Abstract
Gut microbiota participates in diverse metabolic and homeostatic functions related to health and well-being. Its composition varies between individuals, and depends on factors related to host and microbial communities, which need to adapt to utilize various nutrients present in gut environment. We profiled fecal microbiota in 63 healthy adult individuals using metaproteomics, and focused on microbial CAZy (carbohydrate-active) enzymes involved in glycan foraging. We identified two distinct CAZy profiles, one with many Bacteroides-derived CAZy in more than one-third of subjects (n = 25), and it associated with high abundance of Bacteroides in most subjects. In a smaller subset of donors (n = 8) with dietary parameters similar to others, microbiota showed intense expression of Prevotella-derived CAZy including exo-beta-(1,4)-xylanase, xylan-1,4-beta-xylosidase, alpha-l-arabinofuranosidase and several other CAZy belonging to glycosyl hydrolase families involved in digestion of complex plant-derived polysaccharides. This associated invariably with high abundance of Prevotella in gut microbiota, while in subjects with lower abundance of Prevotella, microbiota showed no Prevotella-derived CAZy. Identification of Bacteroides- and Prevotella-derived CAZy in microbiota proteome and their association with differences in microbiota composition are in evidence of individual variation in metabolic specialization of gut microbes affecting their colonizing competence.
Subject terms: Classification and taxonomy, Databases, Proteome informatics, Computational biology and bioinformatics, Microbial communities, Metabolism
Introduction
Human gut microbiota has evolved to live in commensalism and in beneficial mutualism with the host. It consists of hundreds of microbial species, which collectively function to provide the host protection against pathogens, augment digestion of nutrients, synthesize vitamins and stimulate immune system1. Besides many aspects of health and diseases, variations in its composition associate with differences in dietary preferences and body mass index2,3. A low Bacteroidetes-to-Firmicutes ratio and high prevalence of Faecalibacterium associate with high dietary energy intake and overweight, whereas high representation of Bacteroides-species in microbiota associates with diets high in fat and animal-derived protein content; and although not consistently, according to some studies it associates with a higher risk to develop metabolic disease4. Diets poised towards plant-derived foods often favor Prevotella abundance in microbiota. Much of this variation in microbiota depends on differences in metabolic competence of individual microbes adapted to forage different nutrients available in the gut milieu, namely complex polysaccharides of plant hemicelluloses and pectin, animal-derived glycans and host mucus5.
PUL (polysaccharide utilization loci) are gene clusters organized around susC and susD (starch utilization system) genes in bacteria6. PUL encode multiprotein complexes responsible for binding structurally complex polysaccharides to outer membrane of bacteria and for initiation of their depolymerisation7,8. A single PUL may contain various numbers of enzymes (carbohydrate active enzymes, CAZy) required for initial degradation of plant-derived complex polysaccharides or animal glycans (plant fiber and animal glycan degraders), or degradation of the arising less complex glycans (secondary degraders). Degradation of complex polysaccharides requires many steps and a large number of CAZy, which are often organized to several PUL, as recently characterized for rhamnogalactonuronan II degradation in Bacteroides thetaiotamicron9,10. Species of the genus Prevotella have also been identified to encode similar PULs with glycosyl hydrolases (GH) and other CAZy. Profiling of CAZy genes present in Prevotella-isolates representing 50 different species originally isolated from gut, oral cavity or rumen revealed robust differences in their abilities to degrade animal- and/or plant-derived glycans11. Thus, Prevotella have adapted to degrade glycans from different sources at least on species-level, and apparently depending on their preferred habitat in humans and cattle.
In metagenomic profiling of carbohydrate-active enzymes (CAZy) in gut microbiotas, Prevotella-annotated CAZy genes differed between individuals, and this associated with microbiota response to diet modification12. Although several important aspects of fecal microbiota and gut homeostasis have been addressed by proteome analyses13–17, CAZy in fecal microbiota have so far been analyzed only on a few occasions on genomic12 and transcriptomic18 level. In this study, we studied CAZy in fecal microbiota on whole proteome level by newly designed analysis tools19,20, and related their CAZy expression to their microbiota composition. We found individual differences between donors, as the expression of various CAZy clustered into several groups of CAZy co-occurring more likely together in same samples. We also identified two distinct profiles among members of gut microbiota in association with high or low abundance of Bacteroides in microbiota. Enhanced expression of CAZy participating in metabolism of plant cell-wall –derived glycans in a few donors and its association with high abundance of Prevotella in their microbiota suggests exceptional metabolic competence of Prevotella present in some donors to utilize particular carbohydrates including complex plant-derived glycans.
Results
Gut microbiota proteomes identify proteins derived from various commensals of human gut microbiota
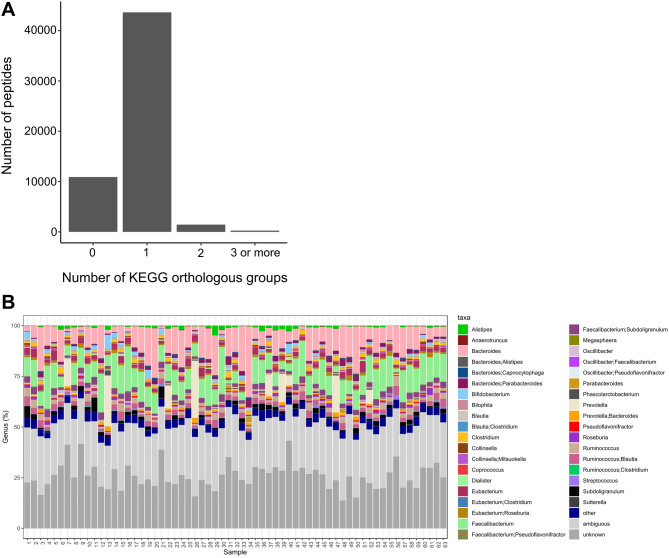
Metaproteomes of gut microbiota extracted from fecal samples were analyzed by mass spectrometry and searched against protein databases. Samples were from a cohort of overweight and obese female donors (n = 63) derived from a dietary intervention study 21 and taken at entry to the study (see Suppl. Table 1 for donor characteristics). A single KEGG orthologous group annotation was assigned to 43,636 peptides (Fig. 1), which represented 1,446 different KEGG orthologous groups. Furthermore, 1,989 peptides annotated to two or more orthologous groups, while 10,901 remained unannotated. The total number of peptides identified ranged from 5,415 to 17,904 peptides per sample (median 11,868 peptides/sample).
Figure 1.
Numbers of functional (A) and taxonomical (B) annotation of the identified peptides. (A) Most of the identified peptides belong to a single orthologous group. (B) At genus level, appr. 20–25% of peptides were unanimously annotated to one bacterial genus, while appr. 40–60% of peptides was shared by two or more genera. Peptides remaining without taxonomic annotation constituted 13.8–43.2% of peptides. (For a particular genus, see color codes).
Genus level taxonomical annotations were assigned to the peptides by utilizing taxonomic annotations of the integrated reference catalog of the human microbiota16. Depending on individual microbiota, one particular genus was assigned to 14.6–27.5% of the peptides. A further 38.5–59.0% of peptides were listed ambiguous between two taxa, reflecting their origin from either of two defined bacterial genera (Fig. 1), while 13.8–43.2% of peptides remained without a taxonomic annotation.
Depending on the donor, three different genera dominated the gut metaproteome. In the majority of donors, most of the genus-specific peptides were annotated either as Bacteroides or Faecalibacterium –specific peptides. A few donors were distinguishable by a high proportion of Prevotella-specific peptides in their microbiota. Genus-specific annotations included also many other genera including Bifidobacterium, Ruminococcus, Alistipes, Clostridium, Collinsella and Parabacteroides (Fig. 1).
Glycoside hydrolases and other bacterial CAZy co-occur in groups according to their bacterial annotations
CAZy enzymes are organized in families of glycoside hydrolases (GH), polysaccharide lyases (PL), carbohydrate esterases (CE) and glycosyl transferases (GT) according to sequence homology with one or more biochemically characterized enzymes. Glycosyl hydrolases are the most diverse group of enzymes, currently divided to 162 families. Correlation analysis of CAZy enzymes distinguished several clusters of glycosyl hydrolases that co-occurred in the samples. Most notable were a cluster formed by Bacteroides-annotated glycosyl hydrolases, and another cluster formed by Prevotella-annotated glycosyl hydrolases (Fig. 2). In addition, a few smaller clusters of glycosyl hydrolases with genus-level annotations were identified, including those annotated to Faecalibacterium, Bifidobacterium and Eubacterium.
Figure 2.
Co-occurrence network of CAZy families of particular bacterial genera in the samples. Bacteroides- and Prevotella-derived glycosyl hydrolases (GH) cluster together in distinct sets of samples. Clusters containing fewer GH enzymes can be identified for Faecalibacterium, Eubacterium and Bifidobacterium- derived enzymes. Each node represents a genus–CAZy family pair, and the edges represent a significant co-occurrence relationship (see text for details). The top 500 correlating pairs were included in the graph.
Abundance of Bacteroides in microbiota associates with expression of Bacteroides-derived CAZy enzymes
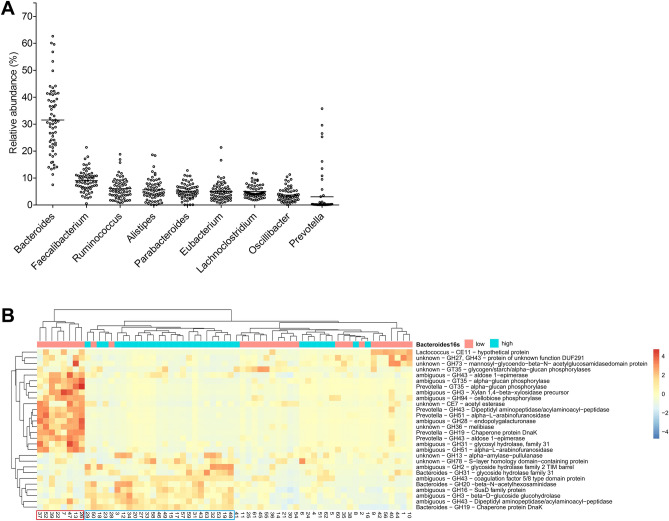
Typical to microbiota in developed countries, Bacteroides was the most abundant genus among our donors. According to 16S-RNA gene sequencing results, Bacteroides-genus constituted between 7 and 62% (median 35%) of the whole microbiota (Fig. 3A). To identify individual CAZy annotations and their potential association with Bacteroides abundance in microbiota, we compared CAZy expression between donors with high abundance (above median) of Bacteroides to donors, in which Bacteroides represented a less prominent proportion of whole microbiota. This comparison identified 25 donors, whose microbiota proteome expressed a number of Bacteroides-derived CAZy enzymes (Fig. 3B and Suppl. Figure 1). This proteome pattern associated with high abundance of Bacteroides in most donors, although not all donors showing high abundance (above median) of Bacteroides in microbiota showed this CAZy pattern either at a false discovery rate (FDR) < 0.01 or < 0.05 (Fig. 3B and Suppl. Figure 1).
Figure 3.
Donors with high abundancy of Bacteroides and donors with high abundance of Prevotella differ in their expression of CAZy. (A) Relative abundance of the most prominent bacterial groups on genus-level according to 16S-RNA parallel sequencing. (B) A heatmap of CAZy enzymes identifies two distinct profiles of CAZy enzyme expression among donors (in vertical columns). CAZy enzymes (horizontal lines) annotated as Bacteroides- or Prevotella-derived (or ambiguous) are expressed at a higher level in two distinct groups of donors (consisting of 25 and 8 donors, demarcated with blue or red frames, respectively). In (A), each dot represents the value of abundance for one donor. Enzymes with a FDR < 0.01 are included in the heatmap.
According to 16S rRNA gene sequencing results, the abundance of Bacteroides in microbiota correlated negatively with several other genera, most strongly with Ruminococcus, Faecalibacterium, and Prevotella, but also to Alistipes and Oscillibacter (not shown). Similar to samples with high Bacteroides abundance, we profiled samples with high abundance of Faecalibacterium as the second most abundant genus in our donors (see Fig. 3A) against all other samples. Although well over one hundred CAZy were differentially expressed between individual samples, donors with high abundance of Faecalibacterium in their microbiota did not present any CAZy profile distinct from other donors (not shown).
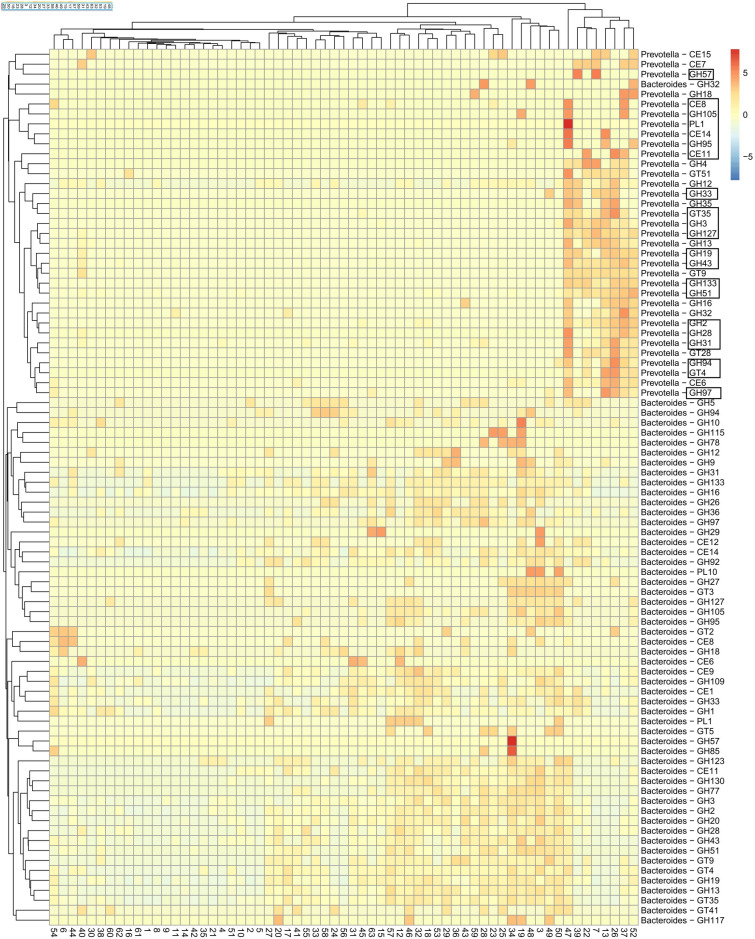
Profiling of donors with Bacteroides-dominated microbiota against other donors made also another CAZy profile apparent. A cluster of CAZy enzymes, most of which were annotated as Prevotella-derived enzymes, was expressed in 8 donors (13% of all donors) more intensely compared to other donors (Fig. 3). We next profiled expression of all CAZy families annotated as Prevotella and Bacteroides to compare CAZy expression between Bacteroides and Prevotella. Without setting any prior selection criteria, we retrieved 88 CAZy families, of which 54 were Bacteroides- and 34 Prevotella -annotated, organizing donors in Bacteroides- and Prevotella clusters as before (Fig. 4). Of these CAZy families, Bacteroides and Prevotella shared only 21 CAZy families, suggesting that Prevotella and Bacteroides express partially distinct enzymes. We then profiled Bacteroides and Prevotella-annotated CAZy on the level of enzyme name annotations, and identified 13 enzymes belonging to these shared 21 CAZy families. Of these 13 enzymes in shared CAZy families, only 3 were the same enzymes, while 10 were different (Suppl. Figure 2). Thus, Prevotella and Bacteroides-CAZy were partially distinct in our donors, suggesting metabolic specialization of Prevotella in individuals with high abundance of Prevotella in their microbiota. Donors with high abundance of Prevotella differed in their microbiota composition (based on 16S rRNA gene parallel sequencing) as well as CAZy enzyme composition compared to other donors (PERMANOVA, p < 0.001 and p < 0.01, respectively) Thus, a unique microbiota composition in these donors associated with a unique CAZy profile (Fig. 5).
Figure 4.
(A) A list of glycosyl hydrolase (GH) and other CAZy families with unambiguous Prevotella or Bacteroides-annotation in the form of a heatmap. Of the 88 families found in this heatmap (horizontal lines), 21 are shared (framed with black for their Prevotella-annotated identifications) and appear in both clusters and groups of donors. Inclusion of CAZy families is based purely on peptide annotations without any profiling of donors.
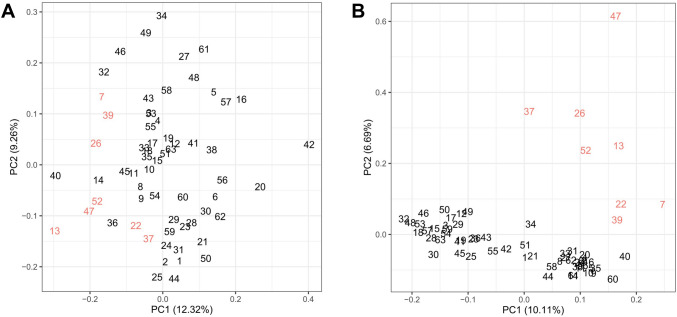
Figure 5.
Prevotella representation in microbiota of study subjects and its relationship to Prevotella-CAZy proteome. (A) Principal component analysis (PCA) of microbiota composition based on 16S-RNA sequencing at genus level and (B) based on intensity-weighed CAZy enzyme expression. In both PCA-plots, donors with high abundance of Prevotella (red) differ significantly from all others (black) according to permutational multivariate analysis of variance (PERMANOVA) (p < 0.001 and p < 0.01, for (A) and (B), respectively). Numbers refer to the number of each donor in heatmaps (Figs. 3B, 4).
Bacteroides and Prevotella CAZy-profiles suggest adaptation to metabolism of different substrates
Enzymes identified in Bacteroides and Prevotella CAZy profiles suggest differences in their substrate specificity. Prevotella CAZy profile contained several enzymes with predicted activities in metabolism of xylan and other complex polysaccharides derived from plants, such as GH51 alpha-l-arabinofuranosidase, GH28-family endopolygalacturonase, GH43 Beta-xylosidase and GH3 Xylan-1,4-beta-xylosidase (Suppl. Figure 1 and Suppl. Figure 2). This profile also included a CAZy belonging to GH13 family involved in digestion of resistant starch and glycogen. Alpha-amylase, alpha-glucosidase and beta-fructofuranosidase identified in this CAZy profile also suggest activities involved in hydrolysis of disaccharides and starch.
Compared with Prevotella CAZy profile, enzymes identified within Bacteroides CAZy profile suggested a different spectrum of specificities. Annotations in the latter included enzymes belonging to glycosyl hydrolase families GH18, GH20 and GH92, which contain enzymes active against animal glycans22, and a SusD family protein belonging to GH16, which contains CAZymes of various substrate specificities (Suppl. Figure 1 and Suppl. Figure 2). Enzymes such as, GH3 beta-d-glucosidase, GH2 beta-galactosidase and aldose-1-epimerase are involved in breakdown of oligo- and disaccharides ().
After identifying a number of CAZy highly expressed in Prevotella-rich microbiotas we performed a pathway analysis for enzymes identified in this enzyme cluster using their KEGG-ortholog codes23–25 and GOMixer pathway analysis (https://www.raeslab.org/omixer/visualisation/map). Accordingly, a number of enzymes in this CAZy cluster are involved in degradation of complex plant-derived polysaccharides and several of them in pectin degradation pathways, including 5-dehydro-4-deoxy-d-glucuronase and endopolygalacturonase (Table 1) and many in xylan degradation pathways. Other degradation pathways in which enzymes of this Prevotella CAZy cluster participate include starch, lactose, galactose and melibiose degradation pathways.
Table 1.
Degradation pathways related to enzymes in the Prevotella CAZy-cluster. KEGG ortholog codes23–25 were analyzed with GOMixer pathway analyzer. See text for details.
| Module ID | Module name | Orthologs |
|---|---|---|
| MF0064 | Pectin degradation | K01051 |
| K01184 | ||
| K01213 | ||
| MF0065 | Pectin degradation-5-dehydro-4-deoxy-d-glucuronate degradation | K01728 |
| K01730 | ||
| K01815 | ||
| K00874 | ||
| K01625 | ||
| MF0091 | Beta-d-glucuronide and d-glucuronate degradation | K00040 |
| K00874 | ||
| K01195 | ||
| K01686 | ||
| K01812 | ||
| MF0071 | d-Galacturonate degradation | K01812 |
| K00041 | ||
| K01685 | ||
| K00874 | ||
| MF0062 | Starch degradation | K01176 |
| K01200 | ||
| K01187 | ||
| MF0047 | Lactose and galactose degradation (PTS) | K02786 + K02787 + K02788 |
| K01220 | ||
| K01819 | ||
| K00917 | ||
| K01635 | ||
| K08302 | ||
| MF0048 | Lactose degradation | K01190 |
| MF0066 | Glycogen metabolism | K00975 |
| K00703 | ||
| K00700 | ||
| K00688 | ||
| K01187 | ||
| K00705 | ||
| MF0056 | Galactose degradation (Leloir pathway) | K01785 |
| K00849 | ||
| K00965 | ||
| K01784 | ||
| K01838 | ||
| MF0050 | Melibiose degradation | K07407 |
| MF0003 | Acetylglucosamine degradation | K00884 |
| K01443 | ||
| K02564 | ||
| MF0093 | Homoacetogenesis (acetate production) | K05299 + K15022 |
| K01938 | ||
| K01491 | ||
| K00297 | ||
| K15023 | ||
| K00191 K00192 + K14138 K00196 + K14138 K00198 + K14138 K03518 + K03519 + K03520 + K14138 | ||
| MF002 | Ethanol production (CO2 pathway) | K01568 |
| K00001 K00121 K04072 K11440 K13953 K13954 |
High abundance of Prevotella does not correlate with increased intake of fibers or other dietary factors
High abundance of Prevotella associates typically with diets rich in plant-derived glycans and dietary fibers. We therefore compared dietary parameters calculated from recorded food diaries. The overall dietary quality26 did not differ between donors displaying Prevotella-CAZy profile and donors not displaying this CAZy profile, yet having intermediate levels of Prevotella in microbiota (P = 0.397). However, when inspecting the individual IDQ (index of diet quality)26 food components reflecting the intake of fiber, the frequency of whole grain intake was in fact lower in individuals with Prevotella-CAZy profile, while there were no differences in frequency of vegetable or fruit consumption between the two groups (Table 2). We also compared energy intake and intake of various dietary components but found no differences in the diet to explain high abundancy of Prevotella in the group with Prevotella-CAZy profile (Suppl. Table 2).
Table 2.
Consumption of whole grains, vegetables, fruits and berries among donors with exceptionally high abundance of Prevotella (and Prevotella-associated CAZy profile, left) and among donors with intermediate levels (and no CAZy profile, right) of Prevotella in their microbiota (median ± interquartile range, IQR). aIndex of diet quality (see text); bthe number of days consumed in a week.
|
Prevotella with CAZy n = 8 |
Prevotella without CAZy n = 7 |
P-value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | P-value/Mann–Whitney U test | |
| IDQa | 9.3 | 6.3–11.8 | 10 | 9.0–13.0 | 0.397 |
| Whole grainsb | 5 | 4.0–6.8 | 7 | 7.0–7.0 | 0.040 |
| Vegetablesb | 6.5 | 6.0–7.0 | 7 | 7.0–7.0 | 0.232 |
| Fruits and berriesb | 5.5 | 5.0–7.0 | 7 | 3.0–7.0 | 0.779 |
Discussion
The knowledge on CAZymes and their role in metabolic activities of gut microbiota is rapidly increasing based on genomic11,12,18, structural6 and biochemical studies10. The CAZy database (https://www.cazy.org) dedicated to compilation of this knowledge makes an important contribution to advance the understanding of the biological significance of carbohydrate-degradation potential in microbiota27,28. To extend the information provided by genomic analyses, these enzymes in microbiota need to be studied also on the level of transcriptome29, proteome and metabolome30, and using biochemical assays31. In this study, we utilized mass spectrometry coupled with a customized set of data analysis tools to characterize the CAZy expression of gut microbiota at proteome level from stool samples from a cohort of overweight (average BMI 30.2) but otherwise healthy females at their early pregnancy21,32.
In CAZy database (https://www.CAZy.org), enzymes participating in degradation of carbohydrates are organized into families of glycosyl hydrolases (GH), polysaccharide lyases (PL) and carbohydrate esterases (CE), in which individual enzymes are defined by sequences that cluster around one or a few biochemically characterized members. Although enzymes within these families vary in their substrate specificities31, the known functions of enzymes within many GH families are often restricted to one or a few types of carbohydrates, thus allowing the prediction of substrate specificity on a general level22,33. The spectral libraries generated from donor microbiotas allowed us to characterize the gut metaproteome to a level sufficient for identification of a number of CAZy and to link many of them to a particular bacterial genus. We identified CAZy across phyla and many bacterial genera. Although the methodological approach of purifying bacteria before protein isolation favors relative yields of cytoplasmic over cell-surface associated proteins34, we chose this approach on the basis of better overall yields of bacteria-associated proteins34. In addition to cytoplasmic CAZy, we identified among Prevotella-annotated CAZy some cell-surface associated proteins including a SusD family CAZy. Many of the CAZy were unanimously identified only on GH family level, but a number of CAZy in Bacteroides and Prevotella CAZy profiles were identified even by their actual enzyme names. According to these annotations, enzymes in both the Bacteroides and Prevotella CAZy profiles were predicted to have activities against complex polysaccharides of either plant cell wall or animal glycan origin6,35, and to some extent, against simpler carbohydrates. Pathway analysis using KEGG orthologs indicated that enzymes in the Prevotella CAZy cluster participated in various carbohydrate degradation pathways, many of them in degradation of pectin. We also identified clusters of CAZy annotated to Firmicutes including Faecalibacterium, but the proteomes did not show a particular CAZy profile analogous to Bacteroides- and Prevotella-CAZy in individuals with high abundance of Faecalibacterium. In spite of overlap of CAZy enzymes in Bacteroides- and Prevotella-CAZy profiles, many of the glycosyl hydrolase (GH) and other CAZy families in heatmaps were distinct, and principal component analysis of CAZy enzyme intensities identified samples with Prevotella-CAZy profile to be unique. This suggests differences between Bacteroides and Prevotella in their utilization of carbohydrate substrates and that Prevotella in these donors were poised towards plant cell wall glycan degradation including xylan degrading enzymes (alpha arabinofuranosidase, xylan 1,4-beta-xylosidase)6,10,22.
Increasing fiber intake in diet promotes changes in microbiota composition in the gut, but not in all individuals36. A recent study demonstrated donor-dependent increase in Prevotella copri in the gut following introduction of barley kernel in the diet, but not in all individuals12. In our donors, the average consumption of fiber did not differ between donors with high abundance of Prevotella and other donors, not even when compared to those with intermediate levels (> 1%) of Prevotella in their microbiota. Their CAZy profile was poised towards enzymes known to participate in foraging of plant-derived glycans and differed from other donors in principal component analysis. This suggests that Prevotella in their gut were particular in CAZy genome5,10,31,35,37 or in the translation of their certain CAZy genes to enzymes. These differences could translate to different levels of success in competition for niche in the gut, allowing their high abundance in gut without a need for particular fiber intake.
Of note, this study consisted of a cohort of pregnant and overweight women21, and as both of these impact on microbiota composition3,38, our results may not be directly generalizable to unselected populations. However, donors with lots of Prevotella in their microbiota showed no bias in age, BMI, duration of pregnancy nor dietary parameters in comparison with other donors. This suggests that the abundance of Prevotella in their microbiota relates either to particular characteristics of their bacteria or to host-related factors not monitored in the cohort.
In summary, by mass-spectrometry based metaproteome analysis we identified two novel CAZy expression profiles in gut microbiota proteome of our donors. These CAZy profiles were mutually exclusive on donor level, included many non-overlapping CAZy enzymes and associated with differences in microbiota composition, suggesting metabolic specialization of individual microbiotas to forage different glycans for energy metabolism. Genomic analyses of Prevotella species isolated from oral cavity and gut have revealed considerable strain-to-strain differences in CAZy gene profiles11, and expression of CAZy genes are also subject to regulation on transcriptional level22,30. Identification of CAZy by metaproteomics provides a possibility to determine metabolic competence of microbiota on a level, which extends metagenomic analyses. Identification of enzyme profiles such as the two reported in this study may pave way to more detailed understanding of the population dynamics of microbiota members.
Materials and methods
Samples and study subjects
Study subjects were overweight or obese but otherwise healthy women in their early pregnancy, and their stool samples were originally collected for an intervention trial published earlier21. Dietary intake was calculated from three-day-food diaries recorded within a week prior to collection of stool sample. The overall dietary quality was measured by the validated index of diet quality (IDQ) questionnaire that reflects adherence to dietary recommendations26. Mean daily intakes of nutrients were calculated using computerized software described earlier21.
Sample preparation for bacterial proteome analyses
For this study, we used parallel stool samples collected at study entry before any intervention. Samples were put at + 4 °C immediately after their collection and an aliquot of the sample was stored at − 80 °C within hours. Thawed fecal material was dissolved in phosphate buffered saline (PBS) at + 4 °C including protease inhibitor (aprotinin) and allowed to dissolve with gentle agitation. Bulk material was removed by spinning the samples at low G force, and supernatant containing bacteria was collected. Bacteria content in the supernatant was determined by in situ labelling of a 16S-RNA consensus sequence to cover the detection of all eubacteria. Bacteria were counted by flow cytometry using a bacterial staining kit (Thermo Fisher) allowing exclusion of dead cells. Following flow cytometry, an aliquot of supernatant containing 108 bacteria was used to prepare each sample. Bacteria were pelleted down and stored as pellets at − 80 °C until protein isolation. Proteins were extracted from pelleted bacteria using NoviPure Microbial Protein kit (MO BIO Laboratories Inc.) according to manufacturer’s instructions. Protease inhibitors (Pierce Protease Inhibitor Tablets, Thermo Scientific) were added to lysis buffer. Mechanical cell lysis was performed by bead-beating using TissueLyser-device (Qiagen) and two 5 min cycles at 50 Hz. Between cycles samples were placed on ice for 5 min. Protein concentrations were determined by DC Lowry (BioRad) method. Fifty microgram proteins were digested by trypsin using filter aided sample preparation (FASP) method39. Peptides were desalted by SepPac C18 96-well plate (Waters), evaporated to dryness and dissolved in 0.1% formic acid. Peptide concentrations were checked with NanoDrop device (Thermo Fisher Scientific), and iRT peptides (Biognosys AG) required for retention time calibration were added to all samples according to manufacturer’s instructions before mass spectrometry (MS) analysis.
Mass spectrometry
Proteins were identified and quantitated using data independent acquisition (DIA) based MS method. A spectral library was created by analyzing seven pooled samples six times with data dependent acquisition method (DDA). All MS analyses were performed on a Q Exactive HF mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a nano-electrospray ionization source and connected to a high performance liquid chromatography (HPLC) system (Easy-nLC1200, Thermo Fisher Scientific).
For DDA analysis 2 µg peptides and for DIA analysis 1 µg peptides were loaded on a C18 column (75 μm × 40 cm, ReproSil-Pur 1.9 μm 120 Å C18-AQ, Dr. Maisch HPLC GmbH, Ammerbuch-Entringen, Germany) with flow rate 200 µl/min. The mobile phase consisted of water with 0.1% formic acid (solvent A), or acetonitrile/water (80:20 (v/v)) with 0.1% formic acid (solvent B). A 75 min gradient from 7 to 25% B, followed by 15 min from 25 to 35% B was used to elute peptides.
MS data were acquired automatically by Thermo Xcalibur 3.1 software (Thermo Fisher Scientific). The DDA method consisted of an Orbitrap MS survey scan of mass range 380–1,200 m/z followed by HCD fragmentation of 20 most intense peptide ions. The DIA MS method covered a mass range from 400 to 1,000 m/z through 40 consecutive windows with isolation width of 15 m/z.
Bacterial proteome analysis
The mass-spectrometry data were analyzed with Diatools software package as described19,20 (Diatools version 1.0 (https://github.com/elolab/diatools) to identify the peptides expressed by fecal microbiota of each donor. The overall protein expression profile was constructed using the integrated gene catalog of the human gut microbiome (IGC)40 covering over 9 million human gut microbiota proteins. The database was further annotated with CAZy family and protein product names by matching corresponding IGC and CAZy database (https://www.cazy.org) sequences with Diamond program41. In total, we identified 5.8% of the IGC sequences as CAZy. Moreover, to focus on bacterial enzymes participating in carbohydrate metabolism, we also searched the mass spectrometry data directly against the CAZy database (https://www.cazy.org).
16S-rRNA gene sequencing
Processing of stool samples for DNA extraction and 16S-rRNA gene sequencing were done as described32. Briefly, primers targeted V3 and V4 regions of the 16S RNA gene and amplicons were sequenced using Illumina platform32. Raw sequences were processed by using the QIIME software package version 1.9.142 (QIIIME: version 1.9.1 (https://qiime.org/). Operational taxonomic units (OTUs) were identified using open-reference OTU picking protocol and chosen at 97% similarity against the Greengenes database (version gg 13 8).
Statistical analyses
The statistical analyses were conducted using R software version 4.0.0. (https://www.R-project.org/). For proteomics, the data was transformed using centered log-ratio transformation (CLR) and differentially expressed peptides between groups (samples with below or above median Bacteroides relative abundance according to 16S rRNA gene sequencing results) were assessed with ROPECA43 using the modified t test with False discovery rate (FDR) cut-off set to 0.01 or 0.05. Heatmaps from the intensities of differentially expressed CAZy enzymes were generated using the Pretty Heatmaps R package. For heatmaps (version 1.0.12; https://CRAN.R-project.org/package=pheatmap), hierarchical clustering of the samples (columns) and the CAZy enzymes (rows) was performed using the euclidean distance metric using the CLR transformed data. For analyzing the co-occurrence of CAZy families of specific bacterial genera in the samples, we used the symmetric modification of the ρ metric utilizing the centered log ratio -transformed data as described43. Similarly, this test was also used for two-parameter comparisons between bacterial abundances derived from the 16S rRNA gene sequencing data.
Principal component analyses (PCA) on microbiota composition and CAZy expression were performed using the prcomp R function on the CLR transformed data. Furthermore, differences in the microbiota composition and CAZy expression between selected donors were assessed by permutational multivariate analysis of variance (PERMANOVA) using the adonis2 function from the vegan R package (https://cran.r-project.org/web/packages/vegan/index.htm). Differences in intake of energy and in dietary variables between donors were analyzed by Mann–Whitney U test.
Ethical considerations
The Ethics Committee of the Hospital District of Southwest Finland approved the clinical study protocol and all participants provided written informed consent for provision of stool sample. The study complies with the Declaration of Helsinki as revised in 2000.
Supplementary information
Acknowledgements
This work was supported by grants from NovoNordisk Foundation (NNF18OC0033880), Päivikki and Sakari Sohlberg Foundation, State research funding for university level health research in Turku University Hospital and Academy of Finland (#285503). The clinical study execution was supported by Academy of Finland (#258606), Expert responsibility Area of Turku University Hospital. Mass spectrometry analyses were performed at Turku Proteomics Facility, supported by Biocenter Finland.
Author contributions
J.A., S.P., R.T. and A.R. generated the results and prepared data for the manuscript, K.M. and K.L. provided donor samples, their 16S rRNA gene sequencing data and analyzed demographic data of donors. J.A., A.R., L.E. and A.H. designed the study. All authors participated in evaluation of the results and in their interpretation and wrote parts of the manuscript. A.H. wrote the final version of the manuscript. L.E., K.L. and A.H. acquired funding.
Data availability
The mass-spectrometry datasets generated during and/or analyzed during the current study are available in the PRIDE (ProteomeXchange) repository. Project Name: Correlation of Prevotella abundance with a particular carbohydrate-active CAZy enzyme profile in healthy gut microbiota. Project accession: PXD017059 Project DOI: Not applicable Reviewer account details: Username: reviewer53498@ebi.ac.uk. Password: ZuHDclhX. 16S-RNA parallel sequencing data is available at https://doi.org/10.5281/zenodo.3608655.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69241-2.
References
- 1.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 2.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. (Berl). 2017;95(1):1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenwright AJ, Pothula KR, Bhamidimarri SP, Chorev DS, Basle A, Firbank SJ, et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 2017;541(7637):407–411. doi: 10.1038/nature20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grondin JM, Tamura K, Dejean G, Abbott DW, Brumer H. Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol. 2017 doi: 10.1128/JB.00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrapon N, Lombard V, Drula E, Lapebie P, Al-Masaudi S, Gilbert HJ, et al. PULDB: the expanded database of polysaccharide utilization loci. Nucleic Acids Res. 2018;46(D1):D677–D683. doi: 10.1093/nar/gkx1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 2018;3(2):210–219. doi: 10.1038/s41564-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndeh D, Rogowski A, Cartmell A, Luis AS, Basle A, Gray J, et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544(7648):65–70. doi: 10.1038/nature21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accetto T, Avgustin G. Polysaccharide utilization locus and CAZYme genome repertoires reveal diverse ecological adaptation of Prevotella species. Syst. Appl. Microbiol. 2015;38(7):453–461. doi: 10.1016/j.syapm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Blakeley-Ruiz JA, Erickson AR, Cantarel BL, Xiong W, Adams R, Jansson JK, et al. Metaproteomics reveals persistent and phylum-redundant metabolic functional stability in adult human gut microbiomes of Crohn's remission patients despite temporal variations in microbial taxa, genomes, and proteomes. Microbiome. 2019;7(1):18. doi: 10.1186/s40168-019-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin PG, Mullaney JA, Loo D, Cao KL, Gottlieb PA, Hill MM, et al. Intestinal metaproteomics reveals host–microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care. 2018;41(10):2178–2186. doi: 10.2337/dc18-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin P, Wang K, Huang C, Nice EC. Mining the fecal proteome: from biomarkers to personalised medicine. Expert Rev. Proteomics. 2017;14(5):445–459. doi: 10.1080/14789450.2017.1314786. [DOI] [PubMed] [Google Scholar]
- 16.Lee PY, Chin SF, Neoh HM, Jamal R. Metaproteomic analysis of human gut microbiota: where are we heading? J. Biomed. Sci. 2017;24(1):36. doi: 10.1186/s12929-017-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtman JS, Sonnenburg JL, Elias JE. Monitoring host responses to the gut microbiota. ISME J. 2015;9(9):1908–1915. doi: 10.1038/ismej.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya T, Ghosh TS, Mande SS. Global profiling of carbohydrate active enzymes in human gut microbiome. PLoS ONE. 2015;10(11):e0142038. doi: 10.1371/journal.pone.0142038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aakko J, Pietila S, Suomi T, Mahmoudian M, Toivonen R, Kouvonen P, et al. Data-independent acquisition mass spectrometry in metaproteomics of gut microbiota—implementation and computational analysis. J Proteome Res. 2020;19:432–436. doi: 10.1021/acs.jproteome.9b00606. [DOI] [PubMed] [Google Scholar]
- 20.Pietila S, Suomi T, Aakko J, Elo LL. A data analysis protocol for quantitative data-independent acquisition proteomics. Methods Mol. Biol. 2019;1871:455–465. doi: 10.1007/978-1-4939-8814-3_27. [DOI] [PubMed] [Google Scholar]
- 21.Pellonpera O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, et al. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care. 2019;42(6):1009–1017. doi: 10.2337/dc18-2591. [DOI] [PubMed] [Google Scholar]
- 22.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11(7):497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 23.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019;47(D1):D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppala J, Lagstrom H, Kaljonen A, Laitinen K. Construction and evaluation of a self-contained index for assessment of diet quality. Scand. J. Public Health. 2010;38(8):794–802. doi: 10.1177/1403494810382476. [DOI] [PubMed] [Google Scholar]
- 27.Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991;280(Pt 2):309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abot A, Arnal G, Auer L, Lazuka A, Labourdette D, Lamarre S, et al. CAZyChip: dynamic assessment of exploration of glycoside hydrolases in microbial ecosystems. BMC Genomics. 2016;17:671. doi: 10.1186/s12864-016-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjursell MK, Martens EC, Gordon JI. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroidesthetaiotaomicron, to the suckling period. J. Biol. Chem. 2006;281(47):36269–36279. doi: 10.1074/jbc.M606509200. [DOI] [PubMed] [Google Scholar]
- 31.Helbert W, Poulet L, Drouillard S, Mathieu S, Loiodice M, Couturier M, et al. Discovery of novel carbohydrate-active enzymes through the rational exploration of the protein sequences space. Proc. Natl. Acad. Sci. U. S. A. 2019 doi: 10.1073/pnas.1815791116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokkala K, Roytio H, Munukka E, Pietila S, Ekblad U, Ronnemaa T, et al. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J. Nutr. 2016;146(9):1694–1700. doi: 10.3945/jn.116.235358. [DOI] [PubMed] [Google Scholar]
- 33.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanca A, Palomba A, Pisanu S, Addis MF, Uzzau S. Enrichment or depletion? The impact of stool pretreatment on metaproteomic characterization of the human gut microbiota. Proteomics. 2015;15(20):3474–3485. doi: 10.1002/pmic.201400573. [DOI] [PubMed] [Google Scholar]
- 35.Accetto T, Avgustin G. The diverse and extensive plant polysaccharide degradative apparatuses of the rumen and hindgut Prevotella species: a factor in their ubiquity? Syst. Appl. Microbiol. 2019;42(2):107–116. doi: 10.1016/j.syapm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, et al. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE. 2014;9(6):e90702. doi: 10.1371/journal.pone.0090702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008;6(2):121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 38.Stanislawski MA, Dabelea D, Wagner BD, Sontag MK, Lozupone CA, Eggesbo M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5(1):113. doi: 10.1186/s40168-017-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6(5):359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014;32(8):834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 41.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 42.Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011;Chapter 10:Unit 10 7. doi: 10.1002/0471250953.bi1007s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suomi T, Elo LL. Enhanced differential expression statistics for data-independent acquisition proteomics. Sci. Rep. 2017;7(1):5869. doi: 10.1038/s41598-017-05949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass-spectrometry datasets generated during and/or analyzed during the current study are available in the PRIDE (ProteomeXchange) repository. Project Name: Correlation of Prevotella abundance with a particular carbohydrate-active CAZy enzyme profile in healthy gut microbiota. Project accession: PXD017059 Project DOI: Not applicable Reviewer account details: Username: reviewer53498@ebi.ac.uk. Password: ZuHDclhX. 16S-RNA parallel sequencing data is available at https://doi.org/10.5281/zenodo.3608655.