Abstract
Background
Calcimimetic treatment of secondary hyperparathyroidism in chronic dialysis patients is often followed by hypocalcemia.
Methods
We investigated the frequency, predictors, consequences and therapeutic responses following cinacalcet-induced hypocalcemia in an incident European hemodialysis cohort of 1068 patients with a cinacalcet prescription.
Results
Of 905 normocalcemic patients initiating cinacalcet, 67% developed hypocalcemia within 12 months: 68% mild, 23% moderate, 9% severe. Compared to persistently normocalcemic patients, those with severe hypocalcemia were more often diabetic, overweight, had cardiovascular disease, shorter dialysis vintage, used a catheter dialysis access, had fewer active vitamin-D sterols, and exhibited higher CRP and iPTH and lower calcium levels. Multivariate predictors of hypocalcemia included a catheter for vascular access, low albumin and high iPTH. Generally, no therapeutic intervention to prevent hypocalcemia was taken prior to cinacalcet initiation. After the hypocalcemic event, the most common clinical response was no change of the dialysis or medical regimen. Following the hypocalcemic event, iPTH remained low even in those with severe hypocalcemia. The number of deaths and cardiovascular events did not differ between patients with and without hypocalcemia within six months following cinacalcet initiation.
Conclusion
Two-thirds of cinacalcet initiated patients experienced hypocalcaemia with 9% being severe. Hypocalcemia was mostly asymptomatic, transient (with and without targeted intervention to correct it) and not associated with an increase in cardiovascular events or deaths.
Electronic supplementary material
The online version of this article (10.1007/s40620-019-00686-z) contains supplementary material, which is available to authorized users.
Keywords: Cinacalcet, Secondary hyperparathyroidism, Parathyroid hormone, Hypocalcemia, Hemodialysis, Calcimimetic
Introduction
Secondary hyperparathyroidism (sHPT) is common in patients on chronic hemodialysis (HD). While some degree of parathyroid hormone (PTH) elevation is considered necessary to maintain normal bone turnover, about 10–20% of dialysis patients have excessively high PTH levels associated with high turnover bone disease, fractures, hypercalcemia episodes and increased mortality [1]. As recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) chronic kidney disease-mineral bone disorder (CKD-MBD) Guideline, therapeutic approaches for dialysis patients with sHPT who have PTH levels above the target range include calcimimetics, calcitriol and active vitamin D sterols, or, in advanced cases, parathyroidectomy [2]. Elevated serum phosphate levels are often difficult to normalize despite dietary advice, prescription of phosphate binders and adjustment of dialysis regimens. Use of active vitamin D sterols for patients with advanced sHPT can lead to hypercalcemia and/or exacerbation of hyperphosphatemia. Thus, calcimimetics, such as cinacalcet, may be the treatment of choice since they do not increase serum calcium or phosphate in contrast to active vitamin D sterols. Moreover, calcimimetics markedly reduce FGF23, a phosphaturic hormone associated with cardiovascular mortality in dialysis patients [3].
In chronic HD patients with severe sHPT, the initiation of cinacalcet is frequently followed by reductions in serum calcium and phosphate that can last one year or longer [4–9]. This has been interpreted as a medically induced equivalent of the “hungry bone syndrome” [10] that is observed after parathyroidectomy for those with advanced sHPT. It is due to rapid and often pronounced incorporation of calcium and phosphate into a previously demineralized bone. Compared to the “hungry bone syndrome”, cinacalcet-induced hypocalcemia is usually milder, more prolonged and usually asymptomatic [4–8, 11]. The asymptomatic nature of cinacalcet induced hypocalcemia was a central reason for the 2017 revision of the KDIGO CKD-MBD guideline that recommends avoidance of hypercalcemia, whereas mild hypocalcemia was considered acceptable, particularly if it is associated with calcimimetic use [2]. Presently it is uncertain whether calcimimetic induced hypocalcemia requires clinical interventions to increase calcium levels in chronic HD patients [12].
To complement findings from the large randomized controlled Evaluation Of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events (EVOLVE) trial where the majority of patients with hypocalcemia resolved spontaneously within 14 days without any therapeutic intervention [11], we assessed cinacalcet-induced hypocalcemia in a large incident European HD cohort in a “real-world” setting. The study objectives were (a) to describe the incidence of hypocalcemia in the 12 months following cinacalcet initiation, (b) to describe discontinuation and re-initiation of cinacalcet following first hypocalcemia episode (Ca < 2.1 mmol/L), (c) to describe factors associated with hypocalcemia, (d) to describe treatment patterns prior to cinacalcet initiation and after hypocalcemia episode; and (e) to describe the number of cardiovascular events and deaths among those who developed hypocalcemia and no hypocalcemia.
Methods
Study design
The Analysing Data, Recognising Excellence and Optimising Outcomes research initiative phase 2 (AROii) is a retrospective longitudinal cohort of incident HD patients (< 6 months on dialysis), who have not undergone prior kidney transplantation, who attended one of > 300 Fresenius Medical Care (FMC) facilities in 14 European countries (Czech Republic, France, Hungary, Ireland, Italy, Poland, Portugal, Romania, Russia, Serbia, Slovak Republic, Slovenia, Spain, Turkey and the United Kingdom). Methods used in analysis of the AROii cohort have been described previously [13, 14]. Longitudinal anonymised individual-level data on medical history (hospitalisation, diabetes, cancer, cardiovascular disease and facture), laboratory (albumin, calcium, c-reactive protein (CRP), ferritin, hemoglobin, phosphate, and PTH), dialysis (frequency, duration per week, dialysis adequacy, dialysis vintage, dialysis calcium, catheter vascular access, and net ultrafiltration), and medication data (calcitriol/alfacalcidol, paricalcitol, phosphate binders), plus ICD-10 coded hospitalisation and death data are available for patients who enrolled in AROii between 1 January 2007 and 31 December 2009 and were followed-up to end of 2014. Data were collected and processed in accordance with local ethical and regulatory requirements for each participating FMC facility. Written informed consent was obtained from all patients, where required.
Study population
Chronic HD patients (> 10 contiguous dialysis sessions) aged ≥ 18 who received a cinacalcet prescription after 1 January 2007, were enrolled in AROii for at least 90 days prior to cinacalcet initiation and had at least 90 days of follow-up after initiation were eligible for study inclusion. Patients who had undergone parathyroidectomy up to and including the first 90 days of follow-up or who had no further cinacalcet prescriptions or who had further prescriptions of less than 15 days were excluded.
The index date was date of cinacalcet initiation. The baseline period was defined as the 90 days prior to cinacalcet initiation. Patients were followed up for 12 months following cinacalcet initiation to determine the incidence of hypocalcemia. Then patients with hypocalcemia were followed-up for an additional 4-months to describe cinacalcet continuation and to describe treatment and management of patients following the episode. Among those who discontinued cinacalcet within the 4-month period, the probability of re-initiation was described in the 12-months following discontinuation. Patients were censored if they died, underwent parathyroidectomy, had a renal transplant, or were lost to follow-up. Patients were considered lost to follow-up if they left a dialysis facility for any reason and did not return to an FMC facility within 45 days.
The main cohort for analysis was restricted to patients with normal uncorrected total serum calcium levels ≥ 2.1 mmol/L at time of cinacalcet initiation. In clinical practice, physicians use uncorrected or albumin-corrected calcium to evaluate hypocalcemia although data suggest that uncorrected is not inferior to corrected calcium [15, 16]; therefore, sensitivity analyses were also carried out based with albumin-corrected calcium. Similar to previous studies, hypocalcemia was defined as mild (calcium 2.0− < 2.1 mmol/L), moderate (calcium 1.87− < 2.0 mmol/L), and severe (calcium < 1.87 mmol/L).
Treatment characteristics (cinacalcet dose, calcitriol/alfacalcidol, paricalcitol, calcium-based phosphate binder, dialysate Ca) were summarized before cinacalcet initiation and after the hypocalcemia episode. Patients were classified as new users or continuing users and their dose change (up-titration, down-titration). For the period before cinacalcet initiation, patients were classified as continuing users if they had a continuous prescription − 60 days up to index date. Dose change was classified as up-titrated if the average daily dose between − 30 days and index date was greater than the average daily dose between − 60 and − 31 days before index date; and down-titrated if the average daily dose between − 30 and index date was less than the average daily dose between − 60 and − 31 days before index date; and stable dose if the average daily dose between − 30 days and index date equaled the average daily dose between − 60 and − 31 days before the index date. Patients were classified as new users if they had a prescription between − 30 days and the index date but there was no prescription between − 60 and − 31 days before index date. For the period after the hypocalcemia episode, treatment characteristics for the 30-days period after the hypocalcemia event (i.e., defined as the intervention period) was compared to the − 30 days period before hypocalcemia. The proportion of patients who achieved normal Ca levels following this treatment intervention period was described at 30, 60 and 90 days afterwards. Sensitivity analyses were also carried out for a subset of subjects with albumin-corrected calcium.
Statistical analysis
Statistical analyses were conducted in SAS (version 9.4; SAS, Cary, NC, USA) and reproduced independently. Baseline characteristics of patients who developed hypocalcemia vs. no hypocalcemia were described using median and interquartile range for continuous variables and counts and proportions for categorical variable parameters. The most recent values available in the 90 days prior to the date of cinacalcet initiation (baseline period) for dialysis, laboratory and medication variables were summarised. To describe factors associated with first hypocalcemia episode in the first 12 months after cinacalcet initiation, Cox regression models were constructed. Variables significant at p < 0.25 level in univariate analysis were entered into a multivariate model using stepwise regression (significance level of p < 0.10 for inclusion/exclusion); and hazard ratios (HR) and 95% confidence intervals (CI) were calculated. To describe time-to-event (first hypocalcemia episode, cinacalcet discontinuation, and cinacalcet re-initiation), Kaplan–Meier curves were plotted, and cumulative monthly probabilities were estimated.
Results
Occurrence of hypocalcemia
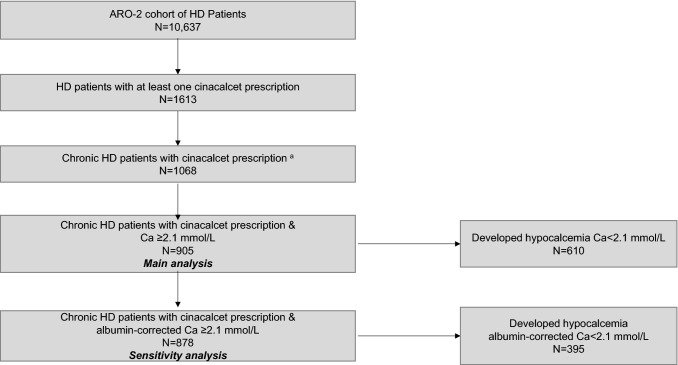
Country-specific cinacalcet prescriptions varied widely ranging from 2% in Turkey and Romania to 35-39% of the HD patients in Spain and Ireland (Table 1). In the AROii cohort we identified 1068 HD patients with a cinacalcet prescription and 905 (84.7%) patients had normal (Ca ≥ 2.1 mmol/L) and 163 patients (15.3%) had hypocalcaemia (Ca < 2.1 mmol/L) at time of first cinacalcet prescription (Fig. 1). Of these 905 patients who had a normal total serum calcium ≥ 2.1 mmol/L at time of cinacalcet initiation, 610 (67%) subsequently developed hypocalcemia (i.e. serum calcium < 2.1 mmol/L) within 12 months of the initiation of the cinacalcet prescription. For the sensitivity analysis, of 878 patients (97%) had an albumin-corrected Ca ≥ 2.1 mmol/L value available at cinacalcet initiation, 395 (45%) subsequently developed hypocalcemia (Online Resource 1).
Table 1.
Number of subjects with cinacalcet prescriptions in the AROii cohort
| Country | Total no. of subjects in AROii cohort | Subjects with at least one prescription, n (%) |
|---|---|---|
| Czech Republic | 688 | 66 (10) |
| France | 92 | 17 (18) |
| Hungary | 654 | 21 (3) |
| Ireland | 23 | 9 (39) |
| Italy | 561 | 62 (11) |
| Poland | 97 | 7 (7) |
| Portugal | 2319 | 443 (19) |
| Romania | 514 | 10 (2) |
| Russia | 102 | 10 (10) |
| Serbia | 88 | 2 (2) |
| Slovak Republic | 9 | 1 (11) |
| Slovenia | 86 | 10 (12) |
| Spain | 2429 | 852 (35) |
| Turkey | 1760 | 27 (2) |
| United Kingdom | 1215 | 76 (6) |
| Total | 10,637 | 1613 (15) |
Fig. 1.
Study flow chart. aChronic HD patients in ARO-2 cohort aged ≥18, filled a cinacalcet prescription after 1 January 2007, enrolled in the cohort for at least 90 days prior to cinacalcet initiation and have at least 90 days follow-up following cinacalcet initiation. Twenty six patients did not meet inclusion criteria (13 patients had a parathyroidectomy and 33 patients only one prescription or no further prescriptions or had further prescriptions of less than 15 days) and were excluded.
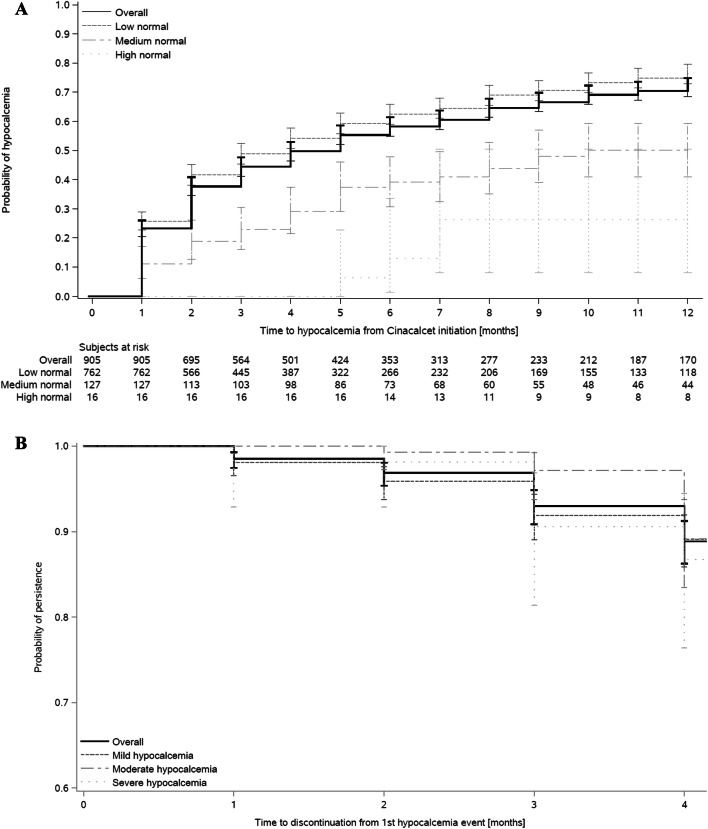
Figure 2a shows the cumulative probability of experiencing a hypocalcemic event (based on uncorrected calcium) at 6 and 12 months as 58% and 72%, respectively, after cinacalcet initiation. Manifestation of hypocalcemia was a function of the baseline calcium and less likely in the few patients with overt hypercalcemia. The 12-month cumulative probability for those who had low-levels of normal calcium (2.1– ≤ 2.5 mmol/L) compared to those who had hypercalcemia (calcium ≥ 2.75 mmol/L) was 76% and 26%, respectively. The median time to the first hypocalcemia episode was 4 months. However, according to corrected calcium, the cumulative probability of experiencing a hypocalcemia episode at 6 and 12 months was lower, 37% and 49%, respectively (Online Resource 2).
Fig. 2.
a Kaplan–Meier curve of time to hypocalcemia episode, overall and by total serum Ca at time of cinacalcet initiation. b Kaplan–Meier curve of time to cinacalcet discontinuation in the 4 months following hypocalcemia episode, overall and by total serum Ca at time of hypocalcemia episode
Of the 610 who developed hypocalcemia after cinacalcet initiation, 68% (n = 415) had mild, 23% (n = 141) had moderate, and 9% (n = 54) had severe hypocalcemia. Following the first hypocalcemic event, 89% of the patients were persistent with their cinacalcet prescription at four months (Fig. 2b). Continuation was similar in those who had mild and severe hypocalcemia, 89% vs. 87%, respectively. Similar persistence was also observed for those in the sensitivity analysis with hypocalcemia (corrected calcium) at four months: 88% overall, 89% for mild and 85% for severe hypocalcemia (Online Resource 3).
Among the 66 patients who discontinued cinacalcet within four months after the hypocalcemia episode, the cumulative probability of re-initiation at 12 months was 40% and 50% in the mild and moderate hypocalcemia groups, respectively (data not shown). This cumulative probability dropped to 20% in the seven patients with severe hypocalcemia. For the sensitivity analysis, the cumulative probability of re-initiation at 12 months was 38% and 56% for the mild and moderate groups and this decreased to 30% in the eight patients with severe hypocalcemia (data not shown).
Characteristics of patients with and without hypocalcemia
Table 2 describes the characteristics of patients who developed hypocalcemia or no hypocalcemia within 12 months of cinacalcet initiation. Generally, characteristics were similar among the two groups. However, in assessing baseline demographics, patients with severe hypocalcemia as compared to those with no hypocalcemia were more likely to be diabetic (37% vs. 30%), overweight (body mass index ≥ 25 kg/m2, 64% vs. 59%), have prior cardiovascular disease (52% vs. 44%), have shorter dialysis vintage (1.7 vs. 2.0 years), more likely to use a dialysis catheter as access (46% vs. 13%), use fewer active vitamin-D sterols (54% vs. 68%), and exhibit higher median CRP (7.4 vs. 4.1 mg/L) and iPTH (677 vs. 545 ng/L) and lower median uncorrected calcium levels (2.3 vs. 2.4 mmol/L). In contrast, when compared to patients with no hypocalcemia according to corrected calcium, (Online Resource 1), severely hypocalcemic patients were younger, more likely to be diabetic, obese, have less cardiovascular disease, similar dialysis vintage, more likely to have a dialysis catheter access, had higher phosphate levels, were more likely to be using active vitamin-D sterols and less likely to be using calcium-based phosphate binders.
Table 2.
Baseline characteristics of patients who developed and did no develop hypocalcemia within 12 months after cinacalcet initiation
| Characteristic | No hypocalcemia N = 295 | Hypocalcemia N = 610 | Patients who develop hypocalcemia within 12 months (N = 610) | ||
|---|---|---|---|---|---|
| Mild Ca 2.0– < 2.1 mmol/L N = 415 | Moderate Ca 1.87– < 2.0 mmol/L N = 141 | Severe Ca < 1.87 mmol/L N = 54 | |||
| Patient age at index date [years]a | 66 (54, 75) | 67 (54, 76) | 67 (54, 77) | 66 (55, 75) | 69 (60, 77) |
| Male, no. (%) | 173 (59) | 352 (58) | 240 (58) | 82 (58) | 30 (56) |
| Geographical area, no. (%) | |||||
| Eastern Europeb | 25 (9) | 56 (9) | 38 (9) | 15 (11) | 3 (6) |
| Western Europec | 33 (11) | 41 (7) | 28 (7) | 8 (6) | 5 (9) |
| Iberian Peninsulad | 237 (80) | 513 (84) | 349 (84) | 118 (84) | 46 (85) |
| BMI [kg/m2]a | 26.9 (24.0, 31.0) | 27.0 (24.3, 30.2) | 26.9 (24.2, 30.2) | 27.2 (24.3, 30.3) | 27.5 (25.5, 30.0) |
| BMI [kg/m2]—category, No. (%) | |||||
| < 18.5 | 4 (1) | 7 (1) | 6 (1) | 1 (1) | 0 (0) |
| ≥ 18.5–< 25 | 79 (27) | 162 (27) | 115 (28) | 37 (26) | 10 (19) |
| ≥ 25–< 30 | 101 (34) | 232 (38) | 154 (37) | 54 (38) | 24 (44) |
| ≥ 30 | 75 (25) | 148 (24) | 102 (25) | 35 (25) | 11 (20) |
| Missing | 36 (12) | 61 (10) | 38 (9) | 14 (10) | 9 (17) |
| Clinical history, no. (%) | |||||
| Hospitalisation | 137 (46) | 310 (51) | 207 (50) | 71 (50) | 32 (59) |
| Diabetes | 89 (30) | 194 (32) | 128 (31) | 46 (33) | 20 (37) |
| Cancer | 26 (9) | 59 (10) | 42 (10) | 11 (8) | 6 (11) |
| Cardiovascular disease | 131 (44) | 304 (50) | 199 (48) | 77 (55) | 28 (52) |
| Fracture | 14 (5) | 29 (5) | 21 (5) | 6 (4) | 2 (4) |
| Dialysis duration per week [hours]a | 12.0 (12.0, 12.4) | 12.0 (12.0, 12.3) | 12.0 (12.0, 12.3) | 12.0 (12.0, 12.4) | 12.0 (12.0, 12.0) |
| Dialysis adequacy [Kt/V]–category, no. (%) | 39 (13) | 88 (14) | 53 (13) | 23 (16) | 12 (22) |
| < 1.2 | 229 (78) | 474 (78) | 320 (77) | 112 (79) | 42 (78) |
| ≥ 1.2 | 27 (9) | 48 (8) | 42 (10) | 6 (4) | 0 (0) |
| Dialysis vintage [years]a | 2.0 (1.0, 3.5) | 1.8 (1.0, 3.0) | 1.8 (1.0, 3.1) | 1.8 (0.9, 3.0) | 1.7 (0.9, 2.3) |
| Dialysate calcium [mmol/L]a | 1.3 (1.3, 1.5) | 1.3 (1.3, 1.5) | 1.3 (1.3, 1.5) | 1.3 (1.3, 1.5) | 1.5 (1.3, 1.5) |
| Catheter vascular access, no. (%) | |||||
| Yes | 37 (13) | 158 (26) | 99 (24) | 34 (24) | 25 (46) |
| No | 246 (83) | 434 (71) | 306 (74) | 101 (72) | 27 (50) |
| Missing | 12 (4) | 18 (3) | 10 (2) | 6 (4) | 2 (4) |
| Net ultrafiltration [L]a | 2.0 (1.4, 2.7) | 2.1 (1.4, 2.7) | 2.0 (1.4, 2.7) | 2.1 (1.5, 2.6) | 2.3 (1.7, 3.0) |
| Blood haemoglobin [g/L]a | 118 (110, 126) | 120 (112, 127) | 119 (111, 127) | 121 (112, 127) | 119 (113, 127) |
| Serum albumin [g/L]a | 41.0 (38.4, 43.0) | 40.0 (37.0, 42.2) | 40.0 (37.9, 42.1) | 39.2 (37.0, 43.0) | 38.4 (36.0, 42.0) |
| Serum ferritin [µg/L]a | 430 (244, 639) | 398 (260, 599) | 410 (267, 620) | 382 (242, 544) | 376 (236, 572) |
| Serum CRP [mg/L]a | 4.1 (1.7, 9.2) | 4.9 (2.0, 11.0) | 4.7 (1.8, 10.0) | 5.0 (2.0, 12.3) | 7.4 (2.8, 17.7) |
| Serum calcium [mmol/L]a | 2.4 (2.3, 2.5) | 2.3 (2.2, 2.4) | 2.3 (2.2, 2.4) | 2.3 (2.2, 2.4) | 2.3 (2.2, 2.3) |
| Serum calcium [mmol/L]—category, no. (%) | |||||
| ≥ 2.10–< 2.50 | 216 (73) | 546 (90) | 367 (88) | 131 (93) | 48 (89) |
| ≥ 2.50–< 2.75 | 67 (23) | 60 (10) | 44 (11) | 10 (7) | 6 (11) |
| ≥ 2.75 | 12 (4) | 4 (1) | 4 (1) | 0 (0) | 0 (0) |
| Serum phosphate [mmol/L]a | 1.7 (1.4, 2.0) | 1.7 (1.4, 2.0) | 1.7 (1.4, 2.0) | 1.7 (1.5, 2.0) | 1.7 (1.3, 1.9) |
| Serum PTH [ng/L]a | 545 (415, 761) | 606 (442, 837) | 580 (434, 833) | 624 (446, 864) | 677 (463, 824) |
| Corrected Ca [mmol/L]a | 2.4 (2.3, 2.5) | 2.3 (2.2, 2.4) | 2.3 (2.3, 2.4) | 2.3 (2.2, 2.4) | 2.3 (2.2, 2.4) |
| Corrected Ca [mmol/L], no. (%) | |||||
| ≥ 2.10–< 2.50 | 190 (64) | 504 (83) | 342 (82) | 119 (84) | 43 (80) |
| ≥ 2.50–< 2.75 | 73 (25) | 68 (11) | 47 (11) | 14 (10) | 7 (13) |
| ≥ 2.75 | 14 (5) | 4 (1) | 4 (1) | 0 (0) | 0 (0) |
| Missing | 18 (6) | 34 (6) | 22 (5) | 8 (6) | 4 (7) |
| Drug use, no. (%) | |||||
| Calcitriol/alfacalcidol | 75 (25) | 149 (24) | 98 (24) | 41 (29) | 10 (19) |
| Paricalcitol | 127 (43) | 265 (43) | 186 (45) | 60 (43) | 19 (35) |
| Phosphate binder use, no. (%) | |||||
| Non-calcium based only | 136 (46) | 257 (42) | 187 (45) | 50 (36) | 20 (37) |
| Calcium based only | 26 (9) | 66 (11) | 41 (10) | 19 (14) | 6 (11) |
| Both calcium based and non-calcium based | 71 (24) | 137 (23) | 96 (23) | 31 (22) | 10 (19) |
| None | 62 (21) | 150 (25) | 91 (22) | 41 (29) | 18 (33) |
aMedian (Q1, Q3)
bEastern Europe: Czech Republic, Hungary, Poland, Romania, Russia, Serbia, Slovak Republic, Slovenia and Turkey
cWestern Europe: France, Ireland, Italy, and the United Kingdom
dIberian Peninsula: Portugal and Spain
Baseline correlates of hypocalcemia
Table 3 shows that in multivariate analysis, baseline predictors for first hypocalcemia episode within 12 months after starting cinacalcet were significant for geographical origin of the patients, having a catheter vascular access, low albumin and high iPTH. Low haemoglobin was associated with a lower risk of hypocalcemia. Table 4 shows significant predictors of severe hypocalcemia which were having a catheter access, high net ultrafiltration, high CRP, and no paricalcitol use. Online Resource 4 and Online Resource 5 describe predictors according to hypocalcemia as defined by corrected calcium. Predictors were generally similar for hypocalcemia vs. no hypocalcemia; whereas having a catheter access was a strong predictor for severe hypocalcemia.
Table 3.
Baseline predictors of time to first hypocalcemia episode in the first 12 months after cinacalcet initiation
| Characteristics | Any hypocalcemia vs no hypocalcemia | ||
|---|---|---|---|
| No. of hypocalcemia events/total no. of subjects (%) | Hazard ratio (95% CI) | p value | |
| Geographical area | 0.01 | ||
| Iberian Peninsulaa | 513/750 (68.4) | References | |
| Eastern Europeb | 56/81 (69.1) | 1.22 (0.87, 1.70) | |
| Western Europec | 41/74 (55.4) | 0.65 (0.46, 0.90) | |
| Catheter vascular access | <0.001 | ||
| No | 434/680 (63.8) | References | |
| Yes | 158/195 (81.0) | 1.59 (1.32, 1.92) | |
| Missing information | 18/30 (60.0) | 0.91 (0.52, 1.57) | |
| Haemoglobin [g/dL] | 0.03 | ||
| ≥ 12 | 306/436 (70.2) | References | |
| 10–< 12 | 276/423 (65.2) | 0.87 (0.74, 1.03) | |
| < 10 | 28/46 (60.9) | 0.61 (0.41, 0.91) | |
| Serum albumin [g/L] | 0.003 | ||
| Q4 | 98/160 (61.3) | References | |
| Q3 | 155/258 (60.1) | 1.07 (0.83, 1.37) | |
| Q2 | 167/222 (75.2) | 1.46 (1.13, 1.87) | |
| Q1 | 156/213 (73.2) | 1.47 (1.14, 1.91) | |
| Missing data | 34/52 (65.4) | 1.25 (0.84, 1.85) | |
| PTH [pg/mL] | 0.005 | ||
| < 600 | 303/476 (63.7) | References | |
| 600–< 1000 | 206/283 (72.8) | 1.32 (1.11, 1.58) | |
| ≥ 1000 | 101/146 (69.2) | 1.26 (0.99, 1.60) | |
Q quartile
aEastern Europe: Czech Republic, Hungary, Poland, Romania, Russia, Serbia, Slovak Republic, Slovenia and Turkey
bWestern Europe: France, Ireland, Italy, and the United Kingdom
cIberian Peninsula: Portugal and Spain
Table 4.
Baseline predictors of time to first severe hypocalcemia episode (Ca < 1.87 mmol/L)
| Severe hypocalcemia vs. No hypocalcemia | |||
|---|---|---|---|
| No. of patients with severe hypocalcemia/ Total no. of subjects | Hazard ratio (95% CI) | p-value | |
| Catheter vascular access | <0.001 | ||
| No | 27/273 (9.9) | References | |
| Yes | 25/62 (40.3) | 5.72 (3.23, 10.13) | |
| Missing | 2/14 (14.3) | 1.07 (0.24, 4.79) | |
| Net Ultrafiltration [L] | 0.004 | ||
| Q1 | 7/89 (7.9) | References | |
| Q2 | 17/85 (20.0) | 4.73 (1.88, 11.93) | |
| Q3 | 12/87 (13.8) | 2.29 (0.88, 5.93) | |
| Q4 | 18/88 (20.5) | 4.16 (1.68, 10.28) | |
| CRP [mg/L] | 0.04 | ||
| Q1 | 3/60 (5.0) | References | |
| Q2 | 12/76 (15.8) | 5.18 (1.42, 18.82) | |
| Q3 | 7/63 (11.1) | 2.87 (0.73, 11.31) | |
| Q4 | 17/63 (27.0) | 5.76 (1.68, 19.75) | |
| Missing | 15/87 (17.2) | 3.32 (0.93, 11.84) | |
| Paricalcitol use | 0.04 | ||
| Yes | 19/146 (13.0) | References | |
| No | 35/203 (17.2) | 1.81 (1.02, 3.24) | |
CRP creatinine reactive protein, Q quartile
Medical therapy immediately prior to the first hypocalcemia episode
To evaluate whether there was any prior intervention to prevent hypocalcemia before initiating cinacalcet, we compared the medical regimen at the time of first hypocalcemia. Generally, no intervention was taken prior to start of cinacalcet prescription. As shown in Table 5, patients developing hypocalcemia vs. no hypocalcemia received a similar dosage of cinacalcet at initiation (median average daily dose 30 mg), similar and stable usage of active vitamin D sterols, and had a similar and stable dialysate calcium concentration. Calcium-containing phosphate binders were used less frequently in patients with high baseline serum calcium, who subsequently developed hypocalcemia. Findings were relatively similar in the sensitivity analysis (Online Resource 6).
Table 5.
Treatment characteristics of chronic haemodialysis patients at time of cinacalcet initiation
| No hypocalcemia within 12 months after cinacalcet initiation N = 295 | Hypocalcemia within 12 months after cinacalcet initiation | |||
|---|---|---|---|---|
| Ca at time of cinacalcet initiation | ||||
| Overall Ca ≥ 2.1 mmol/L N = 610 | Low normal Ca 2.10– < 2.50 mmol/L N = 546 | Moderate to high normal Ca ≥ 2.5 mmol/L N = 64 | ||
| Cinacalcet | ||||
| Average daily dose [mg/day], median (Q1, Q3) | 30.0 (12.9, 30.0) | 30.0 (17.1, 30.0) | 30.0 (17.1, 30.0) | 30.0 (30.0, 30.0) |
| Calcitriol/alfacalcidol | ||||
| Use, n (%) | 60 (20) | 130 (21) | 116 (21) | 14 (22) |
| Daily dose [µg/day], median (Q1, Q3) | 0.4 (0.2, 0.5) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.6) |
| New users, n (%) | 2 (3) | 4 (3) | 4 (3) | 0 (0) |
| Continuing users, n (%) | 58 (97) | 126 (97) | 112 (97) | 14 (100) |
| Stable dose, n (%) | 47 (81) | 106 (84) | 95 (85) | 11 (79) |
| Up-titration, n (%) | 7 (12) | 9 (7) | 9 (8) | 0 (0) |
| Down-titration, n (%) | 4 (7) | 11 (9) | 8 (7) | 3 (21) |
| Paricalcitol | ||||
| Use, n (%) | 113 (38) | 234 (38) | 207 (38) | 27 (42) |
| Daily dose [µg/day], median (Q1, Q3) | 0.9 (0.6, 1.4) | 0.9 (0.6, 1.2) | 0.9 (0.6, 1.1) | 0.9 (0.7, 1.9) |
| New users, n (%) | 2 (2) | 17 (7) | 17 (8) | 0 (0) |
| Continuing users, n (%) | 111 (98) | 217 (93) | 190 (92) | 27 (100) |
| Stable dose, n (%) | 78 (70) | 164 (76) | 146 (77) | 18 (67) |
| Up-titration, n (%) | 23 (21) | 26 (12) | 21 (11) | 5 (19) |
| Down-titration, n (%) | 10 (9) | 27 (12) | 23 (12) | 4 (15) |
| Calcium-based phosphate binder | ||||
| Use, n (%) | 64 (22) | 140 (23) | 132 (24) | 8 (13) |
| Daily dose [mg/day], median (Q1, Q3) | 1943 (715, 2820) | 1980 (1143, 3150) | 1980 (1143, 3000) | 2133 (1240, 4600) |
| New users, n (%) | 1 (2) | 6 (4) | 6 (5) | 0 (0) |
| Continuing users, n (%) | 63 (98) | 134 (96) | 126 (95) | 8 (100) |
| Stable dose, n (%) | 56 (89) | 120 (90) | 113 (90) | 7 (88) |
| Up-titration, n (%) | 4 (6) | 8 (6) | 8 (6) | 0 (0) |
| Down-titration, n (%) | 3 (5) | 6 (4) | 5 (4) | 1 (13) |
| Dialysate calcium [mmol/L] | ||||
| Missing | 11 (4) | 18 (3) | 14 (3) | 4 (6) |
| ≤ 1.00 | 1 (0) | 2 (0) | 2 (0) | 0 (0) |
| > 1.00– < 1.25 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ≥ 1.25– < 1.50 | 146 (49) | 306 (50) | 271 (50) | 35 (55) |
| ≥ 1.50 | 137 (46) | 284 (47) | 259 (47) | 25 (39) |
| Stable concentration, n (%) | 264 (93) | 552 (94) | 497 (94) | 55 (92) |
| Up-titration, n (%) | 8 (3) | 13 (2) | 13 (2) | 0 (0) |
| Down-titration, n (%) | 12 (4) | 24 (4) | 19 (4) | 5 (8) |
Medical therapy, PTH and cardiovascular episodes after the first hypocalcemia episode
Table 6 shows that the most common response to first hypocalcemia within 90 days following detection was no change to the therapeutic regimen with respect to dialysate calcium concentration, or dose of cinacalcet, active vitamin D and calcium containing phosphate binder. Similar observations were made when we assessed responses at 30 and 60 days (data not shown). Findings were similar in the sensitivity analysis (Online Resource 7).
Table 6.
Management of hypocalcemia within 90 days following first hypocalcemia episode
| Management of hypocalcemia following first hypocalcemic event | Overall | First hypocalcemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild Ca 2.0– < 2.1 mmol/L N = 415 | Moderate Ca 1.87– < 2.0 mmol/L N = 141 | Severe Ca < 1.87 mmol/L N = 54 | ||||||||||
| No. at risk | n | % | No. at risk | n | % | No. at risk | n | % | No. at risk | n | % | |
| Dialysate calcium concentration | ||||||||||||
| Increased | 610 | 102 | 17 | 415 | 60 | 15 | 141 | 30 | 21 | 54 | 12 | 22 |
| Stable | 610 | 472 | 77 | 415 | 329 | 79 | 141 | 103 | 73 | 54 | 40 | 74 |
| Decreased | 610 | 15 | 3 | 415 | 13 | 3 | 141 | 2 | 1 | 54 | 0 | 0 |
| Cinacalcet | ||||||||||||
| Increased | 610 | 91 | 15 | 415 | 67 | 16 | 141 | 21 | 15 | 54 | 3 | 6 |
| Stable | 610 | 374 | 61 | 415 | 255 | 61 | 141 | 86 | 61 | 54 | 33 | 61 |
| Discontinued | 610 | 42 | 7 | 415 | 33 | 8 | 141 | 4 | 3 | 54 | 5 | 9 |
| Reduced | 610 | 102 | 17 | 415 | 59 | 14 | 141 | 30 | 21 | 54 | 13 | 24 |
| Calcitriol/alfacalcidol | ||||||||||||
| Initiated | 486 | 41 | 8 | 326 | 20 | 6 | 115 | 13 | 11 | 45 | 8 | 18 |
| Up-titrated | 124 | 30 | 24 | 89 | 23 | 26 | 26 | 5 | 19 | 9 | 2 | 22 |
| Initiated or up-titrated | 610 | 71 | 12 | 415 | 43 | 10 | 141 | 18 | 13 | 54 | 10 | 19 |
| Stable | 124 | 73 | 59 | 89 | 53 | 60 | 26 | 14 | 54 | 9 | 6 | 67 |
| Down-titrated | 124 | 17 | 14 | 89 | 11 | 12 | 26 | 5 | 19 | 9 | 1 | 11 |
| Discontinued | 124 | 4 | 3 | 89 | 2 | 2 | 26 | 2 | 8 | 9 | 0 | 0 |
| Paricalcitol | ||||||||||||
| Initiated | 394 | 65 | 17 | 258 | 39 | 15 | 96 | 18 | 19 | 40 | 8 | 20 |
| Up-titrated | 216 | 54 | 25 | 157 | 40 | 26 | 45 | 11 | 24 | 14 | 3 | 21 |
| Initiated or up-titrated | 610 | 119 | 20 | 415 | 79 | 19 | 141 | 29 | 21 | 54 | 11 | 20 |
| Stable | 216 | 115 | 53 | 157 | 84 | 54 | 45 | 22 | 49 | 14 | 9 | 64 |
| Down-titrated | 216 | 34 | 16 | 157 | 24 | 15 | 45 | 8 | 18 | 14 | 2 | 14 |
| Discontinued | 216 | 13 | 6 | 157 | 9 | 6 | 45 | 4 | 9 | 14 | 0 | 0 |
| Calcium-based phosphate binder | ||||||||||||
| Initiated | 459 | 51 | 11 | 311 | 30 | 10 | 107 | 17 | 16 | 41 | 4 | 10 |
| Up-titrated | 151 | 25 | 17 | 104 | 14 | 14 | 34 | 9 | 27 | 13 | 2 | 15 |
| Initiated or up-titrated | 610 | 76 | 13 | 415 | 44 | 11 | 141 | 26 | 18 | 54 | 6 | 11 |
| Stable | 151 | 106 | 70 | 104 | 78 | 75 | 34 | 18 | 53 | 13 | 10 | 77 |
| Down-titrated | 151 | 14 | 9 | 104 | 8 | 8 | 34 | 5 | 15 | 13 | 1 | 8 |
| Discontinued | 151 | 6 | 4 | 104 | 4 | 4 | 34 | 2 | 6 | 13 | 0 | 0 |
| Any expected responses1 | ||||||||||||
| No treatment intervention | 610 | 255 | 42 | 415 | 188 | 45 | 141 | 51 | 36 | 54 | 16 | 30 |
| Any treatment intervention | 610 | 355 | 58 | 415 | 227 | 55 | 141 | 90 | 64 | 54 | 38 | 70 |
| Any responses2 | ||||||||||||
| No treatment intervention | 610 | 175 | 29 | 415 | 130 | 31 | 141 | 33 | 23 | 54 | 12 | 22 |
| Any treatment intervention | 610 | 435 | 71 | 415 | 285 | 69 | 141 | 108 | 77 | 54 | 42 | 78 |
1Any expected treatment response = for Cinacalcet, discontinued or reduced; for Dialysate Calcium: increased calcium; for other drugs: initiated or up-titrated
2Any treatment = any of the changes listed in table
About one-third (38%) of patients with a hypocalcemia episode had a therapeutic intervention (i.e. discontinued or reduced cinacalcet, increased dialysate calcium or increased vitamin D or calcium-based phosphate binders) within the first 30 days after the hypocalcemia event. This increased to 44% for those who had an episode of severe hypocalcemia (data not shown). Both groups of patients who had a treatment intervention or no intervention were able to achieve a normal calcium at days 30 (after intervention: 43% vs. none: 38%), 60 (64% vs. 60%) and 90 (73% vs. 75%) after the therapeutic intervention period. Findings were similar in the sensitivity analysis (data not shown). When examining the baseline characteristics of hypocalcemia patients who discontinued and did not discontinue within four months following hypocalcemia events, characteristics were relatively similar except baseline PTH at time of cinacalcet initiation was lower among those who discontinued. This suggests that discontinuation was largely dependent on PTH rather than hypocalcemia (Online Resource 8).
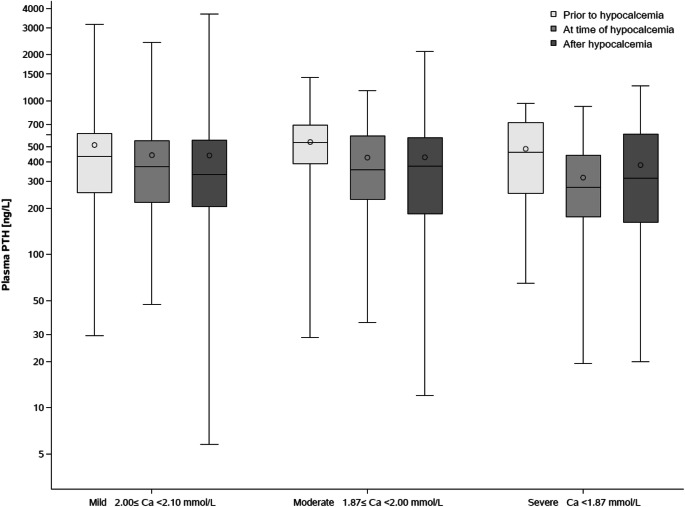
Figure 3 shows closest available iPTH values before, at and after a hypocalcemia episode. No notable changes were observed. iPTH values at time of a hypocalcemia episode were lower than values prior to the episode. Following the hypocalcemia episode, iPTH stayed low even in those in the severe hypocalcemia group. Table 7 shows that the number of deaths and cardiovascular events did not differ in patients who did and did not develop hypocalcemia within 12 months following cinacalcet initiation.
Fig. 3.
Box plot of PTH levels before, at and after first hypocalcemia episode by severity
Table 7.
Number of patients with at least one cardiovascular event or death within 6 months after cinacalcet initiation
| Definition of cardiovascular events and death | Hypocalcemia within 6 months after cinacalcet initiation N = 610 | No hypocalcemia within 6 months after cinacalcet initiation N = 295 | ||
|---|---|---|---|---|
| No. of events within 6 months following cinacalcet initiation | % | No. of events within 6 months following cinacalcet initiation | % | |
| Any cardiovascular event1 | 34 | 5.6 | 16 | 5.4 |
| Coronary artery events1,2 | 12 | 2.0 | 5 | 1.7 |
| Cerebrovascular events1,3 | 4 | 0.7 | 4 | 1.4 |
| Peripheral artery events1,4 | 10 | 1.6 | 4 | 1.4 |
| Congestive heart failure1,5 | 9 | 1.5 | 1 | 0.3 |
| Sudden cardiac death1,6 | 1 | 0.2 | 3 | 1.0 |
| Deaths | 8 | 1.3 | 9 | 3.1 |
1Cardiovascular events as defined in [27]
2Coronary artery events: ICD-10 code I20–I25
3Cerebrovascular events: ICD-10 codes F01, G45, G46, I60, I61, I62, I63, I64, I65, I66, I67, I68, I69
4Peripheral arterial events: ICD-10 codes H34, I51.3, I70, I71, I73.1, I73.9, I74, K55.0, K55.1, N28.0, R02
5Congestive heart failure events: ICD-10 codes I11.0, I13.0, I13.2, I42, I43, I50, I51.5, I51.7
6Sudden cardiac death: ICD-10 codes I46, I49.0
Discussion
In this “real-world” analysis of a large European cohort of chronic HD patients we found that 67% of those initiating cinacalcet subsequently developed hypocalcemia. This frequency is similar to our recent post hoc analysis of the EVOLVE randomized controlled trial, in which cinacalcet-induced hypocalcemia was detected in 58% of patients within a median of 56 days after cinacalcet initiation [11]. This study found that 9% of the hypocalcemia episodes were severe (Ca < 1.87 mmol/L), which is lower than that found in EVOLVE (18%) but comparable to other randomized trials (< 11%) [4, 9, 17]. The high rate of hypocalcemia in EVOLVE may be explained by the systematic assessment of laboratory values in a randomized clinical trial setting, which is more frequent than the recommended interval in routine clinical practice (i.e., 1–3 month interval). Also, the average baseline PTH level was > 700 pg/mL in EVOLVE vs. 600 pg/mL in this study.
Our findings differ from a North-American “real-world” analysis, where cinacalcet led to hypocalcemia in only 47% of patients and only 3% had severe hypocalcemia [18]. In comparing our largely Caucasian European cohort with the North American cohort of Brunelli et al. [18], PTH was similar (mean PTH of 650 pg/mL) but > 50% were African-American, and they had twice as many diabetic patients and the average age was about 10 years younger. All these factors may have substantially influenced treatment and management with cinacalcet. For example, it is thought that Afro-american dialysis patients exhibit less severe high-turnover bone disease at similar PTH levels as compared to Caucasians [19]. Thus, an average PTH level similar to that observed in our study would imply that there was less severe bone disease and a less pronounced “hungry-bone” like scenarios in the North American study. In addition, only 64% of the AROii cohort were prescribed concomitant vitamin D compared to 90% in the North American Cohort.
Prior observational and clinical studies reported that cinacalcet-induced hypocalcemia was transient, usually asymptomatic and self-limited [4, 9, 17, 18, 20, 21]. Indeed, hypocalcemia-induced serious adverse episodes were rare [4, 21] or absent [9, 17] in randomized clinical trials and there are few case reports describing symptomatic patients [22–24]. Our retrospective analysis is limited as hypocalcemia-related events were potentially not systematically captured. However, it is notable that we found no evidence for an increased number of cardiovascular, in particular arrhythmogenic events in the six months immediately following cinacalcet initiation, i.e. the period, when hypocalcemia event was likely to occur (median time to hypocalcemia event was 4 months).
Despite the transient and mostly asymptomatic nature of cinacalcet-induced hypocalcemia, it may still be important to prospectively identify those at risk. In this respect, our present analysis supports prior conclusions that patients with severe sHPT have higher baseline iPTH levels (a consistent predictor of hypocalcemia) and low baseline calcium [14, 18]. It is conceivable that low albumin also predicted hypocalcemia since total calcium rather than ionized calcium was analysed. However, in our sensitivity analyses, there were no differences in our findings when using corrected as opposed to uncorrected calcium. Cumulatively, these observations mirror studies on “hungry bone syndrome” after parathyroidectomy, where the severity of preoperative sHPT was also predictive of occurrence of hypocalcemia [10, 25]. In this study, we had two observations that were difficult to explain, namely having a catheter vascular access was associated with hypocalcemia and having low haemoglobin was associated with a lower risk of hypocalcemia. Although these may represent chance findings further studies need to take them into consideration when protocols are designed. Similarly, risk factors associated with severe hypocalcemia (catheter access, net ultrafiltration, CRP levels, and no paricalcitol use) were of uncertain clinical relevance as we had limited number of severe hypocalcaemia episodes to come to clear conclusions. Even though we assessed albumin-corrected levels, we cannot exclude that inflammation associated with catheter access and high CRP levels, affected protein-bound calcium levels. In addition, we cannot fully exclude that the hypocalcemia risk associated with catheter access might have resulted from inadvertent spillage of citrate locking solution into the systemic circulation. However, citrate locking of catheters was uncommon at the time of our baseline period (2007–2009).
Intrinsic and therapeutic responses to cinacalcet-induced hypocalcemia also closely mirrored data obtained in EVOLVE and in a North American analysis [11, 18] in that PTH levels failed to increase following the hypocalcemic event which is most likely due to persistent suppression of PTH release by the calcimimetic. Therapeutically, although a recent editorial cautioned against inertia [12], we once again observed that the most common response to hypocalcemia was to not change the therapeutic regimen. Indeed, in the about 40% of hypocalcemic patients who received a therapeutic intervention attempting to increase serum calcium, outcomes in terms of normalizing serum calcium were no different as compared to patients with no targeted intervention.
In addition to the limitations discussed above, the present analysis is constrained by a wide range of cinacalcet prescription. Indeed, we noted that, in particular in Eastern European countries, cinacalcet was mostly prescribed to patients with more severe sHPT (Online Resource 9). However, since 83% of our patients were from the Iberian Peninsula, it is unlikely that the different prescription behaviour in Eastern Europe has markedly skewed our analysis towards more severe sHPT cases. Second, our diagnosis of hypocalcemia was based on total rather than ionized serum calcium. While albumin-concentrations were available in 97% of the patients, albumin-corrected total calcium only correlates moderately with ionized serum calcium in advanced CKD [26] and others have concluded that uncorrected calcium is not inferior to corrected calcium in HD patients [15, 16]. Third, whereas short-term adjustments of dialysate calcium might not have been captured in severely hypocalcemic patients, data shown in Table 6 provides little evidence for any systematic trend towards higher dialysate calcium in those with hypocalcemia which is similar to our observations in EVOLVE [11].
In conclusion, in the present “real-world” analysis of incident European HD patients, initiation of cinacalcet led to hypocalcemia in two-thirds of the patients with 9% categorized as severe. Hypocalcemia was mostly asymptomatic, transient with and without targeted intervention to correct it and not associated with an increase in cardiovascular events or deaths. These observations lend further support to the current KDIGO guideline recommendation to avoid hypercalcemia in chronic dialysis patients but to tolerate mild and asymptomatic hypocalcemia in the context of calcimimetic treatment in order to avoid inappropriate calcium loading. However, vigilance will still be necessary in order not to miss the rare case of serious hypocalcemia with potential clinical significance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank participating Fresenius Medical Care Centres for collecting the data.
Author contributions
Research idea and study design: KL, BF and JF; data acquisition: KL and CE; data analysis/interpretation: KL, CE, DCW, PS, BF, and JF; statistical analysis: KL and CE; supervision and or mentorship: BF and JF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
Amgen GmbH, Rotkreuz, Switzerland provided an unrestricted grant for this analysis. This study was funded by Amgen Europe GmbH, Rotkreuz, Switzerland.
Compliance with ethical standards
Conflicts of interest
KL and BF are employees and stockholders of Amgen. CE is an independent contractor providing statistical services to Amgen. DW has received consultancy and speaker fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Janssen, Glaxo Smith Kline, Mundipharam, Napp, Reata and Vifor Fresenius.PS has received consultancy and speaker fees from Reata, Astra Zeneca, Corvidia, Baxter, Astellas and Pfizer. JF has received personal fees from Amgen, Fresenius, Sanofi and Vifor.
Ethical approval
Data were collected and processed in accordance with local ethical and regulatory requirements for each participating FMC facility.
Informed consent
Written informed consent was obtained from all patients, where required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 2.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drueke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J, Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation. 2015;132(1):27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick RD, Newsome BB, Zaun D, Liu J, Solid CA, Nieman K, St Peter WL. Evaluating real-world use of cinacalcet and biochemical response to therapy in US hemodialysis patients. Am J Nephrol. 2013;37(4):389–398. doi: 10.1159/000350213. [DOI] [PubMed] [Google Scholar]
- 6.Messa P, Macario F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazao JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Wilkie M, Zani VJ, Molemans B, Carter D, Locatelli F. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3(1):36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol. 2009;4(2):354–360. doi: 10.2215/CJN.05241008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urena P, Jacobson SH, Zitt E, Vervloet M, Malberti F, Ashman N, Leavey S, Rix M, Os I, Saha H, Ryba M, Bencova V, Banos A, Zani V, Fouque D. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice–the ECHO observational study. Nephrol Dial Transplant. 2009;24(9):2852–2859. doi: 10.1093/ndt/gfp144. [DOI] [PubMed] [Google Scholar]
- 9.Urena-Torres P, Bridges I, Christiano C, Cournoyer SH, Cooper K, Farouk M, Kopyt NP, Rodriguez M, Zehnder D, Covic A. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol Dial Transplant. 2013;28(5):1241–1254. doi: 10.1093/ndt/gfs568. [DOI] [PubMed] [Google Scholar]
- 10.Cruz DN, Perazella MA. Biochemical aberrations in a dialysis patient following parathyroidectomy. Am J Kidney Dis. 1997;29(5):759–762. doi: 10.1016/S0272-6386(97)90131-1. [DOI] [PubMed] [Google Scholar]
- 11.Floege J, Tsirtsonis K, Iles J, Drueke TB, Chertow GM, Parfrey P. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with cinacalcet in the EVOLVE trial. Kidney Int. 2018 doi: 10.1016/j.kint.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Evenepoel P, Shroff R. Facing cinacalcet-induced hypocalcemia: sit back and relax? Kidney Int. 2018;93(6):1275–1277. doi: 10.1016/j.kint.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 13.de Francisco AL, Gillespie IA, Gioni I, Floege J, Kronenberg F, Marcelli D, Wheeler DC, Froissart M, Drueke TB, Cllaborators AROSC. Anti-parathyroid treatment effectiveness and persistence in incident haemodialysis patients with secondary hyperparathyroidism. Nefrologia. 2016;36(2):164–175. doi: 10.1016/j.nefro.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87(5):996–1008. doi: 10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clase CM, Norman GL, Beecroft ML, Churchill DN. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant. 2000;15(11):1841–1846. doi: 10.1093/ndt/15.11.1841. [DOI] [PubMed] [Google Scholar]
- 16.Lian IA, Asberg A. Should total calcium be adjusted for albumin? A retrospective observational study of laboratory data from central Norway. BMJ Open. 2018;8(4):e017703. doi: 10.1136/bmjopen-2017-017703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16(3):800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 18.Brunelli SM, Dluzniewski PJ, Cooper K, Do TP, Sibbel S, Bradbury BD. Management of serum calcium reductions among patients on hemodialysis following cinacalcet initiation. Pharmacoepidemiol Drug Saf. 2015;24(10):1058–1067. doi: 10.1002/pds.3845. [DOI] [PubMed] [Google Scholar]
- 19.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26(6):1368–1376. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Investigators Evolve Trial, Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 21.Wetmore JB, Gurevich K, Sprague S, Da Roza G, Buerkert J, Reiner M, Goodman W, Cooper K. A Randomized Trial of Cinacalcet versus Vitamin D Analogs as Monotherapy in Secondary Hyperparathyroidism (PARADIGM) Clin J Am Soc Nephrol. 2015;10(6):1031–1040. doi: 10.2215/CJN.07050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novick T, McMahon BA, Berliner A, Jaar BG. Cinacalcet-associated severe hypocalcemia resulting in torsades de pointes and cardiac arrest: a case for caution. Eur J Clin Pharmacol. 2016;72(3):373–375. doi: 10.1007/s00228-015-1989-6. [DOI] [PubMed] [Google Scholar]
- 23.Simon F, Bertrand Q, Pirson Y, Flamion B, Loute G. Potentialisation of hyperkalemia effects by cinacalcet-induced hypocalcemia on dialysis. Nephrol Ther. 2012;8(6):476–480. doi: 10.1016/j.nephro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Koubar SH, Qannus AA, Medawar W, Abu-Alfa AK. Hungry bone syndrome two weeks after starting cinacalcet: a call for caution. CEN Case Rep. 2018;7(1):21–23. doi: 10.1007/s13730-017-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viaene L, Evenepoel P, Bammens B, Claes K, Kuypers D, Vanrenterghem Y. Calcium requirements after parathyroidectomy in patients with refractory secondary hyperparathyroidism. Nephron Clin Pract. 2008;110(2):c80–c85. doi: 10.1159/000151722. [DOI] [PubMed] [Google Scholar]
- 26.Gauci C, Moranne O, Fouqueray B, de la Faille R, Maruani G, Haymann JP, Jacquot C, Boffa JJ, Flamant M, Rossert J, Urena P, Stengel B, Souberbielle JC, Froissart M, Houillier P, NephroTest Study G Pitfalls of measuring total blood calcium in patients with CKD. J Am Soc Nephrol. 2008;19(8):1592–1598. doi: 10.1681/ASN.2007040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckardt KU, Gillespie IA, Kronenberg F, Richards S, Stenvinkel P, Anker SD, Wheeler DC, de Francisco AL, Marcelli D, Froissart M, Floege J, Committee AROS. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88(5):1117–1125. doi: 10.1038/ki.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.