Abstract
Genetic testing decision-making for cancer predisposition is inherently complex. Understanding the mechanisms and influencing factors of the decision-making process is essential for genetic counselling and has not yet been investigated in Switzerland. This study’s aim is thus to provide a theory about the individual’s decision-making process regarding genetic testing for cancer predispositions in order to provide medical geneticists and genetic counsellors with insights into the needs and expectations of counsellees. We interviewed at-risk individuals who underwent genetic counselling in a clinical setting in Switzerland, using a grounded theory approach. Based on the interview data, we propose that a control-fate continuum, which is part of the individuals’ life philosophy, importantly influences the decision-making process. Those in need for control decide differently compared with those leaving their future to fate. Several psychosocial factors influence the position on the control-fate continuum: “looking for certainty”; “anticipating consequences”; “being socially influenced”; “simplifying risks”; and “deciding intuitively vs reflectively”. The control-fate continuum theory gives insights into the possible reasons behind decision-making regarding genetic testing for cancer predispositions. It includes both acceptors and decliners of genetic testing. Our theory helps healthcare professionals offering genetic counselling to anticipate problems within at-risk families and adapting their services to people’s needs.
Subject terms: Ethics, Psychology
Introduction
Genetic testing for cancer predispositions was used for the first time in 1993/1994 when testing for hereditary breast and ovarian cancer became possible [1, 2]. Pre-test genetic counselling aims to enable individuals to make an informed decision regarding the genetic test and facilitates self-management. Besides disease-specific information, genetic counselling should provide information regarding possible psychological, social and legal consequences of genetic testing. Healthcare professionals offering genetic counselling should tailor the content of the consultation to the individual needs and expectations of their counsellees [3]. Thus, it is essential for them to anticipate which needs and expectations counsellees have in the decision-making process for or against genetic testing.
The main reasons for undergoing genetic testing for cancer predisposition are the early detection of cancer and a feeling of responsibility towards family members [4–7]. Many at-risk individuals make their decision intuitively and have already decided before genetic counselling to undergo genetic testing [8, 9]. Reasons declining genetic testing for cancer predisposition include lack of knowledge, anxiety, perception of non-relevance, objections from relatives and privacy concerns [10–12]. While people perceive themselves as well informed, they perform poorly on knowledge surveys [13].
Most studies about the decision-making process for genetic testing for cancer predisposition stem from English-speaking countries, and there is a particular lack of data from the German-speaking area. While recent studies have investigated this phenomenon in Southern Europe, particularly in Italy [8, 9], there are considerable differences between European countries regarding legislation [14–16], public discourse [17] and culture [8, 18, 19]. This study aims to fill the existing research gap by interviewing at-risk individuals from the German-speaking part of Switzerland.
In Switzerland, Article 14/1 of the Federal Act on Human Genetic Testing (HGTA) requires professional genetic counselling before and after predictive genetic testing [20]. The decision-making process starts when a person first realises that he or she might be genetically predisposed to cancer. After that, the first genetic consultation takes place, which is mandatory in order to get a test referral. The healthcare professional offering genetic counselling discloses and discusses information about testing procedures and the meaning of test results with the counsellee [3, 20]. That includes implications for the patient and relatives, the meaning of positive and negative test results and the medical consequences of the test. There is a mandatory reflection period (usually several weeks) after the pre-test genetic consultation (Article 14/4 HGTA) [20].
In order to help people through the inherently complex decision-making process of genetic testing for cancer predispositions, it is vital to assess how they make decisions about whether or not to take the genetic test and identify influencing factors. This article aims to explore how at-risk individuals in Switzerland experience the decision-making process of genetic testing for cancer predisposition.
Materials and methods
Strategy
In this qualitative interview study, we used a grounded theory approach for study design, sampling and data analysis [21, 22]. This well-established qualitative method aims to construct a theory grounded on data. We chose this method because we intended to theorise our findings and build new hypotheses, applying a post-positivist research paradigm [23]. One researcher (BZ) has a background in genetics and a positive attitude towards genetic testing. Another team member (IK) is a medical doctor working in general practice and somewhat sceptical towards genetic testing. The different attitudes allowed for a critical and constant reflection on personal views and opinions. We also asked our participants for feedback regarding the results of this study and performed two expert interviews with clinicians after the patient interviews to triangulate data and verify, support and complement our findings. The regional ethics committees of Northwest and Central Switzerland, as well as Bern, approved the study (no. 2017-00316). All at-risk individuals signed an informed consent form before the interview. Experts gave their informed consent verbally, in line with research ethics committee recommendations and Swiss law.
Recruitment
We recruited at-risk individuals through four medical doctors responsible for genetic counselling at Basel and Bern University Hospitals. They informed eligible counsellees about the study and handed over the study information leaflet. Those interested in participation contacted the interviewer proactively, except for four at-risk individuals who permitted the medical doctor to forward their contact details to be contacted directly by the interviewer. The recruitment procedure varied slightly according to doctors’ preferences for recruiting. However, all used the same inclusion criteria. Eligible for the study were men and women aged 18–70 who underwent at least one genetic counselling session for genetic testing for any cancer predisposition, were psychologically stable and not pregnant. Both cancer patients and people opting for presymptomatic genetic testing for cancer predisposition were included. The recruiting medical doctors controlled for these criteria before handing over the study information leaflet. Selection criteria were refined during the study to allow for theoretical saturation: to compensate for the higher percentage of women and those in favour of testing among participating at-risk individuals, we specifically recruited male at-risk individuals and those who decided against testing. For the expert interviews, we recruited healthcare professionals providing genetic counselling.
Data collection
We collected all data between August 2017 and February 2019. The first author (BZ) held all interviews face to face at the university hospital or the participants’ home, according to the participants’ preferences. Using a semi-structured interview guide (Supplementary Material), we asked the at-risk individuals to talk about their experiences regarding genetic counselling and genetic testing. They also answered questions regarding other kinds of testing to assess their more general attitudes, such as predictive genetic testing for nonactionable genetic variants, and predictive genetic testing for diseases with environmental influence (such as diabetes) in the form of hypothetical vignettes. The experts were asked about themes and observations that were identified during the analysis of the patient interviews. The interview guides are provided as Supplementary Material.
Data analysis
All patient and expert interviews were held, recorded and transcribed by the first author. One interview was in French, and three in High German. They were transcribed ad verbatim. The spoken language of the remaining 16 interviews was Swiss-German dialect. The first author translated them to High German during transcription, and all transcripts were anonymised. Following grounded theory, we started our inductive analysis during data collection. We used MaxQDA 2018 (VERBI GmbH) for data analysis. We first analysed all interviews by open coding and discussed them. Reflection on interpretative patterns of the researchers, constant comparison of the content of the different interviews and identification of new concepts and aspects were central for this first analytical step. We built concepts and categories by grouping and connecting initial codes constantly. At the same time, we added abductive steps, asking questions to the data, collecting information on interesting findings in further interviews, and creating and testing hypotheses on our data. We then started connecting different concepts and categories, using the coding paradigm proposed by Strauss and Corbin [21]. Finally, we performed several selective coding steps, where we critically examined and discussed hypotheses and questions raised during data analysis. In all steps, we wrote memos and discussed the analysis.
For this publication, we aimed for an English translation of illustrating quotations that reflects their meaning and context. Three researchers independently checked the quotations’ translation for appropriateness. The two expert interviews took place before the selective coding and were used to inform and confirm the analysis. Since these two expert interviews did not add any new aspect to our analysis, we assumed theoretical saturation. At the end stage of the project, we sent the participating at-risk individuals our preliminary results, asking them to comment on how well they could identify themselves with them. Eleven out of eighteen (61%) replied. Two of them indicated a slightly different position than we would have anticipated from the interview, and we included their additional comments in our analysis.
Results
We interviewed 18 at-risk individuals (Table 1) and two medical doctors specialised in genetics. Most at-risk individuals felt well informed and were happy with the quality and content of the genetic consultation. Criticism focused on clinical procedures and psychological components such as unexpected emotional distress while waiting for the test result. Most at-risk individuals had sustainably consolidated decisions regarding genetic testing and showed little uncertainty in this regard.
Table 1.
Sample characteristics of interviewed counsellees (N = 18).
| Gender | |
| Female | 14 (78%) |
| Male | 4 (22%) |
| Age | |
| 20–29 | 1 (6%) |
| 30–39 | 5 (28%) |
| 40–49 | 3 (17%) |
| 50–59 | 5 (28%) |
| 60+ | 4 (22%) |
| Outcome | |
| Mutation-positive test result | 7 (39%) |
| Mutation-negative test result | 8 (44%) |
| Decided against genetic testing | 3 (17%) |
| Cancer predisposition that was tested for | |
| Lynch syndrome | 2 (11%) |
| Hereditary breast/ovarian cancer | 16 (89%) |
| Participants that had blood-related children | 12 (67%) |
| Participants that were affected by cancer at the moment of genetic counselling | 6 (33%) |
| Participants that had a known mutation in the family at the moment of genetic counselling | 5 (28%) |
| Mean interview duration (min) | 59 (27–101) |
The theory
How at-risk individuals decided regarding genetic testing for cancer predispositions was a reflection of their general life philosophy. Those who preferred to control their life tended to have a positive attitude towards genetic testing. For them, knowledge created certainty and thus gave them a sense of control over their lives, both in the present and in the future. Hence, testing was important and powerful to them. Some of these individuals found it difficult to understand when others decided against testing (see Table 2 for quotation Q1). In contrast, other at-risk individuals preferred to leave their future to fate. They felt more comfortable with living in the present rather than dealing with possible future scenarios and did not wish to gain certainty through genetic knowledge. They tended to reject genetic testing (Q2). We theorise that these two positions are the two extremes of what we term the control-fate continuum. There might be numerous positions within this continuum and we do not claim that we can represent all possible positions. Instead, we provide seven examples that illustrate positions that we identified from our interview data (Box 1). We also identified psychosocial factors influencing the position on the control-fate continuum, and thus the genetic testing decision-making process (Fig. 1).
Table 2.
Quotes supporting the results.
| Code | Quote |
|---|---|
| Q1 | “If they hadn’t done the test, we would only speculate, do I have it, do I not have it, and eh, well, in that case, that’s when fear would actually be present. And now, it’s just normal […] and I, I think knowledge is always important.” (Nick, 50+, healthy carrier) |
| Q2 | “So now it’s like a bit… now I can say, well it’s fate, maybe I will get it [cancer], maybe not. Ehm, I don’t have it in my hands, it is as it is then. And if I tested myself, yes then I’d have a little, yes… I’d be a little more in charge again, although I perhaps couldn’t change anything but… um, nevertheless, I knew it…” (Lea, 30+, untested) |
| Q3 | “[…] I prefer to be informed, I prefer to know if there is something […]. Once I know that there might be something, once I’m told, do you want to know if there is a mutation on that gene, I could no longer say no I don’t want to know.” (Claire, affected non-carrier) |
| Q4 | “Research in the genetic field will be massively improved in the coming years. Well, probably. […] Maybe there will be much better answers in 5, 10 years if you do such a genetic examination because you can take in many more factors, many more influencing factors… And then there will probably be a better answer. And as soon as there is a better answer, I will probably reconsider it [genetic testing].” (Doris, 40+, untested) |
| Q5 | “I won’t do the genetic test. [Because, after the genetic consultation], I thought again about the different aspects of the conversation. What are the results I could really get, specifically, from this genetic test. And namely, results with 100% certainty, so I thought about the certainty the test could provide me with. Or does it give me more uncertainty […] Would it really give me watertight answers. There might be other influencing factors, too. So regarding breast cancer, I couldn’t get it off my mind after all, even if I had a negative genetic test result.” (Doris, 40+, untested) |
| Q6 | “I then got counselling and listened to it all and thought about it, but then I decided against testing at that point. Because of my age. I was still younger than 40 at that time, and I just consciously wanted to take the time, wait until I was 40 and then I can bear the consequences of the test, and until then I just don’t want to know. So I’ve been really, I’ve received good counselling about what are the consequences, what are the advantages, the disadvantages, of this test.” (Daria, 40+, non-carrier) |
| Q7 | “My main consideration […] is how the awareness [of knowing a genetic disease risk] would affect my decisions in life, actually. […] If having the mutation affects my decisions because of a certain sense of responsibility or something, which I would not do of my own free will.” (Aaron, 20+, carrier) |
| Q8 | “Had I been a carrier, I’d have had the surgeries. […] That’s what I’d have done. If there is no such consequence for people I think them doing the test isn’t so important, but for me, that would have been an obvious consequence.” (Silvia, 30+, non-carrier) |
| Q9 | “So I think they [her two daughters] got tested simply because I said so, I think they didn’t know what it meant at the beginning. Because everything was going so fast… I think I overwhelmed them a little bit, to be honest. Well, I don’t regret that, I think it’s important. I also think it’s important that they just watch it afterwards. And are made aware of it.” (Hanna, 50+, affected carrier) |
| Q10 | “I was talking to my mother during the testing process, and otherwise I didn’t really need anybody… I knew what I did, I knew what I was getting into, I knew… what the consequences would be, and so on. Yes.” (Marissa, 50+, healthy) |
| Q11 | “Well, the human psychological aspect, […] I didn’t think that it would have such an impact, I wouldn’t have thought of that, suddenly it came. Over time, when I started to think a little bit.” (Erich, 60+, non-carrier) |
| Q12 | “I pay attention [to protecting my genetic data], yes, but it’s not like it has greatly influenced my decision to do genetic testing.” (Aaron, 20+, carrier) |
| Q13 | “At Cancer Aid, they told me that I would have difficulties insuring myself privately or semi-privately, that health insurance would refuse me because of the carrier test result. Yes, and that was in my head, and then I thought well, I try it anyway, and then, after the genetic consultation it was actually clear to me how to do it. I also asked for the legal text, that was sent to me by the geneticist… And then I called the health insurance company and said that I would like to change my insurance, that I had this problem. Eh, saying that there was the no-discrimination act, sending them all the information. It took about three weeks, and then they agreed, without reservation, without anything.” (Nick, 50+, healthy carrier) |
| Q14 | “But getting this information when you can’t do anything about it anyway. I think I… I don’t want this. Even though I agreed [to genetic testing]. That only occurred to me later. Interviewer: And why did you accept [genetic testing]? Participant: I don’t know! I usually decide spontaneously and think about it afterwards […] Interviewer: And did you consider stopping the process of testing? Participant: No. At home, my wife and daughter have really (laughs) taken sides and said, listen, it is not just for you. It’s for your daughter as well.” (Erich, 60+, non-carrier) |
| Q15 | “Em, but… [if I hadn’t done the genetic test] it would have been a bit like, I couldn’t have taken part in certain things because… If I hadn’t done it, I wouldn’t have known, am I positive or not? […] That’s why it’s actually, yes… I’ve actually wanted to know… uh… yes, how is it for me now, so that I simply know, am I in their group or am I, am I outside.” (Eva, 40+, healthy carrier) |
| Q16 | “I for myself, if my daughter hadn’t asked me because she wanted to know that, I would not have done counselling in that sense. That’s, really, first and foremost for my daughters.” (Emily, 60+, non-carrier) |
| Q17 | “It wouldn’t have left me alone to be told it [genetic testing] would be recommended […] Then I’d preferred not to be told at all, see, now that is coming up again! If they wouldn’t have told me anything, just said, well, we have taken out your cancer now, […] now do these check-ups every five years - have a nice day. That would have been fine for me, yes, that would have been fine with me that alternative that they don’t tell me about genetic testing…” (Erich, 60+, non-carrier) |
| Q18 | “For me, it’s simply a derivation of fate - not fate, but human… needs, so if anything happens and… one lives shorter, or someone else could have protected himself and could not do it, that is careless towards their environment, in my opinion. If my sister died early, and her child lost her mother early, that would not be so cool.” (Rolf, 50+, carrier) |
| Q19 | “Well, for one [sister], it was simply because of the economic expense. If she goes to the gynaecologist twice a year and hasn’t clarified the source of the problem beforehand then she does something useless. This is unnecessary and simply burdens the economy.” (Rolf, 50+, carrier) |
| Q20 | “Yeah, well, if the doctor tells me anything, I’ll do it anyway, won’t I?” (Gloria, 60+) |
| Q21 | “The geneticist knew that I came from the medical field, and then it often happens, you notice that people say, ah you already know that anyway, but he still did it very professionally and as if I was a layperson, I liked that.” (Silvia, 30+, non-carrier) |
| Q22 | “Well, I’d be cautious… to discuss something like this with others. Because […] I would get into a defensive position. […] To defend myself would not be so pleasant. Yes, I decided that way, that’s the way it is, and I wouldn’t want to defend it now.” (Doris, 40+, untested) |
| Q23 | “Well, in the first moment I was completely against it, I didn’t even want to know anything about it, I didn’t even want to think about it. And only after a certain amount of time I remembered it again, when the gynaecologist asked me about it, and then it went around in my head a bit. I also weighed up the advantages and disadvantages, what would happen if I would, or what would it mean, what would be the consequences, and then, yes, no, I don’t want to… And what would be the reasons why I might do it after all… It has been a bit of a back and forth, but the tendency has always been more towards I don’t want to know it.” (Lea, 30+, untested) |
| Q24 | “Yes, I think I already made the decision before the counselling session… And, I don’t know what they could have told me that would have changed my opinion.” (Lea, 30+, untested) |
| Q25 | “Well, in the end, it doesn’t matter what the percentages are… Either I get it or I don’t. Somehow it is always 50:50.” (Daria, 40+, non-carrier) |
| Q26 | “With a genetic cancer predisposition, I feel like, it’s fate somehow, you just wait for the moment it strikes, and no matter what I do… it is predetermined. Yes.” (Lea, 30+, untested) |
| Q27 | “Internally, I anyway expected that it was just a strong family history. If I had feared a genetic cause, I would probably have reacted earlier.” Interviewer: “So you didn’t expect the detection of a genetic cause…” Participant: “Yeah, or… it was more towards the direction that they would not find anything. But that doesn’t necessarily mean that is the case, some perceptions are just repressed easily, aren’t they?” (Emily, 60+, non-carrier) |
| Q28 | “Rationally, the probability of a finding was not very high […] I saw it black on white when going through the pedigree [with the counselling physician]. I’d say I have a big family […] and there are some cases of cancer that alerted me at first, but the majority is healthy. And that is why… Yes, the probability [to detect a function-affecting genetic variant] was reduced due to the many healthy people in my family.” (Doris, 40+, untested) |
| Q29 | “But, so for me, it was clear that there had to be something genetic because… Well, we had to find a cause. […] Well, once I got these results… I could hardly believe it, I was eh, yes, so eh, well, there were no mutations, I, I was really… But I immediately, immediately it came back to me, but, but… what is it then? So there’s another one, there must be something else there!” (Claire, affected non-carrier) |
All names are pseudonyms. The quotes are referred to in the text by the codes in the left column. “Carrier” means being positively tested for a function-affecting genetic variant; “non-carrier” refers to a test result of non-pathogenic variant or a variant of uncertain significance. “Untested” means the person decided against genetic testing. The participants referred to a function-altering genetic variant when using the term “mutation”.
Fig. 1. Model for decision-making regarding genetic testing for cancer predisposition and nonactionable diseases in relation to life philosophy and psychosocial factors.
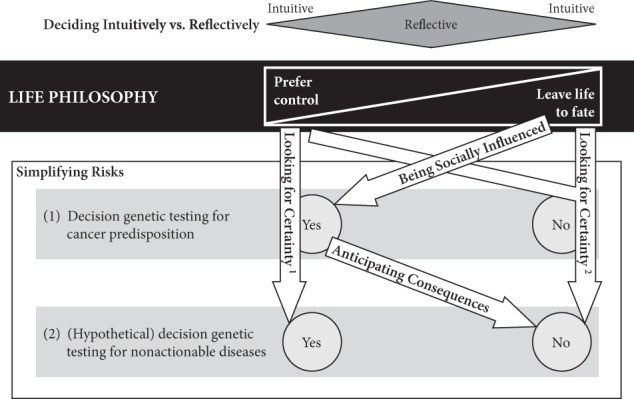
1Perception that genetic testing reduces uncertainty; 2perception that genetic testing increases uncertainty.
Psychosocial factors influencing decision-making
Looking for certainty
At-risk individuals were looking for certainty when deciding whether to take genetic testing. The outcome of the decision depended on their life philosophy. On the one hand, for those who preferred control, genetic testing reduced uncertainty because it provided facts and the possibility to make informed life decisions (example 1, Q3). They were sure that knowing their genetic risk would be beneficial and expressed confidence in scientific progress. On the other hand, genetic testing increased uncertainty for those leaving life to fate (examples 4 and 7). Because the genetic test result only provided disease probabilities, it did not provide certainty. Genetic testing was not considered meaningful as long as scientific knowledge is incomplete (Q4). Moreover, some individuals explained that life was full of uncertainties and risks; therefore, they preferred to live in the present rather than in the unforeseeable future (Q5).
Anticipating consequences
Some at-risk individuals strongly focussed on the consequences of testing. They considered only genetic testing for cancer predisposition beneficial and would not take genetic testing for nonactionable diseases (examples 2 and 3). Therefore, their position on the control-fate continuum is somewhat to the right of those who prefer control in any situation.
Having children and being affected by cancer tended to strengthen at-risk individuals’ focus on consequences. Some cancer patients perceived the testing procedure as intimidating and wanted to postpone it until after cancer treatment because they did not see immediate medical consequences. Moreover, young individuals perceived genetic testing as less urgent, and some postponed genetic testing until they reached the age when their healthcare professionals recommended preventive measures (Q6). Finally, some participants stated that they would refrain from testing if the results would determine their life too much (example 3, Q7).
Four levels of consequences that at-risk individuals anticipated were identified: medical, familial, psychological and discriminatory consequences. First, some individuals based their decision on the potential for medical consequences (examples 2 and 3). These individuals proactively thought about the integration of the test result and its consequences in their life and had already decided on what preventive measures they would take if they were risk variant carriers (Q8). While some perceived frequent screening as a sufficient and satisfying measure, others needed the option of risk-reducing preventive surgery or medication to be available in order to take the test. This might have helped them rationalise and structure their decision-making process, thus reducing uncertainty.
Second, some at-risk individuals strongly anticipated consequences for their family and considered this the main reason for genetic testing (example 5). While some of them accepted that some relatives might exercise their right not to know, others did not reflect on this aspect and motivated their relatives to get genetic testing without further consideration (Q9).
Third, most at-risk individuals anticipated adverse psychological reactions, as discussed in genetic counselling. This anticipation helped them overcome feelings of anxiety and uncertainty (Q10). Still, one participant failed to anticipate psychological consequences and started to brood afterwards (Q11). Fourth, discriminatory consequences were anticipated by some few individuals. They were careful about sharing their test results because they were aware of possible discrimination by employers or private insurance (in Switzerland basic health insurance is obligatory and insurance companies cannot refuse any patients; however, this is not the case for private and semi-private insurance [20]). Still, participants did not refuse genetic testing for cancer predispositions out of privacy concerns (Q12). One interviewee even described a positive experience with his health insurance company (Q13).
Being socially influenced
A sense of duty and social influences from family, healthcare professionals and society left some at-risk individuals with no real alternative but to test even if doing so was against their life philosophy (examples 5 and 6, Q14). First, family considerations had an influence (example 5): some at-risk individuals felt that they would be excluded from their families without genetic testing (Q15). Others felt a duty towards their offspring and other family members despite their sceptical attitude towards genetic testing (Q16). Because of this feeling of obligation, one participant even wished he had never heard about the possibility of testing (Q17). Some at-risk individuals perceived not doing genetic testing as reckless, because to them genetic testing implied taking responsibility for themselves and their social environment (Q18). Some also considered that their genetic test would reduce healthcare costs, which made them advocate the test to family members (Q19).
Second, despite the non-directiveness of genetic counselling, some affected individuals followed the advice of their counselling healthcare professional to be tested (example 6). That happened particularly in the context of a newly diagnosed cancer, where genetic testing might provide therapy-relevant information. The extent to which medical doctors influenced at-risk individuals in their decision-making process depended on their relationships as well as on the at-risk individuals’ general attitudes towards doctors (Q20). One participant stressed that it was important to her to receive genetic counselling that was not influenced by her prior knowledge on the topic (Q21).
Third, we observed various societal influences. For instance, those who had decided against testing did not like to broadcast this decision because they did not want to justify their decision (Q22). By doing a genetic test, “one has done everything one can” (Doris, healthy, untested), which they felt was easier for others to understand. Moreover, online communities, such as Facebook groups or forums, provided another form of external influence. They had variable effects on at-risk individuals, some felt encouraged, others rather scared when reading how carriers dealt with their increased cancer risk. Some were emotionally neutral and used test results as an additional information source in their decision-making process.
Deciding intuitively vs reflectively
Individuals at both extremes of the control-fate continuum tended to decide quickly and intuitively: testing was naturally good for those who gained control through it (example 1), and those who preferred to leave their future life to fate rejected genetic testing equally intuitively (example 7). In addition, those who did testing mainly for their family or on their doctor’s advice intuitively outsourced their decision to these third parties (examples 5 and 6; see also “Being socially influenced”). By contrast, those in the middle of the continuum tended to reflect upon genetic testing intensively, weighing pros and cons. Their decision depended on the circumstances of testing (examples 3 and 4). One participant rejected genetic testing intuitively at first but made a more reflective decision later on, still choosing not to take the test (Q23).
Genetic counselling was vital for a reflective decision-making process (Q6). However, some individuals had already made their decision upfront and thus did not include the content of the genetic consultation in their decision (Q24). Some of them attended the genetic consultation because it was required to get the test. They admitted that it was interesting to get detailed information about the testing procedure as well as the implications and consequences of the test.
Simplifying risks
Because of the complexity of genetic risks, simplification was crucial for an effective decision-making process. While all at-risk individuals simplified risks, they did so to different extents. Some individuals simplified risks and probabilities by breaking them down into a black-and-white picture, thus reinterpreting uncertainty (Q25). Consequently, not finding a function-affecting genetic variant sometimes led to the misconception that the risk of cancer was zero. In contrast, one individual felt that being a carrier of a function-affecting genetic variant would inevitably lead to cancer, even if in reality, the penetrance is not 100% (Q26). She thus rejected genetic testing due to fear.
Another simplification concerned the anticipation of the test result. Some individuals made an intuitive estimation (Q27); others received a risk calculation during genetic counselling (Q28). Those who thought their risk of being a carrier was high tended to decide clearly and intuitively (Q29). Individuals who thought they were probably not carrying a function-affecting genetic variant tended to scrutinise the meaningfulness of genetic testing and tended to reflect more on their decision (see also “Deciding intuitively vs reflectively”).
Box 1. Examples of positions in the control-fate continuum. The numbers of the examples correspond to the numbers in Fig. 1.
|
Example 1: Genetic testing provides me with a unique chance to take control of my life Facts are very important to me. They give me a sense of certainty, a sense of control over my life. Therefore, genetic testing for inheritable cancer risk was self-evident for me. If I am at risk for a disease, I really want to know, I need to know, even if there were no preventive measures available. To me, knowledge is useful as such, even if there is no way to act. I often find it difficult to understand why others in my family do not want to be tested. I think this is irresponsible, especially when they have children. I also strongly believe in scientific progress. It provides me with a lot of hope to learn about recent advances, and I am optimistic that there will be much more progress within the next ten years. |
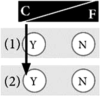
|
|
Example 2: Genetic testing is useful, but only if there is some action I could take Deciding for genetic testing for inheritable cancer risk has been easy for me. I know that cancer runs in my family, and because there are well-established preventive measures, such as screenings or surgeries, I think it is a logical consequence that I perform this test. It might also help my family members. By knowing my genetic cancer risk to be elevated, I am sure that I can prevent myself from getting sick as my mother or father did. However, I would not want to know my genetic fate if there is nothing I can do to prevent it. I do not see the benefits in that case; why should I want to know that? It would only haunt me and make me all nervous and anxious. I do not want a genetic test determining my life in such a way. |
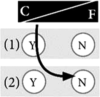
|
|
Example 3: I want genetic testing exclusively if there are immediate consequences for my health strategy Even though I think genetic testing is useful and a necessity for my health, I will wait to get tested until preventive measures are relevant for me. Doing genetic testing too early, before preventive interventions are recommended, would only worry me unnecessarily. Likewise, genetic testing for nonactionable diseases is not an option for me. If there are no direct consequences, I would rather not want to know my genetic risk. However, if I would find out I had an increased risk for cancer, preventive surgery, if applicable, would be mandatory for me. |
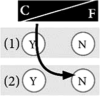
|
|
Example 4: A genetic test has to provide me with 100% certainty I generally think knowledge is useful and helpful for me to handle life, but a genetic test for inheritable cancer risk does not provide me with enough certainty. If the genetic test reveals that I am carrying a function-affecting genetic variant, there is still a good chance that I might not get sick at all. If the test result is negative, it does not mean that I will stay healthy, either. I thus refuse to be tested. If scientific progress led to better tests in the future, I would reconsider my decision and rethink it. Until then, I have enough preventive screenings to feel safe. I do not worry too much. If it gave me 100% certainty, I would even do a genetic test for a disease without preventive measures, because then I know, and feel in better control over my life. |
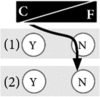
|
|
Example 5: I do genetic testing for my children, not for myself Genetic testing is not for me. I know we have cancer running in our family, but I’d rather not know my exact genetic risk. However, I do it for my children, because they are young and still have the chance to prevent cancer. Thus, I intuitively decided to go for genetic testing. If they wish, I would even consider doing a test for unpreventable diseases, although I find this a difficult decision. |
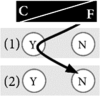
|
|
Example 6: I do genetic testing because others convinced me that it is useful for me On my own initiative, I would not have done genetic testing for inheritable cancer risk. However, my health professionals and my relatives said that it would be useful and important for me to do it. That is mainly why I agreed to do testing. Having done testing does not bother me, I feel good with my decision. However, I would not undergo genetic testing for nonactionable diseases. I do not see the usefulness of this kind of testing; I think learning about a pathogenic genetic variant would bother me too much in that case. |
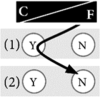
|
|
Example 7: I do not want to know my genetic risk; I will leave my health-related future to fate I know cancer is running in my family, but I do not want to know my genetic risk. I prefer leaving my future life to fate, to live in the present. It is like a gamble: the risk of losing is high, and I fear I could not handle it. Genetic testing cannot provide me with any certainty, and knowing about an elevated genetic risk would only make me miserable. At the same time, learning about a negative test result would not provide me with enough relief to take that risk. |
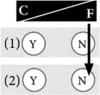
|
In order to illustrate the individuality of the positions, we describe the examples as subjective narratives, which are interpretative summaries of what our participants reported in the interviews. These are the words of the authors and do not correspond to quotes.
Discussion
Our findings illustrate how people undergoing genetic counselling for cancer predisposition in Switzerland perceive the decision-making process for genetic testing. The study provides new findings of decision-making by individuals at risk for hereditary cancer in continental Europe. Our results help healthcare professionals and at-risk individuals to structure the decision-making process. We propose that at-risk individuals position themselves on the control-fate continuum, which derives of their general life philosophy. Medical doctors and genetic counsellors should understand and support individuals in this process. The position on the control-fate continuum is an essential factor for genetic testing decision-making. Those who prefer a control approach to life might benefit from genetic testing, even if the genetic variant that is tested for is not actionable [24]. However, they might also overestimate genetic testing outcomes and feel determined to do genetic testing once it is offered. In contrast, those leaving their future life to fate might refuse genetic testing decisively and be better off without it, even if preventative measures could reduce their disease risk. Several psychosocial factors influence this underlying aspect of life philosophy and thus the decision-making process.
Our findings connect to other studies of decision-making for genetic testing for cancer predisposition, in particular the psychosocial factors identified. First, it has been shown that people are looking for certainty when they do genetic testing [25]. Our findings suggest that the nature and level of certainty at-risk individuals attribute to genetic testing largely depend on their life philosophy. Second, people tend to anticipate consequences more when receiving genetic counselling. In settings without genetic counselling, many people do not anticipate the consequences of genetic testing [26].
Moreover, simplifying risks due to misinterpretation, over- or underestimation is part of human psychology [27] and is thus commonly studied in the context of genetic testing [28–30]. In addition, while intuitive decision makers need genetic counselling mainly for gathering more facts or because it is mandatory to get the genetic test, reflective decision makers might need more psychosocial support and more time to reflect the pros and cons of genetic testing [31, 32]. The social influence on decision-making leads back to the question of whether individuals have a real choice regarding genetic testing. Previous studies also conclude that some people agree to genetic testing out of a feeling of responsibility towards their relatives [4, 33]. While our theory represents this position as well, we suggest that individuals’ life philosophy dominates this motive at the two extremes of the control-fate continuum.
Our theory also accounts for the reasons of those who decline genetic testing. Previous studies showed that even though some decliners do so unreflectively out of fear or lack of knowledge, many refuse genetic testing consciously because they do not see benefits for themselves [11, 34, 35]. We suggest that genetic testing contradicts the life philosophy of some individuals. It is thus essential to counsel those deciding for genetic testing regarding how to inform these family members about the intention of being tested and test results. At-risk individuals have expressed difficulties with uninterested or resistant family members in previous studies [36–38], and our theory helps to explain why different life philosophies can lead to difficulties in communication because those in need of control have difficulties in understanding why others might not want to learn about their genetic risk.
The scope of the control-fate continuum theory covers genetic testing in both healthy and affected individuals provided that genetic testing has predictive components for some disease a person has not developed (yet). For instance, a breast cancer patient with a pathogenic BRCA variant still has an increased risk of ovarian cancer or bilateral breast cancer.
To our knowledge, this is the first study regarding the genetic testing decision-making process from the German-speaking region, which provides a different cultural setting from that of English-speaking countries, which are predominantly studied. Our interviewees were rarely worried about discrimination and did not take this variable into account in their decision-making process, as opposed to previous studies in other regions [39]. However, we acknowledge a possible bias in the sense that those worried regarding discrimination might have refrained from participating in this study. More research is needed to explore this potential cultural difference further.
Clinical implications
Our findings have implications for genetic counselling. It might be useful to discuss individuals’ life philosophy and highlight that these might differ from those of their family members. Our framework can thus be used to support decision-making, anticipate potential conflicts within families and, potentially, helps to understand the reasoning of those declining genetic testing. The finding that some make their decisions intuitively while others need more support for thorough reflection indicates that tailoring genetic counselling resources to actual information needs might be useful. This becomes especially relevant in the context of limited counselling resources and increasing demand for genetic testing. However, more investigations, including quantitative studies, are necessary to assess the appropriateness and consequences of such prioritisation regarding autonomous decision-making.
Limitations
This is a qualitative study with 18 at-risk individuals and two geneticists, and while this is a suitable number to get meaningful qualitative results [40], we propose using our theory to build hypotheses and to test them in more extensive studies. Despite actively searching for 18 months, we could only recruit three genetic testing decliners. Thus, we probably did not reach theoretical saturation for this subgroup. For ethical reasons, at-risk individuals had to contact us proactively in most cases, which gives a bias towards proactive and communicative people interested in genetic testing. Another possible bias results from the recruitment by the counselling doctors, as they might have preferred counsellees with characteristics other than the inclusion criteria. Moreover, qualitative analyses require interpretation, but we minimised personal interpretation bias by frequent team discussions, continuous personal reflection, constant comparison and other methods suggested for the grounded theory approach [21].
Conclusion
Our study illustrates the enormous interpersonal variability of the genetic testing decision-making process. Professional counselling is a way to account for this variability and ensure proper support in the complex decision-making process. The control-fate continuum can help medical geneticists and genetic counsellors to consider this interpersonal variability and provide counselling that respects and anticipates people’s wishes and needs so that counsellees can make informed choices. It also helps their counselling in terms of information dissemination to family members by learning about potential differences in life philosophy, interest and decision-making regarding genetic testing. Moreover, our findings help future research studies for hypothesis testing and result quantification. Because genetic testing is becoming more common, stratifying people regarding their information needs and decision-making strategy helps to rationalise genetic counselling in the future, by saving resources and adapting healthcare services to the needs of those at risk for hereditary cancer.
Supplementary information
Acknowledgements
We acknowledge the contribution of Dr med. Manuela Ragablio and Dr med. Nicole Bürki in patient recruitment and study design.
Funding
This study was financed by the Institute for Biomedical Ethics, University of Basel.
Compliance with ethical standards
Conflict of interest
LK is employed at the University Hospital Bern, and KH is employed at the University Hospital Basel. Both recruited study participants and provided genetic counselling for some of them. They did not have access to the original interviews or transcripts to avoid any conflict of interest in result interpretation. Instead, they were involved in study design and the interpretation of the results. The other authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41431-020-0602-3) contains supplementary material, which is available to authorised users.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Castilla LH, Couch FJ, Erdos MR, Hoskins KF, Calzone K, Garber JE, et al. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994;8:387–91. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- 3.Skirton H, Goldsmith L, Jackson L, Tibben A. Quality in genetic counselling for presymptomatic testing—clinical guidelines for practice across the range of genetic conditions. EJHG. 2013;21:256–60. doi: 10.1038/ejhg.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etchegary H, Miller F, deLaat S, Wilson BJ, Carroll JC, Cappelli M. Decision-making about inherited cancer risk: exploring dimensions of genetic responsibility. J Genet Couns. 2009;18:252–64. doi: 10.1007/s10897-009-9218-z. [DOI] [PubMed] [Google Scholar]
- 5.Hallowell N, Ardern-Jones A, Eeles R, Foster C, Lucassen A, Moynihan C, et al. Communication about genetic testing in families of male BRCA1/2 carriers and non-carriers: patterns, priorities and problems. Clin Genet. 2005;67:492–502. doi: 10.1111/j.1399-0004.2005.00443.x. [DOI] [PubMed] [Google Scholar]
- 6.Claes E, Denayer L, Evers-Kiebooms G, Boogaerts A, Legius E. Predictive testing for hereditary non-polyposis colorectal cancer: Motivation, illness representations and short-term psychological impact. Patient Educ Couns. 2004;55:265–74. doi: 10.1016/j.pec.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Foster C, Watson M, Moynihan C, Ardern-Jones A, Eeles R. Genetic testing for breast and ovarian cancer predisposition: cancer burden and responsibility. J Health Psychol. 2002;7:469–84. doi: 10.1177/1359105302007004627. [DOI] [PubMed] [Google Scholar]
- 8.Godino L, Jackson L, Turchetti D, Hennessy C, Skirton H. Decision making and experiences of young adults undergoing presymptomatic genetic testing for familial cancer: a longitudinal grounded theory study. EJHG. 2018;26:44–53. doi: 10.1038/s41431-017-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battistuzzi L, Franiuk M, Kasparian NA, Rania N, Migliorini L, Varesco L. A qualitative study on decision-making about BRCA1/2 testing in Italian women. Eur J Cancer Care. 2019;28:e13083. doi: 10.1111/ecc.13083. [DOI] [PubMed] [Google Scholar]
- 10.Kanga-Parabia A, Gaff C, Flander L, Jenkins M, Keogh LA. Discussions about predictive genetic testing for Lynch syndrome: the role of health professionals and families in decisions to decline. Fam Cancer. 2018;17:547–55. doi: 10.1007/s10689-018-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keogh LA, Niven H, Rutstein A, Flander L, Gaff C, Jenkins M. Choosing not to undergo predictive genetic testing for hereditary colorectal cancer syndromes: expanding our understanding of decliners and declining. J Behav Med. 2017;40:583–94. doi: 10.1007/s10865-016-9820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlich-Bakker KJ, Kroode HFJ, ten, Wárlám-Rodenhuis CC, van den Bout J, Ausems MG. Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genet Med. 2007;9:766–77. doi: 10.1097/GIM.0b013e318159a318. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RM, Lewis CL, Pignone MP, Couper MP, Barry MJ, Elmore JG, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Mak. 2010;30(Suppl 5):53S–64S. doi: 10.1177/0272989X10378701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalokairinou L, Howard HC, Slokenberga S, Fisher E, Flatscher-Thöni M, Hartlev M, et al. Legislation of direct-to-consumer genetic testing in Europe: a fragmented regulatory landscape. J Community Genet. 2018;9:117–32. doi: 10.1007/s12687-017-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soini S. Genetic testing legislation in Western Europe-a fluctuating regulatory target. J Community Genet. 2012;3:143–53. doi: 10.1007/s12687-012-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper J, Geraedts J, Borry P, Cornel MC, Dondorp WJ, Gianaroli L, et al. Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Hum Reprod. 2014;29:1603–9. doi: 10.1093/humrep/deu130. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann BM, Aebi N, Kolb S, Shaw D, Elger BS. Content, evaluations and influences in newspaper coverage of predictive genetic testing: a comparative media content analysis from the United Kingdom and Switzerland. Public Underst Sci. 2019;28:256–74. doi: 10.1177/0963662518816014. [DOI] [PubMed] [Google Scholar]
- 18.Press N, Fishman JR, Koenig BA. Collective fear, individualized risk: The social and cultural context of genetic testing for breast cancer. Nurs Ethics. 2000;7:237–49. doi: 10.1177/096973300000700306. [DOI] [PubMed] [Google Scholar]
- 19.Binetti G, Benussi L, Roberts JS, Villa A, Pasqualetti P, Sheu C-F, et al. Areas of intervention for genetic counselling of dementia: cross-cultural comparison between Italians and Americans. Patient Educ Couns. 2006;64:285–93. doi: 10.1016/j.pec.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Swiss Federal Council. CC 810.12 Federal Act of 8 October 2004 on Human Genetic Testing (HGTA). Swiss Federal Council; 2004. https://www.admin.ch/opc/en/classified-compilation/20011087/index.html.
- 21.Strauss A, Corbin JM. Basics of qualitative research: grounded theory procedures and techniques. Newbury Park: Calif: SAGE Publications; 1990. [Google Scholar]
- 22.Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. London: SAGE Publications; 2006. [Google Scholar]
- 23.Annells M. Grounded theory method: philosophical perspectives, paradigm of inquiry, and postmodernism. Qual Health Res. 1996;6:379–93. doi: 10.1177/104973239600600306. [DOI] [Google Scholar]
- 24.Wiggins S, Whyte P, Huggins M, Adam S, Theilmann J, Bloch M, et al. The psychological consequences of predictive testing for Huntington’s disease. Canadian Collaborative Study of Predictive Testing. N Engl J Med. 1992;327:1401–5. doi: 10.1056/NEJM199211123272001. [DOI] [PubMed] [Google Scholar]
- 25.Vos J, Menko FH, Oosterwijk JC, van Asperen C, Stiggelbout AM, Tibben A. Genetic counseling does not fulfill the counsellees’ need for certainty in hereditary breast/ovarian cancer families: an explorative assessment. Psycho-Oncology. 2013;22:1167–76. doi: 10.1002/pon.3125. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JS, Gornick MC, Carere DA, Uhlmann WR, Ruffin MT, Green RC. Direct-to-consumer genetic testing: user motivations, decision making, and perceived utility of results. Public Health Genom. 2017;20:36–45. doi: 10.1159/000455006. [DOI] [PubMed] [Google Scholar]
- 27.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. In: Chater N, editor. Judgement and decision making. London: SAGE Publications; 2009. pp. 152–79. [Google Scholar]
- 28.Kelly KM, Leventhal H, Andrykowski M, Toppmeyer D, Much J, Dermody J, et al. Using the common sense model to understand perceived cancer risk in individuals testing for BRCA1/2 mutations. Psycho-Oncology. 2005;14:34–48. doi: 10.1002/pon.805. [DOI] [PubMed] [Google Scholar]
- 29.Cicero G, Luca R, de, Dorangricchia P, Lo Coco G, Guarnaccia C, Fanale D, et al. Risk perception and psychological distress in genetic counselling for hereditary breast and/or ovarian cancer. J Genet Couns. 2017;26:999–1007. doi: 10.1007/s10897-017-0072-0. [DOI] [PubMed] [Google Scholar]
- 30.Goltz HH, Bergman M, Goodson P. Explanatory models of genetics and genetic risk among a selected group of students. Front Public Health. 2016;4:111. doi: 10.3389/fpubh.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard AF, Balneaves LG, Bottorff JL, Rodney P. Preserving the self: the process of decision making about hereditary breast cancer and ovarian cancer risk reduction. Qual Health Res. 2011;21:502–19. doi: 10.1177/1049732310387798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAllister M. Predictive genetic testing and beyond: a theory of engagement. J Health Psychol. 2002;7:491–508. doi: 10.1177/1359105302007005628. [DOI] [PubMed] [Google Scholar]
- 33.Hallowell N, Foster C, Eeles R, Ardern-Jones A, Murday V, Watson M. Balancing autonomy and responsibility: the ethics of generating and disclosing genetic information. J Med Ethics. 2003;29:74–9. doi: 10.1136/jme.29.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White VB, Walsh KK, Foss KS, Amacker-North L, Lenarcic S, McNeely L, et al. Genetic testing for hereditary breast cancer: the decision to decline. Am Surg. 2018;84:154–60. [PubMed] [Google Scholar]
- 35.Peters J, Kenen R, Hoskins LM, Koehly LM, Graubard B, Loud JT, et al. Unpacking the blockers: understanding perceptions and social constraints of health communication in hereditary breast ovarian cancer (HBOC) susceptibility families. J Genet Couns. 2011;20:450–64. doi: 10.1007/s10897-011-9370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrest K, Simpson SA, Wilson BJ, van Teijlingen ER, McKee L, Haites N, et al. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64:317–26. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 37.Daly MB, Montgomery S, Bingler R, Ruth K. Communicating genetic test results within the family: is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer. 2016;15:697–706. doi: 10.1007/s10689-016-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chopra I, Kelly KM. Cancer risk information sharing: the experience of individuals receiving genetic counseling for BRCA1/2 mutations. J Health Commun. 2017;22:143–52. doi: 10.1080/10810730.2016.1258743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wauters A, van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet. 2016;61:275–82. doi: 10.1038/jhg.2015.151. [DOI] [PubMed] [Google Scholar]
- 40.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2017;27:591–608. doi: 10.1177/1049732316665344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


