Abstract
Background
High-molecular-weight kininogen is a cofactor of the human contact system, an inflammatory response mechanism that is activated during sepsis. It has been shown that high-molecular-weight kininogen contributes to endotoxemia, but is not critical for local host defense during pneumonia by Gram-negative bacteria. However, some important pathogens, such as Streptococcus pyogenes, can cleave kininogen by contact system activation. Whether kininogen causally affects antibacterial host defense in S. pyogenes infection, remains unknown.
Methods
Kininogen concentration was determined in course plasma samples from septic patients. mRNA expression and degradation of kininogen was determined in liver or plasma of septic mice. Kininogen was depleted in mice by treatment with selective kininogen directed antisense oligonucleotides (ASOs) or a scrambled control ASO for 3 weeks prior to infection. 24 h after infection, infection parameters were determined.
Findings
Data from human and mice samples indicate that kininogen is a positive acute phase protein. Lower kininogen concentration in plasma correlate with a higher APACHE II score in septic patients. We show that ASO-mediated depletion of kininogen in mice indeed restrains streptococcal spreading, reduces levels of proinflammatory cytokines such as IL-1β and IFNγ, but increased intravascular tissue factor and fibrin deposition in kidneys of septic animals.
Interpretation
Mechanistically, kininogen depletion results in reduced plasma kallikrein levels and, during sepsis, in increased intravascular tissue factor that may reinforce immunothrombosis, and thus reduce streptococcal spreading. These novel findings point to an anticoagulant and profibrinolytic role of kininogens during streptococcal sepsis.
Funding
Full details are provided in the Acknowledgements section.
Keywords: Kininogen, Sepsis, Streptococcus pyogenes
1. Introduction
Kininogens are multifunctional and multidomain glycoproteins, synthetized in the liver and predominantly circulate in the blood, but are also found in other body fluids, organs such as kidneys and in cells such as neutrophils. Two forms of kininogen are found in mammalian plasma: high-molecular-weight kininogen (HK) and low molecular weight kininogen (LK) [for a review see 1]. In humans, both proteins originate from the KNG1 gene through alternative splicing [2]. In contrast to humans’, mice contain two genes for kininogen, KNG1 and KNG2, both produce HK and LK isoforms [3]. Structurally, HK and LK contain an identical heavy chain, which consists of domains 1, 2, and 3. HK contains a 56 kDa light chain that consists of domains 5 (D5H) and 6 (D6). LK contains a unique 4 kDa light chain (D5L). In both proteins, the heavy and light chains are linked by domain 4 (D4), which contains the bradykinin nonapeptide. HK is an important cofactor of the human contact system responsible for inflammatory response against foreign surfaces, proteins and pathogens. Beside HK, the contact system includes two proteases, factor XII (FXII) and plasma prekallikrein (PK). The proteins circulate as zymogens in the bloodstream or are assembled on endothelial cells, neutrophils, and platelets. When blood is exposed to negatively charged artificial or biological surfaces, contact factors bind to it, FXII becomes auto-activated and converts PK to plasma kallikrein (PKa). PK circulates in a noncovalent complex with HK [4] and cleaves it after conversion to PKa, releasing the peptide bradykinin from D4 [5]. Bradykinin is a vasoactive and proinflammatory nonapeptide, which increases the production of nitric oxide and prostaglandins, and causes increased vascular permeability, hypotension, smooth-muscle contraction and fever. HK and LK can also be cleaved by other proteases, including tissue kallikrein and neutrophil-derived proteases [6,7] as well as bacterial proteases (for a review see [8]), to release kinins related to bradykinin. Moreover, contact factors can bind to the surface of pathogenic Gram-positive, such as Staphylococcus aureus or Streptococcus pyogenes and Gram-negative bacteria, such as Escherichia coli and Salmonella [9], become activated and release bradykinin and antimicrobial peptides (AMPs) [10]. AMPs are derived from the proteolysis of kininogen D3 and D5H and are active against a wide range of bacterial species (for a review see [11]).
Kinins were shown to recruit neutrophils [12] and stimulate alveolar macrophages [13], thus potentiate the host response against invading pathogens. However, increased vascular permeability due to bradykinin release may facilitate the systemic spread of the pathogenic microorganisms. Indeed, in one of our previous studies we found that depletion of PK by treatment with antisense oligonucleotides (ASOs), dampens bacterial dissemination and growth in multiple organs in a mouse Streptococcus pyogenes sepsis model [14]. S. pyogenes is a Gram-positive major human pathogen causing mainly local infections of the skin and mucous membranes. Local infections occasionally develop into serious systemic complications, of which streptococcal toxic shock syndrome and necrotizing fasciitis are associated with high morbidity and mortality [15]. S. pyogenes were the first bacteria described to adsorb HK from plasma [16], followed by activation of contact factors [17] and the release of bradykinin [18].
Here we investigated the role of kininogens in a mouse model of streptococcal sepsis and found increased hepatic expression of mouse KNG1/2 genes upon infection. Cleavage of HK in plasma of septic mice indicated a release of bradykinin due to infection. A knockdown of KNG1/2 gene expression, prior to the infection, diminished bacterial spreading and cytokine release, but increased intravascular tissue factor levels and fibrin deposition in kidneys. Thus, the role of kininogen in streptococcal sepsis seems to be profibinolytic rather than procoagulant.
2. Material and methods
2.1. Bacterial strains and culture conditions
The S. pyogenes strain AP1 (40/58) has been described previously [17]. Bacteria were grown overnight in Todd-Hewitt broth (THB; Becton Dickinson) at 37 °C and 5% CO2.
2.2. Human plasma
Pooled plasma was obtained from 20 healthy donors and HK-depleted plasma was purchased from Affinity Biologicals Inc.
2.3. Patient samples
Patients with sepsis, severe sepsis, or septic shock were enrolled from the Intensive Care Medicine Unit at University Medical Center of Rostock as described previously [19]. The protocol had been approved by our Institutional Ethics committee (A 201,151), and informed consent was obtained from the patients or their caring relatives.
2.4. Measurement of kininogen in plasma
The total kininogen amount in plasma of septic patients was quantified by a sandwich ELISA (Coachrom Diagnostica). Briefly, affinity-purified antibody to kininogen (SAKN-IG, produced in sheep), detecting HK and LK in Western blot analysis, was coated onto the wells of a microtiter plate overnight at 4 °C. Any remaining binding sites on the plastic wells were blocked for 1.5 h at room temperature with bovine serum albumin. The plates were washed and plasma samples, in appropriate dilution, were applied overnight at 4 °C. After washing, a peroxidase-conjugated detection antibody to kininogen was added. Peroxidase activity was expressed by incubation with o-phenylenediamine (OPD). After development for 10 min, the reaction was quenched with the addition of H2SO4 and the color produced was quantified using a microplate reader. The color was proportional to the concentration of kininogen present in the samples.
2.5. Kininogen immunoblot analysis
Mouse or human plasma anticoagulated with sodium citrate was fractionated on 4–12% gradient SDS-polyacrylamide gels followed by immunoblotting with mouse antibody detecting the heavy chain of kininogens (sc-25,885, Santa Cruz). Blots were incubated with secondary fluorophore-labelled antibodies (LI-COR) and imaged on Odyssey Imager (LI-COR). HK relative plasma protein levels were determined by densitometry analysis (ImageJ).
2.6. Clot lysis time
A clot was generated in human normal or HK-depleted plasma by the addition of PT-reagent. In some experiments purified HK (50 µg/ml) was added before clot formation was induced. The clot was incubated for 5 min at 37 °C before streptokinase (100 Units), uPA (10 µg) or tPa (10 µg) was added. Time until clot lysis was determined in a coagulometer.
2.7. Clot lysis time in purified clot system
A clot was generated with 15 µl of protein solution with purified fibrinogen (3 mg/ml), plasminogen (200 µg/ml), with or without kininogen (80 µg/ml) by addition of 1 µl thrombin.
The clot was incubated for 5 min at 37 °C before streptokinase (100 Units) was added. Time until clot lysis was determined in a coagulometer.
2.8. d-Dimer Elisa
S. pyogenes AP1 was grown to mid-logarithmic phase (OD620nm = 0.4). 100 μl bacteria (2 × 108CFU/ml) were mixed with 100 μl human normal or HK-depleted plasma and a clot was generated by the addition of thrombin (1 U) and CaCl2 (25 mM). The clot was incubated for 5 min at 37 °C, and overlayed with PBS containing 1% of the original plasma. 50 μl supernatant was taken at indicated time points and stored at −20 °C. The d-Dimers ELISA (Technoclone) was performed according to the manufacturer's instructions.
2.9. Plasmin activity in human plasma
Plasmin activity in human normal or HK-depleted plasma (diluted 1:10 in HEPES-saline, 10 mM HEPES, 137 mM NaCl) was determined after addition of streptokinase (10 Units), uPA (100 µg/ml) or tPa (100 µg/ml) and the chromogenic substrate S-2403 (0.36 mM, Chromogenix) followed by incubation for 3 h at 37 °C, during measurement at 405 nm in an ELISA reader.
2.10. Scanning electron microscopy
A clot was induced with PT reagent (Technoplastin HIS) from 100 or 50 μl mouse or human normal and HK-depleted plasma. The clots were incubated for 5 min at 37 °C, fixed and analysed as described previously [14]. After fixation the clots were washed with distilled water and were dehydrated in an ascending series of acetone. In a final step, acetone was replaced by 1,1,1,3,3,3-hexamethyldisilazane (HMDS; Roth, Germany) for improved air-drying. Samples were mounted on SEM-carriers with adhesive conductive carbon tape (PLANO, Wetzlar, Germany) and were sputter-coated with a gold layer under vacuum (EM SCD 004, BALTEC, Balzers). Samples were analysed by a field emission scanning electron microscope (FE-SEM, MERLIN® VP Compact, Zeiss Microscopy, Germany).
2.11. Antisense-Oligonucleotides (ASOs)
ASOs for KNG1 and KNG2 mRNA knockdown in vivo were provided by Ionis Pharmaceuticals. All oligonucleotides for kininogen mRNA knockdown in vivo were 20 nucleotides in length and chemically modified with phosphorothioate linkages in the backbone and 2′-O-methoxyethyl (MOE) on the wings with a central deoxynucleotide gap (“5–10–5″ design). Oligonucleotides were synthesized and purified as previously described [20]. ASOs were designed to inhibit KNG1 and KNG2 RNA expression simultaneously. ASOs bind and induce cleavage of the KNG1 and KNG2 pre-mRNA in the nucleus. ASO site is located in the exon 8. To identify potent kininogen inhibitors, ASOs were designed and tested in primary mouse hepatocytes for their ability to suppress mRNA levels of the respective target. From these experiments, optimized ASOs were selected for evaluation in mice.
2.12. mRNA analysis
Total RNA was isolated from mouse liver with RNeasy Plus Mini Kit (Qiagen). RNA quality was checked with Agilent RNA 6000 Nano Kit (Agilent Technologies) and RNA concentration determined with Qubit™ RNA HS Assay Kit (Invitrogen). All analyses were performed according to the manufacturer's instructions. 800 ng total RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and the complementary DNA obtained used for real-time quantitative PCR. The reaction mixture (20 µl) containing Assay probe, Taqman Universal PCR Master Mix (Applied Biosystems) and cDNA was amplified as follows: denaturation at 95 °C for 10 min and 45 cycles at 95 °C for 15 s and 60 °C for 1 min. GAPDH (human or rodent) was used as a housekeeping gene. Relative expression was calculated employing the 2−ΔΔct method.
2.13. Animal experiments
Eight-week-old female BALB/c mice with a weight of 16–18 g (Charles River Laboratories) were treated with ASOs through intraperitoneal injections, with a dose of 800 µg/mouse, twice per week for 3 weeks (total 7 injections, each with 800 µg ASO/mouse).
The subcutaneous infection model with S. pyogenes AP1 strain and determination of bacterial dissemination were performed as described previously [14]. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments the Landesveterinär- und Lebensmitteluntersuchungsamt Rostock (Permit Number: 7221.3–1–002/16).
2.14. Multiplex analysis of cytokines
A panel of plasma cytokines (n= 20) was determined according to the manufacturer's instructions of the Procartaplex™ Multiplex Immunoassay (ThermoFisher Scientific, Berlin, Germany). Briefly, plates were coated with magnetic beads, followed by the addition of standards and EDTA plasma samples from infected and healthy control mice (n= 5/group; 25 μl/well). Upon washing, detection antibody was added. Data acquisition was done after incubation with streptavidin-PE. Samples were run on a Bioplex 2000 (Bio-Rad Laboratories GmbH, Munich, Germany). Data were analysed by the Bio-Plex Manager Software and sample concentration (pg/ml) was calculated by plotting against the corresponding standard curve.
2.15. Lendrum (-MSB) staining for Fibrin identification
Mice were killed and organs fixed in buffered 4% formalin at 4 °C. Tissues were dehydrated, embedded in paraffin and cut into sections. After removal of the paraffin, tissues were stained with Martius scarlet blue (MSB Lendrum, Fibrin-Red, Erythrocytes-Yellow, Collagen, Elastic Fibers, Basement Membranes – Blue, Epithelia - Red) according to the manufacturer's instructions (Morphisto). Fibrin areas > 5 μm were counted in each organ and graded on a scale of 0 to 3 (0 = absent; 1 = up to 20; 2 = 20 - 50 and 3 = more than 50 fibrin areas).
2.16. Proteolytic potential for PK/FXIIa activity in mouse plasma
Pooled plasma from 4 mice/group was incubated with Dapptin (Dapptin TC) and FXIIa/PK activity was determined in a microplate reader by chromogenic substrate S-2302 (Chromogenix).
2.17. Tissue factor activity in mouse plasma
The tissue factor chromogenic activity assay (Assaypro LLC) was used to quantify the tissue factor pro-coagulant activity in mouse plasma samples [21].
2.18. Proteome analysis
Proteome analysis was performed with EDTA plasma samples from mice treated with KNG1/2-ASOs or control ASOs (five mice each group) respectively. Samples corresponding to 75 μg of protein were mixed with solubilization buffer (1.5% sodium deoxycholate (SDC), 10 mM dithiothreitol, and 50 mM ammonium bicarbonate (ABC)), incubated at 95 °C for 5 min, and alkylated with 15 mM iodoacetamide for 20 min at room temperature. After dilution with two volumes of 50 mM ABC, sequencing grade trypsin (Promega GmbH, Mannheim, Germany) was added in an enzyme/protein ratio of approximately 1:100 to a final volume of 320 μl. Digestion was performed at 37 °C for 16 h. SDC was removed from the solutions of digested proteins using the phase transfer surfactant method. Finally, the peptide solutions were desalted with OASIS HLB 1cc Vac Cartridges (Waters, Manchester, UK). Peptide samples corresponding to approximately 200 ng of digested protein supplemented with 40 fmol of Hi3 E. coli standard for protein absolute quantification (Waters) were separated using a nanoAcquity UPLC system (Waters). The UPLC system was coupled to a Synapt G2-S mass spectrometer (Waters) operated in data-independent mode (HDMSE) as previously described. Samples were measured once without technical replication. Protein identification and label-free quantification was performed using Progenesis QI for Proteomics version 4.0 (Nonlinear Dynamics, Newcastle upon Tyne, UK) as described. For the database search, a database containing 17,006 reviewed protein sequences from Mus musculus (UniProt release 2019_02) appended with the sequences of ClpB from E. coli (P63284) and porcine trypsin was used. Proteins which showed at least 2-fold increase or decrease, respectively, and ANOVA p-values < 0.01 for the comparison between KNG1/2-ASO mice and control-ASO mice were regarded as significant.
2.19. Statistical analysis
Statistical analysis was performed using GraphPad Prism (Version 8.2.1). The P value was determined by using the unpaired t-test (comparison of two groups), if not otherwise indicated. All samples were analysed in triplicates, and all experiments were performed at least three times, if not otherwise declared. The bars in the figures indicate the standard deviations (SD).
3. Results
3.1. Kininogen expression and degradation in septic mice
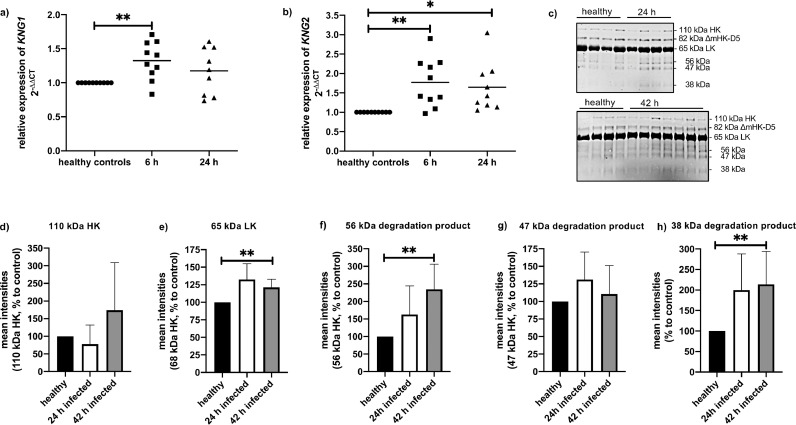
Hepatic expression of KNG1 and KNG2 mRNA during sepsis was investigated in a streptococcal murine sepsis model [14]. Mice were infected subcutaneously with 1.5 - 2 × 107 CFU S. pyogenes and liver samples were collected at 6 h and 24 h after infection. After 6 h of infection, KNG1/2 mRNA levels were significantly increased in infected mice, compared to healthy controls (Fig 1a, b), while 24 h after infection no differences in KNG1 mRNA expression were measured between control and infected animals (Fig 1a). The expression of KNG2 mRNA was still significantly increased at this time point (Fig 1b).
Fig. 1.
Relative gene expression of KNG1 and 2 after subcutaneous (sc.) infection with S. pyogenes.
a, b) Groups of mice (n= 9–10) were infected sc. with 2 × 107 CFU/mouse of S. pyogenes AP1. 6 and 24 h after infection, animals were killed, liver tissue was collected for total RNA isolation and gene expression analysis.
c) Western Blot analysis of mouse plasma with an antibody against kininogen.
d-f) relative levels of HK (d), LK (e) or degradation products (f, g, h) quantified by densitometry from figure 1c. One sample t-test, *p≤ 0.05 ** p≤ 0.001.
Several studies have been published showing that systemic contact activation occurs in patients with severe sepsis and septic shock, combined with a massive release of bradykinin [22,23]. Thus, we performed semiquantitative Western blot analysis in mouse plasma samples 24 h and 42 h after infection with S. pyogenes (Fig 1c). HK (at 110 kDa), the intermediate band (at 82 kD) corresponding to ΔmHK-D5, LK and the heavy chain of HK (at 65 kDa) [3], the light HK chain (at 56 and 47 kDa) and the degradation product at 38 kDa were analyzed by densitometry (Fig 1d-f). Similar to a septic patient cohort in one of our previous studies [19], no significant differences of HK at 110 kDa (Fig 1d), could be observed between plasma of healthy and septic mice. LK and the heavy chain of HK (at 65 kDa) were increased after 42 h of infection (Fig 1e). Degradation products of HK at 56 and 38 kDa also were increased in septic mice (Fig 1f, h). This implicates cleavage of HK and release of BK in the blood of septic mice.
3.2. Knockdown of KNG1/2 in mice by ASO treatment
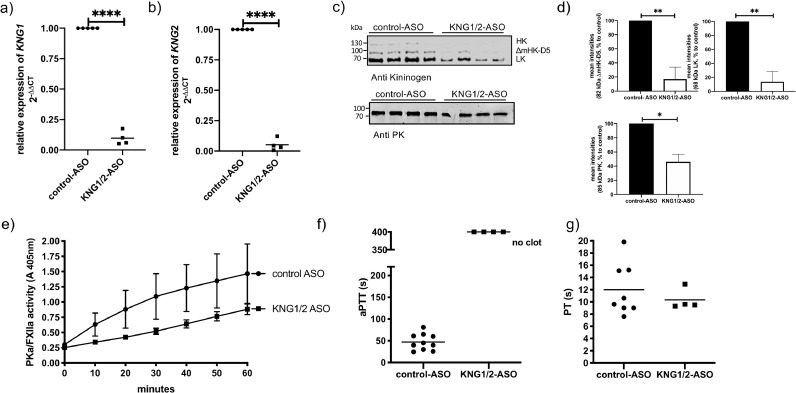
To investigate the functional contribution of kininogen in streptococcal sepsis, expression of KNG1 and 2 were suppressed by treating mice with KNG1/2-ASO for 3 weeks. Control mice received scrambled control-ASO. After 3 weeks of ASO treatment, KNG1/2 mRNA levels in the liver were reduced by over 90% compared to mice treated with control-ASO (Fig 2a, b). HK protein (at 110 kDa) and ΔmHK-D5 (at 82 kD), were almost non-detectable by Western Blotting (Fig 2c). ΔmHK-D5 was reduced to around 17% as determined by densitometry (Fig 2d). LK (at 65 kDa) was equally reduced to 14% in KNG1/2-ASO treated mice, comparing to control-ASO treated mice (Fig 2c, d).
Fig. 2.
Analysis of kininogen gene expression, protein levels and function after KNG1/2-ASO treatment.
A group of mice (n= 4) were treated with KNG1/2-ASO or control-ASO (n= 10) through intraperitoneal injections, with a dose of 800 µg/mouse, twice per week for 3 weeks (total 7 injections, each with 800 µg ASO/mouse).
a, b) After the last ASO dose liver tissue was collected for total RNA isolation and gene expression analysis. One sample t-test, **** p≤ 0.0001
c) Western Blot analysis of mouse plasma with an antibody against kininogen or PK (anti-PK).
d) relative levels of PK, LK and ΔmHK-D5, quantified by densitometry from Fig 2c. One sample t-test, *p≤ 0.05, **p≤ 0.01. e) Plasma samples from 4 mice per group were pooled and proteolytic potential of PKa/FXIIa activity was measured after addition of Dapptin, using chromogenic substrate S-2302.
f) aPTT and g) PT in plasma of ASO-treated animals were measured using a coagulometer.
HK and PK circulate in plasma as a bimolecular complex and previous studies documented low PK levels in patients with hereditary kininogen deficiency [24,25]. Similarly, in mice with inhibited or absent KNG1 expression PK levels were reduced to about 40% [26]. This was confirmed in our study, as blocking expression of KNG1 and 2 reduced also the content of PK in plasma to about 40% (Fig 2c, d). A comparable reduction of the PK concentration to 34% was quantified by mass spectrometry. However other plasma proteins of the contact and the fibrinolysis system, such as FXII or plasminogen, did not significantly change upon ASO-treatment (see suppl. Table 1).
As a consequence of kininogen depletion and PK reduction, proteolytic potential of PKa/FXIIa (Fig 2e), after addition of Dapptin to plasma, was reduced, compared to control mice. Plasma samples from kininogen-depleted mice generated no clot after activation of FXII with a contact activator (aPTT, Fig 2f), however, the prothrombin time (PT) was not affected (Fig 2g).
3.3. Kininogen depletion diminishes bacterial dissemination and cytokine release during streptococcal sepsis
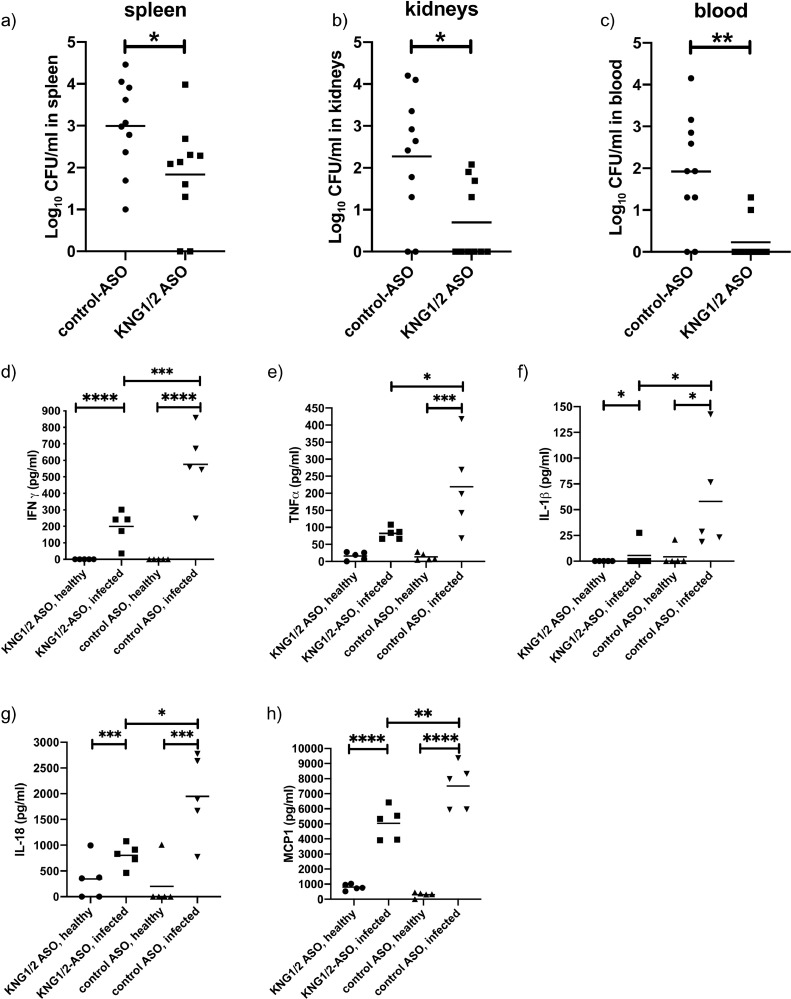
To investigate the role of kininogens in the host response during streptococcal sepsis, kininogen depleted mice were infected as described above. After infection KNG1/2 mRNA levels in the liver and kininogen in plasma stayed reduced in KNG1/2-ASO treated mice (suppl. Fig. 2). 24 h after infection, bacterial dissemination to the organs was determined. Kininogen depleted mice had significantly fewer bacteria in the spleen (Fig 3a), kidneys (Fig 3b) and blood (Fig 3c) comparing to control-ASO treated mice.
Fig. 3.
Bacterial spreading and proinflammatory response of Kininogen-depleted mice infected with S. pyogenes.
After ASO treatment (see methods) groups of mice were injected sc. with 1.5 – 2 × 107 CFU/mouse S. pyogenes AP1. 24 h after infection a) spleen, b) left kidney and c) blood samples were homogenized and the number of CFU quantified. Data are presented as means of 10 mice per group and were obtained from 2 independent experiments. *p≤ 0.05; ** p≤ 0.001. d-H) Mice (n= 5/group) were injected sc. with 2 × 107 CFU/mouse S. pyogenes AP1. 24 h after infection animals were collected and EDTA plasma was analyzed for IFNγ (d), TNFα (e), IL1β (f), IL18 (g) and MCP1 (h), using a Multi-Plex immunoassay. One-way ANOVA *p≤ 0.05, **p≤ 0.01, ***p≤ 0.0002, ****p≤ 0.0001.
Additionally, a panel of 20 cytokines, chemokines and growth factors were measured in healthy or 24 h infected mice, pretreated with control- or KNG1/2-ASOs. Infection boosted the pro-inflammatory response yielding a robust increase of 11 cytokines/chemokines (suppl. Fig 1). In agreement with lower bacterial loads, infected kininogen-depleted mice had significantly lower levels of IFNγ, TNFα, IL-1β, IL-18 and monocyte chemotactic protein 1 (MCP1, Fig 3d-h) compared to infected control mice.
Thus, kininogen depletion restrained bacterial dissemination and cytokinin release significantly in septic mice.
3.4. Kininogen depletion supports a procoagulant phenotype in septic mice
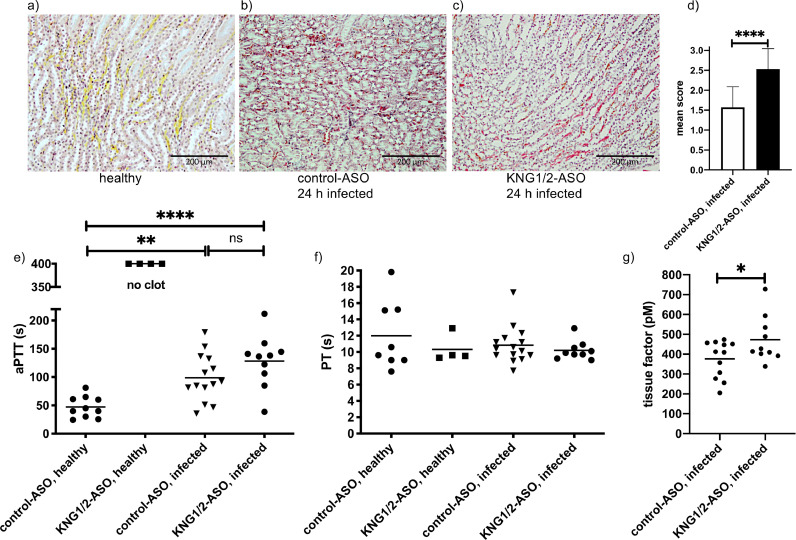
Formation of microvascular fibrin deposition in kidneys was described in human sepsis [27] and was also observed in our sepsis animal model [14]. The quantification of fibrin areas (> 5 µm) on a scale from 0 to 3 (0: absent; 1: ≤ 20 fibrin areas; 2: 20–50 fibrin areas and 3: more than 50 fibrin areas) revealed that the mean score of 2.5 in kininogen-depleted animals was significantly higher in comparison to control group (mean score 1.5, Fig 4a–d).
Fig. 4.
Procoagulant response of Kininogen - depleted mice infected with S. pyogenes.
a-c) Representative kidney tissue sections showing the medullary rays, from non-infected, control-, or KNG1/2-ASO treated animals. Sections were stained (MSB-Lendrum) and fibrin depositions (red) were detected and scored (d) as described in Material and Methods. Bars represent 200 µm
e) aPTT and f) PT in plasma of ASO-treated and infected animals were measured using a coagulometer.
g) Groups of mice were injected sc. with 1.5 – 2 × 107 CFU/mouse S. pyogenes AP1. 24 h after infection plasma samples were collected and tissue factor activity determined. Data are presented as means of 10–12 mice per group and were obtained from two independent experiments. *p≤ 0.05, **p≤ 0.01, ****p≤ 0.0001.
The data indicate that kininogen-depleted animals become more procoagulant in case of streptococcal infection. Clotting times measured in infected animals revealed a prolonged aPTT compared to healthy control mice (Fig 4e). Also, there was no difference in aPTT between control- and KNG1/2-ASO treated animals. This was in contrast to healthy kininogen depleted mice where no clotting after FXII activation could be observed. PT was unchanged in all groups at this time point (Fig 4f)
It has been recently shown that bradykinin inhibits tissue factor expression in vitro and in vivo [28]. Moreover, the content of intravascular tissue factor was shown to be increased in our sepsis animal model [21]. We, therefore, measured plasma tissue factor activity in control- and KNG1/2-ASO-treated animals 24 h after infection. Tissue factor was significantly higher in kininogen-depleted mice after infection, compared to control mice (Fig 4g). Thus, the shortening of aPTT in infected KNG1/2-ASO-treated mice (see Fig 4e) is most likely attributable to increased circulating tissue factor. Taken together, the absence of kininogen leads to a higher content of intravascular tissue factor in septic animals.
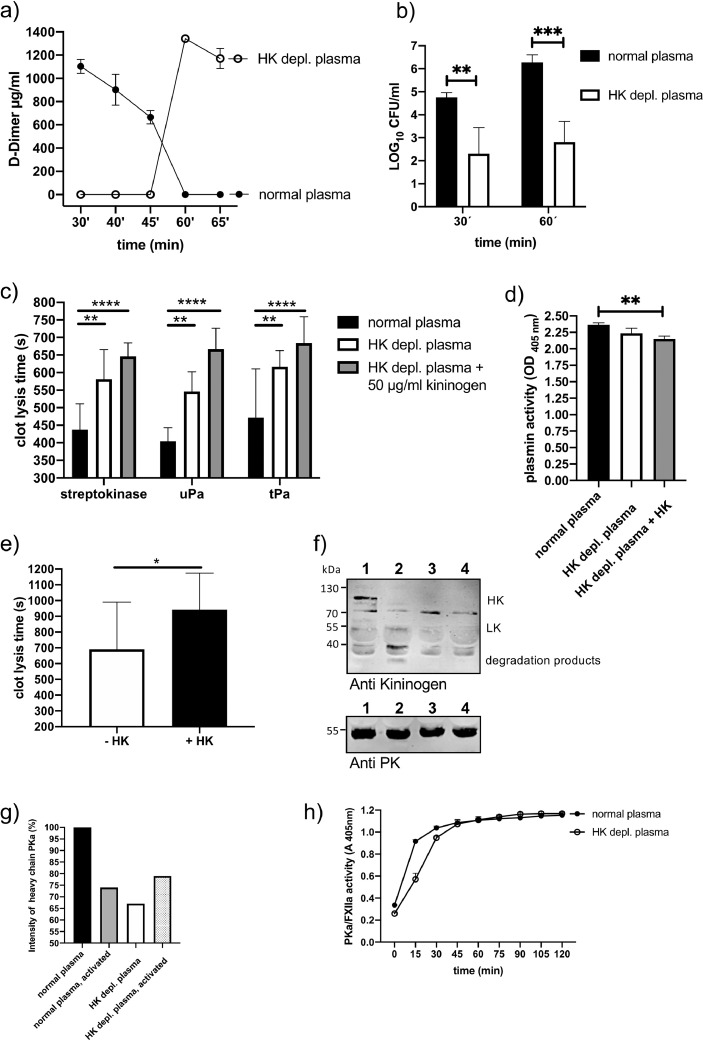
3.5. Role of kininogen in streptococcal-triggered fibrinolysis and clot escape
To test whether kininogens, especially HK, plays a role in S. pyogenes induced fibrinolysis, we measured the content of d-dimer in the supernatant from human plasma clots, which contained S. pyogenes bacteria. Fig. 5a depicts that d-dimer release was significantly delayed due to HK depletion. The time until d-dimer could be detected in supernatants of HK depleted plasma was twice as long (60 min) as in normal plasma (30 min). To prove that delayed fibrinolysis was associated with diminished clot escape of the bacteria, samples from normal or HK-depleted plasma were clotted with thrombin in the presence of S. pyogenes. Clots were covered with PBS containing 1% of the original plasma, incubated at 37 °C and viable S. pyogenes count assays from the supernatants were performed. 30 and 60 min after incubation bacteria could be detected in the supernatant of both plasma clots, but the bacterial number was significantly lower in samples of HK-depleted plasma (Fig 5b). Thus, the absence of HK hindered S. pyogenes-induced fibrinolysis and bacteria clot escape in human plasma.
Fig. 5.
Effect of HK depletion on fibrinolysis induced by streptokinase or S. pyogenes bacteria in human plasma.
a) Growing S. pyogenes (2 × 108 CFU/ml) were mixed with normal or HK-depleted plasma, and thrombin was used to form a stable clot. The plasma clot was overlaid with PBS and d-dimer concentration in the supernatant was measured after different time points, using an ELISA.
b) Growing S. pyogenes (2 × 108 CFU/ml) were mixed with normal or HK-depleted plasma, and thrombin was used to form a stable clot. The plasma clot was overlaid with PBS and the supernatant was plated after 30 and 60 min to determine CFU of escaped bacteria.
c) A clot was induced in normal, HK-depleted plasma, or HK-depleted plasma complemented with HK (50 µg/ml) by PT reagent and time until clot lysis was measured after addition of streptokinase, uPA or tPA.
d) Plasmin activity was determined in normal, HK-depleted or HK-depleted plasma complemented with HK (50 µg/ml), after the addition of streptokinase.
e) A clot was generated with a solution containing purified fibrinogen, plasminogen (200 µg/ml), and kininogen (80 µg/ml) by the addition of thrombin. Streptokinase (100 Units) was added and time until clot lysis was determined in a coagulometer.
f) Western Blot analysis of human plasma with an antibody against kininogen or PK (anti-PK) (lane 1: normal plasma, lane 2: normal plasma activated with Dapptin reagent, lane 3: HK-depleted plasma, lane 4: HK-depleted plasma activated with Dapptin).
g) Relative levels of PK quantified by densitometry from figure 5f.
h) Normal or HK-depleted plasma was activated with Dapptin and PKa/FXIIa activity was measured with a chromogenic substrate (S2303). *p≤ 0.05, **p≤ 0.01, ***p≤ 0.0002, ****p≤ 0.0001.
These findings were supported by plasma clot-lysis assays using bacterial streptokinase or the host plasminogen activators uPA and tPA as fibrinolysis activators (Fig 5c). Clot lysis time in HK-depleted plasma was significantly longer, regardless of what fibrinolysis activator was used. Intriguingly, complementation of HK-depleted plasma with purified HK could not reverse prolongation of clot lysis, on the contrary, the clot lysis time was even more prolonged after the addition of HK (Fig 5c). Plasmin activity after activation with streptokinase was similar in both plasma types, but was reduced in HK-depleted plasma after complementation with purified HK (Fig 5d). Moreover, and similar to the plasma clot experiments, the clot lysis time of a pure fibrin clot was prolonged when HK was added (Fig 5e). The data implicate that purified HK, added to HK depleted plasma or a fibrinogen solution, disturbs clot lysis, and thus, a sole depletion of HK was not responsible for prolonged clot lysis time in plasma or fibrin clots.
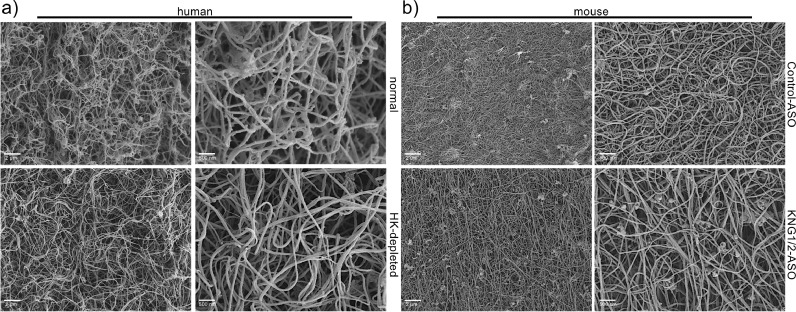
Similar to the kininogen depleted mouse plasma, human HK-depleted plasma had reduced content of PK (to around 65% see Fig 5f, g), and a slightly reduced PKa/FXIIa activity compared to normal plasma (Fig 5h). As we showed recently, PK supports streptococcal fibrinolysis and clot escape [14]. One can assume that the combination of both HK deficiency and reduced PK activity in HK-depleted plasma was responsible for prolonged clot lysis time. We also investigated the structure of human and mouse plasma clots by scanning electron microscopy. Clots derived from kininogen-depleted plasma displayed similar porosity and thickness of fibrin strands compared to normal plasma (Fig 6a, b).
Fig. 6.
Scanning electron microscopy of plasma clots.
a) Human or (b) mouse plasma clots were induced by PT reagent, fixed and analyzed by scanning electron microscopy. Bars represent 2 µm or 500 nm, respectively.
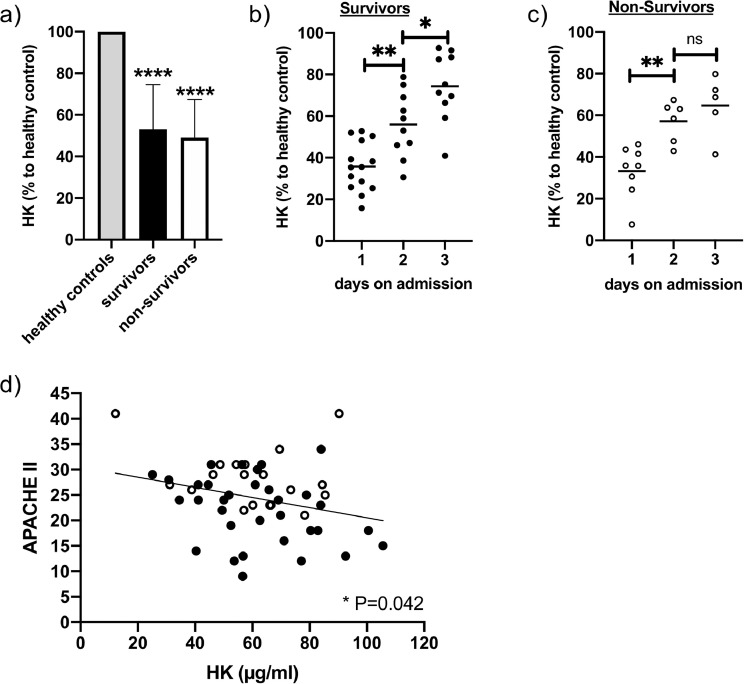
3.6. Kininogen content in plasma of septic patients
Plasma samples collected from 23 patients with sepsis, severe sepsis or septic shock, one, two and three days after admission to the ICU [19] were analyzed for kininogen content. Fig. 7a shows that kininogen levels were significantly reduced in all septic patients, compared to healthy controls. The amount of total kininogen was lowest on the first day after admission to the ICU, but increased significantly from day to day in survivors (Fig. 7b). Also, in non-survivors kininogen increased significantly after 2 days, however, after 3 days kininogen did not continue to rise significant (Fig. 7c). Notably, a low kininogen content correlated significantly with a higher APACHE II score in all patients (Fig. 7d). These data support our findings that kininogen is a positive acute phase protein in sepsis, and indicate that low kininogen concentrations correspond to more severe disease and a higher risk of death.
Fig. 7.
Kininogen content in plasma samples from patients with sepsis, severe sepsis or septic shock.
Blood samples were collected from patients diagnosed with sepsis. Specific sandwich ELISA was performed to detect the levels of kininogen (HK and LK) in plasma of sepsis patients of day 1 to 3 (non-survivors: n= 8, survivors: n= 15, healthy controls: n= 15). a) Kininogen content in plasma samples from all time points. Healthy controls were set 100%. b, c) daily samples from survivors (b) and non-survivors (c) d) simple linear regression of APACHE II score against kininogen concentration from all plasma samples. black dots: samples from survivors, white dots: samples from non-survivors. Statistical significance was calculated by one sample t and Wilcoxon test (a), two-way ANOVA (b, c), and simple linear regression, *p≤ 0.05., **p≤ 0.01, ****p≤ 0.0001, ns - not significant.
4. Discussion
Whether kininogen plays a key role in severe infections or not is possibly dependent on the infectious agent. A recent study by Ding et al. investigated the importance of kininogen in a pneumosepsis mouse model with Klebsiella pneumoniae. In contrast to the present study, they show that depletion of HK in mice, either by ASOs or by KNG1 gene knockout, did not influence bacterial growth in lungs or dissemination to distant body sites. Moreover, HK depletion by ASOs before pneumosepsis did not impact plasma cytokine and chemokine levels or the extent of distant organ injury [26]. Besides the fact that the site of infection is different between them and our sepsis model (lung versus skin), K. pneumoniae are Gram-negative bacteria that, neither in vivo nor in vitro, trigger the activation of the contact system [29]. This in sharp contrast to the Gram-positive S. pyogenes that not only adsorbs and induces cleavage of HK in vitro [30] but also, induces cleavage of HK and prolonged aPTT in vivo, as shown in the present study. HK binds PK in plasma [31], and the HK-PK complexes assemble on the surface of the vascular endothelium, with HK serving as the binding site and as the cofactor for PK activation, resulting in the formation of PKa [32]. Thus, due to the depletion of kininogen by ASOs, PK is reduced to 40%, which might be one reason for reduced bacterial dissemination and plasma cytokine levels, as PK is a host pathogenicity factor in S. pyogenes sepsis [14].
Intriguingly, the present study revealed more fibrin areas in the kidneys of septic animals after the depletion of kininogens. However, kininogen depletion had no effect on disseminated intravascular coagulation (DIC) at 24 h post infection, as the PT, a clinical marker that is elevated in DIC patients [33], was not prolonged in mouse. One explanation for that could be reduced levels of bradykinin, the cleavage product of kininogens. Bradykinin was shown to inhibit expression of tissue factor induced by LPS in vitro, and to inhibit thrombus formation in vivo, associated with lower tissue factor activity in plasma [28]. Our study supports these findings, as intravascular tissue factor was significantly lower in septic mice with normal kininogen levels, despite the fact that these animals had higher levels of several cytokines (e.g. TNFα, IFNγ, IL1β, and MCP1), which were shown to increase tissue factor expression in vitro [34]. These data suggest that kininogen may play a key role to regulate tissue factor expression in sepsis. Future studies will address this hypothesis.
Intravascular tissue factor is the main trigger for immunothrombosis, an innate immune mechanism that generates a scaffold for the recognition, containment and destruction of pathogens [35,36]. Thus, a higher tissue factor production due to the course of streptococcal sepsis [21,37] and the absence of kininogen in KNG1/2 ASO treated mice might support bacterial containment and restrain bacterial dissemination in our model.
Additionally, bradykinin is locally released from HK by S. pyogenes and one assumes that this boosts bacterial spreading due to potent induction of vascular leakage [18]. This assumption was supported by one of our recent studies, showing that S. pyogenes isolates from invasive infections activate the contact system more potently than strains isolated from noninvasive infections [38]. Thus, we suggest that in the absence of kininogens the streptococcal dissemination in our mouse model may be reduced due to several mechanisms 1) lower levels of PK, which may impair bacterial escape from plasma clots [14] 2) lower vascular leakage at the infectious site due to the reduction of kininogen and 3) higher expression of intravascular tissue factor due to the lower levels of kininogen, which reinforces immunothrombosis.
Although KNG1 deficient mice were protected from thrombosis in a Rose-Bengal arterial model [3] and in mouse models of acute ischemic stroke [39], an antithrombotic and profibrinolytic role for kininogens has been earlier described (for a review see [40]). Colman et al., showed that Brown-Norway Katholiek rats – a strain with an absence of plasma HK and LK – have a prothrombotic state [41]. HK also has profibrinolytic functions, it modulates PK-dependent formation of urokinase plasminogen activator (uPA) and consequently generation of plasmin [42], a reaction dependent on the binding of PK to HK domain 6 [43].
HK or cleaved HK also bind directly to plasminogen or vitronectin, which could further amplify plasmin generation [40]. Our in vitro assays demonstrated that bacterial induced fibrinolysis by streptokinase was also impaired in the absence of HK and PK, as d-Dimer release and bacterial escape from a plasma clot were significantly delayed.
Moreover, patients lacking circulating HK are at increased risk for thrombosis and a large prospective human study found recently that higher concentrations of HK and PK were not associated with greater risk for venous thromboembolism (VTE). On the contrary, HK inversely correlated with VTE, supporting an antithrombotic role for HK [44].
Contact system activation in septic patients is often impaired by a reduction of all contact factors, and low levels of PK, FXII, and HK during sepsis correlate with a fatal outcome of the disease (see [1,3,[6], [7], [8]]). Previous studies of human sepsis have shown different outcomes of infection on kininogen plasma levels, e.g. depletion [45], no change [46], or increases [45,47,48]. The data from our patient study reveal a significantly lower plasma levels of kininogens in septic patients, compared to healthy controls, which implies its consumption. At the same time, kininogen levels rose significantly in septic patients who survived on day 2 and 3, however in non-survivors the increase of kininogen from day 2 to 3 was not significantly different. This supports the assumption that kininogen is an acute phase protein and its expression is induced in the liver upon infection [49]. We also detected higher expression of KNG1 and KNG2 mRNA in mouse liver upon invasive infection. There is a trend towards kininogen increase within 42 h of infection in mice plasma, and higher amount of kininogen degradation products suggests consumption that might be compensated in this lethal sepsis model.
Taken all together this is the first study investigating the functional contribution of kininogens in host defense to streptococcal sepsis in a clinically relevant rodent system, where activation of the contact system occurs. The study supports the longstanding hypothesis that kininogen, processed by the pathogen, enhance its spreading [50] but not just through the induction of vascular leakage, but also by its possibly influence on procoagulant mechanisms, by supporting bacterial fibrinolysis, as well as by accompanied reduced circulating levels of PK, an important factor promoting bacterial dissemination.
Research in context
Evidence before this study
Cleavage of kininogen due to contact system activation by Streptococcus pyogenes in vitro leads to the release of proinflammatory bradykinin and may support bacterial spreading by induction of vascular leakage. It has not been investigated yet whether kininogen plays a relevant role in streptococcal sepsis.
Added value of this study
Thought depletion of kininogen in mice reduced streptococcal burdens and plasma cytokine and chemokine levels 24 h after infection, this was accompanied by fibrin deposition in kidney and higher intravascular tissue factor levels. In line with that, low kininogen concentrations in septic patients correlate with higher APACHE II scores. In vitro, kininogen depletion markedly decelerates fibrinolysis induced by the S. pyogenes bacteria.
Implications of all the available evidence
Our findings show that kininogen support dissemination of S. pyogenes and inflammation during sepsis. On the other hand, and in connection with our data from septic patients, a higher kininogen content might decrease the risk for more severe disease and risk of death.
Funding sources
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (project OE 547/ 4–1). SOH was supported by the Federal Excellence Initiative of Mecklenburg Western Pomerania and European Social Fund (ESF) Grant KoInfekt (ESF_14-BM-A55–00xx_16) and by a grant from the Medical Faculty of the University of Rostock in the framework of the FORUN program 2018. CM was supported by grants from the Deutsche Forschungsgemeinschaft (MA5799/2–1 and MA5799/2–2).
Role of the funding source
The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication
Declaration of interests
A.S.R. and A.R.M. are employees and stockholders of Ionis Pharmaceuticals. The remaining authors declare no competing financial interests.
Author contributions
J.K. performed research analysed the data and wrote the paper, C.M. contributed analytic tools and analysed the data, D.K. and M.F. contributed analytic tools; C.S. collected patient material; A.S.R and A.R.M identified and provided reagents, S.M. performed research and analysed the data, BK contributed analytic tools, SOH designed and performed research, analysed the data and wrote the paper.
Acknowledgments
We thank Jana Bull (IMIKRO) and Karoline Schulz (EMZ) for excellent technical assistance and Dr. Ulf Broschewitz for the assessment of histology. We are also grateful to Ionis Pharmaceutical, providing us with ASOs.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102908.
Appendix. Supplementary materials
References
- 1.Lalmanach G., Naudin C., Lecaille F., Fritz H. Kininogens: more than cysteine protease inhibitors and kinin precursors. Biochimie. 2010 Nov;92(11):1568–1579. doi: 10.1016/j.biochi.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura N., Kitagawa H., Fukushima D., Takagaki Y., Miyata T., Nakanishi S. Structural organization of the human kininogen gene and a model for its evolution. J Biol Chem. 1985 Jul 15;260(14):8610–8617. [PubMed] [Google Scholar]
- 3.Merkulov S., Zhang W.M., Komar A.A., Schmaier A.H., Barnes E., Zhou Y. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. American Society of Hematology. 2008 Feb 1;111(3):1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandle R.J., Colman R.W., Kaplan A.P. Identification of Prekallikrein and High-Molecular-Weight Kininogen as a Complex in Human-Plasma. Proc Natl Acad Sci USA. 1976;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman R.W., Schmaier A.H. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997 Nov 15;90(10):3819–3843. [PubMed] [Google Scholar]
- 6.Kahn R., Hellmark T., Leeb-Lundberg L.M.F., Akbari N., Todiras M., Olofsson T. Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J Immunol. Am Assoc Immunol. 2009 Jun 15;182(12):7906–7915. doi: 10.4049/jimmunol.0803624. [DOI] [PubMed] [Google Scholar]
- 7.Kozik A., Moore R.B., Potempa J., Imamura T., Rapala-Kozik M., Travis J. A novel mechanism for bradykinin production at inflammatory sites. Diverse effects of a mixture of neutrophil elastase and mast cell tryptase versus tissue and plasma kallikreins on native and oxidized kininogens. J Biol Chem. Am Soc Biochem Mol Biol. 1998 Dec 11;273(50):33224–33229. doi: 10.1074/jbc.273.50.33224. [DOI] [PubMed] [Google Scholar]
- 8.Oehmcke-Hecht S., Köhler J. Interaction of the human contact system with pathogens-an update. Front Immunol. 2018;9(FEB):312. doi: 10.3389/fimmu.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herwald H., Mörgelin M., Olsen A., Rhen M., Dahlbäck B., Müller-Esterl W. Activation of the contact-phase system on bacterial surfaces - a clue to serious complications in infectious diseases. Nat Med. 1998 Mar;4(3):298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- 10.Frick I.-.M., Åkesson P., Herwald H., Mörgelin M., Malmsten M., Nägler D.K. The contact system–a novel branch of innate immunity generating antibacterial peptides. EMBO J. EMBO Press. 2006 Nov 29;25(23):5569–5578. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frick I.-.M., Björck L., Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007 Sep;98(3):497–502. [PubMed] [Google Scholar]
- 12.Paegelow I., Trzeczak S., Böckmann S., Vietinghoff G. Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology. 2002 Nov;66(3):153–161. doi: 10.1159/000063797. [DOI] [PubMed] [Google Scholar]
- 13.Sato E., Koyama S., Nomura H., Kubo K., Sekiguchi M. Bradykinin stimulates alveolar macrophages to release neutrophil, monocyte, and eosinophil chemotactic activity. J Immunol. 1996;157(7):3122–3129. [PubMed] [Google Scholar]
- 14.Köhler J., Maletzki C., Koczan D., Frank M., Trepesch C., Revenko A.S. The contact system proteases play disparate roles in streptococcal sepsis. Haematologica. Haematologica. 2019 Jul 18 doi: 10.3324/haematol.2019.223545. haematol.2019.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker M.J., Barnett T.C., McArthur J.D., Cole J.N., Gillen C.M., Henningham A. Disease manifestations and pathogenic mechanisms of group a Streptococcus. Clin Microbiol Rev. 2014 Apr;27(2):264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BenNasr A., OLSEN A., Sjöbring U., MullerEsterl W., Björck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol Microbiol. 1996 Jun;20(5):927–935. doi: 10.1111/j.1365-2958.1996.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 17.Oehmcke S., Shannon O., Köckritz-Blickwede von M, Mörgelin M., Linder A., Olin A.I. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood. 2009 Jul 9;114(2):444–451. doi: 10.1182/blood-2008-10-182527. [DOI] [PubMed] [Google Scholar]
- 18.Ben Nasr A., Herwald H., Sjöbring U., Renné T., Müller-Esterl W., Björck L. Absorption of kininogen from human plasma by Streptococcus pyogenes is followed by the release of bradykinin. Biochem J. 1997 Sep 15;326(Pt 3):657–660. [PMC free article] [PubMed] [Google Scholar]
- 19.Trepesch C., Nitzsche R., Glass A., Kreikemeyer B., Schubert J.K., Oehmcke-Hecht S. High intravascular tissue factor-but not extracellular microvesicles-in septic patients is associated with a high SAPS II score. J Intensiv Care. 2016;4:34. doi: 10.1186/s40560-016-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker B.F., Lot S.S., Condon T.P., Cheng-Flournoy S., Lesnik E.A., Sasmor H.M. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. Am Soc Biochem Mol Biol. 1997 May 2;272(18):11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 21.Oehmcke S., Westman J., Malmström J., Mörgelin M., Olin A.I., Kreikemeyer B. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 2013;9(8) doi: 10.1371/journal.ppat.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oehmcke S., Herwald H. Contact system activation in severe infectious diseases. J Mol Med. 2010 Feb;88(2):121–126. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 23.Asmis L.M., Asmis R., Sulzer I., Furlan M., Lämmle B. Contact system activation in human sepsis - 47kD HK, a marker of sepsis severity? Swiss Med Wkly. 2008 Mar 8;138(9–10):142–149. doi: 10.4414/smw.2008.11788. [DOI] [PubMed] [Google Scholar]
- 24.Colman R.W., Bagdasarian A., Talamo R.C., Scott C.F., Seavey M., Guimaraes J.A. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec 1;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson V.H., Kleniewski J., Saito H., Sayed J.K. Prekallikrein deficiency in a kindred with kininogen deficiency and Fitzgerald trait clotting defect. Evidence that high molecular weight kininogen and prekallikrein exist as a complex in normal human plasma. J Clin Invest. Am Soc Clin Investig. 1977 Sep;60(3):571–583. doi: 10.1172/JCI108809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding C., van 't Veer C., Roelofs J.J.T.H., Shukla M., McCrae K.R., Revenko A.S. Limited role of kininogen in the host response during gram-negative pneumonia-derived sepsis. Am J Physiol Lung Cell Mol Physiol. 2018 Mar 1;314(3):L397–L405. doi: 10.1152/ajplung.00288.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aslan A., van den Heuvel M.C., Stegeman C.A., Popa E.R., Leliveld A.M., Molema G. Kidney histopathology in lethal human sepsis. Crit Care. BioMed Cent. 2018 Dec 27;22(1):359. doi: 10.1186/s13054-018-2287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong R., Chen W., Feng W., Xia C., Hu D., Zhang Y. Exogenous Bradykinin Inhibits Tissue Factor Induction and Deep Vein Thrombosis via Activating the eNOS/Phosphoinositide 3-Kinase/Akt Signaling Pathway. Cell Physiol Biochem. 2015;37(4):1592–1606. doi: 10.1159/000438526. [DOI] [PubMed] [Google Scholar]
- 29.Ding C., Scicluna B.P., Stroo I., Yang J., Roelofs J.J.T.H., de Boer O.J. Prekallikrein inhibits innate immune signaling in the lung and impairs host defense during pneumosepsis in mice. J Pathol. 2019 Oct 9 doi: 10.1002/path.5354. John Wiley & Sons, Ltdpath.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oehmcke S., Shannon O., Köckritz-Blickwede von M, Mörgelin M., Linder A., Olin A.I. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood. 2009 Jul 9;114(2):444–451. doi: 10.1182/blood-2008-10-182527. [DOI] [PubMed] [Google Scholar]
- 31.Mandle R.J., Colman R.W., Kaplan A.P. Identification of Prekallikrein and High-Molecular-Weight Kininogen as a Complex in Human-Plasma. Proc Natl Acad Sci USA. 1976;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmaier A.H. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol. 2008 Feb 1;8(2):161–165. doi: 10.1016/j.intimp.2007.08.022. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrashekar V. DIC Score: statistical Relationship with PT, APTT, and Simplified Scoring Systems with Combinations of PT and APTT. ISRN Hematol. Hindawi. 2012;2012(2):579420–579424. doi: 10.5402/2012/579420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Poll T., de Jonge E., Cate an ten H. Madame curie bioscience database [Internet] Landes Bioscience; 2013. Cytokines as Regulators of Coagulation; pp. 2049–2058. [Google Scholar]
- 35.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013 Jan;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 36.Gaertner F., Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol. 2016 Dec;28(6):561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Oehmcke S., Shannon O., Mörgelin M., Herwald H. Streptococcal M proteins and their role as virulence determinants. Clin Chim Acta. 2010;411:1172–1180. doi: 10.1016/j.cca.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 38.Nitzsche R., Rosenheinrich M., Kreikemeyer B., Oehmcke-Hecht S. Streptococcus pyogenes triggers activation of the human contact system by streptokinase. Infect Immun. 2015 Aug;83(8):3035–3042. doi: 10.1128/IAI.00180-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langhauser F., Göb E., Kraft P., Geis C., Schmitt J., Brede M. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012 Nov 8;120(19):4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavakis T., Preissner K.T. Potential pharmacological applications of the antithrombotic molecule high molecular weight kininogen. Curr Vasc Pharmacol. 2003 Mar;1(1):59–64. doi: 10.2174/1570161033386790. [DOI] [PubMed] [Google Scholar]
- 41.Colman R.W., White J.V., Scovell S., Stadnicki A., Sartor R.B. Kininogens are antithrombotic proteins In vivo. Arterioscl Throm Vas. 1999 Sep;19(9):2245–2250. doi: 10.1161/01.atv.19.9.2245. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y., Harris R.B., Yan W., McCrae K.R., Zhang H., Colman R.W. High molecular weight kininogen peptides inhibit the formation of kallikrein on endothelial cell surfaces and subsequent urokinase-dependent plasmin formation. Blood. 1997 Jul 15;90(2):690–697. [PubMed] [Google Scholar]
- 43.Tait J.F., Fujikawa K. Identification of the binding site for plasma prekallikrein in human high molecular weight kininogen. A region from residues 185 to 224 of the kininogen light chain retains full binding activity. J Biol Chem. 1986 Nov 25;261(33):15396–15401. [PubMed] [Google Scholar]
- 44.Folsom A.R., Tang W., Basu S., Misialek J.R., Couper D., Heckbert S.R. Plasma Concentrations of High Molecular Weight Kininogen and Prekallikrein and Venous Thromboembolism Incidence in the General Population. Thromb Haemost. Georg Thieme Verlag KG. 2019 Feb 19 doi: 10.1055/s-0039-1678737. (EFirst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch E.F., Nakajima T., Oshima G., Erdös E.G., Herman C.M. Kinin System Responses in Sepsis After Trauma in Man. J Surg Res. 1974;17(3):147–153. doi: 10.1016/0022-4804(74)90101-2. [DOI] [PubMed] [Google Scholar]
- 46.Hack C.E., Wagstaff J., Strack van Schijndel R.J., Eerenberg A.J., Pinedo H.M., Thijs L.G. Studies on the contact system of coagulation during therapy with high doses of recombinant IL-2: implications for septic shock. Thromb Haemost. 1991 May 6;65(5):497–503. [PubMed] [Google Scholar]
- 47.Colman R.W., Edelman R., Scott C.F., Gilman R.H. Plasma kallikrein activation and inhibition during typhoid fever. J Clin Invest. 1978 Jan 1;61(2):287–296. doi: 10.1172/JCI108938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmaier A.H., Farber A., Schein R., Sprung C. Structural changes of plasma high molecular weight kininogen after in vitro activation and in sepsis. J Lab Clin Med. 1988 Aug;112(2):182–192. [PubMed] [Google Scholar]
- 49.Sriskandan S., Kemball-Cook G., Moyes D., Canvin J., Tuddenham E., Cohen J. Contact activation in shock caused by invasive group A Streptococcus pyogenes. Crit Care Med. 2000 Nov;28(11):3684–3691. doi: 10.1097/00003246-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 50.Herwald H., Morgelin M., OLSEN A., Rhen M., Dahlbäck B., Müller-Esterl W. Activation of the contact-phase system on bacterial surfaces–a clue to serious complications in infectious diseases. Nat Med. 1998 Mar;4(3):298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.