Abstract
Background
One-third of all deaths in hospitals are caused by sepsis. Despite its demonstrated prevalence and high case fatality rate, antibiotics remain the only target-oriented treatment option currently available. Starting from results showing that low-dose anthracyclines protect against sepsis in mice, we sought to find new causative treatment options to improve sepsis outcomes.
Methods
Sepsis was induced in mice, and different treatment options were evaluated regarding cytokine and biomarker expression, lung epithelial cell permeability, autophagy induction, and survival benefit. Results were validated in cell culture experiments and correlated with patient samples.
Findings
Effective low-dose epirubicin treatment resulted in substantial downregulation of the sphingosine 1-phosphate (S1P) degrading enzyme S1P lyase (SPL). Consequent accumulation and secretion of S1P in lung parenchyma cells stimulated the S1P-receptor type 3 (S1PR3) and mitogen-activated protein kinases p38 and ERK, reducing tissue damage via increased disease tolerance. The protective effects of SPL inhibition were absent in S1PR3 deficient mice. Sepsis patients showed increased expression of SPL, stable expression of S1PR3, and increased levels of mucin-1 and surfactant protein D as indicators of lung damage.
Interpretation
Our work highlights a tissue-protective effect of SPL inhibition in sepsis due to activation of the S1P/S1PR3 axis and implies that SPL inhibitors and S1PR3 agonists might be potential therapeutics to protect against sepsis by increasing disease tolerance against infections.
Funding
This study was supported by the Center for Sepsis Control and Care (CSCC), the German Research Foundation (DFG), RTG 1715 (to M. H. G. and I. R.) and the National Institutes of Health, Grant R01GM043880 (to S. S.).
Keywords: Sphingosine 1-phosphate, Epirubicin, Anthracycline, Epithelial barrier, Lung, Autophagy, Cytokine, Lipopolysaccharide, Peritonitis
Research in context.
Evidence before this study
Anthracyclines were shown to induce autophagy mediated protection against severe sepsis. However, anthracyclines are also genotoxic. Sphingolipids are involved in the regulation of autophagy as well, with ceramides inducing strong autophagy leading to cell death and S1P inducing weak autophagy leading to cell survival.
Added value of this study
This study demonstrates an interconnection between the protective effect of genotoxic anthracyclines and S1P signaling. Low-dose epirubicin treatment resulted in decreased SPL expression and subsequent S1P accumulation in local tissues. Consequent activation of S1PR3 entailed protection against severe sepsis similar to low-dose anthracyclines due to reductions in cytokine production, autophagy activation, lung epithelial cell barrier leakage and biomarkers for tissue damage.
Implications of all the available evidence
SPL inhibitors and S1PR3 agonists may serve as protective agents against severe sepsis similar to anthracyclines, but without their genotoxic properties, which could increase their potential range of application compared to anthracyclines.
Alt-text: Unlabelled box
1. Introduction
Sepsis affects about 1.7 million adults in the United States, with nearly 270.000 deaths each year. It is responsible for one-third of all deaths in hospitals. Sepsis is thereby the primary cause of death, particularly in intensive care units, with a higher prevalence than progressive cancer and heart failure [1]. Worldwide, about 48.9 million incident cases of sepsis, with 11 million deaths were recorded in 2017. This represents one-fifth of all global deaths. The most common underlying cause of sepsis-related deaths is lower respiratory infections [2].
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid metabolite involved in many inflammatory processes including the regulation of lymphocyte circulation and positioning [3–5], immune activation [6–8], cytokine release [[8], [9], [10]], cell survival and apoptosis [11,12], pathogen growth [13,14], and endothelial barrier formation [15,16]. It is generated from its precursor sphingosine via phosphorylation by two sphingosine kinases, SphK1 and SphK2, and irreversibly degraded by the S1P lyase (SPL). SPL, encoded by the Sgpl1 gene, is a constitutively active intracellular enzyme that localizes to the endoplasmic reticulum (ER) and mitochondria [17]. Upon SPL inhibition, S1P accumulates intracellularly and can be secreted, enabling it to activate cell surface G protein-coupled S1P-receptors (S1PRs) [18]. Inhibition or genetic depletion of SPL leads to the accumulation of S1P predominantly within tissues [5]. Constitutive global depletion of SPL is pathologic with severe phenotypes in the immune system, lungs, bones, liver, and other organs [19,20]. Short-term incomplete pharmacological inhibition, however, has little side effects and was already clinically tested for the treatment of autoimmune diseases such as rheumatoid arthritis [5,21].
The anthracycline epirubicin is a DNA-intercalating genotoxic drug used for chemotherapy. A recent study suggests that low, non-cytotoxic doses of epirubicin induce adaptive stress responses that protect mice against experimental sepsis [22]. It was suggested that epirubicin raises the tolerance of parenchyma cells against an inflammatory insult via the DNA damage response and autophagy, in a process referred to as disease tolerance [23]. Because S1P and its related metabolite ceramide have been implicated in these processes [24,25], it was of interest to examine the role of S1P in the protective effects of low-dose epirubicin treatment in experimental sepsis.
In the present work, we uncovered a connection between sphingolipid metabolism and S1P signaling to adaptive stress responses by low-dose epirubicin in sepsis. We demonstrated that low-dose epirubicin results in a potent downregulation of SPL, leading to S1P accumulation in peripheral tissues, particularly in the lung. Inhibition of SPL itself was sufficient to provide protective effects similar to those of epirubicin in experimental sepsis on survival and clinical parameters of cytokines, tissue damage markers, and bacterial load. Sepsis prevention was mainly driven by the activation of S1PR3. As expression of SPL increased in sepsis patients, inhibition of SPL or activation of S1PR3, particularly in the lung, may, therefore, serve as new strategies to prevent sepsis-induced organ failure.
2. Material and methods
Detailed descriptions of materials and methods are provided in a data supplement.
2.1. Study design
Dose-dependent effects of the genotoxic agent epirubicin were evaluated in human and mouse lung epithelial cells in vitro upon lipopolysaccharide (LPS) stimulation and in experimental sepsis in vivo. Polymicrobial sepsis was induced by peritoneal contamination and infection (PCI) in mice [26]. All experiments were performed in accordance with German legislation on the protection of animals and with permission of the official animal welfare committee of Thuringia (permit number TVA Reg. Nr. 02–010/15). Animals of the same age were randomly allocated to experimental and control groups of three to five animals per group in two to three independent experiments. The body weight, the survival, and the Clinical Severity Score (CSS), a definitive scoring system from 1 with no signs of illness to 4, which reflects a severe clinical status [26], were monitored twice a day. Injections and evaluation of the animals were conducted in a non-blinded fashion. Data collection and analyses were blinded when possible.
Human data are based on a single-center prospective-observational trial approved by the local Research Ethics Committee (Hamburg Chamber of Physicians: reference PV4550). Sepsis was diagnosed according to the sepsis-3 definitions [27]. The patient cohort was already described in earlier studies [28], [29], [30], and this was a follow-up analysis, including data from selected control and sepsis patients.
2.2. Statistics
At least three mice from two to three different experiments or three independent cell culture experiments were used for statistical analysis. All n values represent biological replicates. Data are expressed as means ± SEM. Statistical analyses were performed with GraphPad Prism 7. One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparisons were performed for analysis of more than two groups. To test for normal distribution, a Kolmogorov–Smirnov test and an Equal Variance Test were utilized. If either failed, Kruskal–Wallis One Way Analysis of Variance on Ranks with post-hoc Dunn's multiple comparisons was performed. For comparisons of two experimental groups, an unpaired, two-tailed t-test was used. The Gehan–Breslow–Wilcoxon test was applied for survival analyses. In all tests, values of p ≤ 0.05 were considered significant.
3. Role of the funding source
The sponsors of the study had not any role in study design, data collection, data analysis, interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
4. Results
4.1. Epirubicin alters S1P metabolism and protects against sepsis
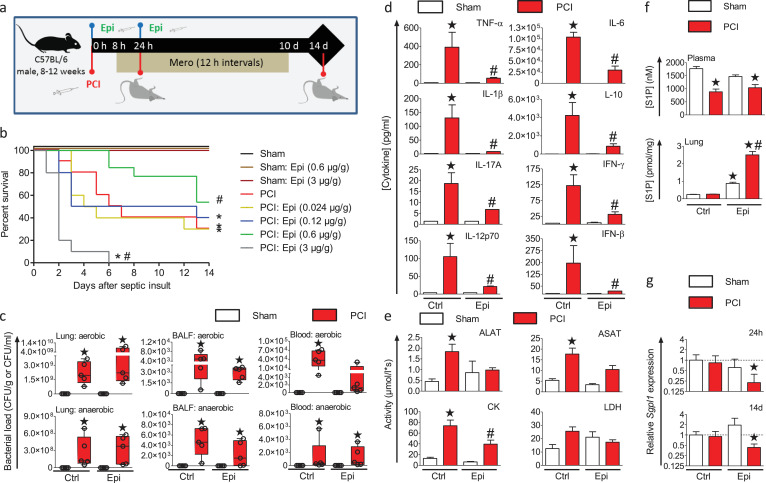
Non-toxic low-dose epirubicin treatment has been shown to protect mice against experimental sepsis induced by cecal ligation and puncture (CLP), particularly by inducing disease tolerance in the lung [22]. Given the pro-survival functions of S1P, we examined the role of S1P in this protective effect. We used the experimental sepsis model of PCI in mice treated with increasing epirubicin concentrations, starting as low as 0.024 µg/g. Sepsis was initiated by intraperitoneal (i.p.) injection of a defined human stool suspension, and antibiotic therapy with meropenem was started 8 h later (Fig. 1a). Meropenem was used to resemble the antibiotic treatment in the clinics as close as possible. The amount of the human stool suspension was adjusted in the way that meropenem would not kill all pathogens, so that a systemic inflammation would still develop. The median survival time of infected mice under these conditions was 6.5 d. Epirubicin was injected twice i.p., at the time of infection and 24 h later. While epirubicin concentrations up to 0.12 µg/g had little effect on the overall survival, a dose of 0.6 µg/g of epirubicin induced a significant survival benefit compared to untreated infected mice with a median survival time of 14 d (Fig. 1b). In contrast, a further increase of the epirubicin dose to 3 µg/g significantly increased the lethality of infected, but not naïve mice, and decreased median survival to 2 d. All infected mice featured a marked bacterial load of aerobes and anaerobes in the lung, bronchoalveolar lavage fluid (BALF) and whole blood 24 h after infection (Fig. 1c) and showed clear signs of sepsis with a loss of body temperature (Fig. S1). Importantly, low-dose epirubicin treatment reduced sepsis-associated levels of inflammatory cytokines, including tumour necrosis factor (TNF)-α, interferon (IFN)-β, IFN-γ, interleukin (IL)−1β, IL-6, IL-10, IL-12p70, and IL-17A in plasma with no statistically difference to non-septic sham animals (Fig. 1d). Furthermore, biomarkers for tissue damage, aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), and creatinine kinase (CK) were significantly increased 24 h after infection in untreated mice, but not in mice treated with 0.6 µg/g epirubicin (Fig. 1e). A similar trend was also seen for lactate dehydrogenase (LDH). Systemic S1P levels in plasma were reduced 24 h after infection and did not change with epirubicin treatment (Fig. 1f). Notably, S1P levels in the lungs remained unchanged in infected control mice but were increased by 10-fold in infected mice treated with 0.6 µg/g epirubicin (Fig. 1f). This local increase of S1P levels correlated with significantly and permanently reduced expression of the S1P-degrading enzyme SPL by 77.6% after 24 h and by 56.9% 14 d after infection, respectively (Fig. 1g). Thus, epirubicin induced disease tolerance, as characterized by reduced cytokine levels, less tissue damage, and unchanged pathogen burden, accompanied by downregulation of the S1P-degrading enzyme SPL and increased S1P levels in the lung.
Fig. 1.
Low dose epirubicin protects mice from sepsis and induces disease tolerance.
(a) Sepsis was induced by i.p. injection of a human stool suspension (PCI). Treatment with the antibiotic meropenem (Mero) was started 8 h after infection and repeated every 12 h for 10 d Indicated concentrations of epirubicin (Epi) or vehicle were injected i.p. immediately after infection and 24 h later. (b) Kaplan–Meier survival plots for a 14-day observational period. n = 5 (sham), n = 10–13 (PCI), *p<0.05 compared to sham, #p<0.05 compared to untreated PCI mice (Gehan–Breslow–Wilcoxon test). (c) Bacterial load of aerobic and anaerobic bacteria in lung tissue, bronchoalveolar lavage fluid (BALF) and whole blood were measured in mice treated without or with 0.6 µg/g Epi 24 h after PCI. n = 5, *p<0.05 compared to sham (Kruskal–Wallis one-way analysis of variance on ranks with post-hoc Dunn's multiple comparison). Box plots: middle bands indicate the median; whiskers indicate minimum and maximum values; symbols indicate individual animals. (d) Cytokine levels and (e) markers of tissue damage were analysed in plasma 24 h after sepsis induction in animals treated with or without 0.6 µg/g Epi, n = 5. (f) Levels of S1P in plasma and lung were measured 24 h after infection by LC-ESI-MS/MS, n = 5. (d-f) mean ± SEM, *p<0.05 compared to sham and #p<0.05 compared to untreated PCI mice tested by unpaired, two-tailed t-test (One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparison). (g) SPL transcript levels were determined by qPCR in total RNA from lungs of treated non‐infected and septic mice sacrificed at 24 h post PCI or 14 days later. *p<0.05, n = 3 (Pair‐wise fixed reallocation randomization test with untreated, non-infected animals as control group).
4.2. Epirubicin raises S1P levels by downregulation of SPL in isolated lung parenchyma cells
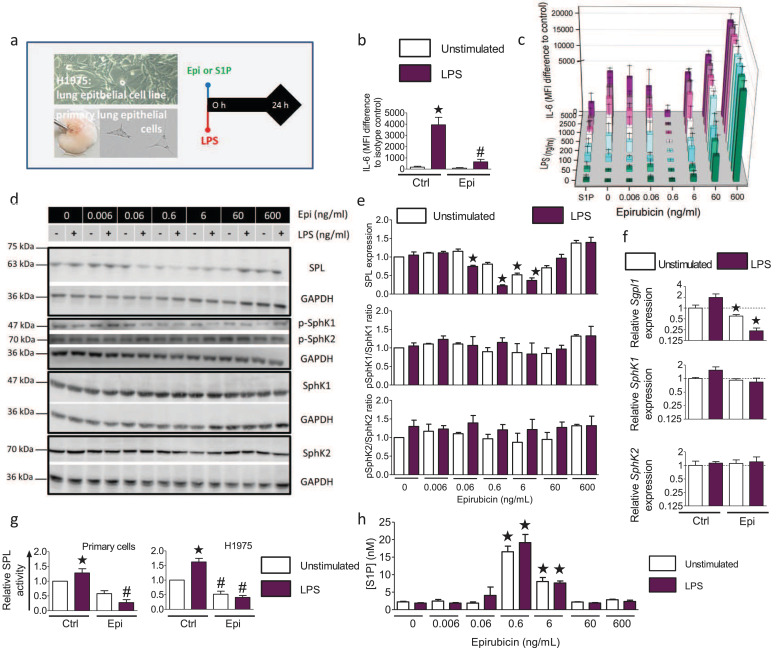
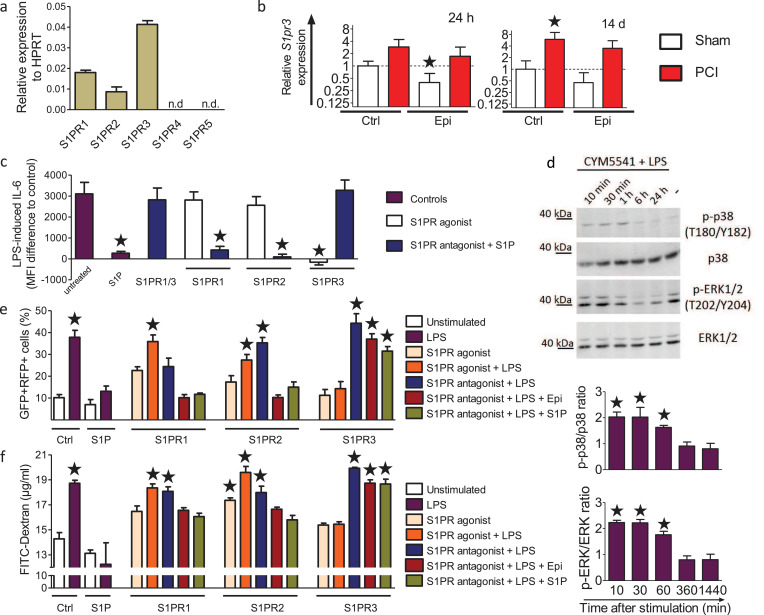
To understand the effects of low-dose epirubicin treatment on sphingolipid metabolism, we performed cell culture experiments with primary murine lung epithelial cells and the human lung epithelial cell line H1975 (Fig. 2a). Primary mouse lung epithelial cells responded to 200 ng/ml LPS, as a surrogate trigger of infection, with a robust increase of intracellular IL-6, which was significantly reduced by treatment with 0.6 ng/ml epirubicin (Fig. 2b). H1975 cells were treated with increasing concentrations of LPS and epirubicin to evaluate their dose-response. Intracellular IL-6 increased concomitantly with the LPS concentration (Fig. 2c). Co-treatment with 0.6 ng/ml epirubicin reduced the intracellular IL-6 levels significantly at all tested LPS-concentrations up to 5 µg/ml. Lower epirubicin concentrations were less effective, and higher concentrations had opposite effects (Fig. 2c). A single application of 0.5 µM S1P prevented increased IL-6 levels at all tested LPS concentrations, reminiscent of the 0.6 ng/ml epirubicin dose. Western blot analysis demonstrated that SPL levels were significantly downregulated following combined treatment with epirubicin and LPS (Fig. 2d) with a maximum observed at 200 ng/ml LPS and 0.6 ng/ml epirubicin. Protein expression and activation of the two S1P-producing sphingosine kinases, SphK1 and SphK2, remained unchanged (Fig. 2e). Quantitative PCR data confirmed the significant downregulation of SPL also on the mRNA levels 24 h after treatment of the H1975 lung epithelial cells with epirubicin and LPS (Fig. 2f), while SphK1 and SphK2 mRNA level remained unchanged. However, SPL activity was significantly increased after stimulation with 200 ng/ml LPS in both primary lung epithelial cells and H1975 cells, and co-treatment with 0.6 ng/ml epirubicin significantly abolished SPL activity (Fig. 2g). As a result, epirubicin treatment of H1975 cells significantly increased S1P secretion by 6-fold at the optimal concentration of 0.6 ng/ml epirubicin, higher and lower concentrations being less effective (Fig. 2h).
Fig. 2.
Protective effect of low-dose epirubicin on LPS–induced inflammatory responses of lung epithelial cells depends on downregulation of S1P lyase and subsequent S1P release.
(a) Experimental protocol: Primary mouse lung epithelial cells and H1975 cells were stimulated for 24 h with 200 ng/ml LPS and indicated concentrations of epirubicin (Epi). (b) IL-6 production of primary lung epithelial cells ± 0.6 ng/ml Epi and 200 ng/ml LPS n = 3–5, *p<0.05 to unstimulated, #p<0.05 to LPS (unpaired, two-tailed t-test). (c) IL-6 production in H1975 cells treated with indicated concentrations of Epi and LPS ± 0.5 µM S1P, mean ± SEM,n = 3–5, p<0.05 for all treatments with 200 ng/ml LPS or higher compared to untreated control except for cells treated with S1P or 0.6 ng/ml Epi (One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparison test). (d) Western Blot analysis of SPL, SphK1, SphK2 and p-SphK1 and p-SphK2 levels in H1975 cells stimulated as indicated. (e) Densitometric quantification of data shown in (d), means ± SEM, n = 3–5. (f) mRNA expression of SPL, SphK1 and SphK2 was determined by qPCR in H1975 without or with 0.6 µg/ml Epi and normalized to HPRT, *p<0.05 to untreated control, n = 3 (Pair‐wise fixed reallocation randomization test). (g) SPL activity in cell lysates of primary murine lung epithelial cells (left) and H1975 cells (right) stimulated for 24 h without or with LPS and 0.6 ng/ml Epi, mean ± SEM, n = 5, *p<0.05 compared to unstimulated control (One-way ANOVA with Bartlett test for equal variances and post-hoc Bonferroni for multiple comparisons), #p < 0.05 compared to LPS-stimulated cells without Epi (Two-tailed, unpaired t-test). (h) S1P secretion in H1975 cells treated for 24 h without or with LPS and indicated concentrations of Epi, mean ± SEM, *p<0.05 n = 3–6 (One-way ANOVA with post-hoc Bonferroni's multiple comparison test to unstimulated control cells).
4.3. Transient inhibition of SPL is sufficient to protect against sepsis
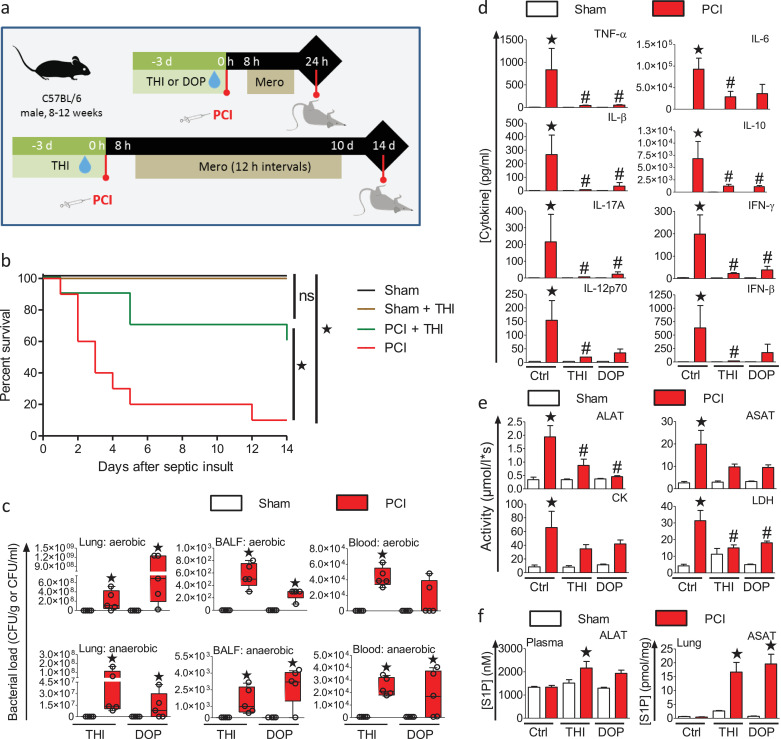
To determine the contribution of SPL inhibition to the observed sepsis protection, mice were treated prior to infection with the SPL-specific inhibitor 2-acetyl-4-tetrahydroxybutyl imidazole (THI) or the vitamin B6 antagonist and SPL inhibitor 4-deoxypyridoxine (DOP) (Fig. 3a). THI pre-treatment significantly increased the survival of infected mice compared to controls with a median survival of at least 14 d compared to 3 d for the infected untreated mice (Fig. 3b). The bacterial burdens were significantly increased in the lungs, BALF, and whole blood of all infected mice, regardless of their treatment (Fig. 3c). All infected animals displayed a loss of body temperature (Fig. S1). Despite the increased bacterial burden, only infected control mice had significantly increased cytokine levels of TNF-α, IFN-β, IFN-γ, IL-6, IL-10, IL-12p70, and IL-17A (Fig. 3d). Treatment with THI or DOP largely prevented increased cytokine release into the plasma. Similarly, the tissue damage markers ALAT, ASAT, CK, and LDH were increased significantly in the infected control mice, but not in mice treated with THI or DOP (Fig. 3e). Moreover, both THI and DOP significantly increased levels of S1P in plasma and lungs of infected mice (Fig. 3f), in accordance with their predicted mode of action. Thus, pharmacological inhibition of SPL mimicked the protective effects of low-dose epirubicin in polymicrobial sepsis.
Fig. 3.
Inhibition of sphingosine 1-phosphate lyase protects from sepsis.
(a) Experimental protocol: Mice were treated with 50 mg/L (~12.5 mg/kg/day) 2-acetyl-4-tetrahydroxybutyl imidazole (THI) or 100 mg/L (~25 mg/kg/day) 4‐deoxypyridoxine (DOP) in drinking water for 3 days prior to sepsis induction by i.p. injection of human faeces as before. Sham treated animals received 0.9% saline solution. Mice were then treated for 10 d with antibiotic starting 8 h after infection and every 12 h (n = 10). Non-infected controls were treated without or with THI or DOP (n = 5). (b) Kaplan–Meier survival plots for a period of 14 days, *p<0.05 compared to sham, #p<0.05 compared to untreated PCI mice (Gehan–Breslow–Wilcoxon test). (c) Aerobic and anaerobic bacteria load in lung tissue, bronchoalveolar lavage fluid (BALF) and whole blood from THI or DOP treated animals, measured 24 h after PCI (n = 4–5), *p<0.05 (Kruskal–Wallis One Way Analysis of Variance on Ranks with a post-hoc Dunn's multiple comparison to untreated sham animals). Box plots: middle band indicates the median; whiskers indicate minimum and maximum values; symbols indicate individual animals. (d) Cytokine levels and (e) markers of tissue damage analysed in plasma 24 h after sepsis induction in animals without or with 50 mg/L (~12.5 mg/kg/day) THI or 100 mg/L (~25 mg/kg/day) DOP treatment (n = 3–5). (f) Levels of S1P in plasma and lung tissue were determined by LC-ESI-MS/MS 24 h after infection (n = 4–5). (d–f) Data are means ± SEM, *p<0.05 compared to sham animals (One-way ANOVA with a post-hoc Bonferroni's multiple comparison test).
4.4. S1P regulates autophagy via activation of ERK1/2 and p38
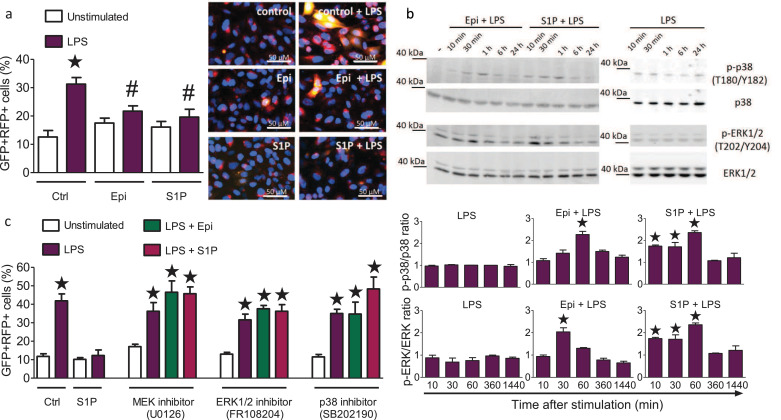
Low-dose epirubicin treatment regulates ATM-dependent autophagy [22]. We, therefore, investigated the impact of epirubicin and S1P on autophagy in H1975 lung epithelial cells transduced with the autophagy reporter RFP-GFP-LC3B [31]. RFP-GFP double-positive autophagosome counts were markedly increased after 24 h stimulation with LPS, illustrating a strong induction of autophagy (Fig. 4a). Treatment with 0.6 ng/ml epirubicin or 0.5 µM S1P alone had no effect on basal autophagy, but significantly reduced the amount of autophagosomes and thereby dampened the induction of autophagy after stimulation with LPS (Fig. 4a). Western blot analysis revealed activation of the mitogen-activated protein kinases (MAPK) ERK1/2 and p38 in H1975 cells challenged with 200 ng/ml LPS and 0.6 ng/ml epirubicin or 0.5 µM S1P, but not with LPS alone (Fig. 4b). These findings indicated that MAPK signaling might mediate the observed reversal of LPS-induced autophagy by epirubicin and S1P. To test this hypothesis, we used inhibitors against MEK1/2 (U0126), ERK1/2 (FR108204), or p38 (SB202190). While the inhibitors did not affect basal autophagy, all three inhibitors prevented S1P- and epirubicin-induced reduction of autophagy (Fig. 4c). Together, these data support the idea that S1P and epirubicin can limit LPS-induced autophagy via ERK and p38-dependent signals.
Fig. 4.
Epirubicin and S1P promote the reversal of LPS-induced autophagy via MAP kinase pathways.
(a) Autophagy in H1975 lung epithelial cells transduced with the Premo™ autophagy tandem sensor RFP-GFP-LC3B (30 particles/cell) after 24 h culture. Cells were treated with 200 ng/ml LPS in the absence or presence of 0.5 µM S1P or 0.6 ng/ml Epi. Representative cell images are shown, scale bars: 50 µm. The number of GFP and RFP double-positive cells per 100 cells was determined in 5 fields of 3 independent samples, means ± SEM, n = 5, *p<0.05 compared to untreated cells, #p<0.05 compared to LPS treated cells (One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparison test). (b) Western blot detection and densitometric quantification of p38, ERK1/2, p-p38 and p-ERK1/2 in H1975 cells treated with 0.5 µM S1P or 0.6 ng/ml Epi along with 200 ng/ml LPS (left panel) or with 200 ng/ml LPS (right panel) for the indicated times, means ± SEM, n = 3, *p<0.05 (One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparison test). (c) Autophagy in H1975 lung epithelial cells transduced with the Premo™ autophagy tandem sensor RFP-GFP-LC3B (30 particles/cell). Cells were stimulated for 24 h without and with LPS and treated with 10 µM U0126 (MEK inhibitor), 50 µM FR108204 (ERK1/2 inhibitor), or 10 µM SB201190 (p38 inhibitor) without and with S1P (0.5 µM) or Epi (0.6 ng/ml) as indicated. The number of both GFP and RFP double-positive cells per 100 cells were counted in 5 fields of 3 wells per stimulation, means ± SEM, n = 3, *p<0.05 (One-way ANOVA with Bartlett´s test for equal variances and post-hoc Bonferroni's multiple comparison test).
4.5. S1P stabilization of the lung epithelial barrier is MAPK dependent
Lung epithelial cells form a barrier and help to maintain homeostasis in the lung. Disruption of the epithelial barrier can promote inflammation and lung injury. To examine the effects of epirubicin and S1P on lung epithelial barrier function, the permeability of H1975 cell layers to fluorescein-labeled (FITC)-dextran was evaluated in Transwell chambers (Fig. S2a). Stimulation with 200 ng/ml LPS significantly increased lung epithelial barrier leakage, which was prevented by treatment with 0.6 ng/ml epirubicin or 0.5 µM S1P (Fig. S2b). Inhibition of MAPKs with the MEK1/2 inhibitor U0126, the ERK1/2 inhibitor FR108204, or the p38 inhibitor SB202190 precluded S1P and epirubicin-induced epithelial cell barrier stabilization (Fig. S2c). The inhibitors themselves did not induce significant FITC-dextran leakage, albeit FITC-dextran levels were slightly elevated compared to controls. The pathophysiological relevance of barrier stabilization in the lung was also investigated in mice that were treated with 0.6 µg/g epirubicin, 100 mg/L (~25 mg/kg/day) DOP, or 50 mg/L (~12.5 mg/kg/day) THI (Fig. S2d). Analysis of BALF 24 h after infection demonstrated increased leakage of serum albumin compared to non-infected controls (Fig. S2e). Of note, the increased serum albumin leakage was prevented by treatment with epirubicin and by inhibition of SPL with THI or DOP (Fig. S2e).
4.6. S1P/S1PR3 signaling reduces cytokine production, stabilizes barrier integrity and prevents excessive autophagy
Next, we investigated the involvement of S1PRs in S1P- and epirubicin-mediated sepsis prevention in more detail. Quantitative PCR analysis showed that S1PR3 was highly expressed in lung epithelial cells together with S1PR1 and S1PR2, while S1PR4 and S1PR5 expression was below detection limits (Fig. 5a). Expression of S1PR3 mRNA was additionally confirmed in lung tissue of mice and was increased 24 h, and 14 d after infection in vehicle- and 0.6 µg/g epirubicin-treated mice (Fig. 5b), while S1PR1 and S1PR2 are rather downregulated (Fig. S3). Stimulation of H1975 cells with LPS for 24 h significantly increased IL-6 production, which was reduced by 0.5 µM S1P (Fig. 5c). This inhibitory effect of S1P was blocked by VPC23019, an antagonist of both S1PR1 and S1PR3. Neither the S1PR1 agonist SEW2781 nor the S1PR2 agonist CYM5520 mimicked the blocking effect of S1P. In line with these results, W146 or JTE-013, antagonists of S1PR1 and S1PR2, respectively, did not prevent the inhibitory effect of S1P on IL-6 production (Fig. 5c). However, the S1PR3 agonist CYM5541 blocked IL-6 production to the same extent as S1P, and the S1PR3 antagonist TY52156 completely blocked S1P-mediated inhibition of IL-6 production, demonstrating a predominant role of S1PR3 (Fig. 5c). Activation of ERK1/2 and p38 was also evident after treatment with LPS and the S1PR3 agonist CYM5541, suggesting that S1PR3 was involved in MAPK activation similarly to S1P (Fig. 5d). In line with these results, only S1PR3 antagonism decreased S1P-mediated regulation of LPS-induced autophagy and epithelial barrier leakage (Fig. 5e, f). Analogously, only the S1PR3 agonist CYM5541 but not the agonists for S1PR1 and S1PR2 recapitulated the effect of S1P (Fig. 5e, f). We conclude that S1P acts via S1PR3 by activating its downstream signaling via MAPK, ERK1/2, and p38 to attenuate LPS-induced cytokine production, autophagy, and epithelial barrier disruption.
Fig. 5.
Protective effects of epirubicin and S1P depend on S1PR3.
mRNA expression of (a) S1PR1–5 in H1975 cells, normalized to HPRT, and (b) S1PR3 in lung samples of Epi-treated control and septic mice sacrificed 24 h and 14 d post infection, normalized to GAPDH, assessed by qPCR, n = 3, *p<0.05 (Pair‐wise fixed reallocation randomization test), n.d. = not detectable. (c) IL-6 production in H1975 cells co-stimulated with 200 ng/ml LPS and 0.5 µM S1P ± 3 µM of the S1PR antagonists VPC23019 (S1PR1/S1PR3), W146 (S1PR1), JTE-013 (S1PR2), or TY52156 (S1PR3). Cells were also stimulated with LPS and with 0.5 µM of the S1PR agonists SEW2781 (S1PR1), CYM5520 (S1PR2) or CYM5541 (S1PR3), n = 3. (d) Western blot analysis and densitometric quantification of MAPK activation in H1975 cells co-stimulated with 200 ng/ml LPS and 0.5 µM CYM5541, n = 3. (e) Autophagy in H1975 lung epithelial cells transduced with the Premo™ autophagy tandem sensor RFP-GFP-LC3B (30 particles/cell) and stimulated with 200 ng/ml LPS, 0.5 µM S1P or 0.6 ng/ml Epi as indicated ± 3 µM of the S1PR antagonists W146 (S1PR1), JTE-013 (S1PR2) or TY52156 (S1PR3), or 0.5 µM of the S1PR agonists SEW2781 (S1PR1), CYM5520 (S1PR2) or CYM5541 (S1PR3). The number of GFP and RFP double-positive cells per 100 cells was determined in 5 fields of 3 independent samples, n = 5. (f) Epithelial barrier stability of H1975 cells was assessed in Transwell inserts with 0.45 µm pore size. Cells were challenged as described in (e). FITC-dextran (70 kDa, 2 mg/ml) was added to the upper chamber, and fluorescence intensity in the lower chamber was measured after 24 h, n = 5. (c-f) Data are means ± SEM, *p<0.05 (One-way ANOVA with post-hoc Bonferroni's multiple comparison test to unstimulated control cells).
4.7. Deletion of S1PR3 mitigates the sepsis protective effect of SPL inhibitors
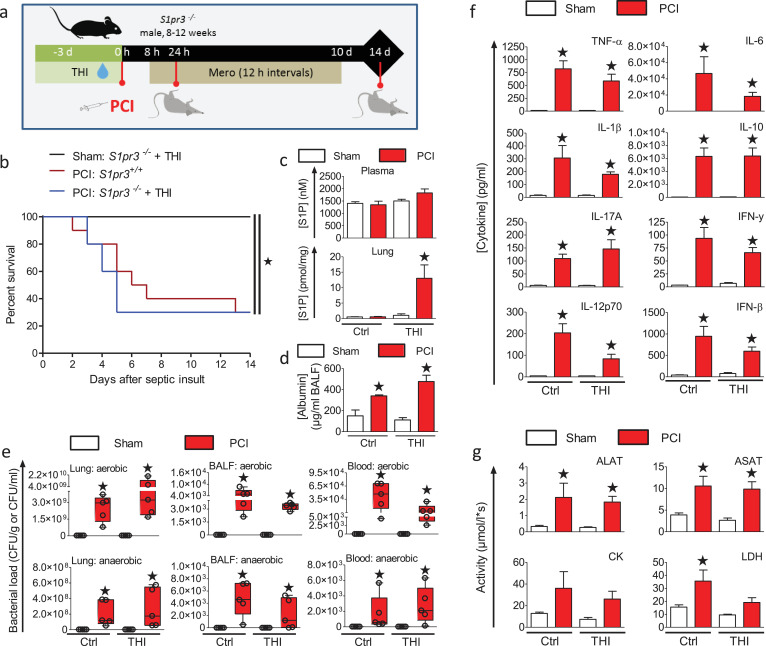
To evaluate the contribution of S1PR3 signaling to the protective effect of SPL-inhibition in experimental sepsis, we utilized S1pr3−/− mice (Fig. 6a). In contrast to wt mice (Fig. 3b), inhibition of SPL by THI did not show any survival benefit in S1pr3−/− mice (Fig. 6b). THI treatment significantly increased S1P levels in the lungs of mice 24 h after infection, consistent with inhibition of S1P lyase (Fig. 6c). The increase in S1P levels was limited to local tissue and not seen in the plasma. Despite this increase of S1P levels, serum albumin leakage into the lung was not prevented in S1pr3−/− mice (Fig. 6d), different from the effects observed in wt mice (Fig. S2e). The bacterial burden was significantly increased in all infected mice in the lung, BALF, and whole blood 24 h after infection and was not affected by SPL inhibition (Fig. 6e). Furthermore, levels of cytokines, including TNF-α, IFN-β. IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, and IL-17A were significantly increased in all infected S1pr3−/− mice 24 h after infection regardless of THI treatment (Fig. 6f) different to the results with wt mice (Fig. 3d). Finally, increased release of the tissue damage markers ALAT, ASAT, and CK into the plasma of infected S1pr3−/− mice was not rescued by THI-induced SPL inhibition except for LDH (Fig. 6g). This was again in sharp contrast to THI-treated wt mice (Fig. 3e). Hence, sepsis prevention by SPL inhibition depends on the presence of S1PR3 (Fig. S4).
Fig. 6.
Deletion of S1PR3 precludes the protective effects of S1P lyase inhibition in polymicrobial sepsis.
(a) S1pr3−/− mice were treated with THI for 3 days in drinking water (50 mg/L, ~12.5 mg/kg/day) prior to PCI induced by i.p. injection of a defined human stool suspension (PCI, 3.5 µl/g, n = 10). Sham treated animals were injected with 0.9% saline solution (n = 5). Mice were then treated for 10 d with antibiotic starting 8 h after infection and every 12 h. (b) Kaplan-Meier survival plots for 14 days, *p<0.05 compared to sham (Gehan–Breslow–Wilcoxon test). (c) Levels of S1P in plasma and lung were measured 24 h after PCI by LC-ESI-MS/MS (n = 5). (d) Leakage of serum albumin into the lung lumen was determined in BALF by ELISA 24 h after sepsis induction (n = 5). (e) Bacterial load of aerobic and anaerobic bacteria in lungs, BALF, and whole blood was measured 24 h after PCI (n = 5), *p<0.05 compared to sham animals (Kruskal-Wallis One Way Analysis of Variance on Ranks with a post-hoc Dunn's multiple comparison). Box plots: middle band indicates median; whiskers indicate minimum and maximum values; symbols indicate individual animals. (f) Cytokine levels and (G) markers of tissue damage were analysed in plasma 24 h after sepsis induction in animals ± THI treatment (n = 5). (c, d, f, g) Data are means ± SEM, *p<0.05 compared to untreated sham animals (One-way ANOVA with a post-hoc Bonferroni's multiple comparison test).
4.8. Septic patients display increased SPL expression and markers of lung damage in the blood
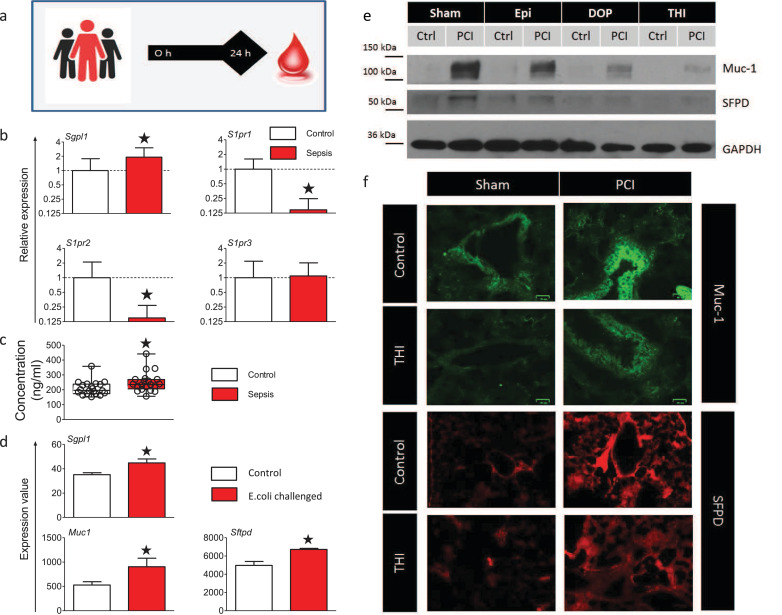
To evaluate SPL and S1PR3 as possible targets for sepsis prevention in humans, we analysed the mRNA of blood cells from septic patients 24 h after admission to the intensive care unit in comparison to healthy controls (Fig. 7a). mRNA levels of SPL were significantly increased, and S1PR3 expression was stable in septic patients, while S1PR1 and S1PR2 were decreased (Fig. 7b). Mucin-1 (Muc-1) levels in plasma, a marker of lung damage[32, 33], were also significantly increased in septic patients (Fig. 7c). Analysis of microarray data from a sepsis study in Papio cyanocephalus baboons infused with E. coli [34] also revealed increased expression of Sgpl1 in the lungs together with Muc-1 and surfactant protein D (Sftpd), another marker of lung damage (Fig. 7d). Muc-1 and surfactant protein D (SFPD) were also increased in the lungs of PCI treated mice (Fig. 7e, f). Finally, the treatment of mice with SPL inhibitors reduced the expression of Muc-1 and SFPD in lung tissue (Fig. 7e, f).
Fig. 7.
Septic patients increase SPL expression and display markers of lung damage.
(a) Blood from septic patients and healthy controls was analysed 24 h after admission to the intensive care unit. (b) SPL and S1PR transcript levels were determined by qPCR in total RNA from blood cells of septic patients (n = 11) and healthy controls (n = 9), *p<0.05 (Pair‐wise fixed reallocation randomization test).. (c) The marker of lung damage mucin-1 was analysed by ELISA (n = 20). #p<0.05 compared healthy controls (Unpaired, two-tailed t-test). Box plots: middle band indicates the median; whiskers indicate minimum and maximum values; symbols indicate individual patients. (d) Analysis of microarray data from a sepsis study in Papio cyanocephalus baboons infused with E. coli (deposited in NCBI's Gene Expression Omnibus accessible through GEO Series accession number GSE23590 [34]). Compared were the lungs of three healthy animals (GSM578550, GSM578551, GSM578552) to three E. coli challenged animals (GSM578553, GSM578554, GSM578555) by Benjamini & Hochberg adjusted p-value for multiple testing. (e) Western blot detection of Muc-1 and SFPD of mouse lungs treated with 0.6 µg/g Epi, THI or DOP under septic and control conditions. A representative blot of n = 3 is depicted. (f) Immunofluorescence of lung sections from mice stained for Muc-1 (green, scale bar= 25 µm) and for SFPD (red, scale bar=100 µm). Representative images are shown (n = 5).
5. Discussion
S1P has a well-known function as an immune modulator that regulates many immunological processes [4, 5, 7–10]. While most studies have focused on the functions of S1P on the immune system, the effects of S1P on parenchyma may be just as important. Here we show that S1P accumulation in lung epithelium promotes disease tolerance in experimental sepsis, leading to decreased levels of cytokines, reduced expression of tissue damage markers, and increased lung epithelial barrier function without significantly affecting bacterial burden. Similar observations were previously reported after treatment with low-dose epirubicin [22]. Importantly, we demonstrate for the first time that S1P accumulation in the lung caused by downregulation of the S1P-degrading enzyme SPL is a major contributor to this genotoxic stress-induced survival benefit. Although the exact mechanism of sepsis lethality is hard to determine and likely varies with different sources of infections, lung function is a critical survival factor in most circumstances and, therefore, of general importance [35]. Reduced lung function has rapid adverse effects on other organs that subsequently suffer mainly from hypoxia. Methods to increase S1P levels in lung tissue have already been suggested to protect lung function in several disease conditions. Intravenously delivered S1P or S1P analogues were shown to reduce vascular leakage in inflammatory lung injury models [16, 36, 37]. However, the route of administration is critical. Intratracheal delivery of S1P, for example, was shown to disrupt the alveolar barrier in mice [38]. Other limitations of exogenously delivered S1P are very rapid turnover, conversion to pro-apoptotic ceramide, bradycardia, and airway hyper-responsiveness [38]. Thus, interfering with intracellular S1P degradation by inhibition of SPL might be a better approach. Moreover, in a mouse model of LPS-induced acute lung injury (ALI), genetic knockdown of SPL, and subsequent S1P accumulation in the lung reduced lung injury [39]. In ALI, increased IL-6 was accompanied by increased pulmonary leakage and increased SPL expression. These observations are similar to our results showing reduced IL-6 production and barrier stabilization following SPL downregulation.
Despite the apparent effects observed in the lung, our findings do not exclude the possibility that S1P accumulation and action in other organs might also contribute to the beneficial effect in sepsis documented here. Surprisingly, the phenotypes of S1pr3−/− mice suffering from PCI-induced sepsis were similar to those of control mice, although it was demonstrated that S1pr3−/− mice were protected from LPS-induced systemic inflammation due to disruption of protease-activated receptor 1 (PAR1) signaling in dendritic cells [8]. In contrast, and consistent with our study, S1pr3−/- mice had reduced survival rates in E. coli- and methicillin-resistant Staphylococcus aureus-induced sepsis [40].
While we found that low-dose epirubicin treatment was protective against polymicrobial sepsis, the effective concentration window appears rather small. 5-times lower than optimal concentrations have almost no effect, and 5-times higher dosages dramatically increase lethality, perhaps due to the unrelated genotoxicity of anthracyclines. Sepsis prevention with SPL inhibitors could be much safer. Inhibitors like THI and DOP have very low toxicity, and are used for their SPL inhibition activity rather than hormetic low concentrations of anthracyclines inducing secondary effects of cellular adaptations [5, 21]. THI was shown to be metabolized possibly by the gut microbes to A6770, which is phosphorylated to A6770-P that acts as a direct SPL inhibitor [41]. The mechanism had been unclear for a long time but led to the development of the synthetic analogues LX2931 and LX2932 (LX3305) for the use in rheumatoid arthritis [21]. LX2931 passed the initial safety trial, but showed only minimal pharmacodynamic effects and failed in a clinical phase II trial [42]. Better and more direct inhibitors, like the SPL inhibitor compound 31 (4-benzylphthalazin-1-yl)-2-methylpiperazin-1-yl] nicotinonitrile, were developed and may serve as new candidates for sepsis prevention [43]. Our study also makes a strong case for S1PR3 agonists as potential drugs for sepsis intervention. S1PR3 promotes the progression of fibrosis [44, 45], but is currently not pursued as a drug target. The rapid upregulation of its expression seen in septic mice and the stable expression in septic patients suggests that S1PR3 agonism in the acute phase of sepsis might be a promising strategy. The allosteric agonist CYM-51736 could be a valuable candidate for that since it was shown to be more specific than previous S1PR3 agonists [46, 47]. In conclusion, our findings reveal decreased SPL expression and concomitant S1P/S1PR3-mediated epithelial barrier control as an integral step of genotoxic-induced disease tolerance in sepsis. Additional studies will be required to test the therapeutic potential of SPL inhibitors and S1PR3 agonists in the human pathophysiology of sepsis.
Acknowledgments
Acknowledgments
We would like to acknowledge PD Dr. Yuan Chen for providing the H1975 cells. We thank Richard Proia (NIH, Bethesda, Maryland, USA) for the generous provision of S1pr3−/− mice. We also would like to thank Mareike Lipinski and Franziska Röstel for excellent technical assistance.
Funding sources
This study was supported by the Center for Sepsis Control and Care (CSCC), the German Research Foundation (DFG), RTG 1715 (to M. H. G. and I. R.) and the National Institutes of Health, Grant R01GM043880 (to S. S.).
Declaration of Competing Interest
Dr. Kluge reports grants and personal fees from Pfizer, personal fees from Biotest, personal fees from Cytosorbents, personal fees from Gilead, personal fees from MSD, personal fees from Bayer, personal fees from Astellas, personal fees from Baxter, personal fees from Fresenius, outside the submitted work. Dr. Nierhaus reports grants and personal fees from CytoSorbents Europe, personal fees from ThermoFisher Scientific, personal fees from Biotest AG, outside the submitted work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102898.
Appendix. Supplementary materials
References
- 1.Rhee C., Jones T.M., Hamad Y., Pande A., Varon J., O'Brien C. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2) doi: 10.1001/jamanetworkopen.2018.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. The Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinamon G., Matloubian M., Lesneski M.J., Xu Y., Low C., Lu T. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5(7):713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 4.Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 5.Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 6.Graler M.H., Huang M.C., Watson S., Goetzl E.J. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174(4):1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 7.Liu G., Burns S., Huang G., Boyd K., Proia R.L., Flavell R.A. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10(7):769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niessen F., Schaffner F., Furlan-Freguia C., Pawlinski R., Bhattacharjee G., Chun J. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452(7187):654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 9.Dorsam G., Graeler M.H., Seroogy C., Kong Y., Voice J.K., Goetzl E.J. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171(7):3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 10.Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuvillier O., Pirianov G., Kleuser B., Vanek P.G., Coso O.A., Gutkind S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza A., Fang V., Chen C., Serasinghe M., Verma A., Muller J. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017;546(7656):158–161. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchet M., Sureau C., Guevin C., Seidah N.G., Labonte P. SKI-1/S1P inhibitor PF-429242 impairs the onset of HCV infection. Antiviral Res. 2015;115:94–104. doi: 10.1016/j.antiviral.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Zilch A., Rien C., Weigel C., Huskobla S., Gluck B., Spengler K. Influence of sphingosine-1-phosphate signaling on HCMV replication in human embryonal lung fibroblasts. Med Microbiol Immunol. 2018;207(3–4):227–242. doi: 10.1007/s00430-018-0543-4. [DOI] [PubMed] [Google Scholar]
- 15.Camerer E., Regard J.B., Cornelissen I., Srinivasan Y., Duong D.N., Palmer D. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119(7):1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia J.G., Liu F., Verin A.D., Birukova A., Dechert M.A., Gerthoffer W.T. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra M., Saba J.D. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50(1):349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stepanovska B., Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2019;154 doi: 10.1016/j.phrs.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Vogel P., Donoviel M.S., Read R., Hansen G.M., Hazlewood J., Anderson S.J. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One. 2009;4(1):e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber C., Krueger A., Munk A., Bode C., Van Veldhoven P.P., Graler M.H. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J Immunol. 2009;183(7):4292–4301. doi: 10.4049/jimmunol.0901724. [DOI] [PubMed] [Google Scholar]
- 21.Bagdanoff J.T., Donoviel M.S., Nouraldeen A., Carlsen M., Jessop T.C., Tarver J. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53(24):8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 22.Figueiredo N., Chora A., Raquel H., Pejanovic N., Pereira P., Hartleben B. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity. 2013;39(5):874–884. doi: 10.1016/j.immuni.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares M.P., Teixeira L., Moita L.F. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17(2):83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 24.Lavieu G., Scarlatti F., Sala G., Carpentier S., Levade T., Ghidoni R. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281(13):8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi M., Kitatani K., Kondo T., Hashimoto-Nishimura M., Asano S., Hayashi A. Regulation of autophagy and its associated cell death by "sphingolipid rheostat": reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem. 2012;287(47):39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonnert F.A., Recknagel P., Seidel M., Jbeily N., Dahlke K., Bockmeyer C.L. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J Surg Res. 2011;170(1):e123–ee34. doi: 10.1016/j.jss.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler M.S., Kluge S., Holzmann M., Moritz E., Robbe L., Bauer A. Markers of nitric oxide are associated with sepsis severity: an observational study. Crit Care. 2017;21(1):189. doi: 10.1186/s13054-017-1782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler M.S., Martz K.B., Nierhaus A., Daum G., Schwedhelm E., Kluge S. Loss of sphingosine 1-phosphate (S1P) in septic shock is predominantly caused by decreased levels of high-density lipoproteins (HDL) J Intensive Care. 2019;7:23. doi: 10.1186/s40560-019-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler M.S., Nierhaus A., Holzmann M., Mudersbach E., Bauer A., Robbe L. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit Care. 2015;19:372. doi: 10.1186/s13054-015-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3(5):452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 32.Eisner M.D., Parsons P., Matthay M.A., Ware L., Greene K., Acute Respiratory Distress Syndrome N. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishizaka A., Matsuda T., Albertine K.H., Koh H., Tasaka S., Hasegawa N. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol – Lung Cell Mol Physiol. 2004;286(6):L1088–L1L94. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 34.Silasi-Mansat R., Zhu H., Georgescu C., Popescu N., Keshari R.S., Peer G. Complement inhibition decreases early fibrogenic events in the lung of septic baboons. J Cell Mol Med. 2015;19(11):2549–2563. doi: 10.1111/jcmm.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czermak B.J., Breckwoldt M., Ravage Z.B., Huber-Lang M., Schmal H., Bless N.M. Mechanisms of enhanced lung injury during sepsis. Am J Pathol. 1999;154(4):1057–1065. doi: 10.1016/S0002-9440(10)65358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McVerry B.J., Garcia J.G. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17(2):131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Peng X., Hassoun P.M., Sammani S., McVerry B.J., Burne M.J., Rabb H. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 38.Sammani S., Moreno-Vinasco L., Mirzapoiazova T., Singleton P.A., Chiang E.T., Evenoski C.L. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43(4):394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y., Gorshkova I.A., Berdyshev E., He D., Fu P., Ma W. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol Biol. 2011;45(2):426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou J., Chen Q., Wu X., Zhao D., Reuveni H., Licht T. S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am J Respir Crit Care Med. 2017;196(12):1559–1570. doi: 10.1164/rccm.201701-0241OC. [DOI] [PubMed] [Google Scholar]
- 41.Ohtoyo M., Machinaga N., Inoue R., Hagihara K., Yuita H., Tamura M. Component of caramel food coloring, THI, causes lymphopenia indirectly via a key metabolic intermediate. Cell Chem Biol. 2016;23(5):555–560. doi: 10.1016/j.chembiol.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Bigaud M., Guerini D., Billich A., Bassilana F., Brinkmann V. Second generation S1P pathway modulators: research strategies and clinical developments. Biochim Biophys Acta. 2014;1841(5):745–758. doi: 10.1016/j.bbalip.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Weiler S., Braendlin N., Beerli C., Bergsdorf C., Schubart A., Srinivas H. Orally active 7-substituted (4-benzylphthalazin-1-yl)-2-methylpiperazin-1-yl]nicotinonitriles as active-site inhibitors of sphingosine 1-phosphate lyase for the treatment of multiple sclerosis. J Med Chem. 2014;57(12):5074–5084. doi: 10.1021/jm500338n. [DOI] [PubMed] [Google Scholar]
- 44.Murakami K., Kohno M., Kadoya M., Nagahara H., Fujii W., Seno T. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takuwa N., Ohkura S., Takashima S., Ohtani K., Okamoto Y., Tanaka T. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85(3):484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerrero M., Poddutoori R., Urbano M., Peng X., Spicer T.P., Chase P.S. Discovery, design and synthesis of a selective S1P(3) receptor allosteric agonist. Bioorg Med Chem Lett. 2013;23(23):6346–6349. doi: 10.1016/j.bmcl.2013.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo E., Bhhatarai B., Repetto E., Guerrero M., Riley S., Brown S.J. Novel selective allosteric and bitopic ligands for the S1P(3) receptor. ACS Chem Biol. 2012;7(12):1975–1983. doi: 10.1021/cb300392z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.