Abstract
When hereditary breast and ovarian cancer (HBOC) due to a BRCA1/BRCA2 germline pathogenic variant is diagnosed, the proband will be asked to inform other at-risk family members. In the Netherlands, a guideline was introduced in 2012 which provided detailed recommendations regarding this proband-mediated procedure. We now evaluated the uptake of predictive BRCA1/BRCA2 testing in 40 consecutive HBOC families diagnosed in our centre in 2014. We performed a retrospective observational study of all 40 families in which a pathogenic BRCA1/BRCA2 germline variant was identified during 2014. We scored the uptake of predictive and confirmatory testing by the end of 2018 and explored factors associated with the level of uptake. Two families were excluded. In the remaining 38 families, among 239 family members ≥18 years at 50% risk of being a mutation carrier or at 25% risk if the family member at 50% risk was deceased, 102 (43%) were tested. Among 108 females 25–75 years of age at 50% risk, 59 (55%) underwent predictive or confirmatory testing, and among 43 males at 50% risk with daughters ≥18 years, 22 (51%) were tested. Factors which complicated cascade screening included family members living abroad, probands not wanting to share information and limited pedigree information. In conclusion, the standard proband-mediated procedure of informing relatives seems to be far from optimal. We suggest a tailored approach for each family, including the option of a direct approach to at-risk family members by the geneticist. In addition, we suggest detailed monitoring and follow-up of families.
Subject terms: Human behaviour, Cancer genetics, Risk factors
Introduction
Germline pathogenic variants in BRCA1 and BRCA2 were identified in 1994 and 1995 as the main causes of autosomal dominant hereditary breast and ovarian cancer (HBOC). Diagnostic testing of patients quickly became available, as did predictive testing of healthy at-risk family members and confirmatory testing of affected relatives [1].
The cancer risks for carriers of pathogenic variants in BRCA1 or BRCA2 are high and include lifetime breast cancer risks of 60–80% for both genes and ovarian cancer risks of 30–60% and 5–20% for carriers of pathogenic germline variants in BRCA1 and BRCA2, respectively [2].
As de novo germline pathogenic variants in BRCA1 and BRCA2 are rare [3], relatives of the proband generally face a high risk of cancer when a pathogenic germline BRCA1/BRCA2 variant is identified.
Informing at-risk relatives has been recognised as an important objective of genetic counselling. If family members are not adequately informed they may present with advanced symptomatic cancers that could have been prevented if they had been notified in time. Accordingly, cost-effectiveness of BRCA1/BRCA2 testing is partly determined by the number of at-risk family members that would benefit from a positive test result in the proband [4].
In professional guidelines on cancer genetics it is generally recommended that at-risk family members are informed via the proband, in a so-called proband-mediated approach. However, it has also been recognised that this standard procedure is ineffective in many families. In a recent review of the literature and using data recorded on HBOC at genetics registries, we found that the uptake of predictive testing ranged from 21 to 44% [5]. The uptake of genetic testing was higher for specific subgroups and was generally higher for close versus distant relatives and for female versus male relatives.
However, the review also showed that a variety of factors contribute to complexity in the scoring of predictive testing. The definition of the pedigree is dependent upon information known to the proband and the efforts of the genetics centre to collect extended pedigree information. In addition, the identification of at-risk relatives is problematic when it is not known if the causative pathogenic variant was inherited from the paternal or maternal side of the family. Furthermore, when family members have not been tested it is often unclear whether they did not receive information or whether they were adequately informed but subsequently chose to refrain from testing. The evaluation of the uptake of testing is also difficult in families in which many relatives of the proband live abroad. Finally, different countries have different healthcare systems, health laws and professional guidelines, as exemplified by recent publications from the United Kingdom, France, Switzerland and Finland [6–10].
In 2012, the Dutch Society for Clinical Genetics published a guideline containing detailed recommendations regarding the proband-mediated procedure. According to this guideline, the importance of information sharing should be discussed in depth with the proband, a family letter containing detailed information aimed at at-risk family members should be sent to the proband, and follow-up counselling by telephone to discuss any difficulties in informing family members is also recommended. In exceptional cases, at-risk family members can be directly contacted by the geneticist (www.vkgn.org [11]).
However, the impact of this guideline on the uptake of testing in cancer families has not been investigated. We therefore evaluated the uptake of predictive and confirmatory BRCA1/BRCA2 testing in all families diagnosed in our centre in 2014, 2 years after the introduction of the guideline. We explored the factors associated with uptake and compared our data with those reported in the studies we recently reviewed.
Patients and methods
The diagnostic laboratory of our institute provided the identification details of all families in which a pathogenic or likely pathogenic BRCA1 or BRCA2 germline variant had been identified in 2014. The resulting number of families was 40. For all families we reviewed the pedigrees, the medical files of family members, and the outcome of diagnostic, predictive and confirmatory DNA testing. Notably, a proportion of family members who were tested for a known familial variant had previously been treated for breast, ovarian or another cancer. We applied the term predictive testing to unaffected and confirmatory testing to affected family members of the proband.
In the Netherlands, as a rule, blood samples for predictive and confirmatory testing are sent to the same laboratory in which initial diagnostic testing in the proband was performed. Therefore, all tested family members were registered at our laboratory, including those who received genetic counselling at our Family Cancer Clinic and family members counselled in other genetics centres in the Netherlands.
Following the identification of a pathogenic or likely pathogenic germline BRCA1/BRCA2 variant in the proband, additional tests were often performed to investigate whether the variant was inherited from the paternal or maternal side of the family. We listed these additional tests under the heading of diagnostic tests and not as predictive or confirmatory tests, since they preceded the sending of a family letter to the proband containing information to be distributed amongst at-risk relatives. We assessed all at-risk relatives according to the pedigree information available by the end of 2018. Ages were calculated with reference to the date of the family letter or estimated when a date of birth was unavailable.
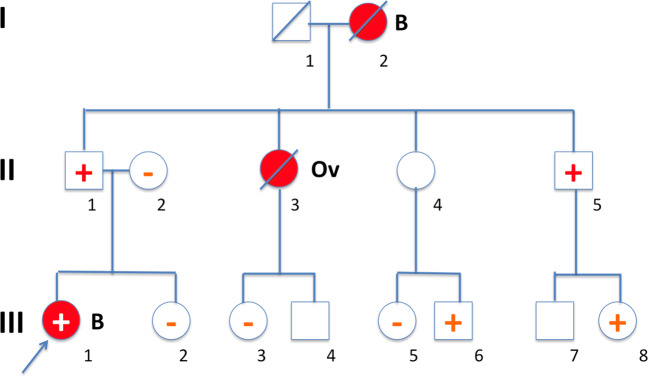
We identified all of the proband’s adult (≥18 years) family members on the affected side of the family and at 50% risk of being a mutation carrier. If a family member at 50% risk was deceased, family members at 25% risk were assessed. Two subgroups were assessed for whom a positive test result (i.e., being a mutation carrier) would have the most important clinical consequences: females at 50% risk aged 25–75 years and males at 50% risk with daughters ≥18 years. The scoring procedure used in this study is illustrated by a fictitious pedigree A depicted in Fig. 1.
Fig. 1. Scoring of at-risk and tested family members.
In this fictitious pedigree (A), a pathogenic BRCA1 germline variant was identified in the proband affected with breast cancer (III-1). Subsequently, both her parents were tested and, as expected based on pedigree data (no breast or ovarian cancer had occurred in the maternal family members, data not shown), the father of the proband proved to be a carrier of the pathogenic germline BRCA1 variant. As a next step, a letter was sent to the family for circulation amongst the proband’s at-risk paternal family members. Testing of the proband and both her parents was labelled as diagnostic testing, while subsequent testing of additional family members was labelled as predictive or confirmatory testing for unaffected and affected at-risk relatives, respectively. When the family first received the letter, five adult relatives were at 50% risk, or at 25% risk if a parent at 50% risk was deceased, comprising the relatives II-4, II-5, III-2, III-3 and III-4. Three of these five relatives were tested. It should be noted that additional family members were tested, including III-5 and III-6 once they learned that their mother did not wish to be tested, and III-8 based on cascade testing after her father was found to be mutation carrier. In the current study, II-4 was scored as untested; however, the fact that the offspring III-5 and III-6 were tested is relevant and is given as extra information in Table 1. The fact that individual III-8 was tested has been added to the total number of tested family members, but individual III-8 was not counted as an at-risk family member since she was originally not at-risk (before her father was tested) according to the criteria used.
Apart from the data obtained for the period 2014–2018, we also evaluated the uptake of testing over the course of time, at quarterly (q) periods between 2014 and 2018.
Results
Among the 40 families, 23 had a pathogenic germline BRCA1 variant, 16 a pathogenic germline BRCA2 variant and one (family 32) a likely pathogenic BRCA2 germline variant. The results for the 40 families are summarised in Table 1 and Table 2. Two families, 5 and 40, were excluded from the general analysis. In family 5, a pathogenic BRCA1 variant had previously been identified in another branch of the family at another centre. In family 40, it appeared that a pathogenic BRCA1 variant had already been identified at our centre in 2007 and the test performed in 2014 showed the same result.
Table 1.
Predictive and confirmatory DNA testing in 40 families with a pathogenic or likely pathogenic BRCA1 or BRCA2 germline variant identified in 2014. Results up to the end of 2018.
| Families | Number of diagnostic and predictive or confirmatory DNA tests performed | At-risk and tested family members | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total number of family members ≥18 years at 50% risk or 25% if at-risk parent was deceased | Number of alive female family members 25–75 years of age at 50% risk | Number of alive at-risk male family members at 50% risk with daughters ≥18 years | ||||||||
| Fam. No. | General remarks | Total | Diagnostic | Predictive and confirmatory | At risk | Tested | At risk | Tested | At risk | Tested |
| 1 | B2a M | 7 | 1 | 6 | 11 | 6 | 3 | 3 | 5 | 2 |
| 2 | B1a M | 3 | 1 | 2 | 3 | 3 | 1 | 1 | 0 | 0 |
| 3 | B1a | 4 | 1 | 3 | 3 | 2 | 1 | 1 | 0 | 0 |
| 4 | B1 M | 3 | 1 | 2 | 10 | 2 | 4 | 1 | 0 | 0 |
| 5 | B1 Excluded (see Text) | |||||||||
| 6 | B2 | 4 | 3 | 1 | 2 | 1 | 1 | 1 | 0 | 0 |
| 7 | B1a M | 2 | 1 | 1 | 4 | 1 | 1 | 1 | 0 | 0 |
| 8 | B2 P | 5 | 1 | 4 | 9 | 4 | 5 | 3 | 2 | 1 |
| 9 | B1 | 8 | 3 | 5 | 10 | 5 | 2 | 1 | 3 | 3 |
| 10 | B2a Mc | 3 | 3 | 0 | 4 | 0 | 1 | 0 | 2 | 0 |
| 11 | B2a M | 10 | 3 | 7 | 5 | 4 | 3 | 2 | 2 | 2 |
| 12 | B2a | 4 | 1 | 3 | 5 | 3 | 2 | 2 | 1 | 0 |
| 13 | B2b | 2 | 2 | 0 | 11 | 0 | 4 | 0 | 2 | 0 |
| 14 | B2 | 5 | 2 | 3 | 3 | 3 | 2 | 2 | 1 | 1 |
| 15 | B2aP | 8 | 1 | 7 | 4 | 4 | 2 | 2 | 2 | 2 |
| 16 | B2 Pc | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| 17 | B2 | 5 | 2 | 3 | 8 | 3 | 1 | 0 | 1 | 1 |
| 18 | B2a | 7 | 1 | 6 | 12 | 5 | 7 | 3 | 1 | 1 |
| 19 | B1c | 2 | 2 | 0 | 11 | 0 | 2 | 0 | 1 | 0 |
| 20 | B2a,b | 1 | 1 | 0 | 11 | 0 | 6 | 0 | 3 | 0 |
| 21 | B1aM | 4 | 1 | 3 | 4 | 3 | 2 | 2 | 1 | 1 |
| 22 | B2a,b | 1 | 1 | 0 | 5 | 0 | 2 | 0 | 1 | 0 |
| 23 | B1 | 5 | 2 | 3 | 5 | 3 | 3 | 2 | 1 | 1 |
| 24 | B1 | 4 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 |
| 25 | B1a | 5 | 1 | 4 | 6 | 3 | 4 | 2 | 1 | 0 |
| 26 | B1 | 17 | 2 | 15 | 6 | 6 | 5 | 5 | 1 | 1 |
| 27 | B1a,b | 1 | 1 | 0 | 6 | 0 | 6 | 0 | 0 | 0 |
| 28 | B2 | 5 | 2 | 3 | 3 | 3 | 2 | 2 | 0 | 0 |
| 29 | B1a,b | 5 | 1 | 4 | 12 | 3 | 6 | 2 | 1 | 0 |
| 30 | B1 | 15 | 1 | 14 | 6 | 5 | 3 | 3 | 2 | 2 |
| 31 | B1 | 7 | 1 | 6 | 5 | 5 | 2 | 2 | 1 | 1 |
| 32 | B2 | 12 | 2 | 10 | 13 | 7 | 5 | 3 | 2 | 1 |
| 33 | B1 | 4 | 2 | 2 | 5 | 2 | 2 | 2 | 0 | 0 |
| 34 | B1a | 5 | 1 | 4 | 3 | 3 | 1 | 1 | 0 | |
| 35 | B1a M | 5 | 1 | 4 | 6d | 3 | 5 | 2 | 0 | 0 |
| 36 | B1a | 5 | 1 | 4 | 12 | 3 | 3 | 3 | 3 | 0 |
| 37 | B1 | 9 | 2 | 7 | 2 | 1 | 1 | 1 | 0 | 0 |
| 38 | B1 | 9 | 2 | 7 | 6 | 4 | 3 | 2 | 2 | 2 |
| 39 | B2aM | 3 | 1 | 2 | 4 | 1 | 4 | 1 | 0 | 0 |
| 40 | B1 Excluded (see Text) | |||||||||
| Total of all 38 families | 206 | 59 | 147 | 239 | 102/239 43% | 108 | 59/108 55% | 43 | 22/43 51% | |
| Total of 33 families (5 familiesb excluded) | 196 | 53 | 143 | 194 | 99/194 51% | 84 | 57/84 68% | 37 | 22/37 59% | |
General remarks: B1/B2: pathogenic BRCA1/BRCA2 germline variant (one likely pathogenic BRCA2 variant in family 32).
aNot known if the mutation was inherited from the (grand-) paternal or the (grand-) maternal side of the family; P: presumably from the (grand-) paternal side, M: presumably from the (grand-) maternal side.
bAll or most family members living abroad.
cThe proband did not wish to inform at-risk relatives.
dIn family 35 one tested relative was <18 years of age.
In family 2 one untested male at-risk family member was scored as tested since he proved to be an obligate variant carrier after his son was shown to be variant carrier. In each of three families (15, 18 and 38) one untested at-risk male was scored as tested since all of their children (one daughter, two daughters and one daughter and one son, respectively) had been tested (all five were negative for the familial variant).
Table 2.
Number and percentages of tested female and male at-risk relatives, all age groups ≥18 years includeda.
| Age (y) | No. at risk | Females | Males | ||||
|---|---|---|---|---|---|---|---|
| No. at risk | Tested | No. at risk | Tested | ||||
| No. | % | No | % | ||||
| 18–20 | 6 | 4 | 1 | 25% | 2 | 0 | 0% |
| 21–25 | 11 | 6 | 4 | 67% | 5 | 1 | 20% |
| 26–30 | 7 | 2 | 2 | 100% | 5 | 1 | 20% |
| 31–35 | 6 | 2 | 2 | 100% | 4 | 1 | 25% |
| 36–40 | 20 | 12 | 9 | 75% | 8 | 3 | 38% |
| 41–45 | 24 | 16 | 11 | 69% | 8 | 2 | 25% |
| 46–50 | 26 | 11 | 5 | 45% | 15 | 5 | 33% |
| 51–55 | 31 | 16 | 9 | 56% | 15 | 5 | 33% |
| 56–60 | 28 | 12 | 6 | 50% | 16 | 7 | 44% |
| 61–65 | 29 | 15 | 6 | 40% | 14 | 6 | 43% |
| 66–70 | 13 | 6 | 2 | 33% | 7 | 4 | 57% |
| 71–75 | 10 | 4 | 4 | 100% | 6 | 3 | 50% |
| 76–80 | 6 | 4 | 0 | 0% | 2 | 0 | 0% |
| 81+ | 10 | 3 | 1 | 33% | 7 | 1 | 14% |
| Total | 227 | 113 | 62 | 55% | 114 | 39 | 34% |
aThe total number is less than the total number in Table 1 due to the fact that for some family members no information on age was available; in family 35 one at-risk female was tested below age 18.
In the remaining 38 families, the number of predictive or confirmatory tests varied from 0 to 15, with a mean number of 3.9 tests per family. In eight families, not a single family member was registered at our laboratory for predictive or confirmatory testing. In three of these eight families—families 10, 16 and 19—the proband did not wish to contact any of his/her at-risk family members. In-depth counselling did not alter that decision and we have not directly contacted the proband’s family members. In the remaining five families, numbers 13, 20, 22, 27 and 29, most or all at-risk family members lived abroad.
Among 239 adult family members at 50% risk of being a mutation carrier (or at 25% risk if the individual at 50% risk was deceased), 102 (43%) were tested. Among 108 females at 50% risk and 25–75 years of age, 59 (55%) underwent predictive or confirmatory testing, and among 43 males at 50% risk with daughters ≥18 years, 22 (51%) were tested. If the five families in which most or all at-risk families members lived abroad are excluded from the analysis, the 33 remaining families showed an uptake of 99/194 (51%) for the total at-risk group, and 57/84 (68%) and 22/37 (59%), respectively, for the specific female and male subgroups.
Information on age was available for 227 at-risk family members. Among 113 at-risk females the uptake was 62/113 (55%) and among 114 males the uptake was 39/114 (34%) (Table 2).
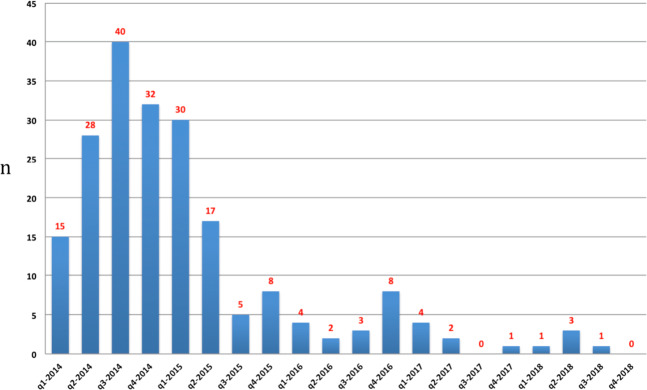
Of the diagnostic and presymptomatic or confirmatory tests performed by the end of 2018, 175/204 (86%) were performed by the end of 2015. The number of tests performed per quarter over this period is depicted in Fig. 2.
Fig. 2. DNA testing in 38 families with a pathogenic BRCA1/BRCA2 germline variant 2014–8.
The number of DNA tests performed in 38 families with a pathogenic BRCA1/BRCA2 germline variant evaluated quarterly (q), based on our laboratory registry. Both diagnostic tests, predictive and confirmatory tests are listed. Two tests performed abroad are listed in Table 2 (total number 206) but not in Fig. 2 since the dates of testing are unknown (total number 204).
Nineteen family members had some form of cancer preceding DNA testing. Most but not all cancers were confirmed by medical or pathology records. In four patients the tumour types were probably not related to the pathogenic germline BRCA1 or BRCA2 variant (cancer of the tongue, cervix, skin (melanoma) and large bowel, respectively). Among the remaining 15 patients, 12 had breast cancer or ductal carcinoma in situ of the breast, two had ovarian cancer and one prostate cancer. The uptake rate of genetic testing among all patients with cancer was 9/19 (47%). Among the patients with tumours probably associated with the germline pathogenic BRCA1 or BRCA2 variant the uptake rate was 8/15 (53%). Notably, in the latter group two breast cancer patients (diagnosed at ages 34 and 56, respectively), were negative for the familial pathogenic variant.
While we did not systematically collect information on the reasons why at-risk family members were not tested, some information was available on family members counselled at our clinic. In two families, at-risk daughters of a patient with ovarian cancer and a BRCA2 mutation decided, after genetic counselling, that “for the moment” they would undergo breast surveillance without predictive testing. We have not recorded either testing or preventive surgery during follow-up. In a family with a BRCA1 variant, a male family member with young daughters underwent genetic counselling and decided that he would undergo testing in the future when the result would be relevant for his offspring.
Discussion
In this retrospective observational study, we evaluated the effects of the most recent national professional guideline concerning informing at-risk family members in families with hereditary cancer syndromes. After 4–5 years of follow-up, the uptake of predictive testing in 38 families with HBOC was 43%. The uptake was higher in women than in men (55% and 34%, respectively). In the two subgroups for whom we considered testing most clinically important—adult females (25–75 years) at 50% risk and males at 50% risk with adult (≥18 years) daughters—the uptake rates, at 55% and 51%, respectively, were more favourable than the overall uptake of 43%.
After exclusion of the five families in which all or most at-risk family members lived abroad, the total and subgroup percentages were higher, although this increase might be somewhat artificial since these five families had the lowest uptake rates at our laboratory and data on possible testing in foreign laboratories was lacking.
In the introduction we discussed the complexity of evaluating the uptake of predictive testing. For example, in an evaluation by Sanz et al. [12] 25 families were excluded from a set of 133 pedigrees due to loss of follow-up data. In a study by Sermijn et al. [13], the counsellors had emphasised that the proband “should not feel obliged to inform relatives, and should only inform relatives if comfortable to do so”. Therefore, we must exercise caution when comparing our results with those reported in literature for HBOC [5]. However, our results imply that, among the families counselled in our clinic, a large proportion of at-risk family members remain untested.
Importantly, we do not know to what extent family members received no information from the proband or, alternatively, were informed but chose not to be tested. Based on literature and illustrative examples registered in our medical files, it is clear that a lack of adequate sharing of information by the proband and postponing or nonparticipation in testing by informed family members both commonly occur. In some studies uptake increased substantially after at-risk relatives were approached directly by a genetics centre [13, 14], suggesting that these relatives had not yet received adequate information. Other authors have reported that some relatives informed by the proband refrained from counselling and testing. It should be noted that some relatives may not have fully understood the implications of the information received [15]. In our clinic some women decided, after in-depth genetic counselling, that they wished to refrain from testing and would undergo periodic mammography.
The main strength of our evaluation is that it is the outcome of a single-centre study performed shortly after the introduction, in 2012, of a new and detailed national guideline on the procedure of informing family members. This implies that the counselling procedures practiced by members of the team were probably quite similar. In addition, extended pedigree information was available for all families.
Our study also had several limitations. First, although we collected as much pedigree information as possible, there are still missing data on the number of at-risk relatives, their ages and offspring. The numbers and percentages presented on the uptake of testing were based on the available pedigree information. Second, we may not have collected all available DNA test results, since diagnostic or predictive tests might have been performed in another centre in the Netherlands if the geneticist in that centre did not know that a branch of the family had previously been evaluated at our clinic. In addition, family members living abroad may have been tested in centres in their country of residence.
The time interval between the identification of the gene variant in the proband and the uptake of testing by an at-risk family member is relevant since during that time interval at-risk family members will probably not take preventive measures. An illustrative example is family 38 in which a causative BRCA1 germline variant was found in a 39-year-old patient with ovarian cancer. A sister of the proband underwent predictive testing and proved to be a variant carrier, which was soon followed by preventive salpingo-oophorectomy. Histologically, an early-stage ovarian cancer was diagnosed and treated by surgery and systemic therapy. At the last follow-up in September 2019 there were no signs of recurrence.
In our cohort we found that the large majority of predictive testing (86%) took place within 1–2 years after the gene defect was diagnosed in the proband. These findings are comparable to the time intervals found in literature [16, 17]. It therefore seems that only a small minority of at-risk family members are subject to an unduly large time interval. Notably, the reasons for a long time interval may differ: late sharing of information by the proband, postponement of testing by the informed family member, or new circumstances, for example, when a daughter of a variant carrier reaches the age of 25, the recommended starting age for breast surveillance in variant carriers.
To summarise, in the families with HBOC evaluated here, only around half of all family members at highest risk of being carrier of a pathogenic BRCA1/BRCA2 variant underwent predictive testing under the current standard proband-mediated procedure.
Clinical genetics is a field in which not only the proband is considered, but also his or her family members. Professional guidelines recommend that adequate information should be provided to all at-risk relatives. However, both our study and data from literature strongly suggest that the current procedure is inadequate.
The subject of informing family members is of increasing importance due to several factors, including broader indications for testing, increased use of gene panel testing, testing shortly after diagnosis to guide cancer management, and the mainstreaming of genetic testing by treating physicians [18–20].
Various procedures have been proposed to enhance cascade screening, including additional counselling of the proband [15, 21], home visits by a genetic field worker [22], and follow-up consultations with family members who undergo surveillance [7]. Another option is that the geneticist directly approaches at-risk family members. This approach has been explored in several studies and is now being considered in several genetics centres and registries [9, 23], including our own.
Progress in this field seems to be hampered by an on-going and complex debate among healthcare professionals that encompasses health law, confidentiality, the right not to know, duties of the patient, duties of the doctor and perceived time restraints [8, 24].
After reviewing all pedigrees, an essential conclusion is that each family is unique and deserves a tailored approach. The structure of each pedigree is unique, as is the medical situation of each proband and her or his relationships with close and distant family members.
In 2019, the national Dutch guideline was updated and now includes, apart from hereditary tumour syndromes, hereditary cardiac and neurological conditions. In this latest guideline the standard procedure remains the proband-mediated sharing of information, but the option of the clinical geneticist directly contacting relatives is emphasised.
In conclusion, we cannot exclusively rely on the standard proband-mediated procedure regarding the informing of at-risk relatives. We suggest that, in addition to the proband-mediated procedure, a tailored approach for each family should be introduced, including the option of direct contact with at-risk family members by the geneticist. We should probably also reconsider the burden of predictive testing on healthy family members and increase efforts with regard to psychosocial support. In addition, we suggest detailed monitoring and follow-up of families in order to improve insight into the information cascade and the uptake of testing and preventive measures.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szabo CI, King MC. Inherited breast and ovarian cancer. Hum Mol Genet. 1995;4:1811–7. doi: 10.1093/hmg/4.suppl_1.1811. [DOI] [PubMed] [Google Scholar]
- 2.Hereditary Tumours. Guidelines for Diagnosis and Prevention. Leiden:The Netherlands Foundation for the Detection of Hereditary Tumours and Dutch Society for Clinical Genetics; 2010. ISBN 978-90-806183-2-9 (in Dutch).
- 3.Golmard L, Delnatte C, Laugé A, Moncoutier V, Lefol C, Abidallah K, et al. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene. 2016;35:1324–7. doi: 10.1038/onc.2015.181. [DOI] [PubMed] [Google Scholar]
- 4.Tuffaha HW, Mitchell A, Ward BL, Connely L, Butler JRG, Norris S, et al. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med. 2018;20:985–94. doi: 10.1038/gim.2017.231. [DOI] [PubMed] [Google Scholar]
- 5.Menko FH, Ter Stege JA, van der Kolk LE, Jeanson KN, Schats W, Moha DA, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127–35. doi: 10.1007/s10689-018-0089-z. [DOI] [PubMed] [Google Scholar]
- 6.Parker M, Lucassen A. Using a genetic test result in the care of family members: how does the duty to confidentiality apply? Eur J Hum Genet. 2018;26:955–9. doi: 10.1038/s41431-018-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derbez B, de Pauw A, Stoppa-Lyonnet D, de Montgolfier S. Supporting disclosure of genetic information to family members: professional practice and timelines in cancer genetics. Fam Cancer. 2017;16:447–57. doi: 10.1007/s10689-017-9970-4. [DOI] [PubMed] [Google Scholar]
- 8.D’Audiffret van Haecke D, de Montgolfier S. Genetic diseases and information to relatives: practical and ethical issues for professionals after introduction of a legal framework in France. Eur J Hum Genet. 2018;26:786–95. doi: 10.1038/s41431-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seppälä TT, Pylvänäinen K, Mecklin J-P. Uptake of genetic testing by the children of Lynch syndrome variant carriers across three generations. Eur J Hum Genet. 2017;25:1237–45. doi: 10.1038/ejhg.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolaidis C, Ming C, Pedrazzani C, van der Horst T, Kaiser-Grolimund A, Ademi Z, et al. Challenges and opportunities for cancer predisposition cascade screening for hereditary breast and ovarian cancer and Lynch syndrome in Switzerland: findings from an international workshop. Public Health Genom. 2018;21:121–32. doi: 10.1159/000496495. [DOI] [PubMed] [Google Scholar]
- 11.Menko FH, Aalfs, Henneman L, Stol Y, Wijdenes M, Otten E, et al. Dutch society for clinical genetics. Informing family members of individuals with Lynch syndrome: a guideline for clinical geneticists. Fam Cancer. 2013;12:319–24. doi: 10.1007/s10689-013-9636-9. [DOI] [PubMed] [Google Scholar]
- 12.Sanz J, Ramón y Cajal T, Torres A, Darder E, Gadea N, Velasco A, et al. Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: a multicenter study in northeastern Spain. Fam Cancer. 2010;9:297–304. doi: 10.1007/s10689-009-9313-1. [DOI] [PubMed] [Google Scholar]
- 13.Sermijn E, Delesie L, Deschepper E, Pauwels I, Bonduelle M, Teugels E, et al. The impact of an interventional counseling procedure in families with a BRCA1/2 gene mutation: efficacy and safety. Fam Cancer. 2016;15:155–62. doi: 10.1007/s10689-015-9854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006;43:665–70. doi: 10.1136/jmg.2005.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly MB, Montgomery S, Bingler R, Ruth K. Communicating genetic test results within the family: is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer. 2016;15:697–706. doi: 10.1007/s10689-016-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks L, Lennard F, Shenton A, Lalloo F, Ambus I, Ardern-Jones A, et al. BRCA1/2 predictive testing: a study of uptake in two centres. Eur J Hum Genet. 2004;12:654–62. doi: 10.1038/sj.ejhg.5201206. [DOI] [PubMed] [Google Scholar]
- 17.Holloway SM, Bernhard B, Campbell H, Lam WWK. Uptake of testing for BRCA1/2 mutations in South East Scotland. Eur J Hum Genet. 2007;16:906–12. doi: 10.1038/ejhg.2008.17. [DOI] [PubMed] [Google Scholar]
- 18.Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–8. doi: 10.1038/nrclinonc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACOG Committee Opinion. Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynaecol. 2018;131:e31–4. doi: 10.1097/AOG.0000000000002457. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs C, Patch C, Michie S. Communication about genetic testing with breast and ovarian cancer patients: a scoping review. Eur J Hum Genet. 2019;27:511–24. doi: 10.1038/s41431-018-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Geus E, Eijzenga W, Menko FH, Sijmons RH, de Haes CJM, Aalfs CM, et al. Design and feasibility of an intervention to support cancer genetic counselees in informing their at-risk relatives. J Genet Couns. 2016;25:1179–87. doi: 10.1007/s10897-016-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116A:11–9. doi: 10.1002/ajmg.a.10868. [DOI] [PubMed] [Google Scholar]
- 23.Katapodi MC, Viassolo V, Caiata-Zufferey M, Nikolaidis K, Bührer-Landolt R, Buerki R, et al. Cancer predisposition cascade screening for hereditary breast/ ovarian cancer and Lynch syndromes in Switzerland: study protocol. JMIR Res Protoc. 2017;6:e184. doi: 10.2196/resprot.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dove ES, Chico V, Fay M, Laurie G, Lucassen AM, Postan E, et al. Familial genetic risk: how can we better navigate patient confidentiality and appropriate risk disclosure to relatives? J Med Ethics. 2019;45:504–7. doi: 10.1136/medethics-2018-105229. [DOI] [PMC free article] [PubMed] [Google Scholar]