Abstract
Objective
Salt-induced kinase 1 (SIK1) acts as a key modulator in many physiological processes. However, the effects of SIK1 on gluconeogenesis and the underlying mechanisms have not been fully elucidated. In this study, we found that a natural compound phanginin A could activate SIK1 and further inhibit gluconeogenesis. The mechanisms by which phanginin A activates SIK1 and inhibits gluconeogenesis were explored in primary mouse hepatocytes, and the effects of phanginin A on glucose homeostasis were investigated in ob/ob mice.
Methods
The effects of phanginin A on gluconeogenesis and SIK1 phosphorylation were examined in primary mouse hepatocytes. Pan-SIK inhibitor and siRNA-mediated knockdown were used to elucidate the involvement of SIK1 activation in phanginin A-reduced gluconeogenesis. LKB1 knockdown was used to explore how phanginin A activated SIK1. SIK1 overexpression was used to evaluate its effect on gluconeogenesis, PDE4 activity, and the cAMP pathway. The acute and chronic effects of phanginin A on metabolic abnormalities were observed in ob/ob mice.
Results
Phanginin A significantly increased SIK1 phosphorylation through LKB1 and further suppressed gluconeogenesis by increasing PDE4 activity and inhibiting the cAMP/PKA/CREB pathway in primary mouse hepatocytes, and this effect was blocked by pan-SIK inhibitor HG-9-91-01 or siRNA-mediated knockdown of SIK1. Overexpression of SIK1 in hepatocytes increased PDE4 activity, reduced cAMP accumulation, and thereby inhibited gluconeogenesis. Acute treatment with phanginin A reduced gluconeogenesis in vivo, accompanied by increased SIK1 phosphorylation and PDE4 activity in the liver. Long-term treatment of phanginin A profoundly reduced blood glucose levels and improved glucose tolerance and dyslipidemia in ob/ob mice.
Conclusion
We discovered an unrecognized effect of phanginin A in suppressing hepatic gluconeogenesis and revealed a novel mechanism that activation of SIK1 by phanginin A could inhibit gluconeogenesis by increasing PDE4 activity and suppressing the cAMP/PKA/CREB pathway in the liver. We also highlighted the potential value of phanginin A as a lead compound for treating type 2 diabetes.
Keywords: Salt-inducible kinase 1, Phanginin A, Phosphodiesterase 4, Gluconeogenesis, cAMP
Highlights
-
•
Phanginin A inhibits gluconeogenesis in primary mouse hepatocytes.
-
•
Phanginin A increases hepatic SIK1 phosphorylation both in vitro and in vivo.
-
•
Activation of SIK1 increases PDE4 activity and suppresses the cAMP signaling pathway.
-
•
Activation of SIK1 inhibits gluconeogenesis by regulating the PDE4/cAMP/PKA/CREB pathway.
-
•
Phanginin A improves metabolic disorders in ob/ob mice.
1. Introduction
Type 2 diabetes is one of the world's leading chronic diseases [1,2]. The liver is the main organ that maintains glucose homeostasis by storing excessive carbohydrates in glycogen following feeding and converting non-carbohydrate precursors into glucose via gluconeogenesis stimulated by glucagon during fasting [3,4]. Glucagon promotes gluconeogenesis by activating the cAMP/PKA pathway, which increases Ser133 phosphorylation of CREB and promotes the expression of the key rate-limiting gluconeogenic enzymes including glucose 6-phosphatase (G6p) and phosphoenolpyruvate carboxykinase (Pepck) [[5], [6], [7]]. Persistent activation of hepatic gluconeogenesis is a main cause of fasting hyperglycemia in patients with type 2 diabetes [1]. Metformin, the first-line drug for the treatment of diabetes, exerts glucose-lowering effects mainly by suppressing gluconeogenesis [8]. Some studies have shown that metformin activates AMPK and then phosphorylates CREB co-activator 2 (CRTC2) and Forkhead box protein O1 (FoxO1), thereby inhibiting gene transcription of PEPCK and G6P, thus leading to gluconeogenesis inhibition [9,10], while other studies reported that energy charge [11] and mitochondrial glycerophosphate dehydrogenase (mGPD) [12] are also involved, indicating that metformin may regulate gluconeogenesis by multiple pathways. Investigating the molecular mechanisms involved in the regulation of gluconeogenesis would be beneficial for exploring novel potential therapeutic targets for the treatment of diabetes.
Salt-inducible kinases (SIKs), composed of three subtypes (SIK1-3), belong to the AMP-activated protein kinase (AMPK)-related kinase (AMPKRK) family. CREB co-activator 2 (CRTC2) and class IIa histone deacetylases (HDACs) are the major substrates of SIKs through which SIKs regulate gene expression and act as key modulators in various metabolic functions [[13], [14], [15]]. Given the important role of CRTC2/CREB in promoting fasting-induced hepatic gluconeogenesis, along with the obvious inhibition of SIKs on this complex, several studies reported that SIKs are involved in the regulation of gluconeogenesis [14,15]. Pan-SIK inhibitor HG-9-91-01 significantly enhanced gluconeogenesis in hepatocytes, suggesting the importance of SIKs in the inhibition of gluconeogenesis [16]. However, whether a major SIK isoform is involved in the regulation of gluconeogenesis is uncertain, and different studies showed controversial results even when they focused on the same SIK isoform [[16], [17], [18], [19]]. One interesting clue showed that hepatic SIK1 mRNA expression and protein levels were rapidly induced under fasting, whereas SIK2 and SIK3 were unaffected [20], indicating that SIK1 might be more important in the regulation of gluconeogenesis.
However, there were only a few reports regarding the function of SIK1 in the liver, and its effects on gluconeogenesis and related mechanisms were under debate. An early study showed that SIK1 acted as a gluconeogenesis suppressor by increasing CRTC2 Ser171 phosphorylation and suppressing cAMP-induced gluconeogenic gene expression [20]. This finding was further supported by increased PEPCK and G6Pase gene expression in SIK-1-deficient cells and the promotion of fasting hyperglycemia in SIK1 knockdown mice. But another study provided sharp contrasting results that liver-specific SIK1 knockout mice showed no alteration in hepatic CRTC2 phosphorylation and blood glucose levels [21]. The contradictory results lead to the inability to reach a consensus on whether SIK1 can act as a key regulator of gluconeogenesis through the CRTC2/CREB pathway. SIK1 was reported to terminate the cAMP signaling pathway by activating phosphodiesterase 4D (PDE4D) in β cells [22,23]. In view of the important role of the cAMP/PKA/CREB pathway in mediating gluconeogenesis, it would be of interest to investigate if SIK1 activates PDE4 in the liver and further regulates gluconeogenesis.
Considering the importance of gluconeogenesis in the maintenance of whole-body glucose homeostasis, a screening of our natural compounds library based on gluconeogenesis was performed in primary mouse hepatocytes. Phanginin A, a natural product isolated from Caesalpinia sappan Linn [24], was found to display potent inhibition of gluconeogenesis. Further studies showed that phanginin A activated AMPK, but knockdown of AMPK had no effect on the suppression of phanginin A on gluconeogenesis, suggesting that the activation of AMPK was not related to phanginin A-reduced gluconeogenesis. However, knockdown of AMPK upstream kinase LKB1 fully reversed the inhibition of phanginin A on gluconeogenesis. Further investigation showed that the inhibition of phanginin A on gluconeogenesis relied on the activation of SIK1, another downstream activator of LKB1. Using phanginin A as a SIK1 activator, we identified a new mechanism that the activation of SIK1 suppressed gluconeogenesis by increasing PDE4 activity and inhibiting the cAMP/PKA/CREB pathway. Similar findings were confirmed by overexpressing SIK1 in primary mouse hepatocytes. In addition, the acute and chronic in vivo effects were evaluated in ob/ob mice to explore the anti-diabetic potential of phanginin A and the underlying value of the activation of SIK1 in the liver as a new therapeutic strategy for the treatment of type 2 diabetes.
2. Methods
2.1. Animals
C57BL/6J male mice were acquired from SLAC Laboratory Animals (Shanghai, China), and fed a normal diet (Shilin, Shanghai, China). B6.V-Lepob/Lepob mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and fed a high-fat diet (Cat. P1400F, Puluteng, Shanghai, China). The animals were housed individually under a 12 h light/12 h dark cycle. All of the animal care and experiments were managed by the guidelines of the Institutional Animal Care and Utilization Committee (IACUC), Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
2.2. Extraction and isolation of phanginin A
Air-dried and powdered seeds of C. sappan (11 kg) were extracted with 95% EtOH (3 × 50 L, v/v, 48 h each time) at room temperature and then filtered. The filtrate was evaporated in vacuo to yield a crude residue (2.1 kg) that was suspended in H2O and then partitioned with EtOAc (3 × 6 L). The EtOAc-soluble fraction (1.2 kg) was subjected to a silica gel column (100–200 mesh) and eluted with a gradient of petroleum ether (PE) acetone (9:1–1:1, v/v) to produce five fractions (A-E). Fraction C (120 g) was fractionated by medium-pressure preparative liquid chromatography (MCI gel) and eluted with MeOH–H2O (50:50 to 100:0) to provide six fractions (A-F). Fraction B (35 g) was chromatographed repeatedly over silica gel eluted with PE-EtOAc (9.5:0.5 to 7:3) to obtain four subfractions (B1–B4). Subfraction B2 (15 g) was recrystallized from methanol to yield 97% pure phanginin A (10 g).
2.3. Hepatocyte culture
Primary mouse hepatocytes were isolated from overnight-fasted male C57BL/6J mice (8–12 weeks old) using a previously described protocol [25]. Isolated hepatocytes were cultured in minimum essential medium (MEM) with 10% FBS (vol/vol, Gibco), insulin (100 nM, Sigma-Aldrich, St. Louis, MO, USA), and dexamethasone (10 nM, Sigma-Aldrich).
2.4. Gluconeogenesis in primary mouse hepatocytes
Primary mouse hepatocytes were seeded in 48-well plates for 4 h and then incubated in glucose-free DMEM with different doses of phanginin A or metformin for 1.5 h. Afterwards, replaced with the DMEM with or without gluconeogenic substrates (2 mM pyruvate and 20 mM lactate), followed by 4 h incubation with or without the stimulation of forskolin (20 μ M) or glucagon (10 nM). For experiments conducted with inhibitors, the inhibitor was incubated in glucose-free DMEM for 0.5 h in advance, and then the previously described procedure was followed. The glucose production was detected by a glucose assay kit (Rongsheng Biotech, Shanghai, China) and normalized to the cellular protein concentration.
2.5. PKA activity and cAMP assay
The PKA activity and intracellular cAMP concentration in primary hepatocytes or liver tissues was measured following the manufacturer's protocol of using a kinase activity kit and a cAMP Complete ELISA kit purchased from Enzo Life Sciences (Farmingdale, NY, USA).
2.6. RNA isolation and real-time PCR
Total RNA was extracted from hepatocytes or liver tissues using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). cDNA was generated by a Primer Script RT reagent kit (TaKaRa Biotechnology, Dalian, China) and analyzed via quantitative PCR. All of the primer sequences used in this study are included in Table S1. The relative amount of individual mRNA was normalized to β-actin mRNA (for primary mouse hepatocytes).
2.7. Western blotting
Cell or tissue lysates were prepared as previously described [26]. Lysate was separated by SDS-PAGE and then blotted with antibodies. Membranes were visualized by an ECL plus Western blotting detection reagent (GE Healthcare, Buckinghamshire, UK) and quantified by densitometry (Bio-Rad). The primary antibodies against phospho-CREB (Ser133) (#9198), CREB (#9197), LKB1 (#3047), phospho-AMPK (Thr172) (#2531), AMPK (#2532), and GAPDH (#5174) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody against phospho-SIK1 (Thr182) (PA5-64610) was purchased from Invitrogen. Antibody against SIK1 (ab217809) was bought from Abcam (Cambridge, UK).
2.8. RNA interference
Primary mouse hepatocytes were transfected with selective siRNA using Lipofectamine 3000 RNAiMAX Transfection Reagent (Thermo Fisher Scientific, Shanghai, China) for 24 or 48 h for follow-up experiments. The siRNA of SIKs was obtained from Life Technologies (Shanghai, China). AMPK siRNA was purchased from Santa Cruz (Shanghai, China). LKB1 siRNA was provided by GenePharma (Shanghai, China). Non-silencing RNA was purchased from GenePharma (Shanghai, China) and used as a negative control.
2.9. PDE4 activity assay
A PDE4 activity assay was conducted as described in a previous study [25,27] and following the manufacturer's protocol (Abcam, Cambridge, UK). The difference between the amount of phosphate cleaved in the absence of roflumilast and the amount in the presence of roflumilast reflected the PDE4 activity. Purified PDE enzymes were used to detect the direct effect of phanginin A on PDE4 activity. Primary mouse hepatocytes and liver extracts were prepared following the manufacturer's protocol. The lysates were incubated with 3′,5′-cAMP substrates and 5′-nucleotidase for 30 min at 30 °C.
2.10. Transient plasmid transfections of SIK1
Primary hepatocytes were transfected with SIK1 plasmid (EX-Mm30183-M02, GeneCopoeia, Shanghai, China) or pEZ expression vector control for 24 h using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's instructions.
2.11. Pyruvate tolerance test in ob/ob mice
Female ob/ob mice (8–9 weeks old) were fasted for 4 h and then divided into three groups according to blood glucose and body weight (n = 8 per group). The mice were orally administered phanginin A (100 mg/kg), metformin (250 mg/kg), or vehicle (0.25% CMC-Na, wt/vol) and injected intraperitoneally with sodium pyruvate (1.5 g/kg) after 2 h. The blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min.
2.12. Acute treatment of phanginin A in ob/ob mice
After 4 h of fasting, the ob/ob mice (male, 7 or 8 weeks old) were assigned to three groups based on body weight (n = 8 per group) and orally administered 100 mg/kg of phanginin A, 250 of mg/kg metformin, or vehicle (0.25% CMC-Na, wt/vol). Two h later, the mice were anesthetized and their livers were dissected for further experiments. The liver glycogen content was measured following the manufacturer's protocol using a glycogen content assay purchased from Cominbio (Suzhou, China).
2.13. Chronic treatment of phanginin A in ob/ob mice
The male ob/ob mice (7 or 8 weeks old) were divided into three groups according to their blood glucose and body weight (n = 8 per group) and orally administered once daily with 100 mg/kg of phanginin A, 250 mg/kg of metformin, or vehicle (0.25% CMC-Na, wt/vol) for 26 days. The random and fast blood glucose levels were measured on days 4, 8, 12, 16, 20, 23, and 26. Body weight was measured regularly throughout the treatment. Oral glucose tolerance (2 g/kg) tests were conducted on 6-h fasted mice on day 23. On the last day, the mice were fasted for 6 h and then anesthetized with an intraperitoneal injection of chloral hydrate. Blood samples were collected for HbA1c measurement. Serum was collected and assayed for triglyceride and cholesterol. The liver was dissected and stored at 80 °C. Hepatic triglyceride and cholesterol were detected using a heptane-isopropanol-Tween mixture (3:2:0.01 by volume) or hexane-isopropanol mixture (3:2 by volume), respectively, and determined by commercial kits (DongOu Jin Ma Biotech, Wenzhou, China).
2.14. Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis between two groups was conducted using a two-tailed unpaired t test. The comparison of multiple groups was carried out using one-way ANOVA followed by Dunnett's test in GraphPad Prism software. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Phanginin A inhibited gluconeogenesis in primary mouse hepatocytes
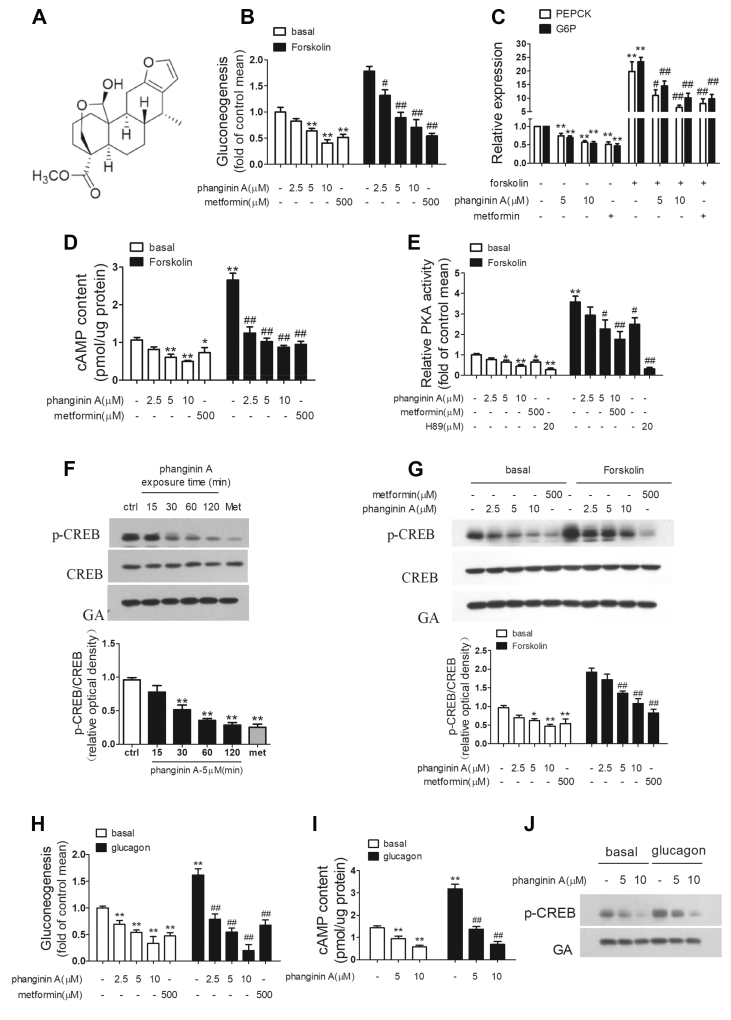
The chemical structure of phanginin A is shown in Figure 1A. Phanginin A suppressed gluconeogenesis in primary mouse hepatocytes in a dose-dependent manner (Figure 1B). Incubation of hepatocytes with 5 and 10 μM of phanginin A significantly decreased glucose production by 36% and 59% under basal conditions and 50% and 60% under forskolin-stimulated conditions, while 500 μM of metformin caused a reduction of 49% and 70% under basal and forskolin-stimulated conditions, respectively. Phanginin A treatment also reduced the mRNA expression of G6P and PEPCK. As shown in Figure 1C, phanginin A at doses of 5 and 10 μM significantly decreased PEPCK mRNA expression by 25% and 43% under basal conditions and 45% and 67% under forskolin-stimulated conditions, respectively. The G6P mRNA expression was also significantly reduced, with 5 and 10 μM of phanginin A resulting in a decrease of 30% and 46% under basal conditions and 38% and 57% under forskolin-stimulated conditions, respectively. We further evaluated the effect of phanginin A on the cAMP/PKA/CREB signaling pathway. As shown in Figures 1D, 5 and 10 μM of phanginin A significantly decreased the intracellular cAMP accumulation by 43% and 54% under basal conditions and 61% and 67% under forskolin-stimulated conditions, respectively. Under the same conditions, PKA activity was profoundly reduced following phanginin A treatment (Figure 1E). Moreover, phanginin A time- and dose-dependently inhibited CREB phosphorylation (Figure 1F,G). Phanginin A also dose-dependently suppressed glucagon-stimulated gluconeogenesis (Figure 1H) and reduced the intracellular cAMP content and CREB phosphorylation (Figure 1I,J). These data demonstrated that phanginin A efficiently inhibited the cAMP/PKA/CREB pathway and reduced gluconeogenesis in primary mouse hepatocytes.
Figure 1.
Phanginin A inhibits gluconeogenesis in primary mouse hepatocytes. (A) Chemical structure of phanginin A. (B) The effect of phanginin A on gluconeogenesis in primary mouse hepatocytes under basal and 20 μM forskolin-induced conditions. (C) The effect of phanginin A on the mRNA expression of G6P and PEPCK in primary mouse hepatocytes under basal and 20 μM forskolin-induced conditions. (D–E) The effect of phanginin A on the cAMP concentration (D) and PKA activity (E) under basal and 20 μM forskolin-induced conditions. (F) Western blotting analysis of CREB phosphorylation in primary mouse hepatocytes incubated with 5 μM of phanginin A for 0–120 min. met = metformin. (G) Western blotting analysis of CREB phosphorylation in primary mouse hepatocytes incubated with phanginin A for 2 h under basal and 20 μM forskolin-induced conditions. (H) The effect of phanginin A on gluconeogenesis in primary mouse hepatocytes under basal and 10 nM glucagon-induced conditions. (I–J) The effect of phanginin A on the cAMP concentration (I) and CREB phosphorylation (J) under basal and 10 nM glucagon-induced conditions. Data are the mean ± SEM (n = 5 for all of the groups, except for n = 8 in Figure C and n = 4 in Figure H and J). ∗P < 0.05 and ∗∗P < 0.01 vs controls under basal conditions. #P < 0.05 and ##P < 0.01 vs controls under forskolin-induced or glucagon-induced conditions.
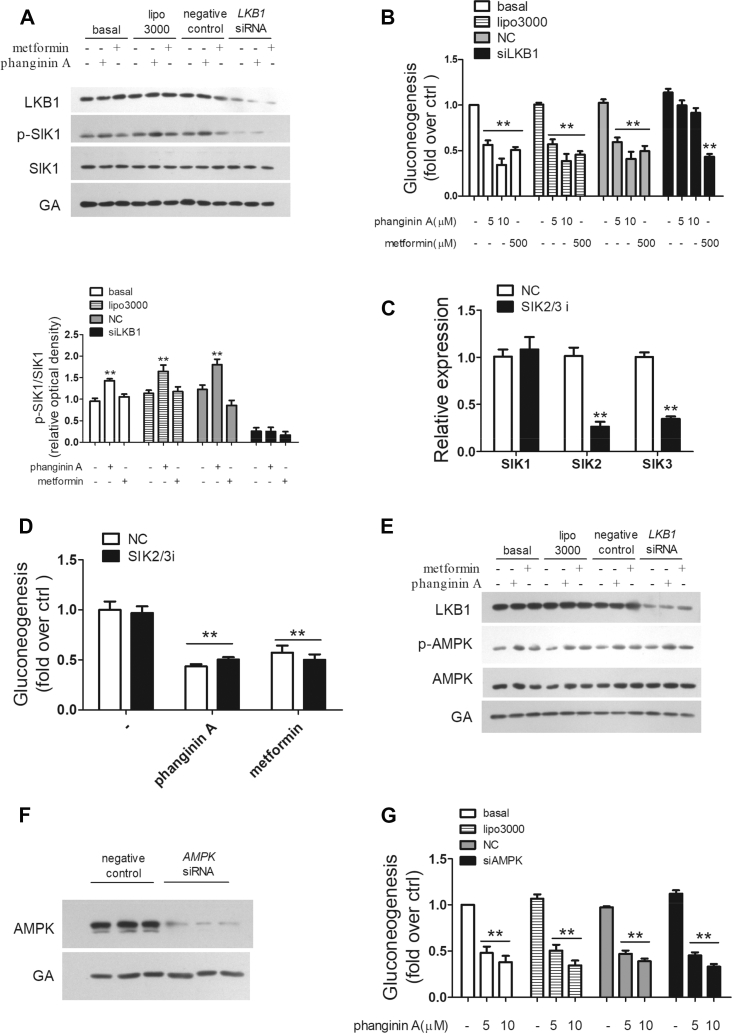
3.2. Phanginin A inhibited gluconeogenesis by activating SIK1 in primary mouse hepatocytes
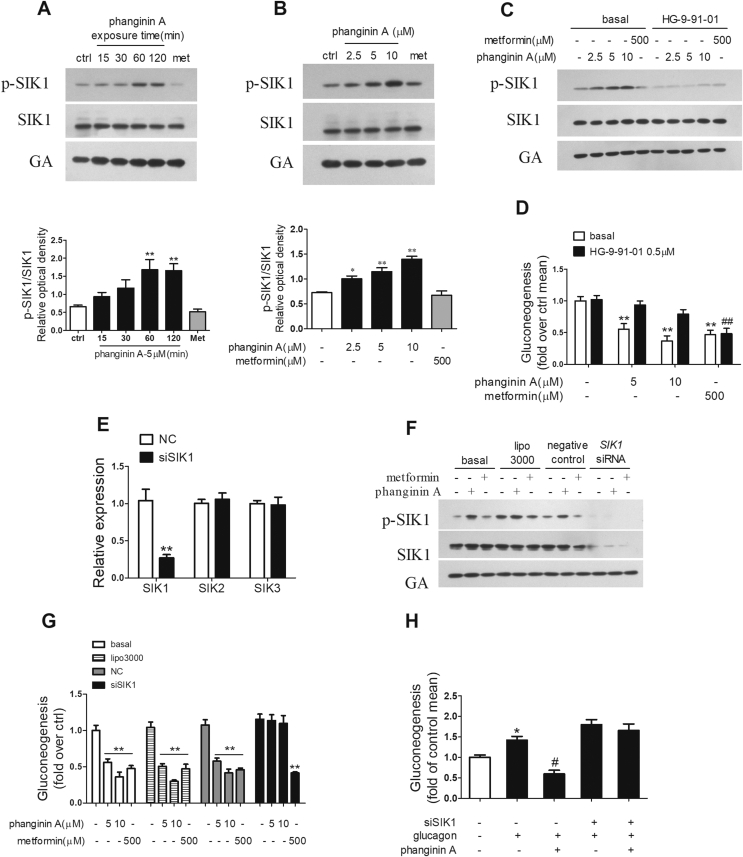
The phosphorylation of Thr182 is essential for the activation of SIK1's kinase catalytic activity [28,29]. Phanginin A increased the Thr182 phosphorylation of SIK1 in a time- and dose-dependent manner, whereas metformin showed no effect (Figure 2A,B). To delineate the role of the activation of SIK1 in phanginin A-inhibited gluconeogenesis, HG-9-91-01, a pan-SIK inhibitor, was used to suppress SIK1 activity. As shown in Figure 2C, the phosphorylation of SIK1 induced by phanginin A was fully blocked by treatment with 0.5 μM of HG-9-91-01. Moreover, the inhibition of phanginin A on gluconeogenesis was abolished by HG-9-91-01, while metformin-induced gluconeogenesis reduction was unaffected (Figure 2D). Since HG-9-91-01 is a pan-SIK inhibitor, and the potential role of SIK2 and SIK3 cannot be ruled out in phanginin A-inhibited gluconeogenesis, SIK1 RNAi was used in the primary mouse hepatocytes. After siRNA interference, the expression of SIK1 genes was reduced by 74% compared with the negative control, and no compensatory changes were observed in the expression of SIK2 and SIK3 (Figure 2E). The protein level of SIK1 was also profoundly knocked down, and the phosphorylation of SIK1 induced by phanginin A was fully blocked (Figure 2F). More importantly, the inhibition of gluconeogenesis in response to phanginin A was fully restored by knockdown of SIK1, whereas the inhibitory effect of metformin on gluconeogenesis was not affected (Figure 2G). SIK1 knockdown also reversed the inhibition of gluconeogenesis by phanginin A under glucagon-stimulated conditions (Figure 2H). These results suggested that phanginin A inhibited hepatic gluconeogenesis in a SIK1-dependent manner.
Figure 2.
Phanginin A inhibits gluconeogenesis by activating SIK1 in primary mouse hepatocytes. (A–B) Primary hepatocytes were incubated with 5 μM phanginin A for different times (A) or with different doses of phanginin A for 2 h (B). SIK1 phosphorylation at Thr182 was detected. met = metformin. (C) Primary hepatocytes were pretreated with or without 0.5 μM of HG-9-91-01 for 30 min and cotreated with 5 μM of phanginin A or 500 μM of metformin for 2 h and then SIK1 phosphorylation was detected. (D) Primary hepatocytes were pretreated with or without 0.5 μM of HG-9-91-01 for 30 min and cotreated with 5 μM or 10 μM of phanginin A or 500 μM of metformin for 4 h and then gluconeogenesis was detected. (E–G) Primary hepatocytes were pretreated with SIK1 siRNA for 48 h followed with or without phanginin A treatment, and the mRNA expression of SIK1-3 (E), SIK1 protein, (F) and gluconeogenesis (G) were detected. (H) The effect of phanginin A on gluconeogenesis in SIK1-knockdown hepatocytes under 10 nM glucagon-induced conditions. All of the results are presented as the mean ± SEM (n = 5 for all of the groups, except for n = 4 in Figure H). ∗P < 0.05 and ∗∗P < 0.01 vs basal controls. ##P < 0.01 vs HG-9-91-01 controls or controls under glucagon-induced conditions.
3.3. Phanginin A inhibited gluconeogenesis by increasing PDE4 activity in primary mouse hepatocytes
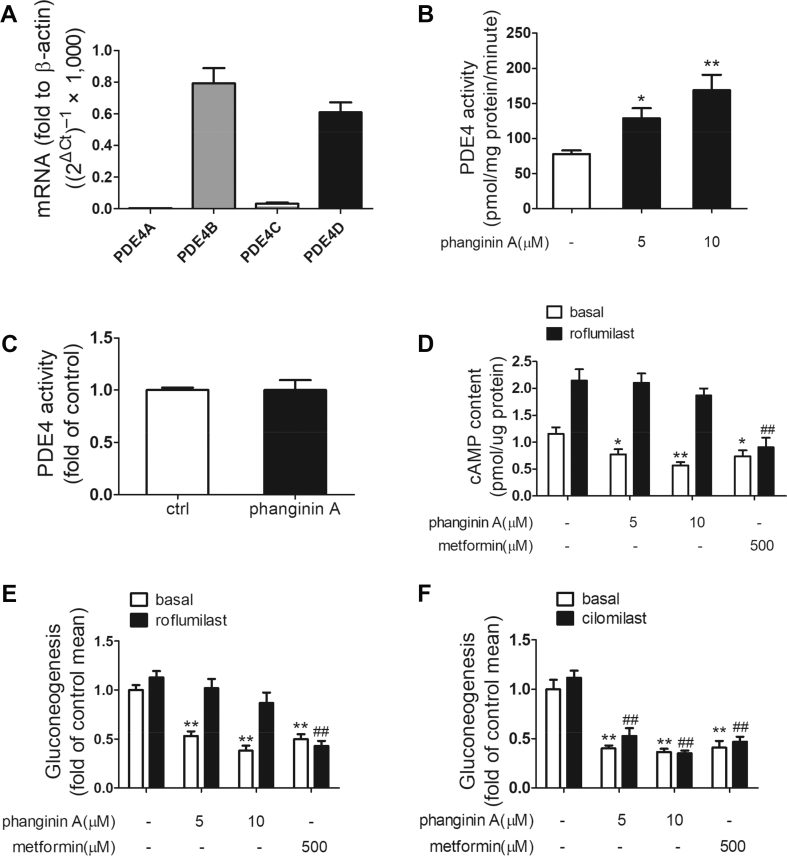
SIK1 has been reported to effectively increase the activity of PDE4D, which in turn promotes the degradation of cAMP in β cells [22]. We first detected the gene expression of the PDE4 isoforms in primary mouse hepatocytes. Real-time PCR revealed that PDE4D and PDE4B were predominant PDE4 isoforms in primary mouse hepatocytes (Figure 3A). We subsequently examined the effect of phanginin A on PDE4 activity. Incubation of primary mouse hepatocytes with 5 and 10 μM of phanginin A increased PDE4 activity of the cell lysate by 65% and 117%, respectively (Figure 3B). However, almost no change was detected when purified PDE enzyme was used to assess the direct impact of phanginin A on PDE4 activity (Figure 3C). In the presence of 1 μM of roflumilast, a selective PDE4 inhibitor, the reduction in cAMP levels induced by phanginin A was abolished, whereas no similar effect was detected in metformin-treated hepatocytes (Figure 3D). Moreover, gluconeogenesis inhibition induced by phanginin A was also reversed by roflumilast, but not PDE3 inhibitor cilomilast (Figure 3E,F). These data suggested that phanginin A suppression of hepatic gluconeogenesis may rely on the activation of PDE4.
Figure 3.
Phanginin A inhibited gluconeogenesis by increasing PDE4 activity in primary mouse hepatocytes. (A) Primary mouse hepatocytes were serum-starved for 4 h and the mRNA levels of PDE4 isoforms were detected (n = 5). (B) Primary mouse hepatocytes were treated with phanginin A for 3 h and then the PDE4 activity was detected (n = 5). (C) The direct effect of 10 μM of phanginin A on PDE4 enzymes (n = 5). (D–E) Primary mouse hepatocytes were pretreated with a PDE4 inhibitor (1 μM of roflumilast) for 30 min and cotreated with 5 or 10 μM of phanginin A and then the cAMP levels (n = 5) (D) and gluconeogenesis (n = 7) (E) were evaluated. (F) Primary mouse hepatocytes were pretreated with a PDE3 inhibitor (100 μM of cilomilast) for 30 min and cotreated with 5 or 10 μM of phanginin A and then gluconeogenesis was measured (n = 5). All of the results are presented as the mean ± SEM. ∗P < 0.05 and ∗∗P < 0.01 vs controls under basal conditions. ##P < 0.01 vs controls under roflumilast or cilomilast conditions.
3.4. SIK1 was indispensable in phanginin A-induced PDE4 activation in primary mouse hepatocytes
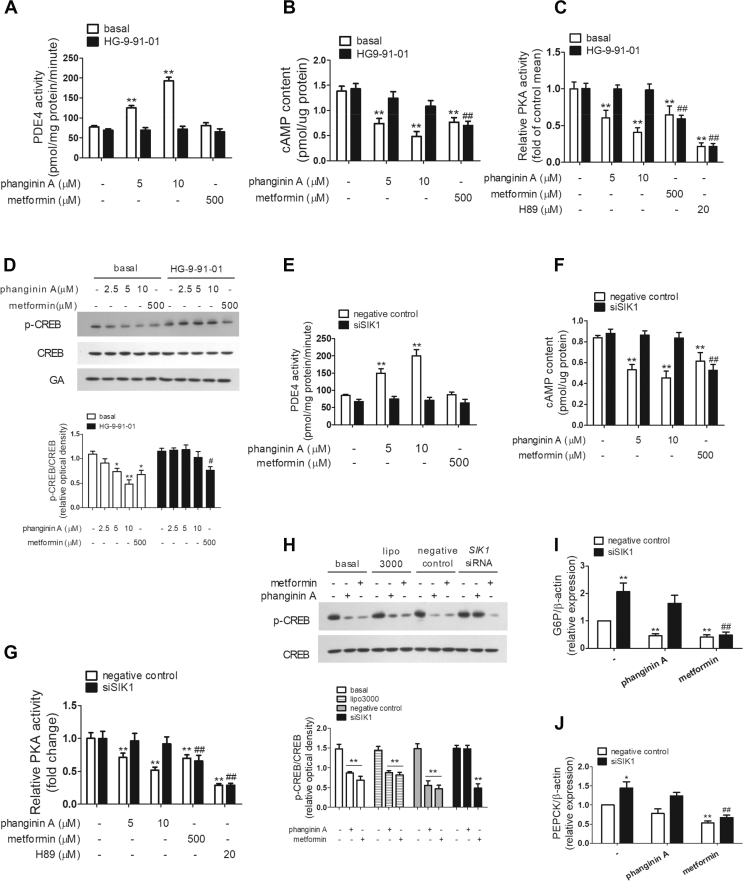
To address whether SIK1 is necessary in phanginin A-induced activation of PDE4, HG-9-91-01 and SIK1 RNAi were used to block SIK1 activation or knock down of SIK1 protein expression in primary mouse hepatocytes. As shown in Figure 4A, the activation of PDE4 by phanginin A was fully abolished in the presence of HG-9-91-01. HG-9-91-01 also abrogated the decrease in cAMP concentration and PKA activity caused by phanginin A (Figure 4B,C). Correspondingly, the reduction in CREB phosphorylation induced by phanginin A was thoroughly blocked in the presence of HG-9-91-01 (Figure 4D). HG-9-91-01 had no influence on the inhibition of metformin in the cAMP/PKA/CREB pathway. These results were further verified in primary mouse hepatocytes that underwent SIK1 siRNA interference. The phanginin A-induced activation of PDE4 was entirely blocked by SIK1 knockdown (Figure 4E). Consistently, the reduction in cAMP accumulation, PKA activity, and CREB phosphorylation caused by phanginin A were completely abolished by the downregulation of SIK1 (Figure 4F–H). Moreover, knockdown of SIK1 led to a 2.1- and 1.4-fold increase in G6p and PEPCK mRNA expression, and the downregulation of phanginin A in gluconeogenic genes was abrogated by knockdown of SIK1 (Figure 4I,J). These results implied that SIK1 was indispensable in phanginin A-induced PDE4 activation in primary mouse hepatocytes.
Figure 4.
Phanginin A increased PDE4 activity and inhibited the cAMP pathway by activating SIK1. (A–D) Primary mouse hepatocytes were pretreated with 0.5 μM of pan-SIK inhibitor HG-9-91-01 for 30 min and cotreated with 5 or 10 μM of phanginin A and then the PDE4 activity (A), cAMP concentration (B), PKA activity (C), and CREB phosphorylation (D) were detected. ∗P < 0.05 and ∗∗P < 0.01 vs controls under basal conditions. #P < 0.05 and ##P < 0.01 vs controls under HG-9-91-01 conditions. (E–J) Primary mouse hepatocytes were pretreated with SIK1 siRNA for 48 h and then treated with 5 μM or 10 μM of phanginin A and then the PDE4 activity (E), cAMP concentration (F), and PKA activity (G) were examined. Primary mouse hepatocytes were treated with 5 μM of phanginin A after SIK1 interference and then the CREB phosphorylation (H), mRNA expression of G6P (I), and PEPCK (J) were evaluated. ∗P < 0.05 and ∗∗P < 0.01 vs controls under negative control conditions. ##P < 0.01 vs controls under siSIK1 conditions. All of the results are presented as the mean ± SEM (n = 5).
3.5. The activation of SIK1 mediated by phanginin A was LKB1-dependent
LKB1 acts as the upstream kinase of the AMPKRK family, including SIKs. To confirm the molecular mechanism by which phanginin A activates SIK1, a LKB1-deletion experiment was conducted. As shown in Figure 5A, the protein level of LKB1 was significantly reduced after LKB1 interference, and the phanginin A-induced phosphorylation of SIK1 was blocked. The inhibition of phanginin A on gluconeogenesis was also reversed in LKB1-deficient primary mouse hepatocytes (Figure 5B). Considering that SIK2, SIK3, and AMPK are also downstream of LKB1, siRNA interference experiments were conducted to further examine their possible effects on the action of phanginin A. After siRNA interference, the expression of SIK2/SIK3 was significantly reduced and the expression of SIK1 was unchanged (Figure 5C), while phanginin A's inhibition of gluconeogenesis was not affected (Figure 5D). Phanginin A increased AMPK phosphorylation, but this effect could not be blocked by the downregulation of LKB1, which suggested that phanginin A activated AMPK independently of LKB1 (Figure 5E). Further studies showed that siRNA interference effectively reduced AMPK protein levels (Figure 5F), but failed to reverse the inhibitory effect of phanginin A on gluconeogenesis (Figure 5G). These results indicated that the activation of SIK1 and inhibition of gluconeogenesis by phanginin A was LKB1-dependent, while SIK2/SIK3/AMPK were unnecessary in the anti-gluconeogenesis effect of phanginin A.
Figure 5.
The activation of SIK1 mediated by phanginin A was LKB1-dependent. (A–B) Primary mouse hepatocytes were pretreated with LKB1 siRNA for 48 h and then treated with 5 μM of phanginin A and then the LKB1 protein and SIK1 phosphorylation levels (A) (n = 4) and gluconeogenesis (B) (n = 5) were examined. (C–D) Primary mouse hepatocytes were pretreated with SIK2 and SIK3 siRNA for 24 h and then treated with or without 5 μM of phanginin A, and the SIK mRNA (C) and gluconeogenesis (D) were tested (n = 5). (E) Primary mouse hepatocytes were pretreated with LKB1 siRNA for 48 h and then treated with 5 μM of phanginin A and then the LKB1 protein and AMPK phosphorylation levels were examined (n = 4). (F–G) Primary mouse hepatocytes were pretreated with AMPK siRNA for 48 h and then treated with or without 5 μM of phanginin A and the AMPK protein level (F) (n = 3) and gluconeogenesis (G) (n = 4) were studied. All of the results are presented as the mean ± SEM. ∗∗P < 0.01 vs individual controls.
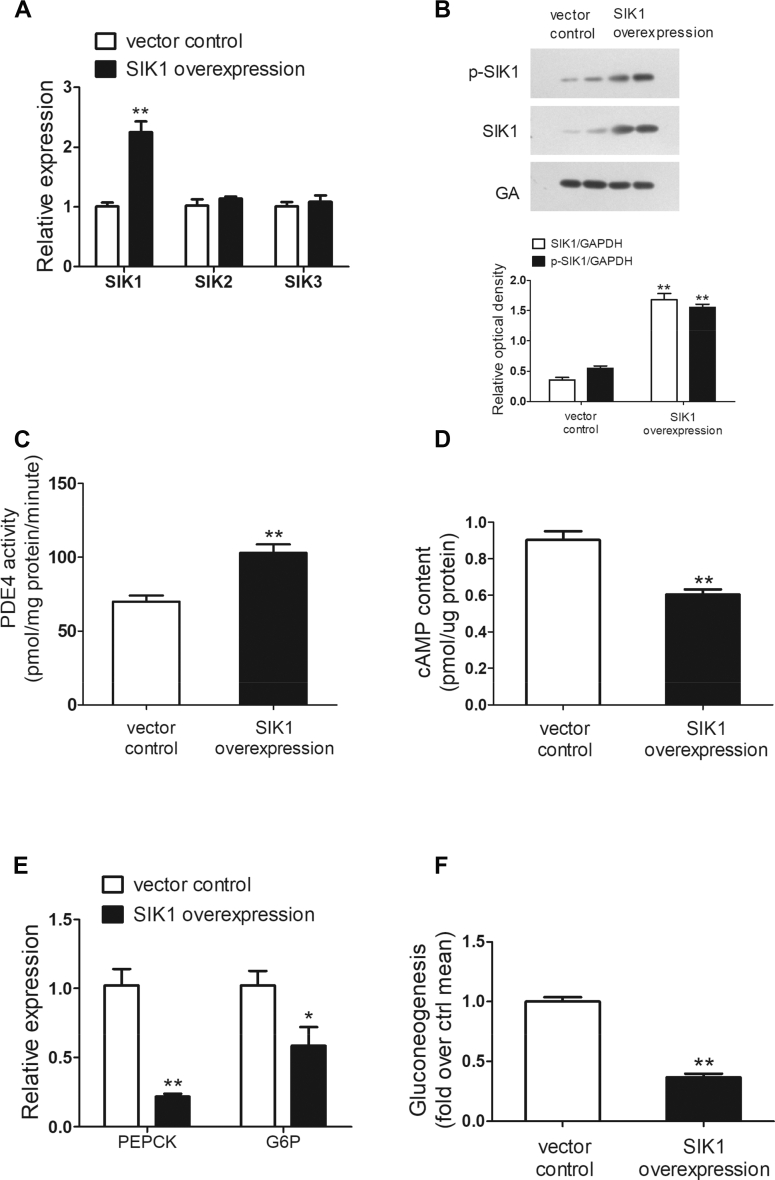
3.6. SIK1 overexpression suppressed gluconeogenesis by activating PDE4 and reducing cAMP levels in primary mouse hepatocytes
To better understand the role of SIK1 in the regulation of hepatic gluconeogenesis, SIK1 overexpression was conducted by transient plasmid transfections in primary mouse hepatocytes. As shown in Figure 6A, the mRNA expression of SIK1 effectively increased in comparison with the vector control, and no compensatory changes were detected in the expression of SIK2 and SIK3. More importantly, the SIK1 protein levels were dramatically enhanced by 3.7-fold, and the phosphorylation of SIK1 was significantly increased by 1.8-fold (Figure 6B). SIK1 overexpression in primary mouse hepatocytes profoundly increased PDE4 activity by 47% and subsequently reduced the intracellular cAMP concentration by 33% (Figure 6C,D). In addition, a profound reduction in mRNA levels of PEPCK and G6P was observed in SIK1-overexpressed hepatocytes (Figure 6E). Furthermore, SIK1 overexpression in primary mouse hepatocytes resulted in a significant reduction in glucose production, which further confirmed the repressive role of SIK1 on hepatic gluconeogenesis (Figure 6F). Taken together, the results suggested that SIK1 could activate PDE4 and thus reduce intracellular cAMP accumulation and inhibit gluconeogenesis in primary mouse hepatocytes.
Figure 6.
SIK1 overexpression inhibited gluconeogenesis and increased PDE4 activity in primary mouse hepatocytes. Primary mouse hepatocytes were overexpressed with SIK1 via transient plasmid transfections for 24 h and then the mRNA expression of SIKs (A) and Western blotting analysis of SIK1 (B) were conducted, and the PDE4 activity (C), cAMP levels (D), mRNA expression of gluconeogenic genes (E), and gluconeogenesis (F) were examined. All of the results are presented as the mean ± SEM (n = 5). ∗P < 0.05 and ∗∗P < 0.01 vs the vector control group.
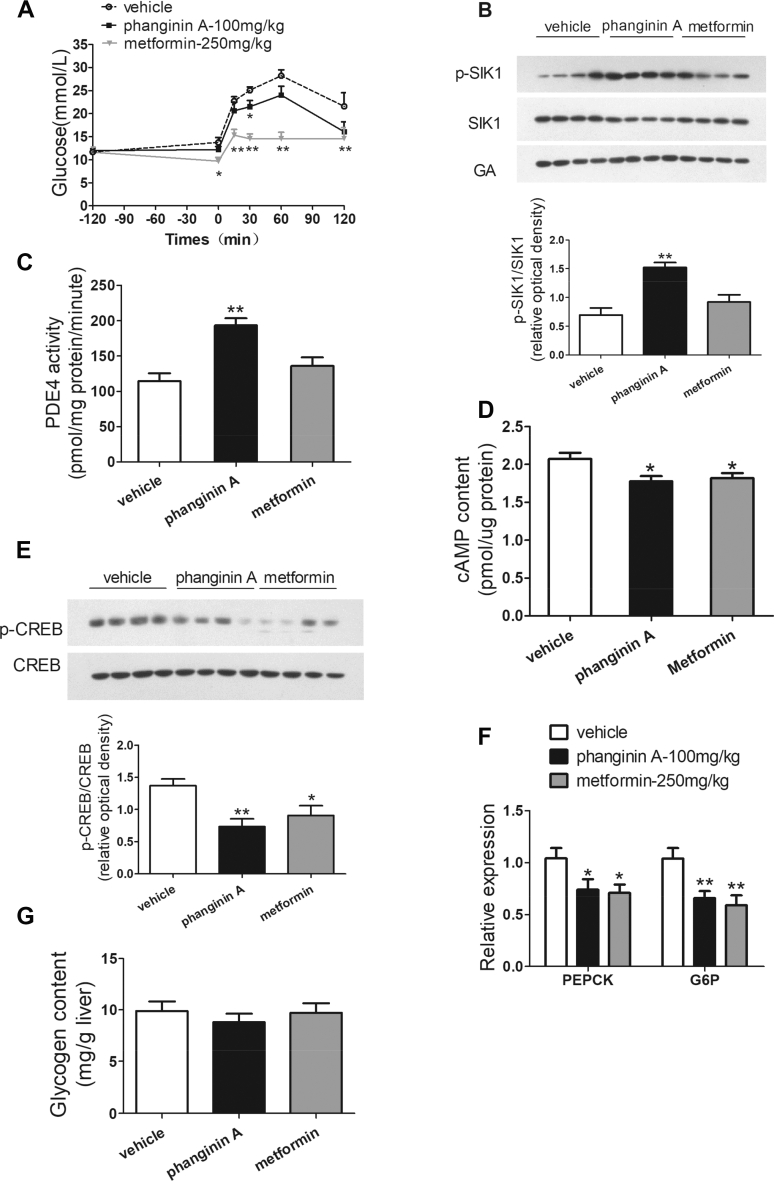
3.7. Acute treatment of phanginin A attenuated gluconeogenesis by inhibiting the cAMP signaling pathway by activating SIK1 and PDE4 in ob/ob mice
A pyruvate tolerance test was conducted to evaluate the effect of phanginin A on gluconeogenesis in vivo, and the effect of the acute administration of phanginin A on hepatic SIK1 phosphorylation and PDE4 activation was investigated in type 2 diabetic ob/ob mice. The pyruvate tolerance test showed that a single oral dose of 100 mg/kg of phanginin A could significantly reduce the blood glucose in the ob/ob mice (Figure 7A). This indicated that phanginin A has anti-gluconeogenesis ability in vivo. Furthermore, a single oral administration of 100 of mg/kg phanginin A profoundly increased the phosphorylation of SIK1 in the liver by 119%, whereas no change was observed under metformin treatment (Figure 7B). PDE4 activity in the liver was elevated by 74% after the administration of phanginin A (Figure 7C). Correspondingly, phanginin A treatment caused a modest but significant decrease in the cAMP concentration along with a 46% decrease in the CREB phosphorylation level in the liver (Figure 7D,E). The hepatic gluconeogenic gene expression was detected, and the mRNA levels of PEPCK and G6P were both significantly attenuated by phanginin A (Figure 7F). In addition, the glycogen content in the liver was measured, and no significant changes could be found after acute treatment with phanginin A in ob/ob mice (Figure 7G). Therefore, the in vivo data are highly concordant with the in vitro outcomes to further support that phanginin A-induced hepatic SIK1 activation leads to the inhibition of gluconeogenesis by increasing PDE4 activity and inhibiting the cAMP signaling pathway.
Figure 7.
Acute treatment with phanginin A attenuated gluconeogenesis by inhibiting the cAMP signaling pathway by activating SIK1 and PDE4 in ob/ob mice. Mice were treated as described in Methods. (A) Blood glucose levels in the pyruvate tolerance test of ob/ob mice were measured. (B) Hepatic SIK1 phosphorylation in ob/ob mice was detected. (C) PDE4 activity and (D) cAMP levels in the liver of ob/ob mice were detected. (E) CREB phosphorylation in the liver of ob/ob mice was analyzed by Western blotting. (F) Hepatic gluconeogenic gene expression in ob/ob mice were evaluated. (G) Glycogen content was measured in the liver of ob/ob mice. All of the results are presented as the mean ± SEM (n = 8 for all of the groups, except for n = 6–7 in Figure A). ∗P < 0.05 and ∗∗P < 0.01 vs the vehicle group.
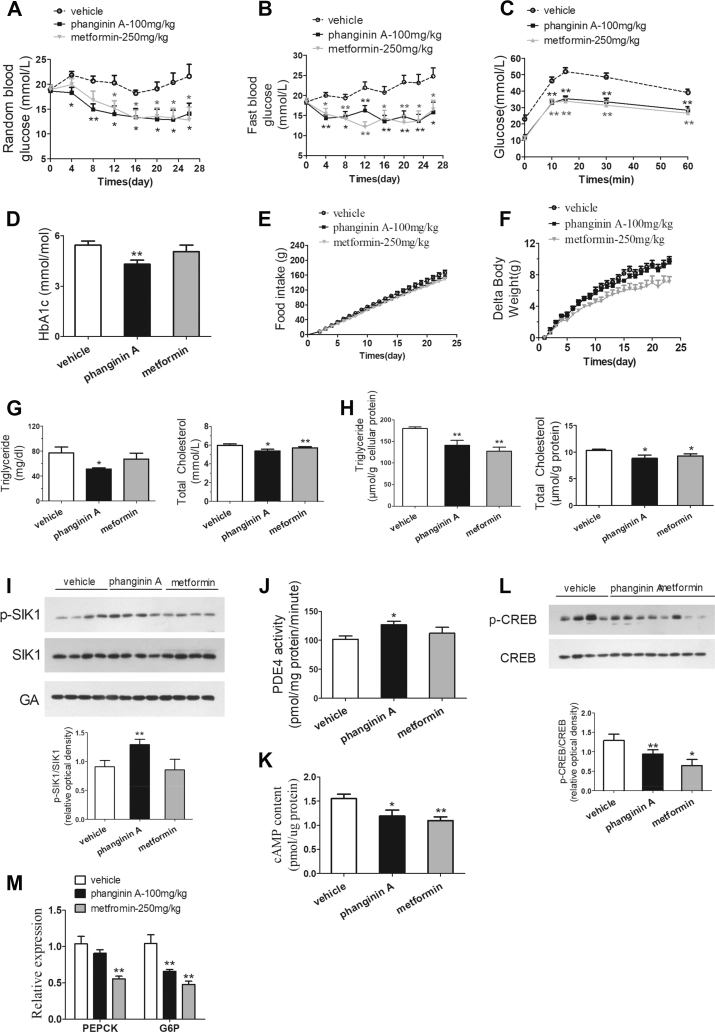
3.8. Chronic treatment of phanginin A ameliorated metabolic disorders in ob/ob mice
The chronic effect of phanginin A (100 mg/kg, orally) was assessed in ob/ob mice along with metformin (250 mg/kg, orally) as a positive control. The random and fast blood glucose levels were significantly reduced in the mice treated with phanginin A. Compared with the vehicle control group, the mice that received phanginin A for 26 days had an average reduction rate of 29% and 32% in random and fast blood glucose, while those in the mice treated with metformin were reduced by 23% and 30%, respectively (Figure 8A,B). After 23 days of treatment of phanginin A, the mice exhibited a marked improvement in glucose tolerance comparable to that of metformin (Figure 8C). The HbA1c level was also significantly reduced by 20% after treatment with phanginin A (Figure 8D). Chronic treatment with phanginin A showed no effect on food intake and body weight (Figure 8E,F). Interestingly, the mice treated with phanginin A had significantly lower levels of triglyceride and total cholesterol in the serum (Figure 8G) and liver (Figure 8H). Moreover, chronic administration of phanginin A significantly increased SIK1 phosphorylation (Figure 8I) and PDE4 activity (Figure 8J), accompanied by decreased cAMP levels and attenuated CREB phosphorylation in the liver (Figure 8K and L). Phanginin A decreased hepatic G6P mRNA expression but showed no obvious effect on PEPCK expression (Figure 8M).
Figure 8.
Chronic treatment of phanginin A improved metabolic disorders in ob/ob mice. Male ob/ob mice were treated with phanginin A (100 mg/kg, once daily, p. o.), metformin (250 mg/kg, once daily, p. o.), or vehicle for 26 days. (A–B) Random (A) and fasting blood glucose levels (B) were detected on days 4, 8, 12, 16, 20, 23, and 26. (C) Oral glucose tolerance tests were conducted on day 23. (D) HbA1c was determined on day 26. (E–F) Food intake accumulation (E) and body weight (F) were regularly measured during treatment. (G–H) Triglyceride and total cholesterol in the serum (G) and liver (H) were detected. (I) SIK1 phosphorylation in the liver was analyzed by Western blotting and quantified as the relative optical density. (J) Hepatic PDE4 activity and (K) cAMP concentration were examined after treatment. (L) CREB phosphorylation in the liver was analyzed by Western blotting. (M) Hepatic gluconeogenesis gene expression in ob/ob mice were evaluated. All of the results are presented as the mean ± SEM (n = 8). ∗P < 0.05 and ∗∗P < 0.01 vs the vehicle group.
4. Discussion
As a member of the SIK family, SIK1 was cloned and identified as a serine/threonine protein kinase from the adrenal glands of rats fed high salt diets [30] and acted as an important component in the regulation of adrenocortical function. SIK1 was recently reported to be involved in many physiological processes [21,22,31,32], including gluconeogenesis. However, the role and underlying mechanism of SIK1 in the regulation of hepatic gluconeogenesis has not been fully clarified. In this study, we found that phanginin A, a natural product extracted from C. sappan Linn [24], significantly inhibited gluconeogenesis in primary mouse hepatocytes. Further studies showed that phanginin A could promote SIK1 phosphorylation, and this effect was strongly correlated with its inhibition of gluconeogenesis. Although phanginin A indirectly activated SIK1 in an LKB1-dependent manner, this compound could still be used as a molecular probe to investigate and clarify the underlying mechanism by which SIK1 inhibits gluconeogenesis.
As a natural compound, phanginin A was isolated and identified in 2008, but only its cytotoxicity and anti-inflammatory activity have been studied to date [33,34]. Herein we reported a breakthrough finding that phanginin A showed outstanding efficacy in inhibiting gluconeogenesis in primary mouse hepatocytes. The cAMP/PKA/CREB pathway is involved in the regulation of key gluconeogenic gene translation under glucagon stimulation during fasting [35,36]. Phanginin A exerted a pronounced inhibition of intracellular cAMP concentration and PKA activity in primary mouse hepatocytes, accompanied by significantly reduced CREB phosphorylation, which led to the suppression of the expression of PEPCK and G6P, indicating that phanginin A could inhibit the expression of key gluconeogenic genes by inhibiting the cAMP/PKA/CREB pathway.
SIKs have recently attracted increasing attention due to their gluconeogenesis regulating potential [37], and the first clue is based on the inhibition of CREB transcriptional activity by SIK1 through suppressing CRTC2 [15,20]. Phosphorylation of SIK1 at Thr182 sites has been suggested to represent the activation of its kinase activity [28,29]. In this study, we demonstrated that phanginin A markedly promoted phospho-SIK1 (Thr182) in primary mouse hepatocytes, indicating the activation of SIK1. HG-9-91-01 is a pan-SIK inhibitor that showed the best selectivity for SIK1, with an IC50 of 0.92, 6.6 and 9.6 nM for SIK1, SIK2, and SIK3, respectively [38]. Phanginin A-reduced gluconeogenesis was completely abolished under the treatment of 0.5 μM of HG-9-91-01, and SIK1 was identified as the major isoform responsible for the inhibition of phanginin A on gluconeogenesis via a SIK1 RNAi experiment. Moreover, the inhibition of phanginin A in glucagon-stimulated gluconeogenesis was also fully blocked by the downregulation of SIK1, indicating the dependence on SIK1. To the best of our knowledge, this is the first report demonstrating that small molecule compounds could activate hepatic SIK1 and exert anti-gluconeogenesis effects.
Although CRTC2 is a widely studied substrate of SIK1 [39], it was recently reported that PDE4D was identified as a new direct substrate for SIK1 in β cells, through which SIK1 terminated cAMP signaling by promoting the degradation of cAMP [22]. Earlier studies suggested that PDE4 was the main PDE isoenzyme responsible for cAMP hydrolysis in the liver, accounting for approximately 80% of total PDE [40], and our data showed that both PDE4D and PDE4B isoforms were highly expressed in the liver. Phanginin A profoundly enhanced PDE4 activity in primary mouse hepatocytes, but there is a limitation in our study that the exact contribution of PDE4D and PDE4B to the increased PDE4 activity was unknown. The inhibition of phanginin A on gluconeogenesis was abrogated following treatment with PDE4 inhibitor roflumilast, but not PDE3 inhibitor cilomilast, further implying that phanginin A suppressed gluconeogenesis in a PDE4-dependent manner. Phanginin A-induced PDE4 activation and cAMP/PKA/CREB pathway inhibition was fully blocked in the presence of HG-9-91-01 or siRNA-mediated downregulation of SIK1, indicating that SIK1 is indispensable in phanginin A-induced PDE4 activation. The downregulation of SIK1 increased the gene expression of PEPCK and G6P, but had no effect on cAMP levels and CREB phosphorylation. This might be due to the decrease in CRTC2 phosphorylation upon SIK1 knockdown, since SIK1 could increase CRTC2 phosphorylation to detach from CREB to inhibit transcription of gluconeogenic genes [20,21]. Taking these results together, although we did not know whether the PDE4D isoform is the specific substrate of SIK1 in hepatocytes as in β cells, we believe that phanginin A can increase PDE4 activity, inhibit the cAMP/PKA/CREB pathway, and further suppress gluconeogenesis by activating SIK1 in hepatocytes.
LKB1, a tumor suppressor kinase, has been reported to be upstream of SIKs. LKB1 could enhance the catalytic activity of SIKs by phosphorylating on their highly conserved threonine residue (the Thr182 of SIK1, Thr175 of SIK2, and Thr279 of SIK3) [38]. We found that both the activation of SIK1 and inhibition of gluconeogenesis by phanginin A were dependent on LKB1. Since the other downstream kinases of LKB1, such as SIK2, SIK3, and AMPK, were also associated with gluconeogenesis [16,19,41], siRNA interference was used to investigate whether they were involved in the gluconeogenesis inhibition induced by phanginin A. When SIK2 or SIK3 were individually used for interference, a compensatory increase in the gene expression of another subtype was observed in our experiments, probably due to the functional redundancy between SIK2 and SIK3 proteins in certain processes. Hence, SIK2/SIK3 double interference was used, and the results showed that SIK2/SIK3 double knockdown had nearly no influence on the anti-gluconeogenesis of phanginin A. Phanginin A increased AMPK phosphorylation, but this effect could not be blocked by the downregulation of LKB1, suggesting that phanginin A activated AMPK independent of LKB1. Moreover, phanginin A-inhibited gluconeogenesis could not be reversed by the downregulation of AMPK, indicating that AMPK was not involved in the inhibition of gluconeogenesis by phanginin A. Therefore, the inhibition of gluconeogenesis by phanginin A was mainly dependent on the enhancement of SIK1 phosphorylation through LKB1, while SIK2/SIK3/AMPK were presumably insufficient and unnecessary. However, a limitation in our research is that the precise way by which phanginin A regulates LKB1 and then affects SIK1 activity has not been thoroughly studied, which is worthy of future investigation.
As a natural compound, the non-specific effect of phanginin A other than the activation of SIK1 could not be excluded. Moreover, phanginin A does not directly activate SIK1. Thus, it is necessary to conduct an overexpression study to identify the PDE4-cAMP axis as the downstream target of SIK1. As expected, the overexpression of SIK1 in primary hepatocytes significantly increased PDE4 activity, reduced intracellular cAMP accumulation, and downregulated the gene expression of PEPCK and G6P. More importantly, significant suppression of gluconeogenesis was observed, which revealed the inhibitory effect of SIK1 on gluconeogenesis. Of note, the PDE4 inhibitor roflumilast could partially reverse the inhibitory effect of SIK1 on gluconeogenesis. Although we could not fully exclude that CRTC2 was also probably involved in the regulation of gluconeogenesis by SIK1, increased PDE4 activity and decreased cAMP levels were observed upon SIK1 overexpression, indicating that PDE4 may have a critical effect on SIK1-mediated inhibition of gluconeogenesis, which suggested a new action mode of SIK1 in hepatocytes.
Gluconeogenesis is one of the most important factors in the maintenance of whole-body blood glucose. Single oral administration of phanginin A attenuated gluconeogenesis and showed no effect on the hepatic glycogen content in ob/ob mice. Both acute and chronic oral administration of phanginin A increased SIK1 phosphorylation and PDE4 activity and suppressed the cAMP pathway and gluconeogenesis gene expression in the liver of ob/ob mice, indicating that phanginin A reduced gluconeogenesis by activation of SIK1 in vivo. Although the liver plays a key role in whole-body glucose homeostasis, we could not exclude that other peripheral tissues might also contribute to improved glucose homeostasis after long-term treatment with phanginin A. Phanginin A showed a strong improvement in glucose tolerance of ob/ob mice, which could not be fully explained only by reduced hepatic gluconeogenesis. Further research should be conducted in the future to investigate the regulation of phanginin A and the activation of SIK1 on the metabolic functions of other peripheral tissues such as skeletal muscle and adipose tissue. Diabetic patients often suffer from dyslipidemia, and improving lipid metabolism is also helpful for ameliorating glucose homeostasis [42]. Apart from the glucose-lowering effects, chronic treatment with phanginin A improved lipid metabolism disorders in ob/ob mice. It was reported that activation of SIK1 modulated hepatic lipid metabolism by suppressing SREBP-1c [28,43]; further research is necessary to investigate the relationship between lipid regulation by phanginin A and its activation of SIK1. Therefore, although we could not rule out the potential regulation of phanginin A on other metabolic tissues (such as the pancreas, skeletal muscle, adipose tissue, and even the immune system), we could still conclude that the suppression of gluconeogenesis caused by the activation of hepatic SIK1 might contribute to the improvement of phanginin A in whole-body glucose homeostasis in ob/ob mice.
5. Conclusion
In conclusion, this is the first report that phanginin A inhibited gluconeogenesis by activation of SIK1 in primary mouse hepatocytes. We discovered that the activation of SIK1 by phanginin A or overexpression of SIK1 in hepatocytes could increase PDE4 activity, inhibit the cAMP/PKA/CREB pathway, and thereby inhibit gluconeogenesis. Acute treatment with phanginin A suppressed in vivo gluconeogenesis accompanied by the activation of SIK1 and PDE4 and reduction in cAMP accumulation in the liver. Chronic administration of phanginin A reduced blood glucose levels and improved glucose tolerance and dyslipidemia in ob/ob mice. These findings demonstrated a novel mechanism of activated SIK1 by phanginin A in suppressing hepatic gluconeogenesis by activating PDE4 and suppressing the cAMP signaling pathway and highlighted the potential value of phanginin A as a leading compound for the treatment of type 2 diabetes.
Author contributions
YL, QSZ, and SLH designed the study. SWL, YF, XDW, and YS conducted the research. SWL, SLH, XDW, and YL analyzed and interpreted the data. SWL, SLH, and YL wrote the paper. All of the authors gave their final approval of the version to be published and wrote and edited the manuscript.
Acknowledgments
This study was financially supported by grants from the National Science and Technology Major Project-Key New Drug Creation and Manufacturing Program, China (2018ZX09711002-006-012), State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences (grant no. SIMM1903KF-08), and the Science and Technology Commission of Shanghai Municipality (19ZR1467600). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101045.
Contributor Information
Qin-shi Zhao, Email: qinshizhao@mail.kib.ac.cn.
Ying Leng, Email: yleng@simm.ac.cn.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rines A.K., Sharabi K., Tavares C.D., Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nature Reviews Drug Discovery. 2016;15(11):786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H.S., Kang G., Kim J.S., Choi B.H., Koo S.H. Regulation of glucose metabolism from a liver-centric perspective. Experimental & Molecular Medicine. 2016;48:e218. doi: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller R.A., Birnbaum M.J. Glucagon: acute actions on hepatic metabolism. Diabetologia. 2016;59(7):1376–1381. doi: 10.1007/s00125-016-3955-y. [DOI] [PubMed] [Google Scholar]
- 5.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nature Reviews Endocrinology. 2017;13(10):572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh K.J., Han H.S., Kim M.J., Koo S.H. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Report. 2013;46(12):567–574. doi: 10.5483/BMBRep.2013.46.12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anyamaneeratch K., Rojvirat P., Sukjoi W., Jitrapakdee S. Insights into transcriptional regulation of hepatic glucose production. International Review Cell and Molecular Biology. 2015;318:203–253. doi: 10.1016/bs.ircmb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Pernicova I., Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature Reviews Endocrinology. 2014;10(3):143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J. Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madiraju A.K., Qiu Y., Perry R.J., Rahimi Y., Zhang X.M., Zhang D. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nature Medicine. 2018;24(9):1384–1394. doi: 10.1038/s41591-018-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.M., Braddock D.T., Albright R.A. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoh Y., Takemori H., Horike N., Doi J., Muraoka M., Min L. Salt-inducible kinase (SIK) isoforms: their involvement in steroidogenesis and adipogenesis. Molecular and Cellular Endocrinology. 2004;217(1–2):109–112. doi: 10.1016/j.mce.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Takemori H., Kajimura J., Okamoto M. TORC-SIK cascade regulates CREB activity through the basic leucine zipper domain. FEBS Journal. 2007;274(13):3202–3209. doi: 10.1111/j.1742-4658.2007.05889.x. [DOI] [PubMed] [Google Scholar]
- 15.Katoh Y., Takemori H., Lin X.Z., Tamura M., Muraoka M., Satoh T. Silencing the constitutive active transcription factor CREB by the LKB1-SIK signaling cascade. FEBS Journal. 2006;273(12):2730–2748. doi: 10.1111/j.1742-4658.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- 16.Patel K., Foretz M., Marion A., Campbell D.G., Gourlay R., Boudaba N. The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nature Communications. 2014;5:4535. doi: 10.1038/ncomms5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dentin R., Liu Y., Koo S.H., Hedrick S., Vargas T., Heredia J. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449(7160):366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 18.Uebi T., Itoh Y., Hatano O., Kumagai A., Sanosaka M., Sasaki T. Involvement of SIK3 in glucose and lipid homeostasis in mice. PloS One. 2012;7(5) doi: 10.1371/journal.pone.0037803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y., Sanosaka M., Fuchino H., Yahara Y., Kumagai A., Takemoto D. Salt-inducible kinase 3 signaling is important for the gluconeogenic programs in mouse hepatocytes. Journal of Biological Chemistry. 2015;290(29):17879–17893. doi: 10.1074/jbc.M115.640821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo S.H., Flechner L., Qi L., Zhang X., Screaton R.A., Jeffries S. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 21.Nixon M., Stewart-Fitzgibbon R., Fu J., Akhmedov D., Rajendran K., Mendoza-Rodriguez M.G. Skeletal muscle salt inducible kinase 1 promotes insulin resistance in obesity. Molecular Metabolism. 2016;5(1):34–46. doi: 10.1016/j.molmet.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M.J., Park S.K., Lee J.H., Jung C.Y., Sung D.J., Park J.H. Salt-inducible kinase 1 terminates cAMP signaling by an evolutionarily conserved negative-feedback loop in beta cells. Diabetes. 2015;64(9):3189–3202. doi: 10.2337/db14-1240. [DOI] [PubMed] [Google Scholar]
- 23.Aikin R., Rosenberg L. A dash of salt-inducible kinase 1 keeps insulin levels in check. Diabetes. 2015;64(9):3061–3062. doi: 10.2337/db15-0692. [DOI] [PubMed] [Google Scholar]
- 24.Yodsaoue O., Cheenpracha S., Karalai C., Ponglimanont C., Chantrapromma S., Fun H.K. Phanginin A-K, diterpenoids from the seeds of Caesalpinia sappan Linn. Phytochemistry. 2008;69(5):1242–1249. doi: 10.1016/j.phytochem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Xie W., Ye Y., Feng Y., Xu T., Huang S., Shen J. Linderane suppresses hepatic gluconeogenesis by inhibiting the cAMP/PKA/CREB pathway through indirect activation of PDE 3 via ERK/STAT3. Frontiers in Pharmacology. 2018;9:476. doi: 10.3389/fphar.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X., Feng Y., Liu X., Zhao X.F., Yu J.H., Yang Y.S. Effect of a novel non-thiazolidinedione peroxisome proliferator-activated receptor alpha/gamma agonist on glucose uptake. Diabetologia. 2007;50(5):1048–1057. doi: 10.1007/s00125-007-0622-3. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh M., Garcia-Castillo D., Aguirre V., Golshani R., Atkins C.M., Bramlett H.M. Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia. 2012;60(12):1839–1859. doi: 10.1002/glia.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song D., Yin L., Wang C., Wen X. Adenovirus-mediated expression of SIK1 improves hepatic glucose and lipid metabolism in type 2 diabetes mellitus rats. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J., Hu X., Yang Z., Takemori H., Li Y., Zheng H. Salt-inducible kinase 1 is involved in high glucose-induced mesangial cell proliferation mediated by the ALK5 signaling pathway. International Journal of Molecular Medicine. 2013;32(1):151–157. doi: 10.3892/ijmm.2013.1377. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z., Takemori H., Halder S.K., Nonaka Y., Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Letters. 1999;453(1–2):135–139. doi: 10.1016/s0014-5793(99)00708-5. [DOI] [PubMed] [Google Scholar]
- 31.Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G.D. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nature Medicine. 2007;13(5):597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 32.Stewart R., Akhmedov D., Robb C., Leiter C., Berdeaux R. Regulation of SIK1 abundance and stability is critical for myogenesis. Proceedings of the National Academy of Sciences of the U S A. 2013;110(1):117–122. doi: 10.1073/pnas.1212676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao H., Zhang L.-L., Liu Q.-Y., Feng L., Ye Y., Lu J.-J. Cytotoxic and pro-apoptotic effects of cassane diterpenoids from the seeds of Caesalpinia sappan in cancer cells. Molecules. 2016;21(6):791. doi: 10.3390/molecules21060791. /791-791/714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu N.-L., Xu X.-D., Hu M.-G., Sun Z.-H., Wu T.-Y., Yuan J.-Q. Bioactive cassane diterpenoids from the seeds of Caesalpinia sappan. Phytochemical Letters. 2017;22:113–116. [Google Scholar]
- 35.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 36.Ali S., Drucker D.J. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2009;296(3):E415–E421. doi: 10.1152/ajpendo.90887.2008. [DOI] [PubMed] [Google Scholar]
- 37.Wein M.N., Foretz M., Fisher D.E., Xavier R.J., Kronenberg H.M. Salt-inducible kinases: physiology, regulation by cAMP, and therapeutic potential. Trends in Endocrinology and Metabolism. 2018;29(10):723–735. doi: 10.1016/j.tem.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakamoto K., Bultot L., Goransson O. The salt-inducible kinases: emerging metabolic regulators. Trends in Endocrinology and Metabolism. 2018;29(12):827–840. doi: 10.1016/j.tem.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Altarejos J.Y., Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature Reviews Molecular Cell Biology. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermsdorf T., Richter W., Dettmer D. Effects of dexamethosone and glucagon after long-term exposure on cyclic AMP phosphodiesterase 4 in cultured rat hepatocytes. Cellular Signalling. 1999;11(9):685–690. doi: 10.1016/s0898-6568(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B.B., Zhou G., Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metabolism. 2009;9(5):407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Wang D.Q., Portincasa P., Neuschwander-Tetri B.A. Steatosis in the liver. Comparative Physiology. 2013;3(4):1493–1532. doi: 10.1002/cphy.c130001. [DOI] [PubMed] [Google Scholar]
- 43.Yoon Y.S., Seo W.Y., Lee M.W., Kim S.T., Koo S.H. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation. Journal of Biological Chemistry. 2009;284(16):10446–10452. doi: 10.1074/jbc.M900096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.