Abstract
Atherosclerotic lesions in the great arteries are frequent findings in the elderly. Numerous studies have shown their strong predictive value for cardiovascular disease, embolic events, and mortality. We sought to determine the risk of all-cause mortality depending on the localization of plaques in the thoracic aorta evaluated by transesophageal echocardiography (TEE). A total of 2,054 patients (median age 65 years, interquartile range 52–73; 58% men) who underwent a TEE examination between 01/2007 and 03/2015 were retrospectively analyzed. For each patient, the presence of atherosclerotic lesions in the ascending aorta, the aortic arch, and in the descending aorta, as well as cardiovascular risk factors and survival were documented. Median follow-up period was 48 months (interquartile range 38–58). Multivariate Cox regression analysis indicated plaque in the ascending aorta (HR of 1.36, 95% CI 1.01–1.83, P = 0.046), the aortic arch (HR of 1.78, 95% CI 1.29–2.45, P < 0.001), the descending aorta (HR of 2.01, 95% CI 1.54–2.77, P < 0.001), and plaque in any part of the thoracic aorta (HR of 1.84, 95% CI 1.42–2.4, P < 0.001), as independent predictors for all-cause mortality after adjusting for age, sex, arterial hypertension, hyperlipidemia, smoking, and diabetes. In this study, we could demonstrate that more than mild plaque at any site of the thoracic aorta predicts all-cause mortality. Assessment of atherosclerotic lesions in all segments of the thoracic aorta should be part of every routine TEE examination.
Electronic supplementary material
The online version of this article (10.1007/s10554-020-01840-6) contains supplementary material, which is available to authorized users.
Keywords: Transesophageal echocardiography, Aortic plaque, Survival
Introduction
Atherosclerosis and its cardiovascular sequelae represent the leading cause of death globally.
A common manifestation of systemic atherosclerosis is atheroma of the thoracic aorta, a well-acknowledged cause of embolic events [1–3]. Numerous studies have shown the strong correlation of severe thoracic aortic plaque and cerebrovascular events [4], especially in patients with cryptogenic or recurrent stroke [5]. Complex and large (> 4 mm) plaques have a particularly high risk for embolism [6]. Furthermore, aortic atheroma is associated with the presence of aortic stenosis [7], atrial fibrillation [8], mitral annular calcification [9], carotid artery disease [10], and coronary artery disease [11]. One small study showed that plaque in the descending aorta predicts cardiovascular events [12], another showed the prognostic value of large plaque lesions in the aortic arch [13].
It is well recognized that plaque may occur at any level of the thoracic aorta and its prevalence increases with age [14]. However, the Stroke Prevention: Assessment of Risk in a Community (SPARC) study identified the descending aorta as the most prevalent site of aortic atherosclerosis (34.4%) followed by the aortic arch (25.6%). The ascending aorta was only affected in 6.2% of included patients [15]. So far, no studies have explored the plaque extent in different levels of the thoracic aorta with long-term survival.
Although there has been evidence for plaque in the thoracic aorta as a risk factor for long-term survival, it is poorly understood whether even small plaque detected in TEE predicts mortality in an unselected population.
We sought to investigate the prognostic value of plaque assessed with TEE depending on its localization in the thoracic aorta.
Methods
Study design, patient selection, and data acquisition
We conducted a retrospective single center cohort study to identify the impact of plaque in the thoracic aorta on all-cause mortality. All patients who received a TEE at our institution between 01/2007 and 03/2015 and where plaque location and extent in the thoracic aorta were clearly stated in the written report were included in the study. The primary endpoint was defined as all-cause mortality. Survival until 12/31/2017 was documented via the “Statistik Austria” death registry. Survival time was calculated from the date of the TOE examination until 12/31/2017 in all patients. Patient demographics, medical diagnoses, and daily medication were retrieved from the centralized patient management system of Vienna (AKIM- AKH-Informationsmanagement). The following clinical data were collected: age, sex, presence of arterial hypertension, dyslipidemia, diabetes mellitus, smoking, and cardiovascular medications (oral anticoagulants, antiplatelet medication, ACE inhibitors/ AT1 blocker, beta blockers, diuretics). Smoking was defined as no-smoker or as current/ former smoking habit. The study was approved by the ethics committee of the Medical University of Vienna (No. 1725/2016) and complies with the 1975 declaration of Helsinki (Fig. 1).
Fig. 1.
Kaplan Meier curves as well as tables with patients at risk showing 4-year-survival of patients with and without plaque in the different regions of the thoracic aorta. a Ascending aorta. b Aortic arch. c Descending aorta. NoAt no atheromatosis, Ath atheromatosis
Echocardiography
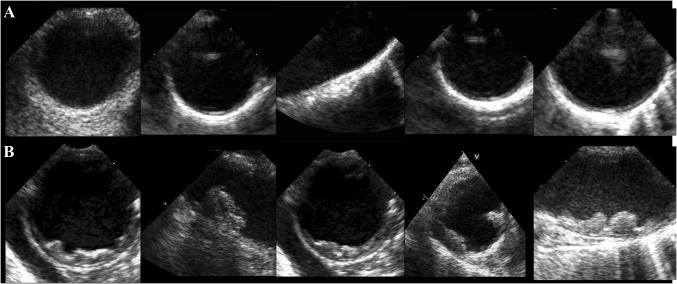
TEE data was retrieved from our echocardiography documentation system. All included patients underwent a complete standardized examination performed by cardiologists experienced in this modality. The plaque extend was assessed by visual estimation and reported as no plaque, mild plaque, mild to moderate plaque, moderate plaque, moderate to severe plaque, and severe plaque. Since data analysis included examinations from a time period of eight years, plaque extent was neither judged by the same readers nor was there a single definition of cut-offs regarding plaque size or plaque thickness. To minimize interobserver variability, we did not differentiate between different degrees of plaque but compared presence or absence of relevant plaque (Fig. 2). Previous studies revealed no increased mortality risk in patients with small plaque [16]. To establish the two study groups relevant and non-relevant plaque, we therefore counted no plaque and mild plaque as non-relevant, any degree of plaque more than mild was counted as relevant plaque.
Fig. 2.
Representative image of patients with none or mild plaque (a) and with more than mild plaque (b: relevant plaque)
The thoracic aorta was divided into three segments: the ascending aorta (proximal the brachiocephalic artery), the aortic arch (between the origin of the brachiocephalic and the left subclavian artery) and the descending aorta (distal from the left subclavian artery).
Statistical analysis
Continuous data are given as median and standard deviation (SD). Discrete data are presented as counts and percentages. Multivariate Cox proportional hazards regression analysis was applied to assess the association between atheroma in the different segments of the thoracic aorta with all-cause mortality. The regression models were adjusted for the following confounders: age, sex, arterial hypertension, diabetes, hyperlipidemia, and smoking. Schoenfeld residuals was calculated to check the proportional hazards assumption. Kaplan Meier Curves are presented for survival analysis. Unpaired student’s t-test was applied to compare categorical data. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS Version 24 (IBM SPSS, USA).
Results
Baseline characteristics
A total of 2054 patients were included in the study, 58% were male. Median age was 65 years (interquartile range 52–73). All patients underwent a complete TEE study. Cardiovascular risk factors were significantly present with 62% suffering from arterial hypertension, 41% had hyperlipidemia, 15% suffered from diabetes mellitus, and 31% were former or active smokers. Cardiovascular risk factors as well as cardiovascular medication were significantly more frequently present in patients with than in those without atheromatosis. Detailed baseline characteristics of all patients are displayed in Table 1.
Table 1.
Patient characteristics
| Patient characteristics | Total | Mild to no atheromatosis of the aorta | > Mild atheromatosis of the aorta | P value |
|---|---|---|---|---|
| Number of patients, n (%) | 2054 | 1190 (58) | 864 (42) | |
| Age, median years (Q1–Q3) | 65 (52–73) | 57 (46–67) | 72 (65–77) | < 0.001 |
| Male sex, n (%) | 1199 (58) | 680 (57) | 519 (60) | 0.155 |
| Cardiovascular risk factors | ||||
| Arterial hypertension, n (%) | 1281 (62) | 609 (51) | 672 (78) | < 0.001 |
| Diabetes mellitus, n (%) | 301 (15) | 108 (9) | 193 (22) | < 0.001 |
| Hyperlipidemia, n (%) | 838 (41) | 413 (35) | 425 (49) | < 0.001 |
| Smoking, n (%) | 633 (31) | 321 (27) | 312 (36) | < 0.001 |
| Medication | ||||
| Anticoagulation, n (%) | 787 (38) | 422 (36) | 365 (42) | 0.003 |
| Antiplatelet therapy, n (%) | 820 (40) | 400 (34) | 420 (49) | < 0.001 |
| ACE inhibitor, n (%) | 1024 (50) | 480 (40) | 544 (63) | < 0.001 |
| Beta blocker, n (%) | 1024 (50) | 502 (42) | 522 (60) | < 0.001 |
| Diuretics, n (%) | 789 (38) | 330 (28) | 459 (53) | < 0.001 |
ACE angiotensin-converting-enzyme
Echocardiographic findings
The thoracic aorta was assessed in all patients. Of the 2054 included patients, 42% had more than mild plaque at any level of the thoracic aorta, 13% had plaque in the ascending aorta, 32% in the aortic arch, and 23% in the descending aorta. The group of patients which did not survive 4-year follow-up had significantly higher numbers of atheromatosis than the group that survived (Table 2).
Table 2.
Patient characteristics and echocardiographic data
| Echocardiographic data | Total | Survived 4 year FU | Did not survive 4 year FU | P value |
|---|---|---|---|---|
| Number of patients, n (%) | 2054 | 1711 (83) | 343 (17) | |
| Plaque thoracic aorta, n (%) | 864 (42) | 634 (37) | 230 (67) | 0.002 |
| Plaque AscAo, n (%) | 268 (13) | 189 (11) | 79 (23) | < 0.001 |
| Plaque AoArch, n (%) | 654 (32) | 485 (28) | 169 (49) | < 0.001 |
| Plaque DesAo, n (%) | 462 (23) | 318 (19) | 144 (42) | < 0.001 |
| Aortic stenosis ≥ mild, n (%) | 342 (17) | 225 (13) | 117 (34) | < 0.001 |
| MAC, n (%) | 188 (9) | 142 (8) | 46 (13) | < 0.001 |
FU follow-up, AscAo ascending aorta, DesAo descending aorta, AoArch aortic arch, MAC mitral annular calcification
Prediction of long-term mortality
Over a median follow-up period of four (SD ± 2,2) years, 17% of patients died. Kaplan–Meier curves for all-cause mortality are shown in Fig. 1.
In a multivariate cox regression analysis adjusting for age, sex, arterial hypertension, hyperlipidemia, smoking, and diabetes, plaque in the thoracic aorta remained independently associated with a dismal outcome regardless of the plaque location (Table 3).
Table 3.
Prognostic value of plaque in the different levels of the Aorta: Multivariate analysis, adjusting for age, sex, cardiovascular risk factors (diabetes, hypertension, hyperlipidemia, smoking), and presence of aortic plaque at different levels as predictors for survival
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Plaque in the AscAo | 1.36 | 1.01–1.83 | 0.046 |
| Plaque in the AoArch | 1.78 | 1.29–2.45 | < 0.001 |
| Plaque in the DescAo | 2.01 | 1.54–2.77 | < 0.001 |
| Plaque in any part of the thoracic aorta | 1.84 | 1.42–2.4 | < 0.001 |
| Plaque in all parts of the thoracic aorta | 0.83 | 0.76–1.25 | 0.829 |
AscAo ascending aorta, AoArch aortic arch, DescAo descending aorta, HR hazard ratio, CI confidence interval
Discussion
The present study demonstrates that more than mild plaque in any of the segments of the thoracic aorta is independently associated with a dismal outcome.
Prognostic value of plaque
Plaque in the thoracic aorta is a common finding in the routinely performed transesophageal echocardiogram, especially in the elderly [17]. An association with cardiovascular risk factors has been demonstrated previously [18]. In the present study, we showed an association with hypertension, diabetes mellitus, dyslipidemia, and smoking.
Most previous studies used severe plaque (> 4/5 mm) as the cut-off and did not take smaller plaque into account [2, 19]. Limited data are available regarding the association of smaller plaque in the thoracic aorta detected by TEE with all-cause mortality. Previous studies were mostly limited to cardiac surgery cohorts [8, 20], patients with coexisting atrial fibrillation [13], or used other modalities such as computed tomography [20] or transthoracic echocardiography [21, 22]. Ferrari et al. demonstrated in a prospective study including 129 patients, that even small plaques (1–3.9 mm) are associated with embolic events and mortality in an unselected population [21]. With our data, we can confirm these findings in a large cohort. Multivariate analysis demonstrated significant prognostic power for all three segments of the thoracic aorta independent of age, sex, and cardiovascular risk factors. Kaplan–Meier curves revealed a significantly elevated mortality risk for patients with plaque in any region of the thoracic aorta.
Different plaque regions
The distribution of plaque along the thoracic aorta in our cohort was similar to previously published reports, which described the arch and the descending aorta as the more frequently affected sites when compared to the ascending aorta [15]. One hypothesis to explain this phenomenon is the differing wall sheer stress along the thoracic aorta [22].
An observational study including more than 22,000 patients demonstrated that plaque of any size in the descending and ascending aorta is a significant risk factor for long-term survival in cardiac surgery patients [23]. However, to the best of our knowledge, ours is the first study to analyze the impact of plaque in the three segments of the thoracic aorta on all-cause mortality in an unselected large population. There are several reports describing the impact of plaque in the different locations of the aorta on cardiovascular diseases. Atheroma in the ascending aorta and the aortic arch are considered main risk factors for stroke [5]. Patients with aortic arch atheroma were shown to have a similar stroke prevalence as patients with atrial fibrillation and carotid stenosis [24]. Furthermore, plaque in the proximal thoracic aorta is considered a major risk factor for recurrent stroke [5] and embolic events related to cardiac procedures (TAVI, cardiac surgery) [23, 25]. Plaque in the descending aorta can also be an indicator for systemic atherosclerotic disease. This assumption is supported by the fact that there is a strong association with age and cardiovascular risk factors [26], and with coronary artery disease [11]. Further investigations are needed to clarify a possible causal link of plaque in the descending aorta with embolic events and mortality.
The knowledge of plaque extent and distribution has several clinical implications. Aortic atheroma is strongly associated with cardiovascular risk factors. Therefore, secondary prevention with optimal treatment of arterial hypertension, diabetes mellitus, and dyslipidemia as well as smoking cessation are of even more importance in this patient group. The knowledge about plaque burden may furthermore provide information to anticipate the patient’s risk for embolic events and mortality during cardiac procedures. Finally, Ferrari et al. were able to demonstrate the superiority of anticoagulation versus antiplatelet therapy in patients with severe aortic plaque (> 4 mm). However, they did not show a beneficial effect in the group with plaque size of 1–3.9 mm [21]. Further studies are necessary to clarify the optimal management of these patients.
Limitations
The present study has several limitations. The data collection was retrospective; the reported values were exported from the hospital’s database and not confirmed by an independent echocardiographer. Our data reflects the experience of a single tertiary care center. However, the potential advantage of a single-center approach is the consistent quality of imaging. We included all consecutive patients undergoing TEE into our study regardless of age, indication, and medical history. However, a cohort with an indication for TEE is not comparable to the general population. Plaque extend in our cohort was assessed by visual estimation by different cardiologists. To minimize this potential limitation, we did not differentiate between different degrees of plaque but compared presence or absence of relevant plaque.
The assessment of the aorta via transesophageal echocardiography is limited by the “blind spot”, which refers to a small area of the distal ascending aorta which cannot be seen due to the interposition of the air-filled trachea between the esophagus and the aorta [27, 28]. In addition, especially in the distal ascending aorta, the aortic arch, and in the proximal descending aorta, due to the proximity of the probe to the aorta, there can be a near field drop out artefact of the aortic wall close to the probe. Even though there are some regions which cannot or can only partly be evaluated, nevertheless the most of the three parts of the aorta are accessible in most patients and give a representative impression on the degree of overall calcification.
Conclusion
In this study, we could demonstrate that more than mild plaque at any site of the thoracic aorta predicts all-cause mortality. Assessment of atherosclerotic lesions in all segments of the thoracic aorta should be part of every routine TEE examination.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Open access funding provided by Medical University of Vienna.
Compliance with ethical standards
Conflict of interest
All authors have read and approved submission of the manuscript and have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tunick PA, Kronzon I. Protruding atherosclerotic plaque in the aortic arch of patients with systemic embolization: a new finding seen by transesophageal echocardiography. Am Heart J. 1990;120(3):658–660. doi: 10.1016/0002-8703(90)90024-R. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery DH, Ververis JJ, McGorisk G, Frohwein S, Martin RP, Taylor WR. Natural history of severe atheromatous disease of the thoracic aorta: a transesophageal echocardiographic study. J Am Coll Cardiol. 1996;27(1):95–101. doi: 10.1016/0735-1097(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 3.Tunick PA, Rosenzweig BP, Katz ES, Freedberg RS, Perez JL, Kronzon I. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23(5):1085–1090. doi: 10.1016/0735-1097(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 4.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, et al. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke. 2010;41(6):1145–1150. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 5.Katsanos AH, Giannopoulos S, Frogoudaki A, Vrettou AR, Ikonomidis I, Paraskevaidis I, et al. The diagnostic yield of transesophageal echocardiography in patients with cryptogenic cerebral ischaemia: a meta-analysis. Eur J Neurol. 2016;23(3):569–579. doi: 10.1111/ene.12897. [DOI] [PubMed] [Google Scholar]
- 6.Sen S, Hinderliter A, Sen PK, Simmons J, Beck J, Offenbacher S, et al. Aortic arch atheroma progression and recurrent vascular events in patients with stroke or transient ischemic attack. Circulation. 2007;116(8):928–935. doi: 10.1161/CIRCULATIONAHA.106.671727. [DOI] [PubMed] [Google Scholar]
- 7.Osranek M, Pilip A, Patel PR, Molisse T, Tunick PA, Kronzon I. Amounts of aortic atherosclerosis in patients with aortic stenosis as determined by transesophageal echocardiography. Am J Cardiol. 2009;103(5):713–717. doi: 10.1016/j.amjcard.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Okura H, Inoue H, Tomon M, Nishiyama S, Yoshikawa T, Yoshida K. Transesophageal echocardiographic detection of cardiac sources of embolism in elderly patients with ischemic stroke. Intern Med. 1999;38(10):766–772. doi: 10.2169/internalmedicine.38.766. [DOI] [PubMed] [Google Scholar]
- 9.Adler Y, Vaturi M, Fink N, Tanne D, Shapira Y, Weisenberg D, et al. Association between mitral annulus calcification and aortic atheroma: a prospective transesophageal echocardiographic study. Atherosclerosis. 2000;152(2):451–456. doi: 10.1016/S0021-9150(99)00497-9. [DOI] [PubMed] [Google Scholar]
- 10.Hueb JC, Bazan R, Pereira Braga G, Fusco DR, Zanati Bazan SG, Bojikian MB. Carotid artery atherosclerotic profile as a predictor of the aorta atherosclerotic profile in patients with cerebrovascular events. Cerebrovasc Dis. 2013;36(1):26–32. doi: 10.1159/000351150. [DOI] [PubMed] [Google Scholar]
- 11.Gu X, He Y, Li Z, Kontos MC, Paulsen WH, Arrowood JA, et al. Relation between the incidence, location, and extent of thoracic aortic atherosclerosis detected by transesophageal echocardiography and the extent of coronary artery disease by angiography. Am J Cardiol. 2011;107(2):175–178. doi: 10.1016/j.amjcard.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Varga A, Gruber N, Forster T, Piros G, Havasi K, Jebelovszki E, et al. Atherosclerosis of the descending aorta predicts cardiovascular events: a transesophageal echocardiography study. Cardiovasc Ultrasound. 2004;2:21. doi: 10.1186/1476-7120-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okura H, Kataoka T, Yoshiyama M, Yoshikawa J, Yoshida K. Aortic atherosclerotic plaque and long-term prognosis in patients with atrial fibrillation-a transesophageal echocardiography study. Circ J. 2013;77(1):68–72. doi: 10.1253/circj.CJ-12-0583. [DOI] [PubMed] [Google Scholar]
- 14.Dávila-Román VG, Phillips KJ, Daily BB, Dávila RM, Kouchoukos NT, Barzilai B. Intraoperative transesophageal echocardiography and epiaortic ultrasound for assessment of atherosclerosis of the thoracic aorta. J Am Coll Cardiol. 1996;28(4):942–947. doi: 10.1016/S0735-1097(96)00263-X. [DOI] [PubMed] [Google Scholar]
- 15.Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O'Fallon WM, Pascoe RD, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74(9):862–869. doi: 10.4065/74.9.862. [DOI] [PubMed] [Google Scholar]
- 16.Hartman GS, Yao FS, Bruefach M, Barbut D, Peterson JC, Purcell MH, et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: a prospective study. Anesth Analg. 1996;83(4):701–708. doi: 10.1213/00000539-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Qiao H, He L, Yuan C, Chen H, Zhang Q, et al. Characterization of atherosclerotic disease in thoracic aorta: a 3D, multicontrast vessel wall imaging study. Eur J Radiol. 2016;85(11):2030–2035. doi: 10.1016/j.ejrad.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331(22):1474–1479. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 19.Izumi C, Takahashi S, Miyake M, Sakamoto J, Hanazawa K, Yoshitani K, et al. Impact of aortic plaque morphology on survival rate and incidence of a subsequent embolic event–long-term follow-up data. Circ J. 2010;74(10):2152–2157. doi: 10.1253/circj.CJ-10-0414. [DOI] [PubMed] [Google Scholar]
- 20.Santos RD, Rumberger JA, Budoff MJ, Shaw LJ, Orakzai SH, Berman D, et al. Thoracic aorta calcification detected by electron beam tomography predicts all-cause mortality. Atherosclerosis. 2010;209(1):131–135. doi: 10.1016/j.atherosclerosis.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari E, Vidal R, Chevallier T, Baudouy M. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol. 1999;33(5):1317–1322. doi: 10.1016/S0735-1097(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12(9):989–999. doi: 10.1097/00004872-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Butler CG, Ho Luxford JM, Huang CC, Ejiofor JI, Rawn JD, Wilusz K, et al. Aortic Atheroma Increases the Risk of Long-Term Mortality in 20,000 Patients. Ann Thorac Surg. 2017;104(4):1325–1331. doi: 10.1016/j.athoracsur.2017.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macleod MR, Amarenco P, Davis SM, Donnan GA. Atheroma of the aortic arch: an important and poorly recognised factor in the aetiology of stroke. Lancet Neurol. 2004;3(7):408–414. doi: 10.1016/S1474-4422(04)00806-3. [DOI] [PubMed] [Google Scholar]
- 25.Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121(7):870–878. doi: 10.1161/CIRCULATIONAHA.109.855866. [DOI] [PubMed] [Google Scholar]
- 26.Katsanos AH, Giannopoulos S, Kosmidou M, Voumvourakis K, Parissis JT, Kyritsis AP, et al. Complex atheromatous plaques in the descending aorta and the risk of stroke: a systematic review and meta-analysis. Stroke. 2014;45(6):1764–1770. doi: 10.1161/STROKEAHA.114.005190. [DOI] [PubMed] [Google Scholar]
- 27.Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11(8):645–658. doi: 10.1093/ejechocard/jeq056. [DOI] [PubMed] [Google Scholar]
- 28.Patil TA, Nierich A. Transesophageal echocardiography evaluation of the thoracic aorta. Ann Card Anaesth. 2016;19:S44–S55. doi: 10.4103/0971-9784.192623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.