Highlights
-
•
Autism spectrum disorder (ASD) & schizophrenia (SZ) have mentalizing deficits.
-
•
Spatially constrained ICA reveals shared deficits in mentalizing default mode activity.
-
•
Mentalizing-related temporoparietal junction activity correlated with ADOS scores in ASD.
-
•
Mentalizing-related precuneus activity correlated with tendency to fantasize in SZ.
-
•
Both categorical and RDoC approaches to study neural deficits in SZ & ASD are supported.
Keywords: Social functioning, Default mode network, Temporoparietal junction, Posterior cingulate cortex, Precuneus, Research domain criteria
Abstract
Schizophrenia and autism spectrum disorder (ASD) are nosologically distinct neurodevelopmental disorders with similar deficits in social cognition, including the ability to form mental representations of others (i.e., mentalizing). However, the extent of patient deficit overlap in underlying neural mechanisms is unclear. Our goal was to examine deficits in mentalizing task-related (MTR) activity modulation in schizophrenia and ASD and the relationship of such deficits with social functioning and psychotic symptoms in patients. Adults, ages 18–34, diagnosed with either ASD or schizophrenia, and typically developed controls (n = 30/group), performed an interactive functional MRI Domino task. Using independent component analysis, we analyzed game intervals known to stimulate mentalizing in the default mode network (DMN), i.e., medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus, and temporoparietal junction (TPJ), for group differences in MTR activity and associations between MTR activity and social and psychosis measures. Compared to controls, both schizophrenia and ASD groups showed MTR activity deficits in PCC and TPJ. In TPJ and MPFC, MTR activity modulation was associated with social communication impairments only in ASD. In precuneus, MTR activity was associated with increased self-reported fantasizing only in schizophrenia. In schizophrenia, we found no indication of over-mentalizing activity or an association between MTR activity and psychotic symptoms. Results suggest shared neural deficits between ASD and schizophrenia in mentalizing-associated DMN regions; however, neural organization might correspond to different dimensional social deficits. Our results therefore indicate the importance of examining both categorical-clinical diagnosis and social functioning dimensional constructs when examining neural deficits in schizophrenia and ASD.
1. Introduction
Schizophrenia (SZ) and Autism Spectrum Disorder (ASD), traditionally conceptualized as separate clinical entities (America Psychiatric Association, 2013), are severe neurodevelopmental disorders that share symptom traits, cognitive deficits and risk factors (King and Lord, 2011). Behaviorally, social processing impairments are central to both ASD and SZ (Couture et al., 2010, King and Lord, 2011, America Psychiatric Association, 2013) and are related to functional outcome (Bell et al., 2009, Couture et al., 2011, Javed and Charles, 2018, Tillmann et al., 2019). Social processing is conceptualized as cognitive processes supporting interaction with conspecifics, which include basic and complex social processes with distinct characteristics and underlying neural circuits (Adolphs, 2010, Yang et al., 2015). Basic processes are automatic and include perception and production of social cues. Complex processes require active inference (Adolphs, 2010, Yang et al., 2015), such as understanding other’s feelings and goals (i.e., mentalizing/theory of mind). While there is evidence that both are impaired in ASD and SZ (Couture et al., 2010, Pepper et al., 2018), meta-analyses demonstrated similar quantitative deficits in mentalizing (Chung et al., 2014, Fernandes et al., 2018), but not in basic emotion perception tasks (Fernandes et al., 2018). Despite phenotypic (i.e., symptomatic) differences between ASD and SZ, studies comparing these patient groups directly confirm similar patterns of mentalizing deficits based on available quantitative social cognitive tasks (Couture et al., 2010, Craig et al., 2004, Pepper et al., 2018). However, it is not known if shared impairments are the manifestation of overlapping or different (disease-specific) underlying neural mechanisms.
Neuroimaging studies suggest specific neural networks subserve different social processes (Schilbach et al., 2008, Yang et al., 2015). The mentalizing network includes the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus (PrC) and temporoparietal junction (TPJ), including superior temporal sulcus (STS) as core regions (Assaf et al., 2009, Yang et al., 2015). Both ASD and SZ, studied separately, show abnormalities in this network, and a review found that while both groups exhibit decreased activations of regions around the STS, they differ in other regions, e.g., MPFC (Sugranyes et al., 2011).
Only three studies, however, compared these groups directly on social tasks. Pinkham et al. (Pinkham et al., 2008) showed similar activation deficits in ASD and paranoid-SZ during a trustworthiness task in the right amygdala, fusiform face area and left ventrolateral PFC. Conversely, Ciaramidaro et al. (Ciaramidaro et al., 2015) and Eack et al. (2017) used mentalizing tasks and found diagnostic specific deficits in PFC and temporal regions, including TPJ and STS. Ciaramidaro et al. (2015) additionally demonstrated that when compared with ASD and controls, SZ patients showed increased activation in the right posterior STS during non-intentional events (i.e., events not involving social interaction). This increased activity in the mentalizing network during non-intentional events in SZ corresponds with the theory that patients with SZ, especially those with prominent positive symptoms such as paranoia, might attribute excess meaning or over-attribute intentions to physical (non-social) events and/or people (Frith, 2004, Martinez et al., 2019). This phenomenon is variably known as “hyper-intentionality” (Ciaramidaro et al., 2015), “hyper-mentalizing” (Bliksted et al., 2019), or “over-mentalizing” (Frith, 2004, Martinez et al., 2019). The number of neuroimaging studies that have examined the neural correlates of the potential over-mentalizing in SZ is small, but these few studies have in common the finding of increased activity in the MPFC in SZ patients, when compared with controls, during non-social fMRI task events (Backasch et al., 2013, Bliksted et al., 2019, Ciaramidaro et al., 2015). Additional neuroimaging studies are required to examine more closely the over-mentalizing theory in SZ and its relationship to positive and negative symptoms.
Other studies that examine the mentalizing network in SZ and ASD employ resting state (RS) fMRI (i.e., when no task is presented) to delineate the default mode network (DMN). This network largely overlaps the mentalizing network and is associated with high-order social processes (Hyatt et al., 2015, Mars et al., 2012). Impaired DMN functional connectivity (FC; a measure of synchronous neural activity between remote brain areas that define neural networks) has been demonstrated in SZ and ASD, each studied separately (Hu et al., 2017, Padmanabhan et al., 2017), and is associated with social functioning and cognitive deficits in these disorders (Assaf et al., 2010, Fox et al., 2017). A meta-analysis showed that RS FC deficits within DMN, and between DMN and task-positive networks, are common to several psychiatric diagnoses, including ASD and SZ, and are related to cognitive impairments (Sha et al., 2019). Additionally, an RS-based classifier of ASD was effective at differentiating SZ (but not ADHD or depression) from controls (Yahata et al., 2016), suggesting a significant overlap in abnormal DMN-FC patterns between ASD and SZ. However, RS imaging studies directly comparing ASD and SZ DMN-FC are scarce. Chen et al. (Chen et al., 2017) demonstrated shared deficits in RS-DMN and salience network (SN) between ASD and SZ, correlating with social deficits in ASD (no social measures in SZ were available). We recently showed that whole brain RS dynamic FC patterns of SZ and ASD have some similar abnormalities, spending more time in a state of weak, intra-network connectivity; however, SZ shows more pervasive deficits (Rabany et al., 2019). Importantly, this work was not specific to either DMN or mentalizing.
Here we aimed to compare mentalizing task-related (MTR) neural activity modulation in the DMN in ASD and SZ during performance of an ecologically valid, social (i.e., interactive) competitive task, a Domino game. We previously showed, in an application of independent component analysis (ICA) to fMRI data from typically developed (TD) adults, that the task interval associated with mentalizing positively modulates activity within specific default mode sub-regions encompassing all of its core regions (i.e., MPFC, PCC/PrC and TPJ) (Hyatt et al., 2015). We now extend this work to young adults with ASD or SZ, along with TD controls, to assess the modulation of MTR activity in default mode subnetworks. In this study, we performed exploratory analyses characterizing the relationships between MTR activity and basic and complex dimensional traits of social abilities (measured with observational, self-reported and performance-based tools) and negative and positive symptoms (SZ) as opposed to clinical categories, to better understand patient abnormalities, in accord with NIMH’s Research Domain Criteria (RDoC) initiative (Cuthbert and Insel, 2013). Our specific hypotheses were that, 1) both patient groups would differ in MTR activity modulation from TD but, due to “over-mentalizing”, only SZ would show increased MTR activity modulation relative to both ASD and TD during task events typically eliciting less mentalizing and, 2) patient neural impairment would be associated with social deficits, and 3) SZ would show a correlation between positive symptoms and DMN neural activity during task events typically eliciting less mentalizing.
2. Methods
2.1. Participants
We present data from 90 participants, 30 per group (high-functioning ASD, SZ and TD) ages 18–34 with estimated full-scale IQ > 80, that completed the Domino fMRI task. We provide inclusion/exclusion criteria and selection process from a larger sample in Supplementary 1.
Participants provided written informed consent after the study had been explained to them and were paid for their time. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the Institutional Review Boards of Hartford Hospital and Yale University.
2.2. Clinical symptoms & social functioning testing
Assessment battery is described in supplementary 2. Psychiatric assessment included the structured clinical interview for DSM-IV axis I disorders (SCID) (First et al., 2002) and the autism diagnosis observation schedule (ADOS)–module 4 (Lord et al., 2000).
We administered the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) to patients and ADOS to all groups to quantify the severity of psychotic and social communication deficits, respectively; TD were excluded from clinical symptom analyses.
To assess social cognition and function, we administered the following tests: 1) Interpersonal Reactivity Index (IRI) (Davis, 1983), 2) Quality of Life Scale (QLS) (Heinrichs et al., 1984), 3) Reading the Mind in the Eyes Task (RMET) (Baron-Cohen et al., 2001), and 4) Social Attribution Test (SAT) (Bell et al., 2010).
2.3. Domino fMRI task
We presented a detailed description of this task previously (Assaf et al., 2013, Assaf et al., 2009, Hyatt et al., 2015) and in supplementary 3; a brief explanation is provided here.
Each participant performed four domino runs, each including multiple games. While all opponent moves were automated and random, at the beginning of each run we told participants they were playing against either a computer, executing automated, random moves, or a human, making strategic decisions. For each game, the participant was given 12 domino playing chips, all of which had to be dispensed by the game’s end for him/her to win. Each game also had a master domino chip that, during each turn, the participant could decide to either match or not match it, placing one of his/her remaining playing chips face down. During this game phase, termed ‘Response to Outcome’ (RTO), the opponent asked the participant to either expose their chip (show event) or not (no-show event). The participant dispensed of the played chip if the opponent elected to ‘no-show’, regardless of whether the participant’s chip matched the master chip and dispensed of the played chip plus an extra chip if an opponent elected to ‘show’ a matching chip. A ‘show’ of a non-matching chip resulted in gaining back the played chip plus one more.
Participants completed a post-scan debriefing that assessed their motivation and playing strategy, using statements scored on a Likert scale, ranging from 1, “does not apply to you at all”, to 5, “applies to you very much”.
2.4. fMRI scan acquisition
We collected BOLD fMRI data with a T2*-weighted echo planar imaging (EPI) sequence (TR/TE = 475/30 msec, flip angle = 60°, FOV = 24 cm, acquisition matrix 80 × 80), using a Siemens Skyra 3 Tesla scanner (Siemens, Malvern, Pennsylvania) at the Olin Neuropsychiatry Research Center (ONRC; Hartford, CT). We acquired forty-eight contiguous axial functional slices of 3.0 mm thickness (interleaved slice order) resulting in 3.0 mm3 voxels. We acquired four ten-minute Domino fMRI runs, each consisting of 1248 images.
2.5. fMRI data preprocessing and motion-artifact correction
We processed functional MRI datasets using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 2008b (Natick, MA). We realigned each subject’s data set to the first ‘non-dummy’ T2* image using the INRIAlign toolbox (http://www-sop.inria.fr/epidaure/software/INRIAlign, A. Roche, EPIDAURE Group) to compensate for any subject head movement. We then screened each subject for excess head movement (>6 mm). We included six motion covariates (x, y, z, roll, pitch, yaw, obtained from the realignment) in the temporal sorting procedure described below. After realignment, we spatially normalized the images to the Montreal Neurological Institute (MNI) standard template (Friston et al., 1995). Finally, we spatially smoothed images with an 8 mm isotropic (FWHM) Gaussian kernel, and then applied a high-pass filter with a cutoff of 128 s to correct for EPI signal low-frequency drift. Note that slice timing correction was not performed due to the multiband short TR sequence, as recommended by the HCP pipeline (Glasser et al., 2013). As a final step, we scrubbed the fMRI data using the ArtRepair toolbox (https://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html, RRID:SCR_005990) (Mazaika et al., 2009) to exclude from analysis fMRI time series sections with excessive movement. Since patients often characterized by high head movement during fMRI, we set ArtRepair at a relatively liberal maximum acceptable movement at 1.0 mm/TR (assuming a 65 mm head radius), and the intensity variation at a maximum percent threshold of 1.3% of the mean global average signal.
We determined that of the participants retained (<6 mm movement) none had >30% of scans repaired from any two common-opponent (computer or human) Domino sessions. The number of participants with >12.5% of scans repaired (but always <30%) for any two common-opponent sessions were as follows: six ASD, three SZ, and one TD. Means and standard deviations of scans repaired were 162 ± 242 (ASD), 94 ± 180 (SZ) and 49 ± 137 (TD) out of a total of 4992 scans. A Kruskal-Wallis test indicated a non-significant difference between groups (χ2 = 5.881, p = 0.053) on the number of repaired scans.
To further reduce the effect of head motion on our results, we included the mean of the root mean square (RMS) of the framewise displacement, or mean FDRMS, for each participant as a second (group) level covariate in all statistical analyses. To calculate mean FDRMS for each participant, we computed a single mean value of the root-mean-square framewise head displacement, determined using the six realignment parameters (over all four Domino sessions) with an assumed head radius of 65 mm for all participants.
We should also note that the GIG-ICA procedure we used has been shown in a previous study (Du et al., 2016) to very effectively reduce the effect of head motion artifact on the data.
2.6. Statistical analyses
Behavioral analyses to assess participants’ engagement in the game, strategies while playing (e.g. risk taking) and engagement in mentalizing are described in Supplementary 5.
Imaging analyses focused on the RTO phase. We previously demonstrated that DMN regions are activated and modulated during this phase due to mentalizing processes, e.g., trying to infer the opponent’s strategy and planning the next play accordingly. This is measured by the show > no-show contrast regardless of the played chip (Assaf et al., 2013, Assaf et al., 2009, Hyatt et al., 2015). As stated in our previous work, although we consider both show and no-show events to involve mentalizing, show events will require significantly greater levels of player mentalizing than no-show events (Assaf et al., 2013). The reason for this is as follows. The opponent obtains new information about the player only during show events (e.g., if the player bluffed or played fairly). The player then knows that the opponent can use this information to change his/her strategy. This in turn requires the player to take more information into account when updating his/her mental representation of the opponent (i.e., requires greater mentalizing).
We calculated subject-level statistics using a general linear model (GLM) design matrix in SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/; RRID:SCR_007037) using the following RTO-phase regressors of interest: ‘show match’, ‘show non-match’, ‘no-show match’ and ‘no-show non-match’ events. Individual GLMs were used for ICA temporal sorting (see below).
2.7. Estimation of subject-level independent components (IC) via spatially constrained ICA
In this study we used spatially constrained ICA (i.e., GIG-ICA) (Du et al., 2018) to extend our earlier work (Hyatt et al., 2015) to clinical populations. Briefly, we used prior group-level ICs from an independent sample (53 TD participants) to guide the extraction of subject-specific ICs while automatically providing labeled components. The benefits of GIG-ICA are that single-subject ICA statistical independence is optimized (Du et al., 2018) and artifact suppression is improved (Du et al., 2016) when compared with traditional single-subject ICA. Note, in contrast to work in Du et al. (Du et al., 2016) which used group maps from the same data, we are using maps derived from independent data, which has the same benefits, but also provides completely independent single-subject results and automatic labeling of components. Using GIG-ICA, we extracted 45 subject-specific ICs, including ten DMN-specific ICs, which matched the 45/10 group-level ICs previously determined to be BOLD-related networks (i.e., not physiological artifacts) and DMN-specific (see Supplementary 6 for details) (Hyatt et al., 2015).
2.8. Group analysis
ICA-based analysis is often applied to resting-state fMRI data. In this study, however, we applied spatially constrained single-subject ICA not to resting-state fMRI data, but to the analysis of a socially interactive fMRI Domino task. We analyzed subject-level components derived from spatially constrained ICA for task-relatedness (RTO task events) using the Group ICA of fMRI Toolbox (GIFT, https://trendscenter.org/software/gift/, RRID:SCR_001953) ‘temporal sort’ multiple-regression feature. Temporal sorting is analogous to GLM regression performed on fMRI voxel timecourses, except regression is performed on subject-level ICA timecourses. The resulting β-weights represent the degree to which component networks were engaged by task events (Kim et al., 2009). Here we were interested in the events-contrast defining the mentalizing effect: show > no-show (Hyatt et al., 2015). Thus, the β-weights were entered into a mixed-model ANCOVA in SPSS, with within-subjects factor being mentalizing (two levels, show and no-show), and between-subject factor being group. The model included the group-by-mentalizing interaction.
To examine the phenomenon of “over-mentalizing” in SZ, we also assessed between-group differences separately for show events and for no-show events, with no-show events here considered as task events typically eliciting less mentalizing in players. We included age, estimated full-scale IQ, and mean framewise head displacement (root mean square; FDRMS) as covariates and results were Bonferroni-corrected for multiple comparisons (Table 3). To test for possible associations between mentalizing and medication intake, sensitivity analyses were performed (Supplementary 8).
Table 3.
Group-by-mentalizing interaction effects. Statistics shown are for the show > no-show contrast for ICs demonstrating this effect (thus, ICs 16 and 19 are excluded).
| Anatomical Region | F [df = 2,627] (p Bonf)* | TD vs. ASD t [df = 627] (p Bonf)^ | TD vs. SZ t [df = 627] (p Bonf)^ | ASD vs. SZ t [df = 627] (p Bonf)^ |
|---|---|---|---|---|
| PCC (IC 8) | 8.849 (0.001) | 3.134 (0.0054) | 3.997 (0.0002) | 0.862 (ns) |
| Precuneus (IC 32) | 2.469 (ns) | – | – | – |
| Right TPJ (IC 27) | 7.133 (0.007) | 2.824 (0.0147) | 3.584 (0.0011) | 0.761 (ns) |
| Left TPJ (IC 33) | 6.391 (0.014) | 1.983 (ns) | 3.568 (0.0012) | 1.584 (ns) |
| dmPFC / ACC (IC 26) | 1.507 (ns) | – | – | – |
| vmPFC (IC 35) | 2.270 (ns) | – | – | – |
| mPFC (IC 38) | 3.009 (ns) | – | – | – |
| dmPFC (IC 40) | 2.615 (ns) | – | – | – |
ACC: anterior cingulate cortex; dmPFC: dorsomedial prefrontal cortex; ns: not significant; PCC: posterior cingulate cortex; TPJ: temporoparietnal junction; vmPFC: ventromedial PFC; * significant p-values are Bonferroni corrected over eight ICs; ^ significant p-values are Bonferroni corrected for three post-hoc pairwise comparisons.
2.9. Exploratory correlation of symptom severity with mentalizing
We assessed the correlation of symptom severity with mentalizing-related neural activity in patients. The temporal sorting beta-weights for show and no-show for each subject were averaged over all four Domino fMRI runs and then subtracted to produce one measure of mentalizing task-related (MTR) neural activity (Δβment = βshow − βno-show). We then used GLM to examine the correlation between the MTR variable (Δβment), and independent variables of symptom severity (clinical testing subscores), and their interactions. Age, IQ and FDRMS were included as covariates and results were false discovery rate (FDR) corrected (Table 4).
Table 4.
Statistics of Group (ASD and SZ only) by ADOS Communication (ADOS-C) score interaction, with the overall show > noshow difference (Δβment) as the dependent variable. Also included is the post hoc analysis of the linear slopes between show > noshow versus ADOS-C, for each group separately.
| Anatomical Region | Group by ADOS-Com interaction F [df = 1,51] (pFDR)* | ASD slopes t [df = 51] (pFDR)* | SZ slopes t [df = 51] (pFDR)* |
|---|---|---|---|
| PCC (IC 8) | 0.849 (ns) | −2.357 (0.026) | −0.947 (ns) |
| Precuneus (IC 32) | 0.175 (ns) | −1.266 (ns) | −1.776 (ns) |
| Right TPJ (IC 27) | 8.431 (0.009) | −2.934 (0.010) | 1.258 (ns) |
| Left TPJ (IC 33) | 7.670 (0.010) | −2.823 (0.011) | 1.177 (ns) |
| dmPFC / ACC (IC 26) | 12.83 (0.003) | −3.007 (0.010) | 2.129 (ns) |
| vmPFC (IC 35) | 12.92 (0.003) | −3.619 (0.003) | 1.570 (ns) |
| mPFC (IC 38) | 8.475 (0.009) | −3.998 (0.002) | 0.264 (ns) |
| dmPFC (IC 40) | 9.211 (0.009) | −2.345 (0.026) | 1.988 (ns) |
*Due to the exploratory nature of this analysis, statistical results were false discovery rate (FDR) corrected over the eight anatomical regions. For additional abbreviations seeTable 3.
3. Results
3.1. Participant characterization
Table 1 lists group demographics and assessments’ scores, including statistical comparisons. Groups matched on gender but not on age and estimated IQ, thus analyses were controlled for the latter. Table 1 shows that both ASD and SZ patients, when compared with TD, had 1) larger mean framewise displacement (indicating greater head motion), 2) larger ADOS scores (on both Communication and Social Interaction subscores), 3) larger IRI Personal Distress subscores, 4) smaller RMET scores and 5) smaller QLS scores. On IRI Perspective Taking subscores, only patients with ASD scored smaller than TD. These results are largely consistent with known clinical symptoms and social cognition deficits of both patient groups.
Table 1.
Participant characterization.
| ASD |
SZ |
TD |
Group comparison |
|||
|---|---|---|---|---|---|---|
| (N = 30) |
(N = 30) |
(N = 30) |
||||
| Mean (SD) | Mean (SD) | Mean (SD) | Statistic | p-value | Post-Hoc tests | |
| Age | 21.7 (3.4) | 26.0 (3.5) | 24.2 (3.6) | F (2,87) = 11.3 | p < 0.001 | TD > ASD*; SZ > ASD** |
| IQ (Estimated) | 110 (14.4) | 101 (13.8) | 115 (12.8) | F (2,87) = 7.78 | p = 0.001 | TD > SZ**; ASD > SZ* |
| Gender (M/F)$ | 26/4 | 19/11 | 22/8 | χ2 (2) = 4.32 | p = 0.115 | _ |
| Mean FDRMS (mm) | 0.103 (0.036) | 0.103 (0.037) | 0.068 (0.010) | F (2,87) = 13.3 | p < 0.001 | SZ > TD**; ASD > TD** |
| ADOS Communication | 3.80 (1.38) | 2.67 (2.14) | 1.14 (0.833) | F (2,86) = 21.8 | p < 0.001 | ASD > TD**; SZ > TD**; ASD > SZ* |
| ADOS Social Interaction | 7.07 (2.30) | 5.06 (3.89) | 0.66 (0.97) | F (2,86) = 46.0 | p < 0.001 | ASD > TD**; SZ > TD** |
| IRI Perspective Taking | 16.1 (6.65) | 18.0 (4.59) | 20.6 (4.41) | F (2,86) = 5.21 | p = 0.007 | TD > ASD* |
| IRI Fantasizing | 17.2 (4.52) | 16.2 (5.38) | 16.6 (4.76) | F (2,86) = 0.317 | p = 0.729 | _ |
| IRI Empathy | 18.6 (3.87) | 19.3 (5.38) | 20.7 (4.29) | F (2,86) = 1.61 | p = 0.206 | _ |
| IRI Personal Distress | 13.1 (5.00) | 13.1 (3.83) | 8.53 (5.00) | F (2,86) = 9.80 | p < 0.001 | ASD > TD**; SZ > TD** |
| RMET | 24.4 (3.51) | 24.3 (4.24) | 27.0 (3.22) | F (2,85) = 5.03 | p < 0.009 | TD > ASD*; TD > SZ* |
| SAT | 14.7 (4.55) | 14.0 (3.76) | 16.3 (3.26) | F (2,86) = 2.92 | p = 0.059# | _ |
| PANSS5 Positive | 10.2 (3.15) | 14.1 (4.70) | _ | t (51.0#) = −3.70 | p = 0.001# | _ |
| PANSS5 Negative | 17.1 (5.63) | 19.8 (8.20) | _ | t (51.5#) = −1.44 | p = 0.157# | _ |
| PANSS5 Cognition | 11.9 (3.69) | 13.6 (3.57) | _ | t (55) = −1.78 | p = 0.081 | _ |
| PANSS5 Hostility | 4.85 (1.06) | 4.90 (1.24) | _ | t (55) = −0.156 | p = 0.876 | _ |
| PANSS5 Emotion | 8.89 (3.18) | 10.4 (3.69) | _ | t (55) = −1.61 | p = 0.113 | _ |
| QLS Total | 82.6 (21.4) | 72.7 (20.7) | 111 (11.4) | F (2,87) = 35.8 | p < 0.001# | TD > ASD**; TD > SZ** |
ASD = Autism spectrum disorder, SZ = Schizophrenia, TD = Typically developed, SD = standard deviation, FDRMS = framewise displacement (root mean square), ADOS = autism diagnosis observation schedule, IRI = interpersonal reactivity index, RMET = reading the mind in the eyes Task, SAT = social attribution test, PANSS = positive and negative syndrome scale , QLS = quality of life scale; $=chi-squared test;#=Equal variances assumption not met (Levene’s test); *p ≤ 0.0.05; **p ≤ 0.0.001
3.2. Behavioral results
Supplementary 5 presents information on games played, post-scan debriefing and risk taking while playing. The post-scan debriefing questions relevant to mentalizing are provided in Table S1 (Supplementary 5). Briefly, there were no significant between-group differences in number of games played, won, or shorter than one-minute. Groups also did not differ on overall game engagement and tendency to engage in mentalizing. Finally, risk taking behavior increased with time (i.e., minutes elapsed) without group differences. Taken together, these results indicate that the ASD and SZ patient groups participated in the Domino task to the same extent as the TD group.
3.3. Default mode network mentalizing task-related (MTR) activity modulation
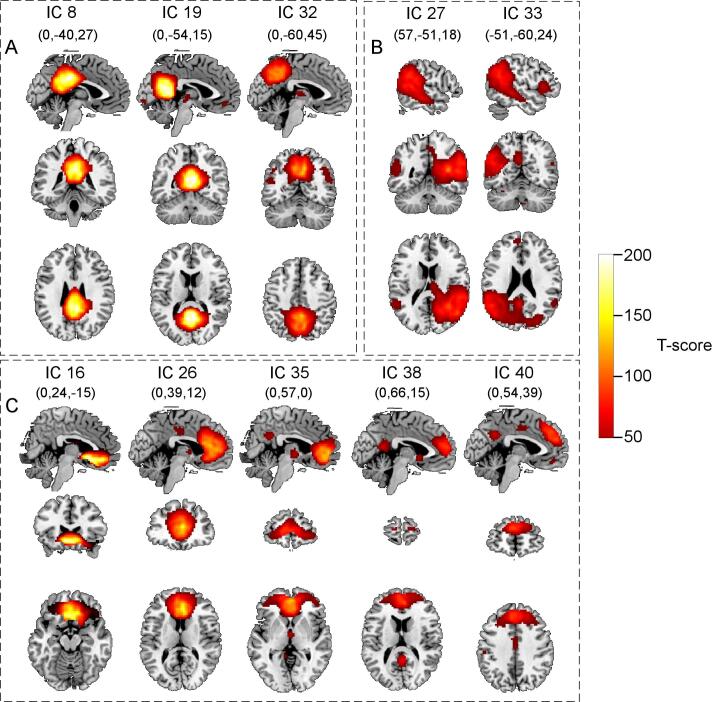
Table 2 lists the ten core DMN ICs examined in this study along with the corresponding ICs from the previous study (Hyatt et al., 2015) (see Supplementary 6 for more information on IC extraction). Fig. 1 shows these same ICs as derived from spatially constrained ICA subject-specific component spatial maps (z-scores). A complete description comparing default mode network ICs in TD participants in this study with a previous study (Hyatt et al., 2015) is provided in Supplementary 6.
Table 2.
Characteristics of the default mode network ICs. Current (n = 30, TD only) versus previous study (n = 53) (see Supplementary 6 for more details).
| Anatomical Region | Curr IC | dR | fALFF | Current study show vs. no-show F-value (pFDR) (df = 1, 205) | Prev IC | dR | fALFF | Previous study show vs. no-show F-value (pFDR) (df = 1, 416) |
|---|---|---|---|---|---|---|---|---|
| PCC | 8 | 0.034 | 2.376 | 17.65 (<0.001) | 18 | 0.024 | 1.935 | 15.39 (<0.001) |
| PCC/Precuneus | 19 | 0.035 | 3.324 | 0.351 (ns) | 36 | 0.032 | 3.842 | 0.070 (ns) |
| Precuneus | 32 | 0.033 | 3.465 | 30.34 (<0.001) | 54 | 0.027 | 2.691 | 10.12 (0.003) |
| Right TPJ | 27 | 0.033 | 3.380 | 109.9 (<0.001) | 49 | 0.025 | 2.393 | 114.1 (<0.001) |
| Left TPJ | 33 | 0.036 | 4.932 | 41.50 (<0.001) | 55 | 0.028 | 3.214 | 60.13 (<0.001) |
| Subgenual ACC | 16 | 0.036 | 2.014 | 0.630 (ns) | 30 | 0.039 | 2.512 | 0.693 (ns) |
| dmPFC/ACC | 26 | 0.032 | 1.746 | 9.690 (0.003) | 46 | 0.028 | 2.697 | 4.177 (0.065) |
| vmPFC | 35 | 0.036 | 2.759 | 6.222 (0.017) | 57 | 0.030 | 2.933 | 1.435 (ns) |
| mPFC | 38 | 0.041 | 4.135 | 12.81 (0.001) | 60 | 0.027 | 2.269 | 0.096 (ns) |
| dmPFC | 40 | 0.032 | 2.630 | 10.37 (0.003) | 64 | 0.032 | 4.222 | 14.16 (<0.001) |
(Curr) Current; (Prev) Previous; (dR) dynamic range; (fALFF) fractional amplitude of low-frequency fluctuations; (FDR) False Discovery Rate; (ns) not significant; (TPJ) temporoparietal junction.
Fig. 1.
The ten ICs identified as DMN components. Maps were created using a SPM8 one-sample t-test from GIG-ICA back-reconstructed subject-specific IC spatial maps from all participants. Panel A shows posterior DMN ICs, panel B, TPJ/STS ICs, and panel C, prefrontal ICs. Threshold was set at a t-score of 50.0.
3.4. DMN task recruitment: MTR activity group differences
A mixed-model ANCOVA of show and no-show event β-weights revealed that three DMN components, of the eight ICs involved in MTR activity, showed a significant (Bonferroni-corrected over eight ICs) group-by-mentalizing interaction: ICs 8 (PCC), 27 (right TPJ) and 33 (left TPJ). Post-hoc pairwise analyses showed that TD had significantly greater MTR activity modulation (i.e., Δβment) than both ASD and SZ in two posterior default mode networks (ICs 8 (PCC) and 27 (right TPJ)), while in IC 33 (left TPJ), TD had significantly greater modulation of MTR activity than SZ only (Fig. 2 and Table 3).
Fig. 2.
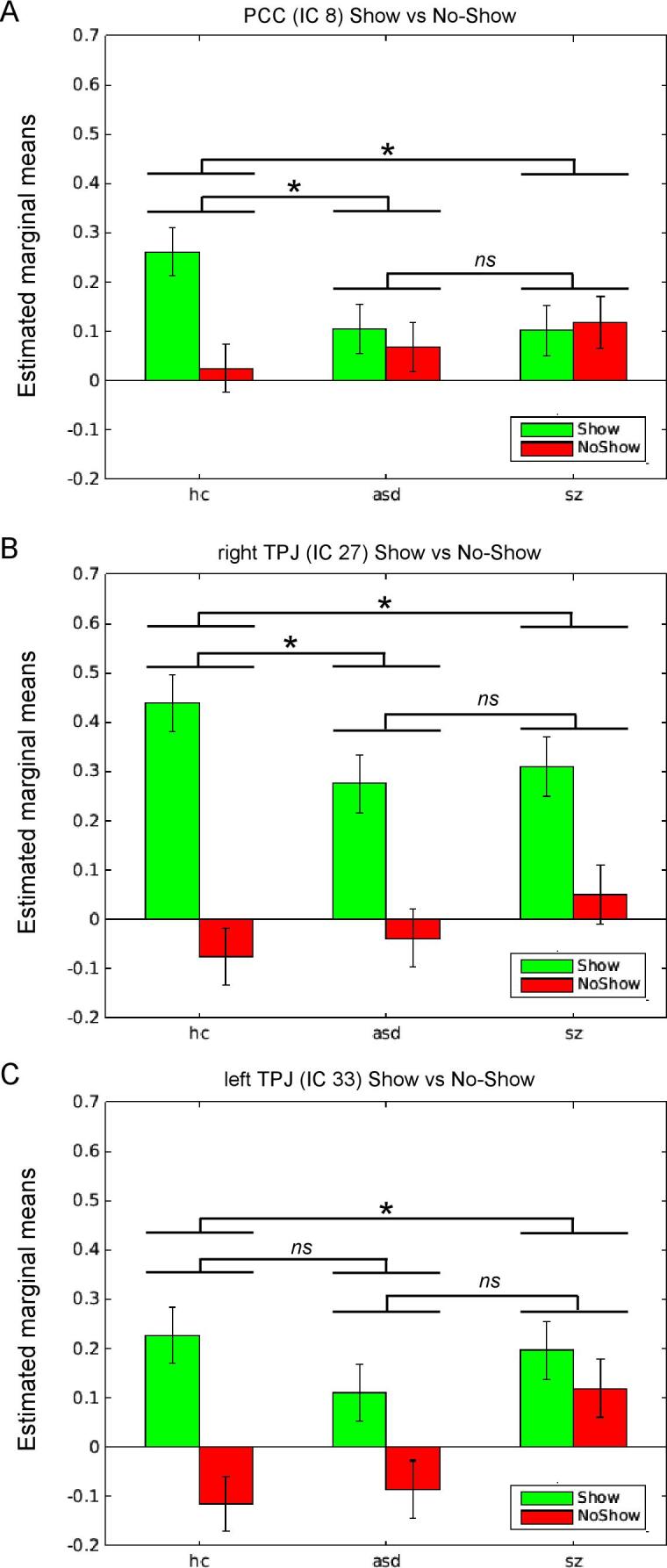
Bar plots showing the three ICs characterized by a significant group-by-mentalizing interaction (show > no-show). Panel A presents estimated means (β-weights) for PCC (IC 8), panel B, right TPJ (IC 27), and panel C, left TPJ (IC 33). For pairwise show > no-show statistics (denoted by brackets) see Table 1. *pBonf < .05; ns: not significant.
The mixed model ANCOVA above included an analysis of show and no-show events beta values separately. Although the bar charts in Fig. 2 appear to show that SZ had greater MTR activity modulation during no-show events (red bars) than ASD and TD, suggesting over-mentalizing per our hypothesis, none of the tests for group differences in no-show event beta values (indicating possible over-mentalizing-related activity) reached statistical significance in any DMN component after correction for multiple comparisons.
3.5. Relationship of symptom severity scores with MTR activity modulation: ASD and SZ differences
GLM of the correlation of symptom severity subscores with MTR activity showed a statistically significant interaction of group-by-ADOS communication subscore (ADOS-C) in six of the eight MTR ICs: ICs 27, 33 (lateral DMN) and ICs 26, 35, 38 and 40 (medial PFC DMN) (see Table 4 for statistical values).
In all six of these ICs, there was a significant negative correlation between MTR activity modulation and ADOS-C for ASD, but not SZ (i.e., correlation slopes not significantly different than zero).
Although the two ICs in posterior DMN (ICs 8 and 32) did not show a significant group-by-ADOS-C interaction (i.e., ASD and SZ slopes were not significantly different), MTR activity in ASD significantly negatively correlated with ADOS-C in IC 8 (PCC) (see Table 4). Conversely, we found no significant interaction of group-by-ADOS social interaction subscore in any MTR ICs. Additionally, we found no significant main effect of ADOS or PANSS subscores, which indicates that there was no correlation between MTR activity modulation in the DMN and behavioral symptom scores that was common to both ASD and SZ diagnoses.
For the IRI scores, we found a significant group-by-IRI Fantasizing subscore interaction in DMN component IC 32, Precuneus, (F(2,71) = 7.849, pFDR = 0.007), where post-hoc analyses showed a significant (negative) slope only for SZ (t = −3.412, df = 71, pFDR = 0.009), with significant group differences in slopes between ASD and SZ (ASD > SZ, F(1,71) = 14.36, pFDR = 0.003) and TD and SZ (TD > SZ, F(1,71) = 10.87, pFDR = 0.012). No other effects were found for the IRI subscores in the MTR ICs. Correlation of SAT, QLS, RMET and PANSS scores with MTR activity showed no significant group-by-test interactions. In an assessment of over-mentalizing in SZ, a linear regression analysis of show event and no-show event beta values with PANSS Positive and Negative symptom scores in SZ showed no significant relationships in any DMN region. Lastly, there was no significant effect of SZ patient duration of illness on the relationship between MTR activity modulation and behavioral scores, or social cognition scores (see Supplementary 4).
4. Discussion
In this study we focused on identifying the neural correlates of social interaction in ASD and SZ. Traditionally considered distinct, SZ and ASD share clinical aspects, including marked social deficits (King and Lord, 2011). We used an ecologically valid, interactive, competitive fMRI Domino game to assess the modulation of mentalizing task-related (MTR) activity within the default mode network in young adults with ASD, SZ and TD. We first established that all groups were similar in understanding and engagement in the Domino game, including taking their opponent’s moves into account (i.e., mentalizing), and in risk-taking behavior over time. Next, we assessed MTR activity modulation in relation to both clinical (categorical) diagnosis and dimensional social functioning. We hypothesized that, when compared with TD, SZ and ASD would have MTR activity deficits in the DMN. In agreement with our hypothesis, both patient groups showed reduced MTR activity modulation in two default mode subnetworks, PCC and bilateral TPJ. We also hypothesized that SZ would show greater MTR activity modulation during no-show events (i.e., events normally eliciting less mentalizing activity) than either TD or ASD, but did not find evidence for this over-mentalizing effect, nor did we find any association between mentalizing activity and positive or negative psychotic symptoms. Importantly, while some behavioral studies have suggested over-mentalizing in SZ with specific association with positive symptoms (e.g., see (Bliksted et al., 2016, Fretland et al., 2015, Martinez et al., 2019, Montag et al., 2011)), only a few neuroimaging studies have shown over-mentalizing-related brain activity in SZ (Backasch et al., 2013, Bliksted et al., 2019, Ciaramidaro et al., 2015). Finally, our exploratory analyses comparing dimensional constructs of social deficits with MTR activity showed diagnosis-specific relationships. Specifically, only patients with ASD showed that MTR activity modulation was associated with social communication deficits in bilateral TPJ and MPFC. Additionally, only in SZ was MTR activity in the PrC associated with the reported tendency to fantasize (i.e., the ability to imaginatively identify oneself with the feelings and actions of fictitious characters (Davis, 1983)). Thus, although our main results suggest shared MTR neural deficits between ASD and SZ in DMN regions, our exploratory analyses potentially point to diagnostic-specific underlying symptom mechanisms, corresponding to either behaviorally observed social deficits (ASD) or perceived affinity to fantasize (SZ).
Our work has been motivated by the current shift in psychiatric research, as exemplified by the RDoC initiative, from emphasizing categorical symptom-based clinical nosology (e.g. DSM-based diagnosis) to exploring dimensional, overlapping constructs that span the range from healthy individuals to individuals with severe psychiatric illnesses (Cuthbert and Insel, 2013). Despite significant research efforts devoted to identifying consistent and valid biological biomarkers for categorical symptom-based clinical disorders to develop objective diagnostic tests and individualized treatments based on neural mechanisms, success has been elusive. In contrast, RDoC emphasizes the heterogeneity within and similarities between clinical diagnoses, taking into account dimensional constructs, such as emotional, neurocognitive, and social cognitive functions. Several groups have taken this approach within diagnostic group or symptom category, such as the psychosis spectrum (Tamminga et al., 2017), anxiety disorders (Oathes et al., 2015, Rabany et al., 2017) and ASD (Feczko et al., 2018), as well as between distinct diagnostic groups, e.g. ASD-SZ (Chen et al., 2017, Eack et al., 2017, Rabany et al., 2019) and ASD-ADHD (Dajani et al., 2019). Notably, the RDoC approach has been criticized as not fully validated, nor proven superior to clinical nosology systems in improving clinical understanding and practice (Weinberger et al., 2015). Our results suggest that rather than being mutually exclusive, these two approaches might capture different aspects of associations between symptoms/behaviors and neural disease mechanisms. This conclusion is in accord with recent work in anxiety disorders (Oathes et al., 2015, Rabany et al., 2017). Further research is required to elucidate the relative significance of categorical vs. dimensional biological markers to the diagnosis, etiology, and treatment of psychiatric disorders.
Behaviorally, our results confirmed previous reports of social deficit overlap between ASD and SZ (Fernandes et al., 2018, Pepper et al., 2018). Both groups showed deficits in observed communication and social interactions (note that ASD-SZ differences on ADOS were expected as it was an inclusion criteria for ASD only), self-reported perspective-taking (i.e., mentalizing), although SZ-TD difference did not reach significance potentially due to self-report bias in this patient group (Lysaker et al., 2013) and personal-distress (self-anxiety experiencing others’ distress), and identifying others’ emotions based on eyes expression. Importantly, although Domino is an interactive game, identified social deficits did not affect patients’ game engagement, strategies, or understanding the game’s rules.
Our imaging analyses focused on DMN functional coherence. Although the DMN has been previously described as being active during task-negative activities (e.g., rest, daydreaming), multiple studies have shown its involvement in social cognitive processes, including mentalizing (Hyatt et al., 2015, Mars et al., 2012). Eight DMN ICs showed neural activity modulated by the opponent’s response, which we ascribe to mentalizing processes (i.e., MTR neural activity) (Assaf et al., 2013, Assaf et al., 2009, Hyatt et al., 2015) covering all three hubs: MPFC, PCC/PrC and TPJ/STS. Of these, posterior and lateral regions had a significant group effect, driven by both patient groups, indicating decreased modulation of MTR activity compared to TD.
As an observational tool, the ADOS quantifies integrated social behavior rather than a specific social process. Therefore, correlations of MTR activity in bilateral TPJ and MPFC with ADOS communication subscore in ASD agree with the suggested critical role of these regions in integrating information from multiple social cognitive processes, including social perception and mentalizing and information about self versus others (Eddy, 2016, van Veluw and Chance, 2014, Yang et al., 2015). Another posterior region, PrC, which was modulated by MTR activity in all groups, showed correlation with self-reported tendency to fantasize or daydream in SZ. This agrees with PrC’s suggested role in directing self-referential processes (Cavanna and Trimble, 2006).
When comparing ASD and SZ each separately to TD, multiple studies have shown abnormal activations and functional connectivity (FC) in DMN during rest and social tasks (Hu et al., 2017, Padmanabhan et al., 2017), which have been concluded to largely overlap in meta-analyses (Sha et al., 2019, Sugranyes et al., 2011). However, our results of similar MTR activity deficits in ASD and SZ are largely inconsistent with two fMRI studies also investigating the neural correlates of mentalizing in these groups concurrently. Ciaramidaro et al. (2015)) used a cartoon mentalizing task with adolescents and young adults, and Eack et al. (2017)) used a visual perspective-taking task in adults. Both studies demonstrated diagnosis-specific activation and FC pattern alternations in TPJ/STS and MPFC regions. While Ciaramidaro et al. (2015) also reported significant correlations between impaired activation and PANSS-Positive scores, neither study reported any additional symptom (e.g., ADOS, PANSS-N) or social cognition (e.g., IRI, RMET) test correlations with brain activity. Thus, direct comparisons to our correlation results are not feasible. Notably, the TPJ/STS clusters reported in both studies are part of ICs 27 and 33 here, which in our study showed similar MTR activity deficits in SZ and ASD. Additionally, one of the frontal clusters that showed impaired activation in SZ in the Eack report (coordinate: 8, 40, 22) is part of IC 26 (dmPFC/ACC), which in our study was associated with MTR activity, but showed no significant group effect. These discrepancies can be attributed to the different tasks used and analysis methods. However, they can also potentially be explained by the notable heterogeneity seen in ASD and SZ (Geschwind, 2009, Tamminga et al., 2017), as even in our study, correlations of social-communication abilities with MTR activity were diagnosis-specific in bilateral TPJ, MPFC and PrC.
Conversely, our results are mostly in agreement with a previous study of DMN in ASD and SZ that used resting state data. Using multivariate pattern analysis (MVPA), Chen et al. (Chen et al., 2017) demonstrated shared impairments in PCC/PrC, right angular gyrus (part of TPJ) and a couple of PFC areas. In contrast, left angular gyrus and vMPFC and few PCC/PrC sub-regions showed diagnosis specific deficits. Additionally, DMN and salience network (SN) shared deficits were associated with ADOS social interaction subscales in ASD (not available for SZ). Our study showed correlation of MTR activity in ASD with the ADOS communication subscale, but not the ADOS social interaction subscale. This inconsistency might be explained by different analysis methods, and inclusion of SN deficits in Chen’s analyses. However, both studies indicate association between shared DMN deficits and observed social communication behaviors in ASD, suggesting a similar neural mechanism underlying these impairments. Notably, Chen et al. also did not demonstrate an association between DMN deficits and PANSS scores in SZ, suggesting dissociation between DMN deficits and psychotic symptoms both during rest and a social task involving mentalizing. Alternative fMRI tasks tapping into psychotic symptoms, such as hallucinations and delusions, might specifically be required to delineate their relationship to MTR activity modulation in the DMN.
We note a minor discrepancy between our previous work in TD (Hyatt et al., 2015) and current results, with the former showing five, as oppose to eight, DMN ICs with significant MTR activity. The discrepancy arises from three additional MPFC ICs (26, 35 and 38) showing this effect in the current sample. These three ICs overlap with dmPFC (current study IC 40, previous study IC 64) a default-mode subnetwork which showed significant MTR activity modulation in both studies. This discrepancy might indicate less specific MTR modulation of activity in the MPFC in the current sample due to different sample characteristics, the previous sample being older (range = 17–60), and including more females (~55% vs. 27%), as associations have been reported between DMN connectivity and aging, including in relation to social cognition and gender (Mak et al., 2017). Our current sample’s narrow age range and small number of females preclude direct testing of these effects. We should also note that we expected greater MTR activity modulation in the DMN for human versus computer opponent for the show versus no-show contrast but did not find any such differences. We observed a similar pattern in our previous study (Hyatt et al., 2015) and theorized that this lack of opponent-type differences is due to possible participant attribution of social reasoning to computers, also known as Computers-Are-Social-Actors (CASA) paradigm (see (Nass and Moon, 2000)).
4.1. Study limitations
Our study has several limitations. First, sample size is relatively small and larger replication studies are essential. Second, the groups did not match on age and IQ, both shown to be related to social cognition and functioning (Henry et al., 2013), and controlling for these parameters might not fully account for their effect. Third, medication effects on group differences in MTR activity in the DMN were not ruled out; however, sensitivity analyses and group comparisons (Supplementary 9) showed no associations between MTR (Δβ-weights) and medication, making this an unlikely confounder. Fourth, the no-show event, consistently showing less modulation of mentalizing activities (Assaf et al., 2013, Hyatt et al., 2015), cannot be considered a truly non-social task event, because an opponent is still involved during this task event. However, no-show events, as comparatively neutral social stimuli, might be better posited conceptually to demonstrate greater “over-mentalizing” responses than physical (non-social) task events used in other studies (Bliksted et al., 2019, Ciaramidaro et al., 2015). Lastly, the mentalizing network might be modulated by activity in other networks in the brain, thus future studies should explore additional cognitive domains and neural networks, as well as examine whole brain activity.
5. Conclusions
The current report describes the modulatory effect of mentalizing processes on the DMN during social interaction in individuals with ASD, SZ and TD. While both patient groups showed similar impaired MTR effect in posterior and lateral DMN regions, they differed in relationship between MTR activity and observed social communication behavior and reported tendency to fantasize. If replicated, these results support the importance of both clinical diagnosis and dimensional constructs related to social functioning in understanding the underlying neural mechanism of social deficits in ASD and SZ.
CRediT authorship contribution statement
Christopher J. Hyatt: Writing - original draft, Writing - review & editing, Formal analysis, Methodology, Software. Vince D. Calhoun: Writing - original draft, Writing - review & editing, Resources, Methodology, Software. Brian Pittman: Formal analysis. Silvia Corbera: Writing - original draft, Resources. Morris D. Bell: Writing - original draft. Liron Rabany: Formal analysis. Kevin Pelphrey: Resources. Godfrey D. Pearlson: Writing - original draft, Conceptualization, Resources. Michal Assaf: Writing - original draft, Writing - review & editing, Conceptualization, Supervision, Project administration, Funding acquisition.
Acknowledgments
Acknowledgments
This work was supported by the National Institutes of Health (R01 MH095888; Assaf), and the National Alliance for Research in Schizophrenia and Affective Disorders (Young Investigator Award 17525; Corbera).
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
Data availability statement
The data that support the findings of this study are available at the National Database for Autism Research (NDAR).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102343.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Diagnostic and Statistical Manual of Mental Disorders (DSM–5). America Psychiatric Association, Washington, DC, 2013. [DOI] [PubMed]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M., Hyatt C.J., Wong C.G., Johnson M.R., Schultz R.T., Hendler T., Pearlson G.D. Mentalizing and motivation neural function during social interactions in autism spectrum disorders. Neuroimage Clin. 2013;3:321–331. doi: 10.1016/j.nicl.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M., Jagannathan K., Calhoun V.D., Miller L., Stevens M.C., Sahl R., O'Boyle J.G., Schultz R.T., Pearlson G.D. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M., Kahn I., Pearlson G.D., Johnson M.R., Yeshurun Y., Calhoun V.D., Hendler T. Brain activity dissociates mentalization from motivation during an interpersonal competitive game. Brain Imaging Behav. 2009;3:24–37. doi: 10.1007/s11682-008-9047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backasch B., Straube B., Pyka M., Klohn-Saghatolislam F., Muller M.J., Kircher T.T., Leube D.T. Hyperintentionality during automatic perception of naturalistic cooperative behavior in patients with schizophrenia. Soc. Neurosci. 2013;8:489–504. doi: 10.1080/17470919.2013.820666. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Bell M., Tsang H.W., Greig T.C., Bryson G.J. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophr. Bull. 2009;35:738–747. doi: 10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.D., Fiszdon J.M., Greig T.C., Wexler B.E. Social attribution test–multiple choice (SAT-MC) in schizophrenia: comparison with community sample and relationship to neurocognitive, social cognitive and symptom measures. Schizophr. Res. 2010;122:164–171. doi: 10.1016/j.schres.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliksted V., Frith C., Videbech P., Fagerlund B., Emborg C., Simonsen A., Roepstorff A., Campbell-Meiklejohn D. Hyper- and hypomentalizing in patients with first-episode schizophrenia: fMRI and behavioral studies. Schizophr. Bull. 2019;45:377–385. doi: 10.1093/schbul/sby027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliksted V., Ubukata S., Koelkebeck K. Discriminating autism spectrum disorders from schizophrenia by investigation of mental state attribution on an on-line mentalizing task: a review and meta-analysis. Schizophr. Res. 2016 doi: 10.1016/j.schres.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen H., Uddin L.Q., Duan X., Zheng J., Long Z., Zhang Y., Guo X., Zhang Y., Zhao J., Chen H. Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism. Res. 2017;10:1776–1786. doi: 10.1002/aur.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.S., Barch D., Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr. Bull. 2014;40:602–616. doi: 10.1093/schbul/sbt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A., Bolte S., Schlitt S., Hainz D., Poustka F., Weber B., Bara B.G., Freitag C., Walter H. Schizophrenia and autism as contrasting minds: neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr. Bull. 2015;41:171–179. doi: 10.1093/schbul/sbu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S.M., Granholm E.L., Fish S.C. A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real-world functioning in schizophrenia. Schizophr. Res. 2011;125:152–160. doi: 10.1016/j.schres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Losh M., Adolphs R., Hurley R., Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol. Med. 2010;40:569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J.S., Hatton C., Craig F.B., Bentall R.P. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger's syndrome and healthy controls. Schizophr. Res. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani D.R., Burrows C.A., Odriozola P., Baez A., Nebel M.B., Mostofsky S.H., Uddin L.Q. Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. Neuroimage Clin. 2019;21 doi: 10.1016/j.nicl.2019.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. [Google Scholar]
- Du Y., Allen E.A., He H., Sui J., Wu L., Calhoun V.D. Artifact removal in the context of group ICA: a comparison of single-subject and group approaches. Hum. Brain Mapp. 2016;37:1005–1025. doi: 10.1002/hbm.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fryer S.L., Lin D., Sui J., Yu Q., Chen J., Stuart B., Loewy R.L., Calhoun V.D., Mathalon D.H. Identifying functional network changing patterns in individuals at clinical high-risk for psychosis and patients with early illness schizophrenia: a group ICA study. Neuroimage Clin. 2018;17:335–346. doi: 10.1016/j.nicl.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S.M., Wojtalik J.A., Keshavan M.S., Minshew N.J. Social-cognitive brain function and connectivity during visual perspective-taking in autism and schizophrenia. Schizophr. Res. 2017;183:102–109. doi: 10.1016/j.schres.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy C.M. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia. 2016;89:465–477. doi: 10.1016/j.neuropsychologia.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Feczko E., Balba N.M., Miranda-Dominguez O., Cordova M., Karalunas S.L., Irwin L., Demeter D.V., Hill A.P., Langhorst B.H., Grieser Painter J., Van Santen J., Fombonne E.J., Nigg J.T., Fair D.A. Subtyping cognitive profiles in Autism Spectrum Disorder using a Functional Random Forest algorithm. Neuroimage. 2018;172:674–688. doi: 10.1016/j.neuroimage.2017.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J.M., Cajao R., Lopes R., Jeronimo R., Barahona-Correa J.B. Social Cognition in schizophrenia and autism spectrum disorders: a systematic review and meta-analysis of direct comparisons. Front. Psychiatry. 2018;9:504. doi: 10.3389/fpsyt.2018.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M.B., Williams, J.B.W., Spitzer, R.L., Gibbon, M., 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). Biometrics Research, New York State Psychiatric Institute, New York.
- Fox J.M., Abram S.V., Reilly J.L., Eack S., Goldman M.B., Csernansky J.G., Wang L., Smith M.J. Default mode functional connectivity is associated with social functioning in schizophrenia. J. Abnorm. Psychol. 2017;126:392–405. doi: 10.1037/abn0000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretland R.A., Andersson S., Sundet K., Andreassen O.A., Melle I., Vaskinn A. Theory of mind in schizophrenia: error types and associations with symptoms. Schizophr. Res. 2015;162:42–46. doi: 10.1016/j.schres.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Frith C.D., Poline J.-B., Heather J.D., Frackowiak R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;3:165–189. [Google Scholar]
- Frith C.D. Schizophrenia and theory of mind. Psychol. Med. 2004;34:385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- Geschwind D.H. Advances in autism. Annu. Rev. Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs D.W., Hanlon T.E., Carpenter W.T., Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Phillips L.H., Ruffman T., Bailey P.E. A meta-analytic review of age differences in theory of mind. Psychol. Aging. 2013;28:826–839. doi: 10.1037/a0030677. [DOI] [PubMed] [Google Scholar]
- Hu M.L., Zong X.F., Mann J.J., Zheng J.J., Liao Y.H., Li Z.C., He Y., Chen X.G., Tang J.S. A Review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt C.J., Calhoun V.D., Pearlson G.D., Assaf M. Specific default mode subnetworks support mentalizing as revealed through opposing network recruitment by social and semantic FMRI tasks. Hum. Brain Mapp. 2015;36:3047–3063. doi: 10.1002/hbm.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A., Charles A. The importance of social cognition in improving functional outcomes in schizophrenia. Front. Psychiatry. 2018;9:157. doi: 10.3389/fpsyt.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim D.I., Mathalon D.H., Ford J.M., Mannell M., Turner J.A., Brown G.G., Belger A., Gollub R., Lauriello J., Wible C., O'Leary D., Lim K., Toga A., Potkin S.G., Birn F., Calhoun V.D. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr. Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.H., Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lysaker P.H., Hasson-Ohayon I., Kravetz S., Kent J.S., Roe D. Self perception of empathy in schizophrenia: emotion recognition, insight, and symptoms predict degree of self and interviewer agreement. Psychiatry Res. 2013;206:146–150. doi: 10.1016/j.psychres.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Mak L.E., Minuzzi L., MacQueen G., Hall G., Kennedy S.H., Milev R. The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect. 2017;7:25–33. doi: 10.1089/brain.2016.0438. [DOI] [PubMed] [Google Scholar]
- Mars R.B., Neubert F.X., Noonan M.P., Sallet J., Toni I., Rushworth M.F. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G., Mosconi E., Daban-Huard C., Parellada M., Fananas L., Gaillard R., Fatjo-Vilas M., Krebs M.O., Amado I. “A circle and a triangle dancing together”: Alteration of social cognition in schizophrenia compared to autism spectrum disorders. Schizophr. Res. 2019;210:94–100. doi: 10.1016/j.schres.2019.05.043. [DOI] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. Methods and software for fMRI analysis for clinical subjects. Hum. Brain Mapp. 2009 [Google Scholar]
- Montag C., Dziobek I., Richter I.S., Neuhaus K., Lehmann A., Sylla R., Heekeren H.R., Heinz A., Gallinat J. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186:203–209. doi: 10.1016/j.psychres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Nass C., Moon Y. Machines and mindlessness: social responses to computers. J. Social Issues. 2000;56:81–103. [Google Scholar]
- Oathes D.J., Patenaude B., Schatzberg A.F., Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol. Psychiatry. 2015;77:385–393. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A., Lynch C.J., Schaer M., Menon V. The default mode network in autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K.L., Demetriou E.A., Park S.H., Song Y.C., Hickie I.B., Cacciotti-Saija C., Langdon R., Piguet O., Kumfor F., Thomas E.E., Guastella A.J. Autism, early psychosis, and social anxiety disorder: understanding the role of social cognition and its relationship to disability in young adults with disorders characterized by social impairments. Transl. Psychiatry. 2018;8:233. doi: 10.1038/s41398-018-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Hopfinger J.B., Pelphrey K.A., Piven J., Penn D.L. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr. Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabany L., Brocke S., Calhoun V., Pittman B.P., Corbera S., Wexler B.E., Bell M.D., Pelphrey K.A., Pearlson G., Assaf M. Dynamic functional connectivity in schizophrenia and autism spectrum disorder: convergence, divergence and classification. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabany L., Diefenbach G.J., Bragdon L.B., Pittman B.P., Zertuche L., Tolin D.F., Goethe J.W., Assaf M. Resting-state functional connectivity in generalized anxiety disorder and social anxiety disorder: evidence for a dimensional approach. Brain Connect. 2017;7:289–298. doi: 10.1089/brain.2017.0497. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S.B., Rotarska-Jagiela A., Fink G.R., Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Sugranyes G., Kyriakopoulos M., Corrigall R., Taylor E., Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga C.A., Pearlson G.D., Stan A.D., Gibbons R.D., Padmanabhan J., Keshavan M., Clementz B.A. Strategies for advancing disease definition using biomarkers and genetics: the bipolar and schizophrenia network for intermediate phenotypes. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:20–27. doi: 10.1016/j.bpsc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Tillmann, J., San Jose Caceres, A., Chatham, C.H., Crawley, D., Holt, R., Oakley, B., Banaschewski, T., Baron-Cohen, S., Bolte, S., Buitelaar, J.K., Durston, S., Ham, L., Loth, E., Simonoff, E., Spooren, W., Murphy, D.G., Charman, T., 2019. Investigating the factors underlying adaptive functioning in autism in the EU-AIMS Longitudinal European Autism Project. Autism Res. [DOI] [PMC free article] [PubMed]
- van Veluw S.J., Chance S.A. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014;8:24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Weinberger D.R., Glick I.D., Klein D.F. Whither research domain criteria (RDoC)?: The good, the bad, and the ugly. JAMA Psychiatry. 2015;72:1161–1162. doi: 10.1001/jamapsychiatry.2015.1743. [DOI] [PubMed] [Google Scholar]
- Yahata N., Morimoto J., Hashimoto R., Lisi G., Shibata K., Kawakubo Y., Kuwabara H., Kuroda M., Yamada T., Megumi F., Imamizu H., Nanez J.E., Sr., Takahashi H., Okamoto Y., Kasai K., Kato N., Sasaki Y., Watanabe T., Kawato M. A small number of abnormal brain connections predicts adult autism spectrum disorder. Nat. Commun. 2016;7:11254. doi: 10.1038/ncomms11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.Y., Rosenblau G., Keifer C., Pelphrey K.A. An integrative neural model of social perception, action observation, and theory of mind. Neurosci. Biobehav. Rev. 2015;51:263–275. doi: 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available at the National Database for Autism Research (NDAR).