Abstract
Accumulating evidence suggests that adipose‐derived stem cell constituent extract (ADSC‐CE) helps hair regrowth in patients with androgenetic alopecia (AGA). However, the effects of ADSC‐CE have not been demonstrated in a randomized, double‐blind, vehicle‐controlled clinical trial. In this randomized, double‐blind, vehicle‐controlled clinical trial, 38 patients (29 men) with AGA were assigned to an intervention group (IG), with twice‐daily self‐application of the ADSC‐CE topical solution over the scalp with fingers, or to a control group (CG). Changes in hair count and thickness at 16 weeks from the baseline were evaluated using a phototrichogram. Overall, 34 (89%) patients (mean age, 45.3 years) completed the study. The phototrichogram at week 8 showed more increase in hair count in the IG than in the CG, and intergroup differences in the change of hair count remained significant until week 16 with overall changes of 28.1% vs 7.1%, respectively. Similarly, a significant improvement in hair diameter was observed in the IG (14.2%) after 16 weeks when compared with hair diameter in the CG (6.3%). Our findings suggest that the application of the ADSC‐CE topical solution has enormous potential as an alternative therapeutic strategy for hair regrowth in patients with AGA, by increasing both hair density and thickness while maintaining adequate treatment safety.
Keywords: adipose‐derived stem cell, androgenetic alopecia, hair growth, hair loss, hair regeneration, mesenchymal stem cell
No randomized controlled trials have reported on the effects of adipose‐derived stem cell constituent extract in patients with androgenetic alopecia. At 16 weeks, hair count was significantly increased in the intervention group (IG; 28.1%) compared with the control group (CG; 7.1%); similarly, hair diameter was significantly increased in the IG (14.2%) compared with the CG (6.3%).

Abbreviations
- ADSC
adipose tissue‐derived stem cell
- ADSC‐CE
adipose‐derived stem cell constituent extract
- ADSC‐CM
adipose‐derived stem cell conditioned media
- AGA
androgenetic alopecia
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CG
control group
- DHT
dihydrotestosterone
- FDA
Food and Drug Administration
- FPHL
female‐pattern hair loss
- HA
hyaluronic acid
- HGF
hepatocyte growth factor
- IG
intervention group
- IGF
insulin‐like growth factor
- ITT
intention‐to‐treat
- MPHL
male‐pattern hair loss
- MSC
mesenchymal stem cell
- PBS
phosphate‐buffered saline
- PP
per protocol
- PRP
platelet‐rich plasma
- RCT
randomized controlled trial
- VEGF
vascular endothelial growth factor
Significance statement.
This study suggests the application of adipose‐derived stem cell constituent extract topical solution has the potential as an alternative therapeutic strategy for hair regrowth in patients with androgenetic alopecia by increasing both hair density and thickness while maintaining adequate treatment safety.
1. INTRODUCTION
Androgenetic alopecia (AGA) is the most common type of hair loss and globally affects approximately 50% of men and 45% of women by 50 years of age. 1 Although it is not a life‐threatening condition, hair loss can be a reason for low self‐esteem and psychological distress by making the patient look less attractive and older than their actual age. 2 , 3 However, despite the efforts of medical experts in seeking effective therapeutic agents, only a few Food and Drug Administration (FDA)‐approved medications are available for these patients.
Age, genetic predisposition, and androgens are the main known driving factors in AGA progression. 1 Dihydrotestosterone (DHT), derived from testosterone, is a major metabolite in hair development. 4 DHT stops the growth of the hair follicle cells and shrinks them, leading to hair loss. 5 Therefore, 5α‐reductase inhibitors that suppress the activity of 5α‐reductase, an enzyme that converts testosterone to DHT, have been commonly used in AGA and are regarded the most effective medications. 6 However, owing to teratogenic risks, 5α‐reductase inhibitors are limited to male patients with AGA. Furthermore, they sometimes result in unexpected side effects such as decreased libido and ejaculate volume and erectile dysfunction, which greatly affects the quality of life in middle‐aged men. 7 These side effects consequently prompt many patients to turn to alternatives with fewer side effects. 8 Therefore, it is necessary to discover agents with anti‐hair loss effects and without serious side effects to expand the range of treatment in AGA.
Mesenchymal stem cells (MSCs), which are mesoderm‐derived immature precursors, have self‐renewal potential and multilineage differentiation capacity. 9 Additionally, as MSCs are found abundantly in the adipose tissue, which is relatively easy to access, recent studies have attempted to identify the medicinal effects of MSCs and apply them to regenerative medicine. 10 , 11 Adipose tissue‐derived stem cells (ADSCs) are a type of MSC that secrete several growth hormones that help cells develop and proliferate. 9 According to laboratory and experimental studies, growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin‐like growth factor (IGF), and platelet‐derived growth factor (PDGF) increase the size of the hair follicle during hair development. 11 , 12 Recent retrospective human studies have shown that ADSCs promote hair growth in both men and women with alopecia. 13 , 14
However, no randomized, placebo‐controlled trial in humans has explored the effects and safety of adipose‐derived stem cell constituent extract (ADSC‐CE) in AGA. Here, we aimed to assess the efficacy and tolerability of ADSC‐CE in middle‐aged patients with AGA. We hypothesized that ADSC‐CE is an effective and safe agent in the treatment of adults with AGA.
2. MATERIALS AND METHODS
2.1. Study design and subjects
This randomized, double‐blind, placebo‐controlled clinical trial was approved by the Institutional Review Board at Busan National University Yangsan Hospital (IRB 02‐2015‐014). The study was performed in accordance with the principles of the Declaration of Helsinki and Korean Good Clinical Products between August 3, 2015, and January 27, 2016. Written informed consents were obtained from all participants before enrollment. This trial is registered with ClinicalTrials.gov (Identifier: NCT02594046).
The candidates were recruited through an advertisement at a tertiary hospital in Yangsan, South Korea. Eligible subjects included those between 18 and 59 years, who were diagnosed with AGA with at least M2, C2, or U1 basic type and V1 or F1 specific type of AGA. 15 Additionally, they maintained a consistent hair style and did not receive any other hair care throughout the 16‐week study period. The following are the exclusion criteria: (a) intake of medications or supplements, including finasteride, dutasteride, steroids, vasodilators, anticonvulsants, beta‐receptor blockers, cell death agents, bronchodilators, diuretics, spironolactone, cimetidine, diazoxide, cyclosporine, ketoconazole, or any other hormonal products, that can affect hair growth in the preceding 30 days; (b) surgical history for hair loss such as hair transplantation or scalp reduction; (c) treatment history of hyperthyroidism or hypothyroidism; (d) currently pregnant or planning conception within the next 6 months or currently breastfeeding; (e) drug history of topical steroids, hair growth solution, or hair restorers to the scalp in the preceding month; (f) uncontrolled blood pressure (BP) and blood glucose levels, infectious skin diseases, or psychiatric disorders in the preceding 6 months; (g) aspartate aminotransferase (AST) or alanine aminotransferase (ALT) serum levels >80 mg/dL or creatinine (Cr) level >1.5 mg/dL; and (h) allergic to the ingredients in this study's interventions.
2.2. Double blinding and randomization
Overall, 44 adults were screened, and 38 (86.4%) participants were finally enrolled. After baseline measurements, they were randomly assigned to one of the following two groups through block randomization method using randomized numbers and provided identification numbers on recruitment: intervention group (IG; n = 19), in which participants received ADSC‐CE, or control group (CG; n = 19), in which participants received the vehicle placebo. Randomization codes were created by an expert in statistics using nQuery Advisor 7.0. The authors responsible for enrolling the participants and conducting the measurements were blinded to the randomization process throughout the study. The test product and vehicle were identical in their external forms and properties, including the label.
2.3. Adipose‐derived stem cell constituent extract
ADSC‐CE used in this study was manufactured and supplied by T‐Stem, Co., Ltd (Changwon‐si, Republic of Korea).
2.3.1. Preparation of adipose tissue
The materials were obtained from the adipose tissue of healthy donors who had consented for their use for research or commercial purposes before liposuction. Donors aged 20 years or above with a body mass index within 25 to 29.9 kg/m2 were selected. Prior to collecting the adipose tissue, the donors were tested for the presence of the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, human T‐cell lymphotropic virus, Epstein‐Barr virus, cytomegalovirus, parvovirus B19, and Treponema pallidum. The physician's examination also confirmed their health status.
2.3.2. Stem cell isolation from adipose tissue
Adipose tissues were exposed to type II collagenase (Sigma‐Aldrich Corp, St. Louis, Missouri) at 37°C, centrifuged, washed, and resuspended in phosphate‐buffered saline (PBS). The ADSCs were, thus, isolated from adipose tissue.
2.3.3. Identification of ADSCs
The cells were fixed in a fixative for 18 minutes, washed with PBS, and incubated with monoclonal antibodies against surface markers for 1 hour at 37°C. Thereafter, the cells were washed with PBS and incubated with fluorescein isothiocyanate‐labeled rabbit anti‐mouse IgG for 1 hour at 37°C. Subsequently, the cells were visualized with a fluorescence microscope. To confirm the identity of the isolated cells as being ADSCs derived from the mesenchyme, immunofluorescence was used to identify the ADSC‐specific cell surface markers, CD105 and CD29, which are mesenchyme‐derived adipose stem cell factors. In contrast, CD34, which is a negative marker, was not found on the cell surface. Thus, the ADSCs maintained their phenotype and mesenchymal‐derived adipose stem cells were isolated.
2.3.4. Culturing of ADSCs
The cells were cultured in serum‐free medium (Power Stem MSC1; PAN‐Biotech, Aidenbach, Germany), which is widely used for stem cell culture. The cells were cultured at 37°C in 5% CO2.
2.3.5. Subculturing of ADSCs
Repeated subculturing was performed using serum‐free medium (Power Stem MSC1; PAN‐Biotech). Thereafter, the culture medium was removed and trypsin/EDTA was added for separating the stem cells, which were then washed several times with PBS to obtain pure ADSCs. Subsequently, the cells were counted and diluted in PBS to obtain 106 ADSCs per milliliter.
2.3.6. Acquiring ADSC‐CE
Prior to disrupting the ADSCs ultrasonically, they were dispersed in distilled water. The ADSC cell membrane could be disrupted using a low frequency of ultrasound wave. The ADSCs were observed under a microscope to ensure complete lysis. Thereafter, the cell membrane debris was removed using centrifugation and successive filtration steps. Finally, we obtained ADSC‐CE enriched with stem cell proteins, which was used for stem cell therapy.
2.3.7. Preparing the test material
A total of 130 mL of test material was prepared in a bottle, for the IG and CG. The material for the IG had 1% ADSC‐CE in distilled water, and that for the CG contained distilled water alone. Both test materials were colorless and odorless. The product conformed to the quality control guidelines released by the Korean Ministry of Food and Drug Safety.
2.4. Intervention
Screening evaluation was conducted at the first visit (visit 1) to assess the eligibility of the candidates for this study. After being assigned to one of the groups, the subjects were given a 130‐mL bottle containing the topical solution, which was colorless and odorless. They were instructed to apply 2 mL of this solution to the hair loss area, twice every day for 16 weeks, gently massaging it into the scalp using their fingers for even absorption. All participants visited the center four times in total (visit 1: for screening; visit 2: for randomization and starting the intervention; visit 3: 8 weeks of intervention; and visit 4: 16 weeks of intervention). They were requested to log when they used the product in a diary, which was turned in along with the bottle to the researcher at every visit. Compliance was assessed by estimating the remaining amount of the product in the bottle; if more than 20% of the product was unused, the participant was considered to have dropped out of the study.
2.5. Measurement of efficacy variables
The primary efficacy variables were the changes from the baseline in total hair number and hair thickness and were confirmed by close‐contact photographs of the subjects taken by phototrichogram (IS‐3000U; BEAUTOPIA Co., Ltd, Seoul, Korea) using a standardized technique, 16 at baseline and after 8 and 16 weeks of using the product. To quantitatively evaluate hair growth in the same spot over the study period, a dot was tattooed on the subject's scalp. The tattoo allowed for a follow‐up phototrichogram at the same spot 16 weeks later. The photographic views in all patients were in a fixed, designated scalp area with identical settings, including the same light, flash, and distance from the lens.
The measurements of secondary efficacy variables included analysis of global photographs of the participants' scalps by an investigator and self‐evaluation of hair growth by the participants.
2.5.1. Investigator assessments
Pictures of the vertex (90°) and frontal hair line (45°) of each patient at baseline and after 8 and 16 weeks of treatment (Figure 1) were taken. An investigator, blinded to the intervention, rated the changes in scalp appearance relative to the baseline using a standardized 7‐point rating scale with the following scores: −3, greatly decreased; −2, moderately decreased; −1, slightly decreased; 0, unchanged; +1, slightly increased; +2, moderately increased; and +3, greatly increased.
FIGURE 1.
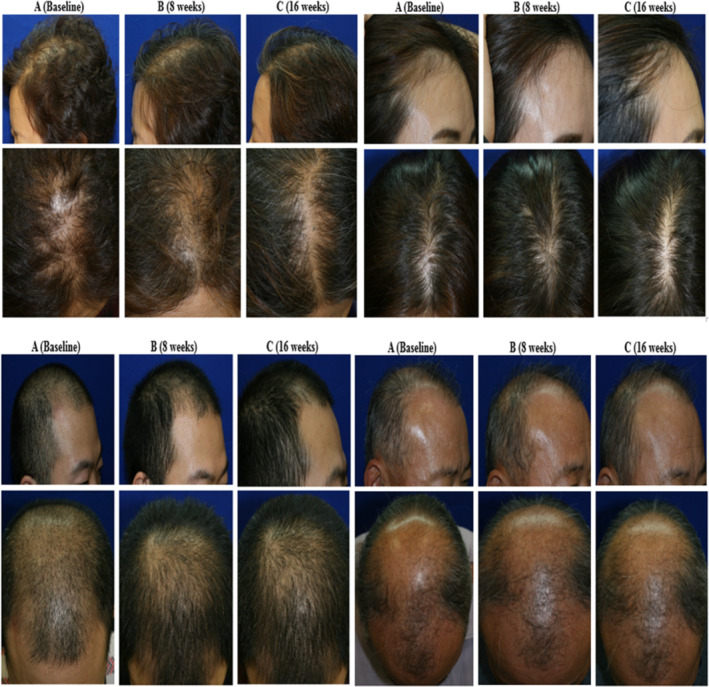
Example of global photographs taken using a phototrichogram at baseline (A), 8 weeks (B), and 16 weeks (C) after ADSC‐CE application in the ADSC‐CE group (left) and the placebo group (right). ADSC‐CE, adipose‐derived stem cell constituent extract
2.5.2. Self‐assessment of the participants
At weeks 8 and 16, the participants performed self‐assessments for the efficacy of treatment (self‐rated improvement score) on a scale of −5 (worsened) to 10 (improved).
2.6. Safety evaluation
To check for local skin reactions owing to the intervention, the participants were asked whether they experienced unpleasant sensations, such as itching, burning, pricking, tingling, and stiffness, on their scalp after using the product. Additionally, an investigator examined the participants for hypersensitive reactions after gently scratching the scalp in the area on which the product was applied and checking for abrasions, irritation, sunburn, or any other skin problem. Levels of fasting glucose, AST, ALT, and Cr in the blood were also measured as safety parameters. Additionally, BP and anthropometric measurements were evaluated at every visit. Reports of any adverse events or unpredicted allergic reactions were collected throughout the study.
2.7. Sample size and statistical analysis
The sample size of the study was calculated based on Lee et al., who tested the effects of traditional oriental hair care products in alopecia. 17 The estimated sample size was 15 subjects per group for 80% power to detect a difference of 6.25 in mean hair count, assuming an SD of 5.866 in the primary outcome variable and an alpha error of 5%. A total of 38 participants (19 per group) were required, with an assumed dropout rate of 20%. When the result of a test was unavailable, the last recorded data entry was included in the analysis (the last observation carried forward method). Efficacy analysis was performed on an intention‐to‐treat (ITT) basis in subjects that received at least one dose of ADSC‐CE or placebo and on those who underwent at least one assessment after baseline and per protocol (PP) by only including data from subjects that completed the study protocol. Shapiro‐Wilk's test was used to test the normality assumption. Intergroup comparisons of baseline characteristics and their changes at week 16 of the trial were performed using the two‐sample t test for continuous variables (or Mann‐Whitney U test for nonparametric continuous variables) and the chi‐square test for categorical variables (or Fisher's exact test for nonparametric categorical variables). Intragroup comparisons were performed using the paired t test for continuous variables (or Mann‐Whitney U test for nonparametric continuous variables). The repeated‐measures analysis of variance was performed to verify the differences in the changes over time. There was a significant difference in hair count between the groups at baseline; therefore, intergroup comparison of the change in the hair count was performed using analysis of covariance to adjust for the difference. A P value of <.05 was considered statistically significant. SPSS version 22.0 (IBM Inc, Armonk, New York) was used for the analysis.
3. RESULTS
3.1. Baseline characteristics of the subjects
Of the 38 initially enrolled patients, 4 dropped out by consent withdrawal (IG = 1, CG = 3); therefore, 34 subjects completed the trial as planned. Three participants refused to continue participation for personal reasons that were not associated with the trial, and one participant failed to visit on time without prior notice (Figure 2). The compliance was satisfactory with more than 95% usage rate in both IG and CG (95.4% ± 4.89% vs 95.2% ± 4.45%, P = .913). Comparison of the baseline characteristics between the two groups is listed in Table 1. There were no significant intergroup differences in the demographic and anthropometric characteristics, drinking habits, and smoking statuses, indicating that the random assignment was statistically appropriate. A majority of the participants were men (76.3%) and the overall mean age was 45.3 years. Despite randomization, at baseline, the total hair count was significantly less in IG than in CG (13.95 ± 4.01 vs 17.58 ± 4.13 counts per cm2; P = .009; Table 2), although there was no significant difference in hair thickness between the groups.
FIGURE 2.
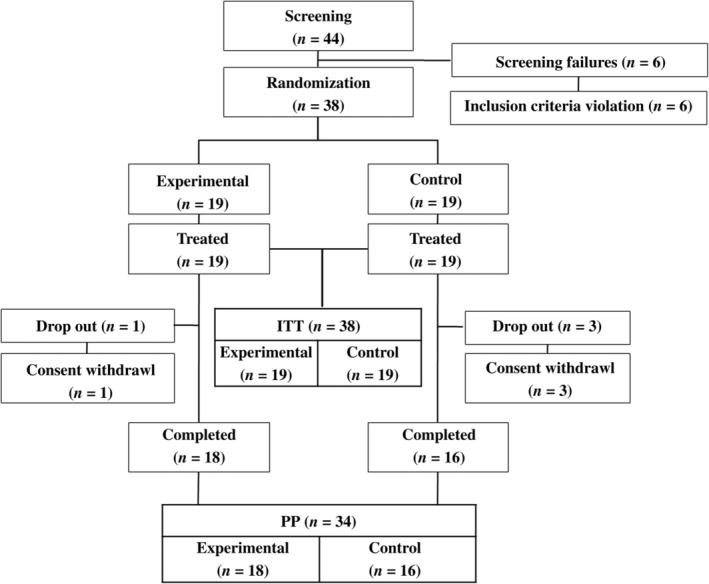
Flow diagram of the study process. Of the 44 enrolled candidates, 38 participants were randomized in a 1:1 ratio to receive adipose‐derived stem cell constituent extract or vehicle solution
TABLE 1.
Baseline characteristics of patients
| Variables | Intention‐to‐treat population | Per‐protocol population | ||||
|---|---|---|---|---|---|---|
| Vehicle (n = 19) | ADSC‐CE (n = 19) | P value | Vehicle (n = 16) | ADSC‐CE (n = 18) | P value | |
| Male | 14 (73.7) | 15 (78.9) | .500 a | 12 (75.0) | 14 (77.8) | .583 a |
| Age, years | 46.21 ± 9.3 | 44.32 ± 8.8 | .313 b | 45.13 ± 9.7 | 43.83 ± 8.7 | .489 b |
| Weight, cm | 73.16 ± 10.6 | 76.61 ± 12.5 | .365 b | 73.24 ± 10.3 | 75.27 ± 11.3 | .590 b |
| Height, cm | 170.1 (163‐176) | 175.9 (165‐177) | .365 c | 169.9 (163‐175) | 171.4 (165‐177) | .370 c |
| BMI, kg/m2 | 25.24 ± 3.1 | 25.79 ± 2.7 | .555 b | 25.4 ± 3.2 | 25.5 ± 2.4 | .930 b |
| Alcohol drinker | 3 (15.8) | 4 (21.1) | .500 a | 2 (12.5) | 4 (22.2) | .389 a |
| Current smoker | 2 (10.5) | 2 (10.5) | .698 a | 2 (12.5) | 2 (11.1) | .652 a |
Notes. Values are expressed as mean ± SD, median (25th‐75th percentile), or frequency (percentage). Shapiro‐Wilk's test was used to test for normality.
Abbreviations: ADSC‐CE, adipose‐derived stem cell constituent extract; BMI, body mass index.
P values according to Fisher's exact test.
P values according to independent t test.
P values according to Mann‐Whitney U test.
TABLE 2.
Changes from the baseline in hair count and diameter at weeks 8 and 16
| ITT | Observed value | Change from baseline | ||||
|---|---|---|---|---|---|---|
| Vehicle (n = 19) | ADSC‐CE (n = 19) | P value | Vehicle (n = 19) | ADSC‐CE (n = 19) | P value | |
| Hair counts, number | ||||||
| Week 0 | 17.58 ± 4.13 | 13.95 ± 4.01 | .009 a | — | — | — |
| Week 8 | 17.05 ± 4.85 | 15.95 ± 4.78 | .011 b | −0.53 ± 2.39 | 2.00 ± 2.79 | .005 a |
| Week 16 | 18.79 ± 4.92 | 17.63 ± 5.41 | .003 b | 1.21 ± 1.87 | 3.68 ± 3.06 | .014 c |
| Hair diameter, mm | ||||||
| Week 0 | 0.055 ± 0.006 | 0.057 ± 0.006 | .508 c | — | — | — |
| Week 8 | 0.059 ± 0.007 | 0.063 ± 0.008 | .140 c | 0.004 ± 0.005 | 0.006 ± 0.007 | .569 c |
| Week 16 | 0.059 ± 0.006 | 0.065 ± 0.009 | .058 c | 0.003 ± 0.004 | 0.008 ± 0.007 | .014 a |
| PP | Observed value | Change from baseline | ||||
|---|---|---|---|---|---|---|
| Vehicle (n = 16) | ADSC‐CE (n = 18) | P value | Vehicle (n = 16) | ADSC‐CE (n = 18) | P value | |
| Hair counts, number | ||||||
| Week 0 | 17.81 ± 4.32 | 13.89 ± 4.11 | .011 a | — | — | — |
| Week 8 | 17.19 ± 5.17 | 16.00 ± 4.91 | .014 b | −0.63 ± 2.60 | 2.11 ± 2.83 | .006 a |
| Week 16 | 19.25 ± 5.12 | 17.78 ± 5.53 | .006 b | 1.44 ± 1.97 | 3.89 ± 3.01 | .025 c |
| Hair diameter, mm | ||||||
| Week 0 | 0.055 ± 0.005 | 0.057 ± 0.006 | .332 c | — | — | — |
| Week 8 | 0.059 ± 0.007 | 0.064 ± 0.008 | .073 c | 0.004 ± 0.006 | 0.007 ± 0.008 | .863 c |
| Week 16 | 0.059 ± 0.005 | 0.066 ± 0.009 | .021 c | 0.004 ± 0.004 | 0.008 ± 0.007 | .023 a |
Notes. Values are expressed as mean ± SD. Shapiro‐Wilk's test was used to test for normality.
Abbreviations: —, no data; ADSC‐CE, adipose‐derived stem cell constituent extract; ITT, intention‐to‐treat; PP, per protocol.
P values according to independent t test.
P values were adjusted for the observed value at baseline by using the baseline values as covariates in analysis of covariance.
P values according to Mann‐Whitney U test.
3.2. Efficacy evaluation
In both ITT and PP analyses, with adjustments for baseline hair count, phototrichograms taken after 8 weeks of ADSC‐CE usage demonstrated a 19.2% increase in hair count in IG compared with CG, and this intergroup difference in hair density was significant until the last visit, with the overall percentage change from baseline of 28.1% vs 7.1% in IG and CG, respectively (Table 2; Figure 3). A significant improvement in hair diameter after 16 weeks was observed in IG compared with that in CG, with the total percentage change from baseline of 14.2% vs 6.3%, respectively (0.008 ± 0.007 vs 0.004 ± 0.004 mm; Table 2; Figure 3).
FIGURE 3.
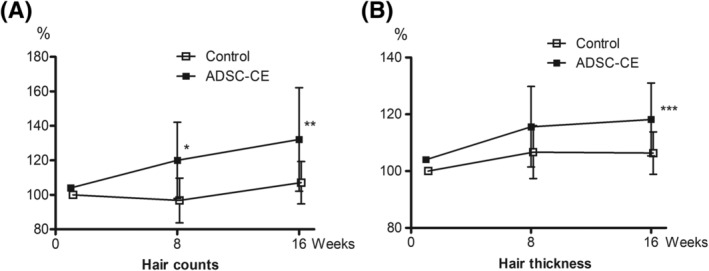
Percentage change from baseline in hair count (A) and diameter (B) over 16 weeks (intention‐to‐treat analysis). Data are means ± SD (Control group n = 19, ADSC‐CE group n = 19). *P = .002, **P = .008, ***P = .026. ADSC‐CE, adipose‐derived stem cell constituent extract
In the investigator assessments using photographs, there were little improvements in both groups during the first 8 weeks, with a mean score of 0.13 in CG and 0.11 in IG. Although slightly higher scores were rated for the change by 16 weeks (0.44 in CG and 0.78 in IG), the intervention failed to show a statistically significant improvement in the investigator evaluation in both groups (Table 3). In the participant self‐assessments, no significant intergroup differences were observed at weeks 8 and 16.
TABLE 3.
Investigator assessment of clinical response
| Vehicle (n = 16) | ADSC‐CE (n = 18) | P value | ||
|---|---|---|---|---|
| Week 8 | 0.13 ± 0.72 | 0.11 ± 0.32 | .556 a | |
| Rating distribution | .269 b | |||
| −3 | 0 (0.0) | 0 (0.0) | ||
| −2 | 1 (6.3) | 0 (0.0) | ||
| −1 | 0 (0.0) | 0 (0.0) | ||
| 0 | 11 (68.8) | 16 (88.9) | ||
| 1 | 4 (25.0) | 2 (11.1) | ||
| 2 | 0 (0.0) | 0 (0.0) | ||
| 3 | 0 (0.0) | 0 (0.0) | ||
| Week 16 | 0.44 ± 1.09 | 0.78 ± 0.73 | .339 a | |
| Rating distribution | .538 b | |||
| −3 | 0 (0.0) | 0 (0.0) | ||
| −2 | 1 (6.3) | 0 (0.0) | ||
| −1 | 1 (6.3) | 0 (0.0) | ||
| 0 | 7 (43.8) | 7 (38.9) | ||
| 1 | 4 (25.0) | 8 (44.4) | ||
| 2 | 3 (18.8) | 3 (16.7) | ||
| 3 | 0 (0.0) | 0 (0.0) | ||
Notes. 7‐point evaluation. −3: greatly decreased; −2: moderately decreased; −1: slightly decreased; 0: no change; 1: slightly increased; 2: moderately increased; 3: greatly increased. Values are expressed as mean ± SD, or frequency (percentage). Shapiro‐Wilk's test was used to test for normality.
Abbreviation: ADSC‐CE, adipose‐derived stem cell constituent extract.
P values according to Mann‐Whitney U test.
P values according to Fisher's exact test.
3.3. Safety evaluation
Most of the subjects completed the protocol without serious adverse symptoms. Throughout the trial, seven adverse findings were reported in five participants. All were mild and resolved naturally without the need for medical intervention. There was no significant difference in the incidence of these side effects between the groups. Additionally, no clinical changes in AST, ALT, Cr, and glucose levels were observed in either group. BP and anthropometric data remained unchanged in both groups, and no intergroup differences in these parameters were observed.
4. DISCUSSION
To the best of our knowledge, this is the first randomized, placebo‐controlled, double‐blind, parallel clinical trial to evaluate the efficacy and safety of ADSC‐CE in patients with AGA. Although the molecular mechanisms underlying the anti‐hair loss effect of ADSC‐CE are now well known, the efficacy of ADSC‐CE on hair regeneration has not been previously demonstrated through a placebo‐controlled, double‐blind clinical trial. We aimed to identify whether topical ADSC‐CE application could help hair growth in humans. Our results demonstrated that ADSC‐CE significantly increased hair count and density compared with the vehicle in both males and females with AGA. Notably, this trial was the first to demonstrate the advantages of a nonintradermal solution of ADSC‐CE that patients can use by themselves without the need for regular visits to the clinic. Additionally, application of ADSC‐CE was well tolerated, and no major adverse effects were observed during the study.
With a primary role in the homeostasis of organs and tissues, MSCs maintain the stem cell niche, help tissue recovery after injuries, and ensure healthy aging. 9 ADSC is known to be one of the most accessible sources of MSCs and has recently emerged as a new therapeutic option for degenerative conditions. 18 In addition to replacing damaged cells in affected tissues, ADSC has beneficial effects through its paracrine action via various cytokines and growth factors. 10 Therefore, the utility of ADSCs isolated from adipose tissue in tissue engineering has been widely expanded when applied alone or in combination with hyaluronic acid (HA), platelet‐rich plasma (PRP), 19 , 20 , 21 , 22 , 23 and fat graft for regenerative surgery strategies in the management of various soft tissue defects. 24 , 25 , 26 Similarly, the use of stem cells has been found to improve hair regrowth in several therapeutic strategies, including reversing the pathological mechanisms that cause hair loss, regeneration of hair follicles, or creating hair using a tissue‐engineering approach. 27 Hair regrowth is regulated primarily by ERK activation and Wnt signaling. Secretory factors derived from ADSCs include PDGF, HGF, VEGF, IGF binding protein precursors, and fibronectin. 27 , 28 PDGF has been shown to induce and maintain the anagen phase in the hair cycle in a mouse model while HGF facilitates hair follicle elongation. 12 , 29 VEGF increases hair growth and size by follicle vascularization, 30 and IGF‐1 improves the migration, survival, and proliferation of hair follicle cells. 27
Although many laboratory experiments and animal studies have investigated the effects of ADSC on hair growth and identified its positive effect in promoting hair regeneration, 11 , 12 , 31 only a few clinical trials have investigated the effects of ADSC‐based therapies on the hair cycle in humans. Hirotaro et al. used intradermal injections of adipose‐derived stem cell conditioned media (ADSC‐CM) with antioxidants or finasteride and illustrated its efficacy on hair density in both males and females. 32 , 33 One of the first recognized retrospective, observational studies in 27 patients with female‐pattern hair loss (FPHL) who were treated with single ADSC‐CM intradermal injection showed that ADSC‐CM application promoted hair density and thickness in these patients without adverse reactions. 13 More recently, the therapeutic potential of ADSC‐CM in male‐pattern hair loss (MPHL) and FPHL was confirmed through a clinical pilot study in 52 patients who received a 12‐week ADSC‐CM monotherapy, using a micro‐needle or mesotherapy gun. 14 However, none of these studies included CGs for comparison. This limitation made the findings insufficient in proving the absolute effects of ADSC‐CM on hair regeneration because manual stimulation, by itself, has a certain effect on hair growth by helping blood flow in the applied area. 34 Therefore, a slight increase in hair density (+7.1%) and hair diameter (+6.3%) was observed in CG in this study after 16 weeks of the intervention.
In the first randomized, placebo‐controlled, double‐blind trial, we explored the efficacy of topical application of ADSC‐CE in hair growth in patients with AGA. Another point that distinguishes our study from other similar studies is the delivery methods that we used for ADSC‐CE application to the scalp. 13 , 14 The distilled water vehicle in combination with the ADSC extract used in this study was hypotonic and absorbed through simple diffusion. The filtering phase during ADSC extraction included micro‐filtering of 200 nm so that simple diffusion could be facilitated by making the particles even and small to the level of peptides. Additionally, the membranes of ADSCs were disrupted by low‐frequency ultrasound waves to obtain a more enriched ADSC‐CE with stem cell proteins. This method may allow for better absorption of the stem cell content into the scalp than that if they had intact cell membranes. Thus, we cautiously assume that ADSC‐CE is likely to penetrate the tissue more than the existing products manufactured using conditioned media (ADSC‐CM). Recent studies have shown that ADSC extract processed this way present regenerative and anti‐inflammatory factors. 35 , 36 Intradermal injection is invasive and practically challenging for long‐term usage when compared with the use of topical agents. In comparison with the findings of previous studies that used injectable preparations, our data showed noninferior outcomes in hair count and thickness with topical ADSC‐CE application, which allows easy access for patients with AGA and frequent use without fear of needle pricks and concerns of pain and possible skin infections.
Compared with healthy individuals, in patients with AGA, the expression of VEGF, KGF, EGF, and transforming growth factor‐β1 is disturbed in the hair follicles, and this affects the hair cycle differently depending on the age and sex. 37 Therefore, it is clinically important to personalize the optimal concentration, dosage, and frequency of ADSC‐based therapies. Indeed, clinical trials have shown that the efficacy of ADSC‐based therapies in AGA treatment varies depending on variables, such as the type of formulation, presence of combined treatments, and delivery methods of ADSC‐based therapies. 13 , 14 , 32 , 33 For example, Shin et al demonstrated that the mean hair density was promoted by 16.4% through micro‐needling with ADSC‐CM weekly for 12 weeks, 13 whereas in our study, the mean hair density increased by 28.1% with a topical solution of ADSC‐CM used twice daily for 16 weeks. Additionally, hair regeneration with the use of ADSC‐CM was found to be enhanced under hypoxic conditions. 38 Given the expanding clinical applications of ADSC‐CM in hair regeneration, it is also necessary to determine the optimal protocol for its administration to maximize the anti‐hair loss effect and minimize the adverse events.
Currently, only a few FDA‐approved agents for AGA treatment are available. Finasteride and minoxidil, either as monotherapy or in combination, are recommended as the gold standard treatment for MPHL. Although finasteride has been proved to enhance hair growth, oral finasteride is frequently shown to cause reduced libido, impotence, and sexual dysfunction. 7 Furthermore, it is only applicable to male patients with AGA owing to its highly teratogenic effects. 6 Topical minoxidil (2%)—another widely used agent—is the only treatment for female patients with AGA, and this has lower efficacy than the 5% minoxidil preparation that is available for male patients, 39 , 40 thus having disappointing outcomes. Furthermore, minoxidil can affect the heart and BP if absorbed excessively through the skin. 41
In IG, the mean hair density and thickness increased by 28.1% and 14.2% by 16 weeks, which were 3.95 and 2.25 times those in CG using the vehicle placebo, respectively. The improvements in IG were quite impressive when compared with results of studies on the efficacy of conventional treatments in AGA. In a previous study of 157 patients with MPHL, 5% topical minoxidil for 48 weeks increased hair density and thickness by only 12.3%. 40 Similarly, in 779 patients with MPHL, finasteride was found to increase hair density by 11.0% after 52 months of treatment. 42 Given that the duration of our intervention was relatively short, we cautiously conclude that the efficacy of topical ADSC‐CE in AGA is not inferior to those of topical minoxidil and finasteride. Furthermore, according to recent data, promotion of hair growth via ASCs can be enhanced by combining it with minoxidil, which stimulates the motility of ASCs and increases the secretion of growth factors and paracrine signaling. 43 Therefore, ADSC‐CE application can be considered as a pragmatic strategy in AGA treatment, or at least as an add‐on therapy with existing treatments, especially in female patients with AGA.
For the secondary efficacy outcomes, the improvement score given by the investigator was higher in IG than in CG, although these improvements were not statistically significant. In contrast, the score of subjective evaluation was lower in IG than in CG. One of the possible reasons is that the intervention duration in our study might not have been sufficient to yield a visually noticeable improvement, even though there was obvious microscopic hair regeneration. Additionally, the self‐rating assessment could have been affected by various levels of expectations toward the intervention that each participant might have had at baseline. The participants should have been inquired regarding the magnitude of improvement they wished for through the intervention after 16 weeks. Furthermore, apart from age and sex, various socioeconomic factors surrounding each individual such as job, marriage status, and age of onset of hair loss, which we failed to consider as covariates, are known to be associated with expectations in AGA treatment. 44
Owing to the limited number of treatments for AGA, PRP and micrografts are also considered effective alternatives to conventional medications. PRP is an autologous preparation of highly concentrated platelets in the plasma; hence, it contains numerous growth factors proved to be actively released by platelets to initiate wound healing, promote tissue repair, and influence the reactivity of vascular and other blood cells in angiogenesis and inflammation. 45 Recent clinical results have shown a potential of PRP application combined with HA 19 , 20 or fat graft in wound healing and reconstruction of bone 46 and soft tissue defects. 21 , 22 The role of PRP in AGA is attributed to numerous growth factors released from platelets that act on the stem cells in hair follicles, promoting neovascularization and stimulating the development of new follicles. 3 , 28 , 47 , 48 , 49 , 50 Although there are several studies showing the clinical efficacy of PRP in hair growth, the results remain debatable, mostly because of the various protocols for the preparation of PRP depending on the number of platelets, availability of growth factors, and chemokines with wide temporal (day‐to‐day) and biological variations between patients. 51 A standardized protocol for PRP preparation and administration in hair regeneration has not yet been established. 52
Although a micrograft is considerably effective in hair loss, it can only be used in cases where sufficient permanent donor hair is available without overlying diffuse telogen effluvium. In addition to high cost, micrografts can leave noticeable donor sites, with the accompanying risk that transplanted hairs will not take to the donor site and die off shortly after transplant. Moreover, the outcome heavily depends on the skill of the team performing the procedure. Furthermore, although there are studies on micrografts, few randomized controlled trials (RCTs) have compared hair transplantation vs no hair transplantation. This is mainly owing to multiple steps involved in the surgical procedure, significant variation in techniques, difficulties in measuring hair growth, difficulties in recruiting patients for an RCT, and insufficient financial support for hair transplantation. 53 A recent study showed the synergic effect of PRP and micrografts enriched with autologous human follicle MSCs on AGA in enhancing Wnt signaling, which is crucial for enhancing hair regrowth, in dermal papilla cells. 50 Additionally, micrografts from scalp tissue, containing human intra‐ and extradermal adipose tissue‐derived hair follicle stem cells, showed a significant improvement in hair regrowth. 54 In comparison with PRP or micrografts, ADSC‐based therapies have more published evidence of their effect on hair regrowth through clinical trials. 3 , 5 Moreover, there is increasing evidence of the positive outcomes of ADSC treatment in combination with human follicle stem cells in hair regrowth. 27 , 54 , 55 Given that current clinical practice guidelines on the treatment of AGA include finasteride 1 mg, dutasteride 0.5 mg, or minoxidil 5% (solution, foam) for men and minoxidil 2% solution or minoxidil 5% foam for women, 53 ADSC‐based therapeutics allow for more options for female patients with AGA. 56 However, owing to the lack of well‐designed clinical trials using ADSCs, there has not been enough evidence of long‐term safety of ADSC treatment.
Our study has some limitations. First, the study duration was 16 weeks, which is a relatively short period of time for a clinical trial. Therefore, our safety‐related data may not be sufficient to conclude the long‐term effect of ADSC‐CE application. Second, although we estimated the total number of participants required for this trial based on a published study, the enrolled number of female participants may be considered small for verifying the efficacy and safety of ADSC‐CE, especially in FPHL. Third, our results may not be generalizable because the data are from a single center. Additionally, we could not measure the type and concentration of growth factors in ADSC‐CE. Further studies on the mechanism of action of growth factors in the hair cycle will help understand the efficacy and safety of ADSC‐CE in AGA treatment. Lastly, our study failed to demonstrate histologically the amount of trial product reaching the hair follicles.
Despite these limitations, our study is valuable and has several strengths. First, to the best of our knowledge, this is the first clinical study with a vehicle placebo‐CG to examine the efficacy and tolerability of ADSC‐CE in hair growth. Second, we tested the efficacy of ADSC‐CE in a nonintradermal method without the need for delivery tools. Lastly, measurements of hair regeneration were confirmed objectively using phototrichograms that allowed for more reliable quantitative assessments than those by global photograph‐based analysis by an investigator or self‐assessments by participants, which have been commonly used in previous studies as the primary efficacy endpoint. 6 , 8 , 38
5. CONCLUSION
There were no toxicities or severe adverse effects associated with the use of ADSC‐CE for hair growth in humans. Compared with the placebo, ADSC‐CE monotherapy for 16 weeks significantly increased both hair count and thickness, and its effect was observable within 8 weeks of application. We suggest that application of the ADSC‐CE solution directly to the scalp can function as a potential and optional agent in AGA treatment and may also be used as an add‐on therapy with either finasteride or minoxidil, while maintaining treatment safety. However, similar studies with large and diverse populations are needed to confirm the beneficial effects of ADSC‐CE on hair growth and elucidate the mechanisms responsible for the action of ADSC‐CE in humans.
conflict of interest
The authors declared no potential conflicts of interest.
author contributions
Y.J.T.: data analysis and interpretation, manuscript writing (original drafting, review, and editing); S.Y.L.: conception and design, collection and assembly of data, data analysis and interpretation; A.R.C.: provision of study material and patients; Y.S.K.: collection and assembly of data.
Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double‐blind, vehicle‐controlled clinical study of hair regeneration using adipose‐derived stem cell constituent extract in androgenetic alopecia. STEM CELLS Transl Med. 2020;9:839–849. 10.1002/sctm.19-0410
Contributor Information
Sang Yeoup Lee, Email: saylee@pnu.edu.
A Ra Cho, Email: zzoara914@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lee W, Lee H. Characteristics of androgenetic alopecia in Asian. Ann Dermatol. 2012;24:243‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han SH, Byun JW, Lee WS, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012;24:311‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Semalty M, Semalty A, Joshi GP, Rawat MSM. Hair growth and rejuvenation: an overview. J Dermatolog Treat. 2011;22:123‐132. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89‐95. [DOI] [PubMed] [Google Scholar]
- 5. Wosicka H, Cal K. Targeting to the hair follicles: current status and potential. J Dermatol Sci. 2010;57:83‐89. [DOI] [PubMed] [Google Scholar]
- 6. Kawashima M, Hayashi N, Igarashi A, et al. Finasteride in the treatment of Japanese men with male pattern hair loss. Eur J Dermatol. 2004;14:247‐254. [PubMed] [Google Scholar]
- 7. Fertig RM, Gamret AC, Darwin E, et al. Sexual side effects of 5‐α‐reductase inhibitors finasteride and dutasteride: a comprehensive review. Dermatol Online J. 2017;23:13030/qt24k8q743. [PubMed] [Google Scholar]
- 8. Cho YH, Lee SY, Jeong DW, et al. Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, double‐blind, placebo‐controlled trial. Evid‐Based Complement Alternat Med. 2014;2014:579721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568‐584. [DOI] [PubMed] [Google Scholar]
- 10. Chu DT, Nguyen TPT, Tien NLB, et al. Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med. 2019;8:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramdasi S, Tiwari SK. Growth factors and cytokines secreted in conditioned media by mesenchymal stem cells‐promising possible therapeutic approach for hair regeneration. J Stem Cells. 2016;11:201‐211. [PubMed] [Google Scholar]
- 12. Lee YR, Yamazaki M, Mitsui S, Tsuboi R, Ogawa H. Hepatocyte growth factor (HGF) activator expressed in hair follicles is involved in in vitro HGF‐dependent hair follicle elongation. J Dermatol Sci. 2001;25:156‐163. [DOI] [PubMed] [Google Scholar]
- 13. Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue‐derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol. 2015;54:730‐735. [DOI] [PubMed] [Google Scholar]
- 14. Shin H, Won CH, Chung WK, et al. Up‐to‐date clinical trials of hair regeneration using conditioned media of adipose‐derived stem cells in male and female pattern hair loss. Curr Stem Cell Res Ther. 2017;12:524‐530. [DOI] [PubMed] [Google Scholar]
- 15. Lee WS, Ro BI, Hong SP, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37‐46. [DOI] [PubMed] [Google Scholar]
- 16. Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713‐721. [DOI] [PubMed] [Google Scholar]
- 17. Lee JG, Lee JS, Park HJ, et al. The effects of traditional oriental hair care products on alopecia. J Korean Med Ophthalmol Otolaryngol Dermatol. 2009;22:145‐152. [Google Scholar]
- 18. Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cervelli V, Lucarini L, Spallone D, et al. Use of platelet‐rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv Skin Wound Care. 2011;24:176‐181. [DOI] [PubMed] [Google Scholar]
- 20. Nicoli F, Balzani A, Lazzeri D, et al. Severe hidradenitis suppurativa treatment using platelet‐rich plasma gel and Hyalomatrix. Int Wound J. 2015;12:338‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scioli MG, Bielli A, Gentile P, Cervelli V, Orlandi A. Combined treatment with platelet‐rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose‐derived stem cells in three‐dimensional collagen scaffolds. J Tissue Eng Regen Med. 2017;11:2398‐2410. [DOI] [PubMed] [Google Scholar]
- 22. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Concise review: the use of adipose‐derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells. 2017;35:117‐134. [DOI] [PubMed] [Google Scholar]
- 23. Gentile P, De Angelis B, Pasin M, et al. Adipose‐derived stromal vascular fraction cells and platelet‐rich plasma: basic and clinical evaluation for cell‐based therapies in patients with scars on the face. J Craniofac Surg. 2014;25:267‐272. [DOI] [PubMed] [Google Scholar]
- 24. Gentile P, Kothari A, Casella D, Calabrese C. Fat graft enhanced with adipose‐derived stem cells in aesthetic breast augmentation: clinical, histological, and instrumental evaluation. Aesthet Surg J. 2019. pii: sjz292. 10.1093/asj/sjz292 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25. Gentile P, Casella D, Palma E, et al. Engineered fat graft enhanced with adipose‐derived stromal vascular fraction cells for regenerative medicine: clinical, histological and instrumental evaluation in breast reconstruction. J Clin Med. 2019;8:E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gentile P, Piccinno MS, Calabrese C. Characteristics and potentiality of human adipose‐derived stem cells (hASCs) obtained from enzymatic digestion of fat graft. Cells. 2019;8:E282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth‐factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8:E466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gentile P, Cole JP, Cole MA, et al. Evaluation of not‐activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43:105‐115. [DOI] [PubMed] [Google Scholar]
- 30. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF‐mediated angiogenesis. J Clin Invest. 2001;107:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Won CH, Yoo HG, Kwon OS. Hair growth promoting effects of adipose tissue‐derived stem cells. J Dermatol Sci. 2010;57:134‐137. [DOI] [PubMed] [Google Scholar]
- 32. Fukuoka H, Suga H, Narita K, Watanabe R, Shintani S. The latest advance in hair regeneration therapy using proteins secreted by adipose‐derived stem cells. Am J Cosmetic Surg. 2012;29:273‐282. [Google Scholar]
- 33. Fukuoka H, Suga H. Hair regeneration treatment using adipose‐derived stem cell conditioned medium: follow‐up with trichograms. Eplasty. 2015;15:e10. [PMC free article] [PubMed] [Google Scholar]
- 34. Koyama T, Kobayashi K, Hama T, et al. Standardized scalp massage results in increased hair thickness by inducing stretching forces to dermal papilla cells in the subcutaneous tissue. Eplasty. 2016;16:e8. [PMC free article] [PubMed] [Google Scholar]
- 35. Venkatarame Gowda Saralamma V, Vetrivel P, Kim SM, et al. Proteome profiling of membrane‐free stem cell components by nano‐LS/MS analysis and its anti‐inflammatory activity. Evid Based Complement Alternat Med. 2019;2019:4683272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee HJ, Lee SM, Moon YG, et al. Membrane‐free stem cell components inhibit interleukin‐1α‐stimulated inflammation and cartilage degradation in vitro and in vivo: a rat model of osteoarthritis. Int J Mol Sci. 2019;20:4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubanov AA, Gallyamova YA, Korableva OA. The study of growth factors in patients with androgenic alopecia. J Biomed Pharmacol. 2017;10:1219‐1228. [Google Scholar]
- 38. Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose‐derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27‐34. [DOI] [PubMed] [Google Scholar]
- 39. Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo‐controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541‐553. [DOI] [PubMed] [Google Scholar]
- 40. Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47:377‐385. [DOI] [PubMed] [Google Scholar]
- 41. Goren A, Naccarato T. Minoxidil in the treatment of androgenetic alopecia. Dermatol Ther. 2018;31:e12686. [DOI] [PubMed] [Google Scholar]
- 42. Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J Am Acad Dermatol. 1998;39:578‐589. [DOI] [PubMed] [Google Scholar]
- 43. Choi N, Shin S, Song SU, Sung JH. Minoxidil promotes hair growth through stimulation of growth factor release from adipose‐derived stem cells. Int J Mol Sci. 2018;19:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim BK, Lee S, Jun M, Chung HC, Oh SS, Lee WS. Perception of hair loss and education increases the treatment willingness in patients with androgenetic alopecica: a population‐based study. Ann Dermatol. 2018;30:402‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anitua E, Andia I, Ardanza B, Nurden P, Nurden A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4‐15. [DOI] [PubMed] [Google Scholar]
- 46. Gentile P, Bottini DJ, Spallone D, Curcio BC, Cervelli V. Application of platelet‐rich plasma in maxillofacial surgery: clinical evaluation. J Craniofac Surg. 2010;21:900‐904. [DOI] [PubMed] [Google Scholar]
- 47. Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet‐rich plasma in hair regrowth: a randomized placebo‐controlled trial. Stem Cells Translational Medicine. 2015;4:1317‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cervelli V, Garcovich S, Bielli A, et al. The effect of autologous activated platelet rich plasma (AA‐PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gentile P, Garcovich S, Scioli MG, Bielli A, Orlandi A, Cervelli V. Mechanical and controlled PRP injections in patients affected by androgenetic alopecia. J Vis Exp. 2018;(131):e56406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gentile P, Scioli MG, Bielli A, et al. Platelet‐rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re‐growth in androgenetic alopecia. Biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oh JH, Kim W, Roh YH, et al. Comparison of the cellular composition and cytokine‐release kinetics of various platelet‐rich plasma preparations. Am J Sports Med. 2015;43:3062‐3070. [DOI] [PubMed] [Google Scholar]
- 52. Stevens J, Khetarpal S. Platelet‐rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2018;5:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blumeyer A, Tosti A, Messenger A, et al. Evidence‐based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(suppl 6):S1‐S57. [DOI] [PubMed] [Google Scholar]
- 54. Gentile P. Micro‐grafts from scalp tissue containing human intra‐ and extra‐dermal adipose tissue‐derived hair follicle stem cells (HD‐AFSCs). Int J Mol Sci. 2019;20:E3446.31337037 [Google Scholar]
- 55. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lockhart RA, Hakakian CS, Birnbaum ZE, et al. Adipose derived stem cell based therapies or male/female pattern hair loss. J Stem Cell Res Med. 2016;1:59‐63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


