Abstract
Skeletal muscle relies on coordination between myogenic and non-myogenic interstitial cells for homeostasis and for regeneration and response to injury. Fibroadipogenic progenitors (FAPs) have recently been recognized as key modulators of signaling to promote myogenesis following injury. FAPs are also responsible for the fibrosis and fatty replacement of muscle tissue seen in many diseased states. While extensive use of surface markers to purify FAPs has been undertaken in the mouse system, in particular PDGFRA, markers for human FAPs are less well understood. Here, we show that CD73 can be used as a single positive marker to purify FAPs from the lineage-negative (CD45-neg, CD31-neg) fraction of skeletal muscle mononuclear cells. Although CD73 was previously found to be expressed in cultured myogenic cells, we find that this marker is only acquired upon culture and that the CD73+ fraction of human skeletal muscle has no myogenic activity. We show that Lin-neg CD73+ cells from human muscle undergo fat differentiation as well as fibrogenesis when exposed to appropriate activating signals in vitro. This simple single positive marker approach effectively enables isolation of human FAPs from fresh human skeletal muscle biopsies.
Keywords: Cell biology, Cell culture, Cell differentiation, Stem cell research, Musculoskeletal system, Human fibroadipogenic progenitors, FAPs, CD73
Cell biology; Cell culture; Cell differentiation; Stem cell research; Musculoskeletal system; Human fibroadipogenic progenitors, FAPs, CD73.
1. Introduction
Skeletal muscle contains numerous non-myogenic supportive cell types which interact with each other and with myogenic cells to maintain muscle function and enable regenerative homeostasis. Using mouse genetic models and cell surface markers, the role of cells with fat and fibroblastic differentiation potential in coordinating the regenerative response of satellite cells to injury has become appreciated in the mouse system (Joe et al., 2010; Murphy et al., 2011; Uezumi et al., 2010; Wosczyna et al., 2019). These cells are commonly referred to as FAPs (fibroadipogenic progenitors), for their ability to differentiate into both fat and matrix-secretory fibroblasts, in vitro and in vivo (Joe et al., 2010). In the murine system, FAPs have been prospectively isolated using a combination of lineage negative (CD45, CD31, and ITGA7) markers together with the positive markers PDGFRA (Uezumi et al., 2010) or CD34 and SCA1 (Joe et al., 2010). After severe injury, such as is generated by myotoxin injection, FAP numbers increase, but return to normal within five days (Joe et al., 2010).
The importance of this cell population in muscle regeneration and injury response has been a topic of intense study in the past decade. However, there are still many unknown questions about FAP biology. One important challenge is the isolation and identification of FAPs in human skeletal muscle. Cell surface markers like PDGFRA and CD201 (Uezumi et al., 2016) have been proposed to be used for FAP identification, but no other candidates have been identified. The best characterized mesenchymal lineage progenitor cells are mesenchymal stem cells (MSC) which are found in a variety of tissues and are known for their plasticity and immunomodulatory properties. In several contexts, MSCs have been found to express CD73 (Breitbach et al., 2018; Dominici et al., 2006). Recent studies have suggested a role for CD73 in regulating stemness of bone marrow MSCs as well as of cancer cells (Lupia et al., 2018; Tan et al., 2019), suggesting that CD73 could be an identifying marker in other stem and progenitor cells. In addition, CD73 is a robust marker to which several FACS-compatible monoclonal antibodies of high quality and specificity are commercially available. Here we investigate whether CD73 can be used as a marker to identify and sort out FAPs from freshly isolated human muscle.
2. Results
2.1. CD73 excludes human cells with myogenic activity but marks cells with adipogenic and fibrogenic activity
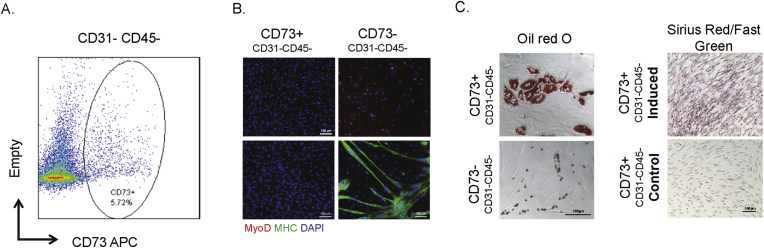
To examine the presence of cells expressing CD73 within human skeletal muscle, and to determine their phenotypic potential, normal human biopsy samples were obtained and stained with CD73, together with the endothelial and hematopoietic markers CD31 and CD45. Out of the four samples examined, the average fraction of CD73+ cells in the lineage negative (CD45– CD31–) population was approximately 5% (Figure 1A and Table 1). This population of cells was sorted out using FACS and cultured on gelatin coated dishes in 5% oxygen. After expansion, cells were tested for differentiation potential by replating under conditions promoting myogenic, adipogenic, or fibrogenic differentiation. Allowing cells to reach confluence and then exposing to myogenic differentiation medium revealed that CD73+ cells do not differentiate into muscle in vitro, evident by the lack myosin heavy chain (MHC) expressing myotubes. In comparison, the CD73– fraction robustly formed myotubes (Figure 1B). Together, these data suggest that CD73 marks a population of cells in the muscle that lacks myogenic differentiation potential. To further establish the identity of this population, CD73+ cells were plated under adipogenic and fibrogenic differentiation conditions. After seven days in their respective differentiation media, the cells were stained for lipid droplets using Oil Red O or for collagen deposition using Sirius Red/Fast Green. While the CD73–lineage– population did not form adipocytes, the CD73+ population readily made adipocytes and cells secreting collagen, under pro-adipogenic or pro-fibrotic conditions (Figure 1C).
Figure 1.
CD73 positive cells are fibrogenic and adipogenic but not myogenic in culture. A. FACS analysis showing CD73 staining of single cells from human muscle biopsy. Average fraction of CD73+ CD45– CD31– cells in wild type muscle is 4.5% (n = 4). B. CD73+ and CD73– cells were cultured in myogenic growth medium and stained with MyoD; or cultured in myogenic differentiation medium, and stained with MHC. C. CD73+ and CD73– cells were cultured in adipogenic differentiation medium, then stained with Oil red O. CD73+ cells were also cultured in fibrogenic differentiation medium, then stained with Sirius Red/Fast Green.
Table 1.
Biopsy sample description.
2.2. Transplanted CD73+ cells engraft in immune-deficient mice, but do not differentiate into muscle
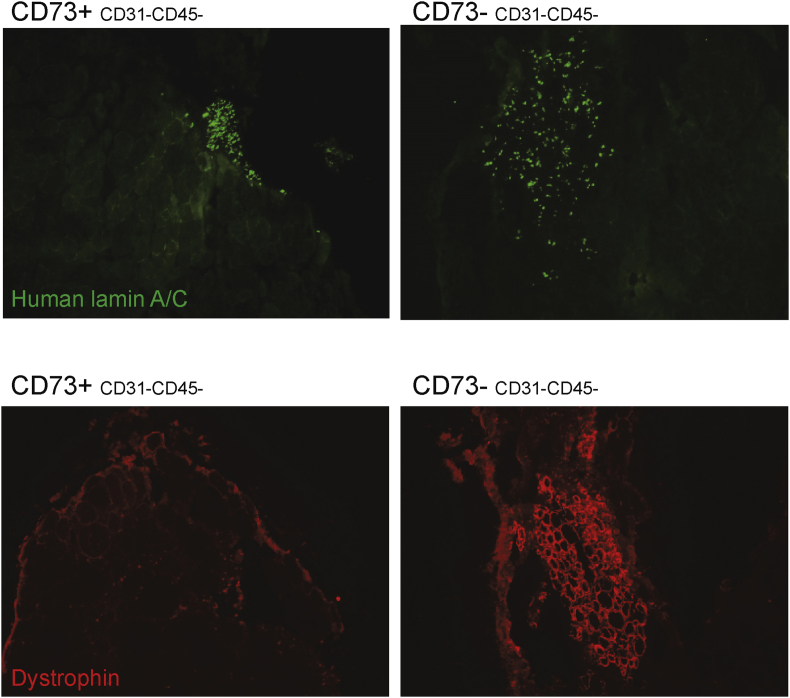
To rigorously confirm the lack of myogenic activity of CD73+ cells, we transplanted freshly isolated CD73+ lineage– cells into pre-injured, irradiated, tibialis anterior muscles of NSG-mdx4Cv mice (Arpke et al., 2013). These animals lack dystrophin, thus donor-derived myofibers can be readily identified by dystrophin staining of muscle sections. As a positive control, the CD73– population was transplanted into the contralateral leg. Two months post-transplantation, whereas the CD73– population engrafted and formed dystrophin+ muscle, the CD73+ population lacked any in vivo myogenic ability (Figure 2). Human-specific lamin A/C staining revealed human mononuclear cells derived from the CD73+ cells were nevertheless present within the transplanted muscle. These data suggest that although these cells are capable of engraftment, they do not have any myogenic potential.
Figure 2.
CD73 positive cells engraft but are not myogenic in vivo. CD73+ and CD73– cells were transplanted into NSG-mdx4Cv mice, which lack dystrophin. Dystrophin (red) staining of muscle cross sections indicates myogenic contribution of injected cells, revealing myogenic differentiation of CD73– cells, but an absence of myogenic contribution from CD73+ cells. Human lamin A/C (green) indicates engraftment of primary cells.
2.3. CD73 expression is induced upon culture of primary myogenic cells
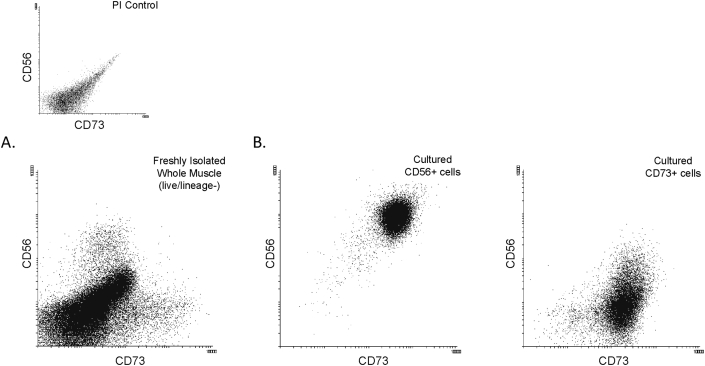
Previous work evaluated cell surface markers on cultured cells derived from human skeletal muscle, screening 332 markers. In this study, it was found that CD73 was expressed by both cultured myogenic progenitors (CD56+) and cultured FAPs (PDGFRA) (Uezumi et al., 2016). As this result seemed contradictory to our observations, we examined this question in more detail, comparing freshly purified myogenic progenitors to their cultured derivatives. Combining CD73 and CD56 antibodies, together with the lineage negative cocktail (CD45– CD31–) we found that in fresh human muscle biopsies, distinct CD73+ and CD56+ populations exist, without overlap (Figure 3A). The CD56+ and CD73+ populations were then sorted by FACS, cultured for two passages, and reevaluated by FACS. This revealed that while the CD73+ population stably retained its CD73+ CD56– expression profile, the CD56+ population had now became double-positive for both CD56 and CD73, demonstrating that CD73 comes on with culture in myogenic cells (Figure 3B).
Figure 3.
CD73 expression is acquired by myogenic cells in culture. A. Freshly isolated cells are single positive for CD73 (FAPs) and CD56 (myogenic cells), B. Upon culture, the CD56+ population gains CD73 expression, whereas the CD73+ population maintains CD73 single positivity.
3. Discussion
In this study we demonstrate that CD73 marks the FAP population in freshly isolated human skeletal muscle. Our data show that freshly isolated CD73+ cells are non-myogenic cells which are capable of both adipogenic differentiation and fibrogenesis in vitro. Furthermore, when transplanted into immunodeficient dystrophin-deficient mice, they engraft but do not form muscle fibers. In aggregate, these data suggest that CD73 marks the resident FAP population of human skeletal muscle. Interestingly, a previous study identified a CD73+ CD105+ mesenchymal population that was likely responsible for heterotopic muscle ossification in freshly isolated skeletal muscle (Downey et al., 2015). The CD73+ population that can undergo both adipogenesis and fibrogenesis in vitro identified here must include this double positive population with bone-forming potential, and supports the notion that FAPs are responsible for heterotopic ossification.
An earlier study examining cell surface markers of mesenchymal and myogenic populations in human skeletal muscle looked at ex vivo expanded CD56+ (myogenic) and PDGFRA+ (mesenchymal) populations (Uezumi et al., 2016). They examined CD73 expression in response to the findings by Downey et al., (2015) but found that both the cultured CD56 and PDGFRA populations were CD73+ (Uezumi et al., 2016), thus considering that CD73 would not be useful as a positive marker for prospective isolation. Our work explains this apparent discrepancy by showing that freshly isolated myogenic cells only gain CD73 expression after ex vivo culture, highlighting the importance of considering how markers may change with culture.
In summary, our data demonstrates that CD73 single positivity identifies a population of mesenchymal cells capable of fibro-adipogenic differentiation, but not capable of muscle differentiation, neither in vitro nor in vivo. While this marker has been used in the past to identify populations of stem and progenitor cells in the mesenchymal lineage, this is the first time that it has been used as a robust single positive marker to isolate FAPs from fresh human muscle. This study also highlights the importance of distinguishing freshly isolated and cultured cells, as the cell surface profile of biopsy-derived cells alters during culture.
4. Materials and methods
4.1. Biopsy tissue processing and flow cytometry
Human muscle tissue samples from the vastus lateralis muscle were obtained after donor consent from surgical waste tissue after diagnostic surgical biopsies. Muscle tissue was mechanically separated and digested with 0.2% collagenase II for 30 min at 37C. Mononuclear cells were washed and resuspended in culture medium (F10 (HyClone) supplemented with 20% FBS, 2-mercaptoethanol, 10−9 M dexamethasone (Sigma), 10 ng/mL bFGF (Peprotech)), Glutamax (GIBCO) and Penicillin/Streptomycin (P/S, GIBCO). Human tissue procurement was conducted under a protocol approved by the University of Minnesota Institutional Review Board. Freshly isolated cells were analyzed and sorted on the BD Aria II flow cytometer using the following antibodies: CD73 (APC, Clone AD2, 17-0739-42), CD56 (APC, Clone CMSSB, 17-0567-42), CD45 (PE-Cy7 Clone H130, BDB557748), CD31 (PE-Cy7 Clone WM-595, BDB563651).
4.2. In vitro differentiation
To examine adipogenic and fibrogenic differentiation potential, CD73+ cells were plated in culture medium until confluency. Then medium was changed to adipogenic medium (DMEM high glucose supplemented with 10% FBS, penicillin-streptomycin, 0.2 mM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/ml recombinant human insulin and 1 μM dexamethasone) or fibrogenic medium (10 ng/ml TGFβ) for 5–7 days. At the termination of differentiation cells were stained with Oil Red O (Sigma, #O0625-25G) and Sirius Red/Fast Green (Chondrex, #9062) to examine adipogenesis and fibrogenesis respectively. Immunostaining was performed on 4% paraformaldehyde fixed cells, treated with 0.2% Triton X-100, blocked with 3% BSA. Primary and secondary fluorochrome conjugated antibodies were diluted in 3% BSA and incubated overnight at 4 °C or for 1 h at room temperature. Antibodies used: mouse anti-MHC (MF20, 1:20, Developmental Studies Hybridoma Bank), Alexa Fluor 488 Goat Anti-Mouse, 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen).
4.3. Transplantation studies
Freshly isolated CD73+ CD45– CD31 cells were transplanted into the tibialis anterior (TA) muscle of NSG-mdx4CV mice (Arpke et al., 2013). Briefly, recipient TA muscles were injected bilaterally with cardiotoxin, then 24-hours later animals were subjected to x-irradiation (1200 cGy), then 24-hours following the irradiation 4 × 104 cells were injected into each pre-injured TA. Freshly isolated CD73– CD45– CD31– cells were injected into the contralateral TA as a myogenic engraftment comparison. The engraftment was repeated with four independent biopsy samples. Three weeks later the muscle was removed and cryosectioned to analyze engraftment. Sections were stained with anti-human lamin A/C and anti-dystrophin to examine engraftment and myogenic contribution. Sections were fixed for 20 min with 4% PFA, permeabilized with 0.2% Triton X-100, blocked for one hour at room temperature with 3% BSA, incubated with primary antibody (mouse anti-human lamin A/C, clone 4C11; rabbit anti-dystrophin, AB15277-1) overnight at 4C. Animal work was conducted under a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee.
Declarations
Author contribution statement
M. Kyba: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
N. Goloviznina: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
N. Xie: Performed the experiments; Analyzed and interpreted the data.
A. Dandapat: Performed the experiments.
P. Iaizzo: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
M. Kyba was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR055685). N. Goloviznina was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F31 AR073642).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Arpke R.W., Darabi R., Mader T.L., Zhang Y., Toyama A., Lonetree C.L., Nash N., Lowe D.A., Perlingeiro R.C.R., Kyba M. A new immuno-, dystrophin-deficient model, the NSG-mdx4Cv mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cell. 2013;31:1611–1620. doi: 10.1002/stem.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach M., Kimura K., Luis T., Fuegemann C., Woll P., Hesse M., Facchini R., Rieck S., Jobin K., Reinhardt J. In vivo labeling by CD73 marks multipotent stromal cells and highlights endothelial heterogeneity in the bone marrow niche. Cell Stem Cell. 2018 doi: 10.1016/j.stem.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating a, Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Downey J., Lauzier D., Kloen P., Klarskov K., Richter M., Hamdy R., Faucheux N., Scimè A., Balg F., Grenier G. Prospective heterotopic ossification progenitors in adult human skeletal muscle. Bone. 2015;71:164–170. doi: 10.1016/j.bone.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Joe A.W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupia M., Angiolini F., Bertalot G., Freddi S., Sachsenmeier K.F., Chisci E., Kutryb-Zajac B., Confalonieri S., Smolenski R.T., Giovannoni R. CD73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Rep. 2018 doi: 10.1016/j.stemcr.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Lawson J., Mathew S., Hutcheson D., Kardon G. Satellite cells , connective tissue fibroblasts and their interactions are crucial for muscle regeneration. 2011;3637:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K., Zhu H., Zhang J., Ouyang W., Tang J., Zhang Y., Qiu L., Liu X., Ding Z., Deng X. CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cell. Int. 2019 doi: 10.1155/2019/8717694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Uezumi A., Nakatani M., Ikemoto-Uezumi M., Yamamoto N., Morita M., Yamaguchi A., Yamada H., Kasai T., Masuda S., Narita A. Cell-surface protein profiling identifies distinctive markers of progenitor cells in human skeletal muscle. Stem Cell Rep. 2016;7:263–278. doi: 10.1016/j.stemcr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M., Konishi C., Perez Carbajal E., Wang T., Walsh R., Gan Q., Wagner M., Rando T. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep. 2019 doi: 10.1016/j.celrep.2019.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]