Abstract
Contagious bovine pleuropneumonia (CBPP) and contagious caprine pleuropneumonia (CCPP) are major infectious diseases of ruminants caused by mycoplasmas in Africa and Asia. In contrast with the limited pathology in the respiratory tract of humans infected with mycoplasmas, CBPP and CCPP are devastating diseases associated with high morbidity and mortality. Beyond their obvious impact on animal health, CBPP and CCPP negatively impact the livelihood and wellbeing of a substantial proportion of livestock-dependent people affecting their culture, economy, trade and nutrition. The causative agents of CBPP and CCPP are Mycoplasma mycoides subspecies mycoides and Mycoplasma capricolum subspecies capripneumoniae, respectively, which have been eradicated in most of the developed world. The current vaccines used for disease control consist of a live attenuated CBPP vaccine and a bacterin vaccine for CCPP, which were developed in the 1960s and 1980s, respectively. Both of these vaccines have many limitations, so better vaccines are urgently needed to improve disease control. In this article the research community prioritized biomedical research needs related to challenge models, rational vaccine design and protective immune responses. Therefore, we scrutinized the current vaccines as well as the challenge-, pathogenicity- and immunity models. We highlight research gaps and provide recommendations towards developing safer and more efficacious vaccines against CBPP and CCPP.
Subject terms: Vaccines, Bacterial infection
Introduction
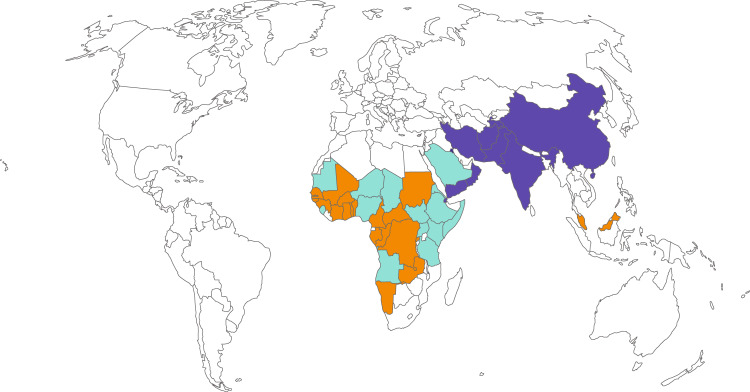
Contagious bovine pleuropneumonia (CBPP) and contagious caprine pleuropneumonia (CCPP) are important transboundary diseases of cattle and goats especially in low and middle-income countries. Both diseases have multiple impacts. Pleuropneumonia is a very painful clinical state that is associated with reduced productivity, and, in its most dramatic outcome, death. Death rates are much higher for CCPP than for CBPP, however, increased mortality rates are associated with CBPP when infected cattle are introduced into naive herds1. The adverse affects on productivity include a reduction in milk production, daily weight gain, draft power, and fertility among others. Several surveys have ranked CBPP and CCPP constantly among the top five ruminant diseases for stakeholders across a range of livestock industry sectors. CBPP and CCPP are diseases that require precise diagnostic procedures in order to be detected. The current World Organization for Animal Health (OIE) prescribed diagnostic tests (cELISA, CFT) are fit-for-purpose at the herd level but are far from being optimal at the individual level. Given the passive surveillance approaches, limited resources, and limited diagnostic capacity in many parts of Africa and Asia, the current prevalence figures are probably an underestimate. Nevertheless, prevalence data retrieved from the OIE specific WAHID interface (Fig. 1 and Supplementary Figure) over the last 10 years suggests that there has not been any progress towards control of CBPP and CCPP, especially on the African continent. Control methods such as movement restrictions, quarantine, antibiotic treatment, and vaccination have had varying success. Reasons for failure to control the diseases are diverse covering social, economic, and political issues. Vaccination-related control strategies have been hampered by variable safety and efficacy of the vaccines and there is an urgent need for an improved vaccine for both diseases. New vaccines for these diseases must not only be safe and efficacious, but also cost-effective, scaleable and accessible to smallholder farmers and this should underpin rational vaccine design. Scientists from the research community reviewed the current knowledge on CBPP and CCPP as well as other mycoplasma discovery research projects to identify research gaps and agree recommendations at a workshop in Switzerland (Supplementary Notes). While control and eradication of these diseases would benefit from new, improved vaccines, there are many other social, economic and political research aspects that need to be addressed, but our focus in this article is the search for new and better vaccines.
Fig. 1. Occurrence of contagious bovine pleuropneumonia (CBPP) and contagious caprine pleuropneumonia (CCPP) from 2010 to 2019.
Countries displayed in orange, purple, and turquoise reported confirmed cases of CBPP, CCPP, or both CBPP & CCPP, respectively. Data were collected from the website of the World Organization of Animal Health (www.oie.int).
History of contagious bovine pleuropneumonia and contagious caprine pleuropneumonia and vaccines used for their control
The timing of the first report on an infectious disease is always difficult to verify in the absence of good diagnostic tools and modern high-speed communications that inform and shape our lives today. CBPP and CCPP were described in the relatively recent past with astonishingly precise descriptions of the clinical manifestations. The first records that mention CBPP originate from Bourgelat in France in 17652, and then more notably from Albrecht von Haller in Switzerland in 17733. Trade of animals from Europe to former colonies in ships, facilitated the entry of CBPP into the southern African continent in 18534, then into Australia in 1858 and into North America in 1871. The causative agent, Mycoplasma mycoides subsp. mycoides, was the first mycoplasma ever cultivated in 18985. These historical accounts have been confirmed and extended by recent molecular studies based on genomic data6, showing the existence of at least two independently imported lineages circulating in Africa6. After introduction and dispersal of CBPP, the disease was curbed by either detection and slaughter, combined with movement restriction (mainly executed by veterinary forces during the colonial period before 1960), or by the use of a combined live vaccine against Rinderpest virus and CBPP during the rinderpest eradication campaign7.
The first report of CCPP dates back to Algeria in 18738. Subsequently, the disease spread to different countries in Europe, Asia, and Africa. For instance, a major outbreak struck South Africa after goats were imported from Turkey in 18819. The fastidious mycoplasma that causes CCPP, namely Mycoplasma capricolum subsp. capripneumoniae, was only identified in 197610. More recently, the Thrace region of Turkey experienced a CCPP outbreak with high mortality in 200211 and the disease now seems to be endemic there12.
There are two vaccines being produced and used in the field for CBPP and CCPP. If manufactured and applied properly they have the capacity to prevent clinical disease and economic loss13,14. Interestingly, the CBPP vaccine is a live attenuated vaccine, while the CCPP vaccine is a bacterin.
The CBPP vaccine was attenuated by serial cultivation in eggs in the 1950’s and is based on the Tanzanian outbreak strain T1, with the 44th passage (T1/44) used as vaccine stock. The vaccine provides a level of protection that can be quantified by reduction in lung lesions in vaccinated and challenged cattle compared to unvaccinated and challenged animals. As it is a live vaccine, it can theoretically be combined with other live vaccines, an approach that was used successfully during the Rinderpest eradication campaign. This live bivalent vaccine containing streptomycin led to a marked reduction in CBPP in Rinderpest-affected countries15. Annual revaccination with the live vaccine is necessary to maintain protective immunity. It is relatively inexpensive and easy to produce at scale, as the cultivation of M. mycoides subsp. mycoides is much easier than cultivation of many other pathogenic Mycoplasma species, such as M. hyopneumoniae (the primary agent of enzootic pneumonia in pigs), M. capricolum subsp. capripneumoniae or M. pneumoniae (the cause of atypical pneumonia in humans). A minimal vaccine dose of 107 colony-forming units of M. mycoides subsp. mycoides is recommended by OIE, so many doses can theoretically be achieved during commercial production from 1 ml of culture (which typically contains 109 CFU/mL). In some instances, severe inflammation at the site of inoculation has been caused from this vaccine, especially after primary vaccination of cattle. This so-called Willems reaction is characterized by massive inflammatory reactions that can lead to skin sloughing at the vaccination site and in the worst case to death. Therefore, in the 1960s, Australian cattle were immunized at the tip of the tail with the V5 live vaccine, so that in the event of severe reactions the only effect was the loss of the tip of the tail. These reactions have an adverse effect on owner acceptance, and on animal welfare and marketing, leading to a low uptake of this vaccine. Anecdotal data suggest breed predispositions to these side effects. In addition, it has been shown that the T1/44 live vaccine strain is not fully attenuated and can cause disease when inoculated endobronchially16. Mass vaccination even with a moderate efficacy and duration of immunity vaccine (such as the current T1/44 live vaccine) alone is unlikely to eliminate CBPP according to an epidemiological model for CBPP transmission in pastoral herds of East Africa17,18. This includes a requirement for strict movement controls and the authors say it is validated by field observation. Furthermore, vaccine-derived immunity of at least 18 months is required to eliminate CBPP from individual herds according to recent study13. A combination of vaccination and antibiotic treatment of diseased cattle turned out to be most promising for CBPP control according to the epidemiological model18.
The current CCPP vaccine is a bacterin, and the OIE provides the guidelines for its production, which is basically culturing any field isolate and inactivating with saponin, which acts as an adjuvant. This vaccine was developed in the 1980s and 1990s19,20. The vaccine is administered as early as 4 months of age and revaccination is recommended every 6 months. The production of this vaccine is rather cumbersome given the fastidious growth requirements of the pathogen. Moreover, the vaccine dose of 150 µg total protein is relatively high, making the vaccine expensive compared to other caprine vaccines. The OIE protocol recommends 3 mg of the adjuvant saponin per vaccine dose, which exceeds standard saponin concentrations (0.3 mg per dose) for small ruminants. The main drawback of this otherwise safe vaccine is the inflammatory reactions at the site of vaccination21, possibly due to the saponin adjuvant, and the short duration of immunity of only 6 months to maximally 1 year. These limitations might be overcome with new adjuvant formulations. Given the envisaged eradication of Peste des Petits Ruminants (PPR), a live CCPP vaccine that can be combined with the PPRV vaccine and other live vaccines would be desirable. Such a vaccine would save cost in terms of production and logistics associated with vaccine delivery. Given the widespread use of the live T1/44-based CBPP vaccine despite its residual pathogenicity16, live strains of M. capricolum subsp. capripneumoniae, which can be safely injected subcutaneously, should be tested for their ability to induce a protective immune response.
The limitations of the current CBPP and CCPP vaccines have led to a search for better vaccines. A lapinized vaccine based on the passaged strain Ben-1 has been used during the eradication of CBPP in China in the framework of a comprehensive eradication program22. This vaccine is reported to provide 95–100% protection over 2 years, which is superior to other mycoplasma vaccines and most commercial ruminant vaccines. However the method used for its production, intraperitoneal inoculation of sheep, is unacceptable under current animal welfare standards.
An experimental vaccine batch based on the lapinized strain but produced in culture media was compared with the commercial T1/44 vaccine in an in vivo trial on African cattle breeds, but its protective capacity was not superior to the commercial T1/44 (https://www.galvmed.org/galvmedat10/05-key-achievements.html). One of the key learnings of eradication of CBPP in China is that the lapinized and its sheep-adapted vaccine were paramount for the establishment of immunity zones around enzootic regions besides the other undoubtedly important control measures such as movement control as well as detection and slaughter.
Recently, using a reverse vaccinology approach researchers identified several candidate vaccine antigens that conferred protective immune responses to experimental CBPP challenge23. Further work has been ongoing to optimize the vaccine (scale up, duration of immunity, field testing, and comparison with T1/44 vaccine) with commercial acceptance in mind.
Any new vaccine should be tested under different field conditions and different control strategies identified using epidemiological models and previous local experience if available, to guide and inform national and regional control policy makers. An additional consideration is the uptake of livestock vaccines by smallholder farmers and marginalized populations which may require different strategies depending on factors such as the type of disease to be tackled, the value of the livestock species, the market size, profitability as well as the support of national and regional vaccine manufacturers among others. Vaccine adoption is influenced by availability of, access to and demand for vaccines. Global and national vaccine campaigns differ from market-driven approaches. The different strategies to increase adoption of animal vaccines by smallholder farmers especially in low and middle-income countries have been very capably reviewed recently24 and, as mentioned for implementation of control strategies, will not be further discussed.
Virulence traits, pathogenicity models, and potential vaccine targets for CBPP and CCPP
Most work on deciphering the factors that drive pathogenicity in these two pathogens has been performed on CBPP, probably because of European outbreaks in the 1980s and 1990s, which subsequently attracted research funding to several European laboratories. It has been proposed that African and European strains differ in virulence, this was based on an in vivo in-contact challenge study employing 2 groups of 2 animals each, which were infected with different M. mycoides subsp. mycoides strains. Strictly speaking only 3 animals were used, since one animals stayed seronegative after the first in-contact challenge and was subsequently reused in the other challenge group. Clinical signs of all 4 in-contact animals were very mild (nasal discharge) and pathomorphological changes were not seen in any of these in-contact challenged animals irrespective of the strain used25. The only difference observed was an earlier onset of seroconversion in animals in-contact with a donor infected with the African strain Afadé26. Later on it was shown by others that experimentally infected cattle developed different seroconversion profiles, described as early high responders, late high responders, and low responders27.
The African and European strains differed in a genetic locus encoding the ATP-binding cassette transporter proteins GtsA, GtsB, and GtsC, which were proposed to be involved in glycerol transport. The European strains missing this locus produced lower concentrations of H2O228. Based on the association between this metabolic difference and the late seroconversion after infection with the European strain L2, it was concluded that H2O2 production is a virulence trait. A mycoplasma metabolic enzyme called glycerol phosphate oxidase (GlpO) was identified as central in the production of peroxide in the presence of physiological glycerol29. This mechanism has been proposed as the main virulence mechanism of CBPP30 without confirmation using ex vivo or in vivo data according to Falkow’s postulates31. More recently, immunization experiments using recombinant GlpO followed by challenge did not demonstrate protection, which may be because either H2O2 production is not a main virulence trait or that GlpO itself is mainly cytoplasmic32, preventing antibodies from inhibiting its function.
Several other virulence traits have been suggested, but mainly based on in vitro systems with little or no data available from ex vivo or in vivo models. Since successful challenge of cattle with M. mycoides subsp. mycoides is difficult (see below) and efficient genetic tools to produce isogenic mutants are unavailable33, researchers have focussed instead on the caprine pathogen M. mycoides subsp. capri, in order to decipher virulence traits in Mycoplasma mycoides and test them in vivo. These studies have used synthetic genomics techniques, which allow unprecedented precise genome engineering of mycoplasmas34–39 and a caprine challenge model40–42. These experiments have provided in vivo evidence that capsular polysaccharide is a virulence trait in Mycoplasma mycoides40, as suggested more than 40 years ago43. They also have enabled the generation of a temperature-sensitive mutant of M. mycoides subsp. capri by targeting the essential gene obg42. However, this mutant did not seem to induce a strong protective immune response against a challenge with the wild type strain.
Another candidate virulence factor is the MIB-MIP system that is proposed to degrade immunoglobulins44. It is not difficult to imagine an extracellular pathogen (the current understanding of the habitat of these pathogens) would be shielded by interference with the function of host immunoglobulins, thus contributing to survival and pathogenicity. The in vitro proteolytic activity of the system has recently been confirmed in vivo41.
Last but not least, lipoproteins and variable surface proteins are candidate virulence factors that definitely deserve more research45. Like the capsular polysaccharide and the MIB-MIP system these cellular components are located on the pathogen’s surface and are therefore the primary molecules interacting with the host. It is known that for certain Mycoplasma species particular lipoproteins act as variable surface antigens [for review see:46] and facilitate evasion of the host’s immune response. Some of the lipoproteins show some degree of amino acid sequence identity across different Mycoplasma species, but their exact role in virulence has not been deciphered for M. mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae. It has been suggested that they contribute to an overwhelming immune response that leads to immunopathological consequences47. The lipid moieties of Mycoplasma pneumoniae lipoproteins have been recently suggested as causative factor of vaccine-enhanced disease using a mouse model48.
Research gaps to be addressed
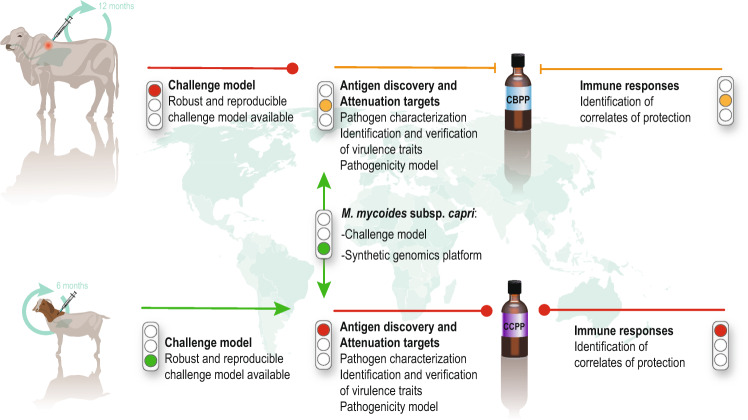
For clarity and a more systematic approach we have subdivided this important area into three topics. We summarized the current state of research blocks (Fig. 2) and a priority list of recommended research needs (Table 1).
Fig. 2. Cartoon displaying the major research blocks that influence rational vaccine design.
The characteristics of the current CBPP and CCPP vaccines are displayed on the left. The state of knowledge is characterized by traffic lights (red—missing; yellow—partly available; green—available).
Table 1.
Five top research priorities for the development of CBPP and CCPP vaccines.
| Priority | Contagious Bovine Pleuropneumonia | Contagious Caprine Pleuropneumonia |
|---|---|---|
| 1 | Development of a robust challenge model | Characterization of surface-localized virulence factors |
| 2 | Ex vivo and in vivo verification of surface-localized virulence factors using harmonized caprine models and M. mycoides subsp. capri (see Table 2) | Generate mutants and confirm attenuation in harmonized caprine ex vivo models |
| 3 | Generate M. mycoides subsp. mycoides mutants based on mutations that led to attenuation in an M. mycoides subsp. capri model (see priority 2) and test them as a live vaccine in vivo | Test candidate mutants for attenuation and induction of immune responses in vivo |
| 4 | Extend and revisit immunological knowledge based on correlates of protection using the novel challenge model (see Priority 1): characterize the innate and adaptive immune responses (local and systemic) after infection of vaccinated and naïve animals | Define immunological correlates of protection: (1) perform adoptive transfer of caprine IgG harvested from immune animals following immunization & challenge; (2) characterize the innate and adaptive immune responses (local and systemic) after infection of vaccinated and naïve animals |
| 5 | Characterize the Willems reaction and the mycoplasma factors that drive it | Applying systems immunology to improve the adjuvant formulation used for the current bacterin vaccine |
Challenge models
A reproducible challenge model is a conditio sine qua non for the development of a vaccine. Most mycoplasmas including M. mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae are host-target tissue specific. We should focus on the natural host and improve, if needed, the available challenge models. But which challenge models are available? For CCPP, of late a novel challenge model has recently been reported49, based on the recent Kenyan outbreak strain ILRI18150. This model is based on a combination of two subsequent inoculations of aerosols of cultures into the nasal cavity of goats followed by a trans-tracheal inoculation. Morbidity of 100% and a mortality of 50–60% was seen in infected animals, closely mimicking natural infection49. Improvements of the challenge model are possible, including titration of the challenge dose, use of an aerosol chamber for infection as has been used for experimental infections with other Mycoplasma species51,52, and whether either the aerosol or the trans-tracheal inoculations are dispensable to allow simplification of the procedure. However, given the robustness of the model thus far and its potential to be implemented in resource-poor settings, improvement of the model is currently a lower priority (Fig. 2). We encourage the development and employment of caprine airway epithelial cell cultures such as used in the human Mycoplasma field53–56.
The current challenge models for CBPP are less advanced and are suboptimal, as they have resulted in a variety of outcomes ranging from no clinical disease26 to a wide spectrum of pathological lesions23,47,57–59. A common feature of these models is the intratracheal inoculation of a Mycoplasma culture using a tube that is inserted via the nasal or oral cavity while the animal is standing or in lateral recumbency. In the past, fresh cultures were used for inoculation, however, it has now been demonstrated that frozen stock cultures work well for both pathogens60,49. The instillation of a solution of agar after inoculation of the mycoplasma broth culture has been show to be dispensable60. Trans-tracheal inoculation is easy to perform in Bos taurus cattle but is difficult in Bos indicus animals, as the dewlap in these animals impedes access and a Willems reaction is likely to occur. Given the range of disease severity with intratracheal inoculation reported so far, systematic development of a robust and reproducible challenge model for CBPP is clearly a priority (Fig. 1, Table 1). It needs to be based on a low passage field isolate, using cattle over 1 year of age and a defined dose of the organism. A good option would be to try aerosolized cultures and repeated inoculations, as this best mimics the natural route of infection. As a starting point, results obtained with M. bovis using an aerosol chamber to induce experimental infection in calves should provide some guidelines for further development51. A standardized challenge model, preferably using a single aliquoted frozen challenge stock, would allow data to be exchanged and compared more easily, a critical requirement given the high costs and logistical challenges associated with large animal trials. This way, the research community could benefit the most from data generated in in vivo trials, which generally trump data derived from in vitro and ex vivo assays.
In-contact infection models were not considered a practicable alternative as the timing of individual infection and disease signs are difficult to synchronize because of differences of interactions between animals, which influence transmission of disease. Therefore, such models are intrinsically more variable, require a large number of animals and are not the best option for the read out of immunological parameters over time. Moreover, recently established ex vivo models61,62 for deciphering pathogenicity mechanisms and identifying virulence traits may prove beneficial (Table 1).
Candidate vaccine antigens and attenuation targets - filling the knowledge gap on Mycoplasma
Mycoplasmas lack a cell wall and due to their inherent minute size one might assume that they are easily characterized, but this is not the case. In fact, we know relatively little about the structure and physiology of M. mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae. This is influenced by the classification as BSL3 pathogens in some countries, restricting many research laboratories from working with them, and by the lack of genetic tools to induce defined mutations. However, the advent of synthetic genomics has paved the way for mutagenesis of a closely related subspecies of M. mycoides subsp. mycoides namely M. mycoides subsp. capri35–38. The availability of diverse next generation sequencing platforms has enabled researchers to characterize the genomes of M. mycoides subsp. mycoides63 and M. capricolum subsp. capripneumoniae50,64, demonstrating relatively little sequence diversity6,65.
What we lack at present is characterization of the pan and core genome of M. mycoides subsp. mycoides, M. capricolum subsp. capripneumoniae and the entire “M. mycoides cluster”. This information is likely to help us identify common genes among mycoplasmas that play a role in fitness and persistence in hosts and to identify new vaccine targets or sites for attenuation. A detailed transcriptomics analysis, preferably combined with proteomics studies is also missing. This would provide baseline data that could be used to tease apart operons and promoters and enable us to better understand the organisms’ physiological pathways as well as improve the annotation of the genomes for the sake of rationale vaccine design.
The mycoplasma metabolism depends on scavenging a wide range of host molecules, as mycoplasmas are unable to synthesize many of the precursors for their macromolecules (including proteins, nucleic acids, and lipids). This scavenging lifestyle requires transporters that span the cell membrane and translocate the scavenged molecules to the enzymatic apparatus inside the mycoplasma cell. Interference with such transporters is likely to affect the growth and viability of mycoplasmas substantially, so essential transporters are likely to be promising targets in future rationale vaccine efforts. Candidates have already been identified in other Mycoplasma species that are present in the “Mycoplasma mycoides cluster”, including the putative oligopeptide/dipeptide (opp/dpp) ATP-binding cassette (ABC) transporter66,67. Therefore, a combination of in silico analysis and OMICS data (proteomics, transcriptomics, metabolomics) is likely to enable us to identify transporters that should be target for future characterization.
However, we need to know more about lipoproteins and other surface-exposed proteins of these mycoplasmas, and particulary their function and abundance in order to identify protective antigens to be tested later on (Table 1). The core in vitro surface proteome of M. mycoides subsp. mycoides has been suggested to include about 50 lipoproteins and other membrane proteins68. Some of these have been shown to be immunogenic69 and even to induce a protective immune response23, while others have been suggested to contribute to immunopathology47. Understanding of the surface proteome of M. capricolum subsp. capripneumoniae is lagging behind. Many genes encoding putative lipoproteins and transmembrane proteins have been identified in silico50,64,70, but data regarding their expression, function and immunogenicity are lacking. It will be important to first confirm the cellular localization of these proteins in M. mycoides and M. capricolum using either available fluorescent molecules (e.g., mCherry, mNeon)71 or tags coupled with electron microscopy among others. This work would allow the research community to build a list of surface-exposed virulence candidates that can be further tested in ex vivo and in vivo systems discussed above. In an effort to initiate such a process, we have listed the candidate virulence traits that we believe require immediate attention (Table 2).
Table 2.
Prioritization of candidate virulence factors for investigation according to Falkow’s postulates.
| Priority | Candidate virulence factor | Comment |
|---|---|---|
| 1 | L-alpha-glycerophosphate oxidase (GlpO) | Knock out mutants are available for M. mycoides subsp. capri, the role of this candidate virulence factor requires immediate investigation, as it is dispensable for virulence in M. gallisepticum |
| 2 | MIB-MIP system | Knock out mutants are already available for M. mycoides subsp. capri, in vivo activity has been proven and this is a top candidate virulence factor |
| 3 | Different DUF groups of lipoproteins | Individual lipoproteins and pathogen specific DUF groups of lipoproteins need to be tested for their role in virulence |
| 4 | Variable surface proteins | Have been shown in vitro and in vivo to be functional in mycoplasmas |
| 5 | Oligopeptide/dipeptide (opp/dpp) ATP-binding cassette (ABC) transporter | Has been shown in M. agalactiae to be a candidate virulence factor |
| 6 | 6-phospho-beta-glucosidase (Bgl) | Correlations indicate a role in cytotoxicity |
Some of the mycoplasma lipoproteins identified so far can be classified into different families, as they possess conserved domains of unknown functions (DUFs). Some of these families are found throughout the phylum Firmicutes (which includes the class Mollicutes) while others appear to be “mycoplasma-specific”. Functional studies of these proteins are not trivial as functional redundancy is likely to occur between the different family members. Until efficient genetic tools are available for M. mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae, we suggest using the closely related pathogens M. mycoides subsp. capri and M. capricolum subsp. capricolum, respectively, as a model to characterize these lipoproteins (Fig. 2). The capacity to delete multiple genes or even subgenomic fragments in the genomes of M. mycoides subsp. capri and M. capricolum subsp. capricolum will allow the generation of strains in which all members of a DUF family have been removed. Using such a mutagenesis system, five genomic regions of M. mycoides subsp. capri strain GM12 comprising 68 genes were deleted recently41. In addition to the glycerol metabolism-related genes, the deleted genes encoded 18 lipoproteins and 21 transmembrane proteins. This resulted in the complete attenuation of this otherwise pathogenic strain. The same synthetic genomics tools have been used to graft heterologous subgenomic Mycoplasma fragments into M. mycoides subsp. capri (unpublished data), generating a useful tool for future characterization of a subset of M. mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae target molecules.
Another interesting feature of mycoplasmas is their exosecretion of bacterial compounds that can interact with their hosts. M. mycoides cell membranes have been shown to have several protrusions that can themselves have several constrictions63. Extracellular vesicles have also been shown to be released by mycoplasmas72. Other mycoplasmas, including M. hyopneumoniae, shed extracellular domains of proteins73 and this has also been observed for M. capricolum subsp. capripneumoniae and M. mycoides subsp. mycoides74. The role of these secreted vesicles and proteins in the pathogenesis of mycoplasmoses, if any, needs to be determined in future studies.
In the interest of maximizing the benefit obtained from limited resources we suggest focusing research activities on a defined set of mycoplasma strains in order to generate synergies and facilitate comparisons between in vivo, ex vivo, and in vitro data (Table 1).
Defining correlates of immunity and characterizing immune responses after infection
Most of the immunological data generated this far has been collected by the research groups at CIRAD, the International Livestock Research Institute and the Kenya Agricultural & Livestock Research Organization, who have been undertaking vaccine development work. In the interest of time and space we do not include in vivo data that have been collected from rodent models, which are not likely to be as informative about protective immune responses in domestic ruminants. In their natural hosts, immunizations with a live CBPP vaccine or a CCPP bacterin induce a protective immune response. This immunity needs to be dissected to foster rational design of improved vaccines (Table 1).
We suggest performing adoptive transfer experiments with purified antibodies from convalescent, diseased, and healthy animals to animals that are subsequently infected experimentally. Such activities should be initiated with CCPP, as the infection model is well established, and large amounts of sera have been collected from convalescent animals at necropsy during a recent trial49. If the adoptively transferred antibodies are protective, we can focus on characterization of the spectrum of specificities of the antibodies using bead-based assays and bioinformatic network analysis of B-cell responses in protected animals. A similar experiment should be done with CBPP, when a better challenge model is available.
Traditional methods of determining the protective role of T-cell mediated immunity, by depleting specific subsets of T cells have been performed for CBPP57. Such depletion experiments require large quantities of monoclonal antibodies directed against markers on ruminant T cells, and often necessitate repeated administration. Their interpretation can be misleading because T cells are a component of an interactive system and removing any one element can have an impact on the whole system. Furthermore, many cell surface markers are not unique to one population. Therefore, alternative approaches may be useful, such as those arising from the field of systems immunology75 and systems vaccinology76, which have recently been adapted from humans to livestock species77,78. High-throughput technologies such as RNA sequencing coupled with data analysis allow the interrogation of hosts’ intracellular and intercellular interactions to understand the entire immune system better. It permits exploration of mechanistic insights, which enables vaccines to induce protective immune responses. The principle of such analyses is the collection of very large data sets by examining responses of immune cells using advanced immunological techniques and transcriptomic analyses. Changes in the transcriptional modules and changes in the immune responses can be analysed for any correlations to identify elements of the innate and adaptive immune response that promote protection in ruminants. It can be used to analyse both live and inactivated vaccine responses and correlate them with protection and to identify a suitable adjuvant that promotes longlasting immunity76. Applying such technologies to the current bacterin CCPP vaccine and novel CBPP and CCPP vaccine candidates will inform on the mechanism of protection, including both antibody responses and responses by different types of T cells, including the role of T helper (Th)1, Th2, and Th17 or regulatory T cells (Table 1). Although these approaches are resource intensive and require bioinformatic and immunological expertise, they will enable a more rational design of vaccine and accelerate the development of improved vaccines. Future research focusing on components of mucosal immunity such as secretory IgA levels that can prevent infection is also required, as it is known with other mycoplasma vaccines that systemic antibody does not necessarily correlate with protection against lung pathology79.
The characterization of the innate immune response is also paramount, as the innate immune response is likely to determine the outcome of infection or immunization by directing the type of adaptive immune response that ensues, and is also the first line of defence against the pathogen. It is self-evident that granulocytes80 and macrophages81 infiltrating the site of infection shape the outcome of disease and the pathogenicity. Therefore, their interaction with the pathogen and its components needs to be closely followed to generate a more complete picture of the mechanisms underlying the development of lesions initiated by either the pathogen or the host immune response. New and improved adjuvants and immune modulators are available that can increase the duration of immunity and direct the immune response towards more protective responses. These should be evaluated, using the data generated in the experiments described above to guide selection of the most appropriate candidate vaccines.
Prioritization of research efforts towards a novel prophylactic vaccine
Prioritization largely depends on the point of view of individual researchers, with microbiologists favouring characterization of the pathogen and immunologists favouring the characterization of the immune responses. However, a closer interaction between these two groups is needed to best tackle pathogens that kill our livestock in such large numbers. After rigorous scientific discussions, our suggested priority actions are shown in Table 1. We believe these approaches will guide development of better vaccines that can be used as tools in the control of these two important pathogens.
However, even the most efficacious vaccine does not alone imply its adoption and successful disease control. The Rinderpest eradication taught us that any vaccination campaign needs to involve many stakeholders such as epidemiologists and social scientists to develop and implement policies and strategies to achieve successful disease control7.
Supplementary information
Acknowledgements
An international workshop entitled ‘Contagious bovine and caprine pleuropneumonia, an update on the current knowledge base’ was held at the University of Bern in 2018. This workshop was funded by the Bill & Melinda Gates Foundation, the UK Department for International Development (DFID) through GALVmed and the University of Bern. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation nor DFID. Jörg Jores, Alain Blanchard, Fabien Labroussaa, and Sanjay Vashee were supported by the International Development Research Centre (Grant ID: 108625) or the National Science Foundation (Grant ID: IOS-1110151). Special thanks to the administrative support we received from University of Bern and GALVmed staff during the workshop. We thank all participants for their participation in the discussions. We thank Jeanne Peter Zocher for her help on the figures.
Author contributions
J.J. and J.S. conceived the article, J.J. drafted the article. All authors contributed substantially to the content and reviewed or edited the manuscript. All authors approved the manuscript.
Data availability
All relevant data are included in the manuscript and Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41541-020-00214-2.
References
- 1.Hubschle O, Aschenborn O, Godinho K, Nicholas R. Control of CBPP–a role for antibiotics? Vet. Rec. 2006;159:464. doi: 10.1136/vr.159.14.464-b. [DOI] [PubMed] [Google Scholar]
- 2.Curasson, G. Traité de pathologie exotique vétérinaire et comparée, Vol. 2276-353 (Vigot frères, 1942).
- 3.von Haller, A. De Lue Bovilla Agri Bernensis Commentatio. In: Novi commentarii Societatis Regiae ScientiarumGottingensis, 25–43 (Gouvernement général civil de l’Algérie, 1773).
- 4.Provost A, et al. Contagious bovine pleuropneumonia. Rev. Sci. Tech. Int. Epiz. 1987;6:625–679. [Google Scholar]
- 5.Nocard E, Roux E. Le microbe de la péripneumonie. Ann. Inst. Pasteur. 1898;12:240–262. [Google Scholar]
- 6.Dupuy V, et al. Evolutionary history of contagious bovine pleuropneumonia using next generation sequencing of Mycoplasma mycoides Subsp. mycoides “Small Colony”. PLoS ONE. 2012;7:e46821. doi: 10.1371/journal.pone.0046821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariner JC, et al. Rinderpest eradication: appropriate technology and social innovations. Science. 2012;337:1309–1312. doi: 10.1126/science.1223805. [DOI] [PubMed] [Google Scholar]
- 8.Thomas, P. Rapport médical sur le Bou Frida. In Publication du gouvernement général civil de l’Algérie (ed Jourdan, A.) (Gouvernement général civil de l’Algérie, Alger, 1873).
- 9.Hutcheon D. Contagious pleuro-pneumonia in angora goats. Vet. J. 1881;13:171–180. [Google Scholar]
- 10.MacOwan KJ, Minette JE. A mycoplasma from acute contagious caprine pleuropneumonia in Kenya. Trop. Anim. Health Prod. 1976;8:91–95. doi: 10.1007/BF02383376. [DOI] [PubMed] [Google Scholar]
- 11.Ozdemir U, Ozdemir E, March JB, Churchward C, Nicholas RA. Contagious caprine pleuropneumonia in the Thrace region of Turkey. Vet. Rec. 2005;156:286–287. doi: 10.1136/vr.156.9.286. [DOI] [PubMed] [Google Scholar]
- 12.Nicholas RAJ, Sayi O, Turkyilmaz MA, Erpek SH. Survey of contagious caprine pleuropneumonia in goat herds in the Thrace region of Turkey. Rev. Sci. Tech. 2018;37:831–836. doi: 10.20506/rst.37.3.2889. [DOI] [PubMed] [Google Scholar]
- 13.Ssematimba A, Jores J, Mariner JC. Mathematical modelling of the transmission dynamics of contagious bovine pleuropneumonia reveals minimal target profiles for improved vaccines and diagnostic assays. PLoS ONE. 2015;10:e0116730. doi: 10.1371/journal.pone.0116730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas R, Churchward C. Contagious caprine pleuropneumonia: new aspects of an old disease. Transbound. Emerg. Dis. 2012;59:189–196. doi: 10.1111/j.1865-1682.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiaucourt F, Aboubakar Y, Wesonga H, Manso-Silvan L, Blanchard A. Contagious bovine pleuropneumonia vaccines and control strategies: recent data. Dev. Biol. (Basel) 2004;119:99–111. [PubMed] [Google Scholar]
- 16.Mbulu RS, et al. Contagious bovine pleuropneumonia (CBPP) caused by vaccine strain T1/44 of Mycoplasma mycoides subsp. mycoides SC. Vet. Microbiol. 2004;98:229–234. doi: 10.1016/j.vetmic.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Mariner JC, McDermott J, Heesterbeek JA, Thomson G, Martin SW. A model of contagious bovine pleuropneumonia transmission dynamics in East Africa. Prev. Vet. Med. 2006;73:55–74. doi: 10.1016/j.prevetmed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Mariner JC, et al. A heterogeneous population model for contagious bovine pleuropneumonia transmission and control in pastoral communities of East Africa. Prev. Vet. Med. 2006;73:75–91. doi: 10.1016/j.prevetmed.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Rurangirwa FR, McGuire TC, Kibor A, Chema S. An inactivated vaccine for contagious caprine pleuropneumonia. Vet. Rec. 1987;121:397–400. doi: 10.1136/vr.121.17.397. [DOI] [PubMed] [Google Scholar]
- 20.Rurangirwa FR, McGuire TC, Mbai L, Ndung’u L, Wambugu A. Preliminary field test of lyophilised contagious caprine pleuropneumonia vaccine. Res Vet. Sci. 1991;50:240–241. doi: 10.1016/0034-5288(91)90114-4. [DOI] [PubMed] [Google Scholar]
- 21.Salt J, et al. Vaccination against CCPP in East Africa. Vet. Rec. 2019;185:272. doi: 10.1136/vr.l5353. [DOI] [PubMed] [Google Scholar]
- 22.Xin J, et al. A history of the prevalence and control of contagious bovine pleuropneumonia in China. Vet. J. 2012;191:166–170. doi: 10.1016/j.tvjl.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Nkando I, et al. Recombinant Mycoplasma mycoides proteins elicit protective immune responses against contagious bovine pleuropneumonia. Vet. Immunol. Immunopathol. 2016;171:103–114. doi: 10.1016/j.vetimm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Donadeu M, Nwankpa N, Abela-Ridder B, Dungu B. Strategies to increase adoption of animal vaccines by smallholder farmers with focus on neglected diseases and marginalized populations. PLoS Negl. Trop. Dis. 2019;13:e0006989. doi: 10.1371/journal.pntd.0006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miserez, R. et al. in Mycoplasmas of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics Vol. 2 (eds Frey. J. & K. Sarris) 147–149 (Office for official publications of the European Communities, Luxembourg, 1996).
- 26.Abdo E-M, et al. Humoral and bronchial immune responses in cattle experimentally infected with Mycoplasma mycoides subsp. mycoides small colony type. Vet. Microbiol. 1998;59:109–122. doi: 10.1016/S0378-1135(97)00184-3. [DOI] [PubMed] [Google Scholar]
- 27.Schubert E, Sachse K, Jores J, Heller M. Serological testing of cattle experimentally infected with Mycoplasma mycoides subsp. mycoides Small Colony us-ing four different tests reveals a variety of seroconversion patterns. BMC Vet. Res. 2011;7:72. doi: 10.1186/1746-6148-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilei EM, Frey J. Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: its impact on H2O2 production and virulence. Clin. Diagn. Lab Immunol. 2001;8:85–92. doi: 10.1128/CDLI.8.1.85-92.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilo P, et al. A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J. Bacteriol. 2005;187:6824–6831. doi: 10.1128/JB.187.19.6824-6831.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilo P, Frey J, Vilei EM. Molecular mechanisms of pathogenicity of Mycoplasma mycoides subsp. mycoides SC. Vet. J. 2007;174:513–521. doi: 10.1016/j.tvjl.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falkow S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev. Infect. Dis. 1988;10(Suppl 2):S274–S276. doi: 10.1093/cid/10.Supplement_2.S274. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher M, et al. Evidence for the cytoplasmic localization of the L-alpha-Glycerophosphate oxidase in members of the “Mycoplasma mycoides Cluster”. Front. Microbiol. 2019;10:1344. doi: 10.3389/fmicb.2019.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labroussaa F, et al. Impact of donor-recipient phylogenetic distance on bacterial genome transplantation. Nucleic Acids Res. 2016;44:8501–8511. doi: 10.1093/nar/gkw688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benders GA, et al. Cloning whole bacterial genomes in yeast. Nucleic Acids Res. 2010;38:2558–2569. doi: 10.1093/nar/gkq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lartigue C, et al. Creating bacterial strains from genomes that have been cloned and engineered in yeast. Science. 2009;325:1693–1696. doi: 10.1126/science.1173759. [DOI] [PubMed] [Google Scholar]
- 36.Lartigue C, et al. Genome transplantation in bacteria: changing one species to another. Science. 2007;317:632–638. doi: 10.1126/science.1144622. [DOI] [PubMed] [Google Scholar]
- 37.Tsarmpopoulos I, et al. In-yeast engineering of a bacterial genome using CRISPR/Cas9. ACS Synth. Biol. 2016;5:104–109. doi: 10.1021/acssynbio.5b00196. [DOI] [PubMed] [Google Scholar]
- 38.Chandran S, et al. TREC-IN: gene knock-in genetic tool for genomes cloned in yeast. BMC Genomics. 2014;15:1180. doi: 10.1186/1471-2164-15-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noskov V, et al. A genetic system for direct selection of gene-positive clones during recombinational cloning in yeast. Nucleic Acids Res. 2002;30:E8. doi: 10.1093/nar/30.2.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jores J, et al. In vivo role of capsular polysaccharide in Mycoplasma mycoides. J. Infect. Dis. 2019;219:1559–1563. doi: 10.1093/infdis/jiy713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jores J, et al. Removal of a subset of non-essential genes fully attenuates a highly virulent mycoplasma strain. Front. Microbiol. 2019;10:664. doi: 10.3389/fmicb.2019.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lartigue, C. et al. Attenuation of a pathogenic mycoplasma strain by modification of the obg gene by using synthetic biology approaches. mSphere.10.1128/mSphere.00030-19 (2019). [DOI] [PMC free article] [PubMed]
- 43.Buttery SH, Lloyd LC, Titchen DA. Acute respiratory, circulatory and pathological changes in the calf after intravenous injections of the galactan from Mycoplasma mycoides subsp. mycoides. J. Med. Microbiol. 1976;9:379–391. doi: 10.1099/00222615-9-4-379. [DOI] [PubMed] [Google Scholar]
- 44.Arfi Y, et al. MIB-MIP is a mycoplasma system that captures and cleaves immunoglobulin G. Proc. Natl Acad. Sci. USA. 2016;113:5406–5411. doi: 10.1073/pnas.1600546113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browning GF, Marenda MS, Noormohammadi AH, Markham PF. The central role of lipoproteins in the pathogenesis of mycoplasmoses. Vet. Microbiol. 2011;153:44–50. doi: 10.1016/j.vetmic.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Citti C, Nouvel LX, Baranowski E. Phase and antigenic variation in mycoplasmas. Future Microbiol. 2010;5:1073–1085. doi: 10.2217/fmb.10.71. [DOI] [PubMed] [Google Scholar]
- 47.Mulongo M, et al. Vaccination of cattle with the N terminus of LppQ of Mycoplasma mycoides subsp. mycoides results in type III immune complex disease upon experimental infection. Infect. Immun. 2015;83:1992–2000. doi: 10.1128/IAI.00003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mara AB, Gavitt TD, Tulman ER, Geary SJ, Szczepanek SM. Lipid moieties of Mycoplasma pneumoniae lipoproteins are the causative factor of vaccine-enhanced disease. NPJ Vaccines. 2020;5:31. doi: 10.1038/s41541-020-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liljander A, et al. Reproduction of contagious caprine pleuropneumonia reveals the ability of convalescent sera to reduce hydrogen peroxide production in vitro. Vet. Res. 2019;50:10. doi: 10.1186/s13567-019-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falquet, L. et al. Complete genome sequences of virulent mycoplasma capricolum subsp. capripneumoniae Strains F38 and ILRI181. Genome Announc.10.1128/genomeA.01041-14 (2014). [DOI] [PMC free article] [PubMed]
- 51.Kanci A, et al. Reproduction of respiratory mycoplasmosis in calves by exposure to an aerosolised culture of Mycoplasma bovis. Vet. Microbiol. 2017;210:167–173. doi: 10.1016/j.vetmic.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Czaja T, et al. Induction of enzootic pneumonia in pigs by the administration of an aerosol of in vitro-cultured Mycoplasma hyopneumoniae. Vet. Rec. 2002;150:9–11. doi: 10.1136/vr.150.1.9. [DOI] [PubMed] [Google Scholar]
- 53.Prince, O. A., Krunkosky, T. M., Sheppard, E. S. & Krause, D. C. Modelling persistent Mycoplasma pneumoniae infection of human airway epithelium. Cell Microbiol.10.1111/cmi.12810 (2018). [DOI] [PMC free article] [PubMed]
- 54.Prince OA, Krunkosky TM, Krause DC. In vitro spatial and temporal analysis of Mycoplasma pneumoniae colonization of human airway epithelium. Infect. Immun. 2014;82:579–586. doi: 10.1128/IAI.01036-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y, et al. Mycoplasma pneumoniae modulates STAT3-STAT6/EGFR-FOXA2 signaling to induce overexpression of airway mucins. Infect. Immun. 2014;82:5246–5255. doi: 10.1128/IAI.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krunkosky TM, Jordan JL, Chambers E, Krause DC. Mycoplasma pneumoniae host-pathogen studies in an air-liquid culture of differentiated human airway epithelial cells. Micro. Pathog. 2007;42:98–103. doi: 10.1016/j.micpath.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Sacchini F, et al. A minor role of CD4+ T lymphocytes in the control of a primary infection of cattle with Mycoplasma mycoides subsp. mycoides. Vet. Res. 2011;42:77. doi: 10.1186/1297-9716-42-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulongo MM, et al. Cattle immunized against the pathogenic L-alpha-glycerol-3-phosphate oxidase of Mycoplasma mycoides subs. mycoides fail to generate neutralizing antibodies and succumb to disease on challenge. Vaccine. 2013;31:5020–5025. doi: 10.1016/j.vaccine.2013.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wesonga HO, Thiaucourt F. Experimental studies on the efficacy of T1 sr and T1/44 vaccine strains of Mycoplasma mycoides subspecies mycoides (small colony) against a field isolate causing contagious bovine pleuropneumonia in Kenya—effect of a revaccination. Rev. d.'élevage et. de. m.édecine v.étérinaire des. pays tropicaux. 2000;53:313–318. [Google Scholar]
- 60.Schieck E, et al. High antibody titres against predicted Mycoplasma surface proteins do not prevent sequestration in infected lung tissue in the course of experimental contagious bovine pleuropneumonia. Vet. Microbiol. 2014;172:285–293. doi: 10.1016/j.vetmic.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 61.Weldearegay, Y. B. et al. Host-pathogen interactions of mycoplasma mycoides in Caprine and Bovine precision-cut lung slices (PCLS) models. Pathogens.10.3390/pathogens8020082 (2019). [DOI] [PMC free article] [PubMed]
- 62.Di Teodoro G, et al. Respiratory explants as a model to investigate early events of contagious bovine pleuropneumonia infection. Vet. Res. 2018;49:5. doi: 10.1186/s13567-017-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer A, et al. High quality draft genomes of the Mycoplasma mycoides subsp. mycoides challenge strains Afadé and B237. Stand Genom. Sci. 2015;10:89. doi: 10.1186/s40793-015-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dupuy, V. & Thiaucourt, F. Complete genome sequence of Mycoplasma capricolum subsp. capripneumoniae strain 9231-abomsa. Genome Announc.10.1128/genomeA.01067-14 (2014). [DOI] [PMC free article] [PubMed]
- 65.Dupuy V, Verdier A, Thiaucourt F, Manso-Silvan L. A large-scale genomic approach affords unprecedented resolution for the molecular epidemiology and evolutionary history of contagious caprine pleuropneumonia. Vet. Res. 2015;46:74. doi: 10.1186/s13567-015-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu, L. et al. Comparative genomic analyses of Mycoplasma synoviae vaccine strain MS-H and its wild-type parent strain 86079/7NS: implications for the identification of virulence factors and applications in diagnosis of M. synoviae. Avian Pathol.10.1080/03079457.2019.1637514 (2019). [DOI] [PubMed]
- 67.Masukagami Y, et al. Metabolite profiling of Mycoplasma gallisepticum mutants, combined with bioinformatic analysis, can reveal the likely functions of virulence-associated genes. Vet. Microbiol. 2018;223:160–167. doi: 10.1016/j.vetmic.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Krasteva I, et al. Characterization of the in vitro core surface proteome of Mycoplasma mycoides subsp. mycoides, the causative agent of contagious bovine pleuropneumonia. Vet. Microbiol. 2014;168:116–123. doi: 10.1016/j.vetmic.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Casal J, et al. Analysis of immune responses to recombinant proteins from strains of Mycoplasma mycoides subsp. mycoides, the causative agent of contagious bovine pleuropneumonia. Vet. Immunol. Immunopathol. 2015;168:103–110. doi: 10.1016/j.vetimm.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 70.Li, Y. et al. Comparative genomics analysis of Mycoplasma capricolum subsp. capripneumoniae 87001. Genomics.10.1016/j.ygeno.2019.04.013 (2019). [DOI] [PubMed]
- 71.Bonnefois T, et al. Development of fluorescence expression tools to study host-mycoplasma interactions and validation in two distant mycoplasma clades. J. Biotechnol. 2016;236:35–44. doi: 10.1016/j.jbiotec.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Gaurivaud P, et al. Mycoplasmas are no exception to extracellular vesicles release: revisiting old concepts. PLoS ONE. 2018;13:e0208160. doi: 10.1371/journal.pone.0208160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tacchi JL, et al. Post-translational processing targets functionally diverse proteins in Mycoplasma hyopneumoniae. Open Biol. 2016;6:150210. doi: 10.1098/rsob.150210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganter, S. et al. Proteases as secreted exoproteins in mycoplasmas from ruminant lungs and their impact on surface-exposed proteins. Appl. Environ. Microbiol.10.1128/AEM.01439-19 (2019). [DOI] [PMC free article] [PubMed]
- 75.Davis MM, Tato CM, Furman D. Systems immunology: just getting started. Nat. Immunol. 2017;18:725–732. doi: 10.1038/ni.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hagan T, Nakaya HI, Subramaniam S, Pulendran B. Systems vaccinology: enabling rational vaccine design with systems biological approaches. Vaccine. 2015;33:5294–5301. doi: 10.1016/j.vaccine.2015.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthijs AMF, et al. Systems immunology characterization of novel vaccine formulations for mycoplasma hyopneumoniae bacterins. Front Immunol. 2019;10:1087. doi: 10.3389/fimmu.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun RO, et al. System immunology-based identification of blood transcriptional modules correlating to antibody responses in sheep. NPJ Vaccines. 2018;3:41. doi: 10.1038/s41541-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6:107–112. [Google Scholar]
- 80.Di Federico M, et al. Pro-inflammatory response of bovine polymorphonuclear cells induced by Mycoplasma mycoides subsp. mycoides. Front Vet. Sci. 2020;7:142. doi: 10.3389/fvets.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jungi TW, Krampe M, Sileghem M, Griot C, Nicolet J. Differential and strain-specific triggering of bovine alveolar macrophage effector functions by mycoplasmas. Micro. Pathog. 1996;21:487–498. doi: 10.1006/mpat.1996.0078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript and Supplementary Information.