Abstract
Background
This was a randomized, open‐label, controlled phase II clinical trial to investigate the safety, efficacy, and outcomes of intrarenal artery infusion of autologous peripheral‐blood‐derived CD34+ cells for patients with chronic kidney disease (CKD; ie, stage III or IV).
Materials and Methods
Between October 2016 and July 2018, 52 consecutive patients with CKD at stage III or IV were randomly allocated into a treatment group (TG; 2.5 × 107 cells for each intrarenal artery; n = 26) and a control group (CG; standardized pharmacotherapy only; n = 26). The primary endpoints included safety and change of creatinine level/creatinine clearance. The secondary endpoints were 12‐month combined unfavorable clinical outcomes (defined as dialysis or death), improvement in proteinuria, and CD34+ cell‐related adverse events.
Results
All patients were uneventfully discharged after CD34+ cell therapy. The baseline endothelial progenitor cell (EPC) populations did not differ between TG and CG (P > .5). Flow cytometric analysis showed increases in circulating EPC (ie, CD34+KDR+CD45dim/ CD34+CD133+CD45dim/CD31+CD133+CD45dim/CD34+CD133+KDR+/CD133+) and hematopoietic stem cell (CD34+) populations after granulocyte‐colony stimulating factor treatment (all P < .001). Besides, Matrigel assay of angiogenesis was also significantly enhanced (all P < .001). Renal‐venous blood samplings (ie, at 0, 5, 10, and 30 minutes after CD34+ cell infusion) demonstrated significant progressive increases in EPC level (P for trend <.001) among the TG patients. One‐year combined unfavorable clinical outcomes were significantly lower in TG than those in CG (0% [0] vs 13.3% [4], P = .038). By 12 months after CD34+ cell therapy, circulating creatinine level, ratio of urine protein to urine creatinine, and creatinine clearance showed no difference between TG and CG (all P > .1).
Conclusion
CD34+ cell therapy was safe and improved 1‐year outcome.
Keywords: angiogenesis, CD34+ cell therapy, chronic kidney disease, circulating endothelial progenitor cells
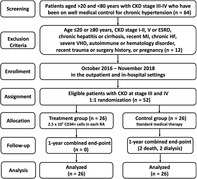
Flowchart illustrating the screening, exclusion, enrollment, assignment, allocation, ?follow‐up, and analysis of this phase II clinical trial. CDK, chronic kidney disease; ESRD, ?end‐stage? renal disease; HF, heart failure; MI, myocardial infarction.
Significance statement.
The results of this phase II clinical trial provide important clinical information about the impact of intrarenal artery infusion of autologous peripheral‐blood‐derived CD34+ cells for patients with chronic kidney disease.
1. INTRODUCTION
Chronic kidney disease (CKD) remains a major and growing public health problem worldwide 1 , 2 , 3 , 4 not only because of its ultimate progression to end‐stage renal disease (ESRD) 1 , 3 but also owing to the high morbidity and mortality commonly associated with patients with CKD hospitalized for other disease entities, especially those with coexistence of cardiovascular diseases (ie, cardiorenal syndrome). 5 , 6 , 7 , 8 Intriguingly, despite the state‐of‐the‐art pharmacotherapy as well as advances in diagnosis criteria and treatment guidelines for CKD, progressive deterioration in renal function with subsequent requirement for renal replacement therapy (ie, dialysis or transplantation) is not uncommon. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 These issues 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 highlight the need for the development of a new treatment modality with safety and efficacy for patients with CKD, especially those who are refractory to the conventional therapy.
Growing evidence from preclinical studies and clinical trials has demonstrated that stem cell therapy effectively protects different organs against ischemia, ischemia‐reperfusion injury, atherosclerosis‐associated endothelial dysfunction, sepsis, and acute respiratory distress syndrome. 18 , 19 , 20 , 21 , 22 , 23 Interestingly, our previous preclinical study has demonstrated that circulatory‐derived EPCs therapy preserved the residual renal function in rats with CKD through enhancement of angiogenesis and blood flow as well as suppression of oxidative stress, inflammation, and fibrosis. 24 Based on the findings of our preclinical study, 24 we had performed a phase I clinical trial using autologous CD34+ cells to treat patients with stages III and IV of CKD. 25 Our results showed that administration of autologous circulatory‐derived CD34+ cells to patients with CKD was safe. 25 However, the sample size was not big enough to provide information about efficacy and clinical outcome. 25 Accordingly, we designed a phase II clinical trial to test the hypothesis that circulatory CD34+ cell therapy may be effective for preserving renal function and improving clinical outcomes in patients with CKD.
2. MATERIALS AND METHODS
2.1. Ethics and study design protocol
This was a phase II clinical trial that was given official approval by the Ministry of Health and Welfare, Taiwan, Republic of China (Institutional Review Board 1086603723) and the Institutional Review Committee on Human Research at Chang Gung Memorial Hospital (201600371A0C603) in 2016.
This phase II clinical trial was a prospective, randomized, open‐label controlled trial to test the safety and efficacy of circulatory‐derived CD34+ cell treatment for patients with CKD at stage III or IV at a single medical center. This study was designed to consecutively enroll 56 (ie, evaluation could be up to n = 70 and enrolled sample sized was 56 study subjects) study patients who had received optimal medical care. The patients were double‐blindly enrolled to either receive CD34+ cells (2.5 × 107) per renal artery (group 1; ie, total 5.0 × 107 cells for one patient) or serve as control subjects with only standard pharmacotherapy (group 2; ie, 1:1 randomization). Such a dosage for one patient was equally transfused into the left and renal artery was strongly recommended by the Ministry of Health and Welfare, Taiwan, Republic of China, and the Institutional Review Committee on Human Research at Chang Gung Memorial Hospital for essential consideration of patient safety.
The primary endpoints of this clinical trial were to test the safety and efficacy (ie, preservation of creatinine clearance rate [Ccr], suppression of serum creatinine level). Secondary endpoints included combined major adverse clinical outcomes (ie, dialysis or death), incidence of cell infusion‐related serious adverse events, and improvement in proteinuria and ratio of urine albumin to urine creatinine.
2.2. Sample‐size calculation for secondary endpoints
We assumed an improvement of Ccr by 10 mL/minute or higher in group 1 patients compared with those in group 2 at 1 year after randomization. An estimated sample size of 26 patients in each group was calculated on the basis of the effective size = 0.83, α = .05, power = 80%, an SD of 12 mL/minute in both groups, and a 5.0% rate for protocol violations or incomplete follow‐up.
2.3. Randomization method
Fifty‐six sealed envelopes were used for randomization. Inside the envelopes included 28 patients assigned to receive CD34+ cell treatment. On the other hand, 28 patients served as the control group, that is, 1:1 homogenous distribution. These 56 sealed envelopes were then put into a box after well preparation. The sealed envelopes were randomly drawn out one by one for each participant.
2.4. Inclusion and exclusion criteria
The criteria have been described in our phase I clinical trial. 25 In detail, the recruitment criteria included patients within 20 and 80 years, with stages III and IV of CKD, with a recognized hypertension history prior to development of CKD, and having regular and optimal antihypertension drugs, including those of angiotensin converting enzyme inhibitors (ACEIs), angiotensin II type I receptor‐blocker (ARB) agents, calcium‐channel blockades, or ß‐blockers. In addition, the serum level of creatinine and Ccr were preserved within 30% of the baseline in recent 6 months. Besides, patients were willing to participate in this phase II clinical study.
Those candidates with a history of the following issues were excluded from this study: hepatitis B or C carrier, major surgery or trauma or acute myocardial infarction within the previous 3 months, liver cirrhosis, hematology or immune diseases, stage V of CKD (defined as creatinine clearance <15 mL/minute) or stage I and stage II of CKD, malignancy, febrile disorders, acute or chronic inflammatory disorders at study entry, severe mitral or aortic insufficiency, advanced heart failure (ie, New York Heart Association Functional Classification ≥3), expected life expectancy <2.0 years, age <20 or ≥80 years, or pregnancy.
Between October 2016 and July 2018, patients who fit the above enrollment criteria were evaluated for eligibility at our hospital. Over an enrollment interval of 10 months, 64 patients with CKD were consecutively screened. Twelve (18.8%) of the 64 patients were excluded because of hesitation or repudiation (ie, screen failed for 7 in treatment and 5 in control group). Accordingly, 52 patients were finally enrolled into the study and prospectively randomized into group 1 (ie, CD34+‐treated patients, n = 26) and group 2 (ie, control group, n = 26; refer to the flowchart of Figure 1).
FIGURE 1.
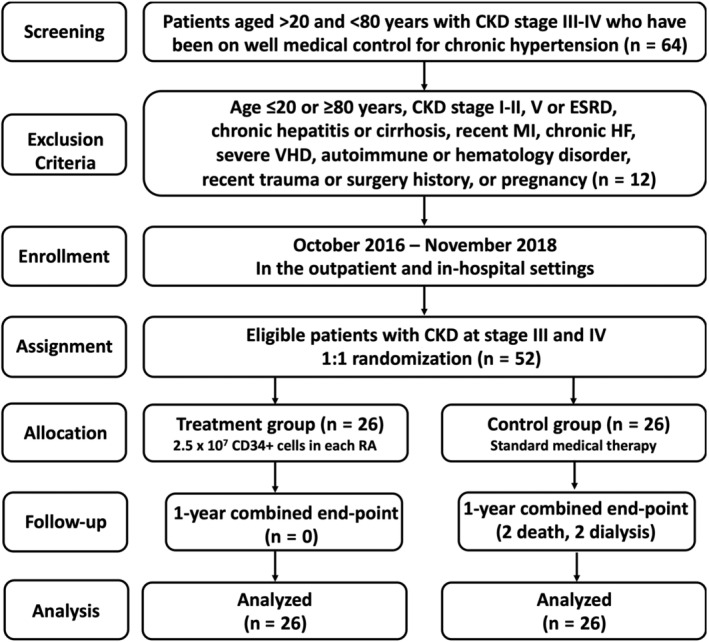
Flowchart illustrating the screening, exclusion, enrollment, assignment, allocation, follow‐up, and analysis of this phase II clinical trial. CDK, chronic kidney disease; ESRD, end‐stage renal disease; HF, heart failure; MI, myocardial infarction
2.5. Procedure and protocol for isolation of circulatory CD34+ cells and intrarenal artery injection
The procedure and protocol for the isolation of circulatory CD34+ cells were based on our previous reports. 19 , 25 In detail, prior to the isolation of circulatory‐derived CD34+ cells, granulocyte‐colony stimulating factor (G‐CSF; 5 μg/kg, every 12 hours for eight doses) was subcutaneously administered to group 2 patients to enhance the number of circulatory CD34+ cells for subsequent collection through leukapheresis. After the final dose of G‐CSF, the mononuclear cells isolated during the procedure of leukapheresis were enriched for CD34+ cells by using a commercially available machine (COBE Spetra 6.1; Terumo BCT, Inc., Lakewood, Colorado) at 8:00 am via a double‐lumen catheter that was implanted into the right femoral vein. After a time interval of approximately 4 hours, adequate circulatory‐derived CD34+ cells were collected and well prepared for intrarenal artery administration.
Based on the International Society of Hematotherapy and Grafting Engineering Guidelines for CD34+ cell determination by flow cytometry to quantitate number of CD34+ cells in systemic circulation, hematological stem cells were characterized by the presence of the surface markers CD34high/CD45dim/SSClow that were employed for quantification of the number of isolated CD34+ cells. The formula for calculation of the number of circulatory‐derived CD34+ cells was as follows: Number of CD34+ cells = (percentage of CD34+ cells) × white blood cell count (ie, CD45+ cells) × 103 × collected peripheral‐blood stem cell volume (mL). After identifying the number of CD34+ cells in 1 mL of leukapheresis product, we were then able to calculate 5.0 × 107 CD34+ cells in how many volumes of leukapheresis product. This estimated volume (ie, leukapheresis product) containing 5.0 × 107 CD34+ cells was then transfused into bilateral renal arteries equally.
In the present study, the flow cytometric analysis was performed according to the College of American Pathology current guideline with a performance coefficient of variation: <4.0% (3.4 ± 2.5; by definition of <10.0% considered acceptable).
After accomplishing the CD34+ cell collection, patients were immediately taken to the cardiac catheterization room for accepting bilateral intrarenal transfusion of CD34+ cells. Left transbrachial arterial method was used for each patient for renal‐arterial angiographic examination, followed by CD34+ cell slow injection for about 5.0 to 7.0 minutes through a fine guiding catheter. Additionally, the puncture of right femoral vein and engagement of right renal vein by guiding catheter were performed for assessing the time courses of EPCs in renal vein.
2.6. Laboratory investigation of circulating and renal‐vein EPC populations by flow cytometry
The procedure and protocol have been described in our previous study. 19 , 25 In detail, EPC populations in circulation and right renal vein were quantified by flow cytometry (FACSCalibur system; Beckman Coulter Inc, Brea, California) using double staining as described in our recent report. 19 , 25 Each examination recruited 300 000 cells per sample. The assessment for circulatory and renal‐vein EPC populations in each sample was performed twice and mean levels were reported. Intra‐assay variability based on repeated examinations of the same blood sample collection was low with a mean coefficient of variance of 3.9% in the study subjects.
One blood sample collection was obtained at 8:00 am, prior to G‐CSF administration, and the other was drawn following G‐CSF therapy for flow cytometric assessment. Additionally, to verify the serial changes of EPC population in right renal vein, several time points of blood samples were collected from the renal vein at 0 minutes prior to CD34+ cell injection and at 5, 10, and 30 minutes after CD34+ cell administration for flow cytometric analysis.
2.7. Collection of circulatory venous blood, EPC culturing, and angiogenesis assessment using Matrigel assay
For verifying the angiogenesis capacity of EPCs, a blood sample collection (10 mL) was obtained from each group 1 patient prior to G‐CSF treatment. Blood samples from group 2 patients were also obtained at the same time point for comparison. After G‐CSF treatment, 10 mL of blood was again acquired from each patient in group 1. From the plasma with isolated CD34+ cells ready for transfusion after G‐CSF treatment in group 1 patients, 10 mL was sampled. All samples were subject to 21‐day culturing for the assessment of angiogenesis ability. The isolated mononuclear cells were cultured in a 100‐mm‐diameter dish with 10 mL EGM‐2 culture medium containing 10% fetal bovine serum. By 21‐day culturing, abundant cobblestone‐like cells, a typical feature of EPCs, were obtained from each study subject. Flow cytometric analysis was performed for identification of cellular characteristics (ie, EPC surface markers) with appropriate antibodies on day 21 of cell cultivation.
The culture‐derived EPCs were well prepared and further incubated on 96‐well plates at 1.0 × 104 cells/well in 150 μL serum‐free EGM‐2 culture medium mixed with 50 μL cold Matrigel (Chemicon International) for a 3‐hour incubation at 37°C in 5% CO2. Three randomized microscopic images (×200) were obtained at each well for calculating the cluster, tube, and network formations with the mean values acquired (Figure 4).
FIGURE 4.
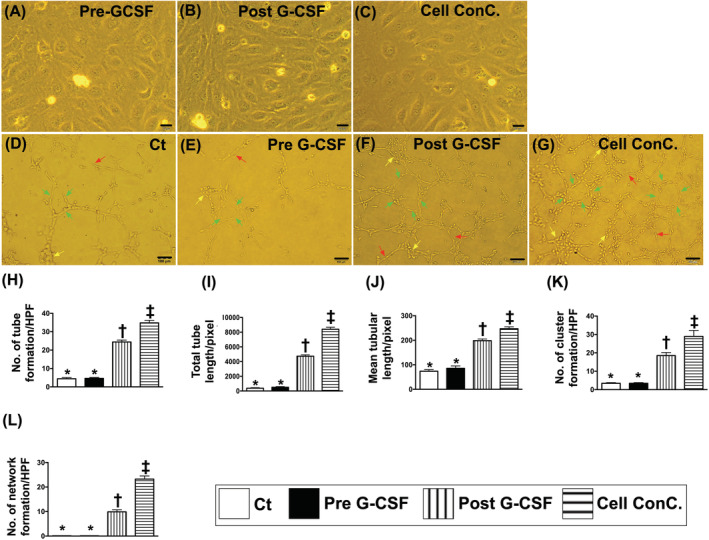
Morphologic feature of cultured EPCs and Matrigel assay for determinant of angiogenesis. A‐C, Illustrating the typically morphological feature (ie, cobblestone‐like) (×400) of 21‐day cell culturing in EPCs in different situations: A, indicated prior to G‐CSF treatment; B, indicated after G‐CSF treatment; C, indicated plasma contained isolated EPCs. Scale bars in the right lower corner of (A) to (C) represent 20 μm. D‐G, Showing the Matrigel assay of angiogenesis features (×100) of tubular length (red arrows), cluster formation (yellow arrows), and network formation (green arrows). Scale bars in the right lower corner of (A) to (C) represent 100 μm. H, Number of tubule formation, * vs other groups with different symbols (†, ‡), P < .0001. I, Total tubular length, * vs other groups with different symbols (†, ‡), P < .0001. J, Mean tubular length, * vs other groups with different symbols (†, ‡), P < .0001. K, Number of cluster formation, * vs other groups with different symbols (†, ‡), P < .0001. L, Number of network formation, * vs other groups with different symbols (†, ‡), P < .0001. All statistical analyses were performed by one‐way analysis of variance, followed by Bonferroni multiple comparison post hoc test (n = 26 for each group). Symbols (*, †, ‡) indicate significance (at .05 level). Group 1 = CD34+‐treated patients; group 2 = control patients. EPC, endothelial progenitor cell; G‐CSF, granulocyte‐colony stimulating factor; HPF, high‐power field (×100)
2.8. Examinations of blood urea nitrogen, creatinine, and urine protein and albumin levels
The procedure and protocol have been described in our previous study. 25 In detail, blood samples from each patient were serially collected before (ie, at hospitalization of days 6 and 7 for group 1 patients) and at 1 week and at 1, 3, 6, 9, and 12 months after the administration of autologous CD34+ cells into the renal artery for the determination of circulatory levels of creatinine and blood urea nitrogen (BUN). Additionally, the ratios of urine total protein (uTP) and urine albumin (uAL) to urine creatinine (uCre) were also serially measured as the time points of measurement for creatinine and BUN. Concentrations of circulatory levels of creatinine, BUN, and urine protein (one spot urine was used) were calculated by the standard method in the Department of Clinical Biochemistry and Pathology of our hospital. Furthermore, the Cockcroft‐Gault equation was used for the measurement of Ccr (mL/minute) in this phase II clinical trial.
2.9. Abdominal ultrasonography for serial assessment of renal parenchyma, size, and cortical echogenicity
Abdominal ultrasound examination was performed by a nephrologist blinded to the treatment protocol. The renal size and feature of renal parenchyma and architecture were serially carefully examined at time intervals prior to and at 1, 3, 6, and 12 months after CD34+ cell transfusion. The parameters were averaged and entered into a computer for further analysis.
Assessment of cortical echogenicity was based on the previous report. 26 In detail, scoring of the renal cortical echogenicity was compared and graded with the echogenicity of the liver and renal medulla with grade 0 to grade 4, respectively. Grade 0 was defined as normal echogenicity less than that of the liver with clear maintenance of corticomedullary differentiation. Grade 1 was defined as the echogenicity identical to that of the liver with maintained corticomedullary differentiation. Grade 2 was defined as the echogenicity greater than that of the liver with maintenance of corticomedullary differentiation. Grade 3 was defined as the echogenicity greater than that of the liver with poorly maintained corticomedullary differentiation. Grade 4 was defined as the echogenicity greater than that of the liver with a loss of corticomedullary differentiation.
2.10. Regular pharmacotherapy and medicine during CD34+ cell injection
The 3000 IU heparin was slowly administered via the intrarenal artery to each patient at the start of the CD34+ cell transfusion, and the heparin's effect was immediately reversed by intravenous administration of protamine (20 mg) after CD34+ cell injection.
Other regular medications, including ACEI, ARB, calcium‐channel blockades, statins, oral hypoglycemia drugs, and beta‐blockers for those patients with hypertension, diabetes mellitus, and hypercholesterolemia, and antiplatelet agents for those patients with coronary artery disease or history of ischemic stroke, were routinely prescribed in the present study.
2.11. Clinical information from follow‐ups
Besides regular monitoring of each patient at the outpatient clinic, a case report form that recorded all clinical issues of each patient, including the presence or absence of acute or subacute events, was designed for each patient and accomplished by a research nurse regularly after each visit and on readmission as well as through regular telephone interviews.
2.12. Statistical analysis
Each value is expressed as the mean ± SD, number, or percentage, where appropriate. Differences in continuous variables between the two groups were analyzed by independent t test for parametric data or Mann‐Whitney U test for nonparametric data. Categorical variables were analyzed with chi‐square test or Fisher's exact test. Continuous variables over different time points within the same group were compared with repeated measures analysis of variance. Additionally, the independent t test was performed for comparison of the continuous variables between the study and control groups, including baseline data and follow‐up results. Descriptive statistics were conducted for reporting the characteristics of efficacy and safety endpoints. Intention‐to‐treat analysis was used for evaluation of clinical outcomes. For the efficacy endpoints, the analysis employed an independent t test at the 5% significance level to evaluate the null hypotheses of no difference in the baseline Ccr between the two groups. An independent t test and a chi‐square test were used for the analysis of continuous and categorical endpoints, respectively. Regarding safety analysis, full descriptive statistics including event rates and their standard errors were reported and summarized in tables for all adverse events and serious adverse events. Statistical analysis was performed using the SPSS statistical software for Windows version 19 (SPSS for Windows, version 19; Chicago, Illinois). A value of P < .05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics among the two groups of patients (Table 1)
TABLE 1.
The baseline characteristics of the study group and the control group
| Variables | Study group (n = 26) | Control group (n = 26) | P value |
|---|---|---|---|
| Age, years | 61.88 ± 10.46 | 66.85 ± 10.21 | .060 |
| Female gender | 9 (34.6%) | 3 (11.5%) | .048 |
| DM | 12 (46.2%) | 12 (46.2%) | 1.000 |
| Hypertension | 26 (100.0%) | 26 (100.0%) | 1.000 |
| Hyperlipidemia | 17 (65.4%) | 17 (65.4%) | 1.000 |
| Current smoking | 2 (7.7%) | 7 (26.9%) | .140 |
| ACEIs/ARB use | 23 (83.5%) | 21 (80.8%) | .703 |
| Stain use | 17 (65.4%) | 18 (69.2%) | .768 |
| Old stroke | 0 (0.0%) | 2 (7.7%) | .490 |
| Old MI | 6 (23.1%) | 9 (34.6%) | .358 |
| History of CAD | 16 (64.0%) | 11 (44.0%) | .156 |
| History of PCI | No: 23 (92.0%) | No: 15 (68.2%) | .063 |
| Stage of CKD |
CKD3: 20 (76.9%) CKD4: 6 (23.1%) |
CKD3: 20 (76.9%) CKD4: 6 (23.1%) |
1.000 |
| Body height, cm | 160.27 ± 7.56 | 164.10 ± 6.14 | .053 |
| Body weight, kg | 68.05 ± 11.92 | 71.07 ± 13.47 | .396 |
| Body mass index | 26.40 ± 3.70 | 26.69 ± 5.21 | .821 |
| Creatinine level, g/dL | 2.00 ± 0.80 | 2.00 ± 0.69 | .647 |
| BUN, g/dL | 27.50 ± 6.56 | 33.58 ± 16.86 | .096 |
| CCr, cc/min | 38.99 ± 12.70 | 39.23 ± 12.27 | .944 |
| Ratio of uTP to uCre, mg/g | 933.9 ± 1464.7 | 1316.5 ± 2086.0 | .956 |
| Ratio of uAL to uCre, mg/g | 548.5 ± 879.9 | 690.9 ± 1093.1 | .701 |
Note: Study group = group 1; control group = group 2.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; BUN, blood urea nitrogen; CAD, coronary artery disease; Ccr, creatinine clearance rate; CKD, chronic kidney disease; DM, diabetes mellitus; MI, myocardial infarction; uAL, urine albumin; uCre, urine creatinine; uTP, urine total protein.
The proportion of females was significantly lower in group 2 (ie, control group) than in group 1 (ie, study group). However, age, the incidences of diabetes mellitus, hyperlipidemia, hypertension, current smoking, statin and ACEI/ARB use, old stroke, and previous myocardial infarction did not differ between the two groups. Additionally, the incidences of obstructive coronary artery disease and catheter‐based interventions also did not differ between these two groups.
The mean stage of CKD, body height, body weight, and body mass index did not differ between groups 1 and 2. Additionally, there was no notable difference in baseline serum creatinine and blood urea nitrogen level, Ccr, and ratio of uTP and uAL to uCre between the two groups.
3.2. Comparison of serial changes in Ccr, creatinine, BUN levels, and ratios of uTP and uAL to uCre between study and control groups, renal ultrasound findings, and 12‐month follow‐up clinical outcomes
The Ccr did not differ among the time points of prior to and at 1, 3, 6, 9, and 12 months (ie, serial changes) among the group 1 or group 2 patients (Figure 2A; Table 2). Further analysis of this parameter showed no difference between patients in group 1 and those in group 2 prior to and at any time point after CD34+ treatment (Figure 2A; Tables 2 and 3).
FIGURE 2.
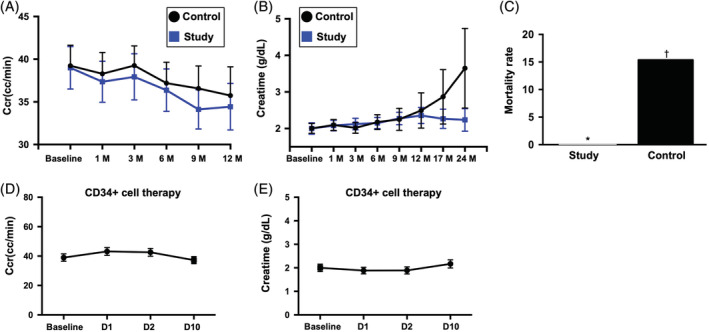
The time courses of Ccr and serum level of creatinine and combined untoward clinical outcome in study and control patients. A, Illustrating the time courses (ie, from baseline to 12 months) of Ccr between group 1 (ie, CD34+ cell–treated group) and group 2 (ie, control group). The analytical results showed that the Ccr did differ among the time points of prior to and at 1, 3, 6, 9, and 12 months (ie, serial changes) among the group 1 or group 2 patients or between groups 1 and 2 patients prior to and at any time point after CD34+ treatment. No significant difference was found at any time points between group 1 and group 2. B, Demonstrating the time courses (ie, from baseline to 12 months) of creatinine level between group 1 (ie, CD34+ cell–treated group) and group 2 (ie, control group). The analytical results showed that this parameter did differ among the time points of prior to and at 1, 3, 6, 9, and 12 months (ie, serial changes) among the group 1 or group 2 patients or between groups 1 and 2 patients prior to and at any time point after CD34+ treatment. C, Showing the combined endpoint of untoward clinical outcome (ie, dialysis or death during 12‐month follow‐up was significantly lower in group 1 than in group 2, * vs †, P = .038. D, Displaying the short‐term intervals of Ccr prior to and after CD34+ cell therapy. As compared with the baseline, this parameter was relatively lower after (ie, at days 1 to 10 after cell therapy) the cell therapy. E, Showing the short‐term intervals of creatinine level prior to and after CD34+ cell therapy. As compared with the baseline, this parameter was also relatively lower after (ie, at days 1 and 2 after cell therapy) the cell therapy. Ccr, creatinine clearance rate; D, day; M, month
TABLE 2.
Comparisons of serial changes of renal function, Ccr, albuminuria, and proteinuria among the study and control groups
| Variables | Baseline | 1 month | 3 months | 6 months | 9 months | 12 months | P value |
|---|---|---|---|---|---|---|---|
| Ccr, mL/min | 39.1 ± 12.4A | 37.8 ± 12.4A,B | 38.6 ± 12.6A,B | 36.7 ± 12.0A,B | 35.2 ± 11.7A,B | 35.0 ± 13.6B | .032 a |
| Study group | 39.0 ± 12.7 | 37.4 ± 12.3 | 37.9 ± 13.8 | 36.4 ± 12.7 | 34.1 ± 11.5 | 34.4 ± 13.4 | .833 b |
| Control group | 39.2 ± 12.3 | 38.3 ± 12.7 | 39.3 ± 11.5 | 37.2 ± 11.5 | 36.6 ± 12.1 | 35.7 ± 14.3 | |
| BUN, mg/dL | 30.5 ± 13.0 | 30.6 ± 13.4 | 29.6 ± 11.8 | 30.5 ± 13.2 | 33.0 ± 17.5 | 32.8 ± 13.2 | .105 a |
| Study group | 27.5 ± 6.6 | 27.6 ± 9.2 | 28.1 ± 10.9 | 27.8 ± 7.7 | 31.0 ± 10.3 | 31.7 ± 9.5 | .274 b |
| Control group | 33.6 ± 16.9 | 33.6 ± 16.2 | 31.1 ± 12.7 | 33.6 ± 17.2 | 35.3 ± 23.5 | 34.2 ± 17.2 | |
| Creatinine, mg/dL | 2.00 ± 0.74A | 2.09 ± 0.74A,B | 2.07 ± 0.76A,B | 2.16 ± 0.85B | 2.27 ± 1.11A,B | 2.42 ± 1.55A,B | .029 a |
| Study group | 2.00 ± 0.80 | 2.08 ± 0.73 | 2.12 ± 0.80 | 2.15 ± 0.78 | 2.28 ± 0.86 | 2.36 ± 1.07 | .977 b |
| Control group | 2.00 ± 0.68 | 2.09 ± 0.76 | 2.02 ± 0.73 | 2.17 ± 0.95 | 2.25 ± 1.38 | 2.49 ± 2.05 | |
| Ratio of uTP to uCre (mg/g) | 1125 ± 1795 | 1089 ± 1549 | 1074 ± 1599 | 1077 ± 1491 | 1176 ± 1908 | 1331 ± 2172 | .255 a |
| Study group | 934 ± 1465 | 975 ± 1403 | 1114 ± 1551 | 1304 ± 1629 | 1622 ± 2315 | 1803 ± 2606 | .241 b |
| Control group | 1316 ± 2086 | 1202 ± 1702 | 1033 ± 1679 | 808 ± 1294 | 620 ± 1039 | 700 ± 1205 | |
| Ratio of uAL to uCre (mg/g) | 620 ± 985 | 631 ± 955 | 606 ± 941 | 570 ± 771 | 650 ± 1154 | 771 ± 1404 | .310 a |
| Study group | 549 ± 880 | 597 ± 929 | 625 ± 920 | 700 ± 832 | 979 ± 1433 | 1084 ± 1738 | .123 b |
| Control group | 691 ± 1093 | 664 ± 997 | 585 ± 980 | 414 ± 679 | 238 ± 412 | 354 ± 590 | |
| Log (ratio of uTP to uCre) | 5.76 ± 1.74 | 5.79 ± 1.71 | 5.74 ± 1.71 | 5.77 ± 1.75 | 5.68 ± 1.83 | 5.78 ± 1.83 | .437 a |
| Study group | 5.74 ± 1.64 | 5.76 ± 1.64 | 5.87 ± 1.70 | 6.06 ± 1.77 | 6.05 ± 1.94 | 6.12 ± 1.95 | .186 b |
| Control group | 5.78 ± 1.86 | 5.83 ± 1.80 | 5.60 ± 1.75 | 5.44 ± 1.70 | 5.23 ± 1.61 | 5.33 ± 1.60 | |
| Log (ratio of uAL to uCre) | 4.66 ± 2.27 | 4.58 ± 2.36 | 4.57 ± 2.32 | 4.57 ± 2.35 | 4.47 ± 2.36 | 4.48 ± 2.54 | .641 a |
| Study group | 4.82 ± 2.07 | 4.68 ± 2.28 | 4.79 ± 2.24 | 4.98 ± 2.34 | 5.06 ± 2.45 | 4.97 ± 2.55 | .119 b |
| Control group | 4.50 ± 2.49 | 4.49 ± 2.48 | 4.33 ± 2.43 | 4.09 ± 2.33 | 3.72 ± 2.07 | 3.83 ± 2.44 |
Abbreviations: BUN, blood urea nitrogen; Ccr, creatinine clearance rate; Log, logarithmized uAL, urine albumin; uCre, urine creatinine;
Serial changes of parameter value were calculated with repeated measures analysis of variance. Alphabetic characters (A, B) in superscript indicate statistical significance of P value. Only creatinine clearance rate and serum level of creatinine demonstrated significantly worsening progression at 12 and 6 months, respectively.
To compare the values between study and control groups with independent t test at different time points. P values show no significant differences between groups.
TABLE 3.
Net change of deterioration from baseline to month 12
| Variables | Study group | Control group | P value |
|---|---|---|---|
| Ccr, mL/min | −4.58 ± 7.03 | −3.02 ± 8.75 | .446 |
| BUN, mg/dL | 3.96 ± 8.59 | 1.28 ± 9.25 | .339 |
| Creatinine, mg/dL |
0.37 ± 0.43 0.34 (−0.02, 0.57) |
0.51 ± 1.46 0.08 (−0.09, 0.42) |
.269 |
| Ratio of uTP to uCre, mg/g |
841.7 ± 2176.4 7.1 (−39.7, 1038.2) |
−167.9 ± 748.2 −11.8 (−277.3, 45.2) |
.178 |
| Ratio of uAL to uCre, mg/g |
522.0 ± 1533.1 1.1 (−24.9, 677.3) |
−24.1 ± 350.4 −4.7 (−82.3, 11.7) |
.322 |
| Log, ratio of uTP to uCre |
0.37 ± 1.01 0.15 (−0.15, 1.00) |
−0.06 ± 0.77 −0.14 (−0.65, 0.47) |
.170 |
| Log, ratio of uAL to uCre |
0.14 ± 1.19 0.08 (−0.55, 0.63) |
−0.05 ± 1.12 −0.26 (−0.75, 0.92) |
.594 |
Note: Study group = group 1; control group = group 2.
Abbreviations: Ccr, creatinine clearance rate; BUN, blood urine nitrogen; Log, logarithmized; uAL, urine albumin; uCre, urine creatinine; uTP, urine total protein.
Moreover, when we looked at the serial changes in creatinine levels, we found no difference in this parameter at any time point both among group 1 or group 2 patients and between groups 1 and 2 (Figure 2B; Tables 2 and 3). However, by the end of this study period, the incidences of unfavorable clinical outcomes (ie, combined endpoint) were significantly lower in group 1 than those in group 2 (Figure 2C). Two patients died as a result of acute respiratory distress syndrome and sudden death with suspicious etiology of cardiovascular disease, respectively. Additionally, the causal etiologies of hemodialysis in those two patients were due to use of Chinese herbal drugs in one patient and after receiving percutaneous coronary stenting in another patient due to contrast medium induced acute renal failure.
Similarly, no difference was noted in serum BUN levels among group 1 or group 2 patients and between groups 1 and 2 during the course of follow‐up (Tables 2 and 3).
The ratios of uTP and uAL to uCre were not significantly different at any time point both among group 1 or group 2 patients and between groups 1 and 2 (Table 2). On the other hand, the net change of deterioration of these parameters (ie, comparison between the baseline and at 12 months) were notably relative higher in group 1 than in group 2 patients (Table 3). The findings could be attributed to the subsequent exclusion of four patients in group 2 who met the definition of combined major adverse events; two of the patients succumbed and two others ended up on dialysis.
To elucidate the anatomical feature, architectural integrity, and size of both kidneys (ie, long axis and short axis), renal sonography was performed for each patient at baseline and 1, 3, 6, and 12 months after CD34+ cell transfusion. The results demonstrated no differences in length and width of kidney, grade of cortical echogenicity, parenchymal thickness, presence of calyceal dilatation, kidney stone, kidney cyst, and tumorigenesis among patients in each group or between groups 1 and 2 (Table 4).
TABLE 4.
Comparisons of bilateral renal ultrasound findings at different time intervals between the study group and the control group
| Variables | Baseline | 1 month | 3 months | 6 months | 12 months | P value |
|---|---|---|---|---|---|---|
| Length, cm | 10.23 ± 1.24 | 10.20 ± 1.20 | 9.99 ± 1.38 | 10.17 ± 1.18 | 10.04 ± 1.26 | .134 a |
| Study group | 10.28 ± 1.28 | 10.28 ± 1.34 | 10.30 ± 1.31 | 10.34 ± 1.21 | 10.28 ± 1.35 | .134 b |
| Control group | 10.18 ± 1.21 | 10.11 ± 1.05 | 9.66 ± 1.40 | 9.97 ± 1.13 | 9.72 ± 1.09 | |
| Width, cm | 4.77 ± 0.74 | 4.83 ± 0.69 | 4.67 ± 0.94 | 4.76 ± 0.80 | 4.66 ± 0.75 | .325 a |
| Study group | 4.53 ± 0.74 | 4.82 ± 0.71 | 4.82 ± 0.87 | 4.80 ± 0.81 | 4.79 ± 0.82 | .398 b |
| Control group | 5.01 ± 0.68 | 4.83 ± 0.68 | 4.52 ± 0.99 | 4.71 ± 0.79 | 4.49 ± 0.64 | |
| Grade of cortical echogenicity | 1.31 ± 0.48 | 1.37 ± 0.54 | 1.29 ± 0.53 | 1.33 ± 0.48 | 1.46 ± 0.52 | .200 a |
| Study group | 1.23 ± 0.47 | 1.29 ± 0.59 | 1.31 ± 0.58 | 1.35 ± 0.56 | 1.52 ± 0.60 | .786 b |
| Control group | 1.38 ± 0.48 | 1.44 ± 0.50 | 1.28 ± 0.48 | 1.30 ± 0.36 | 1.39 ± 0.40 | |
| Parenchymal thickness, cm | 1.28 ± 0.28 | 1.28 ± 0.25 | 1.25 ± 0.30 | 1.29 ± 0.25 | 1.27 ± 0.23 | .457 a |
| Study group | 1.26 ± 0.32 | 1.33 ± 0.26 | 1.32 ± 0.22 | 1.34 ± 0.25 | 1.30 ± 0.23 | .080 b |
| Control group | 1.30 ± 0.23 | 1.23 ± 0.23 | 1.18 ± 0.36 | 1.23 ± 0.24 | 1.24 ± 0.25 | |
| Pelvicalyceal dilatation (−/+) | 1 (1.9%) | 2 (3.8%) | 1 (1.9%) | 1 (2.1%) | 2 (4.8%) | — |
| Study group | 1 (3.8%) | 1 (3.8%) | 1 (3.8%) | 0 (0.0%) | 0 (0.0%) | — |
| Control group | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 1 (4.5%) | 2 (11.1%) | |
| Kidney stone (−/+) | 8 (15.4%) | 7 (13.5%) | 7 (13.7%) | 4 (8.3%) | 7 (16.7%) | — |
| Study group | 2 (7.7%) | 3 (11.5%) | 3 (11.5%) | 1 (3.8%) | 4 (16.7%) | — |
| Control group | 6 (23.1%) | 4 (15.4%) | 4 (16.0%) | 3 (13.6%) | 3 (16.7%) | |
| Kidney cyst (−/+) | 21 (41.2%) | 21 (40.4%) | 21 (41.2%) | 21 (43.8%) | 18 (42.9%) | — |
| Study group | 9 (34.6%)A | 8 (30.8%)A | 9 (34.6%)A | 9 (34.6%)A | 7 (29.2%)B | <.05 b |
| Control group | 12 (48.0%)A | 13 (50.0%)A | 12 (48.0%)A | 12 (54.5%)A | 11 (61.1%)B | <.05 b |
| Mass formation (−/+) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | — |
| Study group | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | — |
| Control group | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Note: Study group = group 1; control group = group 2. Alphabetic characters (A, B) in superscript indicate statistical significance of P value.
Serial changes of parameter value in each group were calculated with repeated measures analysis of variance.
Independent t test or chi‐square test was used to compare the values between the study and control groups at different time points.
3.3. Circulating EPC populations between group 1 and group 2 patients and in group 1 after G‐CSF treatment as well as the time courses of EPC populations in right renal vein among group 1 patients
As expected, the baseline levels of circulatory EPCs (ie, CD34+KDR+CD45dim, CD34+CD133+CD45dim, CD31+CD133+CD45dim, CD34+CD133+KDR+ and CD133+; Figure 3A‐E) and hematopoietic stem cells (HSCs; CD34+; Figure 3F) did not differ between groups 1 and 2. However, these parameters were significantly higher in G‐CSF‐treated group 1 patients and more significantly increased in the plasma with isolated EPCs (Figure 3A‐F).
FIGURE 3.
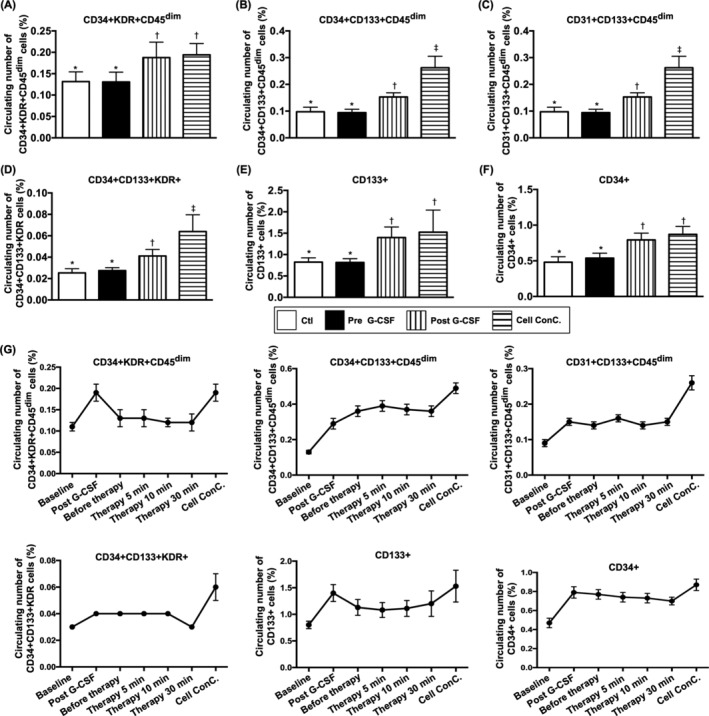
Circulating endothelial progenitor cell (EPC) populations between groups 1 and 2 patients and in group 1 after G‐CSF treatment as well as the time courses of EPC populations in right renal vein among group 1 patients. A, Circulating number of CD34+KDR+CD45dim cells, * vs †, P < .001. B, Circulating number of CD31+CD133+CD45dim cells, * vs other groups with different symbols (†, ‡), P < .0001. C, CD31+CD133+CD45dim, * vs other groups with different symbols (†, ‡), P < .0001. D, Circulating number of CD34+CD133+KDR+ cells, * vs other groups with different symbols (†, ‡), P < .0001. E, Circulating number of CD133+ cells, * vs †, P < .001. F, Circulating number of CD34+ cells, * vs †, P < .001. G, Flow cytometric analyses of serial changes of EPCs population in circulation (ie, at baseline and after G‐CSF treatment) and in right renal vein prior to and after CD34+ cell transfusion (ie, from time intervals of 0, 5, 10, and 30 minutes) in group 1 patients as well as in plasma contained isolated CD34+ cells (ie, concentrated cells). The results showed that the EPCs and hematopoietic stem cells (HSCs) were continuously drained from right renal vein to circulation after intrarenal artery transfusion, but they showed significant difference among the time intervals (0, 5, 10, and 30 minutes), suggesting the cells were retained in intrarenal arteries. On the other hand, the circulating numbers of EPCs and HSCs were notably higher after G‐CSF treatment and even higher in cell concentration than that prior to G‐CSF treatment (P for trend <.0001). All statistical analyses were performed by one‐way analysis of variance, followed by Bonferroni multiple comparison post hoc test (n = 26 for each group). Symbols (*, †, ‡) indicate significance (at .05 level). Group 1 = CD34+‐treated patients; group 2 = control patients. G‐CSF, granulocyte‐colony stimulating factor
To assess the shedding rate of EPCs from the right renal vein after CD34+ cell injection, flow cytometric analysis of EPCs and HSCs was used at different time intervals. The results exhibited that EPCs and HSCs were continuously drained from the right renal vein to the circulation after intrarenal artery administration (refer to Figure 3F). Additionally, among group 1 patients, the circulatory numbers of EPCs and HSCs were remarkably increased after G‐CSF administration (Figure 3G). This finding implicates that G‐CSF mobilized EPCs and HSCs from bone marrow to circulation.
3.4. Assessment of angiogenesis by Matrigel assessment in group 1 and 2 patients (Figure 4)
The results of Matrigel assay demonstrated that angiogenesis capacity (ie, including cluster formation, tubular length, and network formation) was similar between groups 1 and 2 prior to G‐CSF treatment. However, the capacity was significantly augmented in group 1 patients after receiving G‐CSF therapy compared with that in those of both groups prior to G‐CSF treatment, suggesting once again that G‐CSF would augment the mobilization of functional EPCs from bone marrow to circulation. One distinctive finding was that the parameters of angiogenesis were highest in plasma containing isolated EPCs.
4. DISCUSSION
It is well recognized that application of cell‐based therapy is rapidly growing worldwide to cover different disease entities. 19 , 20 , 23 , 25 , 27 , 28 Surprisingly, except for our previous study that tested the safety issue of CD34+ cell therapy for patients with CKD, the efficacy of cell therapy in this patient population has not been addressed. As far as we know, this is the first phase II clinical trial to address the therapeutic impact of the CD34+ cells on patients with CKD at stage III and IV.
The most distinctive finding of the current study was that the incidence of dialysis or death at 1‐year follow‐up (ie, combined endpoint of clinical outcome) was significantly lower in patients with CKD with CD34+ cell therapy than that in those without, highlighting potential extrarenal benefits of intrarenal artery infusion of autologous CD34+ cells in the setting of advanced kidney disease.
Two essential findings in the present study were the 100% safety (ie, one of primary endpoints) and the absence of serious adverse events (one of secondary endpoints). The findings of the present study were also supported by those of our previous studies, showing that administration of circulatory‐derived autologous CD34+ cells to patients with CKD 25 or coronary artery disease 19 , 28 is safe without serious side effects.
The serial changes in Ccr and circulatory creatinine level were the two secondary endpoints to be investigated in this phase II clinical trial. Unexpectedly, the two parameters did not show significant fluctuation at the end of this phase II clinical trial (ie, after 12‐month follow‐up), suggesting that intrarenal artery infusion of CD34+ cells did not offer additional benefit in patients with CKD up to a follow‐up period of 12 months. Interestingly, we found that the combined adverse clinical events (ie, either dialysis or mortality) all occurred within 6 months, probably because of the severity of the patients’ condition. This finding together with the relatively small sample size and the fact that the parameters (ie, Ccr, creatinine level, and ratios of uTP and uAL to uCre) were not regularly followed at the 6th, 9th, and 12th months in these four untoward clinical outcome patients may distort the statistical significance of our findings.
The follow‐up period of this phase II clinical trial was originally designed to be 12 months because of two reasons: (a) the limitation of financial support and (b) the recommendation of TFDA. Nevertheless, purely clinical follow‐up alone with only regular measurements of the serum level of creatinine for 5 years were permitted by TFDA, and up to now, only 12 patients in each group were completely followed up for 2 years. Surprisingly, when we looked at the serum creatinine level, we found a tendency of statistical significance (ie, P = .285) of this parameter between group 1 and group 2 patients (refer to Figure 2B) at this late time point (ie, at 2 years). This could, at least in part, be explained by the relatively slow reduction in Ccr and elevation in serum creatinine level (ie, usually <3.0% to 4.0% per year) when the patients were under optimal medical control (eg, AICEs and ARB). Therefore, long‐term follow‐ups are warranted to clarify the effectiveness of CD34+ for treating patients with CKD.
Progressive endothelial cell and arteriolar dysfunction followed by the development of obstructive atherosclerosis 9 , 29 , 30 have been suggested as a mechanism underlying the pathogenesis of CKD. Our previous study has further demonstrated marked reduction in the number of circulating EPCs in patients with CKD compared with that in healthy controls. 31 Our previous findings and those from other studies 9 , 29 , 30 , 31 suggest that EPC mobilization to the circulation may help in preserving the residual renal function in patients with CKD. Consistently, in the present study, we found that the circulating EPC population was remarkably increased and angiogenesis was substantially enhanced in our patients after receiving G‐CSF treatment. Therefore, the results may at least partly explain the significantly lower incidence of 1‐year combined untoward clinical outcomes and 2‐year serum creatinine level in the study group than those in the control group.
Safety is always the utmost concern of physicians, patients, and scientists engaged in cell‐based therapy. In the current study, there were no serious cell infusion‐related side effects, such as immune reaction or embolic event (ie, reflected by transient improvement in Ccr and reduction in serum creatinine level after CD34+ cell transfusion; Figure 1D,E). Additionally, ultrasound examination of the kidneys at the end of the study period (ie, 12 months) showed no evidence of tumorigenesis in all patients, supporting the safety of CD34+ therapy in the present clinical setting.
4.1. Study limitations
This study has limitations. First, the sample size was relatively small, so the statistical significance of differences in some parameters may be distorted. Second, the present study did not provide data on long‐term follow‐up. Third, the truly double‐blinded design (ie, another control group treated by G‐CSF+ intrarenal artery transfusion of plasm only without CD34+ cells) was not permitted by TFDA. Therefore, whether the observed preservation of renal function was the result of G‐CSF infusion or that of CD34+ infusion or the combination of these two regimens remains unclear. Fourth, it is well recognized that anomaly of renal‐arterial branch usually exists in 20% to 30% frequency. However, no renal‐arterial anomaly was identified in our patients, suggesting that it could be under evaluation. Another reason could be due to the fact that we did not routinely evaluate the anomaly of renal‐arterial branches. The CD34+ cells were always transfused into the main trunks of left and right renal arteries, which could minimize the bias of the results. Finally, it is well known that the time course of renal function greatly depends on the cause of CKD. However, the true etiology of CKD including glomerulonephritis, diabetic kidney disease, polycystic kidney disease, nephrosclerosis, or other was not known in this study.
5. CONCLUSION
Intrarenal artery administration of circulatory‐derived autologous CD34+ cells notably reduced the incidence of 1‐year combined untoward clinical outcomes and 2‐year creatinine level in patients with CKD, suggesting its therapeutic potential in this clinical setting.
conflict of interest
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
C.‐C.Y.: recruitment, collection and/or assembly of data, data analysis and interpretation, financial support; P.‐H.S.: recruitment, collection and/or assembly of data, data analysis and interpretation, manuscript writing; B.‐C.C.: recruitment, collection and/or assembly of data, data analysis and interpretation; Y.‐C.L., Y.‐L.C.: collection and/or assembly of data, data analysis and interpretation; M.S.L., H.‐K.Y.: conception and design, manuscript writing, final approval of manuscript.
acknowledgment
This study was funded by programs grants from the Ministry of Science and Technology, Taiwan, Republic of China (104‐2325‐B‐182A‐004, 105‐2325‐B‐182A‐002) and Chang Gung Memorial Hospital, Chang Gung University (NMRPG8E0021, NMRPG8F0181).
Yang C‐C, Sung P‐H, Cheng B‐C, et al. Safety and efficacy of intrarenal arterial autologous CD34+ cell transfusion in patients with chronic kidney disease: A randomized, open‐label, controlled phase II clinical trial. STEM CELLS Transl Med. 2020;9:827–838. 10.1002/sctm.19-0409
Funding information Ministry of Science and Technology, Taiwan, Republic of China, Grant/Award Numbers: 104‐2325‐B‐182A004, 105‐2325‐B‐182A‐002; Chang Gung Memorial Hospital, Chang Gung University, Grant/Award Numbers: NMRPG8E0021, NMRPG8F0181
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hsu CC, Hwang SJ, Wen CP, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48:727‐738. [DOI] [PubMed] [Google Scholar]
- 2. Jayatilake N, Mendis S, Maheepala P, Mehta FR, CKDu National Research Project Team . Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuo HW, Tsai SS, Tiao MM, Yang CY. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46‐55. [DOI] [PubMed] [Google Scholar]
- 4. Lunyera J, Mohottige D, Von Isenburg M, et al. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol. 2016;11:379‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowie MR, Komajda M, Murray‐Thomas T, Underwood J, Ticho B, POSH Investigators . Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J. 2006;27:1216‐1222. [DOI] [PubMed] [Google Scholar]
- 6. Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61‐67. [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296‐1305. [DOI] [PubMed] [Google Scholar]
- 8. Metra M, Nodari S, Parrinello G, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188‐195. [DOI] [PubMed] [Google Scholar]
- 9. Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974‐980. [DOI] [PubMed] [Google Scholar]
- 10. Elliott MJ, Gil S, Hemmelgarn BR, et al. A scoping review of adult chronic kidney disease clinical pathways for primary care. Nephrol Dial Transplant. 2017;32:838‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure‐‐trials and tribulations. Clin J Am Soc Nephrol. 2013;8:1816‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84‐104. [DOI] [PubMed] [Google Scholar]
- 13. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin‐angiotensin system in patients with hypertension, microalbuminuria, and non‐insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratcliffe LE, Thomas W, Glen J, et al. Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis. 2016;67:548‐558. [DOI] [PubMed] [Google Scholar]
- 15. Shroff GR, Solid CA, Herzog CA. Response to letter regarding article, “Long‐term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug‐eluting and bare metal stents in the United States”. Circulation. 2013;128:e407. [DOI] [PubMed] [Google Scholar]
- 16. Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure‐independent effect. Circulation. 2002;106:672‐678. [DOI] [PubMed] [Google Scholar]
- 17. Zafari N, Churilov L, MacIsaac RJ, et al. Diagnostic performance of the chronic kidney disease epidemiology collaboration (CKD‐EPI) equation at estimating glomerular filtration rate in adults with diabetes mellitus: a systematic review and meta‐analysis protocol. BMJ Open. 2019;9:e031558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee FY, Chen KH, Wallace CG, et al. Xenogeneic human umbilical cord‐derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget. 2017;8:45626‐45642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee FY, Chen YL, Sung PH, et al. Intracoronary transfusion of circulation‐derived CD34+ cells improves left ventricular function in patients with end‐stage diffuse coronary artery disease unsuitable for coronary intervention. Crit Care Med. 2015;43:2117‐2132. [DOI] [PubMed] [Google Scholar]
- 20. Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sung PH, Chang CL, Tsai TH, et al. Apoptotic adipose‐derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res Ther. 2013;4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sung PH, Chiang HJ, Wallace CG, et al. Exendin‐4‐assisted adipose derived mesenchymal stem cell therapy protects renal function against co‐existing acute kidney ischemia‐reperfusion injury and severe sepsis syndrome in rat. Am J Transl Res. 2017;9:3167‐3183. [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang TH, Chen YT, Sung PH, et al. Peripheral blood‐derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res. 2015;7:804‐824. [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MS, Lee FY, Chen YL, et al. Investigated the safety of intra‐renal arterial transfusion of autologous CD34+ cells and time courses of creatinine levels, endothelial dysfunction biomarkers and micro‐RNAs in chronic kidney disease patients‐phase I clinical trial. Oncotarget. 2017;8:17750‐17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siddappa JK, Singla S, Al Ameen M, et al. Correlation of ultrasonographic parameters with serum creatinine in chronic kidney disease. J Clin Imaging Sci. 2013;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borakati A, Mafi R, Mafi P, Khan WS. A systematic review and meta‐analysis of clinical trials of mesenchymal stem cell therapy for cartilage repair. Curr Stem Cell Res Ther. 2018;13:215‐225. [DOI] [PubMed] [Google Scholar]
- 28. Sung PH, Lee FY, Tong MS, et al. The five‐year clinical and angiographic follow‐up outcomes of intracoronary transfusion of circulation‐derived CD34+ cells for patients with end‐stage diffuse coronary artery disease unsuitable for coronary intervention‐phase I clinical trial. Crit Care Med. 2018;46:e411‐e418. [DOI] [PubMed] [Google Scholar]
- 29. Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta‐analysis. Kidney Int. 2001;59:260‐269. [DOI] [PubMed] [Google Scholar]
- 30. Seliger SL, Salimi S, Pierre V, Giffuni J, Katzel L, Parsa A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016;17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen YT, Cheng BC, Ko SF, et al. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:83‐91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


