Abstract
Metal hydrides have been rarely used in biomedicine. Herein, we fabricate titanium hydride (TiH1.924) nanodots from its powder form via the liquid-phase exfoliation, and apply these metal hydride nanodots for effective cancer treatment. The liquid-phase exfoliation is an effective method to synthesize these metal hydride nanomaterials, and its efficiency is determined by the matching of surface energy between the solvent and the metal hydrides. The obtained TiH1.924 nanodots can produce reactive oxygen species (ROS) under ultrasound, presenting a highly efficient sono-sensitizing effect. Meanwhile, TiH1.924 nanodots with strong near-infrared (NIR) absorbance can serve as a robust photothermal agent. By using the mild photothermal effect to enhance intra-tumoral blood flow and improve tumor oxygenation, a remarkable synergistic therapeutic effect is achieved in the combined photothermal-sonodynamic therapy. Importantly, most of these TiH1.924 nanodots can be cleared out from the body. This work presents the promises of functional metal hydride nanomaterials for biomedical applications.
Subject terms: Cancer therapy, Nanoscale materials, Nanobiotechnology
Dynamic therapy is attracting attention for cancer treatment. Here, the authors report that metal hydride nanodots can be used for sonodynamic therapy, which can be further enhanced by photothermal heating to increase tissue oxygenation.
Introduction
Sonodynamic therapy (SDT) triggered by ultrasound (US) is a non-invasive therapeutic strategy that can be applied to treat deeply-seated tumors1–4. During SDT, sono-sensitizers are able to interact with surrounding oxygen and even water molecules to produce cytotoxic reactive oxygen species (ROS) to kill tumor cells2,4–7. However, the limitations of current sono-sensitizers have substantially hindered the extensive clinical applications of SDT. Traditional organic sono-sensitizers (e.g., photofrin8, phthalocyanine9, and chlorophyll derivative10), which are often derived from photo-sensitizers, often show photo-toxicity toward the skin11,12. The most representative paradigm of inorganic sono-sensitizers is semiconductor titanium dioxide (TiO2)13–15, whose quantum yield of US-triggered ROS generation, however, is relatively low due to the fast combination of the electron (e−) and holes (h+) (50 ± 30 ns)16,17.
Titanium hydride (TiH1.924) has been explored for applications in hydrogen storage18,19, and is frequently used as a foaming agent in the production of metallic foams (e.g., Zn, Al foams)20,21, as well as a raw material for producing highly purified titanium and titanium alloys22,23. Considering the unique valence status of Ti (containing Ti0, Ti2+, Ti3+, and Ti4+) in TiH1.924,24 we hypothesize that it might be easily activated by external stimuli (e.g., light, ultrasound, and microwave) for applications in photo-catalysis and sono-catalysis25,26. However, nano-structured TiH1.924 has not yet been synthesized to our best knowledge.
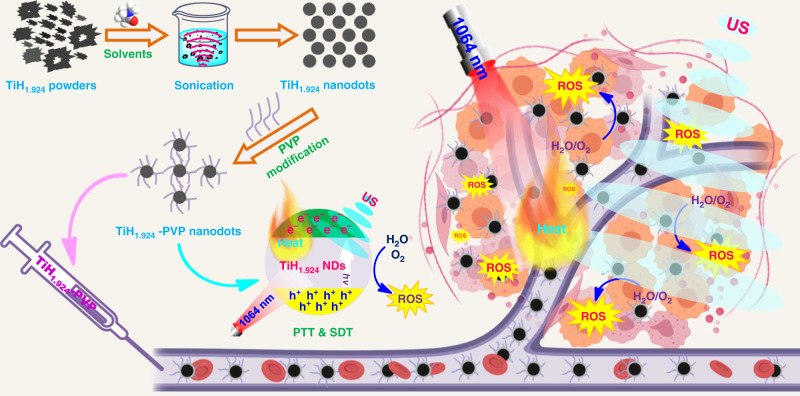
Liquid-phase exfoliation usually by sonicating bulk materials in appropriate solvents is a simple top-down route to produce various types of nanomaterials. In this work, we successfully exfoliate TiH1.924 powder into ultrasmall nanodots via the liquid-phase exfoliation technology, and find that the surface energy plays an important role in the formation of the ultrasmall TiH1.924 nanodots. In addition, this liquid-phase exfoliation is an effective method to synthesize various types of metal hydride nanomaterials (e.g., TiH1.924, ZrH2, CaH2, and HfH1.983). Taking TiH1.924 nanodots for example, they have highly effective US-triggered ROS generation capability, which is superior to the sono-sensitizing effect of titanium dioxide (TiO2), the classical inorganic sono-sensitizer, likely owing to the reduced bandgap in TiH1.924. Moreover, these black TiH1.924 nanodots with strong near-infrared (NIR) absorption can also use as an excellent photothermal agent. Taking the advantage of mild photothermal effect to enhance intra-tumor blood flow and improve oxygen supply, a remarkably synergistic photothermal-sonodynamic therapeutic outcome has been achieved with TiH1.924 nanodots (Fig. 1). In a mouse tumor model, the complete tumor eradication without recurrence is achieved after intravenous injection of TiH1.924 nanodots and exposure of tumors to light and ultrasound, sequentially. Importantly, these TiH1.924 nanodots with ultra-small sizes show efficient body excretion and no appreciable toxicity to the treated animals. This work highlights the potential of metal hydride nanomaterials as physical stimuli-triggered nanoagents for cancer treatment.
Fig. 1. The preparation and application of TiH1.924 nanodot.
Schematic illustration to show the preparation of TiH1.924 nanodots by liquid-phase exfoliation and their applications for combined photothermal-sonodynamic cancer therapy.
Results
Preparation and characterization of TiH1.924 nanodots
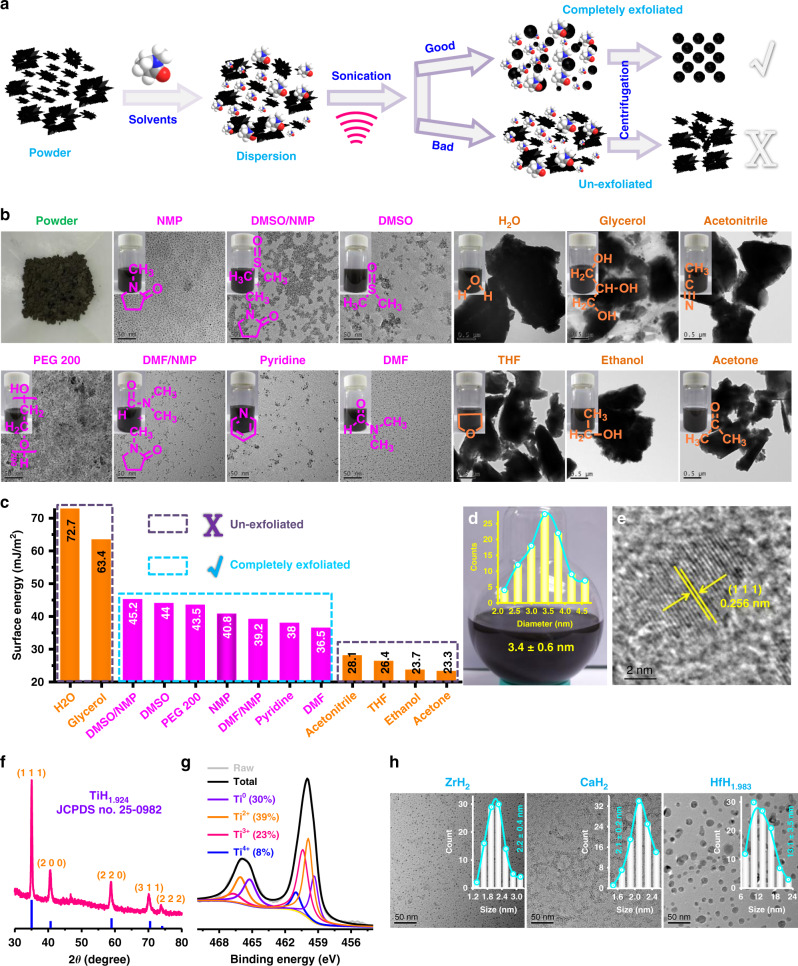
Liquid-phase exfoliation technology has been widely reported for the preparation of mono- or few-layered two-dimensional nanosheets27–30. In this work, we unexpectedly found that metal hydrides powder could be easily exfoliated into nanodots using liquid-phase exfoliation technology in the presence of appropriate solvents (Fig. 2a). Taking TiH1.924 for example, we initially sonicated commercial TiH1.924 powder in a number of exfoliation solvents (Fig. 2a, b). Among these 13 solvents including water, glycerol, dimethyl sulfoxide/N-methyl pyrrolidone (DMSO/NMP), DMSO, polyethylene glycol 200 (PEG 200), NMP, N,N-dimethylformamide (DMF)/NMP, pyridine, DMF, acetonitrile, tetrahydrofuran (THF), ethanol and acetone, 6 solvents of them (water, glycerol, acetonitrile, THF, ethanol, and acetone) showed no exfoliation effects to the TiH1.924 powder, while the other 7 solvents (DMSO/NMP, DMSO, PEG 200, NMP, DMF/NMP, pyridine, and DMF) could efficiently exfoliate the TiH1.924 powder into small nanoparticles (Fig. 2b). The different exfoliation results might be attributed to the surface energy of these solvents (Fig. 2c)27,31–33. For these 13 solvents, when the surface energy is too high (H2O, 72.7; glycerol, 63.4 mJ m−2) or too low (acetonitrile, 28.1; THF, 26.3; ethanol, 23.7; acetone, 23.3 mJ m−2), TiH1.924 powder could not be effectively exfoliated. When the surface energy of the solvent (DMSO/NMP, 45.2; DMSO, 44; PEG 200, 43.5; NMP, 40.8; DMF/NMP, 39.2; pyridine, 38; DMF, 36.5 mJ m−2) reaches a range of 41 ± 5 mJ m−2, successful exfoliation of the TiH1.924 powder into small nanoparticles could be achieved. Therefore, we proposed that the successful exfoliation might be owing to the matching of surface energy between the applied solvents and the TiH1.924 powder.
Fig. 2. Preparation and characterization of TiH1.924 nanodots.
a Schematic illustration to show light-phase exfoliation to prepare TiH1.924 nanodots. b A photograph of commercial TiH1.924 powder, the TEM images and corresponding photographs of exfoliated dispersions using various solvents (H2O, glycerol, dimethyl sulfoxide/N-methyl pyrrolidone (DMSO/NMP) DMSO/NMP, DMSO, polyethylene glycol 200 (PEG 200), NMP, N,N-dimethylformamide (DMF)/NMP, pyridine, DMF, acetonitrile, tetrahydrofuran (THF), ethanol, and acetone) for TiH1.924 exfoliation. c The surface energies of various solvents used to exfoliate TiH1.924. d A photograph of exfoliated TiH1.924 nanodots in NMP. Inset is the particle-size distribution (PSD) of TiH1.924 nanodots determined by the TEM image (n = 100 nanodots examined over TEM images). e High-resolution TEM (HRTEM) image of TiH1.924 nanodots. f XRD spectra of TiH1.924 nanodots. g XPS spectra to show Ti 2p peaks for the TiH1.924 nanodots sample. h TEM images and PSD of ZrH2 nanodots, CaH2 nanodots, and HfH1.983 nanoparticles exfoliated in NMP (n = 100 nanomaterials examined over TEM images). A representative image of three biological replicates from each group is shown in b, e.
Among these 13 solvents, NMP offered excellent exfoliation efficiency and the obtained TiH1.924 nanodots showed very uniform sizes and morphology (Fig. 2b). Thus, we employed NMP as the representative solvent to investigate the liquid-phase exfoliation of TiH1.924 powder. After exfoliation of TiH1.924 powder by sonication in NMP for different periods of time (Supplementary Fig. 1A–C), we found that the intensities of the X-ray diffraction (XRD) characteristic peaks decreased significantly by a time-dependent manner (Supplementary Fig. 1D). Particularly, after 20 min of ultrasonication, the TiH1.924 powder was entirely exfoliated into nanodots. Most importantly, this sample could be scaled-up to prepare large amounts of TiH1.924 nanodots with high quality, and the obtained TiH1.924 nanodots with an average diameter of 3.4 ± 0.6 nm could be well dispersed in NMP (Fig. 2d). The high-resolution TEM (transmission electron microscope) determined the lattice spacing to be 0.256 nm (Fig. 2e), which could be assigned to the (1 1 1) lattice plane of TiH1.924 (JCPDS No. 25-0982) (Fig. 2f)34. The energy dispersive spectrometer (EDS) spectrum also confirmed the existence of Ti elements (Supplementary Fig. 2). Based on the X-ray photoelectron spectroscopy (XPS, Supplementary Fig. 3), Ti with various valence states including Ti0 (30%), Ti2+ (39%), Ti3+ (23%), and Ti4+ (8%) were found in the obtained TiH1.924 nanodots (Fig. 2g)24. Apart from the TiH1.924 powder, we also successfully exfoliated zirconium hydride (ZrH2), calcium hydride (CaH2), and hafnium hydride (HfH1.983) powder using the liquid-phase exfoliation technology with the assistance of NMP (Fig. 2h, Supplementary Fig. 4). The obtained ZrH2 nanodots (2.2 ± 0.4 nm), CaH2 nanodots (2.1 ± 0.2 nm), and HfH1.983 nanoparticles (13.1 ± 3.5 nm) all showed uniform morphology, suggesting that the liquid-phase exfoliation technology could be a simple and universal method to prepare various types of metal hydrides nanomaterials.
Sonodynamic and photothermal performance of TiH1.924 nanodots
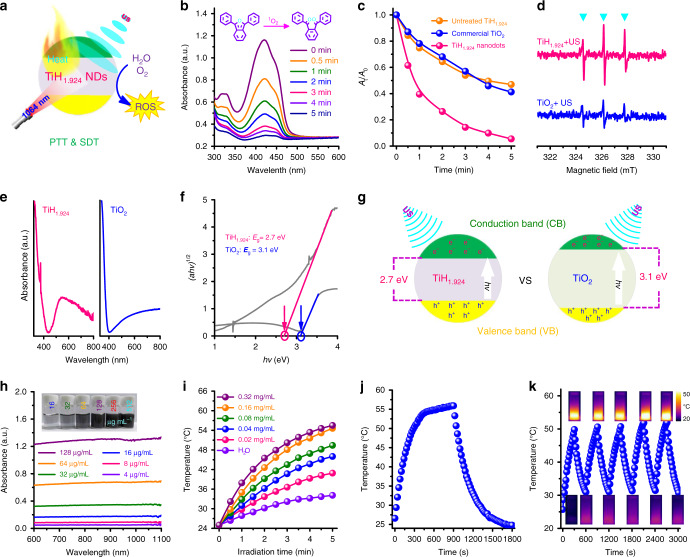
The special valence structure of TiH1.924 nanodots indicates that they might be activated under US irradiation as a sono-sensitizer (Fig. 3a). Thus, to explore whether TiH1.924 nanodots could enhance sono-catalysis, 1,3-diphenylisobenzofuran (DPBF), a reactive oxide species (ROS) probe, was employed to detect the ROS generation by US-activated TiH1.924 nanodots. After mixing TiH1.924 nanodots with the DPBF probe, the UV-vis absorption spectrum of the mixture was monitored after different periods of ultrasound (US) irradiation (Fig. 3b, Supplementary Fig. 5). Undergoing a series of US irradiation time, the intensity of DPBF characteristic absorption peak at 420 nm showed significant decrease, suggesting the quenching of probe by the generated ROS. Compared with commercial TiO2 nanoparticles and untreated TiH1.924 powder, the exfoliated TiH1.924 nanodots exhibited a higher DPBF oxidation rate under the same US irradiation (Fig. 3c, Supplementary Fig. 6), indicating that TiH1.924 nanodots could serve as a stronger sono-sensitizer than TiO2. In addition, TiH1.924 sono-sensitizers with higher exfoliation degrees showed better sonodynamic performance (Supplementary Fig. 7). Electron spin resonance (ESR) detection was also performed to compare the generated ROS (1O2, ·O2−, and ·OH) between TiH1.924 and TiO2 sono-sensitizer. (Fig. 3d, Supplementary Figs. 8 and 9). The characteristic peak intensities of the TiH1.924 plus US showed a great increase than that of TiO2, further demonstrating that TiH1.924 nanodots could be activated to generate large amounts of ROS under US irradiation.
Fig. 3. Sonodynamic and photothermal performance of TiH1.924 nanodots.
a Schematic illustration of sonodynamic and photothermal properties of TiH1.924 nanodots. b Time-dependent oxidation of DPBF indicating ROS generation by US-activated TiH1.924 nanodots. c Comparison of DPBF oxidation by TiH1.924 nanodots, untreated TiH1.924, and commercial TiO2 under US irradiation for 5 min. d ESR spectra demonstrating ROS (1O2) generation for TiH1.924 and TiO2 under US irradiation for 1 min. e, f Normalized absorption spectra (e) and optical bandgaps (f) of TiH1.924 nanodots and TiO2. g Schematic illustration of the activation mechanism of TiH1.924 and TiO2 under US irradiation. h UV-vis-NIR absorbance spectra at different concentrations of TiH1.924 nanodots (4, 8, 16, 32, 64, and 128 µg mL−1). The inset is the photograph of TiH1.924 nanodots with different concentrations. i Concentration-dependent photothermal heating curves of TiH1.924 nanodots (0, 0.02, 0.04, 0.08, 0.16, and 0.32 mg mL−1). j The photothermal profile after laser exposure to reach a steady temperature and then to cool down by turning the laser off. k Heating/cooling profiles for five repeated ON-OFF cycles of laser irradiations.
To understand the mechanism of sono-sensitization effect of TiH1.924 nanodots, the optical absorbance spectra of solid TiH1.924 nanodots and the commercial TiO2 nanoparticles were measured (Fig. 3e). Based on the optical absorbance spectra and the kubelka-munk theory (Fig. 3f)35,36, the optical bandgap of TiO2 was calculated to be ~3.1 eV, which was consistent with the previous reports37–39. Interestingly, the bandgap of TiH1.924 nanodots was determined to be ~2.7 eV, much lower than that of TiO2. The bandgap is related to the required minimum energy to realize electron excitation40,41. Thus, the lower bandgap means easier activation and would result in more ROS generation under external stimuli42,43. Based on the above discussion, the possible mechanism is proposed in Fig. 3g. Under the US irradiation, the valence electron receives energy and could transit from the valence band (VB) to the conduction band (CB), resulting in the generation of the electron-hole pairs and excess energy, which are captured by surrounding O2 and H2O molecules to generate ROS (e.g., 1O2, ·O2−, ·OH). With a lower bandgap compared to that of TiO2, TiH1.924 nanodots thus could be easier to be activated to produce more ROS under US irradiation, useful for applications in SDT.
We then studied the optical properties of the obtained TiH1.924 nanodots. The as-made TiH1.924 nanodots showed black color and strong optical absorbance, which appeared to be independent to the wavelength and was extended to the second NIR (NIR-II) region (Fig. 3h), in which light would have much higher tissue-penetrating capability in comparison to that in the NIR-I window14,44. On this ground, we presented that TiH1.924 nanodots could use as a photothermal agent for effective NIR-II PTT (1064 nm). The extinction coefficient of TiH1.924 at 1064 nm was tested to be ~10.27 L g−1 cm−1 (Supplementary Fig. 10), which was higher than that of black titania nanoparticles (B-TiO2-x, 5.54 L g−1 cm−1)14, traditional graphene oxide (GO, 3.6 L g−1 cm−1)45, and carbon nanodots (CQs, 0.35 L g−1 cm−1)46. Then, the photothermal performance of the TiH1.924 aqueous solution was further evaluated under 1064-nm NIR II laser. Significant concentration-dependent and laser-power-dependent photothermal heating effect was observed for these TiH1.924 nanodots (Fig. 3i, Supplementary Fig. 11). The photothermal conversion efficiency (η) of TiH1.924 nanodots was calculated to be ~58.6% (Fig. 3j, Supplementary Fig. 12), much higher than those of widely reported photothermal agents, like gold nanorods (21%)47, copper selenide (Cu2-xSe) nanocrystals (22%)48, copper sulfide (Cu9S5) nanocrystals (25.7%)49, and prussian blue (41.4%)50. In addition, there was almost no change of photothermal performance post five times ON/OFF laser cycles, showing the high photothermal stability of these TiH1.924 nanodots (Fig. 3k).
In order to increase their stabilities in the physiological environment, as-made TiH1.924 nanodots were modified with polyvinyl pyrrolidone (PVP), which could stabilize TiH1.924 nanodots likely via the chelating-coordination between the O atoms of PVP and Ti atoms of TiH1.924 (Supplementary Fig. 13)44,51. The amount of PVP coated on the surface of TiH1.924 nanodots was measured by thermogravimetric analysis (TGA) to be ~26.4%. Unlike as-made TiH1.924 nanodots which could be well dispersed in water but would aggregate in the presence of salt (e.g., in phosphate-buffered saline, PBS), TiH1.924-PVP nanodots showed great dispersity in both water, PBS, and cell culture medium for a week. Notably, the photothermal and sonodynamic performance of TiH1.924 nanodots did not change after surface modification with PVP or additional H2O2 treatments (Supplementary Figs. 14–16).
In vitro mild PTT-enhanced SDT
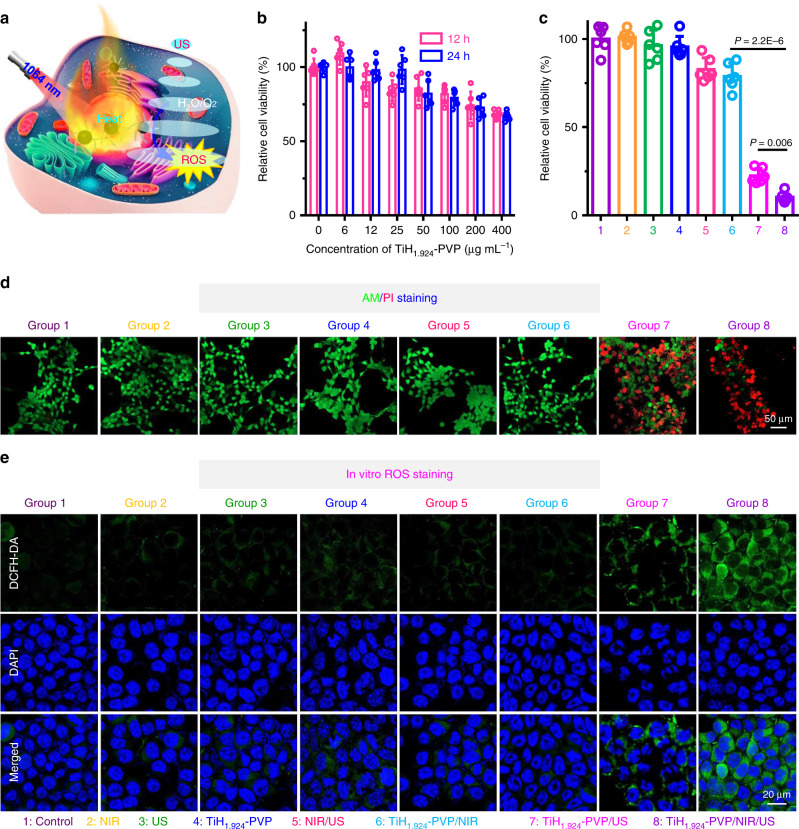
With strong NIR-II absorbance and effective sono-sensitizing ability, we expected the utilization of TiH1.924-PVP for synergistic photothermal-sonodynamic cancer therapy (Fig. 4a, Supplementary Fig. 17). Firstly, the standard methyl thiazolyl tetrazolium (MTT) assay demonstrated that TiH1.924-PVP nanodots show negligible cytotoxicity even at high concentrations (400 µg mL−1) toward 4T1 tumor cells (Fig. 4b). Next, the in vitro PTT-enhanced SDT induced by TiH1.924-PVP was evaluated (Fig. 4c). After the mild PTT using the 1064-nm laser at the power density of 0.8 W cm−2 for 10 min, the cell culture temperature increased to ~42 °C and the cell viability incubated with TiH1.924-PVP showed a slight decrease (~80.9%). When further US irradiation was conducted (40 kHz, 3 W cm−2, 1 min per cycle, 5 cycles), the 4T1 cell viabilities significantly decreased to ~10.6%, presenting increased cell killing compared to TiH1.924-PVP treated cells exposed to US alone without pre-treatment by the NIR-II laser. In addition, the excellent cancer cell killing effect of mild PTT-enhanced SDT using TiH1.924-PVP was also confirmed by live/dead co-staining (live cells, calcein-AM, AM; dead cells, propidium iodide, PI) (Fig. 4d). This increased SDT performance may be ascribed to the mechanism that the laser treatment could change the cell membrane permeability and enhance the cell uptake of TiH1.924-PVP nanodots52,53.
Fig. 4. In vitro mild PTT-enhanced SDT via TiH1.924-PVP.
a Schematic illustration of TiH1.924-PVP for mild PTT-enhanced sonodynamic therapy. b Relative viabilities of 4T1 cells after incubation with various concentrations of TiH1.924-PVP for 12 h and 24 h (n = 6 biologically independent samples). c Relative viabilities of 4T1 cells after different treatments, including control, TiH1.924-PVP, NIR, US, NIR/US, TiH1.924-PVP/NIR, TiH1.924-PVP/US, and TiH1.924-PVP/NIR/US (n = 6 biologically independent samples). d Confocal images of 4T1 cells stained with calcein AM (green, live cells) and propidium iodide (red, dead cells) after different treatments. e Confocal images of 4T1 cells stained with DCFH-DA after various treatments. The nuclei and intracellular ROS were stained by DAPI (blue) and DCFH-DA (green), respectively. TiH1.924-PVP: 50 µg mL−1, NIR laser: 1064 nm, 0.8 W cm−2, 10 min, T < 42 °C; US irradiation: 40 kHz, 3 W cm−2, 1 min per cycle, 5 cycles. Data are presented as mean values ± SD. Statistical significance was calculated with two-tailed Student’s t test (c). A representative image of three biological replicates from each group is shown in d, e.
Next, 2,7-dichlorofluorescein diacetate (DCFH-DA, green color) and dihydroethidium (DHE, red color) staining assays were also performed to determine intracellular ROS generation and verify the mechanism of TiH1.924-PVP as a sono-sensitizer to kill cancer cells under ultrasound (Fig. 4e, Supplementary Figs. 18 and 19)54. Cells in the control group, TiH1.924-PVP only group, laser only group, US only group, laser/US group, and TiH1.924-PVP/NIR group (mild PTT), all showed weak intracellular ROS-related fluorescence. In contrast, strong fluorescent signals were clearly observed in cells from the TiH1.924-PVP/US and TiH1.924-PVP/NIR/US groups, demonstrating the effective intracellular ROS generation by TiH1.924-PVP under US stimulation.
Mild PTT-defeated tumor hypoxia
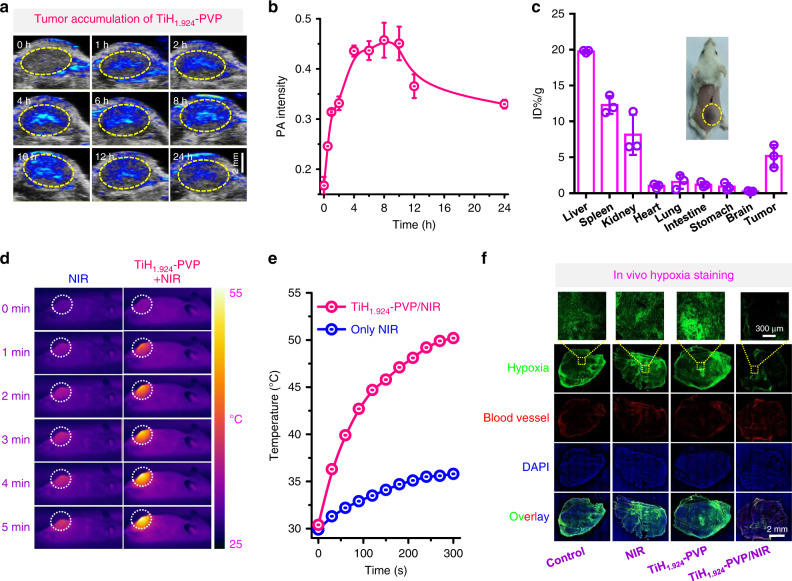
After in vitro experiments, the in vivo behaviors of TiH1.924-PVP were studied using photoacoustic (PA) imaging and it could monitor the tumor uptake of NIR-absorbing TiH1.924-PVP nanodots. After intravenous (i.v.) injection of TiH1.924-PVP into 4T1-tumor-bearing balb/c mice for 8 h, much obvious PA signals were clearly appeared in the tumor site (Fig. 5a, b), verified the tumor uptake of TiH1.924-PVP via the enhanced permeability and retention (EPR) effect. At the following time points, the PA signals gradually decrease, likely due to the clearance of the ultrasmall TiH1.924-PVP from the tumor. In addition, the biodistribution of nanodots in the tumor was then quantitatively studied by measuring the content of titanium ions through inductively coupled plasma optical emission spectrometry (ICP-OES) at 8 h post injection (p.i.). The tumor uptake of TiH1.924-PVP was determined to be ~5.2%ID g−1, further confirming the efficient tumor accumulation of these nanodots (Fig. 5c).
Fig. 5. In vivo tumor accumulation and mild PTT-defeated tumor hypoxia via TiH1.924-PVP.
a In vivo PA imaging of 4T1 tumor-bearing mice after intravenously injected with TiH1.924-PVP. b Time-dependent tumor PA signals at 900 nm based on PA imaging data in a (n = 3 biologically independent mice). c Biodistribution of TiH1.924-PVP in mice (n = 3 biologically independent mice). d, e IR thermal images (d) and temperature change curves (e) of 4T1 tumors under the 1064-nm laser irradiation, for untreated mice and TiH1.924-PVP injected mice (irradiated at 8 h p.i.). f Representative immunofluorescence images of tumor slices after hypoxia staining. The nuclei, blood vessels, and hypoxia areas were stained by DAPI (blue), anti-CD31 antibody (red), and antipimonidazole antibody (green), respectively. TiH1.924-PVP: 20 mg kg−1; NIR laser: 1064 nm, 0.8 W cm−2, 20 min. A representative image of three biological replicates from each group is shown in f. Data are presented as mean values ± SD.
Afterward, the in vivo photothermal performance of TiH1.924-PVP for NIR II-induced hyperthermia was investigated own to the strong NIR absorbance and high tumor accumulation of TiH1.924-PVP nanodots. And the surface temperature of tumors was recorded by infrared (IR) thermal imaging. 4T1 tumors-bearing mice post i.v. injection of TiH1.924-PVP for 8 h were exposed to the 1064-nm laser irradiation (0.8 W cm−2, 5 min) (Fig. 5d, e). Obviously, the tumor temperatures for TiH1.924-PVP treated mice quickly increased to 50 °C, while that of the control group showed much less significant temperature increase.
Due to aberrant cancer cell proliferation and distorted blood tumor vessels, hypoxia arises in a wide variety of solid tumors and often causes the failure of cancer therapies, especially for those that consume oxygen in the cell killing process such as radiotherapy, photodynamic therapy (PDT), and SDT55–57. Based on previous reports, the mild photothermal effect may increase intra-tumoral blood flow and then overcome tumor hypoxia58–60. To confirm this effect, immune-fluorescence hypoxia staining assay was conducted (Fig. 5f). Obviously, TiH1.924-PVP plus NIR-II irradiation group showed a significantly decrease of the hypoxia signals, suggesting that the mild photothermal effect could efficiently overcome the tumor hypoxia, favorable for defeating hypoxia-associated SDT resistance.
In vivo mild PTT-enhanced SDT
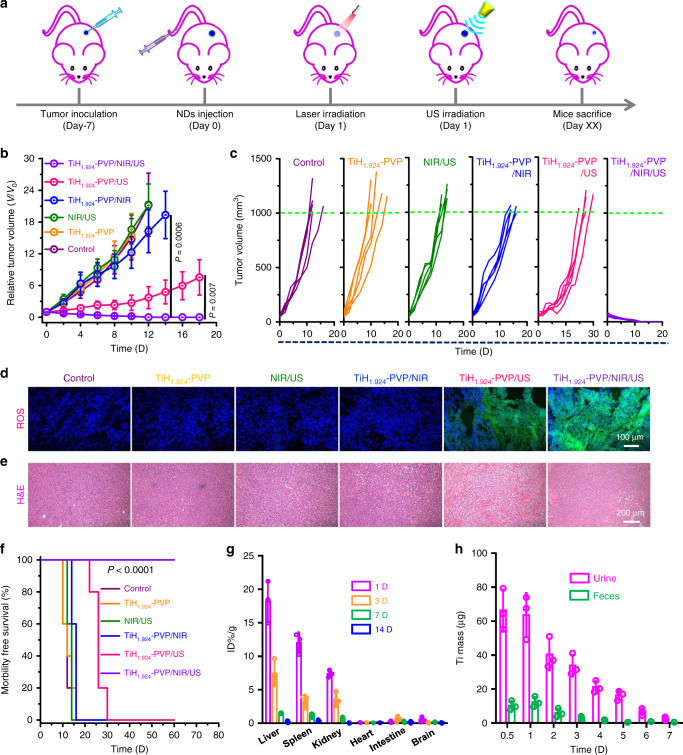
Then, we conducted the mild PTT-enhanced SDT on 4T1 tumor-bearing mice using TiH1.924-PVP. All of the mice were divided into five groups: (1) Control; (2) TiH1.924-PVP (i.v. injection, 20 mg kg−1); (3) NIR (1064 nm, 0.8 W cm−2, 20 min, T < 45 °C) + US (40 kHz, 3 W cm−2, 1 min per cycle, 20 cycles); (4) TiH1.924-PVP + NIR; (5) TiH1.924-PVP + US; (6) TiH1.924-PVP + NIR + US. At 8 h post i.v. injection of TiH1.924-PVP, the tumors were treated with 1064 nm laser and subsequent US irradiation (Fig. 6a). After various treatments, the tumor growth on different groups of mice was monitored. Compared with control, TiH1.924-PVP injection alone, or NIR/US treated for saline injected mice showed no appreciable effect to the tumor group (Fig. 6b, c, Supplementary Fig. 20A). The mild PTT with TiH1.924-PVP could only partially inhibit the tumor growth. The tumor growth of the SDT group (TiH1.924-PVP/US) was remarkably suppressed, suggesting the excellent SDT performance of TiH1.924-PVP. Interestingly, the TiH1.924-PVP/NIR/US group showed the most remarkable therapeutic outcome, and the tumor tissues were completely eradiated without recurrence during two months. The survival time of mice in the SDT group (TiH1.924-PVP/US) was prolonged compared to the other four groups (control, TiH1.924-PVP, NIR/US, and mild PTT group) (Fig. 6f). More importantly, the mice in the mild PTT-enhanced SDT group showed 100% survival for two months, further demonstrating an obvious synergistic therapeutic outcome for the combined PTT-SDT with TiH1.924-PVP in comparison to the single-modality SDT or mild PTT.
Fig. 6. In vivo mild PTT-enhanced SDT via TiH1.924-PVP.
a Schematic illustration to show the combination of PTT and SDT with TiH1.924-PVP nanodots. b, c Average tumor growth curves (b) and individual tumor growth curves (c) on mice after different treatments, including control, TiH1.924-PVP, NIR/US, TiH1.924-PVP/NIR, TiH1.924-PVP/US, and TiH1.924-PVP/NIR/US (n = 5 biologically independent mice). d Micrograph of DCFH-DA stained tumor slices collected for mice receiving different treatments. e H&E stained tumor slices collected from different treatment groups. f Survival curves of mice after various treatments. g Biodistribution of TiH1.924-PVP post i.v. injection in mice on different days (n = 3 biologically independent mice). h The detected Ti mass in urine and feces at different time points post i.v. injection of TiH1.924-PVP (n = 3 biologically independent mice). The Ti contents were measured by ICP-OES. TiH1.924-PVP: 20 mg kg−1; NIR laser: 1064 nm, 0.8 W cm−2, 20 min, T < 45 °C; US irradiation: 40 kHz, 3 W cm−2, 1 min per cycle, 20 cycles. A representative image of three biological replicates from each group is shown in d, e. Data are presented as mean values ± SD. Statistical significance was calculated with two-tailed Student’s t test (b) and Logrank test (two-sided) for trend (f).
To further understand the mechanism of synergistic therapy, ROS staining of tumor slices was conducted to evaluate the ROS levels in the tumor post different treatments (Fig. 6d). Compared with the weak green fluorescence in tumor slices from control, TiH1.924-PVP, NIR/US, and TiH1.924-PVP/NIR groups, the TiH1.924-PVP/US group showed obvious green fluorescence, and the strongest ROS-related fluorescence was observed in the combined laser plus US treatment group (TiH1.924-PVP/NIR/US). Our results indicated the mild PTT could overcome tumor hypoxia and facilitate the SDT-triggered ROS production. In addition, hematoxylin and eosin (H&E) staining were conducted at 24 h after different treatments. Tumor cells were severely damaged in the SDT group and mild PTT-enhanced SDT group, while the other four groups showed little cell dead (Fig. 6e). These results confirmed the efficient synergistic effects induced by mild PTT-enhanced SDT, in the presence of TiH1.924-PVP as a concurrent sono-sensitizer and photothermal nanoagent.
The body clearance behaviors
In two weeks after the treatment, the body weights of mice showed no significant change, indicating no apparent acute toxicity of TiH1.924-PVP (Supplementary Fig. 20B). Next, we further investigated the body clearance behaviors of TiH1.924-PVP after systemic injection, and a time-dependent biodistribution study was conducted after i.v. injection of TiH1.924-PVP nanodots (Fig. 6g). Relatively high retention of TiH1.924-PVP was observed in the liver (18.2 ± 3.1%ID g−1), spleen (12.1 ± 1.5%ID g−1), and kidney (7.4 ± 0.5%ID g−1) at 24 h p.i. Importantly, rapid decrease of Ti levels in these organs was observed over time, indicating the efficient clearance of TiH1.924-PVP. After 14 days, the Ti retention in major organs drastically decreased to be <0.5%ID g−1, indicating the nearly complete clearance of TiH1.924-PVP. To further investigate the clearance pathway, the Ti concentrations in the urine and feces were also measured. High levels of Ti were observed in the urine, strongly evidencing the elimination of TiH1.924-PVP nanodots via the renal filtration pathway (Fig. 6h). In addition, H&E staining of the major organs (heart, liver, spleen, kidney, heart, lung, and brain) also confirmed the negligible toxicity of TiH1.924-PVP to mice at this therapeutic dose (Supplementary Fig. 21). With efficient clearance and no acute toxicity, such TiH1.924-PVP nanodots could be safe for in vivo use without long-term toxicity within the appropriate dose range.
Discussion
In summary, nano-structured TiH1.924 materials were synthesized via the liquid-phase exfoliation method. It was found that when the surface energy of the applied solvent had a good match with that of the TiH1.924 powder, efficient exfoliation of such metal hydride powder into nanoparticles could be realized. Using the same method, a series of metal hydrides powders (TiH1.924, ZrH2, CaH2, and HfH1.983) were successfully exfoliated into small nanoparticles. With strong NIR-II absorbance and efficient US-triggered ROS production ability, TiH1.924 nanodots with PVP modification were further applied for the combined PTT-SDT therapy with great in vivo tumor destruction efficacy. Such TiH1.924 nanodots present the following advantages as therapeutic nano-agent. (1) These TiH1.924-PVP nanodots exhibit excellent sonodynamic performance in US-triggered ROS generation due to the reduced band-gap in TiH1.924 compared to that of TiO2. (2) The strong NIR absorption of TiH1.924-PVP could enable enhanced photothermal-sonodynamic therapy, in which the mild hyperthermia-induced tumor hypoxia relief would lead to improved sonodynamic tumor killing. (3) Containing biocompatible elements (Ti and H), TiH1.924-PVP nanodots with ultra-small sizes could allow their efficient body excretion without appreciable toxicity. Moreover, this work illustrates the promises of nano-structured metal hydrides nanomedicine platform against cancer and possibly other types of diseases.
Methods
Materials
Titanium hydride (TiH1.924), zirconium hydride (ZrH2), calcium hydride (CaH2), hafnium hydride (HfH1.983), and N-methyl pyrrolidone (NMP) were purchased from Aladdin reagent Co., Ltd. (Shanghai, China). 1,3-diphenylisobenzofuran (DPBF), Polyvinyl pyrrolidone (PVP, MW 10 k), 2,2,6,6-tetramethylpiperidine (TEMP), and 5,5-dimethyl-pyrroline-N-oxide (DMPO) were obtained from Sigma-Aldrich. All chemicals were of analytical grade and used without further purification.
Synthesis of TiH1.924 nanodots
100 mg commercial TiH1.924 powder was dispersed in 20 mL NMP. The mixture was treated under ultrasonication for different periods of time (Ultrasonic Cleaner, KQ-100KDB, power: ~100 W, temperature: ~15 °C). After ultrasonic treatment for 20 min, TiH1.924 nanodots were obtained and further purified by centrifugation (64 k × g, 10 min) and washing repeatedly with anhydrous ethanol. Via the same method, zirconium hydride (ZrH2) nanodots, calcium hydride (CaH2) nanodots, and hafnium hydride (HfH1.983) nanoparticles were also synthesized.
Modification of TiH1.924 nanodots
The as-synthesized TiH1.924 nanodots were modified by polyvinylpyrrolidone (PVP)44,51. Briefly, 20 mg TiH1.924 and 200 mg PVP (MW 10 k) were dissolved in 50 mL anhydrous ethanol and refluxed at 50 °C for 8 h. After collecting by centrifugation (64 k × g, 10 min) and washing with water and ethanol, the final TiH1.924-PVP nanodots were dispersed in deionized water, and stored at 4 °C for further use (concentration, 2 mg mL−1).
Characterization
Transmission electron microscope (TEM) imaging and elemental mapping were carried out by FEI Tecnai F20 TEM. Powder X-ray diffraction (XRD) measurement was conducted by a PANalytical X-ray diffractometer equipped with CuKα radiation (λ = 0.15406 nm). XPS analysis was performed by the PHI Quantera SXM X-ray photoelectron spectrometer with an Al Ka monochromator source. ROS was quantified by an ESR spectrometer (Bruker EMXplus). UV-vis-NIR absorbance spectra were recorded by PerkinElmer Lambda 750 UV-vis-NIR spectrophotometer. The ultrasonic generator was made by Hainertec (Suzhou) Co., Ltd. The absolute Ti contents were determined by ICP-OES (inductively coupled plasma optical emission spectrometry).
Quantitative analysis of the generation of ROS
1 mL TiH1.924 (20 µg mL−1) was mixed with 20 μL DPBF (1 mg mL−1). After different US (40 kHz, 3 W cm−2) durations, the absorbance changes of DPBF at 420 nm were recorded to quantify the generation of ROS by US-activated TiH1.924. ESR technology combined with TEMP (for 1O2 detection) or DMPO (for ·OH detection) was employed to detect different types of the generated ROS. In this case, 1 mL TiH1.924 (20 µg mL−1) was mixed with 20 µL TEMP (1 M) or 10 µL DMPO (1 M) and exposed to US irradiation (40 kHz, 3 W cm−2) for 1 min. The characteristic peak signals were detected by the ESR spectrometer. The settings for the EPR spectrometer were as follows: center field, 3520 G; sweep width, 100 G; microwave frequency, 9.77 GHz; modulation frequency, 100 kHz; power, 20.00 mW.
Photothermal performance of TiH1.924 nanodots
The photothermal performance of TiH1.924 was analyzed by irradiating a glass cuvette containing a dispersion of TiH1.924 nanodots. The extinction coefficient and the photothermal conversion efficiency were calculated according to the previous studies44.
Cellular experiments
Murine breast cancer 4T1 cells were cultured in the standard cell culture medium at 37 °C under 5% CO2. For the in vitro cytotoxicity test, 4T1 cells seeded in 96-well plates were incubated with different concentrations (0-400 µg mL−1) of TiH1.924-PVP for 12 h and 24 h. Relative cell viabilities were tested by the standard MTT assay.
For in vitro mild PTT-enhanced SDT, 4T1 cells were incubated with TiH1.924-PVP (50 µg·mL−1) for 8 h, followed by laser irradiation (1064 nm, 0.8 W cm−2, 10 min, T < 42 °C) or US irradiation (40 kHz, 3 W cm−2, 1 min per cycle, 5 cycles). The cell viabilities were determined afterward by the MTT assay.
For live/dead staining, 4T1 cells under different treatments (including control, TiH1.924-PVP, NIR, US, NIR/US, TiH1.924-PVP/NIR, TiH1.924-PVP/US and TiH1.924-PVP/NIR/US) were stained with calcein AM (AM, live cell) and propidium iodide (PI, dead cell). For ROS detection, the treated 4T1 cells were stained with DCFH-DA (20 μM) for 30 min. All the images were acquired by a confocal laser scanning microscope (CLSM, Zeiss Axio-Imager LSM-800).
Tumor model
Balb/c mice were purchased from Nanjing Sikerui Biological Technology Co. Ltd, and all animal experiments were carried out under the permission by Soochow University Laboratory Animal Center. Six-week-old male Balb/c mice (18 ± 2 g) were used as the animal model in this work. Mice were housed in groups of 5 mice per individually ventilated cage in a 12-h light–dark cycle (8:00–20:00 light; 20:00–8:00 dark), with constant room temperature (21 ± 1 °C) and relative humidity (40-70%). All mice had access to food and water ad libitum.
Hypoxia tumor analysis
For immunohistochemistry analysis, 4T1 tumor-bearing mice were intravenously injected with TiH1.924-PVP (20 mg·kg−1). At 8 h p.i., tumors on these mice were exposed to the 1064-nm laser irradiation for 20 min with their temperature maintained at ~45 °C. Then immediately, tumors were surgically excised for hypoxia staining assay using the Hypoxyprobe-1 plus kit (Hypoxyprobe Inc) following the standard protocol61,62. Anti-pimonidazole mouse monoclonal antibody conjugated with FITC (FITC-Mab1, Hypoxyprobe Inc.; Cat. No.: HP2-100Kit; Lot No.: 04-11-19; Clone: 4.3.11.3; Dilution: 1:200) and Alex 488-conjugated goat anti-mouse secondary antibody (Jackon Inc., Cat. No.: 115-545-003, Lot No.: 146108, RRID: AB_2338840; dilution: 1:200) for hypoxia staining. Rat anti-CD31 mouse monoclonal antibody (Biolegend Inc., Cat. No.: 102402, Lot No.: B226360, Clone: 390; dilution: 1:100) and Rhodamine-conjugated donkey anti-rat secondary antibody (Jackon Inc. Cat. No.: 712-025-150, Lot No.: 147079, RRID: AB_2340635; Dilution: 1:200) for blood vessel staining.
In vivo mild PTT enhanced SDT
Mice bearing 4T1 tumors (~100 cm3) were divided into six groups (n = 5 per group): (1) control; (2) TiH1.924-PVP (i.v. injection, 20 mg kg−1); (3) NIR (1064 nm, 0.8 W cm−2, 20 min, T < 45 °C) + US (40 kHz, 3 W cm−2, 1 min per cycle, 20 cycles); (4) TiH1.924-PVP (i.v. injection, 20 mg kg−1) + NIR (1064 nm, 0.8 W cm−2, 20 min, T < 45 °C); (5) TiH1.924-PVP (i.v. injection, 20 mg kg−1) + US (40 kHz, 3 W cm−2, 1 min per cycle, 20 cycles); (6) TiH1.924-PVP (i.v. injection, 20 mg kg−1) + NIR (1064 nm, 0.8 W cm−2, 20 min, T < 45 °C) + US (40 kHz, 3 W cm−2, 1 min per cycle, 20 cycles). At 8 h after i.v. injection, the tumors were treated with laser irradiation, or US irradiation, or laser irradiation + US exposure, sequentially. Tumor temperature and thermal images were monitored and recorded by an IR thermal camera (Infrared Cameras. Inc). Tumor sizes and body weight were monitored every two days. The tumor volumes were calculated by the formula: volume = length × width2/2. For in vivo H&E staining, tumors in different groups were collected on the second day post treatment.
In vivo metabolism study
Healthy mice after i.v. injection with TiH1.924-PVP (20 mg·kg−1) was sacrificed at 1, 3, 7, and 14 days, respectively. The major organs were collected, with one halves used for H&E staining, and the other halves used for detection of Ti levels by ICP-OES after these organs were solubilized by aqua regia. To study the excretion pathway, mice after i.v. injection with TiH1.924-PVP nanodots were kept in metabolic cages to collect their feces and urine at various time points, which were solubilized by aqua regia measured by ICP-OES to determine Ti levels.
Software
All statistical analyses were performed on Origin 8.5, Excel 2010 and GraphPad Prism 6. Fluorescent images were collected by Confocal Microscopy (Zeiss LSM 880) and analyzed by LAS AF Lite 3.2.0 Image J 1.74v. IR thermal images were collected by Infrared Camera (Fotric 255). Photoacoustic imaging data was processed by PA Tomography (Vevo LAZR). All other characterization of TiH1.924 was conducted by these instruments as indicated in the Characterization section.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This article was partially supported by the National Basic Research Programs of China (973 Program) (2016YFA0201200), the National Natural Science Foundation of China (51525203, 51761145041, 51572180), Collaborative Innovation Center of Suzhou Nano Science and Technology, a Jiangsu Natural Science Fund for Distinguished Young Scholars (BK20170063), and a Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. L.C. was supported by the Tang Scholarship of Soochow University. In particular, we sincerely thank Hainertec (Suzhou) Co., Ltd. for providing the ultrasonic generator.
Author contributions
Z.L. oversaw all research; Z.L., L.C., and F.G. designed the experiments; L.C. and F.G. synthesized the materials; F.G., N.Y., and Y.G. performed the sonodynamic and photothermal experiments; F.G., Y.N., and S.B. performed the cells experiments; F.G., X.W., M.C., and Q.C. performed animal experiments; Z.L., L.C., and F.G. wrote the paper; All authors reviewed and edited the paper.
Data availability
The authors declare that all data needed to evaluate the conclusion of this work are presented in the paper and the Supplementary Information. Other data related to this work are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Jae Hyung Park and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liang Cheng, Email: lcheng2@suda.edu.cn.
Zhuang Liu, Email: zliu@suda.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-17485-x.
References
- 1.Qian X, Zheng Y, Chen Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): breaking the depth shallow of photoactivation. Adv. Mater. 2016;28:8097–8129. doi: 10.1002/adma.201602012. [DOI] [PubMed] [Google Scholar]
- 2.Gong F, et al. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater. 2019;31:1900730. doi: 10.1002/adma.201900730. [DOI] [PubMed] [Google Scholar]
- 3.Lin X, Song J, Chen X, Yang H. Ultrasound activated sensitizers and applications. Angew. Chem. Int. Ed. 2020;59:2–24. doi: 10.1002/anie.201906823. [DOI] [PubMed] [Google Scholar]
- 4.Pan X, et al. Metal-organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv. Mater. 2018;30:1800180. doi: 10.1002/adma.201800180. [DOI] [PubMed] [Google Scholar]
- 5.Cui X, Han X, Yu L, Zhang B, Chen Y. Intrinsic chemistry and design principle of ultrasound-responsive nanomedicine. Nano Today. 2019;28:100773. [Google Scholar]
- 6.Huang P, et al. Metalloporphyrin-encapsulated biodegradable nanosystems for highly efficient magnetic resonance imaging-guided sonodynamic cancer therapy. J. Am. Chem. Soc. 2017;139:1275–1284. doi: 10.1021/jacs.6b11846. [DOI] [PubMed] [Google Scholar]
- 7.Zhong X, et al. GSH-depleted PtCu3 nanocages for chemodynamic-enhanced sonodynamic cancer therapy. Adv. Funct. Mater. 2020;30:1907954. [Google Scholar]
- 8.Xu Z-Y, et al. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells. Ultrasonics. 2013;53:232–238. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Xu H-N, et al. Preparation and sonodynamic activities of water-soluble tetra-α-(3-carboxyphenoxyl) zinc (II) phthalocyanine and its bovine serum albumin conjugate. Ultrason. Sonochem. 2015;22:125–131. doi: 10.1016/j.ultsonch.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, et al. Detection and comparison of reactive oxygen species (ROS) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011;18:1028–1034. doi: 10.1016/j.ultsonch.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Pang X, Xu C, Jiang Y, Xiao Q, Leung AW. Natural products in the discovery of novel sonosensitizers. Pharmacol. Ther. 2016;162:144–151. doi: 10.1016/j.pharmthera.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, et al. A single-step multi-level supramolecular system for cancer sonotheranostics. Nanoscale Horiz. 2018;4:190–195. doi: 10.1039/c8nh00276b. [DOI] [PubMed] [Google Scholar]
- 13.Deepagan V, et al. Long-circulating Au-TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Lett. 2016;16:6257–6264. doi: 10.1021/acs.nanolett.6b02547. [DOI] [PubMed] [Google Scholar]
- 14.Han X, et al. Oxygen-deficient black titania for synergistic/enhanced sonodynamic and photoinduced cancer therapy at near infrared-II biowindow. ACS Nano. 2018;12:4545–4555. doi: 10.1021/acsnano.8b00899. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. Site-specific sonocatalytic tumor suppression by chemically engineered single-crystalline mesoporous titanium dioxide sonosensitizers. J. Mater. Chem. B. 2017;5:4579–4586. doi: 10.1039/c7tb00938k. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa K, et al. Electron-hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett. 2014;5:1953–1957. doi: 10.1021/jz500770c. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch M, et al. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011;3:489. doi: 10.1038/nchem.1048. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Choi YJ, Fang ZZ, Sohn HY, Rönnebro E. Hydrogen storage properties of nanosized MgH2-0.1TiH2 prepared by ultrahigh-energy-high-pressure milling. J. Am. Chem. Soc. 2009;131:15843–15852. doi: 10.1021/ja906340u. [DOI] [PubMed] [Google Scholar]
- 19.Choi YJ, Xu Y, Shaw WJ, Rönnebro EC. Hydrogen storage properties of new hydrogen-rich BH3NH3-metal hydride (TiH2, ZrH2, MgH2, and/or CaH2) composite systems. J. Phys. Chem. C. 2012;116:8349–8358. [Google Scholar]
- 20.Banhart J. Light-metal foams-History of innovation and technological challenges. Adv. Eng. Mater. 2013;15:82–111. [Google Scholar]
- 21.Orovčík Ľ, et al. Effect of the TiH2 pre-treatment on the energy absorption ability of 6061 aluminium alloy foam. Mater. Lett. 2015;148:82–85. [Google Scholar]
- 22.Joshi VV, et al. Development of Ti-6Al-4V and Ti-1Al-8V-5Fe alloys using low-cost TiH2 powder feedstock. J. Mater. Eng. Perform. 2013;22:995–1003. [Google Scholar]
- 23.Oh J-M, Heo K-H, Kim W-B, Choi G-S, Lim J-W. Sintering properties of Ti-6Al-4V alloys prepared using Ti/TiH2 powders. Mater. Trans. 2013;54:119–121. [Google Scholar]
- 24.Zhang Y, et al. Surface characterizations of TiH2 powders before and after dehydrogenation. Appl. Surf. Sci. 2017;410:177–185. [Google Scholar]
- 25.Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013;52:7372–7408. doi: 10.1002/anie.201207199. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Ioccozia J, Sun L, Lin C, Lin Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014;7:2182–2202. [Google Scholar]
- 27.Coleman JN, et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science. 2011;331:568–571. doi: 10.1126/science.1194975. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez Y, et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008;3:563. doi: 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- 29.Nicolosi V, Chhowalla M, Kanatzidis MG, Strano MS, Coleman JN. Liquid exfoliation of layered materials. Science. 2013;340:1226419. [Google Scholar]
- 30.Hanlon D, et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015;6:8563. doi: 10.1038/ncomms9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen J, et al. Liquid phase exfoliation of two-dimensional materials by directly probing and matching surface tension components. Nano Lett. 2015;15:5449–5454. doi: 10.1021/acs.nanolett.5b01842. [DOI] [PubMed] [Google Scholar]
- 32.Coleman JN. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 2009;19:3680–3695. [Google Scholar]
- 33.Nguyen EP, et al. Investigation of two-solvent grinding-assisted liquid phase exfoliation of layered MoS2. Chem. Mater. 2014;27:53–59. [Google Scholar]
- 34.Sandim HRZ, Morante BV, Suzuki PA. Kinetics of thermal decomposition of titanium hydride powder using in situ high-temperature X-ray diffraction (HTXRD) Mater. Res. 2005;8:293–297. [Google Scholar]
- 35.Aydın C, et al. Determination of optical band gap of ZnO:ZnAl2O4 composite semiconductor nanopowder materials by optical reflectance method. J. Electroceram. 2013;31:265–270. [Google Scholar]
- 36.Abdullahi SS, Güner S, Musa YKIM, Adamu BI, Abdulhamid MI. Simple method for the determınatıon of band gap of a nanopowdered sample usıng kubelka munk theory. J. Niger. Assoc. Math. Phys. 2016;35:241–246. [Google Scholar]
- 37.Shanga X, Lia B, Zhanga T, Lib C, Wanga X. Photocatalytic degradation of methyl orange with commercial organic pigment sensitized TiO2. Procedia Environ. Sci. 2013;18:478–485. [Google Scholar]
- 38.Swetha S, Santhosh S, Geetha RB. Synthesis and comparative study of nano-TiO2 over Degussa P-25 in disinfection of water. Photochem. Photobio. 2010;86:628–632. doi: 10.1111/j.1751-1097.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Wen L, Zhao X. The photoluminescence spectroscopic study of anatase TiO2 prepared by magnetron sputtering. Mater. Chem. Phys. 2007;106:350–353. [Google Scholar]
- 40.Zaanen J, Sawatzky G, Allen J. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 1985;55:418. doi: 10.1103/PhysRevLett.55.418. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka A, Hashimoto K, Kominami H. Visible-light-induced hydrogen and oxygen formation over Pt/Au/WO3 photocatalyst utilizing two types of photoabsorption due to surface plasmon resonance and band-gap excitation. J. Am. Chem. Soc. 2014;136:586–589. doi: 10.1021/ja410230u. [DOI] [PubMed] [Google Scholar]
- 42.Khan MA, Akhtar MS, Woo SI, Yang O-B. Enhanced photoresponse under visible light in Pt ionized TiO2 nanotube for the photocatalytic splitting of water. Catal. Commun. 2008;10:1–5. [Google Scholar]
- 43.Irie H, Washizuka S, Hashimoto K. Hydrophilicity on carbon-doped TiO2 thin films under visible light. Thin. Solid. Films. 2006;510:21–25. [Google Scholar]
- 44.Lin H, Gao S, Dai C, Chen Y, Shi J. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017;139:16235–16247. doi: 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- 45.Robinson JT, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 46.Fang C-Y, Chang C-C, Mou C-Y, Chang H-C. Preparation and characterization of ion-irradiated nanodiamonds as photoacoustic contrast agents. J. Nanosci. Nanotechnol. 2015;15:1037–1044. doi: 10.1166/jnn.2015.9741. [DOI] [PubMed] [Google Scholar]
- 47.Zeng J, Goldfeld D, Xia Y. A plasmon-assisted optofluidic (PAOF) system for measuring the photothermal conversion efficiencies of gold nanostructures and controlling an electrical switch. Angew. Chem. Int. Ed. 2013;52:4169–4173. doi: 10.1002/anie.201210359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hessel CM, et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011;11:2560–2566. doi: 10.1021/nl201400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian Q, et al. Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano. 2011;5:9761–9771. doi: 10.1021/nn203293t. [DOI] [PubMed] [Google Scholar]
- 50.Cai X, et al. A versatile nanotheranostic agent for efficient dual-mode imaging guided synergistic chemo-thermal tumor therapy. Adv. Funct. Mater. 2015;25:2520–2529. [Google Scholar]
- 51.Wu C, et al. Biodegradable Fe(III)@WS2-PVP nanocapsules for redox reaction and TME-enhanced nanocatalytic, photothermal, and chemotherapy. Adv. Funct. Mater. 2019;29:1901722. [Google Scholar]
- 52.Fisher JW, et al. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010;70:9855–9864. doi: 10.1158/0008-5472.CAN-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgart J, et al. Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells. Biomaterials. 2012;33:2345–2350. doi: 10.1016/j.biomaterials.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 54.Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 56.Ding H, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Controlled Release. 2011;156:276–280. doi: 10.1016/j.jconrel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y-L, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773–1783. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen S, et al. Bottom-up preparation of uniform ultrathin rhenium disulfide nanosheets for image-guided photothermal radiotherapy. Adv. Funct. Mater. 2017;27:1700250. [Google Scholar]
- 59.Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. 2018;57:11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 60.Song G, et al. Core-shell MnSe@Bi2Se3 fabricated via a cation exchange method as novel nanotheranostics for multimodal imaging and synergistic thermoradiotherapy. Adv. Mater. 2015;27:6110–6117. doi: 10.1002/adma.201503006. [DOI] [PubMed] [Google Scholar]
- 61.Song X, Feng L, Liang C, Yang K, Liu Z. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett. 2016;16:6145–6153. doi: 10.1021/acs.nanolett.6b02365. [DOI] [PubMed] [Google Scholar]
- 62.Zhu W, et al. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv. Funct. Mater. 2016;26:5490–5498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data needed to evaluate the conclusion of this work are presented in the paper and the Supplementary Information. Other data related to this work are available from the corresponding authors upon reasonable request.