Abstract
Objectives
To evaluate β-hCG concentration in vaginal fluid as a biochemical marker for PPROM in suspected cases and its correlation with onset of labour.
Materials and Methods
This is a prospective case–control study carried out in tertiary care centre in 1 year. Total 150 pregnant women of gestational age 28–36 week + 6 days were included and were divided into two groups: control (Group 1) (n = 50) normal antenatal patients. Group 2 cases with history of leaking per vaginum subdivided into two groups—Group 2A—(n = 50) with no detectable leakage of amniotic fluid present on per speculum examination and Group 2B—(n = 50) with minimal leaking per vaginum present upon per speculum examination (frank leaking were excluded). β-hCG level was measured by chemiluminescent microparticle assay, and all women were followed till onset of labour.
Results
Mean β-hCG level in vaginal fluid was measured as 6.10 ± 8.52 mIU/mL, 57.10 ± 30.86 mIU/mL and 111.35 ± 36.01 mIU/mL in Group 1, Group 2A and Group 2B, respectively. By taking 21.5 mIU/ml as cut-off, receiver operating characteristic curve shows sensitivity 100%, specificity 92.0%, positive predictive value 92.6%, negative predictive value 100% and diagnostic accuracy 96%. Regarding the correlation of β-hCG level with onset of labour if the β-hCG level is < 21.5 mIU/ml, 100% pregnancy continued beyond 4 weeks and 56% women delivered within 4 weeks when β-hCG level is > 75 mIU/ml.
Conclusion
β-hCG in vaginal fluid is a reliable biochemical marker for diagnosing suspected cases of PPROM and is well correlated with onset of labour.
Keywords: β-hCG, Vaginal fluid, PPROM
Introduction
Premature rupture of membranes (PROM) is defined as rupture of the amniotic membranes before the onset of labour, regardless of gestational age [1]. Its prevalence ranges from 8 to 19.53% of term pregnancies and 2–25% of all pregnancies and is responsible for preterm birth which is one of the most important cause of perinatal mortality and morbidity throughout the world [2]. Preterm premature rupture of membranes (PPROM) refers to rupture of the amniotic membranes before 37 weeks of pregnancy [3].
PPROM is largely a clinical diagnosis based on history of watery discharge from vagina and confirmed on sterile per speculum examination. The traditional method for the diagnosis of PPROM relies on clinician ability to document three clinical signs on sterile per speculum examination: visual pooling of clear fluid from vagina, an alkaline pH of the cervicovaginal discharge detected by nitrazine test or microscopic ferning of the cervicovaginal discharge on drying [1].
β-hCG is a glycoprotein, biosynthesized by the syncytiotrophoblast, and is present in varying concentration in maternal serum, urine, and amniotic fluid during pregnancy. It is also secreted by the cervical glands and is present at a certain level in vaginal fluid. It is found in high concentration until 20 weeks of gestation, but after 20 weeks, it remains at a very low stable level in second and third trimester of pregnancy [2]. The mean level of β-hCG in the vaginal fluid in a pregnancy with intact membranes varies throughout the pregnancy from 37.9 mIU/mL in the first trimester to 9.5 mIU/mL in the second trimester, and from 6.3 to 7.71 mIU/mL in the third trimester. On the other hand, the β-hCG levels in pregnancies with PROM in the literature ranges from 330.88 to 468.06 mIU/mL [4].
The present study has been done to determine whether β-hCG in vaginal fluid can be used as a biochemical marker for PPROM in suspected cases; its correlation with onset of labour; its role in modifying the management and finally its role in maternal and neonatal outcome.
Materials and Methods
The present study was conducted in the Department of Obstetrics and Gynaecology in a Queen Mary Hospital from July 2018 to June 2019 in collaboration with Department of Pathology in King George’s Medical University Lucknow, India.
Inclusion Criteria
The study includes 150 healthy antenatal subjects with singleton pregnancy whose period of gestation was between 28 and 36 week + 6 days (confirmed by USG and LMP) and willing to participate in the study.
Exclusion Criteria includes confirmed rupture of foetal membranes, gestational age < 28 weeks or ≥ 37 weeks, polyhydramnios, multiple pregnancy, abruptio placentae, placenta previa, presence of gross blood in vagina, symptoms of intraamniotic infections, cervical dilation of > 4 cm, congenital anomalies and preeclampsia.
Study Design
The study was a prospective case–control study comprising 150 pregnant women. The detailed history of all subjects including menstrual history, obstetrics history, presenting complaints, general examination, per abdominal and per speculum examination was done. These 150 subjects were divided into two groups: Group 1 (Control, n = 50) normal antenatal patients. Group 2 (Cases, n = 100) with history of leaking per vaginum subdivided into two groups—Group 2A—(n = 50) with no detectable leakage of amniotic fluid on per speculum examination and Group 2B—(n = 50) with minimal leaking per vaginum present on per speculum examination (subjects having frank leaking were excluded).
Study Procedure
Patient was laid in lithotomy position in good illumination. Sterile vaginal examination using a sterile speculum was done. In patients of Group 2B (leaking present) vaginal fluid from posterior fornix was aspirated directly through sterile 5-ml syringe, while in patient of Group 1 and Group 2A (leaking absent), vaginal fluid was aspirated 3 min after injecting 5 cc of normal saline. The samples were collected in plain vial, stored at temperature of 4–8 °C and were transported to laboratory in Department of Pathology for estimation of β-hCG level in a vaginal fluid. The sample were centrifuged for 5 min with 2500 r.p.m, and β-hCG titre was determined by Chemiluminescent microparticle assay (CMIA). Total duration of the test was 15–20 min.
Statistical Analysis
Data were collected and analysed by using Chi-square tests. It was expressed as mean ± SD. The optimum cut-off for the marker was determined by area under the receiver operating curve (a ROC). Using these cut-off points, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy were calculated. Significance level was set at p value of < 0.05.
Results
Demographic characteristics of cases and control showed no statistical significant difference (p > 0.005) (Table 1).
Table 1.
Comparison of demographic characteristics of subjects in term of age, parity, religion, residence, booking status and gestational age at time of admission
| Control | Case | p value | ||
|---|---|---|---|---|
| GROUP 1 | Group 2A | Group 2B | ||
| Mean age ± SD (years) | 24.60 ± 3.36 | 24.78 ± 3.49 | 24.63 ± 3.27 | 0.996 |
| Parity | ||||
| Gravida 1 | 30 (60%) | 30 (60%) | 25 (50%) | 0.833 |
| Gravida 2 | 14 (28%) | 13 (26%) | 17 (34%) | |
| Gravida 3 or more | 6 (12%) | 7 (14%) | 8 (16%) | |
| Religion | ||||
| Hindu | 37 (74%) | 35 (70%) | 38 (76%) | 0.849 |
| Muslim | 13 (26%) | 15 (30%) | 12 (24%) | |
| Residence | ||||
| Rural | 31 (62%) | 26 (52%) | 31 (62%) | 0.591 |
| Urban | 19 (38%) | 24 (48%) | 19 (38%) | |
| Booking status | ||||
| Booked | 8 (16%) | 12 (24%) | 13 (26%) | 0.500 |
| Unbooked | 42 (84%) | 38 (76%) | 27 (74%) | |
| Gestational age at time of admission | ||||
| 28–31 weeks + 6 days | 20 (40%) | 29 (58%) | 30 (60%) | 0.080 |
| 32–36 weeks + 6 days | 30 (60%) | 21 (42%) | 20 (40%) | |
The mean value of β-hCG in vaginal fluid in Group 2B was 111.35 ± 36.01 which was significantly higher in comparison with Group 2A (57.10 ± 30.86) and Group 1 (6.10 ± 8.52) (p < 0.001) (Fig. 1).
Fig. 1.

Mean β-hCG levels (mIU/mL) obtained from vaginal fluid of pregnant women
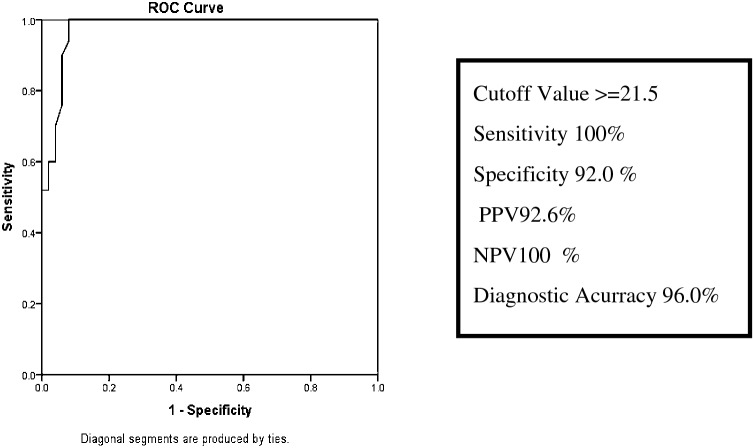
Receiver operating characteristic (ROC) curve established the optimal cut-off of β-hCG in vaginal fluid as 21.5 mIU/ml. At this cut-off value, sensitivity was found to be 100%, specificity 92.0%, positive predictive value 92.6%, negative predictive value 100% and diagnostic accuracy 96% (Fig. 2).
Fig. 2.
Receiver–operator curve (ROC) analysis for the optimal cut-off point for vaginal washing fluid β-human chorionic gonadotrophin
Obstetrics characteristics among cases and control showed statistically significant difference in relation to gestational age at time of delivery, interval between complaints and delivery, type of delivery, birth weight and APGAR score. The mean birth weight and APGAR score in Group 2B was significantly lower in comparison with Group 2A and Group 1 (Table 2).
Table 2.
Comparison between obstetrics characteristic in between control group and study group
| Control | Group 2A | Group 2B | Chi sq | p value | |
|---|---|---|---|---|---|
| GA at delivery | |||||
| 28–31 weeks + 6 days | 4 (8%) | 9 (18.0%) | 12 (24.0%) | 52.40 | < 0.001 |
| 32–36 weeks + 6 days | 3 (6%) | 12 (24.0%) | 30 (60.0%) | ||
| ≥ 37 week | 43 (86%) | 29 (58.0%) | 8 (16.0%) | ||
| Interval between complaint and delivery | |||||
| <2 week | 1 (2%) | 8 (16%) | 28 (56%) | 71.05 | < 0.001 |
| 2–3 weeks + 6 days | 3 (6%) | 11 (22%) | 12 (24%) | ||
| 4–5 weeks + 6 days | 8 (16%) | 8 (16%) | 9 (18%) | ||
| ≥ 6 week | 38 (76%) | 23 (46%) | 1 (2%) | ||
| Type of delivery | |||||
| Fullterm | 44 (88%) | 30 (60%) | 7 (14%) | 43.98 | < 0.001 |
| Preterm | 6 (12%) | 20 (40%) | 43 (86%) | ||
| Mean birth weight | 2.52 ± 0.47 | 2.23 ± 0.55 | 1.94 ± 0.51 | 34.99 | < 0.001 |
| APGAR | |||||
| 1 min | 6.06 ± 1.68 | 4.73 ± 1.60 | 4.31 ± 1.65 | 14.81 | < 0.001 |
| 5 min | 7.54 ± 1.31 | 6.82 ± 1.31 | 6.49 ± 1.31 |
Our results showed that the value of β-hCG less than cut-off (21.5 mIU/ml) guarantees delivery after 4 weeks (NPV-100%), while the value more than 75 mIU/ml showed chances of delivery within 4 weeks in 56% women. So as the concentration of β-HCG increases duration of interval between complaints and delivery decreases (Table 3).
Table 3.
Correlation of time interval between sampling and delivery and β-hCG values
| β-hCG level versus duration | Control (n = 50) | Case | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 2A (N = 50) | Group 2B (N = 50) | |||||||||||
| < 2 weeks | 2–4 weeks | 4–6 weeks | > 6 weeks | < 2 weeks | 2–4 weeks | 4–6 weeks | > 6 weeks | < 2 weeks | 2–4 weeks | 4–6 weeks | > 6 weeks | |
| < 25 mIU/mL | ||||||||||||
| No. | 0 | 0 | 8 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| % | 0.0 | 0.0 | 16.0 | 76.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 25–49 mIU/mL | ||||||||||||
| No. | 1 | 3 | 0 | 0 | 0 | 1 | 5 | 22 | 0 | 1 | 0 | 0 |
| % | 2.0 | 6.0 | 0.0 | 0.0 | 0.0 | 2.0 | 10.0 | 44.0 | 0.0 | 2.0 | 0.0 | 0.0 |
| 50–74 mIU/mL | ||||||||||||
| No. | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 1 |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 4.0 | 2.0 | 0.0 | 0.0 | 2.0 | 2.0 |
| 75–99 mIU/mL | ||||||||||||
| No. | 0 | 0 | 0 | 0 | 3 | 6 | 1 | 0 | 9 | 6 | 7 | 0 |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 12.0 | 2.0 | 0.0 | 18.0 | 12.0 | 14.0 | 0.0 |
| 100–124 mIU/mL | ||||||||||||
| No. | 0 | 0 | 0 | 0 | 5 | 3 | 0 | 0 | 9 | 2 | 1 | 0 |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 10.0 | 6.0 | 0.0 | 0.0 | 18.0 | 4.0 | 2.0 | 0.0 |
| 125–149 mIU/mL | ||||||||||||
| No. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 | 6.0 | 0.0 | 0.0 |
| ≥ 150 mIU/mL | ||||||||||||
| No. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.0 | 0.0 | 0.0 | 0.0 |
If β-hCG level < 21.5 mIU/mL—100% patient delivered after 4 week
If β-hCG level > 75 mIU/mL—56% patients delivered within 4 weeks
The bold values signify number of patients who were delivered within the specified level of beta-hcG
Discussion
β-hCG in vaginal fluid is a very good biochemical marker to determine suspected cases of PPROM and its correlation with onset of labour. Other biochemical markers used for detection of PPROM are: alpha-fetoprotein (AFP) [1, 5], foetal fibronectin (fFN) [6], insulin-like growth factor binding protein 1 (IGFBP-1) [7], prolactin [1], Creatinine [1, 8], urea [8] and placental alpha-macroglobulin 1 (PAMG-1) [7], but since the method using β-hCG is cost-effective, rapid, simple and easy to use, it is preferred over its other counterparts.
Similar study was conducted by Sak et al. [9] showed that mean neonatal birth weight and APGAR scores of neonates in the preterm group were significantly lower in comparison with the control group which is comparable with our study (p < 0.001) Al-Bayati et al. [10] concluded that value of β-hCG sampling in vaginal fluid was inversely proportional to duration of onset of labour. Tigga et al. [1] studied the duration from PROM to onset of labour and concluded that β-hCG is a better predictor of onset of labour in confirmed cases of PROM. Bahasadri et al. [3] and Tamer et al. [11] which showed that mean β-hCG value in vaginal fluid was significantly higher in suspected cases of PPROM as compared to control. Abbas et al. [12] in addition to mean β-hCG values also pointed to the fact that mean gestational age of study group at time of delivery was lower in suspected cases of PPROM as compare to control. Similarly Elmahalawi et al. [13] concluded that the β-hCG level in cervicovaginal washing was higher in confirmed cases of PPROM as compare to normal antenatal patients (no history of leaking present).
Conclusion
β-hCG is a very sensitive marker to detect PPROM in suspected cases and can be used as an adjunctive test. It is cost-effective, simple and rapid test. Patient can be sent home, and it helps to reduce hospital stay and financial burden and avoid unnecessary interventions like administration of antibiotics, corticosteroids, tocolysis therapy and induction of labour.
Dr. Soumya Jain
is working in the Department of Obstetrics and Gynaecology KGMU, Lucknow, as a junior resident. She has completed her MBBS from Motilal Nehru Medical College Allahabad. She has presented various papers and posters in State level conference. She plans to strive and continue her work in the field of infertility.
Compliance with Ethical Standards
Conflict of interest
There are no conflicts of interest in the study.
Research Involving Human Participants and/or Animals
Yes and an informed consent were taken from the patients.
Ethical Statement
A prior approval was obtained from the King George’s Medical University (K.G.M.U.) of Lucknow, India ethics committee vide letter no-794/Ethics/18 to conduct this research.
Footnotes
Dr. S. P. Jaiswar is an Professor & Unit Head, Dept. of Obs & Gynae, KGMU; Dr. Soumya Jain is an MD, Dept. of Obs & Gynae, KGMU; Dr. Nisha Singh is an Professor, Dept. of Obs & Gynae, KGMU; Dr. Sujata Deo is an Professor, Dept. of Obs & Gynae, KGMU; Dr. Monica Agarwal is an Assistant Professor, Dept. of Obs & Gynae, KGMU; Dr. Wahid Ali is an Associate Professor, Dept. of Pathology, KGMU.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Soumya Jain, Email: soumyajain91@gmail.com.
S. P. Jaiswar, Email: spjaiswar59@gmail.com
Nisha Singh, Email: nisha.kgmc@gmail.com.
Sujata Deo, Email: dr.sujata.2008@rediffmail.com.
Monica Agarwal, Email: dr.monikaagrawal@gmail.com.
References
- 1.Tigga MP, Malik S. Comparative analysis of four biomarkers in diagnosing premature rupture of membranes and their correlation with onset of labour. Int J Reprod Contracept Obstet Gynecol. 2015;4:1070–1075. doi: 10.18203/2320-1770.ijrcog20150429. [DOI] [Google Scholar]
- 2.Dartibale CM, Uchimura NS, Nery L, et al. Qualitative determination of human chorionic gonadotropin in vaginal washings for the early diagnosis of premature rupture of fetal membranes. Rev Bras Ginecol Obstet. 2017;39:317–321. doi: 10.1055/s-0037-1603939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahasadri S, Kashnian M, Khalili S. Evaluation of vaginal fluid β- human chorionic gonadotrophin for diagnosis of preterm premature rupture of membranes. J Obstet Gynaecol Res. 2013;39(4):777–778. doi: 10.1111/jog.12012. [DOI] [PubMed] [Google Scholar]
- 4.Anai T, Tanaka Y, Hirota Y, et al. Vaginal fluid hCG levels for detecting premature rupture of membranes. Obstet Gynecol. 1997;89(2):261–264. doi: 10.1016/S0029-7844(96)00448-6. [DOI] [PubMed] [Google Scholar]
- 5.Verma P, Mathur S. Role of alpha feto protien as a marker in diagnosis of premature rupture of membranes in rural population. J Evol Med Dent Sci. 2015;4(64):11182–11186. doi: 10.14260/jemds/2015/1610. [DOI] [Google Scholar]
- 6.Abdelazim IA. Fetal fibronectin (Quick Check fFN test®) for detection of premature rupture of fetal membranes. Arch Gynecol Obstet. 2013;287(2):205–210. doi: 10.1007/s00404-012-2548-3. [DOI] [PubMed] [Google Scholar]
- 7.Abdelazim IA, Al-Sherbeeny MM, Ibrahim MEM, et al. Insulin-like growth factor binding protein-1/alpha-fetoprotein versus placental alpha microglobulin-1 for diagnosis of premature fetal membranes rupture. Acta Med Int. 2016;3(1):69–74. doi: 10.5530/ami.2016.1.15. [DOI] [Google Scholar]
- 8.Osman OM, Elghazaly M. Can vaginal washing fluid urea, creatinine and qualitative β-hCG diagnose suspected premature rupture of membranes? Open J Obstet Gynecol. 2014;4:967–972. doi: 10.4236/ojog.2014.415136. [DOI] [Google Scholar]
- 9.Erdal S, Sak S, Gul T. Beta human chorionic gonadotropin concentration in cervicovaginal secretion as an early marker of preterm delivery. J Clin Exp Investig. 2010;1(1):16–20. [Google Scholar]
- 10.Al-Bayati M, Al-Kazaly E, Athrab S. Vaginal washing fluid B-HCG levels for detecting premature rupture of membranes. Iraqi Postgrad Med J. 2011;10(1):61–66. [Google Scholar]
- 11.Tamer F, Fouad MS, Mohammed IN. Evaluation of vaginal fluid Β-Hcg for the diagnosis of premature rupture of membranes. World J Pharm Res. 2017;6(6):1716–1723. [Google Scholar]
- 12.Abbas AM, El-Shorbagy SH, El-Bandary AS, et al. Evaluation of vaginal fluid β-human chorionic gonadotropin for diagnosis of premature rupture of membranes. Med J Cairo Univ. 2018;86(1):597–603. [Google Scholar]
- 13.Elmahalawi MN, Ibrahim MI, Mohamed RM, et al. The accuracy of β-hCG assay in vaginal fluid for diagnosis of premature rupture of membrane. J Wom Heal Gyne Obes 2019:IWHGO-104.