Abstract
Until recently, data supporting the tissue-resident status of mesenchymal stromal cells (MSC) has been ambiguous since their discovery in the 1950–60s. These progenitor cells were first discovered as bone marrow-derived adult multipotent cells and believed to migrate to sites of injury, opposing the notion that they are residents of all tissue types. In recent years, however, it has been demonstrated that MSC can be found in all tissues and MSC from different tissues represent distinct populations with differential protein expression unique to each tissue type. Importantly, these cells are efficient mediators of tissue repair, regeneration, and prove to be targets for therapeutics, demonstrated by clinical trials (phase 1–4) for MSC-derived therapies for diseases like graft-versus-host-disease, multiple sclerosis, rheumatoid arthritis, and Crohn’s disease. The tissue-resident status of MSC found in the lung is a key feature of their importance in the context of disease and injuries of the respiratory system, since these cells could be instrumental to providing more specific and targeted therapies. Currently, bone marrow-derived MSC have been established in the treatment of disease, including diseases of the lung. However, with lung-resident MSC representing a unique population with a different phenotypic and gene expression pattern than MSC derived from other tissues, their role in remediating lung inflammation and injury could provide enhanced efficacy over bone marrow-derived MSC methods. Through this review, lung-resident MSC will be characterized, using previously published data, by surface markers, gene expression patterns, and compared with bone-marrow MSC to highlight similarities and, importantly, differences in these cell types.
Keywords: lung inflammation, lung-resident MSC, mesenchymal stem cells, mesenchymal stromal cells, tissue repair
INTRODUCTION
Defining Mesenchymal Stromal Cells
Identifying the characteristics and molecular mechanisms of adult cell lineages with multipotent differentiation potential provides a unique and promising opportunity in tissue regeneration and the treatment of many diseases. The tissue parenchyma is maintained by many supportive cellular residents of the surrounding stroma, and failure of these supportive cells results in disease and tissue dysfunction (34, 35, 119, 146, 148). Stromal residents of particular interest in wound repair are mesenchymal stromal cells (MSC). In the mid-20th century, Friedenstein et al. (54) first identified a cell in bone-marrow extracts, a tissue with high turnover and regenerative capacity, that has multipotent and self-renewing potential. Culturing this population of bone marrow-derived multipotent cells yields a clonogenic and plastic adherent phenotype with a fibroblastic morphology, coined “mesenchymal stem cells” by Caplan (24) in 1991. These colonies derive from a clonogenic cell known as colony-forming unit (CFU)-fibroblast, which additionally outlines a defining assay of this cell type, the colony-forming assay. In the literature, MSC are referred to as mesenchymal stem cells, “mesenchymal stromal cells,” “multipotent stromal cells,” and “mesenchymal progenitor cells” but are most widely identified as “MSC.” It should be noted that the term mesenchymal stem cells typically refers to the in vitro proliferation of these cells types, particularly when the population is CD271pos/CD140aneg, meeting stringent criteria for stem cell classification, which are considered mesenchymal stromal cells in vivo, as limited data on the physiologic relevance of their differentiation in vivo is currently available (55, 146). There has been a recent call to rename MSC as “Medicinal Signaling Cells” to more clearly define their functionality in therapeutics, thus demonstrating the quickly evolving nature of this field (25, 42). Like other stem cells, MSC undergo self-renewal while maintaining their multipotency and have the capacity to differentiate into cell types of mesodermal lineage such as chondrocytes, osteocytes, and adipocytes. More recently, in vitro studies have suggested that MSC may also transdifferentiate into cell types of ectodermal and endodermal lineages, such as through mesenchymal to epithelial transition (MET); however, this has yet to be validated in vivo (33, 112). These outlined features of MSC contribute to their role in tissue repair and wound healing and serve as the rationale for the ongoing characterization and potential application of MSC and their products in therapeutics.
Phenotype
The conflict in nomenclature for MSC throughout the literature galvanized the release of a position statement from the International Society for Cellular Therapy (ISCT) in defining the criteria for MSC classification, listed as follows: cells must be enriched for CD73, CD90, and CD105 surface proteins while being negative for the hematopoietic markers (CD11b, CD34, CD45) as well as CD14, CD19, CD79a, and human leukocyte antigen-1 (HLA)-DR, and they must be plastic-adherent and successfully differentiate into adipocytes, chondrocytes, and osteocytes in vitro (46). The lack of myosin heavy chain (MHC) markers contributes to their ability to seemingly “evade” immune detection, an important phenotypic feature of MSC that contributes to their therapeutic potential. In fact, there are promising data on the success of MSC allografts. However, these data are not universally obtained, since some reports of poor engraftment are noted, and current studies on MSC allograft rejection suggest the term “immunoprivileged” more accurately describes this phenomenon (6, 146).
Role in Tissue Regeneration and Maintenance
MSC define the stromal microenvironment and modulate tissue regeneration via the following functions: differentiation into essential cell types; extracellular matrix deposition; and paracrine actions targeting surrounding epithelial, endothelial, and immune cells (86, 146). MSC are capable of homing to injury sites where they integrate and elicit modulatory effects on the surrounding cells and direct efforts toward tissue repair (79). Specifically, MSC sense and respond to stress signals in the tissue, effectively becoming “activated,” and promote cell survival, regeneration, and angiogenesis with the release of an abundance of growth factors while modifying the activation and transcription of nearby cells for cytoprotection, proliferation, and repair (19, 26, 38, 44, 114, 117). MSC have an important role in maintaining tissue integrity by inhibiting reactive oxygen species, a common byproduct of inflammation and injury, thereby preventing fibrosis secondary to oxidative stress-induced tissue damage (105). The secretion of antimicrobial peptides by MSC also contributes to the protection of tissue from damaging microbial interactions, and, for this reason, MSC have been implicated in the treatment of sepsis (88, 93, 107, 140). Notably, MSC also project protrusions to cells in need and directly pass mitochondria to the target cytoplasm. Interestingly, mitochondrial donation by MSC plays an important role in recovering cellular function and in repairing damaged tissue (98, 134).
Contributing to their effectiveness in therapeutics is the ability of MSC to differentiate into chondrocytes, adipocytes, and osteocytes. While there is limited data to suggest this differentiation in vivo, the cell types that MSC can differentiate into are particularly relevant to their role in regenerative medicine (27, 113, 118). Chondrocytes produce type II collagen, which serves as a scaffold within the extracellular matrix, an essential step in ameliorating tissue damage. The scaffold promotes a supportive matrix for the seeding of new cells to repopulate the damaged tissue region (80). In contrast, the role of adipocytes in wound healing is characterized by the release of adipokines, such as adiponectin and leptin, which are critical in angiogenesis and restoring the epithelial cell populations within damaged tissue (132). Osteocytes are critical for maintaining the delicately orchestrated homeostasis of bone, which is constantly undergoing remodeling in osteoblastic and osteolytic cycles. Osteocytes extend multiple processes that reach throughout the matrix, expanding their surface area to sense the status of surrounding cells, which underscores the influence of osteocytes in maintaining homeostasis in bone (29). With more thorough evidence for in vitro differentiation of MSC to many cell types, such as insulin-producing cells, epithelium, and endothelium, further in vivo characterization of MSC differentiation potential is needed (36, 59, 103, 112, 129, 135).
Immunomodulation by MSC
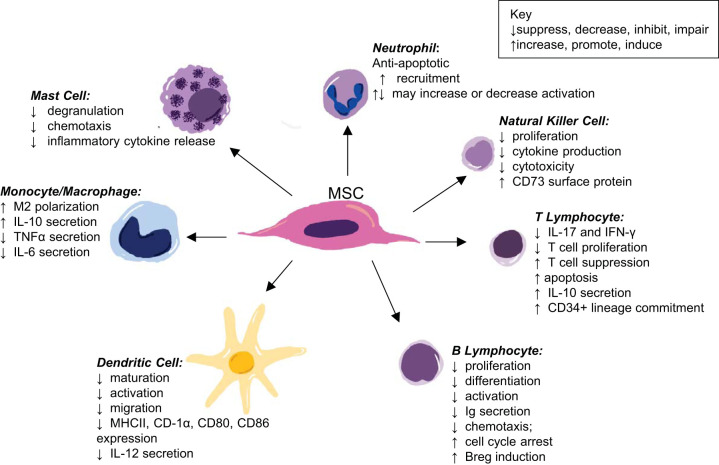
MSC regulate tissue homeostasis in their multipotent state via their secretome, through direct cell-to-cell interactions, as well as a combination of the two, ultimately exerting immunoregulatory functions. MSC can assist the innate immune response due to their expression of a variety of Toll-like receptors and have been documented as proinflammatory; however, the majority of their documented functions are anti-inflammatory, likely due to the interest of using these cells for treatments of inflammatory and autoimmune diseases (82, 109, 146, 154). Waterman et al. (154) describes the potential for MSC polarization, such as is documented in macrophages, as MSC1 or MSC2 phenotypes based on the pro- or anti-inflammatory effects of the activated MSC. MSC can elicit direct effects on immune cells such as neutrophils, macrophages, dendritic cells, lymphocytes, mast cells, and natural killer (NK) cells (83, 111, 146, 156). As summarized in Fig. 1, MSC drive anti-inflammation directly through modulating immune cell activities. MSC suppress the chemotaxis, inflammatory cytokine release, and degranulation of mast cells. Macrophage polarization toward an anti-inflammatory M2-like phenotype is mediated by MSC interaction. This ultimately promotes the synthesis and secretion of anti-inflammatory cytokine IL-10 while suppressing TNF-α and IL-6 release. The proliferation of NK cells and T and B lymphocytes is inhibited by MSC, which also drives a shift in the secretion of inflammatory cytokines to anti-inflammatory cytokines. Additionally, MSC can suppress the activation of B lymphocytes, dendritic cells, NK cells, and neutrophils. Taken together, these downstream effects of MSC actions result in a dampening of inflammation and polarization toward the inflammation-resolution state (1, 32, 70, 156).
Fig. 1.
A schematic of the immunomodulatory effects of mesenchymal stromal cells (MSC) on target immune cells (1, 32, 70, 156).
MSC GAIN RESIDENT STATUS IN THE LUNG
MSC Are Found in All Organs
With MSC first isolated from bone marrow (BM-MSC) in the 1960s, their presence in other tissue types was assumed to be the result of intravascular migration from bone marrow to sites of tissue injury. To date, BM-MSC remain the best-characterized MSC in the literature. These populations are numerous and more readily available for study of their differentiation capacity, phenotype, and roles in immunomodulation and tissue homeostasis. In 1973, MSC were additionally derived from adipose tissue; however, their multipotency was not defined until 2001 (116, 161). Finally, in 2006, da Silva Meirelles et al. (41) described murine mesenchymal stromal cells derived from nearly all postnatal tissues; more specifically, MSC were identified in all the murine tissues they collected. This paper demonstrated a potential role of MSC populations in every tissue type and substantiated the hypothesis that MSC might have resident status in all tissues. MSC have since been isolated repeatedly from other tissues, including placenta, dental pulp, amnion, synovial fluid, cartilage, brain, and skin to name a few. While MSC populations represent a smaller percent of cells in these tissue types compared with bone marrow, there are many papers documenting their presence in tissues (3, 8, 37, 41, 66, 72, 82, 95, 122, 128, 137).
MSC Are a Self-Renewing Population in the Lung
In 2005, human bronchial fibroblast cultures were derived from human bronchial biopsies. These cultured cell types resembled MSC phenotype and multipotency, but this was only a step in the direction of discovering lung-resident MSC (127). In 2007, a study of lung allografts found MSC within the bronchoalveolar lavage fluid (BALF) obtained from transplanted lungs. Multipotent MSC populations were discovered in the BALF with 97% of the sex-mismatched MSC populations having the XY chromosomal status of the donor (91). Therefore, MSC derived from lung allografts are clearly either long lived or self-renewing. Using a different in vivo approach, Jun et al. administered genetically green fluorescent protein (GFP)-marked bone marrow grafts to assess the tissue status of MSC in the lung in a bleomycin-induced fibrosis murine model (77). These findings identified a majority GFP-negative MSC population in the lung homogenates 20 wk following the bone marrow graft, as assessed by flow cytometry, providing evidence supporting the hypothesis of a lung-resident MSC population. While these data provided strong evidence in the case of lung-resident MSC, the limitations of these studies are that MSC derived from BALF or homogenates do not adequately nor directly demonstrate lung tissue residence, since they did not identify the precise location of MSC in the lung. In fact, da Silva et al. (40) proposed a need to determine the true identity of these cell types in vivo.
Lung-Resident MSC Are Perivascularly Located
It was not until 2014 that Rolandsson et al. (126) demonstrated that MSC isolated from bronchial wall and parenchymal tissue biopsies of allografted lungs were found to be perivascular in location and enriched for CD90 and CD105 surface proteins. These data substantiated the identity, phenotype, and perivascular localization of lung-resident MSC (lr-MSC). Additionally, as with previous lung allograft data, these lr-MSC populations were donor matched up to 16 yr posttransplant, contributing further evidence that these populations are long-lived and/or self-renewing residents of lung stroma. In demonstrating a lung tissue-resident population of MSC, directed efforts to characterize this population relative to the well-characterized BM-MSC began.
SPECIFIC CHARACTERISTICS OF LR-MSC
Enriched Surface Proteins
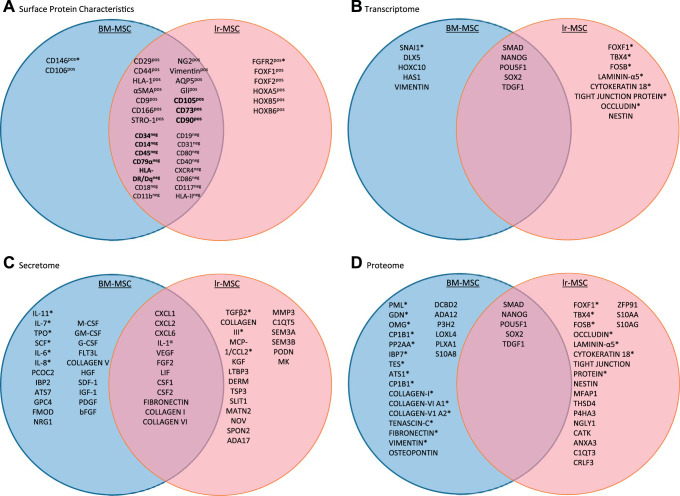
The classification of MSC is defined by specific minimal criteria, the first of which comprises distinct surface protein characterization. Most data attained for characterization of lr-MSC sought to establish a phenotypically similar identity to BM-MSC, the most well-characterized MSC population. Much of the literature, therefore, consists of data defining the enriched surface protein profile consistencies between BM-MSC and lr-MSC, with more recent efforts made at outlining major differences. Meeting the minimal criteria for MSC classification by surface proteins, as outlined by the ISCT, lr-MSC have been described as enriched for CD73, CD90, and CD105 in addition to being negative for hematopoietic markers. The role of these enriched proteins has yet to be fully characterized in the context of lr-MSC-lung interactions. However, it is generally accepted that CD73 has a specific role in MSC-driven suppression of the adaptive immune response. CD73-enriched MSC promote the expression of anti-inflammatory genes, decrease the expression of proinflammatory genes in macrophages, and attenuate the recruitment of proinflammatory macrophages (12). Additionally, CD90 expression has been identified in stem cell populations and certain fibroblast and endothelial cell populations. The immunosuppressive effects of MSC have been attributed to their enriched CD90 expression (21). Specifically, CD90 has a defined role in inflammation and wound healing by promoting secretion of extracellular matrix molecules as well as cytokines and as an adhesion molecule on a variety of cell types involved in the production and release molecular mediators at the site of inflammation (96). CD105, also referred to as endoglin, is important in angiogenesis. Interestingly, the CD105 expression of MSC has been considered representative of only a subpopulation of MSC, with conflicting hypotheses as to the nature of this heterogenicity (5, 45, 76, 97, 159). Both lr-MSC and BM-MSC populations have also been shown to consist of subpopulations that are Gli+ and implicated in the development of tissue fibrosis; however, no data have been published comparing the expression levels between the two (14, 22, 130). MSC derived from bone marrow and lung have no significant differences in expression of CD9, CD29, CD44, CD166, stromal cell surface marker-1 (STRO-1), neuron-glial antigen 2 (NG2), aquaporin-5 (AQP5), HLA-1, α-smooth muscle actin (α-SMA), HLA class I, or vimentin (124). However, although both CD146 and CD106 are enriched surface markers on both, they are expressed at significantly higher levels on BM-MSC. Conversely, the expression of fibroblast growth factor (FGF) receptor-2 (FGFR2) is significantly greater on lr-MSC compared with BM-MSC, to which FGF10 binds, facilitating the mobilization of lr-MSC (49, 106, 124, 144). Additionally, lr-MSC and BM-MSC are equally negative for the hematopoietic surface proteins assessed in each of the publications. These surface protein findings are summarized in Fig. 2A.
Fig. 2.
Venn diagram comparisons of lung-resident mesenchymal stromal cells (lr-MSC) and bone marrow-derived mesenchymal stromal cells (BM-MSC) highlighting their reported similarities and differences. Proteins or genes that are exclusively found on one cell population are listed in the appropriate circle. *Statistically significant difference in expression between shared proteins, with the protein listed within the circle of the cell type with higher expression. The bolded terms represent protein criteria for mesenchymal stromal cell classification. A: surface protein differences. B: transcriptome differences within cells. C: secretome differences within conditioned cell culture media. D: proteome differences within cells (10, 41, 55, 78, 91, 124, 125, 133, 144, 148).
Proteome and Secretome
The response of MSC to the microenvironment is mediated by dynamic proteomic and secretomic profiles by which BM-MSC and lr-MSC can be distinguished. These marked differences are reported in the literature of MSC of other tissue origins as well, although many of these reports do not include lr-MSC in their analyses (17). Contributing to these vast proteomic and transcriptomic differences are the microenvironments by which each tissue of origin alters the resident MSC; thus, there is considerable evidence supporting tissue specificity of MSC populations (89, 123). The driving force of these secretome expression profiles is the ability of MSC to sense stress and changes in the tissue microenvironment. In response, MSC secrete soluble factors that have paracrine activity and modulate cytokine production, differentiation, activation, and the secretome of target cells. Given this, it stands to reason that protein expression of BM-MSC and lr-MSC varies in comparative studies that assay the proteome and secretome of each cell type (10, 91, 121, 124, 125). In 2016, Rolandsson Enes et al. (125) determined that BM-MSC secreted significantly less monocyte chemoattractant protein-1 (MCP-1, CCL2), a monocyte chemotactic protein essential for macrophage recruitment, than lr-MSC, while there were no significant differences in immunosuppressive effects on lymphocytes between the two subpopulations. In the 2017 study by Rolandsson Enes et al. (124), BM-MSC cell layers and conditioned medium were compared with those of lr-MSC cultures by data-independent acquisition-mass spectrometral analysis. Importantly, lr-MSC had nearly 1,000 more proteins expressed and 288 significantly enriched proteins within the cell layer (CL) compared with BM-MSC CL. Additionally, lr-MSC had 36 significantly enriched proteins in the conditioned medium (CM) compared with the CM of BM-MSC cultures. In contrast, BM-MSC were significantly enriched for 64 CL proteins and 37 CM proteins when compared with lr-MSC cultures (124). These data created two distinct clusters upon hierarchical clustering analyses, distinguishing BM and lr-MSC subpopulations. To further assess the protein data from publications on BM- and lr-MSC where pathway analysis was not conducted, we have curated two separately compiled lists, one list each for BM-MSC and lr-MSC, containing significantly upregulated proteins that were then entered into the pathway analysis software Reactome (51). Among the proteins entered in the software, those that yielded upregulated pathways reaching statistical significance (as determined by Reactome algorithms) were used to generate Table 1. These data highlight some unique differences among these populations of MSC. Of note, there are more pathways involving Wnt, elasticity, motility, MET signaling, and integrins among the genes upregulated in lr-MSC compared with BM-MSC.
Table 1.
Pathway analyses of proteomic data
| Pathways Downregulated in lr-MSC | Ref. No. | Pathways Upregulated in lr-MSC | Ref. No. |
|---|---|---|---|
| YAP1 (TAZ)-stimulated gene expression | 121, 125 | RUNX3 regulates WNT signaling | 121, 125 |
| Interferon-γ signaling | 121, 125 | Elastic fiber formation | 121, 125 |
| MPS IIID–Sanpilippo syndrome D | 121, 125 | Nuclear signaling by ERBB4 | 121, 125 |
| Antagonism of activin by follstatin | 121, 125 | MET promotes cell motility | 121, 125 |
| Gene and protein expression by JAK-STAT signaling after IL-12 stimulation | 121, 125 | MET activates PTPN11 | 121, 125 |
| Interferon signaling | 121, 125 | MET activates STAT3 | 121, 125 |
| IL-12 signaling | 121, 125 | Degradation of extracellular matrix | 121, 125 |
| IL-12 family signaling | 121, 125 | MET activates PTK2 signaling | 121, 125 |
| Glycoprotein hormones | 121, 125 | MET receptor activation | 121, 125 |
| Regulation of active cofactor, UDP-glucuronate | 121, 125 | MET activates PI3K/AKT signaling | 121, 125 |
| Peptide hormone biosynthesis | 121, 125 | Potassium transport channels | 121, 125 |
| Transcriptional regulation by RUNX3 | 121, 125 | MET interacts with TNS proteins | 121, 125 |
| Signaling by activin | 125 | MET activates PTPN11 | 121, 125, 148 |
| Peptide hormone metabolism | 121, 125, 148 | MET activates STAT3 | 121, 125, 148 |
| Regulation of PTEN localization | 121, 125, 148 | Regulation of IGF transport and uptake by insulin-like growth factor-binding proteins | 121, 125, 148 |
| Cell proliferation | 124 | Posttranslational protein phosphorylation | 121, 125, 148 |
| Cell growth | 124 | Extracellular matrix organization | 121, 125, 148 |
| Response to wound healing | 124 | IL-4 and IL-13 signaling | 121, 125, 148 |
| Oxidation-reduction processes | 124 | Laminin interactions | 121, 125, 148 |
| Positive regulation of gene expression | 124 | Chemokine receptors bind chemokines | 121, 125, 148 |
| Activation of GABAB receptors | 121, 125, 148 | ||
| Degradation of extracellular matrix | 121, 125, 148 | ||
| Nonintegrin membrane-ECM interactions | 121, 125, 148 | ||
| GABA receptor activation | 121, 125, 148 | ||
| Formation of β-catenin: TCF transactivating complex | 121, 125, 148 | ||
| Platelet degranulation | 121, 125, 148 | ||
| Response to elevated platelet cytosolic Ca2+ | 121, 125, 148 | ||
| ECM proteoglycans | 121, 125, 148 | ||
| Activation of G protein-gated potassium channels | 121, 125, 148 | ||
| G protein-gated potassium channels | 121, 125, 148 | ||
| Inhibition of voltage-gated Ca2+ channels via Gβ/γ-subunits | 121, 125, 148 | ||
| Platelet activation, signaling, and aggregation | 121, 125, 148 | ||
| Neurodegenerative diseases | 121, 125, 148 | ||
| Deregulated CDK5 triggers multiple degenerative pathways in Alzheimer’s | 121, 125, 148 | ||
| Myogenesis | 121, 125, 148 | ||
| Inwardly rectifying K+ channels | 121, 125, 148 | ||
| Oxidation-reduction process | 124 | ||
| Cell proliferation | 124 | ||
| Response to drug | 124 | ||
| RNA splicing | 124 | ||
| Protein folding | 124 | ||
| Positive regulation of IκB/NF-κB signaling | 124 | ||
| Metabolic process | 124 | ||
| Reg mRNA stability | 124 |
Table comprises significantly different pathways between lung-resident mesenchymal stromal cells (lr-MSC) and bone marrow-derived mesenchymal stromal cells (BM-MSC), downregulated in lr-MSC compared with BM-MSC (left column) and upregulated in lr-MSC compared with BM-MSC (right column), determined by proteomic analysis. If enrichment analyses were not provided, we conducted our own analyses of the differentially expressed proteins, with P ≤ 0.05. These analyses were conducted using Reactome (https://reactome.org) using a cutoff of P ≤ 0.05 and are listed in italics above. YAP1, yes-associated protein 1; MPS IIID, mucopolysaccharidosis type IIID; JAK, Janus kinase; STAT, signal transducer and activator of transcription; IL, interleukin; PTEN, phosphatase and tensin homolog; RUNX3, runt-related transcription factor 3; WNT, wingless and Int-1; ERBB4, Erb-B2 receptor tyrosine kinase 4; MET, MET proto-oncogene, receptor tyrosine kinase; PTPN11, protein tyrosine phosphatase nonreceptor type 11; PTK2, protein tyrosine kinase 2; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; TNS, tensin proteins; IGF, insulin-like growth factor; GABAB, γ-aminobutyric acid B receptors; ECM, extracellular matrix; GABAA, γ-aminobutyric acid A receptors; TCF, T cell factor; CDK5, cyclin-dependent kinase 5; IκB, inhibitor of κB; NF-κB, nuclear factor-κB.
Transcriptome
There are increasing reports of distinct gene expression patterns that distinguish MSC of different tissue origin as unique subpopulations (55, 121, 124, 125). The gene expression of these differing MSC subtypes is modulated by their interactions with their respective microenvironments and defined role within their tissue of origin. To visualize identified similarities and differences between the transcriptomes of BM-MSC and lr-MSC, please refer to Fig. 2D and Table 2. Interestingly, the transcriptome and proteome of lr-MSC are of closer resemblance to epithelial cell types than BM-MSC, with pathways upregulated in lr-MSC following the pattern from Table 1. There are more pathways involved in cell adhesion and motility, as well as integrin signaling. From the 2016 paper by Rolandsson Enes et al. (125), among 89 differentially expressed genes in lr-MSC compared with BM-MSC, genes related to epithelial-to-mesenchymal transition [SNAI2 (snail family transcriptional repressor 2), HGF (hepatocyte growth factor), TGF-β2 and 3, TCF4 (transcription factor 4), and CDH2 (cadherin 2)] had significantly higher expression in lr-MSC. Additionally, this paper reported significantly increased expression of the genes commonly expressed in lung-resident cells [FOXF1 (forkhead box 1) and HOXB5 (homeobox B5)] along with the Wnt-signaling protein secreted frizzled-related protein 1 (SFRP1) in lr-MSC compared with BM-MSC (125). The importance of Wnt signaling in lr-MSC subpopulations has been attributed to the regulation of epithelial and myofibroblast differentiation in lr-MSC and the consequential suppression of pulmonary fibrosis (22, 23, 30, 31, 101, 131, 150). The myofibroblast differentiation of lr-MSC is induced by TNF-α and TGF-β1. Kruppel-like factor 4 (Klf4), inhibitor of growth family member 5 (Ing5), and nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) are downstream targets of TNF-α and TGF-β1 with a potential role in attenuating this differentiation (69, 150). These data illuminate the potential role of lr-MSC in the lung due to the need for frequent epithelial regeneration to maintain homeostasis and barrier function of the pulmonary system while evading further functional damage through tissue fibrosis. Additionally, lr-MSC exhibit a greater expression of genes related to cell migration, cell adhesion, and extracellular matrix organization, features that integrate seamlessly with the physiology of the lung in particular, where maintaining the structure of the tissue is critical for proper function, since many airway structures are often a few cell layers thick and must remain elastic (144). These data suggest significant differences among these two MSC populations that shed light on their tissue-specific roles and provide evidence to the impact of tissue specificity of the phenotype, genotype, and function of these cell types.
Table 2.
Enrichment analyses of transcriptomic data
| Pathways Upregulated in BM-MSC | Ref. No. | Pathways Upregulated in lr-MSC | Ref. No. |
|---|---|---|---|
| Response to lipopolysaccharide | 144 | Cell adhesion | 144 |
| Response to drug | 144 | Integrin-mediated signaling pathways | 144 |
| Cell-to-cell adhesion | 144 | Positive regulation of epithelial cell proliferation | 144 |
| Response to hypoxia | 144 | Extracellular matrix organization | 144 |
| Aging | 144 | Cell migration and morphogenesis | 55, 144 |
| Embryonic development | 55, 121, 125, 144, 148 | Negative regulation of ossification | 144 |
| Signal transduction | 144 | Embryonic organ system morphogenesis | 55, 121, 125, 144, 148 |
| Response to activity | 144 | Signal transduction: small GTPase mediated | 55, 144 |
| Negative regulation of apoptotic process | 144 | Cellular response to growth factor stimulus | 144 |
| Response to organic cyclic compound | 144 | Collagen biosynthetic process | 144 |
| Positive regulation of angiogenesis, blood vessel development | 55, 144 | Positive regulation of transcription from RNA | 55, 144 |
| Response to γ-irradiation | 144 | Activation of MAPK activity | 144 |
| Inflammatory response | 55, 121, 125, 144, 148 | Heart development | 144 |
| Negative regulation of cell development | 55, 121, 125, 144, 148 | Positive regulation of cell proliferation | 144 |
| Proximal distal pattern formation | 55, 121, 125, 144, 148 | Negative regulation of mitotic division | 55, 121, 125, 144, 148 |
| Skeletal system development | 55, 121, 125, 144, 148 | Meiotic nuclear division | 55, 121, 125, 144, 148 |
| Negative regulation of multicellular organismal process | 55, 121, 125, 144, 148 | Chromosome segregation | 55, 121, 125, 144, 148 |
| Cell division | 55, 121, 125, 144, 148 | ||
| Digestive tract development | 55, 121, 125, 144, 148 | ||
| Mitotic cell cycle process | 55, 121, 125, 144, 148 |
Table comprises significantly different gene ontologies (GO) between lung-resident mesenchymal stromal cells (lr-MSC) and bone marrow-derived mesenchymal stromal cells (BM-MSC), downregulated in lr-MSC compared with BM-MSC (left column) and upregulated in lr-MSC compared with BM-MSC (right column), determined by enrichment analyses on genes. If analyses were not already provided, we conducted our own analyses of the differentially expressed genes, with P ≤ 0.05 from expression results and a false discovery rate P ≤ 0.05 using Panther (http://pantherdb.org). Analyses conducted by us are italicized and bolded where there is overlap between our analyses and those published previously (55, 121, 125, 144, 148).
ROLE AND POTENTIAL OF LR-MSC IN THE REGULATION OF TISSUE HOMEOSTASIS
Role of lr-MSC in Lung Biology
The pulmonary epithelium, in providing barrier function to the lungs that are continually exposed to pathogens and damaging inhalants, requires continual renewal to maintain pulmonary epithelial integrity. Additionally, the lung epithelium has a unique role as a primary defense in immunity evidenced by its frequent exposure to pathogens and the infrequent rate of pulmonary infections (11). Although there are major voids in the literature outlining the precise mechanisms of action of lr-MSC within the lung, many studies of BM-MSC provide a foundation for understanding how lr-MSC might effectively contribute to lung homeostasis. Overall, MSC mediate anti-inflammatory effects by driving down inflammatory cytokine production and release, preventing the activation and proliferation of proinflammatory immune cells, promoting microvascular remodeling, and differentiating into cell types that contribute to tissue repair and regeneration (34, 55, 57, 60, 65, 74, 105, 158). MSC ameliorate oxidative damage through secreted products and additionally alter the gene expression of surrounding cells through the release of miRNAs (contained in exosomes) that serve to dampen inflammation that is exacerbated by more widespread tissue damage (39, 47, 52, 67, 104). Extracellular vesicles (EV) have been specifically identified as secretory products of MSC, and the characterization of these MSC-derived EV has identified cargo, such as miRNAs and functional proteins, often of an anti-inflammatory nature, which highlights their potential use in cell-free therapeutics (142, 143). It has been reported that EV derived from adipose-MSC and BM-MSC promote an M2-like macrophage polarization in vitro, and both MSC-derived EV or conditioned media attenuate inflammatory lung diseases (73, 84, 99, 100, 120). Ultimately, the function of lr-MSC in the lung can only be inferred through these data until further studies are performed.
In a study by Abreu et al. (1), human BM-MSC were exposed to human BALF from patients with acute respiratory distress syndrome (ARDS) or other lung diseases, such as acutely exacerbated cystic fibrosis. The response of BM-MSC was dependent on the disease from which the BALF was derived, with other lung diseases promoting anti-inflammation in BM-MSC cultures more effectively than BALF from ARDS. This work has alluded to the specific interactions between lr-MSC and the pulmonary microenvironment, evidenced by the phenotypic alterations of MSC resulting from lung inflammation (1). When comparing the immunomodulatory effects of lr-MSC with BM-MSC, Ricciardi et al. (121) determined lr-MSC to exert similar effects on NK cells and B cells to those of BM-MSC cocultures. Furthermore, this study identified an important distinction between the two populations, since lr-MSC more remarkably differentiated to epithelial cells in retinoic acid treatment while also expressing epithelial genes at a higher basal amount (121).We have previously detailed changes in lr-MSC resulting from exposure to organic dust extract, a model of agriculture occupational dust exposure, among which include decreased proliferation and migration of dust-exposed human lr-MSC and increased release of both pro- and anti-inflammatory cytokines: TNF-α, IL-6, IL-8, and AREG, FGF10 (109). Additionally, lr-MSC have been shown to encourage prorepair fates of immune cells and increase T-helper 2 cytokines IL-4 and IL-13 while secreting receptors that neutralize proinflammatory cytokines and inhibiting further inflammatory signaling and cytokine release, including TNF-α and IFN-γ (81, 158). Human lr-MSC isolated from allografts were used for in vitro characterization of their interactions with T cells. These isolated lr-MSC were unable to activate allogenic T cells; however, they were able to inhibit CD4 and CD8 T cell proliferation in response to allogenic and mitogenic stimuli through the release of prostanoids (74).
Role of lr-MSC in Lung Pathology
It is important to note that MSC derived from BALF constitute the majority of lung-MSC studies in diseases of the lung, and ultimately disease may result from either too much or too little action by MSC. For example, bronchoalveolar lavage (BAL)-derived donor-matched MSC from lung transplant patients are implicated in patients with chronic graft rejection, namely, bronchiolitis obliterans syndrome (BOS) (148). In this pathology, fibrotic obliteration of small airways is linked with significant increases in BAL-derived MSC with clear variations of gene expression and phenotype compared with BAL-derived MSC from those without BOS. lr-MSC derived from the BAL of BOS patients exhibit increased collagen deposition and increased α-SMA expression associated with a profibrotic phenotype, and FOXF1 expression directly correlates with lr-MSC in lung tissue and increased numbers of lr-MSC in BALF (148). While Walker et al. (148) hypothesize these cells are mobilized to promote tissue in repair, it is unclear if these cells may also contribute to disease pathogenesis in other cases. Vella et al. (147) determined that expression changes in BAL-derived MSC from BOS patients include increased mRNAs associated with histone deacetylases and methyltransferases, as well as genes involved in protein binding, catalytic activity, transferase activity, and macromolecular complex binding to name a few. Although these data suggest a profibrotic phenotype of MSC that might contribute to the fibrosis that is characteristic of BOS, it still stands that BAL-derived MSC are identified only as a predictor of BOS and less clearly as a direct contributor to the pathology. Another paper identified multipotent perivascular Gli1+ cells, expressing MSC markers, in bone marrow, muscle, heart, liver, kidneys, and lung that proliferate and differentiate into myofibroblasts upon tissue injury (87). The identification of Gli+ MSC as drivers of tissue fibrosis has been documented elsewhere (23, 130). These findings allude to the potential role of tissue-specific MSC in organ fibrosis and more specifically how the presence of these cell types in the lung might contribute to injury-induced pulmonary fibrosis.
Identifying the role of lr-MSC in lung homeostasis within the context of bleomycin-induced pulmonary fibrosis has been of interest, since this disease has devastating impacts on individuals treated with bleomycin for their cancer. Hou et al. determined that increased TNF-α as well as Wnt/β-catenin and NF-κB signaling, which are characteristics of bleomycin-induced pulmonary fibrosis, contribute to myofibroblast differentiation of lr-MSC in vivo (69). In this mouse model of bleomycin-induced pulmonary fibrosis, the inhibition of NF-κB prevented TNF-α activation and ultimately reduced the development of fibrosis. This outcome was measured by collagen deposition in tissue sections as well as collagen protein expression. Additionally, the replacement of lr-MSC subsequently resolved fibrosis in this model (77). Furthermore, the importance of lr-MSC in tissue repair has also been documented in an in vivo model of LPS-induced acute lung injury (ALI). Using this model, Wang et al. (153) determined that lr-MSC impacted T cell fate by regulating the balance between regulatory T cells and Th17 cells. In this model of LPS-induced ALI, administration of MSC resolved the increase in vascular permeability, ameliorated inflammation, and decreased collagen deposition within the interstitium. Finally, in a review by Möbius and Rüdiger (108), the loss of lr-MSC in neonates is highlighted as a contributory mechanism to the development of bronchopulmonary dysplasia (BPD), and studies using administration of exogenous MSC seem to attenuate this disease. This review hypothesizes that many factors associated with the immature lung alter lr-MSC function, which in turn impairs pulmonary homeostasis and contributes to BPD pathogenesis. Additionally, this review discusses the rescue effect of MSC administration in driving an adequate therapeutic response and recovery in BPD. Altogether, it still remains to be seen if the epigenetic and phenotypic changes in lr-MSC identified in lung injury or disease contribute to disease etiology, pathogenesis, or sequalae and ultimately how these roles and mechanisms may vary relative to a particular disease.
BAL-derived MSC have been distinguished from lr-MSC in vivo, and this distinction is of physiologic importance (126, 133). This evidence is critical to our understanding of MSC subpopulations, since these are equally considered lr-MSC but have markedly different phenotypes and transcriptomes and since BAL-derived-MSC differentiate into a profibrotic phenotype compared with tissue-resident lr-MSC (133). This work by Sinclair et al. (133) demonstrates that BAL-derived lr-MSC express more surface markers typical of fibroblasts, such as α-smooth muscle actin and collagen 1A1, than the tissue-derived lr-MSC. Ultimately, these data suggest that lr-MSC are recruited into alveolar spaces in lung disease states and additionally differentiate to a profibrotic phenotype. It is this profibrotic dysfunction of lr-MSC recruited to the airways that is hypothesized to play a key role in pulmonary diseases like BOS, BPD, and interstitial lung disease (ILD), since the scale tips away from the prorepair phenotype of tissue-resident lr-MSC. Furthermore, the presence of lr-MSC in the airways and their respective proliferative capacity is a measure of disease status used in the clinical determination of the prognosis of BOS. Therefore, teasing apart the differences in tissue-derived and BAL-derived MSC populations is an essential step in understanding their role in pulmonary homeostasis, and further study is warranted.
Unique Therapeutic Potential of lr-MSC
MSC cell therapies are currently implicated in many clinical trials such as those for graft versus host disease, chronic obstructive pulmonary disease, acute lung injury, cardiac ischemic injury and regeneration, radiation damage, traumatic brain injury, idiopathic pulmonary fibrosis, tuberculosis, cancer, and other diseases related to chronic inflammation and autoimmune disorders (2, 7, 58, 61, 85, 90, 102, 149). Of particular importance is identifying the therapeutic potential of these endogenous mediators of repair and renewal for the treatment of disease specific to their host tissue and potentially in diseases of differing tissue types (162). The majority of these current and previous clinical applications use BM-MSC, regardless of the organ implicated in disease (141). These therapies include direct injection of MSC into circulation, in auto- and allograft-based treatments, as well as cell-free therapies using MSC-derived secretory products. A major pitfall of intravenously administered MSC is their inability to migrate across pulmonary capillaries to the lung stroma (48, 53). Furthermore, these injected MSC have short half-lives. These shortcomings have generated greater interest in using cell-free therapies via the MSC-derived secretome. Among these secreted products are proteins, cytokines, and EV with varying cargo that mediate repair and modulate inflammation within tissue targets. EV are particularly stable packages containing receptors, immunoglobulins, and microRNAs. They have been found in milk and serum while effectively withstanding homogenization and heat inactivation, respectively, and can impact the inflammatory response of the consumer (15, 16, 94, 110, 115). With these cell-free-based therapies, there is no risk of allograft rejection, and the products can be administered intravenously or aerosolized. While worth mentioning, these MSC and MSC-derived cell-free therapies are beyond the scope of this review, which aims to highlight the importance of lung-resident MSC specifically but have been extensively reviewed elsewhere (7, 9, 56, 58, 102, 138, 145, 158).
Zulueta et al. (162) published one of the few studies examining the specific impacts of therapies derived from lr-MSC; here, IB3–1 cells (an in vitro model of cystic fibrosis) were treated with EV derived from human lr-MSC, leading to anti-inflammatory and antioxidant effects. These effects included transcriptional and protein-level decreases in proinflammatory cytokines, such as IL-1β, IL-6, and IL-8 with EV treatment when the IB3–1 cells were stimulated with TNF-α. The mechanism of action in this regulation was determined to be through peroxisome proliferator-activated receptor-γ (PPARγ), leading to downregulation of NF-κB signaling and upregulation of heme oxygenase-1 (HO-1) (162). Further foundational work on characterizing these tissue-resident populations of MSC, including lr-MSC, will contribute a greater understanding toward the development of specialized and targeted approaches to the treatment of disease. How lr-MSC mediate their effects on specific cell types is poorly understood, and therefore more robust data on the interactions between tissue-resident MSC and their host tissue are needed to identify mechanisms by which resident populations can be stimulated or activated without the need for cell-based therapies, and ultimately dictated by their potential roles in the pathogenesis of disease.
CONCLUDING REMARKS
Limitations and Current Needs in the Field
An important limitation in this field is the similarity between MSC populations and fibroblasts. There is debate among scientists as to whether MSC and fibroblasts are distinct cell lineages, or merely separate stages along a continuum of differentiation, especially since both of these cell types may contribute to fibrotic diseases via their differentiation to myofibroblasts (13, 50, 75, 92, 151). Fibroblasts are also characterized as plastic-adherent and have even demonstrated a similar capacity to differentiate into chondrocytes, osteocytes, and adipocytes, although these data are varied (4, 18, 28, 139). Some have even proposed the use of fibroblasts as an alternative to MSC as they also have immunoregulatory properties (64, 71). In fact, there are a multitude of papers comparing MSC to fibroblasts on the basis of proliferation potential, differentiation capacity, immunomodulation, and gene expression and DNA-regulatory patterns (43, 62–64, 68, 136). Even cell surface markers are largely nonspecific, although some papers have identified markers with the potential to distinguish the two (20, 43, 62). An interesting consideration that is discussed in some of these studies and reviews that may be contributing to the likeness of results is that MSC and fibroblast populations studied in vitro may be heterogeneous and actually contain a mix of both, even in commercially available lines, since they are not easily separated (139). Other studies have shown that fibroblasts are targets of MSC, demonstrating an interplay between the two. Conditioned media from and cocultures with MSC have been shown to suppress the proliferation and myofibroblast differentiation of fibroblasts (155, 157, 160). Everything considered, while necessary and informative, in vitro work must always be taken with a grain of salt as not all culture conditions are physiologically relevant or reproducible in vivo, and culture conditions can even make a cancer cell appear phenotypically normal (152). Thus, better characterization of these populations in vivo is incredibly important.
To date, the data on MSC populations are heavily reliant on in vitro and ex vivo studies. While in vitro work helps characterize cells and expand general knowledge of the role of that cell, in vitro studies lack the complexity of in vivo physiology. Ultimately, the characteristics of MSC populations are more complex than we can determine from in vitro and ex vivo studies alone, and, moving forward, considerable effort must be invested into examining these nuances. Furthermore, in vitro and in vivo characterization of lr-MSC, as well as MSC of other tissue origins, is required to broaden the understanding of MSC-directed disease pathologies and ultimately to improve the efficacy of MSC-based therapeutics. In particular, in vivo work can better illuminate the interplay of MSC within their unique tissue microenvironment to more accurately characterize these unique populations and potentially identify more targeted therapies.
Consideration of Tissue Specificity in Defining MSC Populations
As of now, most studies contributing to preclinical and clinical trials are understood within the context of BM-MSC, which leaves much to be discovered within more tissue-specific approaches. There are only a handful of papers that aim to characterize lr-MSC more specifically and even fewer examining the therapeutic potential of this subpopulation. While BM-MSC and lr-MSC share similarities, which at the very least allow for their classification as MSC, there are striking differences between these cell populations. Many of the similarities determined arise from efforts to apply what is known of BM-MSC to other MSC populations, but this approach is parochial and contributes to a limited understanding of how the tissue microenvironment alters MSC biology. As demonstrated by phenotypic markers, proteomics, transcriptomics, and secretomic data, there is a distinct relationship between these MSC and their respective tissue of origin. Therefore, identifying these functional differences is paramount in addressing how these MSC subtypes impact tissue dysfunction or promote tissue repair and homeostasis.
Ultimately, characterizing the similarities of these cell types was critical in identifying the existence of tissue-resident MSC populations; however, it is of considerable importance at this time to put greater effort into identifying inherent differences to advance our understanding of how MSC function in their home tissue and how these functions can be exploited in the treatment of tissue injury and disease. Although these cell types may meet the criteria for MSC classification, these data suggest that lr-MSC and BM-MSC are not equivalent and therefore may present with variation in therapeutic potential. Ultimately, more consideration of how the tissue microenvironment drives MSC fate and biological function in vivo is needed to appropriately characterize MSC so they may be appropriately used in both the understanding of disease pathogenesis and ultimately in the treatment of disease.
Methods
The publications comprising this review were identified on PubMed using the search terms “lung resident mesenchymal stem cells,” “lung resident mesenchymal stromal cells,” “lung mesenchymal cells,” “mesenchymal stromal cells”, and “mesenchymal progenitor cells.” Gene and protein expression data were compiled from papers that specifically stated the use of normal, nondiseased bone marrow and lung tissue. Additionally, in those studies in which primary cells were derived, we extracted data from papers that used at least two additional methods of confirming MSC populations by the ISCT definition (e.g., CFU assay, surface protein expression, flow cytometry). For the Pathway and Enrichment analyses, gene and protein lists were sorted by significantly (P < 0.05) upregulated and downregulated, only where data comparing BM-MSC and lr-MSC were published together to limit potential bias in pooling data from many different sources. A significance and false discovery rate (FDR) cutoff of P ≤ 0.05 was used when conducting our analyses.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Grant R00ES025819.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N.S. prepared figures; S.N.S. drafted manuscript; S.N.S. and T.M.N. edited and revised manuscript; S.N.S. and T.M.N. approved final version of manuscript.
REFERENCES
- 1.Abreu SC, Rolandsson Enes S, Dearborn J, Goodwin M, Coffey A, Borg ZD, Dos Santos CC, Wargo MJ, Cruz FF, Loi R, DeSarno M, Ashikaga T, Antunes MA, Rocco PRM, Liu KD, Lee JW, Matthay MA, McKenna DH, Weiss DJ. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am J Physiol Lung Cell Mol Physiol 317: L823–L831, 2019. doi: 10.1152/ajplung.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, Bonnet D, Janes SM. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One 4: e8013, 2009. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50: 1522–1532, 2004. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 4.Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, Vykoukal D, Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 103: 197–208, 2011. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 5.Anderson P, Carrillo-Gálvez AB, García-Pérez A, Cobo M, Martín F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One 8: e76979, 2013. doi: 10.1371/journal.pone.0076979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32: 252–260, 2014. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes MA, Laffey JG, Pelosi P, Rocco PR. Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem 115: 1023–1032, 2014. doi: 10.1002/jcb.24783. [DOI] [PubMed] [Google Scholar]
- 8.Appaix F, Nissou MF, van der Sanden B, Dreyfus M, Berger F, Issartel JP, Wion D. Brain mesenchymal stem cells: the other stem cells of the brain? World J Stem Cells 6: 134–143, 2014. doi: 10.4252/wjsc.v6.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustine S, Avey MT, Harrison B, Locke T, Ghannad M, Moher D, Thébaud B. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl Med 6: 2079–2093, 2017. doi: 10.1002/sctm.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol 45: 809–816, 2011. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 23: 327–333, 2004. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 12.Barancelli GV, Camargo TM, Gagliardi NG, Porto E, Souza RA, Campioni F, Falcão JP, Hofer E, Cruz AG, Oliveira CA. Pulsed-field gel electrophoresis characterization of Listeria monocytogenes isolates from cheese manufacturing plants in São Paulo, Brazil. Int J Food Microbiol 173: 21–29, 2014. doi: 10.1016/j.ijfoodmicro.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts: busy multitaskers. Am J Pathol 186: 2519–2531, 2016. doi: 10.1016/j.ajpath.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benmoussa A, Diallo I, Salem M, Michel S, Gilbert C, Sévigny J, Provost P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci Rep 9: 14661, 2019. doi: 10.1038/s41598-019-51092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benmoussa A, Ly S, Shan ST, Laugier J, Boilard E, Gilbert C, Provost P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J Extracell Vesicles 6: 1401897, 2017. doi: 10.1080/20013078.2017.1401897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, Graumann J. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep 6: 21507, 2016. doi: 10.1038/srep21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, Canzi L, Cristini S, Invernici G, Parati EA, Alessandri G. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell 3: 5, 2011. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 27: 670–681, 2009. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brendel C, Kuklick L, Hartmann O, Kim TD, Boudriot U, Schwell D, Neubauer A. Distinct gene expression profile of human mesenchymal stem cells in comparison to skin fibroblasts employing cDNA microarray analysis of 9600 genes. Gene Expr 12: 245–257, 2005. doi: 10.3727/000000005783992043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campioni D, Rizzo R, Stignani M, Melchiorri L, Ferrari L, Moretti S, Russo A, Bagnara GP, Bonsi L, Alviano F, Lanzoni G, Cuneo A, Baricordi OR, Lanza F. A decreased positivity for CD90 on human mesenchymal stromal cells (MSCs) is associated with a loss of immunosuppressive activity by MSCs. Cytometry B Clin Cytom 76: 225–230, 2009. doi: 10.1002/cyto.b.20461. [DOI] [PubMed] [Google Scholar]
- 22.Cao H, Chen X, Hou J, Wang C, Xiang Z, Shen Y, Han X. The Shh/Gli signaling cascade regulates myofibroblastic activation of lung-resident mesenchymal stem cells via the modulation of Wnt10a expression during pulmonary fibrogenesis. Lab Invest 100: 363–377, 2020. doi: 10.1038/s41374-019-0316-8. [DOI] [PubMed] [Google Scholar]
- 23.Cao H, Wang C, Chen X, Hou J, Xiang Z, Shen Y, Han X. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci Rep 8: 13644, 2018. doi: 10.1038/s41598-018-28968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplan AI. Mesenchymal stem cells. J Orthop Res 9: 641–650, 1991. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6: 1445–1451, 2017. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084, 2006. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 27.Chan J, Waddington SN, O’Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells 25: 875–884, 2007. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 28.Chen FG, Zhang WJ, Bi D, Liu W, Wei X, Chen FF, Zhu L, Cui L, Cao Y. Clonal analysis of nestin(-) vimentin(+) multipotent fibroblasts isolated from human dermis. J Cell Sci 120: 2875–2883, 2007. doi: 10.1242/jcs.03478. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol 48: 61–68, 2015. doi: 10.1007/s00795-015-0099-y. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Shi C, Cao H, Chen L, Hou J, Xiang Z, Hu K, Han X. The hedgehog and Wnt/β-catenin system machinery mediate myofibroblast differentiation of LR-MSCs in pulmonary fibrogenesis. Cell Death Dis 9: 639, 2018. doi: 10.1038/s41419-018-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Shi C, Meng X, Zhang K, Li X, Wang C, Xiang Z, Hu K, Han X. Inhibition of Wnt/β-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-β1 and FGF-2. Exp Mol Pathol 101: 22–30, 2016. doi: 10.1016/j.yexmp.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YC, Chang YW, Tan KP, Shen YS, Wang YH, Chang CH. Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS One 13: e0205563, 2018. doi: 10.1371/journal.pone.0205563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiabotto G, Bruno S, Collino F, Camussi G. Mesenchymal stromal cells epithelial transition induced by renal tubular cells-derived extracellular vesicles. PLoS One 11: e0159163, 2016. doi: 10.1371/journal.pone.0159163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow K, Fessel JP, Kaoriihida-Stansbury, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ 3: 31–49, 2013. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins JJ, Thébaud B. Lung mesenchymal stromal cells in development and disease: to serve and protect? Antioxid Redox Signal 21: 1849–1862, 2014. doi: 10.1089/ars.2013.5781. [DOI] [PubMed] [Google Scholar]
- 36.Crisan M. Transition of mesenchymal stem/stromal cells to endothelial cells. Stem Cell Res Ther 4: 95, 2013. doi: 10.1186/scrt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294: C675–C682, 2008. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 39.Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsialis SA, Kourembanas S, Thane K, Hoffman AM, McKenna DH, Rocco PR, Weiss DJ. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates Aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med 4: 1302–1316, 2015. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287–2299, 2008. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 41.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213, 2006. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 42.de Windt TS, Vonk LA, Saris DBF. Response to: mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6: 1747–1748, 2017. doi: 10.1002/sctm.17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denu RA, Nemcek S, Bloom DD, Goodrich AD, Kim J, Mosher DF, Hematti P. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol 136: 85–97, 2016. doi: 10.1159/000445096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 4: 201, 2013. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, James J, Pillai RM. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther 3: 57, 2012. doi: 10.1186/scrt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res 363: 114–120, 2018. doi: 10.1016/j.yexcr.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3: 297, 2012. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, Bellusci S. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141: 296–306, 2014. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, Bellusci S. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell 21: 166–177, 2017. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Fabregat A, Korninger F, Viteri G, Sidiropoulos K, Marin-Garcia P, Ping P, Wu G, Stein L, D’Eustachio P, Hermjakob H. Reactome graph database: efficient access to complex pathway data. PLOS Comput Biol 14: e1005968, 2018. doi: 10.1371/journal.pcbi.1005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep 8: 1419, 2018. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18: 683–692, 2009. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedenstein AJCR, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3: 393–403, 1970. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 55.Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, Suzuki S, Suzuki T, Yamada M, Kondo T, Kato H, Yamaya M. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest 91: 363–378, 2011. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22: 824–833, 2018. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep 11: 280–287, 2015. doi: 10.1007/s12015-014-9583-3. [DOI] [PubMed] [Google Scholar]
- 58.Ghadiri M, Young PM, Traini D. Cell-based therapies for the treatment of idiopathic pulmonary fibrosis (IPF) disease. Expert Opin Biol Ther 16: 375–387, 2016. doi: 10.1517/14712598.2016.1124085. [DOI] [PubMed] [Google Scholar]
- 59.Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro differentiation potential of mesenchymal stem cells. Transfus Med Hemother 35: 228–238, 2008. doi: 10.1159/000124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong X, Sun Z, Cui D, Xu X, Zhu H, Wang L, Qian W, Han X. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol Int 38: 405–411, 2014. doi: 10.1002/cbin.10240. [DOI] [PubMed] [Google Scholar]
- 61.Grauss RW, Winter EM, van Tuyn J, Pijnappels DA, Steijn RV, Hogers B, van der Geest RJ, de Vries AA, Steendijk P, van der Laarse A, Gittenberger-de Groot AC, Schalij MJ, Atsma DE. Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 293: H2438–H2447, 2007. doi: 10.1152/ajpheart.00365.2007. [DOI] [PubMed] [Google Scholar]
- 62.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev 20: 53–66, 2011. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 63.Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 94: 258–263, 2009. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 179: 1595–1604, 2007. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 65.Harrell CR, Sadikot R, Pascual J, Fellabaum C, Jankovic MG, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-based therapy of inflammatory lung diseases: current understanding and future perspectives. Stem Cells Int 2019: 4236973, 2019. doi: 10.1155/2019/4236973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12, 2011. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 20: 591–600, 2015. doi: 10.1111/nep.12490. [DOI] [PubMed] [Google Scholar]
- 68.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 14: 516–521, 2012. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 69.Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, Xiang Z, Han X. TNF-α-induced NF-κB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J Cell Physiol 233: 2409–2419, 2018. doi: 10.1002/jcp.26112. [DOI] [PubMed] [Google Scholar]
- 70.Hyvärinen K, Holopainen M, Skirdenko V, Ruhanen H, Lehenkari P, Korhonen M, Käkelä R, Laitinen S, Kerkelä E. Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front Immunol 9: 771, 2018. doi: 10.3389/fimmu.2018.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ichim TE, O’Heeron P, Kesari S. Fibroblasts as a practical alternative to mesenchymal stem cells. J Transl Med 16: 212, 2018. doi: 10.1186/s12967-018-1536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102: 1548–1549, 2003. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 73.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303: L967–L977, 2012. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181: 4389–4396, 2008. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2: 787–792, 2014. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu Y, Zhang WJ, Cao Y, Zhou G. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials 31: 3564–3571, 2010. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 77.Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, Tadjali M, Lara A, Nozik-Grayck E, Malkoski S, Sorrentino B, Meyrick B, Klemm D, Rojas M, Wagner DH Jr, Majka SM. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 29: 725–735, 2011. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karoubi G, Cortes-Dericks L, Breyer I, Schmid RA, Dutly AE. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest 89: 1100–1114, 2009. doi: 10.1038/labinvest.2009.73. [DOI] [PubMed] [Google Scholar]
- 79.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4: 206–216, 2009. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Kessler MW, Grande DA. Tissue engineering and cartilage. Organogenesis 4: 28–32, 2008. doi: 10.4161/org.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khatri M, O’Brien TD, Chattha KS, Saif LJ. Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell Res Ther 6: 222, 2015. doi: 10.1186/s13287-015-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 14: 11692–11712, 2013. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37: 1445–1453, 2009. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol 302: L891–L908, 2012. doi: 10.1152/ajplung.00288.2011. [DOI] [PubMed] [Google Scholar]
- 85.Klein D, Schmetter A, Imsak R, Wirsdörfer F, Unger K, Jastrow H, Stuschke M, Jendrossek V. Therapy with multipotent mesenchymal stromal cells protects lungs from radiation-induced injury and reduces the risk of lung metastasis. Antioxid Redox Signal 24: 53–69, 2016. doi: 10.1089/ars.2014.6183. [DOI] [PubMed] [Google Scholar]
- 86.Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int 2016: 4285215, 2016. doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, Lee S, Kim JM, Shin JC, Park IY. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep 6: 23544, 2016. doi: 10.1038/srep23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6: 481–492, 2011. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 91.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117: 989–996, 2007. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lecarpentier Y, Schussler O, Sakic A, Rincon-Garriz JM, Soulie P, Bochaton-Piallat ML, Kindler V. Human bone marrow contains mesenchymal stromal stem cells that differentiate in vitro into contractile myofibroblasts controlling T lymphocyte proliferation. Stem Cells Int 2018: 6134787, 2018. doi: 10.1155/2018/6134787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 187: 751–760, 2013. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehrich BM, Liang Y, Khosravi P, Federoff HJ, Fiandaca MS. Fetal bovine serum-derived extracellular vesicles persist within vesicle-depleted culture media. Int J Mol Sci 19: 19, 2018. doi: 10.3390/ijms19113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemos DR, Duffield JS. Tissue-resident mesenchymal stromal cells: implications for tissue-specific antifibrotic therapies. Sci Transl Med 10: 10, 2018. doi: 10.1126/scitranslmed.aan5174. [DOI] [PubMed] [Google Scholar]
- 96.Leyton L, Díaz J, Martínez S, Palacios E, Pérez LA, Pérez RD. Thy-1/CD90 a bidirectional and lateral signaling scaffold. Front Cell Dev Biol 7: 132, 2019. doi: 10.3389/fcell.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leyva-Leyva M, Barrera L, López-Camarillo C, Arriaga-Pizano L, Orozco-Hoyuela G, Carrillo-Casas EM, Calderón-Pérez J, López-Díaz A, Hernandez-Aguilar F, González-Ramírez R, Kawa S, Chimal-Monroy J, Fuentes-Mera L. Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev 22: 1275–1287, 2013. doi: 10.1089/scd.2012.0359. [DOI] [PubMed] [Google Scholar]
- 98.Li C, Cheung MKH, Han S, Zhang Z, Chen L, Chen J, Zeng H, Qiu J. Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep 39: 39, 2019. doi: 10.1042/BSR20182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li QC, Liang Y, Su ZB. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 316: L1107–L1117, 2019. doi: 10.1152/ajplung.00391.2018. [DOI] [PubMed] [Google Scholar]
- 100.Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med 6: 1018–1028, 2017. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu Y, Zhang T, Shan S, Wang S, Bian W, Ren T, Yang D. MiR-124 regulates transforming growth factor-β1 induced differentiation of lung resident mesenchymal stem cells to myofibroblast by repressing Wnt/β-catenin signaling. Dev Biol 449: 115–121, 2019. doi: 10.1016/j.ydbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019: 9628536, 2019. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo L, Hu DH, Yin JQ, Xu RX. Molecular mechanisms of transdifferentiation of adipose-derived stem cells into neural cells: current status and perspectives. Stem Cells Int 2018: 5630802, 2018. doi: 10.1155/2018/5630802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lv H, Liu Q, Sun Y, Yi X, Wei X, Liu W, Zhang Q, Yi H, Chen G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann Transl Med 8: 334, 2020. doi: 10.21037/atm.2020.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maria AT, Toupet K, Bony C, Pirot N, Vozenin MC, Petit B, Roger P, Batteux F, Le Quellec A, Jorgensen C, Noël D, Guilpain P. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis Rheumatol 68: 1013–1025, 2016. doi: 10.1002/art.39477. [DOI] [PubMed] [Google Scholar]
- 106.McQualter JL, McCarty RC, Van der Velden J, O’Donoghue RJ, Asselin-Labat ML, Bozinovski S, Bertoncello I. TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res (Amst) 11: 1222–1233, 2013. doi: 10.1016/j.scr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 107.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047–1057, 2010. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 108.Möbius MA, Rüdiger M. Mesenchymal stromal cells in the development and therapy of bronchopulmonary dysplasia. Mol Cell Pediatr 3: 18, 2016. doi: 10.1186/s40348-016-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nordgren TM, Bailey KL, Heires AJ, Katafiasz D, Romberger DJ. Effects of agricultural organic dusts on human lung-resident mesenchymal stem (stromal) cell function. Toxicol Sci 162: 635–644, 2018. doi: 10.1093/toxsci/kfx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, Romberger DJ. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J Nutr Biochem 64: 110–120, 2019. doi: 10.1016/j.jnutbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2: 59–68, 2009. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Păunescu V, Deak E, Herman D, Siska IR, Tănasie G, Bunu C, Anghel S, Tatu CA, Oprea TI, Henschler R, Rüster B, Bistrian R, Seifried E. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med 11: 502–508, 2007. doi: 10.1111/j.1582-4934.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pei Z, Zeng J, Song Y, Gao Y, Wu R, Chen Y, Li F, Li W, Zhou H, Yang Y. In vivo imaging to monitor differentiation and therapeutic effects of transplanted mesenchymal stem cells in myocardial infarction. Sci Rep 7: 6296, 2017. doi: 10.1038/s41598-017-06571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA 92: 4857–4861, 1995. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, van Lent PL, van den Berg WB, de Vries M, van der Kraan PM, van de Loo FA. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS One 10: e0121123, 2015. doi: 10.1371/journal.pone.0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poznanski WJ, Waheed I, Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab Invest 29: 570–576, 1973. [PubMed] [Google Scholar]
- 117.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74, 1997. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 118.Quinn C, Flake AW. In vivo differentiation potential of mesenchymal stem cells: prenatal and postnatal model systems. Transfus Med Hemother 35: 239–247, 2008. doi: 10.1159/000129129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Redondo J, Sarkar P, Kemp K, Heesom KJ, Wilkins A, Scolding NJ, Rice CM. Dysregulation of mesenchymal stromal cell antioxidant responses in progressive Multiple sclerosis. Stem Cells Transl Med 7: 748–758, 2018. doi: 10.1002/sctm.18-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, Zhao X, Lu C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res 38: 62, 2019. doi: 10.1186/s13046-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, Nwabo Kamdje AH, Chilosi M, Krampera M. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One 7: e35639, 2012. doi: 10.1371/journal.pone.0035639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riekstina U, Muceniece R, Cakstina I, Muiznieks I, Ancans J. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 58: 153–162, 2008. doi: 10.1007/s10616-009-9183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int 2017: 5173732, 2017. doi: 10.1155/2017/5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rolandsson Enes S, Åhrman E, Palani A, Hallgren O, Bjermer L, Malmström A, Scheding S, Malmström J, Westergren-Thorsson G. Quantitative proteomic characterization of lung-MSC and bone marrow-MSC using DIA-mass spectrometry. Sci Rep 7: 9316, 2017. doi: 10.1038/s41598-017-09127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rolandsson Enes S, Andersson Sjöland A, Skog I, Hansson L, Larsson H, Le Blanc K, Eriksson L, Bjermer L, Scheding S, Westergren-Thorsson G. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci Rep 6: 29160, 2016. doi: 10.1038/srep29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rolandsson S, Andersson Sjöland A, Brune JC, Li H, Kassem M, Mertens F, Westergren A, Eriksson L, Hansson L, Skog I, Bjermer L, Scheding S, Westergren-Thorsson G. Primary mesenchymal stem cells in human transplanted lungs are CD90/CD105 perivascularly located tissue-resident cells. BMJ Open Respir Res 1: e000027, 2014. doi: 10.1136/bmjresp-2014-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroché V, Rossi GA, Brouty-Boyé D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 85: 962–971, 2005. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 128.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 23: 220–229, 2005. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 129.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180: 2581–2587, 2008. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 130.Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, Bindels EM, Heckl D, Busche G, Fleck D, Muller-Newen G, Wongboonsin J, Ventura Ferreira M, Puelles VG, Saez-Rodriguez J, Ebert BL, Humphreys BD, Kramann R. Gli1(+) mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell 20: 785–800.e8, 2017. doi: 10.1016/j.stem.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi C, Lv T, Xiang Z, Sun Z, Qian W, Han X. Role of Wnt/β-catenin signaling in epithelial differentiation of lung resident mesenchymal stem cells. J Cell Biochem 116: 1532–1539, 2015. doi: 10.1002/jcb.25069. [DOI] [PubMed] [Google Scholar]
- 132.Shook B, Rivera Gonzalez G, Ebmeier S, Grisotti G, Zwick R, Horsley V. The role of adipocytes in tissue regeneration and stem cell niches. Annu Rev Cell Dev Biol 32: 609–631, 2016. doi: 10.1146/annurev-cellbio-111315-125426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sinclair KA, Yerkovich ST, Chen T, McQualter JL, Hopkins PM, Wells CA, Chambers DC. Mesenchymal stromal cells are readily recoverable from lung tissue, but not the alveolar space, in healthy humans. Stem Cells 34: 2548–2558, 2016. doi: 10.1002/stem.2419. [DOI] [PubMed] [Google Scholar]
- 134.Sinclair KA, Yerkovich ST, Hopkins PM, Chambers DC. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther 7: 91, 2016. doi: 10.1186/s13287-016-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 18: 980–982, 2004. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 136.Soundararajan M, Kannan S. Fibroblasts and mesenchymal stem cells: two sides of the same coin? J Cell Physiol 233: 9099–9109, 2018. doi: 10.1002/jcp.26860. [DOI] [PubMed] [Google Scholar]
- 137.Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, Giudice LC. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 86: 58, 2012. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25: 829–848, 2016. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 139.Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 25: 1610–1617, 2007. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- 140.Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell Microbiol 18: 424–436, 2016. doi: 10.1111/cmi.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 142.Tan SS, Chen TS, Tan KH, Lim SK. An overview of the proteomic and miRNA cargo in MSC-derived exosomes. In: Mesenchymal Stem Cell Derived Exosomes. The Potential for Translational Nanomedicine. Cambridge, MA: Academic, 2015, chapt. 2, p. 21–36. [Google Scholar]
- 143.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 46: 843–853, 2018. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tong L, Zhou J, Rong L, Seeley EJ, Pan J, Zhu X, Liu J, Wang Q, Tang X, Qu J, Bai C, Song Y. Fibroblast growth factor-10 (FGF-10) mobilizes lung-resident mesenchymal stem cells and protects against acute lung injury. Sci Rep 6: 21642, 2016. doi: 10.1038/srep21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turnbull MT, Zubair AC, Meschia JF, Freeman WD. Mesenchymal stem cells for hemorrhagic stroke: status of preclinical and clinical research. NPJ Regen Med 4: 10, 2019. doi: 10.1038/s41536-019-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 147.Vella S, Conaldi PG, Cova E, Meloni F, Liotta R, Cuzzocrea S, Martino L, Bertani A, Luca A, Vitulo P. Lung resident mesenchymal cells isolated from patients with the Bronchiolitis Obliterans Syndrome display a deregulated epigenetic profile. Sci Rep 8: 11167, 2018. doi: 10.1038/s41598-018-29504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach PH, Keshamouni VG, Peters-Golden M, Lama VN. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol 178: 2461–2469, 2011. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walker PA, Shah SK, Jimenez F, Aroom KR, Harting MT, Cox CS Jr. Bone marrow-derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery 152: 790–793, 2012. doi: 10.1016/j.surg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 150.Wang C, Cao H, Gu S, Shi C, Chen X, Han X. Expression analysis of microRNAs and mRNAs in myofibroblast differentiation of lung resident mesenchymal stem cells. Differentiation 112: 10–16, 2020. doi: 10.1016/j.diff.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Gu S, Cao H, Li Z, Xiang Z, Hu K, Han X. miR-877-3p targets Smad7 and is associated with myofibroblast differentiation and bleomycin-induced lung fibrosis. Sci Rep 6: 30122, 2016. doi: 10.1038/srep30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst 94: 1494–1503, 2002. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, Chen C, Jiang J, Bai C, Zhou J, Song Y. Lung-resident mesenchymal stem cells promote repair of LPS-induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation 42: 199–210, 2019. doi: 10.1007/s10753-018-0884-6. [DOI] [PubMed] [Google Scholar]
- 154.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5: e10088, 2010. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watson SL, Marcal H, Sarris M, Di Girolamo N, Coroneo MT, Wakefield D. The effect of mesenchymal stem cell conditioned media on corneal stromal fibroblast wound healing activities. Br J Ophthalmol 94: 1067–1073, 2010. doi: 10.1136/bjo.2009.165837. [DOI] [PubMed] [Google Scholar]
- 156.Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol 10: 1191, 2019. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu Y, Peng Y, Gao D, Feng C, Yuan X, Li H, Wang Y, Yang L, Huang S, Fu X. Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int J Low Extrem Wounds 14: 50–62, 2015. doi: 10.1177/1534734614568373. [DOI] [PubMed] [Google Scholar]
- 158.Xu S, Liu C, Ji HL. Concise review: therapeutic potential of the mesenchymal stem cell derived secretome and extracellular vesicles for radiation-induced lung injury: progress and hypotheses. Stem Cells Transl Med 8: 344–354, 2019. doi: 10.1002/sctm.18-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci 48: 43–52, 2007. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 160.Zhuang L, Xia W, Hou M. Co-culturing with hypoxia pre-conditioned mesenchymal stem cells as a new strategy for the prevention of irradiation-induced fibroblast-to-myofibroblast transition. Oncol Rep 42: 1781–1792, 2019. doi: 10.3892/or.2019.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228, 2001. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 162.Zulueta A, Colombo M, Peli V, Falleni M, Tosi D, Ricciardi M, Baisi A, Bulfamante G, Chiaramonte R, Caretti A. Lung mesenchymal stem cells-derived extracellular vesicles attenuate the inflammatory profile of Cystic Fibrosis epithelial cells. Cell Signal 51: 110–118, 2018. doi: 10.1016/j.cellsig.2018.07.015. [DOI] [PubMed] [Google Scholar]