Abstract
Oxysterol-binding protein like protein 3 (OSBPL3) has been shown involving in the development of several human cancers. However, the relationship between OSBPL3 and colorectal cancer (CRC), particularly the role of OSBPL3 in the proliferation, invasion and metastasis of CRC remains unclear. In this study, we investigated the role of OSBPL3 in CRC and found that its expression was significantly higher in CRC tissues than that in normal tissues. In addition, high expression of OSBPL3 was closely related to poor differentiation, advanced TNM stage and poor prognosis of CRC. Further experiments showed that over-expression of OSBPL3 promoted the proliferation, invasion and metastasis of CRC in vitro and in vivo models. Moreover, we revealed that OSBPL3 promoted CRC progression through activation of RAS signaling pathway. Furthermore, we demonstrated that hypoxia induced factor 1 (HIF-1A) can regulate the expression of OSBPL3 via binding to the hypoxia response element (HRE) in the promoter of OSBPL3. In summary, Upregulation of OSBPL3 by HIF1A promotes colorectal cancer progression through activation of RAS signaling pathway. This novel mechanism provides a comprehensive understanding of both OSBPL3 and the RAS signaling pathway in the progression of CRC and indicates that the HIF1A–OSBPL3–RAS axis is a potential target for early therapeutic intervention in CRC progression.
Subject terms: Colorectal cancer, Cell signalling
Introduction
Colorectal cancer (CRC) is one of the most common digestive malignant tumor with high morbidity and mortality in the world. The incidence of CRC is on rise year by year, and the age of onset is becoming younger and younger1. Carcinogenesis of CRC is a process with multistep and multiple molecular processes, it is accompanied by activation of oncogenes, inactivation of tumor suppressor genes, apoptosis-regulating genes and DNA repair genes change2. The key issues in CRC initiation include mutational of oncogene KRAS3,4 and PIK3CA5, inactivation or deletion of suppressor genes APC6, PTEN7 and p538, and activation of the canonical Wnt pathway9,10. Although survival rates of CRC patients have improved in the last few years, the clinical outcome of advanced stage CRC patients still remains poor11. Therefore, it is necessary to further explore the mechanisms of the occurrence and development of CRC, and to find more effective treatments for improving the life quality of CRC patients.
OSBPL3 belongs to Oxysterol-binding protein (OSBP) family, which consists of twelve members (OSBP, OSBPL1-OSBPL11)12,13. OSBP is a family of closely related gene sequences with two major structures: a highly conserved C-terminal oxysterol domain, and in most cases, an N-terminal pleckstrin homology (PH) domain. It is speculated that OSBP plays a role in cellular lipid metabolism, vesicle transport, and cell signaling14,15. OSBPL3 mainly expressed in kidney and lymphatic tissues in human. High levels of ORP3 mRNA expression in blood leukocytes, such as B-cells, T-cells and macrophages have also been observed16,17. Moreover, OSBPL3 expression was found to be upregulated in malignancies, such as Burkitt’s lymphoma and CRC18. However, the relationship between OSBPL3 and CRC, particularly the role of OSBPL3 in the proliferation, invasion and metastasis of CRC remains unclear. Our results show that upregulation of OSBPL3 by hypoxia inducible factor 1 subunit alpha (HIF1A) promotes colorectal cancer progression through activation of RAS signaling pathway. This novel mechanism provides a comprehensive understanding of both OSBPL3 and the RAS signaling pathway in the progression of CRC and indicates that the HIF1A–OSBPL3–RAS axis may be a potential target for early therapeutic intervention in CRC progression.
Materials and methods
Clinical samples
133 samples, which were histopathologically and clinically diagnosed at Southern Medical University Institutional Board (Guangzhou, China), were collected between 2015 and 2018. Prior patient consent and approval were obtained from the Institutional Research Ethics Committee. And 24 CRC tissues and the matched adjacent normal tissues were obtained between 2016 and 2017. All the tissue biopsies used here were freshly frozen in liquid nitrogen and stored until further use. The medical records of the patients were reviewed to collect the following clinicopathological information: age, gender, TNM stage, differentiation and DUCKS stage.
Cell Culture
The human CRC cell lines SW480, HCT116, RKO and HCT15 were purchased from American Type Culture Collection (ATCC, USA). SW480 and HCT116 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA); RKO and HCT15 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS (Gibco). All the cells were cultured at 37 °C with 5% CO2.
RNA extraction, real-time PCR, plasmid construction and transfection
RNA extraction, real-time quantitative PCR (RT-PCR), plasmid construction and transfection were conducted according to previously described methods19. Further details are provided in the Materials and Methods section.
Western blot and IHC
Western blotting (WB) and IHC were conducted according to previously described methods20. Further details are provided in the Materials and Methods section.
Immunofluorescence
Cells (5 × 104/well) were seeded on coverslips for 48 h and then probed with primary antibodies against OSBPL3 (Bethyl Laboratories, Inc, MT) or Flag (Sigma, Saint Louis, MO, USA), and then incubated with rhodamine-conjugated or fluorescein isothiocyanate (FITC)-conjugated goat antibodies against rabbit or mouse IgG (Jackson Laboratory, West Grove, PA). The cover slips were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI, Sigma, Saint Louis, MO) and imaged with a confocal laser-scanning microscope (Olympus FV1000).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were carried out using a kit (ACTIVE MOTIF, ChIP-IT Express, catalog #53008). Briefly, cells (2 × 107) in a 10 cm culture dish were treated with 1% formaldehyde to cross-link chromatin-associated proteins to DNA. The cell lysates were subjected to ultrasound for 9–10 sets of 10-s pulses at 40% output to shear the DNA into fragments between 200 and 1000 bps. Equal cell lysates were respectively incubated with 1 μg of anti-Flag antibody (Sigma) and anti-IgG antibody (Millipore) as negative control. All the above chromatin supernatants were incubated with 20 μl magnetic proteinG beads overnight at 4 °C with rotation. Second day, the protein-DNA complexes were reversed and purified for pure DNA. The human SNAI1 promoter was amplified with RT-PCR.
MTT assay, colony formation assay, soft agar assay
MTT assay, colony formation assay and soft agar assay were performed as previously described21. Further details are provided in the Materials and Methods section.
Transwell, wound-healing assay and three-dimensional morphogenesis assay
The transwell, wound-healing assay and the three-dimensional morphogenesis assay were conducted according to previously described methods21,22. Further details are provided in the Materials and methods section.
Flow-cytometry analysis
Flow-cytometry assay was performed to detect the cell cycle. Cells were seeded into sixwell plates (4 × 105 cells/well). For cell cycle, in order to synchronize cells into the G2/M phase of the cell cycle, cells were treated with 0.1 μm colchicine for 12 h, then cells were harvested and fixed with 70% cold ethanol. Next, cells were added bovine pancreatic RNAase to remove total RNA, incubated for 30 min at 37 °C, added 20 μg/ml propidium iodide (Sigma-Aldrich) and incubated at room temperature for 20 min. At last, flowcytometry was used to detect the cell cycle for prepared cells. All experiments were performed 3 times. Data were averaged for statistical analysis.
Tumorigenesis in nude mice
CRC cells (2 × 106), including RKO-Vector and RKO-OBPL3, SW480-Scramble, SW480-OBPL3 shRNA#1 and SW480-OBPL3 shRNA#2 were subcutaneous injected (n = 6 for each group), on the hind limbs of 4–6week-old Balb/C athymic nude mice (nu/nu) achieved from Animal Center of Southern Medical University, Guangzhou, China. All mice were raised and fed under SPF conditions, and all experiments were under the approvement of the Use Committee for Animal Care and proceeded on the basis of institutional guidelines. Tumor size was measured with a slide caliper and tumor volume was calculated by the formula 0.44 × A × B2 (A represents the base diameter of tumor and B represents the corresponding perpendicular value). The tumors were excised, then fixed with 10% neutral buffered formalin and 4μm sections were cut. The sections were stained with hematoxylin and eosin according to standard protocols, then further under IHC staining using antibody against Ki-67.
Orthotopic mouse metastatic model
CRC cells (2 × 106), including RKO-Vector and RKO-OBPL3, SW480-Scramble, SW480-OBPL3 shRNA#1 and SW480-OBPL3 shRNA#2 were subcutaneous injected (n = 6 for each group), on the hind limbs of 4–6 week-old Balb/C athymic nude mice (nu/nu) achieved from Animal Center of Southern Medical University, Guangzhou, China. Two weeks later, the animals were sacrificed, and the tumors were excised. Tumor was divided into small pieces approximately 1 mm in diameter. Surgical orthotopic implantation of the CRC tumor fragments onto the mesentery of the cecum was performed in nude mice after anesthesia was administered. The mice were euthanized 60 days after surgery, the individual organs were excised, and metastases were observed by histological analysis.
Selective inhibitor of R-Ras: geranylgeranyltransferase I (GGTI-2133)
We treated RKO cells with a R-Ras inhibitor (GGTI-2133) for 24 h with 38 nM (IC50 = 38 nM, Sigma Biotechnology St. Louis, MO), geranylgeranyltransferase I (GGTI-2133) that inhibits R-Ras but not H-Ras. Control samples were treated with equal volumes of DMSO, the GGTI carrier23.
Statistical analysis
All statistical analyses were carried out using the SPSS20.0 for Windows. Statistical tests included the Fisher exact test, log-rank test, χ2 test, ANOVA and Student’s t-test. Bivariate correlations between study variables were calculated by Spearman’s rank correlation coefficients. Survival curves were plotted by the Kaplan-Meier method and were compared by the log-rank test. Data represent the mean ± SD. p < 0.05 was considered significant. Statistically significant data were indicated by asterisks: *p < 0.05, **p < 0.01.
Accession numbers for the data sets
The GEO database (GSE39582 and GSE17538) and the TCGA data were used to analyze the relationship between the expression of OSBPL3 and the 5-year overall survival of the CRC patients. The GEO databases (GSE13294 and GSE13067) were used for the GSEA analysis of the “Rac1 signaling pathways” gene sets in the study.
Results
High expression of OSBPL3 was correlated with advanced progression and poorer prognosis of CRC
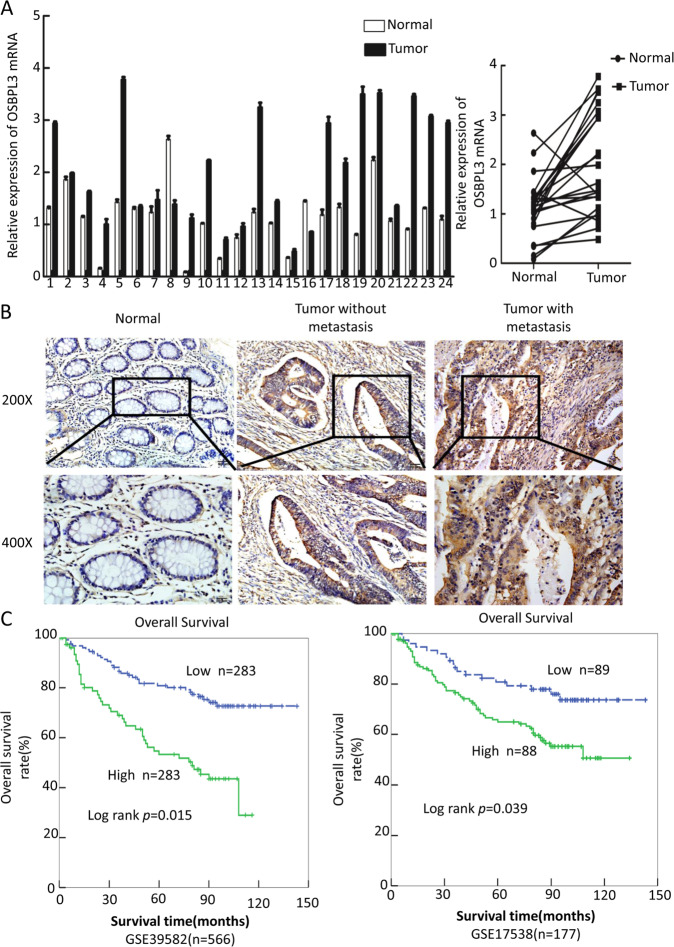
OSBPL3 is a differential expression gene that we screened using transcriptome gene expression chip (Affymetrix, HG-U133_Plus 2) in our earlier experiments, and the results show that OSBPL3 mRNA expression levels in colorectal cancer tissue and liver metastasis lesions are significantly higher than normal intestinal mucosa tissue (Supplementary Fig. S1A). Next, we used a public database (http://gepia.cancer-pku.cn/index.html) to detect OSBPL3 expression in a variety of tumors and normal tissues, we found that OSBPL3 expression was significantly higher than normal in 21 malignancies, including colorectal cancer (Supplementary Fig. S1B, C). Consistent with the results of the public database, we detected the expression of OSBPL3 in 24 cases of fresh primary human CRC biopsies and their paired adjacent normal tissues by real-time PCR. We found that OSBPL3 was up-regulated in 95.8% (23/24) of CRC tissue samples (T) compared to their matched adjacent normal tissues (N) (Fig. 1a).
Fig. 1. High expression of OSBPL3 was correlated with advanced progression and poorer prognosis of CRC.
a Expression of OSBPL3 in 24 cases of fresh human CRC tissues and their matched adjacent normal tissues by real-time PCR analyses (2-ΔΔCT, n = 24, **p < 0.01). b Representative images of OSBPL3 expression in normal intestinal epithelium and CRC specimens with or without metastasis examined by IHC. c Kaplan–Meier survival analysis and log-rank tests of OSBPL3 using bioinformatics data (Fig. 1c, log-rank, p < 0.05).
To further investigate the clinicopathological significance of OSBPL3, we analyzed the expression of OSBPL3 by stage in CRC, the results showed that the expression of OSBPL3 was positively correlated with the stage of the CRC, this result is also consistent with the results of public data (Supplementary Fig. S1C, D). Next, immunohistochemistry staining was used to detect the protein expression level and subcellular localization of OSBPL3 in 133 cases of paraffin-embedded CRC tissues. The results showed that OSBPL3 displayed as cytoplasmic or cytoplasmic/membrane localization in CRC cells. Moreover, the OSBPL3 expression was up-regulated in CRC cancer tissues compared to their matched adjacent normal tissues, especially higher in those with distant metastasis (Fig. 1b). Analyses of the results indicated that high expression of OSBPL3 was positively associated with poor differentiation, advanced TNM stage and Dukes stage (Table 1). Kaplan–Meier survival analyses of two published CRC data sets (GSE39582, GSE17538) revealed that the higher OSBPL3 expression was significantly correlated with the poorer survival of patients (Fig. 1c, log-rank, p < 0.05).
Table1.
Correlation between OSBPL3 expression levels and CRC clinicopathological parameters.
| Characteristics | OSBPL3 expression | χ2-values | p-values | |
|---|---|---|---|---|
| Low | High | |||
| Age(years) | ||||
| <60 | 28 | 37 | 0.156 | 0.693 |
| ≥60 | 27 | 41 | ||
| Gender | ||||
| Male | 26 | 42 | 0.012 | 0.912 |
| Female | 28 | 47 | ||
| Differentiation | ||||
| Well | 16 | 17 | 6.103 | 0.047 |
| Moderate | 18 | 25 | ||
| Poor | 14 | 43 | ||
| Dukes stage | ||||
| A | 10 | 12 | 8.013 | 0.046 |
| B | 16 | 24 | ||
| C | 10 | 29 | ||
| D | 5 | 27 | ||
| T stage | ||||
| T1 | 1 | 2 | 9.49 | 0.023 |
| T2 | 8 | 10 | ||
| T3 | 12 | 30 | ||
| T4 | 9 | 61 | ||
| N classification | ||||
| N0 | 24 | 35 | 9.950 | 0.002 |
| N1-2 | 12 | 62 | ||
| M classification | ||||
| M0 | 39 | 40 | 6.199 | 0.013 |
| M1 | 15 | 39 | ||
Overexpression of OSBPL3 promotes progression of CRC cells
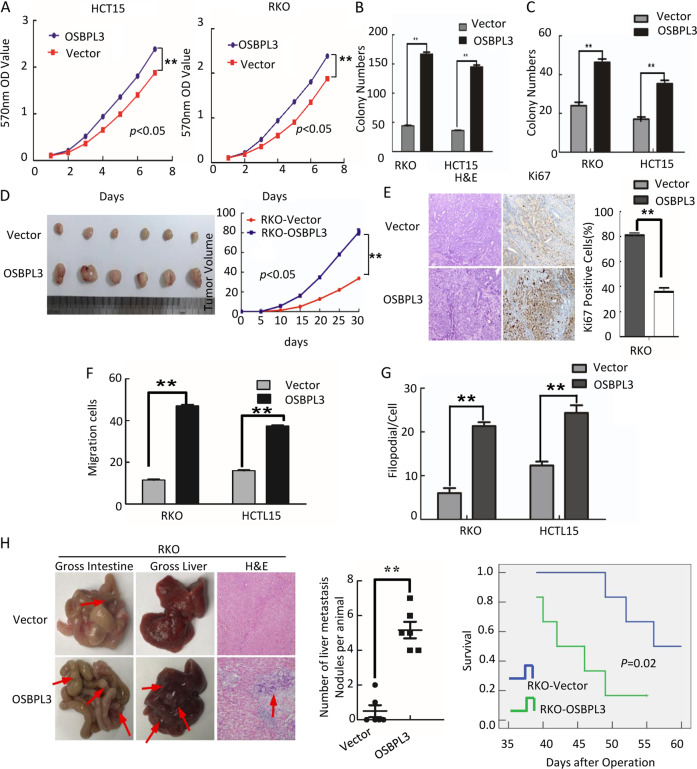
To investigate whether OSBPL3 plays a role in the progression of CRC cells, we established stable OSBPL3-expressing CRC cells (RKO/OSBPL3 and HCT15/ OSBPL3) (Supplementary Fig. S2A). Overexpression of OSBPL3 accelerated the cell growth rate as determined by MTT assays (Fig. 2a) and colony formation assays (Fig. 2b, and Supplementary Fig. S2B). We next examined the effect of OSBPL3 on the anchorage-independent growth ability of CRC cells, using the soft agar assay. Overexpression of OSBPL3 significantly promoted the growth of HCT15 and RKO in comparison with control cells (Fig. 2c and Supplementary Fig. S2C). Flow-cytometry analysis of cell cycle showed a significant decrease in the percentage of cells in the G1/G0 peak and an increase in the percentage of cells in the S and G2/M peak in RKO/OSBPL3 and HCT15/ OSBPL3 (Supplementary Fig. S2D). These results suggested that OSBPL3 could promote cell proliferation by facilitating the tumor cells into S and G2/M phase. We next detected the effect of OSBPL3 overexpression on tumor growth in nude mice. In comparison with control cells (RKO/Vector), RKO/OSBPL3 cells implanted in nude mice exhibited more rapid growth speed and significantly larger tumor volumes (Fig. 2d). In addition to the difference of tumor volume, we also found that the tumors formed by RKO/OSBPL3 cells displayed a higher Ki-67 index than that in tumors formed by RKO/Vector cells (Fig. 2e).
Fig. 2. Overexpression of OSBPL3 promotes progression of CRC cells.
a Overexpression of OSBPL3 promotes cell growth in MTT assays. b Colony formation assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. c Soft agar assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. d Tumorigenesis in nude mice. Tumor volumes were measured on the indicated days. Data points are the mean tumor volumes ± SD. e The sections of tumor were under H&E staining or subjected to IHC staining using an antibody against Ki-67. f Transwell assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. g Three-dimensional morphogenesis assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. h Orthotropic transplantation assay. The representative gross images of the intestines and livers from different experimental groups are shown. Sections of the liver were stained with H&E. Arrows indicate the metastases in the intestines and livers(left). Box-scatter plots show the number of metastatic nodules in the liver as observed in each group (middle) and the overall survival time of each group (log-rank test, p < 0.05) (right).
Furthermore, wound healing assay, transwell assay and three-dimensional morphogenesis assay showed that OSBPL3 overexpression promoted the invasion and metastasis of CRC cells compared with control cells (Fig. 2f–g and Supplementary Fig. S2E–G). Orthotopic mouse metastatic model showed that the mice injected with RKO/OSBPL3 cells exhibited more visible metastatic nodules in the liver than RKO/Vector group. Histological staining confirmed that the nodules in the liver were metastatic CRC. CRC cells with over-expressed OSBPL3 markedly reduced the overall survival of the mice (Fig. 2h).
Knocking down of OSBPL3 inhibits progression of CRC cells
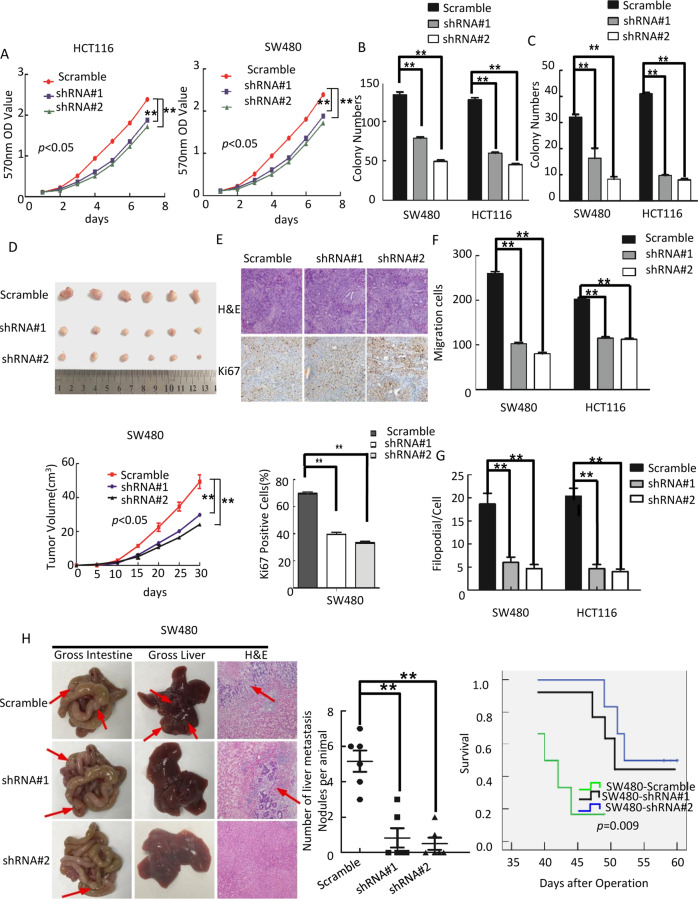
To further confirm the impact of OSBPL3 on proliferation, invasion and tumorigenesis of CRC cells, we knockdown endogenous OSBPL3 in SW480 and HCT116 CRC cells using shRNAs specifically targeting OSBPL3 (Supplementary Fig. S3A). MTT assay and colony formation assay show that downregulation of OSBPL3 obviously inhibited the cell growth rate (Fig. 3a, b, Supplementary Fig. S3B). Soft agar assay shows that downregulation of OSBPL3 inhibited the anchorage-independent growth ability of CRC cells (Fig. 3c, Supplementary Fig. S3C). Flow-cytometry analysis of cell cycle showed that knockdown of endogenous OSBPL3 increased the percentage of cells in the G1/G0 peak and a decrease in the percentage of cells in the S peak significantly (Supplementary Fig. S3D). These results suggested that knockdown of endogenous OSBPL3 could inhibit cell proliferation by arresting the tumor cells at G1/G0 phase. In vivo tumorigenesis assay exhibited that knockdown of endogenous OSBPL3 expression in SW480 cells caused significant inhibition of tumor growth. IHC staining showed that the tumors of SW480/shOSBPL3 group displayed much lower Ki-67 index than that in control group (Fig. 3d, e).
Fig. 3. Knocking down of OSBPL3 inhibits progression of CRC cells.
a Knocking down of OSBPL3 inhibited cell growth in MTT assays. b Colony formation assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. c Soft agar assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. d Tumorigenesis in nude mice. Tumor volumes were measured on the indicated days. Data points are the mean tumor volumes ± SD. e The sections of tumor were under H&E staining or subjected to IHC staining using an antibody against Ki-67. f Transwell assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. g Three-dimensional morphogenesis assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. h Orthotropic transplantation assay. The representative gross images of the intestines and livers from different experimental groups are shown. Sections of the liver were stained with H&E. Arrows indicate the metastases in the intestines and livers (left). Box-scatter plots show the number of metastatic nodules in the liver as observed in each group (middle) and the overall survival time of each group (log-rank test, p < 0.05) (right).
Meanwhile, wound healing assay, transwell assay and three-dimensional morphogenesis assay showed that OSBPL3 knockdown inhibited the invasion and metastasis of CRC cells compared with control cells (Fig. 3f, g and Supplementary Fig. S3E–G). Orthotopic mouse metastatic model showed that the mice injected with SW480/shOSBPL3 cells exhibited less visible CRC metastatic nodules in the liver than SW480/Vector group. CRC cells with OSBPL3 knockdown markedly increased the overall survival of the mice (Fig. 3h).
Activation of RAS signaling pathway plays an important role in OSBPL3-promoted progression of CRC
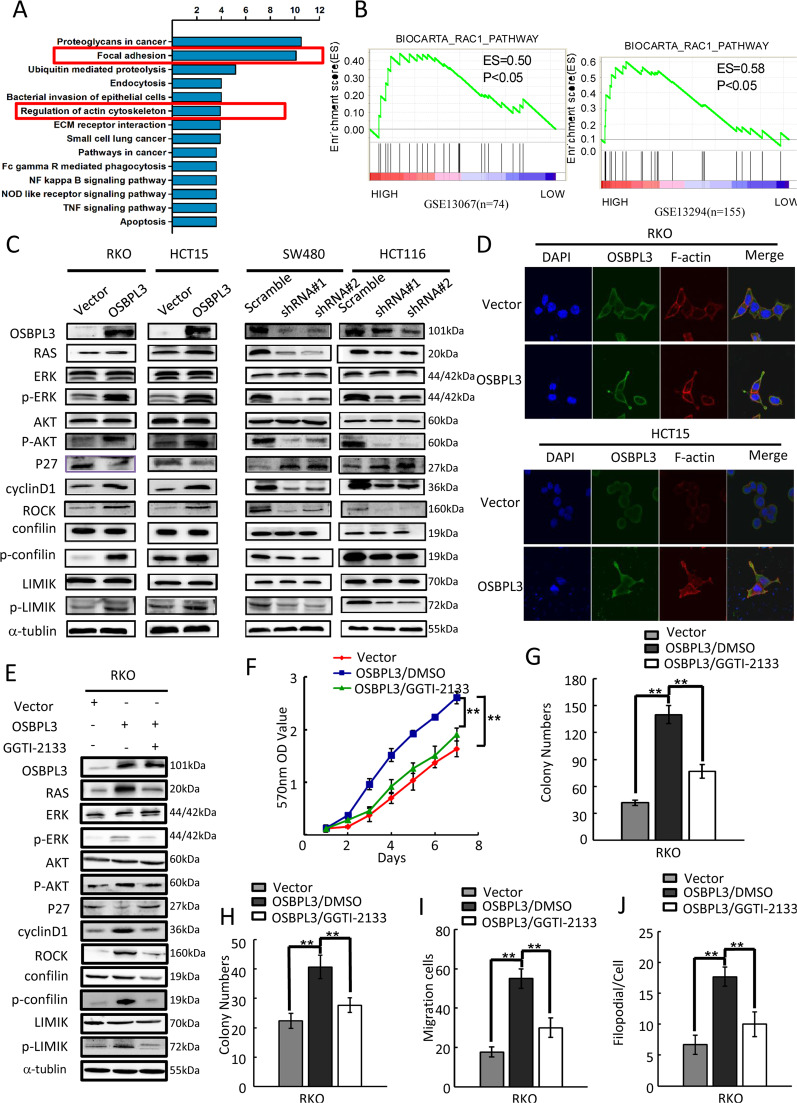
GSEA bioinformatics analysis revealed that many biological processes and pathways were potentially involved in the OSBPL3-promoted CRC progression, the results showed that these genes with co-expression characteristics of OSBPL3 were mainly involved in cell focal adhesion, cytoskeleton regulation and other tumor-related signaling pathways (Supplementary Fig. S4A and Fig. 4a). Further GSEA analysis results show significant enrichment of RAC1 signaling pathways (p < 0.05) in OSBPL3 high expression group colorectal cancer (Fig. 4b).
Fig. 4. OSBPL3 upregulation activates RAS signaling pathway in CRC.
a GO and KEGG analysis of genes with co-expression characteristics of OSBPL3 in human tumors, including lung, breast and colorectal cancers. b GSEA plot showed that high expression of OSBPL3 was positively correlated with the RAC1 pathway in published CRC patient gene expression profiles (GSE13067, n = 74, and GSE13294, n = 155). c Western blot analysis in indicated cells of protein products of RAS signaling pathway involved genes. d Confocal laser scanning microscopy analysis of the actin cytoskeletal remodeling of CRC cells with OSBPL3 overexpression. e RKO/ OSBPL3 cells were treated with the R-RAS inhibitor GGTI-2133 (38 nM) or DMSO for 24 h, then harvested to examine the expression levels of the indicated proteins by Western blotting. f–h RKO/ OSBPL3 cell proliferation was determined by MTT (f), colony formation (g) and soft agar assays (h) after treatment with GGTI-2133 or DMSO. i, j RKO/ OSBPL3 cell invasion and metastasis was determined by transwell (i) and 3D morphogenesis (j) after treatment with GGTI-2133 or DMSO. Error bars represent mean ± SD from 3 independent experiments; **p < 0.01.
Next, we detected the expression of target genes of RAS signaling. Western blot analysis showed that overexpression of OSBPL3 in RKO and HCT15 cells increased the levels of RAS, p-ERK, p-AKT, cyclin D1, ROCK, p-confilin and p-LIMK, but decreased the levels of p27. Meanwhile, knockdown of OSBPL3 in SW480 and HCT116 cells decreased the levels of RAS, p-ERK, p-AKT, cyclin D1, ROCK, p-confilin and p-LIMIK, but increased the levels of p27 (Fig. 4c).
Immunofluorescence analysis showed that up-regulated expression of OSBPL3 in RKO and HCT15 cells, promoted elongating of cells and the formation of more lamellipodia and protrusions. Moreover, knock down of OSBPL3 in SW480 and HCT116 cells, inhibited the cells elongated and the lamellipodia and protrusions formation (Fig. 4d and Supplementary Fig. S4C). Moreover, we found that OSBPL3 may regulate the organization of the actin cytoskeleton by interacting directly with Ras-related protein R-Ras in protein-protein interaction networks (Supplementary Fig. S4B).
To investigate whether OSBPL3 affects the expression of downstream signaling pathway-related proteins through targeted R-RAS, we treated OSBPL3-overexpressed RKO cells with a selective inhibitor of geranylgeranyltransferase I (GGTI-2133) that inhibits R-Ras but not H-Ras. As shown in Fig. 4e, the expression levels of the levels of RAS, p-ERK, p-AKT, cyclin D1, ROCK, p-confilin and p-LIMK were significantly reduced by GGTI-2133 in RKO/ OSBPL3 cells, while the expression of P27 was partly rescued by treatment with the GGTI-2133 compared to control cells treated with DMSO.
To further validate OSBPL3 promotes progression of CRC through activation of the RAS pathways, we examined the growth and invasion ability of OSBPL3-overexpressed RKO cells after inhibition of this pathway using GGTI-2133. MTT, colony formation and soft agar assays showed that the growth of RKO/ OSBPL3 cells and transwell and three-dimensional morphogenesis assay showed that the invasion and metastasis of RKO/OSBPL3 were both significantly compromised by treatment with the R-RAS inhibitors compared to control cells treated with DMSO (Fig. 4g–j and Supplementary Fig. S4D–G).
HIF1A is involved in RAS signaling pathways by regulated expression of OSBPL3 in the CRC
Bioinformatics analysis (cBioPortal for Cancer Genomics (http://www.Cbioportal.org) showed that in TCGA (Nature 2012, n = 212, Provisional, n = 220) colorectal cancer respectively, the mutant rate of OSBPL3 is only 3.8% and 3.4%, and neither gene deletion nor gene amplification was discovered. These results suggested that neither OSBPL3 mutant nor deletion plays an important role in the progression of colorectal cancer (Supplementary Fig. S5A).
Furthermore, we analyzed the coexpression genes with OSBPL3 in human tumors, the results of gene set enrichment analysis (GSEA) and GO analysis showed that many genes were co-expressed with OSBPL3 in CRC, among which HIF1A was a highly correlated gene. Therefore, we speculate that HIF1A may be an upstream regulatory molecule of OSBPL3 (Supplementary Fig. S5B).
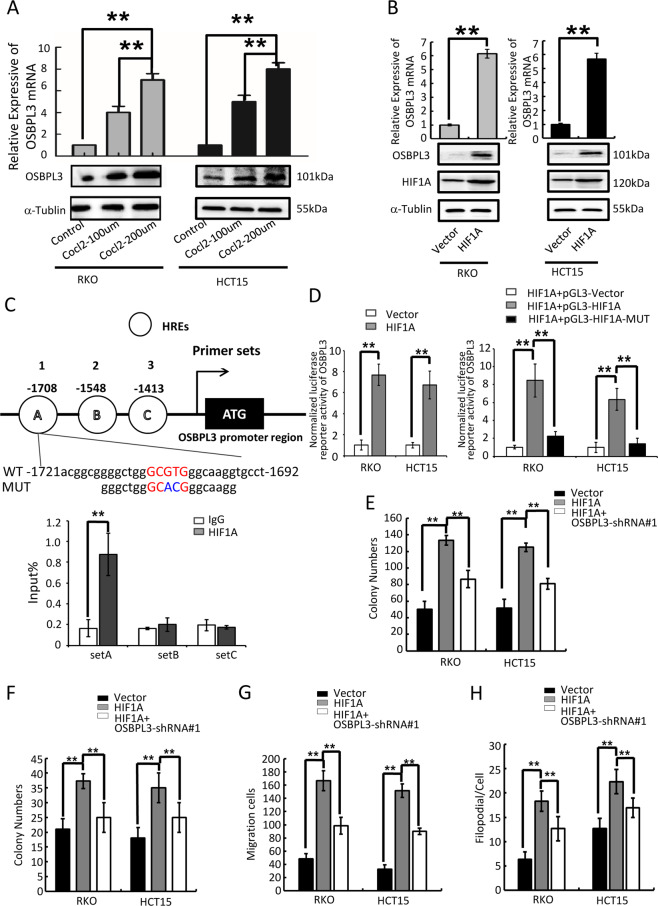
To further verify the regulatory effect of HIF1A on OSBPL3, we treated CRC cells with CoCl2 (simulated hypoxia).The results showed that, compared with the control group, the expression levels of OSBPL3 in CRC cell lines RKO and HCT15 were significantly increased with different concentrations of CoCl2, showing a concentration-dependent relationship (Fig. 5a). Overexpression of HIF1A increased OSBPL3 expression, inferring that HIF1A is a regulatory factor of OSBPL3 (Fig. 5b). It is well established that Hypoxia factors (HIFs) bind to hypoxia inducible response elements (HRE) with consensus sequence 5′-A/GCGTG-3′ in the promoter of the genes they regulate24. In silico analysis identified that OSBPL3 promoter (−2000bp to +1 bp) contained 3 putative HREs (Fig. 5c, up). Therefore, we investigated whether OSBPL3 could be regulated by HIF1A. Chromatin immunoprecipitation (ChIP) assays revealed that endogenous HIF1A protein was bound to the set A region of the OSBPL3 promoter (Fig. 5c, down). Moreover, overexpression HIF1A activated the wild-type OSBPL3 promoter but did not affect the mutant promoter (Fig. 5d).
Fig. 5. HIF1A regulated the expression of OSBPL3.
a Detection of the influence of Cocl2 on the expression of OSBPL3 in CRC. b Levels of OSBPL3 in HIF1A overexpressing cells were determined by quantitative RT-PCR and western blot. c Schematic depiction of the OSBPL3 promoter with three HIF1A binding sites, as indicated A, B and C, and the HIF1A B binding motif in the set A proximal promoter and its mutant containing altered nucleotides in set A (top). ChIP analysis of HIF1A binding to the OSBPL3 promoter in RKO cells. Primers against the −1721 to −1692 base pairs in the promoter region (set A) showed significant enrichment after normalization to the input control (bottom). RT-PCR experiments were performed. d Relative expression of a WT OSBPL3 promoter–driven luciferase reporter in Vector control or HIF1A-overexpression CRC cells (left) and the relative expression of WT or MUT OSBPL3 promoter–driven luciferase reporters in HIF1A -overexpression CRC cells (right). Error bars represent the mean ± SD of 3 independent experiments; **p < 0.01. e Colony formation assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. f Soft agar assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. g Transwell assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01. h Three-dimensional morphogenesis assay. Error bars represent mean ± SD from three independent experiments; **p < 0.01.
Moreover, colony formation assay, soft agar assay, healing assay, transwell assay and three-dimensional morphogenesis assay showed that overexpression of OSBPL3 could antagonize the effect of HIF1A interference on the progression of CRC in vitro (Fig. 5e–h and Supplementary Fig. S6A-E), which further light on the relation of OSBPL3 with the regulation of HIF1A. Finally, bioinformatics analysis showed that HIF1A and OSBPL3 were expressed in colon cancer with a clear positive correlation (p = 0.001, TCGA, Supplementary Fig. S5C).
Discussion
The main treatments for CRC patients are surgery, radiotherapy and chemotherapy. Patients in early stage can be cured by surgery, but up to 20% of patients are found with distant metastasis25. Therefore, discovery of new molecular indicators is of great clinical significance for the early diagnosis of CRC. Here, we showed that the expression of OSBPL3 in tumor with or without metastasis were significantly higher than that in adjacent normal intestinal mucosa, and its high expression was significantly correlated with the differentiation, TNM stage, Dukes stage. These results suggest that OSBPL3 playing a role as an oncogene in the development of CRC. Furthermore, we investigated the molecular mechanism of OSBPL3 promoting the proliferation, invasion and metastasis of CRC. By bioinformatics analysis, we found that RAS pathway activity was upregulated in CRC. RAS is an oncogene, which maintains a high degree of conservation in evolution and plays a crucial role in the cell proliferation, differentiation, growth and development26. Ras/Raf and PI3K/Akt are the two most important downstream signaling pathways of Ras27. CyclinD1 is a downstream target gene of Ras/Raf pathways. Studies have shown that CyclinD1 is the key factor which controls the cells from G1 to S phase, and finally promote cell proliferation28. PI3K/Akt is the third downstream of Ras signaling pathway. Activated Akt inhibits apoptosis-related proteins Caspase9 and Bad29. The Rho/ROCK pathway is another downstream signaling pathway of Ras. It also plays a key role in cell migration. Large concentrations of Rac can be found around migrating cells, which can regulate the aggregation of microfilaments into pseudopodia and promote cell migration22,30.
In our study, we found that OSBPL3 can affect cell proliferation by changing the cell cycle of CRC. In addition, we showed that OSBPL3 can regulate cytoskeleton reconstruction through RAS pathway, by immunofluorescence analysis, we observed that OSBPL3 can change shape of CRC cells and the number of lamellar pseudopodia, thereby promoting the movement and migration of CRC cells. This conclusion is consistent with the views of Weber-Boyvat M31 and Lehto M32.
Then, we showed that hypoxia in cancer microenvironment can increase the expression of HIF1A, which further up-regulate the expression of OSBPL3 in CRC. In most solid tumors, the body loses its normal regulation on the proliferation and apoptosis of tumor cells, leading to rapid tumor growth. When the growth rate of tumor is faster than the growth rate of blood vessels, ischemia and hypoxia can be caused. Hypoxia in cancer microenvironment is a common feature of most malignant tumors. In the hypoxia microenvironment, the expression level of hypoxia inducible factor l (HIF l) in tumor cells was significantly increased33. HIF1 protein is composed of alpha and beta subunits. HIF1A is an important oxygen-regulating subunit, and it also is one of the key transcription activators that regulate cancer cells to adapt to hypoxic environment34,35. A large number of studies have revealed that HIF1A is highly expressed in gastric cancer, small-cell lung cancer and other cancer. It is closely related to malignant biological behavior of cancer36–39. Another important effect of anoxic microenvironment is the suppression of host immune response40–43. In addition, the lipid metabolism of cancer cells also changes. The breakdown of fatty acids requires oxygen, so the body uses various methods to prevent the breakdown of fatty acids. We founded that the c-terminal of OSBPL3 mainly binds oxidized cholesterol derivatives and other unknown ligands, so OSBPL3 also plays an important role in lipid metabolism. We hypothesized that the increased HIF1A expression caused by hypoxia may promote the progression of CRC through the upregulation of OSBPL3 expression just by affecting lipid metabolism. This needs to be further verified by our later work.
In summary, our findings suggest that OSBPL3 is upregulated in CRC, and may affect cell progression in CRC through activation of RAS signaling pathway. HIF1A participates in the proliferation, invasion and metastasis of CRC by regulating the expression of OSBPL3 (Fig. 6). OSBPL3 may represent a useful therapeutic approach for targeting CRC.
Fig. 6. Molecular mechanism of OSBPL3 in progression of CRC.
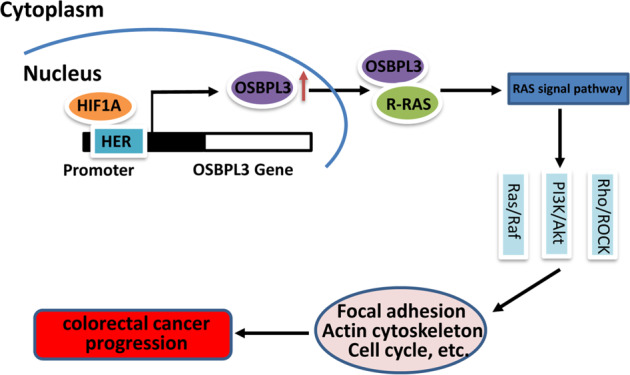
Upregulation of OSBPL3 by HIF1A activates the RAS signaling pathway, and further promotes colorectal cancer progression by regulating the cell cycle and cytoskeleton.
Supplementary information
Acknowledgements
We thank the Central Laboratory of Southern Medical University for technical supports.
Funding
This work was supported by the National Basic Research Program of China (973 program, 2015CB554002), the National key R&D program of China (2017YFC1309002), the National Natural Science Foundation of China (81972756, 81902476, 81874194, 81702915, 81773101, 81773196, 81472313, 81672886, 81472710), the Postdoctoral Science Foundation of China (2018M633080), the Guangdong Provincial Natural Science Foundation of China (2017A030313583, 2016A030310395, 2017A030313463, 2016A030310392, 2017A030310117, 2017A030313707, 2016A030310394).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
CRC tissue samples were acquired from patients undergoing a surgical procedure at the Nanfang Hospital, Southern Medical University. Patient consent was obtained prior to the initiation of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by A. Stephanou
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41419-022-05367-7
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hong-li Jiao, Bin-shu Weng and Shan-shan Yan
Change history
11/29/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-022-05367-7
Change history
11/2/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-022-05367-7
Contributor Information
Yan-qing Ding, Email: dyqsmu@126.com.
Ya-ping Ye, Email: 149941225@qq.com.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-02793-3).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douillard JY, Rong A, Sidhu R. RAS mutations in colorectal cancer. N. Engl. J. Med. 2013;369:2159–2160. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 4.Ye, YP et al. miR-450b-5p induced by oncogenic KRAS is required for colorectal cancer progression. Oncotarget, 10.18632/oncotarget.11016 (2016). [DOI] [PMC free article] [PubMed]
- 5.Ogino S, et al. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene. 2014;33:2949–2955. doi: 10.1038/onc.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TH, et al. The prognostic significance of APC gene mutation and miR-21 expression in advanced-stage colorectal cancer. Colorectal Dis. 2013;15:1367–1374. doi: 10.1111/codi.12318. [DOI] [PubMed] [Google Scholar]
- 7.Davies EJ, Marsh Durban V, Meniel V, Williams GT, Clarke AR. PTEN loss and KRAS activation leads to the formation of serrated adenomas and metastatic carcinoma in the mouse intestine. J. Pathol. 2014;233:27–38. doi: 10.1002/path.4312. [DOI] [PubMed] [Google Scholar]
- 8.Cooks T, et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 10.Li T, et al. MicroRNA-224 sustains Wnt/beta-catenin signaling and promotes aggressive phenotype of colorectal cancer. J. Exp. Clin. Cancer Res. 2016;35:21. doi: 10.1186/s13046-016-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 12.Lehto M, et al. The OSBP-related protein family in humans. J. Lipid Res. 2001;42:1203–1213. doi: 10.1016/S0022-2275(20)31570-4. [DOI] [PubMed] [Google Scholar]
- 13.Jaworski CJ, Moreira E, Li A, Lee R, Rodriguez IR. A family of 12 human genes containing oxysterol-binding domains. Genomics. 2001;78:185–196. doi: 10.1006/geno.2001.6663. [DOI] [PubMed] [Google Scholar]
- 14.Olkkonen VM, Levine TP. Oxysterol binding proteins: in more than one place at one time? Biochem. Cell Biol. 2004;82:87–98. doi: 10.1139/o03-088. [DOI] [PubMed] [Google Scholar]
- 15.Lehto M, Olkkonen VM. The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim. Biophys. Acta. 2003;1631:1–11. doi: 10.1016/S1388-1981(02)00364-5. [DOI] [PubMed] [Google Scholar]
- 16.Johansson M, et al. The two variants of oxysterol binding protein-related protein-1 display different tissue expression patterns, have different intracellular localization, and are functionally distinct. Mol. Biol. Cell. 2003;14:903–915. doi: 10.1091/mbc.e02-08-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomisto TT, Riekkinen MS, Viita H, Levonen AL, Yla-Herttuala S. Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading-cDNA and protein array study. Atherosclerosis. 2005;180:283–291. doi: 10.1016/j.atherosclerosis.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Lehto M, Tienari J, Lehtonen S, Lehtonen E, Olkkonen VM. Subfamily III of mammalian oxysterol-binding protein (OSBP) homologues: the expression and intracellular localization of ORP3, ORP6, and ORP7. Cell Tissue Res. 2004;315:39–57. doi: 10.1007/s00441-003-0817-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, et al. The tumor-suppressor gene LZTS1 suppresses colorectal cancer proliferation through inhibition of the AKT-mTOR signaling pathway. Cancer Lett. 2015;360:68–75. doi: 10.1016/j.canlet.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, et al. FOXF1 promotes angiogenesis and accelerates bevacizumab resistance in colorectal cancer by transcriptionally activating VEGFA. Cancer Lett. 2018;439:78–90. doi: 10.1016/j.canlet.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Jiao, HL et al. Down-regulation of SAFB sustains the NF-kappaB pathway by targeting TAK1 during the progression of colorectal cancer. Clin. Cancer Res., 10.1158/1078-0432.CCR-17-0747 (2017). [DOI] [PubMed]
- 22.Ye YP, et al. Hypermethylation of DMTN promotes the metastasis of colorectal cancer cells by regulating the actin cytoskeleton through Rac1 signaling activation. J. Exp. Clin. Cancer Res. 2018;37:299. doi: 10.1186/s13046-018-0958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths GS, Grundl M, Allen JS, Matter ML. R-Ras interacts with filamin a to maintain endothelial barrier function. J. Cell. Physiol. 2011;226:2287–2296. doi: 10.1002/jcp.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, et al. The bidirectional regulation between MYL5 and HIF-1alpha promotes cervical carcinoma metastasis. Theranostics. 2017;7:3768–3780. doi: 10.7150/thno.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Nordlinger B, Cervantes A, Group EGW. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann. Oncol. 2010;21:v93–v97. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 26.Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J. Pathol. 2011;223:219–229. doi: 10.1002/path.2796. [DOI] [PubMed] [Google Scholar]
- 27.Grant S. Cotargeting survival signaling pathways in cancer. J. Clin. Invest. 2008;118:3003–3006. doi: 10.1172/JCI36898E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Vogt PK, Class I. PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wozniak MA, Kwong L, Chodniewicz D, Klemke RL, Keely PJ. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol. Biol. Cell. 2005;16:84–96. doi: 10.1091/mbc.e04-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber-Boyvat M, et al. OSBP-related protein 3 (ORP3) coupling with VAMP-associated protein A regulates R-Ras activity. Exp. Cell Res. 2015;331:278–291. doi: 10.1016/j.yexcr.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Lehto M, et al. The R-Ras interaction partner ORP3 regulates cell adhesion. J. Cell Sci. 2008;121:695–705. doi: 10.1242/jcs.016964. [DOI] [PubMed] [Google Scholar]
- 33.Semenza G. Intestinal digestion and absorption of sugars. Biochem Soc. Trans. 1975;3:221–223. doi: 10.1042/bst0030221. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen LK, et al. A dynamic model of the hypoxia-inducible factor 1a (HIF-1a) network. J. Cell Sci. 2015;128:422. doi: 10.1242/jcs.167304. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, et al. LASP1 is a HIF1alpha target gene critical for metastasis of pancreatic cancer. Cancer Res. 2015;75:111–119. doi: 10.1158/0008-5472.CAN-14-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan J, et al. HIF-1alpha effects on angiogenic potential in human small cell lung carcinoma. J. Exp. Clin. Cancer Res. 2011;30:77. doi: 10.1186/1756-9966-30-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morine Y, et al. Hypoxia inducible factor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2011;58:1439–1444. doi: 10.5754/hge11156. [DOI] [PubMed] [Google Scholar]
- 38.Kitajima Y, Miyazaki K. The critical impact of HIF-1a on gastric cancer biology. Cancers (Basel) 2013;5:15–26. doi: 10.3390/cancers5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeber LM, et al. The role of hypoxia inducible factor-1alpha in gynecological cancer. Crit. Rev. Oncol. Hematol. 2011;78:173–184. doi: 10.1016/j.critrevonc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Chang LY, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- 41.Conroy H, Galvin KC, Higgins SC, Mills KH. Gene silencing of TGF-beta1 enhances antitumor immunity induced with a dendritic cell vaccine by reducing tumor-associated regulatory T cells. Cancer Immunol. Immunother. 2012;61:425–431. doi: 10.1007/s00262-011-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berchem G, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5:e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.