Abstract
Fibrosis occurs in most human organs including the liver, lung, heart and kidney, and is crucial for the progression of most chronic diseases. As an indispensable catabolic process for intracellular quality control and homeostasis, autophagy occurs in most mammalian cells and is implicated in many biological processes including fibrogenesis. Although advances have been made in understanding autophagy process, the potential role of autophagy in fibrotic diseases remains controversial and has recently attracted a great deal of attention. In the current review, we summarize the commonalities of autophagy affecting different types of fibrosis in different organs, including the liver, lung, heart, and kidney as well as in cystic fibrosis, systematically outline the contradictory results and highlight the distinct role of autophagy during the various stages of fibrosis. In summary, the exact role autophagy plays in fibrogenesis depends on specific cell types and different stimuli, and identifying and evaluating the pathogenic contribution of autophagy in fibrogenesis will promote the discovery of novel therapeutic strategies for the clinical management of these fibrotic diseases.
Keywords: Fibrosis, autophagy, TGF-β pathway, cell senescence, protein degradation.
Fibrosis
Under the influence of exogenous stimuli, such as pathogens and toxins, and endogenous stimuli, including cytokines and certain metabolites, fibrosis gradually occurs in most human organs including the liver, lung, kidney, and heart, and participates in the progression of almost all chronic diseases, which are associated with high morbidity and mortality due to unclear pathogenesis and limited therapeutic options. Following tissue injury, epithelial and endothelial cells release chemokines, cytokines, damage-associated molecular patterns (DAMPs) and growth factors, leading to increased vessel permeability and infiltration of immune cells. In the initial inflammation phase, resident fibroblasts, fibrocytes and epithelial cells undergoing epithelial-mesenchymal transition (EMT) transform into α-smooth muscle actin (α-SMA)-expressing and extracellular matrix (ECM)-producing myofibroblasts. Stimulated myofibroblasts, epithelial and endothelial cells also produce matrix metalloproteinases (MMPs), contributing to the balance between ECM deposition and degradation. In the subsequent remodeling phase, myofibroblasts continuously produce ECM proteins and trigger wound contraction accompanied by angiogenesis 1. The physiological degradation of ECM and re-epithelialization are crucial features of tissue repair and remodeling, while under pathological conditions, persistent myofibroblast activation caused by chronic injury and unresolved inflammation results in the persistent production of ECM as well as tissue inhibitor of metalloproteinases (TIMPs), inhibitors of MMPs 2. The excessive deposition of ECM leads to a progressively irreversible fibrotic healing response, which not only results in the replacement of normal parenchymal tissue with fibrotic tissue but also leads to organ dysfunction and even failure.
Autophagy
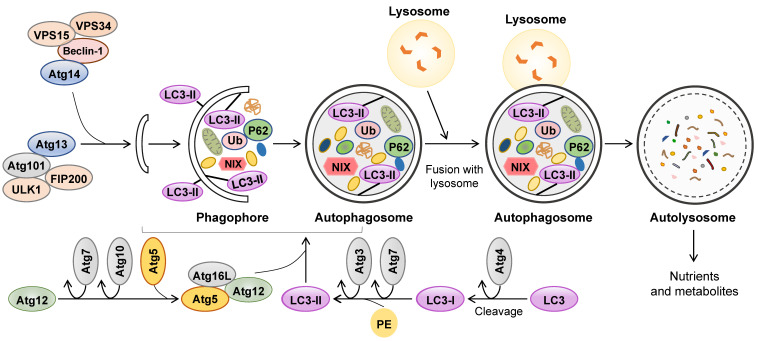
Autophagy is a self-eating catabolic process involving intracellular degradation and recycling that plays a housekeeping role in the maintenance of cellular homeostasis, especially when cells are undergoing starvation or other stress stimulation 3. Autophagy participates in energy metabolism, regulates cellular growth and differentiation, and is responsible for the clearance of misfolded proteins, damaged organelles and lipid droplets (LDs) 4. Autophagy occurs in most mammalian cells and involves a series of specific steps: autophagosome formation, secretion of cargo, formation and degradation of autophagosome. There are three primary types of autophagy depending on lysosomal functions, including macroautophagy, microautophagy and chaperone-mediated autophagy 5. Generally, autophagy is often referred to as macroautophagy, which majorly eliminates damaged cellular organelles and debris. In the process of macroautophagy, a vesicular structure called an autophagosome is formed after a phagophore, which is a double or multimembrane structure and swallows organelles sequestrated by cytoplasm (Figure 1). Conserved factors called autophagy-related gene (Atg) proteins, including Atg3, Atg5, Atg6, Atg7, Atg9, Atg12, Atg14 and Atg16, are required for autophagosome formation, the first step of autophagy. Light chain 3 (LC3) including LC3A, LC3B and LC3C, as well as Beclin-1, autophagy substrate p62 (p62/SQSTM1) and cathepsin B (CTSB) also play significant roles in autophagosome formation 6. Upon stimulation, LC3 binds to Atg4 and cleavages into LC3-I, which subsequently binds to Atg4 and Atg7 and is cleaved to LC3-II. Subsequently, LC3-II, together with Atg5, Atg7, Atg10, Atg16 and Atg12, as well as Beclin-1 accelerate the formation of the phagophore. Later, p62 functions as a selective autophagy adaptor by simultaneously capturing ubiquitinated cargo and interacting with LC3-II, after which the LC3-II-p62-cargo complex is anchored to the autophagosomal membrane 7. Autophagy flux is a dynamic process involving the formation and clearance of autophagosomes, the phagocytosis of cargo and the formation and degradation of autolysosomes. The increased ratios of LC3-II/LC3-I and LC3-II/p62 are believed to the hallmarks of autophagy flux 8. After devouring damaged proteins and organelles, mature autophagosomes are transited to fuse with the lysosomes and form a single membrane autolysosome mediated by microtubules. Autolysosomes further degrade cargos and release nutrients and metabolites to the cytoplasm for cellular replenishment.
Figure 1.
The feature of autophagy. Autophagy involves the formation of autophagosomes, their fusion with lysosomes and subsequent degradation of autophagolysosomal contents. The formation of phagophore is depending on the interactions between lipid bilayers and recruited ULK complex (composed of Atg13, Atg101, ULK1 and FIP200) and PI3K complex (composed of Atg14, Beclin-1, VPS34 and VPS15). At the same time, two different conjugation systems, LC3 and the complex of Atg12-Atg5-Atg16L are involved in the assembling of autophagosome. After devouring damaged proteins and organelles, mature autophagosome fuses with lysosome to form autolysosome. Finally, the inner contents are degraded by lysosomal hydrolases and released as nutrients and metabolites. Atg: autophagy-related gene; LC3: Light chain 3.
Although advances have been achieved in elucidating the role of autophagy in cell signaling, the clearance of misfolded proteins, lipid metabolism and inflammation progression, the importance of the contradictory roles of autophagy-mediated processes in fibrogenesis in different organs have attracted a great deal of attention. However, there is a dispute regarding how the activation or inhibition of autophagy is involved in the progression of fibrotic diseases and whether tissue/cell-specific autophagy-related genes can be therapeutic targets for the treatment of fibrosis. In this review, we summarize the latest findings with regards to the correlation between autophagy and fibrosis in different main organs, including the liver, lung, heart, kidney as well as cystic fibrosis, and highlight the existing evidence supporting autophagy as a potential target for the treatment of chronic multiorgan fibrotic diseases.
Liver fibrosis and autophagy
It has been globally accepted that liver fibrosis is a common process of chronic or iterative liver diseases, such as hepatitis, cirrhosis, and other hepatic dysfunctions. Different types of hepatic cells, especially hepatic stellate cells (HSCs), are involved in the process of liver fibrosis 9, 10. Triggered by persistent liver parenchymal injury and the recruitment of immune cells, HSCs transform into active myofibroblasts and express α-SMA, vimentin and desmin. Myofibroblasts continuously produce ECM and collagen type I (Col-1) and secrete pro-fibrogenic cytokines, including transforming growth factor beta (TGF-β) 1 and tumor necrosis factor-alpha (TNF-α), into the hepatic microenvironment 11. Emerging evidence suggests that complicated interactions occur between autophagy and liver fibrosis, which has become a major focus of research (Figure 2 and 3).
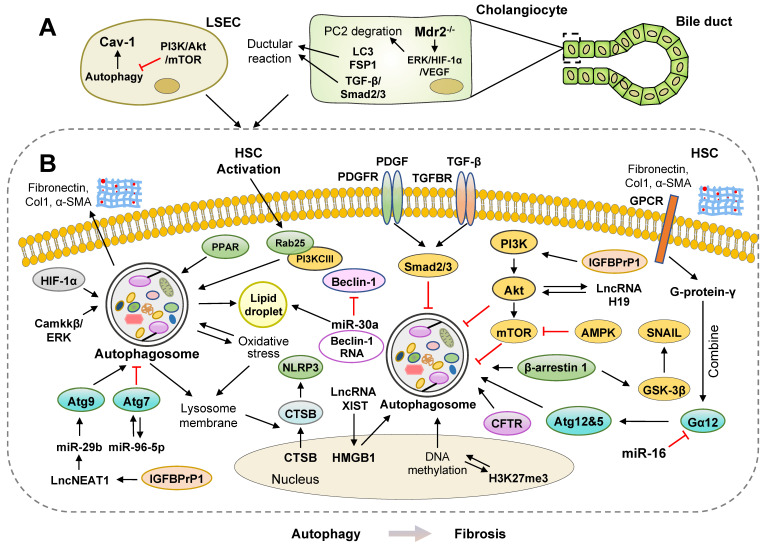
Figure 2.
Pro-fibrosis roles of autophagy in the liver. Multiple studies have shown a positive correlation between activated autophagy and liver fibrosis. (A) Ductular reaction is usually associated with hepatic fibrogenesis. During ductular reaction, increased LC3-II is mainly located in cholangiocytes isolated from human and mouse fibrotic livers, which requires the activation of TGF-β/Smad2/3 signaling in an autophagy-dependent manner. Activated ERK/HIF-1α/VEGF pathways promote proteasomal PC2 degradation; however, blockade of autophagosome formation prevents PC2 degradation and ductular reaction. (B) The activation of autophagy promotes liver fibrosis by accelerating the digestion of LDs and upregulating the levels of several ncRNAs, including miR-96-5p, miR-29b, lncRNA XIST, lncRNA NEAT1 and lncRNA H19. During oxidative stress induced by ethanol or chemicals, autophagy is also rapidly activated, resulting in the elevated formation of NLRP3 inflammasomes and subsequent HSC activation. Furthermore, panel B represents how autophagy-related signaling pathways, including PI3K/Akt/mTOR, β-arrestin 1/GSK-3β/SNAIL, PDGF/TGF-β/Smads, promote liver fibrosis. AMPK: AMP-activated protein kinase; Akt: protein kinase B; LD: lipid droplet; mTOR: mammalian target of rapamycin; GPCR: G protein-coupled receptor; PDGFR: platelet-derived growth factor receptor; PPAR: peroxisome proliferator-activated receptor; TGFBR: transforming growth factor beta receptor.
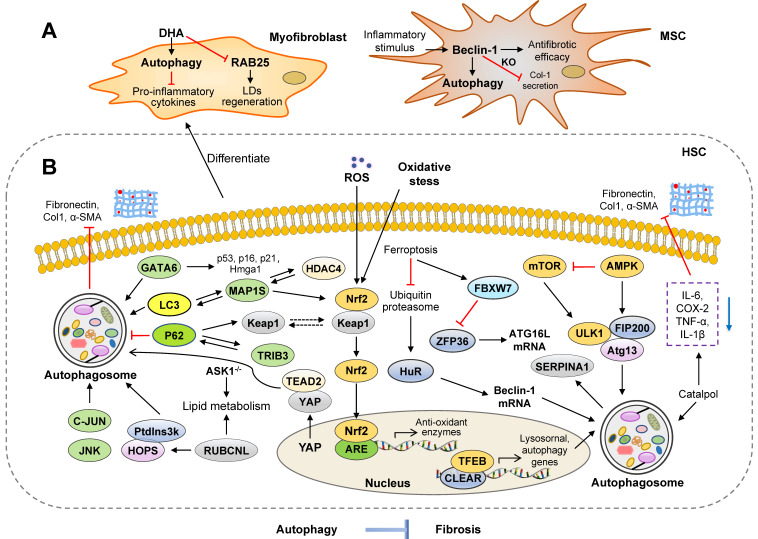
Figure 3.
Anti-fibrosis roles of autophagy in the liver. Several studies suggest that the activation of autophagy may protect against liver fibrotic diseases through different mechanisms. (A) In myofibroblasts, the activation of autophagy inhibits liver fibrosis by promoting LD regeneration and downregulating pro-inflammatory cytokines. The knockdown of Beclin-1 suppresses autophagy and decreases collagen 1 secretion in mesenchymal stem cells (MSCs). (B) The upregulation of YAP, GATA6, MAP1S and complex of Ptdlns3k and HOPS activate autophagy and inhibit HSC activation. Furthermore, panel B represents how autophagy-related signaling pathways, including mTOR/ULK1, Nrf2/Keap1, JNK/c-JUN, protect against liver fibrosis. ARE: AU-rich element; JNK: c-Jun NH2-terminal kinases; HOPS: homotypic fusion and protein sorting; TFEB: transcription factor EB; HuR: human antigen R; RUBCNL: rubicon like autophagy enhancer; SERPINA1: serpin peptidase inhibitor, clade A 1; YAP: yes-associated protein.
Pro-fibrosis roles of autophagy in the liver
Multiple studies have shown a positive correlation between activated autophagy and liver fibrosis (Figure 2). Cassidy et al. developed a mouse model incorporating a doxycycline (dox)-inducible shRNA system targeting Atg5 (Atg5i mice) and observed that the depletion of Atg5 induced by a dox-infused diet resulted in an abnormally large liver but showed no signs of fibrosis. Of note, hepatic fibrosis was observed in livers of Atg5i mice after autophagy restoration by changing the dox-infused diet to a standard diet for 6 weeks 12.
Recently, activated HSCs were observed to exhibit the features of autophagy in carbon tetrachloride (CCL4)- and thioacetamide (TAA)-induced liver fibrosis mouse model 13. In an alcoholic liver fibrosis mouse model, ethanol accelerated autophagy flux as indicated by downregulated p62 expression and upregulated LC3-II/LC3-I, which promoted oxidative stress and HSC activation 14. During oxidative stress induced by NaAsO2, autophagy was also rapidly activated and promoted the release of cytoplasmic CTSB from the nucleus into the cytoplasm following disruption of the lysosomal membrane. CTSB further mediated the formation of the NOD-like receptors containing pyrin domain 3 (NLRP3) inflammasome and activated rat primary HSCs and HSC-T6 cell lines 15. Recently, both hypoxia and lipopolysaccharide (LPS) induced autophagy in a hypoxia-inducible factor 1α (HIF-1α) dependent manner in LX-2 cells (human HSC lines), as indicated by the upregulation of the autophagy markers Atg3, p62 and LC3 16. Significant autophagy was also observed in a mouse model with combined diabetes and liver fibrosis. The researchers further suggested that acid-sensing ion channel 1α (ASIC1α) was crucial for the activation of autophagy in this model by regulating the Camkkβ/ERK pathway, and both pharmacological inhibition and genetic deletion of ASIC1α not only attenuated liver fibrosis in mice but also abrogated platelet-derived growth factor-β (PDGF-B)-induced HSC activation and proliferation 17. A previous study further demonstrated that in the hyperhomocysteinemia mouse model and HL-7702 cells (human normal hepatic cells), homocysteine inhibited cystic fibrosis transmembrane conductance regulator (CFTR) expression, activated autophagy and induced liver injury via interaction between histone H3 lysine 27 trimethylation (H3K27me3) and DNA methylation. The overexpression of CFTR inhibited the expression of Beclin-1, LC3-II/I and Atg12 and the formation of autophagosomes 18. Overall, by employing autophagy inhibitors, such as 3-Methyladenine (3-MA) and LY294002, or siRNAs targeting autophagic genes, the results from the above experimental models have verified that malfunction of autophagy obstructs the progression of liver fibrosis. Further investigations on how activated autophagy aggravates liver fibrosis by affecting multifarious signaling pathways are still required.
mTOR-mediated inhibition of autophagy
Mammalian target of rapamycin (mTOR) has been well established as a negative regulator of autophagy. AMP-activated protein kinase (AMPK) is an endogenous sensor of the AMP/ATP ratio and functionally maintains energy homeostasis. In general, AMPK activation triggers the inhibition of mTOR and thereby facilitates autophagy activation. However, it is noteworthy that 5-aminoimidazole-4-carboxamide-1-4-ribofuranoside (AICAR), a pharmacological activator of AMPK, inhibited autophagy flux and restricted the fibrotic potential by activating the protein kinase B (Akt) pathway in TGF-β-induced LX-2 cells 19. After TGF-β treatment, P62 aggradation was reduced, while autophagosomes, autolysosomes and autophagy flux were increased. Ursodeoxycholic acid (UDCA) inhibited autophagy flux and suppressed collagen aggradation in TGF-β-induced LX-2 cells and CCL4-induced mouse model 20. These UDCA-induced effects were completely abolished by an autophagy inducer, rapamycin, suggesting a crucial role of Akt/mTOR-mediated autophagy inhibition in the progression of liver fibrosis.
In addition, insulin-like growth factor binding protein-related protein 1 (IGFBPrP1), belongs to IGFBP family, is a novel TGF-β1-interacting profibrotic cytokine involved in the upregulation of TGF-β1 expression and ECM 21. Activated IGFBPrP1 promoted autophagy and activation of primary HSCs and HSC cell lines, as demonstrated by increasing long non-coding RNA (lncRNA) NEAT1/ microRNA-29b (miR-29b)/Atg9a axis and downregulating phosphatidylinositide3-kinase (PI3K)/Akt/mTOR signaling, which was verified by IGFBPrP1 overexpression or knockdown 22, 23. As expected, IGFBPrP1 expression and autophagy levels were observed to be concomitantly increased in bile duct ligation (BDL) mouse livers. This observation was further confirmed by Huang and colleagues that activated IGFBPrP1 stimulated autophagy and HSC activation in both IGFBPrP1 adenovirus-induced fibrotic mouse livers and JS-1 cells. Furthermore, the activation of lncRNA H19 promoted autophagy and HSC activation by interacting with the PI3K/Akt/mTOR pathway during IGFBPrP1-induced HSC activation 24. Caveolin-1 (Cav-1) is known as a structural protein that is associated with autophagy in liver sinusoidal endothelial cells (LSECs). Autophagic degradation of Cav-1 and F-actin remodeling were increased in CCL4-induced LSEC defenestration and liver fibrosis by inhibiting the NO-dependent PI3K-Akt-mTOR pathway. Additionally, inhibition of autophagy, including 3-MA, bafilomycin and Atg5-siRNA treatment, alleviated Cav-1 depletion and maintained LSEC fenestrae 25.
Autophagy and digestion of lipid droplets
Autophagy regulates lipid accumulation by maintaining the balance between LD synthesis and degradation. Physiologically, quiescent HSCs are fat-storing cells (FSCs) and vitamin A-storing cells, whereas under pathological conditions, activated HSCs lose lipid droplets and vitamin A. The activation of autophagy accelerates the catabolic process of LDs in transforming HSCs, leading to the release of free fatty acids (FFAs), adenosine triphosphate (ATP) and triglycerides (TG), which provide extra energy for HSC activation. Both 3-MA, a chemical inhibitor of autophagy, and transfection with siRNAs targeting Atg3 and Atg7 inhibited autophagy in HSCs and in the livers of CCL4- or TAA-treated mice and reduced lipid metabolism and energy production, which then inhibited ECM production and liver fibrosis 13. Another study further suggested that LD disappearance during HSC activation is associated with increased autophagy. HSC activation was shown to promote the autophagic recognization, wrapping and elimination of LDs by triggering the overexpression of Rab25 and accelerating the binding of Rab25 to PI3KCIII. Antioxidants, such as glutathione and N-acetyl cysteine, inhibited Rab25-induced autophagy and increased intracellular LDs, which subsequently arrested HSC activation 26. Atg2A was recently found to be localized on LDs, and its release was required for autophagosome formation and HSC activation. Compared to the changes in LDs in activated HSCs, Atg2A deficiency resulted in enlarged LDs, suppressed autophagy flux and decreased HSC activation in LX-2 cells 27. Additionally, Beclin-1 downregulation, mediated by the binding of miR-30a to its 3'-UTR regions, inhibited HSC autophagy, increased lipid accumulation and decreased the expression of α-SMA and Col-1 in the livers of BDL-induced fibrotic mice. Consistently, knockdown of Beclin-1 using siRNA simultaneously prevented HSC activation and autophagy in LX-2 cells 28.
LPS, another stimulator for HSC activation, obviously increased autophagy flux, reduced the number of LDs and lipid staining areas in HSCs and CCL4-induced mice, which was attenuated by autophagy inhibitor chloroquine (CQ) or silencing Atg5 29. Peroxisome proliferator-activated receptor α (PPARα) plays a crucial role in regulating fatty acid transport and β-oxidation, lipid metabolism and inflammation in livers. After arsenic trioxide (As2O3) treatment, HSCs were activated by reducing LD levels and activating the PPARα and autophagy pathways 30.
Interaction between autophagy and the TGF-β pathway
After TGF-β stimulation, phosphorylated Smad2/3 bind to Smad4 to form a Smad2/3/4 complex and then translocate into the nucleus, which regulates gene expression and induces HSC activation. PDGF-B is documented as an essential contributor to the phosphorylation of Smad2/3 by binding to its receptor PDGFR and synergizing with TGF-β-mediated profibrotic signaling. Recently, it has been reported that quercetin and salidroside reduced autophagy and suppressed liver fibrosis by increasing PI3K/Akt signaling pathway and alleviating TGF-β/Smads signaling pathways in a BDL-induced mouse model, respectively 31, 32. Consistently, isorhamnetin blocked autophagy and inhibited HSC activation in different fibrotic mouse models by downregulating the p38 mitogen-activated protein kinase (MAPK) and TGF-β1-mediated Smad3 signaling pathways 33. Furthermore, B. abortus infection induced HSC activation with a fibrogenic phenotype and promoted the activation of autophagic pathways in HSCs. On the other hand, activated autophagy by rapamycin also increased the B. abortus-mediated the secretion of TGF-β1 and collagen deposition in HSCs, which was reversed by a lysosomal protease inhibitor 34.
Recently, it has been reported that β-arrestin 1 was increased in human and mouse fibrotic livers and promoted autophagy by recruiting Beclin-1 in the presence of TGF-β signaling. β-arrestin 1 knockout (KO)- or 3-MA treatment-mediated autophagy inhibition led to an accumulation of glycogen synthase kinase-3β (GSK-3β), which phosphorylated Snail and decreased its nuclear translocation. On the other hand, by facilitating TGF-βRII, β-arrestin 1 promoted autophagy, rescued Snail by inhibiting GSK-3β and thus promoted Snail-dependent HSC growth and activation 35.
Non-coding RNAs and autophagy
The regulatory role of other newly discovered non-coding RNAs involved in autophagy has gradually attracted a great deal of attention. High-mobility group box-1 (HMGB1) is a nuclear DNA-binding protein and DAMP that is implicated in innate immunity. Recently, researchers found that the overexpression of HMGB1 increased the number of autophagic vacuoles, upregulated levels of autophagy marker LC3-II and induced liver fibrosis, which was alleviated in LX-2 cells transfected by LC3-siRNA 36. As with other lncRNAs, lncRNA XIST functioned as a competitive endogenous RNA (ceRNA) and harbored putative binding sites of miR-29b, which directly targeted and inhibited the expression of HMGB1. Hence, lncRNA XIST upregulated HMGB1 expression, promoted ethanol-induced autophagy and eventually activated HSCs 37. G protein-coupled receptors (GPCRs) play complex roles in cellular signal transduction in HSC activation. Both G protein-γ, ligands of GPCRs coupling with Gα12, and Gα12 were overexpressed in HSCs and fibrotic livers and facilitated autophagy by promoting Atg12-5 formation. Considering as a direct inhibitor of Gα12, miR-16 was abundant in quiescent HSCs, while disordered miR-16 led to HSC activation by triggering Gα12 accumulation-related autophagy 38. Furthermore, Yu et al. first identified that miR-96-5p was an abnormally upregulated miRNA in fibrotic tissues. By using computational predictions and luciferase assays, they suggested that miR-96-5p directly interacted with Atg7, which then blocked autophagy and inhibited HSC activation. After reintroducing Atg7, miR-96-5p mimics-mediated suppression of α-SMA, Col-1 and autophagy activity were completely reversed in LX-2 cells 39.
Autophagy in ductular reaction
Ductular reaction (DR), characterized by intensive bile duct proliferation, is usually associated with persistent inflammation and fibrosis. Recently, accumulating evidence has suggested that autophagy plays a critical role in DR and the fibrogenesis in cholangiopathies. A previous study demonstrated that compared with non-cirrhotic livers, co-localization of LC3-II and lysosome-associated membrane protein-1 (LAMP-1), increased levels of LAMP-2 and enhanced maturation of lysosomal cathepsin D were detectable in cirrhotic livers. Besides, by using immunofluorescence staining of cytokeratin 19 (CK-19), Hung et al. illustrated that in human cirrhotic livers and AAF- and CCL4-induced mouse livers, increased LC3-II was primarily located in cholangiocytes during DR, which was markedly attenuated by CQ inhibitor, suggesting that autophagy was positively correlated with DR in cirrhosis 40. They further demonstrated that ductular cells from fibrotic livers exhibited increased autophagy and underwent a mesenchymal transition, which required the activation of TGF-β/Smad2/3 signaling in an autophagy-dependent manner. Importantly, ductular cells were not only positive for LC3-II but also showed increased expression of TGF-β and fibroblast-specific protein-1 (FSP1) in cirrhotic human livers 41. As a nonselective calcium channel, the downregulation and loss of function of polycystin-2 (PC2) is associated with polycystic liver diseases, characterized by the formation of multiple cysts and fibrotic scars. In multidrug resistance gene 2 (Mdr2) KO, BDL- and 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced mouse model, activated extracellular signal-regulated kinase 1/2 (ERK1/2)/HIF-1α/vascular endothelial growth factor (VEGF) pathways promoted proteasomal PC2 degradation and downregulated PC2 protein expression. Researchers further confirmed these in vivo observations in proinflammatory cytokine-, nitric oxide donor- and endoplasmic reticulum (ER) stressor-induced primary mouse cholangiocytes. Consistently, blockade of autophagosome formation by PI3K inhibitors and CQ or inhibition of proteasomes with bortezomib prevented PC2 degradation, DR and liver fibrosis 42. These studies suggest the potential involvement of autophagy in DR and liver injury, but further investigation is urgently required to assess this possibility.
Anti-fibrosis roles of autophagy in the liver
Several studies suggest that activation of autophagy may protect against liver fibrotic diseases through different mechanisms (Figure 3). In contrast with Atg5 knockdown results from Cassidy 12, Ni et al. generated liver-specific Atg5 KO (L-Atg5 KO) mice, L-Atg5/mTOR, and L-Atg5/Raptor double knockout (DKO) mice and further found that L-Atg5/mTOR DKO and L-Atg5/Raptor DKO mice had increased hepatic inflammation and fibrosis at 6 months of age and even developed liver tumors at 9 months of age, suggesting the importance of autophagy in the progression of liver fibrosis 43. Furthermore, in Atg5 liver-specific KO mice, profibrotic genes, including Col-1, α-SMA, connective tissue growth factor (CTGF), and TGF-β were increased in hepatocytes 44. In the wild type (WT) mice, Src inhibitors (saracatinib and PP2) markedly suppressed the expression of α-SMA in HSCs and decreased the TGF-β-induced CTGF expression by increasing autophagy flux in hepatocytes. However, in primary hepatocytes of hepatocyte-specific Atg7 KO mice (Atg7f/f Alb-Cre+), Src inhibitors-induced autophagy and increased CTGF levels did not occur 45. Thus, there is still a substantial gap between the current understanding of the potential roles of autophagy deficiency in the progression of liver fibrosis and whether Atg5 and Atg7 can be used as effective targets for the treatment of liver diseases.
Autophagy and liver fibrosis mediated by multiple pathways
The crosstalk between autophagy and HSC activation determines the fibrotic process and is governed by multiple signaling pathways. Yes-associated protein (YAP) is responsible for cell proliferation and angiogenesis through regulation of TEAD family transcription factors. In the BDL-induced mouse model and rat primary HSCs, dihydrotanshinone increased autophagy flux and suppressed ECM accumulation by inhibiting YAP nuclear translocation, preventing the formation of YAP and TEAD2 complex and downregulating fibrogenic genes, including survivin and CTGF 46. Among Rab GTPase family proteins, Rab18 is known as a mediator of stellate cell activation. A previous study demonstrated that Rab18 downregulated autophagy flux and inhibited HSC activation in LX-2 cells, which was further illustrated by Rab18 knockdown, overexpression and pharmacologic inhibition analyses 47. In addition to cell proliferation and activation, other signaling pathways have been shown to play crucial roles in regulating autophagy-associated HSC senescence and fibrosis. It has been reported that docosahexaenoic acid (DHA) treatment promoted autophagy flux and senescence of activated HSCs in rat fibrotic livers and increased the expression of senescence markers including p53, p16, p21 and HMGa1 by promoting GATA binding protein 6 (GATA6) accumulation in LX-2 cells. Furthermore, treatment with various autophagy inhibitors, such as 3-MA and CQ, prevented DHA-induced p62 degradation, which in turn inhibited GATA6 accumulation and HSC senescence 48.
Several studies reported that microtubule-associated protein 1S (MAP1S) interacted with LC-3 and positively promoted autophagy flux. Driven by binding with LC3, fibronectin was translated and the resulting protein was exported to the cell surface for its assembly. Subsequently, fibronectin was degraded depending on autophagy and lysosomal activities. Notably, in LC3-overexpressing MAP1S KO mice, overexpression of LC3 enhanced the synthesis of fibronectin and MAP1S depletion caused the impairment of autophagy flux, synergistically resulting in fibronectin accumulation and aggravated liver fibrosis 49. Upon exposure to oxidative stress, Nrf2 forms a complex with Keap 1 and regulates the transcription of cytoprotective enzymes by binding to antioxidant response element (ARE). A previous study further suggested that MAP1S stability was also regulated by its interaction with histone deacetylase (HDAC4). Spermidine, a non-canonical Nrf2 inducer, increased MAP1S stability and promoted autophagy flux through depletion of cytosolic HDAC4, which in turn reduced liver fibrosis and extended lifespan in MAP1S-deficient mice 50. In addition to the selective degradation of ubiquitinated cargo, p62 is involved in the degradation of Keap1 51. MAP1S was also reported to enhance Nrf2 stabilization, compete with Keap1 for combining to Nrf2 and eventually promote the degradation of Keap1 by inducing autophagy. Consistently, spermidine suppressed liver fibrosis by activating Nrf2 and increasing MAP1S-mediated autophagy, which was partly reversed in Nrf2 or p62 single KO mice and completely inhibited in Nrf2 and p62 DKO mice 52. These observed anti-fibrotic impacts of MAP1S rely upon the promotion of autophagy flux, once again, showing the importance of increased autophagy flux in the development of liver fibrosis.
Recently, it has been reported that high levels of tribbles pseudokinase 3 (TRIB3) and p62 were positively correlated in livers of patients with cirrhosis. Liver-specific knockdown of TRIB3 restored autophagy activity in livers and protected the liver against TAA- and BDL-induced liver fibrosis. These findings further demonstrated that TRIB3 interacted with p62, hindered its binding to LC3 complexes and further resulted in p62 accumulation and impaired autophagy flux, eventually leading to decreased late endosome degradation and accelerated INHBA/Activin A-enriched exosomes-caused HSC activation and proliferation 3. A recent study demonstrated that in liver fibrosis, fibrogenic extracellular vesicles (EVs), including exosomes, induced HSC migration in an autocrine manner. Unlike TGF-β stimulation, PDGF and its downstream molecule Src homology 2-containing protein tyrosine phosphatase 2 (SHP2) exerted a pro-fibrotic effect in HSCs by enhancing the release of fibrogenic EVs via autophagic inhibition and activation of the mTOR pathway 53.
Farnesoid X receptor (FXR), known as a bile acid nuclear receptor, has been reported to be involved in HSC activation and autophagy regulation. In addition, probucol inhibited LPS- and CCL4-induced activation of HSCs both in vitro and in vivo by increasing the expression of FXR and accelerating autophagy, which was verified using the FXR antagonist Z-guggulsterone, and the FXR agonist GW4064 54. It has been reported that iron overload accelerated liver damage by reducing the expression of LC3-II, LC3-I and Beclin-1 and inhibiting the activation of Atg3, 5, 7 and 12 in ethanol- and high-fat diet (HFD)-induced mouse model 55. Unc-51-like kinase 1 (ULK1) forms a protein complex with FIP200 and the autophagy protein Atg13 to mediate the initiation of autophagy and is regulated by the mTOR and AMPK pathways 56. Recent studies reported that norUDCA improved alpha-1-antitrypsin (α1AT) deficiency-induced accumulation of a1AT mutant Z and liver fibrosis in mice by promoting autophagy-mediated degradation of a1ATZ protein and activating the AMPK/ULK1 signaling pathway, which could be abolished by CQ and the ULK1 inhibitor SBI-0206965 57, 58. Transcription factor EB (TFEB) is a key transcriptional factor driving lysosomal gene expression and is the primary player involved in coordinating the formation of autophagosomes and autophagosome-lysosome fusion 59. Serpin peptidase inhibitor, clade A (SERPINA1) deficiency is a common genetic cause of chronic liver disease. Liver-directed gene transfer of TFEB attenuated hepatic fibrosis by accelerating the autophagy-dependent degradation of SERPINA1 polymer in autolysosomes and decreasing SERPINA1 monomer expression in SERPINA1 deficient mice 60. Furthermore, Liu et al. reported that platycodin D mediated the activation of autophagy and suppressed activation of HSCs by promoting phosphorylation of C-Jun NH2-terminal kinases (JNK) and C-Jun, which was reversed by JNK inhibitor P600125 61.
Autophagy and lipid accumulation in hepatic cells
Although a previous study reported that autophagy contributed to the recognization, wrapping and elimination of LDs by upregulating Rab25 expression in HSCs 26, a more recent study identified that the exact role autophagy played in LDs was dependent on specific cell types and different stimuli. Unlike quiescent HSCs, DHA increased autophagosome generation and autophagy flux, however, it paradoxically induced LD regeneration in activated HSCs and improved liver fibrosis by inhibiting Rab25 expression 62.
Interestingly, an increasing number of studies have pointed out that the induction of autophagy may offer benefits to fibrotic livers, probably because it alleviates steatosis without directly targeting the process of liver fibrosis itself. Autophagy dysfunction-associated p62 accumulation is also regarded as one of the histological features of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). The number of hepatocytes with p62 aggregation was positively correlated with an increased number of autophagosomes, the M1-polarization of macrophages and liver fibrosis in livers from NAFLD patients 63. Silent information regulator 1 (SIRT1), an NAD+ dependent histone deacetylase, regulated energy and lipid homeostasis in the liver, and miR-34a-5p was the direct inhibitor of SIRT1. Melatonin reversed HFD-caused liver fibrosis and NAFLD by increasing the ratio of LC3-II/I, decreasing cytoplasmic p62 levels and reducing the expression of miR-34a-5p in WT, but not SIRT1 heterozygous (HET) mice 64. Similar to previous results, the activation of autophagy by rapamycin alleviated methionine choline-deficient (MCD) diet-caused liver fibrosis in a NASH mouse model 65. Interestingly, ASMase KO mice were resistant to HFD-induced hepatic ER stress, fibrosis and NASH but sensitive to that induced by tunicamycin. Impaired autophagy, lower autophagy flux and increased ER stress were observed in primary mouse hepatocytes from ASMase KO mice 66. Rubicon like autophagy enhancer (RUBCNL/Pacer) is a vertebrate-specific autophagy regulator that contributes to the formation of autophagosomes. Previous studies demonstrated that homotypic fusion and protein sorting (HOPS) complexes had an initial recognition and fusion capabilities in the process of membrane fusion. Activated RUBCNL promoted autolysosome formation by engaging the class III phosphatidylinositol 3-kinase (PtdIns3K) and HOPS complexes in the cytoplasm 67. Cheng et al. further demonstrated that RUBCNL acetylation markedly promoted HOPS complex recruitment, caused autophagosome maturation and lipid metabolism. Additionally, hepatocyte-specific RUBCNL KO mice exhibited impaired autophagy flux, increased LD accumulation and severe liver fibrosis 68, 69. It has been identified that apoptosis signal-regulating kinase 1 (ASK1) was a critical suppressor of NASH and liver fibrosis. HFD- and chow-fed liver-specific ASK1-KO mice developed more severe hepatic steatosis and fibrosis due to blunted autophagy. Consistently, in hepatocytes, depletion of ASK1 impaired autophagy and elevated lipid accumulation. On the other hand, conditional liver-specific ASK1 overexpression protected mice against HFD- and CCL4-induced liver fibrosis. Remarkably, in lean and obese human livers, the expression of ASK1 was positively correlated with the autophagy marker LC3-II and negatively associated with liver fat content and NASH scores 70.
Autophagy and RNA-binding proteins
Emerging studies demonstrated that human antigen R (HuR), an RNA-binding protein, plays a critical role in ferroptosis and liver fibrosis 71. After exposing HSCs to ferroptosis stimulators, HuR was significantly increased by inhibiting the ubiquitin-proteasome pathway and enhanced classical ferroptosis through increased autophagosome generation and autophagy flux in HSCs. This treatment also improved BECN1/Beclin-1 mRNA stability and accelerated the activation of autophagy by binding the AU-rich elements (AREs) within the F3 of the 3'-untranslated region of Beclin-1 mRNA, which was prevented by internal deletion of the F3 region 72. Additionally, HSC-specific knockdown of HuR impaired sorafenib-promoted HSC ferroptosis, and finally, aggravated liver fibrosis in mice. More recently, another RNA-binding protein, ZFP36 ring finger protein (ZFP36) was reported to play a crucial role in regulating ferroptosis in HSCs. After treatment with ferroptosis-inducing compounds, the ubiquitin ligase F-box and WD repeat domain containing 7(FBXW7)/CDC4 reduced the expression of ZEP36 by recognizing the SFSGLPS motif in HSCs. Interestingly, the overexpression of ZEP36 not only inhibited autophagy activation by destabilizing ATG16L1 mRNA but also promoted ATG16L1 mRNA decay through binding to AREs within its 3'-untranslated region. Consistently, the overexpression of ZEP36 prevented erastin- and sorafenib-induced HSC ferroptosis and aggravated liver fibrosis. Clinically, fibrotic patients with HCC receiving sorafenib monotherapy showed lower expression of ZEP36 and ferroptosis activation 73. In accordance with the above findings, it has been revealed that autophagy-dependent ferroptosis in HSCs functions as a potential target for the treatment of liver fibrosis.
Autophagy and anti-inflammatory effects
Correlative evidence has provided insights into the anti-fibrotic role of autophagy under inflammatory conditions. In response to inflammatory stimuli including cytokines TNF-α, interferon gamma (IFN-γ) and TGF-β1, Beclin-1 was increased and then triggered autophagy in mesenchymal stem cells (MSCs). Knockdown of Beclin-1 not only suppressed autophagy but also promoted the antifibrotic efficacy of MSCs and decreased collagen 1 secretion 74. Although autophagy in macrophages promoted the production of inflammatory cytokines, especially interleukin 1α (IL-1α) and IL-1β during chronic liver injury, Atg5 deficiency aggravated CCl4 administration-triggered excessive release of cytokines associated with increased ECM accumulation and liver fibrosis. Conditional cultured medium of macrophage isolated from Atg5 KO mice contained more ROS-induced IL-1α and IL-1β and upregulated profibrogenic gene expression in hepatic myofibroblasts 75. As expected, DHA increased autophagy flux and autophagosome generation in activated HSCs and thus suppressed the expression of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, in the activated HSCs and inhibited inflammation in CCL4-induced rat model 76. It has been indicated that catalpol upregulated the expression of autophagy markers, such as LC3-II and Beclin-1, and reduced the expression of α-SMA and Col-1 and decreased ECM accumulation by downregulating the levels of the inflammatory cytokines, IL-1β, TNF-α, IL-18, IL-6 and cyclooxygenase-2 (COX-2) in CCL4-induced rat models. Furthermore, the anti-inflammatory and anti-fibrotic effects of catalpol toward HSCs were abolished by autophagy inhibitor LY294002 or Atg5-siRNA 77.
Lung fibrosis and autophagy
Idiopathic pulmonary fibrosis (IPF), known as a chronic and progressive fibro-proliferative lung disease, leads to severe breathlessness, respiratory failure and even death. Alveolar epithelium injury, differentiation of resident fibroblast-to-myofibroblast and accumulation of myofibroblast, which further results in ECM deposition and lung fibrosis, are involved in the process of IPF 78. Interestingly, advances have been achieved in our understanding of dual roles of autophagy played in pulmonary fibrosis (Figure 4).
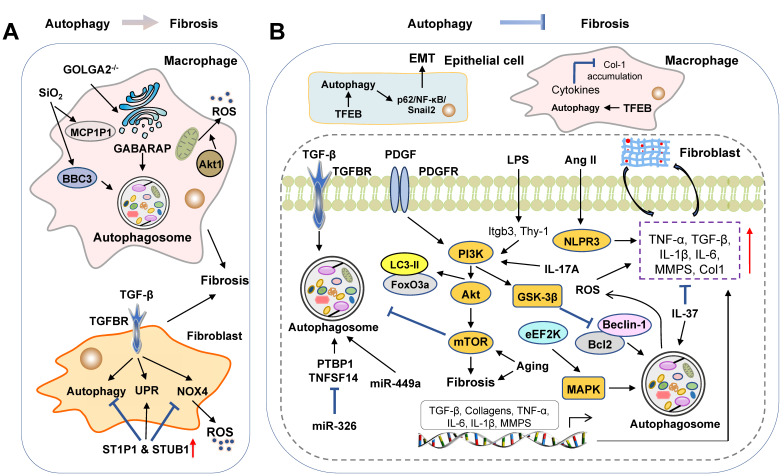
Figure 4.
Molecular targets of autophagy in regulating lung fibrosis. Advances have been achieved in our understanding of dual roles of autophagy in pulmonary fibrosis. (A) In macrophages, the upregulation of BBC3 and MCP1P1 and GABARAP release promote the activation of autophagy and fibrosis. In addition, UPR and the release of ROS induced by TGF-β contribute to the activation of lung fibroblasts. (B) Accumulating evidence suggests that the activation of autophagy improves lung fibrosis through several signaling pathways, including NLPR3, GSK-3β, PI3K/Akt/mTOR and MAPK. Furthermore, several microRNAs have also been demonstrated to perform essential functions in regulating lung fibrosis. Increased expressions of miR-326 and miR-449α promote autophagy activity of fibroblasts and alleviate lung fibrosis. eEF2K: eukaryotic elongation factor-2 kinase; PTBP1: polypyrimidine tract-binding protein; Sio2: silicon dioxide; TNFSF14: tumor necrosis factor superfamily-14; UPR: unfolded protein response.
Pro-fibrosis roles of autophagy in the lung
It is well established that autophagy flux is involved in the progression and severity of lung fibrosis. Fibroblast activation during lung fibrosis is driven not only by the release of cell contents and ROS from epithelial cells but also by pro-inflammatory mediators secreted by macrophages. Current studies demonstrated that activated macrophages might contribute to lung fibrosis by activating autophagy. After treatment with silica, increased monocyte chemotactic protein-1-induced protein 1 (MCP1P1) facilitated macrophage apoptosis and promoted the proliferation and migration of fibroblasts by activating autophagy, which was reversed by 3-MA 78. Furthermore, silica increased the autophagy and cell apoptosis of alveolar macrophages by elevating the expression of BCL-2 binding component 3 (BBC3) in the mouse model, which was significantly enhanced by rapamycin and was mitigated by BBC3-siRNA and 3-MA 79.
Unfolded protein response (UPR) is responsible for restoring defective proteostasis and attenuating protein synthesis upon ER stress, which in turn promotes the capacity of ER in misfolded protein processing. There have been significant advances in understanding the role of dysregulated autophagy flux and UPR in various human fibrotic diseases during the last decade. TGF-β-induced autophagy flux, as illustrated by LC3B accumulation and p62 degradation, UPR and ECM accumulation were significantly suppressed by treating with bafilomycin A1, an autophagy flux inhibitor in primary lung fibroblasts 80. NADPH oxidase 4 (NOX4)-induced ROS production is involved in TGF-β-mediated cell differentiation and apoptosis. The previous study demonstrated that the treatment of azithromycin was reported to inhibit TGF-β-induced NOX4 and myofibroblast differentiation in the human lung fibroblasts. Furthermore, it inhibited autophagy flux and enhanced UPR by increasing the expression of STIP1 homology and U-box containing protein 1 (STUB1) and promoting NOX4 ubiquitination 81.
Furthermore, some recent researches applied KO animals to investigate the relationship between autophagy and pulmonary fibrosis. In addition, previous studies also demonstrated that Akt1 suppressed cell apoptosis, induced mitochondrial ROS and mitophagy in macrophages and promoted lung fibrosis. Deletion of macrophage Akt1 kinase was observed to induce impaired mitophagy and reduce TGF-β expression in the macrophages (Akt1-/-Lyz2-cre) and Parkin 2 (PARK2) KO mice 82. Phosphoglycerate mutase family member 5 (PGAM5), a mitochondrial protein, regulates mitochondrial homeostasis and participates in lung fibrosis. In PGAM5 KO lung fibrosis mouse model, pulmonary fibrosis and mitophagy were reduced compared to the PGAM5-proficient control 83. The formation of autophagosome is initiated from the assembly of phagophore and Golgi serves as a double-membraned source of the phagophore. It has been reported that the deletion of Golgin A2 (GOLGA2), encoding a cis-Golgi protein, induced the formation of the autophagosome via the releases of GABARAP from the Golgi 84. Recently, researchers have revealed that the deletion of GOLGA2 induced autophagy and lung fibrosis by disrupting the Golgi function, increasing alveolar macrophages and reducing subcellular lipid storage in GOLGA2 KO mice 85.
Anti-fibrosis roles of autophagy in the lung
Emerging evidence supports that the dysfunction of autophagy promotes the progression of IPF. Kesireddy and colleagues generated LC3B KO mice and observed that LC3B KO mice displayed increased lung epithelial cell apoptosis, ER stress and susceptibility to bleomycin (BLM)-induced lung fibrosis 86. Furthermore, after BLM administration, Atg4b deficient mice displayed a higher inflammatory response and severe lung fibrosis, associated with the increased production of proinflammatory cytokines, neutrophilic infiltration, alveolar and bronchiolar epithelial cell apoptosis and collagen accumulation 87. Reduced autophagy flux, altered mitophagy and defected mitochondrial function were also observed in BLM- and TGF-β-induced IPF mouse models as well as TGF-β-mediated fibroblast differentiation in vitro model 88. Additionally, in a silica nanoparticle (SiNPs)-induced IPF model, after engulfed by alveolar epithelial cells, SiNPs inhibited autophagy flux, induced autophagosome accumulation and then promoted IPF by suppressing lysosomal degradation and promoting cyclic-3',5'-adenosine monophosphate (cAMP) in IPF mouse model. Enhanced autophagy flux by rapamycin improved lung fibrosis in these mice 89.
Plenty of studies have identified that the proliferation, migration, invasion and EMT of fibroblasts play a crucial role in the progression of IPF. Protein phosphatase 1 regulatory subunit 13B (PPP1R13B), a major member of the apoptosis-stimulating protein of p53 family, was upregulated and its upstream circRNA-012091 was downregulated in silica-treated fibroblasts. The overexpression of PPP1R13B promoted the proliferation and migration of lung fibroblasts by increasing ER stress and autophagy, which was reversed by 3-MA 90. Intemperate and pathogenic invasiveness of lung fibroblasts is a representation of IPF that has been increasingly recognized and studied over the last decades. Inhibition of vimentin intermediate filaments (VimIFs) was shown to suppress the invasiveness of fibroblasts and further protect mice from fibrosis. Specifically, with the treatment of a VimIF inhibitor, withaferin (WFA), autophagy was increased and invasiveness of fibroblasts was restricted in the BLM-induced mouse model and in the 3D lung organoids and pulmospheres derived from IPF patients 91. Most recently, it was revealed that the expressions of signal transducer and activator of transcription 3 (STAT3) and Janus kinase 2 (JAK2) were upregulated in the lung tissue of IPF patients. Dual inhibition of p-JAK2/p-STAT3 ameliorated BLM-induced lung fibrosis and collagen deposition by preventing fibroblast to myofibroblast transition and fibroblast migration and ameliorating impaired autophagy 92. Furthermore, it has been shown that Snail superfamily, including snail1, snail2 and snail3, promoted EMT process by repressing the levels of intracellular E-cadherin. Notably, inhibition of autophagy by HCQ accelerated EMT by increasing the p62-nuclear factor κB (NF-κB)-Snail2 pathway in alveolar epithelial cells 93.
Similar to findings in liver fibrosis, many studies have consistently indicated that TFEB activation and nuclear translocation were closely associated with autophagy and rescued fibrotic diseases. Annexin A2 (ANXA2) was observed to alleviate lung fibrosis caused by BLM by combining with BLM and accelerating TFEB-mediated autophagy flux in vitro and in vivo, which was abolished by CQ and bafilomycin A1 in the ANXA2 genetic depletion mice and CRISPR-Cas9-engineered ANXA2 mutation lung epithelial cells 94. More recently, it has been reported that the overexpression of TFEB or treatment with a TFEB activator, trehalose, suppressed the levels of inflammatory cytokine and prevented lung fibrosis by attenuating lysosomal dysfunction and augmenting autophagy flux in alveolar macrophages, which was abolished by CQ, bafilomycin A1 and TFEB knockdown 95. Collectively, these findings support that the impairment of autophagy in IPF progression is tightly regulated by the interplay between TFEB location and lysosomal dysfunction.
Synthesized by Sphks, sphingosine-1-phosphates (S1Ps) act on the S1PR receptor and S1P signaling plays a critical role in the progression of IPF. It has been demonstrated that S1P lyase (S1PL) was increased in the BLM-induced mouse model and patients with IPF. The overexpression of S1PL suppressed expression of α-SMA and fibronectin and S1P-induced fibroblast differentiation by upregulating the expression of LC3 and Beclin-1 and inhibiting Smad3 expression. Consistent with these results, the knockdown of S1PL in mice augmented BLM-induced pulmonary fibrosis 96. Eukaryotic elongation factor-2 kinase (eEF2K), a negative modulator of protein synthesis, regulates the process of autophagy under environmental stress. A recent study reported that eEF2K activated autophagy and ameliorated the activation of lung myofibroblasts via upregulating P38 MAPK signaling, which was abolished by a P38 MAPK inhibitor SB203580 and eEF2K-siRNA transfection 97.
Notably, several microRNAs have also been demonstrated to perform an essential function in regulating lung fibrosis. Previous studies have identified that miR-449α was significantly reduced in the silica-induced mouse model and TGF-β treated fibroblasts. The overexpression of miR-449α alleviated lung fibrosis through the upregulation of autophagy activity both in vitro and in vivo 98. Furthermore, the level of miR-326 was down-regulated in silica-induced pulmonary fibrosis mouse, silicon monoxide-stimulated lung epithelial cells, HBE and A549, and TGF-β-treated lung fibroblast cells, MRC-5 and NIH/3T3. Increased expression of miR-326 promoted autophagy activity of fibroblasts and alleviated silica-induced pulmonary fibrosis by downregulating the expressions of polypyrimidine tract-binding protein (PTBP1) and tumor necrosis factor superfamily-14 (TNFSF14) 99.
Autophagy and the PI3K-Akt-mTOR pathways
Previously, researchers have shown that activated M-CSF receptor (M-CSF-R) stimulated PI3K and activated Akt in macrophages and then activated Akt promoted collagen production in the BLM-induced mouse model. Reduced expression of Beclin-1, LC3A and LC3B and increased of p62 were also observed in a transgenic mouse model that expressed a constitutively-active form of Akt 100, indicating the pathogenic role of activated Akt in the regulation of autophagy and lung fibrosis. Forkhead box O3a (FoxO3a), a direct target of Akt, transcriptionally activated LC3-II by binding with the promoter region of LC3B. Of note, the expressions of FoxO3a and LC3-II were significantly reduced in fibroblasts from IPF patients, which subsequently reduced autophagy, promoted cell death and IPF progression 101. It has been reported that the expression of HDAC6 was observed to be increased in the BLM-treated mouse lungs and IPF lung tissues. As an HDAC6 inhibitor, tubastatin reversed the BLM-induced LC3B inhibition, S6k phosphorylation and HIF-1α expression, which ameliorated lung fibrosis by inhibiting the PI3K-Akt pathway in WT mice, but not in HDAC6 KO mice 102. Furthermore, recent studies also demonstrated a crucial role of the PDGFR-PI3K-Akt pathway in the activation of autophagy and regulation of myofibroblast differentiation. Genetic deletion of PARK2 inhibited mitophagy and enhanced myofibroblast differentiation and proliferation by activating the PDGFR-PI3K-Akt pathway in the BLM-induced mouse model 103. Similarly, pirfenidone, a PARK2 promoter, was observed to induce autophagy and inhibit myofibroblast differentiation by inducing PARK2 expression and reducing PDGFR-PI3K-Akt activation in BLM-treated WT mice, but not in PARK2 KO mice 104.
Aging is a significant risk factor contributing to higher mortality of IPF and dysregulated autophagy is believed to be involved in the progression of aging-related diseases. Compared with health control, LC3-II expression in fibroblasts from old IPF patients was downregulated due to increased S6K1 phosphorylation and hyperactivity of the mTOR pathway. Furthermore, modified starvation-induced autophagy, which further resulted in apoptosis resistance, was observed in the aging fibroblasts but not in the young fibroblasts. Altogether, mediated by mTOR activity, aging influenced the response of lung fibroblasts to autophagy and enhanced the vulnerability to lung fibrosis 105. More recent studies reported that sustained autophagy promoted fibroblast senescence and prevented the differentiation of fibroblasts to myofibroblasts through the regulation of mTOR complex 2 (mTORC2) 106. However, decreased degradation of p62 and impaired autophagy flux were also observed in senescent cells 107. In addition to aging and cell senescence, several obesity genes are also involved in the pathogenesis of IPF by regulating the mTOR pathway-related autophagy. Leptin promoted the EMT of lung epithelial cells and the TGF-β-induced production of Col-1 and α-SMA through the inhibition of autophagy. It was further demonstrated that leptin elevated the expression of p62, decreased autophagosome formation and inhibited lipidation of LC3-I to LC3-II by activating the PI3K/Akt/mTOR pathways 108. Intriguingly, LPS was also reported to inhibit autophagy and accelerate lung fibroblast proliferation by activating the PI3K-Akt-mTOR pathway, which was reversed by LY294002 and rapamycin 109. Wan and colleagues further found that LPS reduced autophagy and activated the PI3K/Akt/mTOR pathway via upregulating integrin β3 (Itgb3) and downregulating thymocyte differentiation antigen-1 (Thy-1) in the LPS-induced lung fibrotic mouse model and human lung fibroblast cells. Additionally, the effects of LPS on lung fibroblasts were respectively abolished by Thy-1 overexpression, a chemical inhibitor and specific shRNA of Itgb3 110.
Akt/mTOR-regulated autophagy may serve as a potential therapeutic option for the treatment of lung fibrosis. As mentioned above, ULK1 was regulated by the mTOR and AMPK pathways. A recent study has suggested that LKB1-AMPK-ULK1 axis-mediated autophagy plays a critical role in the BLM-induced lung fibrosis 111. Several studies have shown that several antioxidant agents and bioactive natural products, such as coenzyme Q 10 (CoQ10), celastrol and ligustrazin, promoted autophagy, reduced expression of TGF-β and Col-1 and protected against different chemical compounds-induced IPF through the downregulation of the PI3K/Akt/mTOR pathways in animal models 112-114. Du et al. further suggested that dioscin activated autophagy, reduced the secretion of inflammatory factors and subsequently alleviated the abnormal collagen accumulation. These effects of dioscin were abolished in alveolar macrophages when Atg5 was genetically depleted 115.
Interaction between activated autophagy and inflammation
Several studies suggest that inflammatory responses play essential roles in the interaction between IPF and autophagy. Angiotensin (Ang) II has been shown to aggravate lung fibrosis by activating the NLRP3 pathways. Meng et al. reported that activated autophagy attenuated the Ang II-induced activation of NLRP3 by eliminating ROS and alleviating mitochondrial dysfunction in the BLM-induced lung fibrosis, which was verified by rapamycin and 3-MA 116. In contrast to the promotive effects of Ang II on fibrosis, Ang-(1-7) attenuated smoking-induced lung fibrosis by activating autophagy flux and reducing NOX4-dependent ROS production in the passive smoking rat model and cigarette smoke extract-treated fibroblasts. These protective effects of Ang-(1-7) were abolished by autophagy inhibitors, 3-MA and bafilomycin A1 117. Toll-like receptor 4 (TLR4) is a critical player in the regulation of innate immunity. Recently, it has been revealed that activated TLR4 improved lung function and pulmonary fibrosis in the BLM-induced mouse model, which was abolished by TLR4 inhibitor TAK-242. Rapamycin treatment promoted the anti-fibrotic effects of TLR4 agonists, and in contrast, 3-MA treatment contributed to the effects of TLR4 antagonism 118. Furthermore, IL-17A was observed to promote the progression of lung fibrosis by regulating the interaction between inflammatory responses and autophagy. Physiologically, the phosphorylation of Ser70 of B-cell CLL/lymphoma 2 (Bcl-2) accelerated its dissociation from Beclin-1 so as to activate autophagy 119. IL17A inhibited the phosphorylation of Bcl-2 by activating PI3K-GSK-3β signaling cascade and thus blocked the activation of autophagy. Pharmacological inhibition of IL-17A suppressed lung fibrosis and accelerated collagen degradation by activating autophagy 120. On the other hand, as an anti-inflammatory cytokine, IL-37 was expressed in alveolar epithelial cells and alveolar macrophages in healthy controls but was significantly reduced in IPF patients. A recent study demonstrated that IL-37 decreased inflammation and inhibited collagen accumulation through the activation of autophagy in the BLM-induced mouse lung tissues 121.
Cardiac fibrosis and autophagy
Cardiac fibrosis is a clinical manifestation of various cardiovascular diseases, including heart failure, cardiac arrhythmias and myocardial infarction. During the development of cardiac fibrosis, activation of cardiac fibroblasts and differentiation of fibroblast-to-myofibroblast result in the secretion of proinflammatory cytokines and fibrotic factors and ECM deposition 122. Increased α-SMA protein is assembled to stress fibers and remodel ECM, and consequently accelerates cardiac remodeling and heart failure. Notably, mounting evidence shows that autophagy plays a contradictory role in cardiac fibrosis (Figure 5). Given that almost all heart diseases show fibrosis phenomenon and we mainly focus on the role of autophagy in fibroblast activation and cardiac fibrosis, articles regarding only fibrosis phenomenon involved in other heart diseases were excluded.
Figure 5.
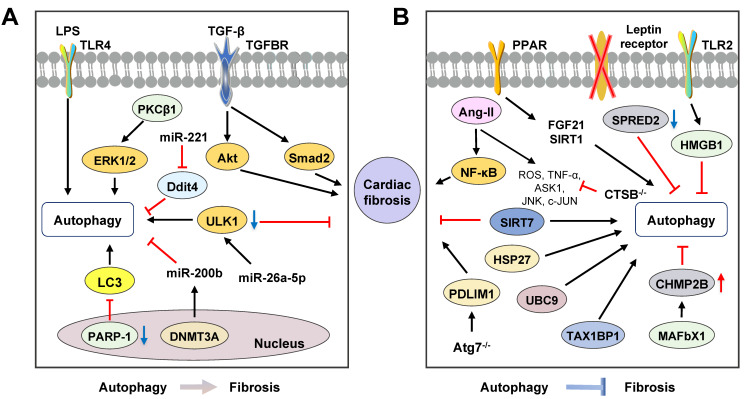
Molecular links between autophagy and cardiac fibrosis. Mounting evidence shows that autophagy plays contradictory roles in cardiac fibrosis. (A) The overexpression of several miRNAs, including miR-26a-5p and miR-221, or the downregulation of miR-200b suppress the activation of cardiac fibroblasts and autophagy flux. Furthermore, the activation of autophagy promotes cardiac fibrosis by regulating PARP-1, TGF-β/Smad and PKCβ1/ERK1/2 signaling pathways. (B) Accumulating evidence suggests that the activation of autophagy improves cardiac fibrosis illustrated by a variety of studies using specific-gene KO (CTSB, p8, Atg7 and SIRT7) mouse models. Panel B represents how autophagy-related signaling pathways, including SPRED2, PPAR, HMGB1, JNK/c-JUN, HSP27 and TAX1BP1/NF-κB pathways, and unfolded protein reactions contribute to cardiac fibrosis. CHMP2B: charged multivesicular body protein 2B; Ddit4: damage inducible transcript 4; DNMT3A: DNA methyltransferases 3A; HMGB1: high-mobility group box-1; PDLIM1: PDZ and LIM domain 1; SPRED2: sprouty-related protein with an EVH domain 2.
Pro-fibrosis roles of autophagy in the heart
Emerging evidence suggests that the activation of autophagy is accompanied by cardiac fibrosis and targeting autophagy is a potential therapeutic option. A previous study reported that poly polymerase 1 (PARP-1), an extensive nuclear enzyme, accelerated the proliferation, migration and differentiation of cardiac fibroblasts induced by TGF-β. Genetic and pharmacological inhibition of PARP-1 decreased α-SMA expression and improved cardiac fibrosis through the downregulation of LC3 and Beclin-1 in the TGF-β-stimulated cardiac fibroblasts 122. Transverse aortic constriction (TAC) treatment was reported to increase the levels of Atg5, Atg16, LC3-II and Beclin-1, induce high levels of Ang II and heart fibrosis in the heart. Besides, aliskiren inhibited autophagy and thus attenuated heart fibrosis via downregulating the Ang II- protein kinase C β1 (PKCβ1)-ERK1/2 pathway in the TAC-induced mouse model, which was confirmed by 3-MA and PKCβ1 inhibitor LY333531 123. WD repeat and five domain containing 1 (WDFY1) is downstream of neuropilin-2, an important receptor in immune regulation. Cyclosporin A promoted myocardial fibrosis and increased the expressions of α-SMA, MMP-2/9, Col-1, TNF-α, LC3-II, Beclin-1 and ERK1/2 by activating autophagy, downregulating neuropilin-2 and upregulating WDFY1 in cardiac fibroblasts, which was abolished by 3-MA 124. Furthermore, a recent study demonstrated that LPS treatment upregulated the expression of autophagy markers and α-SMA in the cardiac fibroblasts after myocardial infarction. However, in TLR4 KO mice, the expressions of autophagy markers Atg5, Atg7, LC3-II, Beclin-1 were reduced and decreased autophagy was associated with the downregulation of fibrotic markers, such as α-SMA and vimentin 125.
Autophagy and microRNAs
Researchers have pointed out that miRNAs act as crucial regulators of cardiac homeostasis in heart diseases. In human cardiac fibroblasts, TGF-β treatment induced Col-1 and fibronectin synthesis by activating autophagy, which was facilitated by rapamycin and Atg5 overexpression and abolished by 3-MA and Beclin-1 knockdown 126. TGF-β receptor II (TGFBRII) was reported to transduce TGF-β1/2/3 signaling from cell membrane to cytoplasm. MiR-19a-3p suppressed ECM accumulation, cell invasion and phosphorylation of Smad2 and Akt through the deactivation of autophagy and downregulation of TGF-β RII signaling, which was confirmed by 3-MA and reversed by rapamycin 127. ULK1, a key player in the regulation of autophagy, is the target of miR-26a-5p. The overexpression of miR-26a-5p suppressed the expression of Col-1 and activation of LC3-I to LC3-II via downregulating the mRNA stability of ULK1 in rat primary cardiac fibroblasts 128. Coincidentally, recent studies confirmed that miR-221 reduced fibroblast activation and cardiac fibrosis by inhibiting damage inducible transcript 4 (Ddit4)-mediated autophagy in the hypoxia and reoxygenation-induced rat cardiac fibroblast activation, suggesting potential therapeutic effects of miR-221 on myocardial infarction-related fibrosis 129. Furthermore, miR-200b is another miRNA possessing anti-fibrotic effects by regulating autophagy. A previous study suggested that the expression of DNA methyltransferases 3A (DNMT3A), a protein of DNA methyltransferases family, was upregulated and miR-200b expression was decreased in rat cardiac fibroblast and fibrosis tissues. DNMT3A accelerated cardiac fibroblasts autophagy caused by rapamycin and cardiac fibrosis through the downregulation of miR-200b, which was abolished by miR-200b mimics and inhibition of DNMT3A in an abdominal aortic coarctation-induced cardiac fibrosis mouse model 130. Compared with fibrosis in other organs, although both the significance of miRNAs in the regulation of autophagy and the pathological role of excessive autophagy in the progression of cardiac fibrosis have been reported, the regulatory networks and interplay of miRNAs remain to be elucidated.
Anti-fibrosis roles of autophagy in the heart
Autophagy dysfunction in myocardial hypertrophy-related cardiac fibrosis
Despite controversies, accumulating evidence suggests that the induction of autophagy may improve cardiac fibrosis as illustrated by a variety of pharmacological agonists/antagonists or specific-gene KO mouse models. Ang II contributes to cardiac fibrosis and graver cardiac remodeling, largely because of its promotive effects on myocardial hypertrophy. Ang II treatment induced the activation of rat cardiac fibroblasts by upregulating the expressions of Col-1 and fibronectin, increased ratios of LC3-II/LC3-I and LC3-II/p62 and the co-localization of LC3 puncta with vimentin in a mouse model. Pharmacological inhibition of autophagy by CQ not only enhanced the pro-fibrotic effects of Ang II but also augmented cardiac dysfunction, which was reversed by rapamycin treatment 131. Genetic abolishment of adiponectin (APN) increased the expressions of α-SMA, IL-1β, TNF-α and Col-1, promoted macrophage infiltration and decreased LC3-positive autophagosomes in the cardiac macrophages isolated from Ang II-induced mouse model. On the other hand, APN promoted macrophage autophagy and suppressed the Ang II-induced inflammatory responses and cardiac fibrosis through the activation of AMPK, which was abolished by AMPK inhibitor compound C 132. Recently a study showed that CTSB, a substrate of autophagy, was expressed in murine and human hearts, linking to myocardial infarction. Insufficient CTSB was shown to attenuate the cardiac remodeling and activation of TNF-α, ASK1, JNK and c-Jun signalings induced by Ang II in the CTSB KO mice 133. Autophagy-specific gene KO animals are also used to study the relationship between autophagy and myocardial hypertrophy-related cardiac fibrosis. Zhao et al. generated Atg5+/- mouse model and confirmed that Ang II induced cardiac fibrosis and inflammation via increasing ROS production, macrophage accumulation, and the activation of NF-κB in macrophages was aggravated in Atg5+/- mouse model 134. In recent years, it has become increasingly clear that MMPs play a crucial role in the pathogenesis of cardiovascular diseases. Transcriptional regulator p8 was observed to be increased in failing human hearts and stimulated MMP-9 induction. Georgescu et al. established p8 KO mouse model and confirmed that the deficiency of p8 attenuated cardiac fibrosis, decreased MMP-9 production and upregulated the expressions of MMP-2 and MMP-3 via accelerating expression of LC3-II and Atg12 in the TAC-stimulated mouse model 135. The involvement of TLR2 in Ang II-induced macrophage activation and cardiac fibrosis also has been noticed. It has been demonstrated that HMGB1 and TLR2 were co-localized in cardiac fibroblasts, accelerated the expressions of α-SMA and Col-1 and subsequently promoted cardiac fibrosis by inhibiting autophagy flux in the cardiac fibroblasts from the isoproterenol-induced myocardial hypertrophy-related cardiac fibrosis mouse model, which was prevented by silencing TLR2 136. Additionally, a novel cardiac hypertrophy mouse model was constructed by inducible endothelium-specific deleting of leptin receptors (End.LepR-KO). After applying transverse aortic constriction, activated autophagy, illustrated by elevated LC3-I/-II conversion and LC3-II positive endothelial cells in hearts, improved cardiac angiogenesis and suppressed cardiomyocyte hypertrophy were observed in End.LepR-KO mouse model. Interestingly, the inhibition of mTOR by rapamycin activated autophagy and the spontaneous cardiac hypertrophy in End.LepR-KO mice was improved 137.
Autophagy dysfunction contributes to fibrosis in proteotoxic cardiac diseases
The balance of protein synthesis and degradation is essential for cardiomyocyte homeostasis and is regulated by a large number of signaling cascades. Indeed, autophagy, UPR and ubiquitylation are key factors for this balance, whereas imbalance will inevitably result in cardiac dysfunction and fibrosis. Sprouty-related protein with an EVH domain 2 (SPRED2), an intracellular repressor of ERK-MAPK signaling, was expressed in the human heart and interacted with p62, a neighbor of BRCA1 (NBR1) and lysosomal cathepsin D thus participated in autophagolysosome formation. Insufficient SPRED2 reduced autophagy flux, dysregulated vesicle handing and promoted cardiac fibrosis by impairing the phagophore formation and inducing the accumulation of autophagosomal and lysosomal marker proteins in the SPRED2 KO hearts 138. Desmin-related cardiomyopathy (DRC), derived from interstitial fibrosis, is characterized by mutations in desmin or associated proteins and subsequent accumulation of cytotoxic misfolded proteins. Bhuiyan et al. established an enhanced Atg7 transgenic DRC mouse model and observed that the expression of Atg7 was increased in the DRC mouse model by crossing the two kinds of mice. They further confirmed that the Atg7-crossed DRC mouse model showed significantly reduced interstitial fibrosis and ventricular dysfunction accompanied by increased autophagy 139. Furthermore, ubiquitin/proteasome system (UPS) has been reported to mediate cardiomyocyte proteostasis. Ubiquitin-conjugating enzyme 9 (UBC9) is a small ubiquitin-like modifier (SUMO) E2 ligase and its expression improves SUMOylation-mediated and UPS-dependent cardiac protein quality control. Of note, the overexpression of UBC9 decreased cardiac fibrosis and improved cardiac function by increasing autophagy flux in the transgenic mice that overexpressed UBC9 in cardiomyocytes 140. Charged multivesicular body protein 2B (CHMP2B) is a protein participated in autophagosome formation. Mice lacking muscle-specific ubiquitin ligase atrogin-1 (also known as MAFbx1) failed to degrade CHMP2B, leading to autophagy impairment, UPR activation, cardiomyocyte apoptosis and subsequent cardiac fibrosis. The knockdown of CHMP2B restored autophagy flux and thus protected against atrogin-1 deficiency-induced cardiomyocyte death and interstitial fibrosis in the atrogin-1 KO mice 141.
Activation of autophagy improves cardiac fibrosis with different etiologies
Activation of autophagy is reported to attenuate myocardial infarction-related cardiac fibrosis. PDZ and LIM domain 1 (PDLIM1/CLP36), a negative cytoskeleton organization regulator, was accumulated in ischemia-reperfusion (IR) treated cardiomyocytes. Deficiency of Atg7 aggravated myofibrillar disarray, contractile dysfunction and severe cardiac fibrosis by inducing impaired degradation and accumulation of PDLIM1 in cardiomyocytes that specific disruption of Atg7 142. The activation of autophagy was found to reduce fibroblast differentiation and myocardial infarction-related cardiac fibrosis were observed in the SIRT7 KO mice and SIRT7 KO mouse-derived cardiac fibroblasts. Loss of Sirt7 downregulated the expressions of TGF-β RI, Smad2/3 and fibrosis-related genes, including α-SMA and fibronectin by increasing LC3 expression and activating autophagy flux, which was then abolished by CQ in SIRT7-siRNA treated cardiac fibroblasts 143.
Additionally, autophagy improves aging-related cardiac fibrosis. Heat shock protein 27 (HSP27) protected cardiac function from ischemia or chemical challenge and a mouse model with cardiac-specific expression of HSP27 was employed in the experiment of Lin et al. Studies with respect to HSP27 reported that it alleviated the aging-related interstitial fibrosis by increasing the expression of Atg13 and activating mitophagy in the HSP27 transgenic mouse model 144. Rho-associated coiled-coil-containing protein kinases (ROCKs), including ROCK1 and ROCK2, act as physiological regulators for vascular remodeling throughout aging. In ROCK1 and ROCK2 DKO mouse model, aging-related cardiac fibrosis was ameliorated accompanied with increased expression of LC3-II and suppressed Akt-mTOR pathway 145. Moreover, the development of cardiac aging was promoted by reducing the expressions of Atg5, Atg7, LC3-II and Beclin-1 and increasing p62 expression in Akt and AMPK DKO mice 146.
Furthermore, activation of autophagy attenuated fibrosis in diabetes-related cardiopathy. Decades of study have reported that numerous potential factors, including high circulating glucose, HFD, stress and inflammation in diabetes, contribute to susceptibility and progression of cardiopathy and cardiac fibrosis. Expression of tax1 binding protein 1 (TAX1BP1), a ubiquitin-binding protein that restricted NF-κB activation and inhibited inflammation, was decreased in diabetic mouse heart. The overexpression of TAX1BP1 ameliorated cardiac fibrosis and improved cardiac function by accelerating ratios of LC3-II/LC3-I and LC3-II/p62, which was reversed by 3-MA, CQ and bafilomycin A1 in the streptozotocin-induced diabetic cardiomyopathy mouse model 147. High glucose treatment, mimicking diabetes-related cardiopathy, was observed to increase the pro-fibrotic and inflammatory response, trigger oxidative stress and suppress autophagy in rat cardiomyocytes. Administration of fenofibrate (FF), a PPAR-α agonist, induced activation of autophagy and inhibited the fibrotic effects induced by high glucose through the upregulation of fibroblast growth factor 21 (FGF21)/SIRT1, which was reversed by 3-MA and SIRT1 inhibition 148.
Kidney fibrosis and autophagy
Kidney fibrosis is a histological hallmark and the final step of chronic kidney disease (CKD). Differentiation of resident fibroblast-to-myofibroblast, interstitial myofibroblast accumulation and ECM deposition are involved in kidney fibrosis 149, 150. Furthermore, renal tubular epithelial cells are perturbed in the pathological condition of acute kidney injury and contribute to kidney fibrosis by multiple mechanisms, including secreting inflammatory cytokines, growth and apoptosis factors and producing fibrogenic protein 150. Consistent with results in the liver, lung and heart, the function of autophagy in renal fibrosis remains ambiguous (Figure 6).
Figure 6.
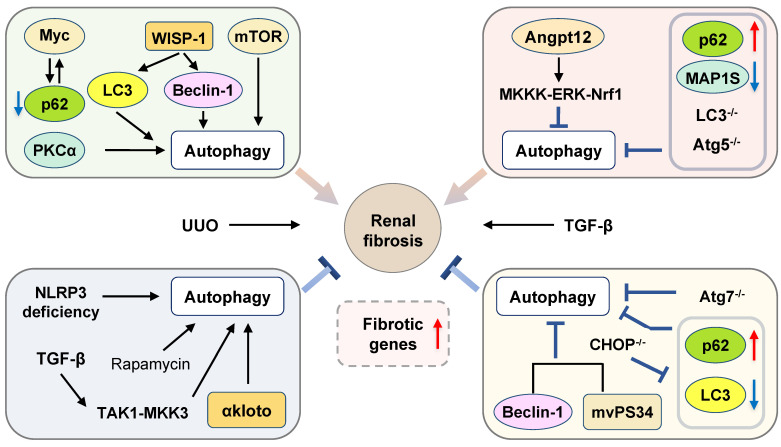
Scheme diagram of autophagy in renal fibrosis. This diagram represents contradictory roles of autophagy played in the pathogenesis of renal fibrosis depending on different experimental models, including pharmacological regulation and genetic modulation. Angpt12: angiopoietin-like protein 12; CHOP: C/EBP homologous protein; MAP1S: microtubule-associated protein 1S; mVps34: mammalian homologue of yeast vacuolar protein sorting defective 34; NLRP3: NOD-like receptors containing pyrin domain 3.
Pro-fibrosis roles of autophagy in the kidney
As many fibrogenic pathways are conserved across tissues, recent findings about autophagy flux in the liver, lung and heart could be further extended to studies of fibrosis in the kidney. Unilateral ureteral obstruction (UUO) is commonly used for the induction of renal fibrosis. Pharmacological inhibition of autophagy by CQ and 3-MA and autophagy disruption induced by genetic deletion of Atg7 in kidney proximal tubule cell prevented UUO-associated renal fibrosis. Consistent with these results, enhancement of autophagy by rapamycin promoted TGF-β-stimulated tubular cell death, interstitial inflammation and the production of α-SMA, fibronectin and vimentin in renal fibroblasts, which were reversed by CQ and 3-MA 149. Previous studies have also demonstrated that WNT family member 1 (WNT1)-induced signaling pathway protein-1 (WISP-1) was involved in fibrosis development in various organs. The overexpression of WISP-1 significantly increased the expression of LC3 and Beclin-1 and aggravated renal fibrosis both in the UUO-induced mouse model and TGF-β-stimulated tubular epithelial cells. Anti-WISP-1 antibody treatment suppressed the activation of autophagy and improved renal injury in the UUO-stimulated rat model 151. mTORC2 and PKCα, one of the major sub-pathways of mTORC2, play a crucial role in the fibroblast activation and renal fibrosis. A recent study demonstrated that activated PKCα accelerated renal fibrosis development by increasing the expression of LAMP-2, autophagosome-lysosome fusion and autophagy flux, which was abolished by PKCα inhibitor Go6976 and PKCα-siRNA in the TGF-β-induced fibroblasts and UUO-stimulated mouse model 150. Myc gene was activated and interacted with p62 to induce functional metabolic derangement and following activation of kidney stromal cells after UUO-induced renal injury. IL-1β treatment stimulated autophagy flux, as indicated by the increased ratios of LC3-II/LC3-I and LC3-II/p62, resulted in the degradation of p62 and accumulation of Myc and subsequently induced tubular fibrosis, which was reversed by the inhibition of IL-1R signal transducer kinase 4 (IRAK4) or Myc 152. Furthermore, in C/EBP homologous protein (CHOP) KO mice, Noh et al. demonstrated that the expression of Beclin-1, LC3-I, LC3-II and p62, and renal cell apoptosis caused by UUO treatment were reduced and renal fibrosis was alleviated 153.
Autophagy is also implicated in the regulation of IR injury-related kidney fibrosis. Baisantry et al. developed a transgenic mouse model with specific deletion of Atg5 in the proximal tubular S3 segments and demonstrated that these mice were protected against IR injury-induced tubular senescence, cell death, and interstitial fibrosis. Interestingly, due to autophagy-mediated the malfunction of fibrosis-related tissue repair, the long-term outcome of these mice after IR injury was worse than WT control mice, as indicated by severe inflammation and development of senescent phenotype 154. Previous studies suggested that proximal epithelial cell (PTC) arrested at G2/M phase is critical for the secretion of fibrotic factors during kidney injury. Recently, Canaud et al. observed that autophagy-dependent formation of mTOR-autophagy spatial coupling compartments (TASCCs) and following mTORC1 activation contributed to the increased secretion of inflammatory and fibrotic proteins in PTC in both IR-induced kidney injury and human chronic kidney diseases. Genetic depletion of Rapter, a key component in the formation of TASCCs, attenuated kidney fibrosis 155.
The promotive effects of autophagy on kidney injury-related fibrosis in other experimental models have also been investigated. The mammalian homologue of yeast vacuolar protein sorting defective 34 (mVps34), a class III PI3K, was reported to combine with Beclin-1 and participate in the regulation of autophagy. It has been shown that specific KO of mVps34 in the podocytes resulted in complemental increased autophagosome and lysosomes and these mice developed obvious podocyte vacuolization, glomerulosclerosis and interstitial fibrosis by 6 weeks of age and died before 9 weeks of age 156. In addition, sodium hydrosulfide protected against chronic renal injury by ameliorating oxidative status, reducing the mRNA levels of LC3-II and Beclin-1 and apoptosis in a 5/6 nephrectomy rat model 157.
Anti-fibrosis roles of autophagy in the kidney
Pharmacological activation of autophagy by rapamycin attenuated Col-1 accumulation and kidney fibrosis in the UUO-induced mouse model and Ang II-treated epithelial cells. Additionally, several whole body or cell-specific autophagy gene KO animals were developed to identify the role of autophagy in renal fibrosis. Genetic inhibition of autophagy by proximal tubular epithelial cell-specific Atg5 KO mice were highly susceptible to UUO-induced kidney fibrosis, concomitant with cellular senescence including mitochondrial dysfunction, mitochondrial DNA abnormalities, nuclear DNA damage, and cell cycle arrest at the G2/M phase 158, 159. Concurrently, Peng et al. applied the same cell-specific Atg5 KO mice and confirmed that the deficiency of Atg5 markedly impaired autophagy, produced more cytokines and accelerated macrophage and lymphocyte infiltration by inducing the nuclear translocation of p65 and transcriptional activity of NF-κB in the obstructed kidneys after UUO treatment 160. Furthermore, endothelial-specific Atg5 KO mice displayed IL-6-dependent endothelial mesenchymal transition and severe renal fibrosis. Neutralization of IL-6 by monoclonal antibody promoted autophagy and alleviated fibrosis, which was reversed by 3-MA 161. In addition to specific Atg5 KO mice, Nam et al. further constructed a tubular epithelial cell-specific Atg7 deletion mouse model and demonstrated that impaired autophagy accelerated renal tubulointerstitial fibrosis and distal tubules EMT-like phenotype change via increasing the expression of TGF-β, IL-1β and NLRP3 in the kidney after UUO treatment 162. Mitochondrial dysfunction and ER stress were reported to be involved in the progression of focal segmental glomerular sclerosis (FSGS), a heterogeneous kidney disease. In the renal epithelium cell-specific Atg5 or Atg7 KO mice, mitochondrial dysfunction was observed in association with ER stress and oxidative stress and the incidence of spontaneous FSGS was elevated 163.
Similar to the findings in the liver, a recent study further identified that MAP1S-related autophagy is involved in the regulation of renal fibrosis. The MAP1S levels were significantly reduced in the renal fibrotic tissues from renal failure patients and the autophagy flux was impaired. As expected, the depletion of MAP1S disrupted autophagy-dependent clearance of fibronectin and induced fibronectin accumulation during renal fibrosis 164. In renal tubular cells, hypoxia induced mitochondrial localization of NLRP3 and its binding to mitochondrial antiviral signal protein (MAVS), which facilitated mitochondrial ROS production and cell apoptosis. The deletion of NLRP3 suppressed the UUO-induced renal fibrosis and decreased the renal tubular cell apoptosis by increasing the autophagy-dependent ROS clearance 165.
Notably, recent studies have yielded many important advances in the understanding of the role of age-dependent autophagy in cellular senescence and fibrosis 166, 167. A previous study suggested that although basal autophagy activity was higher in the aged kidney, autophagy flux and protein turnover were inhibited with aging, as illustrated by the accumulation of p62 and ubiquitin-positive proteins. Accumulation of misfolded proteins further contributed to cell senescence and potentiated renal fibrosis 158. α-Klotho, a type 1 transmembrane protein, is originally identified as an aging suppressor and can be released into circulation and exert multiple effects on target organs.α-Klotho expression deficiency was shown to be responsible for endothelial dysfunction, microangiopathy and fibrosis 168. Similarly, a recent article showed that αKlotho activation attenuated renal fibrosis, protected renal cells from oxidative stress and suppressed the progression of AKI to CKD through the upregulation of autophagy flux, which was reversed in α-Klotho deficiency mouse model 169. Autophagy was also implicated in the fibrogenesis in diabetic nephropathy. Angiopoietin-like protein 12 (Angpt12), a member of the Angptl family, has a critical function in physiological tissue remodeling. Activated Angptl2 downregulated autophagy, increased pro-inflammatory factors, accelerated renal fibrosis by activating the MKKK-ERK-Nrf1 pathway, which was completely reversed by Angptl2 knockdown in a diabetic rat model 170. Additionally, calorie restriction decreased the accumulation of oxidized GSH, ameliorated tubulointerstitial macrophage infiltration and renal fibrosis by promoting autophagy in ob/ob diabetic mice 171.
Autophagy and the TGF-β-related pathways
Emerging evidence suggests that TGF-β treatment not only stimulates collagen deposition but also induces autophagy in the primary mouse and human tubular epithelial cells. Ding et al. further constructed LC3-/- or Beclin-1+/- mouse model and identified that the disruption of autophagy promoted collagen accumulation and the expression of TGF-β in the UUO mouse model 172. Previous studies also demonstrated a crucial role of autophagy played in the transcriptional regulation of collagen expression. TGF-β-activated kinase 1 (TAK1), a serine/threonine kinase, belongs to the MAPK kinase kinase (MKKK) family. In addition to the Smad signaling pathways, TGF-β phosphorylates and activates TAK1 in a Smad-independent manner and subsequently triggers downstream signaling cascades. Recently, researchers reported dual roles of TGF-β that TGF-β not only induced Col-1 accumulation but also promoted autophagy and Col-I degradation by inducing the TAK1-MAPK kinase 3 (MKK3) pathway in primary mouse mesangial cells, which was confirmed in the MKK KO mice. Dysfunction of autophagy induced by heterozygous deletion of Beclin-1 increased the protein levels and accumulation of Col-1 in primary mouse mesangial cells, indicating that the intracellular degradation of Col-I and insoluble pro-collagen I was at least partly dependent on autophagy 173.
Autophagy and cystic fibrosis
Cystic fibrosis, as a most frequent lethal genetic disorder, is induced by the mutations in the CFTR gene and affects multiple organs, including the lung, liver, kidney, pancreas and intestine 174. The most common observed mutation of CFTR is a phenylalanine deletion at position 508, which leads to protein misfolding, incomplete glycosylation, impaired cell-surface trafficking and eventually abolished the function of CFTR as a plasma membrane located ion channel. Although rare, mutation-mediated CFTR dysfunction is implicated in the dysregulation of glucose metabolism, impairment of insulin release, complications in lung and airway and subsequent multiorgan failure, which contribute to the high mortality of cystic fibrosis 175.
Pro-fibrosis roles of autophagy in cystic fibrosis
Cystic lung fibrotic disease is considered as a hypersecretory inflammatory disease characterized by airway obstruction and treatments aiming to control chronic inflammation and decrease excess mucus are effective strategies to reverse cystic fibrosis. In a recent report, IL-13 aggravated cystic fibrosis-induced airway inflammatory by accelerating the formation of airway goblet cells that secreted excess mucus. Notably, the inhibition of autophagy by 3-MA and bafilomycin A1 attenuated mucus secretion in the IL-13-induced mouse model, which was further confirmed by Atg5 or Atg16 depleting 175.
Anti-fibrosis roles of autophagy in cystic fibrosis
It has been well-established that the failure of autophagy is closely associated with the pathogenesis of cystic fibrosis. It has been reported that autophagy was impaired in cystic fibrosis patients and cystic fibrosis mice, as illustrated by downregulated intracellular expression of essential autophagy molecules including LC3-II, Beclin-1 and Atg5 176. On the other hand, defective CFTR function further impaired autophagy in cystic fibrosis patients. In the airway epithelial cells of patients with cystic fibrosis, dysfunction of mutated CFTR stimulated Beclin-1 reduction and p62 accumulation via upregulating ROS and tissue transglutaminase (TG2) 177.
A growing number of studies suggested that restoring autophagy presented beneficial effects against cystic fibrosis development. Under cellular ER stress and an ER-to-Golgi transport blockade, immature mutated CFTR was transported to cytomembrane from ER through an unconventional route bypassed Golgi, which partially restored the function of mutated CFTR as an ion channel. Interestingly, this unconventional trafficking is dependent on the activation of autophagy. Autophagy inhibition mediated by 3-MA, wortmannin, and genetic deletion of Atg5 and Atg7 significantly abolished the unconventional secretion of CFTR 178. The binding of Bcl2-associated athanogene (BAG) 3 with HSP70 is critical for the UPS- and autophagy-dependent misfolded proteins degradation. The knockdown of BAG3 by specific siRNA not only restored autophagy in cystic fibrosis but also functionally corrected mutated CFTR by enhancing its stability and facilitating its trafficking. Compromising autophagy by Atg7 siRNA inhibited the protective effects of BAG3 silencing 179. It has been reported that Atg7 and Atg16 were targets of miR-17-92 and downregulation of miR-17-92 improved CFTR channel function and reversed the compromised ability of transport halide through the activation of autophagy in macrophages 174.
Cystic fibrosis patients are more susceptible to infection induced by bacteria, including P seudomonas aeruginosa and B.cenocepacia. Emerging evidence suggested that the dysfunction of mutated CFTR and defective autophagy synergistically compromised internalization and clearance of bacteria by macrophages and epithelial cells. Ferrari et al. demonstrated that proteostasis regulator cysteamine partly rescued the function of mutated CFTR, enhanced macrophages-mediated bacteria clearance and ameliorated inflammation through the upregulation of Beclin-1 and activation of autophagy 180. Furthermore, pyocyanin is a well-described P. aeruginosa virulence factor playing an important role in the airway infection and initial inflammation in cystic fibrosis patients and significantly induces autophagy through eIF2α-ATF4 pathway. Interestingly, inhibition of autophagy by CQ significantly increased bacteria colonization, and the mortality rate of P. aeruginosa infection, suggesting that activation of autophagy is an adaptive and protective mechanism 181. Furthermore, the activation of autophagy induced by epigallocatechin-3-gallate restricted the B.cenocepacia replication and subsequently ameliorated cystic fibrosis by suppressing the methylation of Atg12 promoter and increasing Atg12 expression 176.
Conclusion and future prospection
Fibrosis is a chronic disease characterized by an excessive accumulation of ECM and is a hallmark feature of pathologic remodeling in numerous tissues. Although the specific symptoms of fibrosis are diverse in each organ, the pathogenesis across organs shares several common mechanisms, including but not limited to excessive activation of fibroblasts, conversion of fibroblasts to myofibroblasts and the proliferation of ECM-producing myofibroblasts. As a complex and essential catabolic process, autophagy occurs in most mammalian cells and is considered to be a crucial regulator of multiorgan fibrotic diseases. However, based on all the evidence summarized above, it is not difficult to see the controversial role of autophagy in the regulation and development of fibrotic diseases (Figure 7). Some studies have shown that autophagy participates in lipid droplet digestion and energy supply for fibroblast activation and can aggravate fibrosis, whereas other studies have revealed that activated autophagy may attenuate inflammatory responses and ameliorate fibrosis in multiple organs. Considering the contradictory role of autophagy in the pathogenesis of almost all types of fibrotic diseases, the identification of downstream specific targets or signaling pathways of autophagy remains crucial for the treatment of fibrosis.
Figure 7.
Generalized relations between autophagy and fibrosis. Fibrosis: liver fibrosis, lung fibrosis, cardiac fibrosis, renal fibrosis and cystic fibrosis.
Fibrotic diseases are commonly progressive, and their pathological manifestations at different stages of fibrosis vary dramatically. LDs are stored in quiescent fibroblasts at an early step in fibrosis but not in activated myofibroblasts. Concurrently, the breakdown of intracellular lipids and the satisfaction of energy requirements are indispensable to establish an activated fibroblast phenotype. Thus, functions and susceptibility to activation of autophagy may be different in the diverse stages of fibrosis. Moreover, given that aging may affect the alteration of autophagy activity and concurrently increase the risk of fibrosis, comparing and analyzing experimental results between adult and aged mice is unpersuasive and less rigorous. In addition, numerous studies have demonstrated that multiple signaling pathways are involved in the activation and development of fibrosis. Considering the simultaneous effects of these diverse signaling pathways on the regulation of autophagy, it is essential to distinguish the autophagy-mediated effects from secondary compensating actions. Taking liver fibrosis as an example, although lncRNA H19 activation accelerated liver fibrosis and concurrently triggered autophagy by activating the PI3K/Akt/mTOR pathway, it is inappropriate to tell whether activated autophagy in this mouse model is the pathogenic mechanism or a compensatory response against fibrosis. Similarly, due to the specific anti-fibrotic effects of TGF-β inhibition itself, it is inaccurate and subjective to conclude that inhibition of autophagy can ameliorate liver fibrosis in studies that simultaneously blocked autophagy and TGF-β pathways. In addition, the exact role of autophagy in fibrogenesis is dependent on specific cell types and different stimuli. Gao et al. indicated that autophagy was inhibited in PDGF- but not TGF-β-stimulated HSCs, leading to enhanced release of fibrogenic EVs 53. Consistent with these results, Allaire et al. confirmed that the role of autophagy in fibrosis is more complicated than originally thought and probably depends on specific cell types 5. Further identification and evaluation of the function of autophagy in different cell types and specific stimuli will improve the understanding of the role of autophagy in fibrosis.
Mounting evidence showed that autophagy flux was considered to be a dynamic process of autophagy, involving the formation and secretion of autophagosomes, the phagocytosis of cargo and the formation and degradation of autolysosomes. Compared with the expression of autophagy markers, the change in autophagy flux, as assessed by ratios of LC3-II/LC3-I and LC3-II/p62, more adequately represent the activity of autophagy. Interestingly, several studies demonstrated that although LC3-II accumulation was activated, blocking autophagy flux and inhibited autophagy activity were observed in vivo and in vitro 182, 183, suggesting the need to measure autophagy flux in future studies. As reviewed above, the suppressive effects of increased autophagy flux on fibrosis in multiple organs, including the liver, lung, heart and kidney have been consistently observed. However, the contrasting results of experiments have described the promoting effects of increased autophagy on liver and kidney fibrosis, where only the expression of autophagy markers was examined and autophagy flux was rarely assessed, indicating that these studies were not comprehensive and objective.
Certainly, experimental evidence obtained from the specific-autophagy genes, like Atg genes, KO mice are necessary and convincing to elucidate the role of autophagy in the fibrosis process. Considering that embryonic or neonatal lethality has been detected in conventional Atg gene KO mice, conditional tissue/cell-specific or whole-body gene KO was performed in adult mice 184. Interestingly, due to the lower mortality of Atg5 and Atg7 KO mice compared with that of mice with other gene deletions, previous studies used Atg KO mice, mostly targeting Atg5 and Atg7, but rarely used LC3 and Atg4b KO mice. Most of these studies showed that impaired autophagy promoted inflammation, cell apoptosis, ROS production and subsequently aggravated multiorgan fibrosis, while a few studies reported that autophagy inhibition ameliorated liver fibrosis and cell senescence in the kidney. It is noteworthy that Atg gene KO, regardless of whether they are global or tissue/cell-specific, induce complete and irreversible abrogation of autophagy, which does not generally exhibit the etiology of human diseases. Thus, these issues bring substantial obstacles to translate observations from Atg KO mouse experiments to clinical therapies of fibrotic diseases.
Collectively, emerging evidence has confirmed and made substantial strides in promoting a better understanding of the role of autophagy in multiorgan fibrosis, including liver, lung, cardiac, renal and cystic fibrosis. Although the anti-fibrotic function of autophagy remains a controversial topic, it is indisputable that autophagy has increasingly become a promising therapeutic target of fibrotic diseases. Therefore, characterizing and emphasizing the function of autophagy in fibrosis not only provides a novel framework for the appreciation and exploration of pathogenesis in fibrosis but also is urgently required for the discovery of potential clinical treatments of multiorgan fibrosis.
Acknowledgments
This work was supported by grants from Beijing Nova Program of Science and Technology (Grant NO. Z191100001119088 to XL); Research grant from Beijing University of Chinese Medicine (Grant NO. 2020-JYB-ZDGG-038 to RL); Beijing University of Chinese Medicine Specific Grant for "double top construction" (Grant NO. 1000041510168 to XL, 1000041510166 to RL).
Authors' contributions
Dr. X. Li conceived the original idea. Dr. X. Li, Dr. R. Liu and Ms. Y. Li wrote and revised the manuscript. Ms. Y. Li and Ms. J. Wu revised figures.
Abbreviation
- AMPK
AMP-activated protein kinase
- Ang II
angiotensin II
- Akt
protein kinase B
- Atg
autophagy-related gene
- BDL
bile duct ligation
- BLM
bleomycin
- CCl4
carbon tetrachloride
- CQ
chloroquine
- Col-1
collagen type I
- CFTR
cystic fibrosis transmembrane conductance regulator
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK1/2
extracellular signal-regulated kinase1/2
- HSC
hepatic stellate cell
- IPF
idiopathic pulmonary fibrosis
- LC3
light chain 3
- LD
lipid droplet
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositide3-kinase
- TAK1
TGF-β-activated kinase 1
- TGF-β
Transforming growth factor-beta
- TLR
toll-like receptor
- UUO
unilateral ureteral obstruction
References
- 1.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XW, Zhou JC, Peng D, Hua F, Li K, Yu JJ. et al. Disrupting the TRIB3-SQSTM1 interaction reduces liver fibrosis by restoring autophagy and suppressing exosome-mediated HSC activation. Autophagy. 2020;16:782–96. doi: 10.1080/15548627.2019.1635383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravanan P, Srikumar IF, Talwar P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Allaire M, Rautou PE, Codogno P, Lotersztajn S. Autophagy in liver diseases: Time for translation? J Hepatol. 2019;70:985–98. doi: 10.1016/j.jhep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Cavalli G, Cenci S. Autophagy and protein secretion. J Mol Biol. 2020;432:2525–45. doi: 10.1016/j.jmb.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Czaja MJ, Ding WX, Donohue TM Jr, Friedman SL, Kim JS, Komatsu M. et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–58. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y, Sun G, Li E, Kiselyov K, Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev. 2017;34:3–14. doi: 10.1016/j.arr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X. et al. Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis. Hepatology. 2019;70:1317–35. doi: 10.1002/hep.30662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Liu R, Huang Z, Gurley EC, Wang X, Wang J. et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology. 2018;68:599–615. doi: 10.1002/hep.29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy LD, Young AR, Perez-Mancera PA, Nimmervoll B, Jaulim A, Chen HC. et al. A novel Atg5-shRNA mouse model enables temporal control of Autophagy in vivo. Autophagy. 2018;14:1256–66. doi: 10.1080/15548627.2018.1458172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z. et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–46. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie ZY, Xiao ZH, Wang FF. Inhibition of autophagy reverses alcohol-induced hepatic stellate cells activation through activation of Nrf2-Keap1-ARE signaling pathway. Biochimie. 2018;147:55–62. doi: 10.1016/j.biochi.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Tao Y, Qiu T, Yao X, Jiang L, Wang N, Jia X. et al. Autophagic-CTSB-inflammasome axis modulates hepatic stellate cells activation in arsenic-induced liver fibrosis. Chemosphere. 2019;242:124959. doi: 10.1016/j.chemosphere.2019.124959. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Huang Q, Wang Y, Shen P, Guan F, Li J. et al. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem Biophys Res Commun. 2014;454:328–34. doi: 10.1016/j.bbrc.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Sun Y, Zuo L, Wang Y, Huang Y. ASIC1a promotes high glucose and PDGF-induced hepatic stellate cell activation by inducing autophagy through CaMKKbeta/ERK signaling pathway. Toxicol Lett. 2019;300:1–9. doi: 10.1016/j.toxlet.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Yang A, Jiao Y, Yang S, Deng M, Yang X, Mao C. et al. Homocysteine activates autophagy by inhibition of CFTR expression via interaction between DNA methylation and H3K27me3 in mouse liver. Cell Death Dis. 2018;9:169. doi: 10.1038/s41419-017-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Liu J, Yang L, Ling W. AMP-activated protein kinase regulates lipid metabolism and the fibrotic phenotype of hepatic stellate cells through inhibition of autophagy. FEBS Open Bio. 2017;7:811–20. doi: 10.1002/2211-5463.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye HL, Zhang JW, Chen XZ, Wu PB, Chen L, Zhang G. Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy. Life Sci. 2020;242:117175. doi: 10.1016/j.lfs.2019.117175. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Zhang Y, Zhang Q, Guo X, Zhang H, Zheng G. et al. Insulin-like growth factor binding protein-related protein 1 (IGFBPrP1) contributes to liver inflammation and fibrosis via activation of the ERK1/2 pathway. Hepatol Int. 2015;9:130–41. doi: 10.1007/s12072-014-9578-9. [DOI] [PubMed] [Google Scholar]
- 22.Kong Y, Huang T, Zhang H, Zhang Q, Ren J, Guo X. et al. The lncRNA NEAT1/miR-29b/Atg9a axis regulates IGFBPrP1-induced autophagy and activation of mouse hepatic stellate cells. Life Sci. 2019;237:116902. doi: 10.1016/j.lfs.2019.116902. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Zhang Q, Kong Y, Guo X, Zhang H, Fan H. et al. Insulin-Like Growth Factor Binding Protein-Related Protein 1 Activates Primary Hepatic Stellate Cells via Autophagy Regulated by the PI3K/Akt/mTOR Signaling Pathway. Dig Dis Sci. 2020;65:509–23. doi: 10.1007/s10620-019-05798-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Huang TJ, Ren JJ, Zhang QQ, Kong YY, Zhang HY, Guo XH. et al. IGFBPrP1 accelerates autophagy and activation of hepatic stellate cells via mutual regulation between H19 and PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2019;116:109034. doi: 10.1016/j.biopha.2019.109034. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, Wang D, Zhu X, Wang G, You Y, Ning Z. et al. Autophagic degradation of caveolin-1 promotes liver sinusoidal endothelial cells defenestration. Cell Death Dis. 2018;9:576. doi: 10.1038/s41419-018-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhao S, Yao Z, Wang L, Shao J, Chen A. et al. Autophagy regulates turnover of lipid droplets via ROS-dependent Rab25 activation in hepatic stellate cell. Redox Biol. 2017;11:322–34. doi: 10.1016/j.redox.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Hong Y, Li S, Wang J, Li Y. In vitro inhibition of hepatic stellate cell activation by the autophagy-related lipid droplet protein ATG2A. Sci Rep. 2018;8:9232. doi: 10.1038/s41598-018-27686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Yu Y, Li S, Liu Y, Zhou S, Cao S. et al. MicroRNA-30a ameliorates hepatic fibrosis by inhibiting Beclin1-mediated autophagy. J Cell Mol Med. 2017;21:3679–92. doi: 10.1111/jcmm.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Liu J, Yang W, Ling W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy. 2017;13:1813–27. doi: 10.1080/15548627.2017.1356550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Tao Y, Qiu T, Yao X, Jiang L, Wang N. et al. Taurine protected As2O3-induced the activation of hepatic stellate cells through inhibiting PPARalpha-autophagy pathway. Chem Biol Interact. 2019;300:123–30. doi: 10.1016/j.cbi.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Zhang Q, Mo W, Feng J, Li S, Li J. et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng J, Chen K, Xia Y, Wu L, Li J, Li S. et al. Salidroside ameliorates autophagy and activation of hepatic stellate cells in mice via NF-kappaB and TGF-beta1/Smad3 pathways. Drug Des Devel Ther. 2018;12:1837–53. doi: 10.2147/DDDT.S162950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y. et al. Isorhamnetin Inhibits Liver Fibrosis by Reducing Autophagy and Inhibiting Extracellular Matrix Formation via the TGF-beta1/Smad3 and TGF-beta1/p38 MAPK Pathways. Mediators Inflamm. 2019;2019:6175091. doi: 10.1155/2019/6175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriola Benitez PC, Pesce Viglietti AI, Herrmann CK, Dennis VA, Comerci DJ, Giambartolomei GH. et al. Brucella abortus Promotes a Fibrotic Phenotype in Hepatic Stellate Cells, with Concomitant Activation of the Autophagy Pathway. Infect Immun. 2017;86:e00522–17. doi: 10.1128/IAI.00522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan S, Lu Y, Xu M, Huang X, Liu H, Jiang J. et al. beta-Arrestin1 enhances liver fibrosis through autophagy-mediated Snail signaling. FASEB J. 2019;33:2000–16. doi: 10.1096/fj.201800828RR. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Zeng C, Zheng B, Liu C, Tang M, Jiang Y. et al. HMGB1-induced autophagy facilitates hepatic stellate cells activation: a new pathway in liver fibrosis. Clin Sci (Lond) 2018;132:1645–67. doi: 10.1042/CS20180177. [DOI] [PubMed] [Google Scholar]
- 37.Xie ZY, Wang FF, Xiao ZH, Liu SF, Lai YL, Tang SL. Long noncoding RNA XIST enhances ethanol-induced hepatic stellate cells autophagy and activation via miR-29b/HMGB1 axis. IUBMB Life. 2019;71:1962–72. doi: 10.1002/iub.2140. [DOI] [PubMed] [Google Scholar]
- 38.Kim KM, Han CY, Kim JY, Cho SS, Kim YS, Koo JH. et al. Galpha12 overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J Hepatol. 2018;68:493–504. doi: 10.1016/j.jhep.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu K, Li N, Cheng Q, Zheng J, Zhu M, Bao S. et al. miR-96-5p prevents hepatic stellate cell activation by inhibiting autophagy via ATG7. J Mol Med (Berl) 2018;96:65–74. doi: 10.1007/s00109-017-1593-6. [DOI] [PubMed] [Google Scholar]
- 40.Hung TM, Yuan RH, Huang WP, Chen YH, Lin YC, Lin CW. et al. Increased Autophagy Markers Are Associated with Ductular Reaction during the Development of Cirrhosis. Am J Pathol. 2015;185:2454–67. doi: 10.1016/j.ajpath.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Hung TM, Huang YJ, Lin YC, Chen YH, Wu YM, Lee PH. A critical role of autophagy in regulating the mesenchymal transition of ductular cells in liver cirrhosis. Sci Rep. 2019;9:10673. doi: 10.1038/s41598-019-46764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spirli C, Villani A, Mariotti V, Fabris L, Fiorotto R, Strazzabosco M. Posttranslational regulation of polycystin-2 protein expression as a novel mechanism of cholangiocyte reaction and repair from biliary damage. Hepatology. 2015;62:1828–39. doi: 10.1002/hep.28138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Ni HM, Chao X, Yang H, Deng F, Wang S, Bai Q. et al. Dual Roles of Mammalian Target of Rapamycin in Regulating Liver Injury and Tumorigenesis in Autophagy-Defective Mouse Liver. Hepatology. 2019;70:2142–55. doi: 10.1002/hep.30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP. et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61:617–25. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo HY, Lee SH, Lee JH, Kang YN, Hwang JS, Park KG. et al. Src Inhibition Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation and Decreasing Connetive Tissue Growth Factor. Cells. 2020;9:558. doi: 10.3390/cells9030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge M, Liu H, Zhang Y, Li N, Zhao S, Zhao W. et al. The anti-hepatic fibrosis effects of dihydrotanshinone I are mediated by disrupting the yes-associated protein and transcriptional enhancer factor D2 complex and stimulating autophagy. Br J Pharmacol. 2017;174:1147–60. doi: 10.1111/bph.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.BasuRay S. RAB18 modulates autophagy in human stellate cells. J Clin Lipidol. 2019;13:832–8. doi: 10.1016/j.jacl.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Yao Z, Zhao S, Shao J, Chen A, Zhang F. et al. Interaction between autophagy and senescence is required for dihydroartemisinin to alleviate liver fibrosis. Cell Death Dis. 2017;8:e2886. doi: 10.1038/cddis.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Zou J, Yue F, Song K, Chen Q, McKeehan WL. et al. Defects in MAP1S-mediated autophagy cause reduction in mouse lifespans especially when fibronectin is overexpressed. Aging Cell. 2016;15:370–9. doi: 10.1111/acel.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yue F, Li W, Zou J, Jiang X, Xu G, Huang H. et al. Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res. 2017;77:2938–51. doi: 10.1158/0008-5472.CAN-16-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobi AJ, Huber ST, Mortensen SA, Schultz SW, Palara A, Kuhm T. et al. Structural basis of p62/SQSTM1 helical filaments and their role in cellular cargo uptake. Nat Commun. 2020;11:440. doi: 10.1038/s41467-020-14343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu P, de la Vega MR, Dodson M, Yue F, Shi B, Fang D. et al. Spermidine Confers Liver Protection by Enhancing NRF2 Signaling Through a MAP1S-Mediated Noncanonical Mechanism. Hepatology. 2019;70:372–88. doi: 10.1002/hep.30616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020. S0168-8278: 30290-7. [DOI] [PMC free article] [PubMed]
- 54.Yang R, Hu Z, Zhang P, Wu S, Song Z, Shen X. et al. Probucol ameliorates hepatic stellate cell activation and autophagy is associated with farnesoid X receptor. J Pharmacol Sci. 2019;139:120–8. doi: 10.1016/j.jphs.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Tan TC, Crawford DH, Jaskowski LA, Subramaniam VN, Clouston AD, Crane DI. et al. Excess iron modulates endoplasmic reticulum stress-associated pathways in a mouse model of alcohol and high-fat diet-induced liver injury. Lab Invest. 2013;93:1295–312. doi: 10.1038/labinvest.2013.121. [DOI] [PubMed] [Google Scholar]
- 56.Alers S, Loffler AS, Paasch F, Dieterle AM, Keppeler H, Lauber K. et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–33. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Fickert P, Trauner M, Marcus N, Blomenkamp K, Teckman J. Autophagy induced by exogenous bile acids is therapeutic in a model of alpha-1-AT deficiency liver disease. Am J Physiol Gastrointest Liver Physiol. 2016;311:G156–65. doi: 10.1152/ajpgi.00143.2015. [DOI] [PubMed] [Google Scholar]
- 58.Tang Y, Blomenkamp KS, Fickert P, Trauner M, Teckman JH. NorUDCA promotes degradation of alpha1-antitrypsin mutant Z protein by inducing autophagy through AMPK/ULK1 pathway. PLoS One. 2018;13:e0200897. doi: 10.1371/journal.pone.0200897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao RQ, Ren C, Xia ZF, Yao YM. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2020. p: 1-17. [DOI] [PMC free article] [PubMed]
- 60.Pastore N, Ballabio A, Brunetti-Pierri N. Autophagy master regulator TFEB induces clearance of toxic SERPINA1/alpha-1-antitrypsin polymers. Autophagy. 2013;9:1094–6. doi: 10.4161/auto.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu YM, Cong S, Cheng Z, Hu YX, Lei Y, Zhu LL. et al. Platycodin D alleviates liver fibrosis and activation of hepatic stellate cells by regulating JNK/c-JUN signal pathway. Eur J Pharmacol. 2020;876:172946. doi: 10.1016/j.ejphar.2020.172946. [DOI] [PubMed] [Google Scholar]
- 62.Qiu S, Xu H, Lin Z, Liu F, Tan F. The blockade of lipophagy pathway is necessary for docosahexaenoic acid to regulate lipid droplet turnover in hepatic stellate cells. Biomed Pharmacother. 2019;109:1841–50. doi: 10.1016/j.biopha.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Fukushima H, Yamashina S, Arakawa A, Taniguchi G, Aoyama T, Uchiyama A. et al. Formation of p62-positive inclusion body is associated with macrophage polarization in non-alcoholic fatty liver disease. Hepatol Res. 2018;48:757–67. doi: 10.1111/hepr.13071. [DOI] [PubMed] [Google Scholar]
- 64.Stacchiotti A, Grossi I, García-Gómez R, Patel GA, Salvi A, Lavazza A. et al. Melatonin Effects on Non-Alcoholic Fatty Liver Disease Are Related to MicroRNA-34a-5p/Sirt1 Axis and Autophagy. Cells. 2019;8:1053. doi: 10.3390/cells8091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen R, Wang Q, Song S, Liu F, He B, Gao X. Protective role of autophagy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice. Eur J Pharmacol. 2016;770:126–33. doi: 10.1016/j.ejphar.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 66.Fucho R, Martinez L, Baulies A, Torres S, Tarrats N, Fernandez A. et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J Hepatol. 2014;61:1126–34. doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding X, Jiang X, Tian R, Zhao P, Li L, Wang X. et al. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy. 2019;15:1774–86. doi: 10.1080/15548627.2019.1596478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng X, Sun Q. RUBCNL/Pacer and RUBCN/Rubicon in regulation of autolysosome formation and lipid metabolism. Autophagy. 2019;15:1120–1. doi: 10.1080/15548627.2019.1596500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043–53. doi: 10.1016/j.biopha.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 70.Challa TD, Wueest S, Lucchini FC, Dedual M, Modica S, Borsigova M. et al. Liver ASK1 protects from non-alcoholic fatty liver disease and fibrosis. EMBO Mol Med. 2019;11:e10124. doi: 10.15252/emmm.201810124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu R, Wu K, Li Y, Sun R, Li X. Human antigen R: A potential therapeutic target for liver diseases. Pharmacol Res. 2020;155:104684. doi: 10.1016/j.phrs.2020.104684. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A. et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2019. p: 1-24. [DOI] [PMC free article] [PubMed]
- 74.Wang HY, Li C, Liu WH, Deng FM, Ma Y, Guo LN. et al. Autophagy inhibition via Becn1 downregulation improves the mesenchymal stem cells antifibrotic potential in experimental liver fibrosis. J Cell Physiol. 2019;235:2722–37. doi: 10.1002/jcp.29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lodder J, Denaes T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM. et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–92. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Guo M, Zhao S, Shao J, Zheng S. ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic Biol Med. 2016;101:272–83. doi: 10.1016/j.freeradbiomed.2016.10.498. [DOI] [PubMed] [Google Scholar]
- 77.Liu Z, Zhu P, Zhang L, Xiong B, Tao J, Guan W. et al. Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed Pharmacother. 2018;103:1262–71. doi: 10.1016/j.biopha.2018.04.156. [DOI] [PubMed] [Google Scholar]
- 78.Liu H, Fang S, Wang W, Cheng Y, Zhang Y, Liao H. et al. Macrophage-derived MCPIP1 mediates silica-induced pulmonary fibrosis via autophagy. Part Fibre Toxicol. 2016;13:55. doi: 10.1186/s12989-016-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H, Cheng Y, Yang J, Wang W, Fang S, Zhang W. et al. BBC3 in macrophages promoted pulmonary fibrosis development through inducing autophagy during silicosis. Cell Death Dis. 2017;8:e2657. doi: 10.1038/cddis.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghavami S, Yeganeh B, Zeki AA, Shojaei S, Kenyon NJ, Ott S. et al. Autophagy and the unfolded protein response promote profibrotic effects of TGF-beta1 in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2018;314:L493–L504. doi: 10.1152/ajplung.00372.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsubouchi K, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N. et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13:1420–34. doi: 10.1080/15548627.2017.1328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, Carter AB. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity. 2016;44:582–96. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganzleben I, He GW, Gunther C, Prigge ES, Richter K, Rieker RJ. et al. PGAM5 is a key driver of mitochondrial dysfunction in experimental lung fibrosis. Cell Mol Life Sci. 2019;76:4783–94. doi: 10.1007/s00018-019-03133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joachim J, Jefferies HB, Razi M, Frith D, Snijders AP, Chakravarty P. et al. Activation of ULK Kinase and Autophagy by GABARAP Trafficking from the Centrosome Is Regulated by WAC and GM130. Mol Cell. 2015;60:899–913. doi: 10.1016/j.molcel.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park S, Kim S, Kim MJ, Hong Y, Lee AY, Lee H. et al. GOLGA2 loss causes fibrosis with autophagy in the mouse lung and liver. Biochem Biophys Res Commun. 2018;495:594–600. doi: 10.1016/j.bbrc.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 86.Kesireddy VS, Chillappagari S, Ahuja S, Knudsen L, Henneke I, Graumann J. et al. Susceptibility of microtubule-associated protein 1 light chain 3beta (MAP1LC3B/LC3B) knockout mice to lung injury and fibrosis. FASEB J. 2019;33:12392–408. doi: 10.1096/fj.201900854R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M. et al. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy. 2015;11:670–84. doi: 10.1080/15548627.2015.1034409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell. 2015;14:774–83. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao X, Wei S, Li Z, Lin C, Zhu Z, Sun D. et al. Autophagic flux blockage in alveolar epithelial cells is essential in silica nanoparticle-induced pulmonary fibrosis. Cell Death Dis. 2019;10:127. doi: 10.1038/s41419-019-1340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng Y, Luo W, Li Z, Cao M, Zhu Z, Han C. et al. CircRNA-012091/PPP1R13B-mediated Lung Fibrotic Response in Silicosis via Endoplasmic Reticulum Stress and Autophagy. Am J Respir Cell Mol Biol. 2019;61:380–91. doi: 10.1165/rcmb.2019-0017OC. [DOI] [PubMed] [Google Scholar]
- 91.Surolia R, Li FJ, Wang Z, Li H, Dsouza K, Thomas V. et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight. 2019;4:e123253. doi: 10.1172/jci.insight.123253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milara J, Hernandez G, Ballester B, Morell A, Roger I, Montero P. et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir Res. 2018;19:24. doi: 10.1186/s12931-018-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hill C, Li J, Liu D, Conforti F, Brereton CJ, Yao L. et al. Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis. 2019;10:591. doi: 10.1038/s41419-019-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang K, Zhang T, Lei Y, Li X, Jiang J, Lan J. et al. Identification of ANXA2 (annexin A2) as a specific bleomycin target to induce pulmonary fibrosis by impeding TFEB-mediated autophagic flux. Autophagy. 2018;14:269–82. doi: 10.1080/15548627.2017.1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He X, Chen S, Li C, Ban J, Wei Y, He Y. et al. Trehalose Alleviates Crystalline Silica-Induced Pulmonary Fibrosis via Activation of the TFEB-Mediated Autophagy-Lysosomal System in Alveolar Macrophages. Cells. 2020;9:122. doi: 10.3390/cells9010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang LS, Berdyshev EV, Tran JT, Xie L, Chen J, Ebenezer DL. et al. Sphingosine-1-phosphate lyase is an endogenous suppressor of pulmonary fibrosis: role of S1P signalling and autophagy. Thorax. 2015;70:1138–48. doi: 10.1136/thoraxjnl-2014-206684. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Huang G, Wang Z, Qin H, Mo B, Wang C. Elongation factor-2 kinase acts downstream of p38 MAPK to regulate proliferation, apoptosis and autophagy in human lung fibroblasts. Exp Cell Res. 2018;363:291–8. doi: 10.1016/j.yexcr.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 98.Han R, Ji X, Rong R, Li Y, Yao W, Yuan J. et al. MiR-449a regulates autophagy to inhibit silica-induced pulmonary fibrosis through targeting Bcl2. J Mol Med (Berl) 2016;94:1267–79. doi: 10.1007/s00109-016-1441-0. [DOI] [PubMed] [Google Scholar]
- 99.Xu T, Yan W, Wu Q, Xu Q, Yuan J, Li Y. et al. MiR-326 Inhibits Inflammation and Promotes Autophagy in Silica-Induced Pulmonary Fibrosis through Targeting TNFSF14 and PTBP1. Chem Res Toxicol. 2019;32:2192–203. doi: 10.1021/acs.chemrestox.9b00194. [DOI] [PubMed] [Google Scholar]
- 100.Dakhlallah D, Wang Y, Bobo TA, Ellis E, Mo X, Piper MG. et al. Constitutive AKT Activity Predisposes Lung Fibrosis by Regulating Macrophage, Myofibroblast and Fibrocyte Recruitment and Changes in Autophagy. Adv Biosci Biotechnol. 2019;10:346–73. doi: 10.4236/abb.2019.1010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Im J, Hergert P, Nho RS. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am J Physiol Lung Cell Mol Physiol. 2015;309:L552–61. doi: 10.1152/ajplung.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saito S, Zhuang Y, Shan B, Danchuk S, Luo F, Korfei M. et al. Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta-PI3K-Akt pathway. PLoS One. 2017;12:e0186615. doi: 10.1371/journal.pone.0186615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi K, Araya J, Minagawa S, Hara H, Saito N, Kadota T. et al. Involvement of PARK2-Mediated Mitophagy in Idiopathic Pulmonary Fibrosis Pathogenesis. J Immunol. 2016;197:504–16. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 104.Kurita Y, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N. et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir Res. 2017;18:114. doi: 10.1186/s12931-017-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero Y, Bueno M, Ramirez R, Alvarez D, Sembrat JC, Goncharova EA. et al. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell. 2016;15:1103–12. doi: 10.1111/acel.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernard M, Yang B, Migneault F, Turgeon J, Dieude M, Olivier MA, Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy. 2020. p: 1-13. [DOI] [PMC free article] [PubMed]
- 107.Tai H, Wang Z, Gong H, Han X, Zhou J, Wang X. et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13:99–113. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gui X, Chen H, Cai H, Sun L, Gu L. Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;498:660–6. doi: 10.1016/j.bbrc.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 109.Xie T, Xu Q, Wan H, Xing S, Shang C, Gao Y. et al. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway. Lab Invest. 2019;99:625–33. doi: 10.1038/s41374-018-0160-2. [DOI] [PubMed] [Google Scholar]
- 110.Wan H, Xie T, Xu Q, Hu X, Xing S, Yang H. et al. Thy-1 depletion and integrin beta3 upregulation-mediated PI3K-Akt-mTOR pathway activation inhibits lung fibroblast autophagy in lipopolysaccharide-induced pulmonary fibrosis. Lab Invest. 2019;99:1636–49. doi: 10.1038/s41374-019-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L, Cheng S, Jiang X, Zhang J, Meng P, Tang Q. et al. Pregnancy exposure to carbon black nanoparticles exacerbates bleomycin-induced lung fibrosis in offspring via disrupting LKB1-AMPK-ULK1 axis-mediated autophagy. Toxicology. 2019;425:152244. doi: 10.1016/j.tox.2019.152244. [DOI] [PubMed] [Google Scholar]
- 112.Mohamed DI, Khairy E, Tawfek SS, Habib EK, Fetouh MA. Coenzyme Q10 attenuates lung and liver fibrosis via modulation of autophagy in methotrexate treated rat. Biomed Pharmacother. 2019;109:892–901. doi: 10.1016/j.biopha.2018.10.133. [DOI] [PubMed] [Google Scholar]
- 113.Divya T, Sureshkumar A, Sudhandiran G. Autophagy induction by celastrol augments protection against bleomycin-induced experimental pulmonary fibrosis in rats: Role of adaptor protein p62/ SQSTM1. Pulm Pharmacol Ther. 2017;45:47–61. doi: 10.1016/j.pupt.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Liu MW, Su MX, Tang DY, Hao L, Xun XH, Huang YQ. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing miR-193a expression. BMC Pulm Med. 2019;19:35. doi: 10.1186/s12890-019-0799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Du S, Li C, Lu Y, Lei X, Zhang Y, Li S. et al. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics. 2019;9:1878–92. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meng Y, Pan M, Zheng B, Chen Y, Li W, Yang Q. et al. Autophagy Attenuates Angiotensin II-Induced Pulmonary Fibrosis by Inhibiting Redox Imbalance-Mediated NOD-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation. Antioxid Redox Signal. 2019;30:520–41. doi: 10.1089/ars.2017.7261. [DOI] [PubMed] [Google Scholar]
- 117.Pan M, Zheng Z, Chen Y, Sun N, Zheng B, Yang Q. et al. Angiotensin-(1-7) Attenuated Cigarette Smoking-related Pulmonary Fibrosis via Improving the Impaired Autophagy Caused by Nicotinamide Adenine Dinucleotide Phosphate Reduced Oxidase 4-Dependent Reactive Oxygen Species. Am J Respir Cell Mol Biol. 2018;59:306–19. doi: 10.1165/rcmb.2017-0284OC. [DOI] [PubMed] [Google Scholar]
- 118.Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan HM. et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–92. doi: 10.1016/j.ajpath.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 119.Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy. 2013;9:730–42. doi: 10.4161/auto.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG. et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–14. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 121.Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS. et al. IL-37 Attenuates Lung Fibrosis by Inducing Autophagy and Regulating TGF-beta1 Production in Mice. J Immunol. 2019;203:2265–75. doi: 10.4049/jimmunol.1801515. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Yang X, Yang Q, Gong L, Xu H, Wu Z. PARP-1 inhibition attenuates cardiac fibrosis induced by myocardial infarction through regulating autophagy. Biochem Biophys Res Commun. 2018;503:1625–32. doi: 10.1016/j.bbrc.2018.07.091. [DOI] [PubMed] [Google Scholar]
- 123.Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J, Wang XX. et al. Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin. 2014;35:1005–14. doi: 10.1038/aps.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chi J, Wang L, Zhang X, Fu Y, Liu Y, Chen W. et al. Cyclosporin A induces autophagy in cardiac fibroblasts through the NRP-2/WDFY-1 axis. Biochimie. 2018;148:55–62. doi: 10.1016/j.biochi.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 125.Yang R, Song Z, Wu S, Wei Z, Xu Y, Shen X. Toll-like receptor 4 contributes to a myofibroblast phenotype in cardiac fibroblasts and is associated with autophagy after myocardial infarction in a mouse model. Atherosclerosis. 2018;279:23–31. doi: 10.1016/j.atherosclerosis.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 126.Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH. et al. Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis. 2015;6:e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X. et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-beta R II during TGF-beta1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep. 2016;6:24747. doi: 10.1038/srep24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng L, Lin S, Lv C. MiR-26a-5p regulates cardiac fibroblasts collagen expression by targeting ULK1. Sci Rep. 2018;8:2104. doi: 10.1038/s41598-018-20561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou Y, Richards AM, Wang P. MicroRNA-221 Is Cardioprotective and Anti-fibrotic in a Rat Model of Myocardial Infarction. Mol Ther Nucleic Acids. 2019;17:185–97. doi: 10.1016/j.omtn.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao XD, Qin RH, Yang JJ, Xu SS, Tao H, Ding XS. et al. DNMT3A controls miR-200b in cardiac fibroblast autophagy and cardiac fibrosis. Inflamm Res. 2018;67:681–90. doi: 10.1007/s00011-018-1159-2. [DOI] [PubMed] [Google Scholar]
- 131.Liu S, Chen S, Li M, Zhang B, Shen P, Liu P. et al. Autophagy activation attenuates angiotensin II-induced cardiac fibrosis. Arch Biochem Biophys. 2016;590:37–47. doi: 10.1016/j.abb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Qi GM, Jia LX, Li YL, Li HH, Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155:2254–65. doi: 10.1210/en.2013-2011. [DOI] [PubMed] [Google Scholar]
- 133.Wu QQ, Xu M, Yuan Y, Li FF, Yang Z, Liu Y. et al. Cathepsin B deficiency attenuates cardiac remodeling in response to pressure overload via TNF-alpha/ASK1/JNK pathway. Am J Physiol Heart Circ Physiol. 2015;308:H1143–54. doi: 10.1152/ajpheart.00601.2014. [DOI] [PubMed] [Google Scholar]
- 134.Zhao W, Li Y, Jia L, Pan L, Li H, Du J. Atg5 deficiency-mediated mitophagy aggravates cardiac inflammation and injury in response to angiotensin II. Free Radic Biol Med. 2014;69:108–15. doi: 10.1016/j.freeradbiomed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Georgescu SP, Aronovitz MJ, Iovanna JL, Patten RD, Kyriakis JM, Goruppi S. Decreased metalloprotease 9 induction, cardiac fibrosis, and higher autophagy after pressure overload in mice lacking the transcriptional regulator p8. Am J Physiol Cell Physiol. 2011;301:C1046–56. doi: 10.1152/ajpcell.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu RN, Yu TY, Zhou JC, Li M, Gao HK, Zhao C. et al. Targeting HMGB1 ameliorates cardiac fibrosis through restoring TLR2-mediated autophagy suppression in myocardial fibroblasts. Int J Cardiol. 2018;267:156–62. doi: 10.1016/j.ijcard.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 137.Gogiraju R, Hubert A, Fahrer J, Straub BK, Brandt M, Wenzel P. et al. Endothelial Leptin Receptor Deletion Promotes Cardiac Autophagy and Angiogenesis Following Pressure Overload by Suppressing Akt/mTOR Signaling. Circ Heart Fail. 2019;12:e005622. doi: 10.1161/CIRCHEARTFAILURE.118.005622. [DOI] [PubMed] [Google Scholar]
- 138.Ullrich M, Assmus B, Augustin AM, Habich H, Abesser M, Martin Machado J. et al. SPRED2 deficiency elicits cardiac arrhythmias and premature death via impaired autophagy. J Mol Cell Cardiol. 2019;129:13–26. doi: 10.1016/j.yjmcc.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 139.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM. et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–97. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta MK, McLendon PM, Gulick J, James J, Khalili K, Robbins J. UBC9-Mediated Sumoylation Favorably Impacts Cardiac Function in Compromised Hearts. Circ Res. 2016;118:1894–905. doi: 10.1161/CIRCRESAHA.115.308268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N. et al. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest. 2014;124:2410–24. doi: 10.1172/JCI66339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li S, Liu C, Gu L, Wang L, Shang Y, Liu Q. et al. Autophagy protects cardiomyocytes from the myocardial ischaemia-reperfusion injury through the clearance of CLP36. Open Biol. 2016;6:160177. doi: 10.1098/rsob.160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Araki S, Izumiya Y, Rokutanda T, Ianni A, Hanatani S, Kimura Y. et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-beta Signaling Pathway. Circulation. 2015;132:1081–93. doi: 10.1161/CIRCULATIONAHA.114.014821. [DOI] [PubMed] [Google Scholar]
- 144.Lin S, Wang Y, Zhang X, Kong Q, Li C, Li Y. et al. HSP27 Alleviates Cardiac Aging in Mice via a Mechanism Involving Antioxidation and Mitophagy Activation. Oxid Med Cell Longev. 2016;2016:2586706. doi: 10.1155/2016/2586706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi J, Surma M, Yang Y, Wei L. Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging. FASEB J. 2019;33:7348–62. doi: 10.1096/fj.201802510R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang S, Kandadi MR, Ren J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: Role of autophagy and mitophagy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1865–75. doi: 10.1016/j.bbadis.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao Y, Wu QQ, Duan MX, Liu C, Yuan Y, Yang Z. et al. TAX1BP1 overexpression attenuates cardiac dysfunction and remodeling in STZ-induced diabetic cardiomyopathy in mice by regulating autophagy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1728–43. doi: 10.1016/j.bbadis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y. et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin Sci (Lond) 2016;130:625–41. doi: 10.1042/CS20150623. [DOI] [PubMed] [Google Scholar]
- 149.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy. 2016;12:976–98. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xue X, Ren J, Sun X, Gui Y, Feng Y, Shu B. et al. Protein kinase Calpha drives fibroblast activation and kidney fibrosis by stimulating autophagic flux. J Biol Chem. 2018;293:11119–30. doi: 10.1074/jbc.RA118.002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang X, Wang H, Tu Y, Li Y, Zou Y, Li G. et al. WNT1-inducible signaling protein-1 mediates TGF-beta1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy. J Cell Physiol. 2020;235:2009–22. doi: 10.1002/jcp.29187. [DOI] [PubMed] [Google Scholar]
- 152.Lemos DR, McMurdo M, Karaca G, Wilflingseder J, Leaf IA, Gupta N. et al. Interleukin-1beta Activates a MYC-Dependent Metabolic Switch in Kidney Stromal Cells Necessary for Progressive Tubulointerstitial Fibrosis. J Am Soc Nephrol. 2018;29:1690–705. doi: 10.1681/ASN.2017121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noh MR, Woo CH, Park MJ, In Kim J, Park KM. Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1634–41. doi: 10.1016/j.bbadis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 154.Baisantry A, Bhayana S, Rong S, Ermeling E, Wrede C, Hegermann J. et al. Autophagy Induces Prosenescent Changes in Proximal Tubular S3 Segments. J Am Soc Nephrol. 2016;27:1609–16. doi: 10.1681/ASN.2014111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Canaud G, Brooks CR, Kishi S, Taguchi K, Nishimura K, Magassa S, Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci Transl Med. 2019. 11. [DOI] [PMC free article] [PubMed]
- 156.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol. 2013;24:198–207. doi: 10.1681/ASN.2012010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shirazi MK, Azarnezhad A, Abazari MF, Poorebrahim M, Ghoraeian P, Sanadgol N. et al. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J Cell Physiol. 2019;234:11411–23. doi: 10.1002/jcp.27797. [DOI] [PubMed] [Google Scholar]
- 158.Yamamoto T, Takabatake Y, Kimura T, Takahashi A, Namba T, Matsuda J. et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy. 2016;12:801–13. doi: 10.1080/15548627.2016.1159376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li H, Peng X, Wang Y, Cao S, Xiong L, Fan J. et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy. 2016;12:1472–86. doi: 10.1080/15548627.2016.1190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng X, Wang Y, Li H, Fan J, Shen J, Yu X. et al. ATG5-mediated autophagy suppresses NF-kappaB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 2019;10:253. doi: 10.1038/s41419-019-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takagaki Y, Lee SM, Dongqing Z, Kitada M, Kanasaki K, Koya D. Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis. Autophagy. 2020. p: 1-10. [DOI] [PMC free article] [PubMed]
- 162.Nam SA, Kim WY, Kim JW, Park SH, Kim HL, Lee MS. et al. Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-beta and NLRP3 inflammasome signaling pathway. Cell Death Dis. 2019;10:78. doi: 10.1038/s41419-019-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kawakami T, Gomez IG, Ren S, Hudkins K, Roach A, Alpers CE. et al. Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J Am Soc Nephrol. 2015;26:1040–52. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu G, Yue F, Huang H, He Y, Li X, Zhao H. et al. Defects in MAP1S-mediated autophagy turnover of fibronectin cause renal fibrosis. Aging (Albany NY) 2016;8:977–85. doi: 10.18632/aging.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim SM, Kim YG, Kim DJ, Park SH, Jeong KH, Lee YH. et al. Inflammasome-Independent Role of NLRP3 Mediates Mitochondrial Regulation in Renal Injury. Front Immunol. 2018;9:2563. doi: 10.3389/fimmu.2018.02563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zheng K, He Z, Kitazato K, Wang Y. Selective Autophagy Regulates Cell Cycle in Cancer Therapy. Theranostics. 2019;9:104–25. doi: 10.7150/thno.30308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE. et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics. 2018;8:5713–30. doi: 10.7150/thno.28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi M, Maique J, Shaffer J, Davidson T, Sebti S, Fernandez AF. et al. The tripartite interaction of phosphate, autophagy, and alphaKlotho in health maintenance. FASEB J. 2020;34:3129–50. doi: 10.1096/fj.201902127R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S. et al. alphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J Am Soc Nephrol. 2016;27:2331–45. doi: 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang H, Ni H, Ma K, Zou J. ANGPTL2 regulates autophagy through the MEK/ERK/Nrf-1 pathway and affects the progression of renal fibrosis in diabetic nephropathy. Am J Transl Res. 2019;11:5472–86. [PMC free article] [PubMed] [Google Scholar]
- 171.Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J. et al. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94:912–25. doi: 10.1016/j.kint.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 172.Ding Y, Kim S, Lee SY, Koo JK, Wang Z, Choi ME. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014;25:2835–46. doi: 10.1681/ASN.2013101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem. 2012;287:11677–88. doi: 10.1074/jbc.M111.308460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tazi MF, Dakhlallah DA, Caution K, Gerber MM, Chang SW, Khalil H. et al. Elevated Mirc1/Mir17-92 cluster expression negatively regulates autophagy and CFTR (cystic fibrosis transmembrane conductance regulator) function in CF macrophages. Autophagy. 2016;12:2026–37. doi: 10.1080/15548627.2016.1217370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ. et al. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy. 2016;12:397–409. doi: 10.1080/15548627.2015.1056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Caution K, Pan A, Krause K, Badr A, Hamilton K, Vaidya A. et al. Methylomic correlates of autophagy activity in cystic fibrosis. J Cyst Fibros. 2019;18:491–500. doi: 10.1016/j.jcf.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C. et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–75. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 178.Noh SH, Gee HY, Kim Y, Piao H, Kim J, Kang CM. et al. Specific autophagy and ESCRT components participate in the unconventional secretion of CFTR. Autophagy. 2018;14:1761–78. doi: 10.1080/15548627.2018.1489479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hutt DM, Mishra SK, Roth DM, Larsen MB, Angles F, Frizzell RA. et al. Silencing of the Hsp70-specific nucleotide-exchange factor BAG3 corrects the F508del-CFTR variant by restoring autophagy. J Biol Chem. 2018;293:13682–95. doi: 10.1074/jbc.RA118.002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ferrari E, Monzani R, Villella VR, Esposito S, Saluzzo F, Rossin F. et al. Cysteamine re-establishes the clearance of Pseudomonas aeruginosa by macrophages bearing the cystic fibrosis-relevant F508del-CFTR mutation. Cell Death Dis. 2017;8:e2544. doi: 10.1038/cddis.2016.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang ZS, Ma LQ, Zhu K, Yan JY, Bian L, Zhang KQ. et al. Pseudomonas toxin pyocyanin triggers autophagy: Implications for pathoadaptive mutations. Autophagy. 2016;12:1015–28. doi: 10.1080/15548627.2016.1170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Khabir M, Aliche AZ, Sureau C, Blanchet M, Labonté P. Hepatitis Delta Virus Alters the Autophagy Process To Promote Its Genome Replication. J Virol. 2020;94:e01936–19. doi: 10.1128/JVI.01936-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Y, Jiang K, Zhang Q, Meng S, Ding C. Autophagy in Negative-Strand RNA Virus Infection. Front Microbiol. 2018;9:206. doi: 10.3389/fmicb.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rockey DC, Bell PD, Hill JA. Fibrosis-a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–49. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]