, randomized, single-ascending, and multiple-dose trial. Clin. Transl. Sci. 2017;10:487–495. doi: 10.1111/cts.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenou P., Rossignol J., Pembouong G., Gothland A., Colesnic D., Barbeyron R., Rudiuk S., Marcelin A.-G., Ménand M., Baigl D., Calvez V., Bouteiller L., Sollogoub M. Bridging β-cyclodextrin prevents self-inclusion, promotes supramolecular polymerization, and promotes cooperative interaction with nucleic acids. Angew. Chem. Int. Ed. 2018;57:7753–7758. doi: 10.1002/anie.201802550. [DOI] [PubMed] [Google Scholar]
- Fernández M., Villalonga M.L., Fragoso A., Cao R., Villalonga R. Effect of β-cyclodextrin-polysucrose polymer on the stability properties of soluble trypsin. Enzyme Microb. Technol. 2004;34:78–82. doi: 10.1016/j.enzmictec.2003.09.003. [DOI] [Google Scholar]
- French D. The schardinger dextrins. Adv. Carbohydr. Chem. 1957;12:189–260. doi: 10.1016/S0096-5332(08)60209-X. [DOI] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(2020):01047. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glende J.H., Pfefferle S., Drosten C., Schwegmann-Weßels C., Herrler G. Lipid microdomains are important for the entry process of SARS coronavirus to target cells. FASEB J. 2008;22:282. doi: 10.1096/fasebj.22.2_supplement.282. [DOI] [Google Scholar]
- Goyal G., Vavia P.R. Complexation approach for fixed dose tablet formulation of lopinavir and ritonavir: an anomalous relationship between stability constant, dissolution rate and saturation solubility. J. Incl. Phenom. Macrocycl. Chem. 2012;73:75–85. doi: 10.1007/s10847-011-0022-7. [DOI] [Google Scholar]
- Graham D.R.M., Chertova E., Hilburn J.M., Arthur L.O., Hildreth J.E.K. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with β-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 2003;77:8237–8248. doi: 10.1128/jvi.77.15.8237-8248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grancher N., Venard V., Kedzierewicz F., Ammerlaan W., Finance C., Muller C.P., Le Faou A. Improved antiviral activity in vitro of ribavirin against measles virus after complexation with cyclodextrins. Antiviral Res. 2004;62:135–137. doi: 10.1016/j.antiviral.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Yu., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang Jin-lin, Liang Z., Peng Y., Wei L., Liu Y., Hu Ya.-hua., Peng P., Wang Jian-ming, Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Fisher K.A., Darcy R., Cryan J.F., O’Driscoll C. Therapeutic targeting in the silent era: advances in non-viral siRNA delivery. Mol. BioSyst. 2010;6:1143–1161. doi: 10.1039/c001050m. [DOI] [PubMed] [Google Scholar]
- Guo H., Huang M., Yuan Q., Wei Y., Gao Y., Mao L., Gu L., Tan Y.W., Zhong Y., Liu D., Sun S. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Kiyokawa E., Abrami L., Turelli P., Trono D. Role for human immunodeficiency virus Type 1 membrane cholesterol in viral internalization. J. Virol. 2002;76:10356–10364. doi: 10.1128/jvi.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S., Steinberg S.P., Gershon M.D., Gershon A.A. Cholesterol dependence of varicella-zoster virion entry into target cells. J. Virol. 2007;81:7548–7558. doi: 10.1128/jvi.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T. Cyclodextrin-based molecular accessories for drug discovery and drug delivery. Chem. Pharm. Bull. 2019;67:289–298. doi: 10.1248/cpb.c18-00735. [DOI] [PubMed] [Google Scholar]
- Higashi T., Tajima A., Ohshita N., Hirotsu T., Hashim I.I.A., Motoyama K., Koyama S., Iibuchi R., Mieda S., Handa K., Kimoto T., Arima H. Design and evaluation of the highly concentrated human IgG formulation using cyclodextrin polypseudorotaxane hydrogels. AAPS PharmSciTech. 2015;16:1290–1298. doi: 10.1208/s12249-015-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T., Motoyama K., Arima H. Supramolecular pharmaceutical sciences: a novel concept for future pharmaceutical sciences. Yakugaku Zasshi. 2019;139:175–183. doi: 10.1248/yakushi.18-00168-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Chen D., Wu L., He G., Ye W. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou, China. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3544826. [DOI] [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J.W.-M.J.J.F.-W., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M.W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M.J.J.F.-W., Yan W.-W., Chan W.-M.W.-M., Chan J.W.-M.J.J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannazzo D., Mazzaglia A., Scala A., Pistone A., Galvagno S., Lanza M., Riccucci C., Ingo G.M., Colao I., Sciortino M.T., Valle F., Piperno A., Grassi G. β-Cyclodextrin-grafted on multiwalled carbon nanotubes as versatile nanoplatform for entrapment of guanine-based drugs. Colloids Surfaces B Biointerfaces. 2014;123:264–270. doi: 10.1016/j.colsurfb.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Jeulin H., Venard V., Carapito D., Finance C., Kedzierewicz F. Effective ribavirin concentration in mice brain using cyclodextrin as a drug carrier: evaluation in a measles encephalitis model. Antiviral Res. 2009;81:261–266. doi: 10.1016/j.antiviral.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vuković L., Král P., Kaiser L., Huang S., Constant S., Kirkegaard K., Boivin G., Stellacci F., Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6:eaax9318. doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane C., O’Neil K., Conk M., Picha K. Inhalation delivery of protein therapeutics. Inflamm. Allergy - Drug Targets. 2013;12:81–87. doi: 10.2174/1871528111312020002. [DOI] [PubMed] [Google Scholar]
- Khan N.A., Durakshan M. Cyclodextrin: an overview. Int J Bioassays. 2013;2:858–865. [Google Scholar]
- Kilsdonk E.P.C., Yancey P.G., Stoudt G.W., Bangerter F.W., Johnson W.J., Phillips M.C., Rothblat G.H. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Yun C.-H., Han S.H. Induction of dendritic cell maturation and activation by a potential adjuvant, 2-hydroxypropyl-β-cyclodextrin. Front. Immunol. 2016;7:435. doi: 10.3389/fimmu.2016.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koźbiał M., Gierycz P. Comparison of aqueous and 1-octanol solubility as well as liquid-liquid distribution of acyclovir derivatives and their complexes with hydroxypropyl-β-cyclodextrin. J. Solution Chem. 2013;42:866–881. doi: 10.1007/s10953-013-9995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M.R., Regen S.L. The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc. Chem. Res. 2014;47:3512–3521. doi: 10.1021/ar500260t. [DOI] [PubMed] [Google Scholar]
- Kurkov S.V., Loftsson T. Cyclodextrins. Int. J. Pharm. 2013;453:167–180. doi: 10.1016/j.ijpharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- Kusakabe T., Ozasa K., Kobari S., Momota M., Kishishita N., Kobiyama K., Kuroda E., Ishii K.J. Intranasal hydroxypropyl-β-cyclodextrin-adjuvanted influenza vaccine protects against sub-heterologous virus infection. Vaccine. 2016;34:3191–3198. doi: 10.1016/j.vaccine.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Kwon S., Lee W., Shin H.J., Yoon S. il, Kim Y. Tae, Kim Y.J., Lee K., Lee S. Characterization of cyclodextrin complexes of camostat mesylate by ESI mass spectrometry and NMR spectroscopy. J. Mol. Struct. 2009;938:192–197. doi: 10.1016/j.molstruc.2009.09.025. [DOI] [Google Scholar]
- Lakkakula J.R., Maçedo Krause R.W. A vision for cyclodextrin nanoparticles in drug delivery systems and pharmaceutical applications. Nanomedicine. 2014;9:877–894. doi: 10.2217/nnm.14.41. [DOI] [PubMed] [Google Scholar]
- Larson, N., 2019. Compositions comprising an rna polymerase inhibitor and cyclodextrin for treating viral infections. US20190083525A1.
- Lauder S.N., Jones E., Smart K., Bloom A., Williams A.S., Hindley J.P., Ondondo B., Taylor P.R., Clement M., Fielding C., Godkin A.J., Jones S.A., Gallimore A.M. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq L. Interactions between cyclodextrins and cellular components: towards greener medical applications? Beilstein J. Org. Chem. 2016;12:2644–2662. doi: 10.3762/bjoc.12.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo D., Swaminathan S., Donalisio M., Civra A., Pastero L., Aquilano D., Vavia P., Trotta F., Cavalli R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013;443:262–272. doi: 10.1016/j.ijpharm.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.Y., Chen Y., Chen C.H., Liu Y. Amphiphilic multi-charged cyclodextrins and vitamin K co-assembly as a synergistic coagulant. Chem. Commun. 2019;55:11790–11793. doi: 10.1039/c9cc06545h. [DOI] [PubMed] [Google Scholar]
- Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang C., Li H., Wang X., Goh S.H., Ding J.L., Wang D.Y., Leong K.W. Cationic supramolecules composed of multiple oligoethylenimine-grafted β-cyclodextrins threaded on a polymer chain for efficient gene delivery. Adv. Mater. 2006;18:2969–2974. doi: 10.1002/adma.200600812. [DOI] [Google Scholar]
- Liang S., Li M., Yu X., Jin H., Zhang Y., Zhang L., Zhou D., Xiao S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019;166:328–338. doi: 10.1016/j.ejmech.2019.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T. Cyclodextrins and the biopharmaceutics classification system of drugs. J. Incl. Phenom. Macrocycl. Chem. 2002;44:63–67. doi: 10.1023/A:1023088423667. [DOI] [Google Scholar]
- Loftsson T., Brewster M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- López C.A., de Vries A.H., Marrink S.J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput. Biol. 2011;7 doi: 10.1371/journal.pcbi.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C.A., De Vries A.H., Marrink S.J. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci. Rep. 2013;3:1–6. doi: 10.1038/srep02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorizate M., Kräusslich H.G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011;3:1–20. doi: 10.1101/cshperspect.a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R.M., Hwang Y.C., Liu I.J., Lee C.C., Tsai H.Z., Li H.J., Wu H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020;27:1–30. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahammad S., Parmryd I. Cholesterol depletion using methyl-β-cyclodextrin. Methods Mol. Biol. 2015;1232:91–102. doi: 10.1007/978-1-4939-1752-5_8. [DOI] [PubMed] [Google Scholar]
- Marichal-Gallardo P.A., Álvarez M.M. State-of-the-art in downstream processing of monoclonal antibodies: process trends in design and validation. Biotechnol. Prog. 2012;28:899–916. doi: 10.1002/btpr.1567. [DOI] [PubMed] [Google Scholar]
- Martin J., Díaz-Montaña E.J., Asuero A.G. Cyclodextrin - A Versatile Ingredient. InTech; 2018. Cyclodextrins: past and present. [DOI] [Google Scholar]
- Martín J.J., Holguera J., Sánchez-Felipe L., Villar E., Muñoz-Barroso I. Cholesterol dependence of Newcastle Disease Virus entry. Biochim. Biophys. Acta - Biomembr. 2012;1818:753–761. doi: 10.1016/j.bbamem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F.M., Ljungman P., Chemaly R.F., Maertens J., Dadwal S.S., Duarte R.F., Haider S., Ullmann A.J., Katayama Y., Brown J., Mullane K.M., Boeckh M., Blumberg E.A., Einsele H., Snydman D.R., Kanda Y., DiNubile M.J., Teal V.L., Wan H., Murata Y., Kartsonis N.A., Leavitt R.Y., Badshah C. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl. J. Med. 2017;377:2433–2444. doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- Mazzaglia A., Scala A., Sortino G., Zagami R., Zhu Y., Sciortino M.T., Pennisi R., Pizzo M.M., Neri G., Grassi G., Piperno A. Intracellular trafficking and therapeutic outcome of multiwalled carbon nanotubes modified with cyclodextrins and polyethylenimine. Colloids Surfaces B Biointerfaces. 2018;163:55–63. doi: 10.1016/j.colsurfb.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Mazzon M., Mercer J. Lipid interactions during virus entry and infection. Cell. Microbiol. 2014;16:1493–1502. doi: 10.1111/cmi.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher G., Bhattacharjya S., Chakraborty H. Membrane cholesterol modulates oligomeric status and peptide-membrane interaction of severe acute respiratory syndrome coronavirus fusion peptide. J. Phys. Chem. B. 2019;123:10654–10662. doi: 10.1021/acs.jpcb.9b08455. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers, J.C.M., Bakhtiari, K., Peters, S.L.M., Zollinger, D.P., 2019. Cyclodextrins as procoagulants. WO2017188820A1.
- Mellet C.O., Fernández J.M.G., Benito J.M. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011;40:1586–1608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- Miertus S., Chiellini E., Chiellini F., Kona J., Tomasi J., Solaro R. Modelling of molecular interactions and inclusion phenomena in substituted β-cyclodextrin: from simple probes to proteins. Macromol. Symp. 1999;138:41–55. doi: 10.1002/masy.19991380106. [DOI] [Google Scholar]
- Morra M.E., Van Thanh L., Kamel M.G., Ghazy A.A., Altibi A.M.A., Dat L.M., Thy T.N.X., Vuong N.L., Mostafa M.R., Ahmed S.I., Elabd S.S., Fathima S., Le Huy Vu T., Omrani A.S., Memish Z.A., Hirayama K., Huy N.T. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev. Med. Virol. 2018;28 doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A.B., Attimarad M., Al-Dhubiab B.E., Wadhwa J., Harsha S., Ahmed M. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Deliv. 2014;21:540–547. doi: 10.3109/10717544.2013.853213. [DOI] [PubMed] [Google Scholar]
- Nayak D.P., Hui E.K.W. The role of lipid microdomains in virus biology. Subcell. Biochem. 2004;37:443–491. doi: 10.1007/978-1-4757-5806-1_14. [DOI] [PubMed] [Google Scholar]
- Németh K., Tárkányi G., Varga E., Imre T., Mizsei R., Iványi R., Visy J., Szemán J., Jicsinszky L., Szente L., Simonyi M. Enantiomeric separation of antimalarial drugs by capillary electrophoresis using neutral and negatively charged cyclodextrins. J. Pharm. Biomed. Anal. 2011;54:475–481. doi: 10.1016/j.jpba.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Nicolazzi C., Abdou S., Collomb J., Marsura A., Finance C. Effect of the complexation with cyclodextrins on the in vitro antiviral activity of ganciclovir against human cytomegalovirus. Bioorg. Med. Chem. 2001;9:275–282. doi: 10.1016/S0968-0896(00)00247-9. [DOI] [PubMed] [Google Scholar]
- Nicolazzi C., Venard V., Le Faou A., Finance C. In vitro antiviral efficacy of the ganciclovir complexed with β-cyclodextrin on human cytomegalovirus clinical strains. Antiviral Res. 2002;54:121–127. doi: 10.1016/S0166-3542(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Nishijo J., Moriyama S., Shiota S. Interactions of cholesterol with cyclodextrins in aqueous solution. Chem. Pharm. Bull. (Tokyo) 2003;51:1253–1257. doi: 10.1248/cpb.51.1253. [DOI] [PubMed] [Google Scholar]
- Notario-Pérez F., Martín-Illana A., Cazorla-Luna R., Ruiz-Caro R., Tamayo A., Rubio J., María-Dolores V. Mucoadhesive vaginal discs based on cyclodextrin and surfactants for the controlled release of antiretroviral drugs to prevent the sexual transmission of HIV. Pharmaceutics. 2020;12:321. doi: 10.3390/pharmaceutics12040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O., Rott R., Loyter A. Fusion of influenza virus particles with liposomes: requirement for cholesterol and virus receptors to allow fusion with and lysis of neutral but not of negatively charged liposomes. J. Gen. Virol. 1992;73:2831–2837. doi: 10.1099/0022-1317-73-11-2831. [DOI] [PubMed] [Google Scholar]
- O’Mahony A.M., O’Neill J.M., Godinho B.M.D.C., Darcy R., Cryan J.F., O’Driscoll C.M. Cyclodextrins for non-viral gene and siRNA delivery. Pharm. Nanotechnol. 2013;1:6–14. doi: 10.2174/2211738511301010006. [DOI] [Google Scholar]
- Ohtani Y., Irie T., Uekama K., Fukunaga K., Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989;186:17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Ohvo H., Slotte J.P. Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemistry. 1996;35:8018–8024. doi: 10.1021/bi9528816. [DOI] [PubMed] [Google Scholar]
- Onishi M., Ozasa K., Kobiyama K., Ohata K., Kitano M., Taniguchi K., Homma T., Kobayashi M., Sato A., Katakai Y., Yasutomi Y., Wijaya E., Igarashi Y., Nakatsu N., Ise W., Inoue T., Yamada H., Vandenbon A., Standley D.M., Kurosaki T., Coban C., Aoshi T., Kuroda E., Ishii K.J. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015;194:2673–2682. doi: 10.4049/jimmunol.1402027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A., Freed E.O. Role of lipid rafts in virus replication. Adv. Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- Padmanabhan, S., 2020. Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with Nitazoxanide and Hydroxychloroquine. (Preprint). < 10.13140/RG.2.2.28124.74882>. [DOI]
- Pan Y., Xue Y., Snow J., Xiao H. Tailor-made antimicrobial/antiviral star polymer via ATRP of cyclodextrin and guanidine-based macromonomer. Macromol. Chem. Phys. 2015;216:511–518. doi: 10.1002/macp.201400525. [DOI] [Google Scholar]
- Parkin J., Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- Pharma Advancement, 2020. WuXi Biologics Enables Development of Multiple Neutralizing Antibodies for Novel Coronavirus [WWW Document]. URL <https://www.pharmaadvancement.com/manufacturing/wuxi-biologics-enables-development-of-multiple-neutralizing-antibodies-for-novel-coronavirus/> (accessed 5.18.20).
- Ping Y., Liu C., Zhang Z., Liu K.L., Chen J., Li J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials. 2011;32:8328–8341. doi: 10.1016/j.biomaterials.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Radi A.E., Nassef H.M., El-Naggar A.E. Electrochemical and spectral characterization of the host-guest inclusion complex of the antiparasitic drug nitazoxanide with β-cyclodextrin. Monatsh. Chem. 2014;145:421–426. doi: 10.1007/s00706-013-1109-1. [DOI] [Google Scholar]
- Ramezani V., Vatanara A., Seyedabadi M., Nabi Meibodi M., Fanaei H. Application of cyclodextrins in antibody microparticles: potentials for antibody protection in spray drying. Drug Dev. Ind. Pharm. 2017;43:1103–1111. doi: 10.1080/03639045.2017.1293679. [DOI] [PubMed] [Google Scholar]
- Rane, S., 2017. Formulations comprising 2-amino-2-[2-(4-octylphenyl) ethyl] propane-1, 3-diol. WO2012135561A1.
- Rao M.R.P., Chaudhari J., Trotta F., Caldera F. Investigation of cyclodextrin-based nanosponges for solubility and bioavailability enhancement of rilpivirine. AAPS PharmSciTech. 2018;19:2358–2369. doi: 10.1208/s12249-018-1064-6. [DOI] [PubMed] [Google Scholar]
- Regeneron, 2020. Regeneron Announces Expanded Collaboration with HHS to Develop Antibody Treatments for New Coronavirus | Regeneron Pharmaceuticals Inc. [WWW Document]. URL <https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-announces-expanded-collaboration-hhs-develop-antibody> (accessed 5.18.20).
- Roquette, 2020. Combating Coronavirus: Key Role of Cyclodextrins in Treatment and Prevention [WWW Document]. URL <https://www.roquette.com/-/media/media-centre/press-releases/2020/2020-02-11-combating-viruses/position-paper---coronavirus-combatment-via-cyclodextrins.pdf?la=en> (accessed 5.18.20).
- Roy A., Saha S., Roy D., Bhattacharyya S., Roy M.N. Formation & specification of host–guest inclusion complexes of an anti-malarial drug inside into cyclic oligosaccharides for enhancing bioavailability. J. Incl. Phenom. Macrocycl. Chem. 2020;97:65–76. doi: 10.1007/s10847-020-00984-1. [DOI] [Google Scholar]
- Sagan S.M., Rouleau Y., Leggiadro C., Supekova L., Schultz P.G., Su A.I., Pezacki J.P. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochem. Cell Biol. 2006;84:67–79. doi: 10.1139/o05-149. [DOI] [PubMed] [Google Scholar]
- Sallas F., Darcy R. Amphiphilic cyclodextrins – advances in synthesis and supramolecular chemistry. Euro. J. Org. Chem. 2008;2008:957–969. doi: 10.1002/ejoc.200700933. [DOI] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA - J. Am. Med. Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sanofi, 2020. Sanofi and Regeneron begin global Kevzara® (sarilumab) clinical trial program in patients with severe COVID-19 - Mar 16, 2020 [WWW Document]. URL <http://www.news.sanofi.us/2020-03-16-Sanofi-and-Regeneron-begin-global-Kevzara-R-sarilumab-clinical-trial-program-in-patients-with-severe-COVID-19> (accessed 5.18.20).
- Saokham P., Loftsson T. γ-Cyclodextrin. Int. J. Pharm. 2017;516:278–292. doi: 10.1016/j.ijpharm.2016.10.062. [DOI] [PubMed] [Google Scholar]
- Schmitt A.P., Lamb R.A. Influenza virus assembly and budding at the viral budozone. Adv. Virus Res. 2005;64:383–416. doi: 10.1016/S0065-3527(05)64012-2. [DOI] [PubMed] [Google Scholar]
- Schüle S., Schulz-Fademrecht T., Garidel P., Bechtold-Peters K., Frieß W. Stabilization of IgG1 in spray-dried powders for inhalation. Eur. J. Pharm. Biopharm. 2008;69:793–807. doi: 10.1016/j.ejpb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Serno T., Carpenter J.F., Randolph T.W., Winter G. Inhibition of agitation-induced aggregation of an IgG-antibody by hydroxypropyl-β-cyclodextrin. J. Pharm. Sci. 2010;99:1193–1206. doi: 10.1002/jps.21931. [DOI] [PubMed] [Google Scholar]
- Serno T., Geidobler R., Winter G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv. Drug Deliv. Rev. 2011;63:1086–1106. doi: 10.1016/j.addr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Sevukarajan M., Bachala T., Nair R. Novel Inclusion complexs of oseltamivir phosphate-with [beta] cyclodextrin: physico-chemical characterization. J. Pharm. Sci. Res. 2010;2:583. [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yang D.H., Xiong J., Jia J., Huang B., Jin Y.X. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. 2005;15:193–200. doi: 10.1038/sj.cr.7290286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R.I. Assessment of low-molecular-weight heparin trials in cardiology. Pharmacol. Ther. 2000;87:1–9. doi: 10.1016/S0163-7258(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Song J.C., Wang G., Zhang W., Zhang Y., Li W.Q., Zhou Z. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil. Med. Res. 2020;7:19. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:1525–1531. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Lu X., Xu C., Wang Y., Sun W., Xi J. CD-sACE2 inclusion compounds: an effective treatment for coronavirus disease 2019 (COVID-19) J. Med. Virol. 2020;jmv.25804 doi: 10.1002/jmv.25804. [DOI] [PubMed] [Google Scholar]
- Sun X., Whittaker G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003;77:12543–12551. doi: 10.1128/jvi.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szente L., Szemán J., Sohajda T. Analytical characterization of cyclodextrins: history, official methods and recommended new techniques. J. Pharm. Biomed. Anal. 2016;130:347–365. doi: 10.1016/j.jpba.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Tan L., Zheng T., Li M., Zhong X., Tang Y., Qin M., Sun X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv. Transl. Res. 2020;10:678–689. doi: 10.1007/s13346-020-00725-4. [DOI] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., George A., Taylor T., Hildreth J.E.K. Cholesterol depletion inactivates XMRV and leads to viral envelope protein release from virions: evidence for role of cholesterol in XMRV infection. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0048013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Liu P., Chen M., Qin Y. Virion-associated cholesterol regulates the infection of human parainfluenza virus type 3. Viruses. 2019;11:438. doi: 10.3390/v11050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavornvipas S., Tajiri S., Hirayama F., Arima H., Uekama K. Effects of hydrophilic cyclodextrins on aggregation of recombinant human growth hormone. Pharm. Res. 2004;21:2369–2376. doi: 10.1007/s11095-004-7691-5. [DOI] [PubMed] [Google Scholar]
- Taylor D.R., Hooper N.M. Role of lipid rafts in the processing of the pathogenic prion and Alzheimer’s amyloid-β proteins. Semin. Cell Dev. Biol. 2007;18:638–648. doi: 10.1016/j.semcdb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Thuja Capital, 2019. Alveron Pharma closes series A round to advance a cyclodextrin based procoagulant medicine into the clinic — Thuja [WWW Document]. URL <https://www.thujacapital.com/news/2019/5/16/alveron-pharma-closes-series-a-round-to-advance-a-cyclodextrin-based-procoagulant-medicine-into-the-clinic> (accessed 5.18.20).
- Tian Z., Si L., Meng K., Zhou X., Zhang Y., Zhou D., Xiao S. Inhibition of influenza virus infection by multivalent pentacyclic triterpene-functionalized per-O-methylated cyclodextrin conjugates. Eur. J. Med. Chem. 2017;134:133–139. doi: 10.1016/j.ejmech.2017.03.087. [DOI] [PubMed] [Google Scholar]
- Totura A.L., Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expert Opin. Drug Discov. 2019;14:397–412. doi: 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamaloukas A., Szadkowska H., Slotte P.J., Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 2005;89:1109–1119. doi: 10.1529/biophysj.105.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. (Tokyo) 2004;52:900–915. doi: 10.1248/cpb.52.900. [DOI] [PubMed] [Google Scholar]
- Verma D., Gupta D., Lal S. Host lipid rafts play a major role in binding and endocytosis of influenza a virus. Viruses. 2018;10:650. doi: 10.3390/v10110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiers A. Sur la fermentation de la fécule par l’action du ferment butyrique. Compt. Rend. Acad. Sci. 1891;112:536–538. [Google Scholar]
- Vinetz J.M. Lack of efficacy of hydroxychloroquine in covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m2018. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Yuan, Z., Pavel, M.A., Hansen, S.B., 2020. The role of high cholesterol in age-related COVID19 lethality. bioRxiv 2020.05.09.086249. < 10.1101/2020.05.09.086249>. [DOI]
- Wang Z., Ren L., Zhao X., Hung T., Meng A., Wang J., Chen Y.-G. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J. Virol. 2004;78:7523–7527. doi: 10.1128/jvi.78.14.7523-7527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudiri G.A., Nicola A.V. Cellular cholesterol facilitates the postentry replication cycle of herpes simplex virus 1. J. Virol. 2017;91 doi: 10.1128/jvi.00445-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudiri G.A., Schneider S.M., Nicola A.V. Herpes simplex virus 1 envelope cholesterol facilitates membrane fusion. Front. Microbiol. 2017;8:2383. doi: 10.3389/fmicb.2017.02383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Si L., Tian Z., Jiao P., Fan Z., Meng K., Zhou X., Wang H., Xu R., Han X., Fu G., Zhang Y., Zhang L., Zhou D. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: a dramatic multivalent effect. Biomaterials. 2016;78:74–85. doi: 10.1016/j.biomaterials.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Hussain A., Paulson J., Abbruscato T.J., Ahsan F. Cyclodextrins in nasal delivery of low-molecular-weight heparins: in vivo and in vitro studies. Pharm. Res. 2004;21:1127–1136. doi: 10.1023/B:PHAM.0000032998.84488.7a. [DOI] [PubMed] [Google Scholar]
- Yang S.T., Kreutzberger A.J.B., Lee J., Kiessling V., Tamm L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids. 2016;199:136–143. doi: 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Wang X., Li H., Ling Ding J., Yun Wang D., Li J. A supramolecular gene carrier composed of multiple cationic α-cyclodextrins threaded on a PPO-PEO-PPO triblock polymer. Polymer (Guildf). 2009;50:1378–1388. doi: 10.1016/j.polymer.2009.01.010. [DOI] [Google Scholar]
- Zawada K.E., Wrona D., Rawle R.J., Kasson P.M. Influenza viral membrane fusion is sensitive to sterol concentration but surprisingly robust to sterol chemical identity. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep29842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li T., Fu L., Yu C., Li Y., Xu X., Wang Y., Ning H., Zhang S., Chen W., Babiuk L.A., Chang Z. Silencing SARS-CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J., Ma P.X. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug Deliv. Rev. 2013;65:1215–1233. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Xiao S., Zhou D., Sollogoub M., Zhang Y. Design, synthesis and biological evaluation of water-soluble per-O-methylated cyclodextrin-C60 conjugates as anti-influenza virus agents. Eur. J. Med. Chem. 2018;146:194–205. doi: 10.1016/j.ejmech.2018.01.040. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R., Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta - Biomembr. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses-drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]