Abstract
Background
Although video-assisted thoracic surgery (VATS) can significantly reduce postoperative pain, the incidence is as high as 30–50%. The purpose of this study was to explore the safety and efficacy of ultrasound-guided serratus anterior plane block (SAPB) combined with dexmedetomidine (Dex) for patients undergoing VATS.
Methods
Eighty patients were randomized into two groups (20 mL 0.5% ropivacaine plus 0.5 µg/kg or 1 µg/kg Dex). Primary outcome was the visual analog scale of pain while coughing (VASc) score at 24 h after surgery. Secondary outcomes included hemodynamics, sufentanil consumption, number of patients needing rescue analgesia, time to first rescue analgesic, total dose of rescue analgesic, satisfaction scores of patients and surgeons, time of chest tube removal, length of hospital stay, adverse effects, the prevalence of chronic pain and quality of life.
Results
Compared with D1 group, visual analog scale of pain at rest (VASr) was significantly lower during the first 24 h after surgery, while VASc was significantly lower during the first 48 h after surgery (P<0.05). Mean arterial pressure was significantly decreased from T2 to T8, and heart rate was significantly decreased from T2 to T7 in the D2 group (P<0.05). Consumption of sevoflurane, remifentanil, DEX and the recovery time were significantly reduced in the D2 group (P <0.05). Consumption of sufentanil 8–72 h after surgery was significantly lower in the D2 group (P<0.05). Additionally, the number of patients who required rescue analgesia, the time to the first dose of rescue analgesia, and the total dose of rescue analgesia was significantly lower in the D2 group (P<0.05).
Conclusion
The results of this study show that 1 µg/kg DEX is a beneficial adjuvant to ropivacaine for single-injection SAPB in VATS patients while stable hemodynamics were maintained.
Keywords: serratus anterior plane block, dexmedetomidine, video-assisted thoracic surgery, ultrasound, preemptive analgesia
Introduction
Post-thoracotomy pain is one of the most severe forms of post-operative pain.1 Although video-assisted thoracic surgery (VATS) can significantly reduce postoperative pain, the incidence has been reported to be as high as 30–50% and excellent pain control remains challenging.2 Insufficient analgesia may impair respiratory mechanics and result in complications such as atelectasis, hypoxemia, pneumonia, and venous thromboembolic disease.3
Both systemic and regional analgesic techniques have been used to control post-VATS pain.4 Thoracic epidural block (TEB), paravertebral blocks (PVB), and intercostal nerve blocks (INB) are the commonly used regional analgesic techniques. However, these methods are relatively invasive and associated with a risk of serious complications such as hematoma, pneumothorax, and injury of the central neuraxial structures.5,6 Therefore, it is important to determine an optimal regional analgesic technique, which is less invasive, for patients undergoing VATS.7 Serratus anterior plane block (SAPB) is a technique in which local anesthetics could inject into the plane either above or below the serratus anterior muscle. It can provide analgesia from T2 to T9 by blocking the intercostal nerves and their lateral cutaneous branches.8,9 However, the duration of analgesia can only last for 6–8 h.10,11 The use of local anesthetics along with dexmedetomidine (DEX) has been reported to prolong analgesia in epidural, paravertebral and brachial plexus blocks.12–14 However, it remains unclear whether ropivacaine combined with DEX during ultrasound-guided SAPB could improve postoperative analgesia. The aim of this study was to compare the safety and efficacy of single-injection ultrasound-guided superficial SAPB combined with either 0.5 or 1 µg/kg DEX for patients undergoing VATS.
Patients and Methods
Patients
This randomized controlled trial was designed according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement. We obtained ethical approval from the Institutional Review Boards of both Liaocheng People’s Hospital and The First People’s Hospital of Tianmen to conduct the trial. Written informed consent was obtained from each patient and their guardian. The trial was registered at chictr.org (ChiCTR-TRC-14,004,191).
Eighty patients aged 40–60 years with American Society of Anesthesiologists (ASA) physical status class I or II who were about to undergo three-port VATS under general anesthesia for benign disease were enrolled in this study between May 2018 and April 2019. The exclusion criteria included: smokers; allergy to lidocaine, ropivacaine, or DEX; suspected coagulopathy or injection site infection; severe cardiovascular and cerebrovascular disease; hepatorenal insufficiency; chronic opioid use; inability to communicate; body mass index (BMI) >30 kg/m2; the sensory block did not reach the required effect and converted to open thoracotomy.
Randomization and Blinding
Randomization was performed using a computer-generated randomization sequence by an independent investigator. The allocation results were concealed in sealed opaque envelopes by an anesthesiologist not involved in the study. The anesthetic nurses prepared ropivacaine with two different doses of DEX in 20 mL syringes according to the allocation results and performed the postoperative assessments.
SAPB Procedure
ASA standard monitors (electrocardiography, pulse oximetry, noninvasive blood pressure, and temperature) were attached to all patients after they entered the operating room. 4 L/min oxygen was administered via a nasal cannula. SAPB was performed according to the technique described by Blanco et al.15 After establishing intravenous access, each patient was placed in a supine position and sedated with 1 mg midazolam and 50 µg fentanyl. After aseptic preparation of the skin with iodophor, the ultrasound probe was placed over the midclavicular region of the thoracic cage in the sagittal plane, and then the subcutaneous tissue, latissimus dorsi, serratus anterior, intercostal muscle, and pleura superficial to the fourth and fifth ribs in the midaxillary line were identified. The superficial SAPB was targeted to the interfascial plane between the serratus anterior muscle and the latissimus dorsi muscle. After administering subcutaneous anesthetic (2 mL of 2% lidocaine), SAPB was conducted using an ultrasound-guided in-plane approach with a 22G needle. For each patient, 20 mL of 0.5% ropivacaine along with DEX (0.5 µg/kg DEX in the D1 group and 1 µg/kg DEX in the D2 group) was injected before placement of the needle tip was confirmed by injecting 2 mL normal saline and verifying that no air or blood was aspirated. Using ultrasound, the needle tip placement and the spread of local anesthetic and DEX between the serratus anterior and latissimus dorsi muscles were visualized. Approximately 20 min after SAPB, cold and pinprick tests along the midclavicular, midaxillary, and midscapular lines were performed. Successful sensory block was defined as a markedly reduced perception of cold.
Anesthesia
Propofol 2–2.5 mg/kg, sufentanil 0.3 µg/kg, cisatracurium 0.2 mg/kg, and lidocaine 1–1.5 mg/kg were used to facilitate endotracheal intubation. The position of the tube was verified using fiberoptic bronchoscopy before the patient was turned to a lateral decubitus position. Anesthesia was maintained using sevoflurane 2–3%, remifentanil 0.1–0.2 µg/kg/min, and DEX 0.2–0.7 µg/kg/h titrated according to the Bispectral index (maintained between 40 and 60) and hemodynamics. During two-lung ventilation, the patients were mechanically ventilated using volume-controlled ventilation (VCV) with a tidal volume of 6–8 mL/kg and a ventilatory frequency of 12–14 times/min to maintain the partial pressure of end-tidal carbon dioxide (PETCO2) at 35–40 mmHg. During one-lung ventilation, the patients were mechanically ventilated using pressure-controlled ventilation (PCV) with an airway peak pressure (Ppeak) <25 cmH2O to maintain PETCO2 within 37–45 mmHg. Ketorolac 30 mg and tropisetron 5 mg were injected 30 min before the end of surgery, followed by patient-controlled intravenous analgesia (PCIA) involving sufentanil 0.8 μg/kg to 200 mL. The PCIA was set to 2 mL/h background infusion, 2 mL bolus, 10 min lockout time, and a 4-h limit of 40 mL.
Patients were extubated immediately after surgery and moved to the post-anaesthesia care unit (PACU) for further observation. All patients had a standardized postoperative multimodal analgesia regimen including 1 g oral paracetamol and 400 mg oral ibuprofen every 8 h based on the previous study.16 If the visual analog scale for pain while coughing (VASc) score was ≥4, 2.5 µg intravenous sufentanil was given.
VATS was performed by the same surgical team. All operations involved a standard three-port technique comprising two 1-cm incisions (which were used for inserting the thoracoscopic video camera at the 7th intercostal space and the surgical instruments at the 8th intercostal space) and one 4-cm incision at the 4th intercostal space.17
Data Collection
Both VASc score along with the visual analog scale of pain at rest (VASr) score were measured at 1, 4, 8, 12, 24, 48, and 72 h postoperatively. Hemodynamics (recorded at the following time points: arrival at the operating room (T0), before intubation (T1), after intubation (T2), before incision (T3), 30 min after one-lung ventilation (T4), at extubation (T5), and 1 (T6), 2 (T7), 4 (T8), 8 (T9), and 12 (T10) h postoperatively), sufentanil consumption (at 1, 4, 8, 12, 24, 48, and 72 h postoperatively), number of patients who required rescue analgesia, time to the first rescue analgesic, total dose of rescue analgesic, satisfaction scores of patients and surgeons (5-point scale: 5, most satisfied; 1, most dissatisfied), time of chest tube removal, length of hospital stay, adverse effects, and Riker Sedation–Agitation Scale (SAS, 7-point scale: 7, dangerous agitation; 1, unarousable) at extubation. At 3 months after surgery, the incidence of chronic pain, quality of life (assessed using the Flanagan Quality of Life Scale [QOLS], a 16‐item questionnaire with each item scored from 1 to 7), and activity level (assessed using the Barthel Activities of Daily Living Scale [ADLS], which comprises 10 basic daily activities, each of which is scored as 0 = need complete help, 1 = need some help, or 2 = need no help) were assessed.
Hypotension was defined as systolic arterial pressure (SAP) <90 mmHg or a decrease >20% compared with baseline, and it was treated with 40 μg phenylephrine or 6 mg ephedrine. Bradycardia was defined as heart rate <60 beats/min and/or a decrease >20% compared with baseline, and it was treated with 0.5 mg atropine. Hypoxemia was defined as pulse oximetry <92% in room air, and it was treated with 5 L/min oxygen.6
Statistical Analysis
The sample size was calculated based on our pilot study, in which the mean VASc at 24 h after surgery was 4.8 in the D1 group, with an approximate standard deviation of 1.1. A 1.5-point decrease in the VASc score was considered clinically significant. For a study power of 80% and α value of 0.05, the required sample size per group was calculated to be 34. Given an estimated dropout rate of 15%, we recruited 40 patients (after applying the preoperative exclusion criteria) for each group.
All data were analyzed using SPSS 22.0 statistical software. The normality of the data distribution was assessed using the Kolmogorov–Smirnov test and a Q–Q plot. For normally distributed data, the data were presented as mean±standard deviation, and the groups were compared by analysis of variance (ANOVA) with the Bonferroni correction. For nonnormally distributed data, the data were presented as median (interquartile range) or number of patients, and the groups were compared with the Mann–Whitney U-test, Pearson’s chi-square test, or Fisher’s exact test. P<0.05 was considered as statistically significant.
Results
Demographic and Clinical Characteristics
The CONSORT guidelines were used during patient enrollment, and a CONSORT diagram is presented in Figure 1. Between May 2018 and April 2019, 182 patients were recruited for the study, 102 patients were excluded before surgery (35 were smokers; 4 were allergic to lidocaine, ropivacaine, or DEX; 12 had suspected coagulopathy or injection site infection; 23 had severe cardiovascular and cerebrovascular disease; 5 had hepatorenal insufficiency; 12 were chronic opioid users; 2 could not communicate; and 9 had a BMI >30 kg/m2). Additionally, five patients were excluded because the surgery was converted to open thoracotomy (two in the D1 group and three in the D2 group) and four patients were excluded because the sensory block did not reach the required effect (three in the D1 group and one in the D2 group). Finally, 35 patients in the D1 group and 36 patients in the D2 group were included in the analysis. The two groups were comparable in terms of the demographic and clinical characteristics (P>0.05; Table 1).
Figure 1.
Patient enrollment flow diagram.
Table 1.
Demographic and Clinical Characteristics
| Group D1 (n=35) | Group D2 (n=36) | P-values | |
|---|---|---|---|
| Age (years) | 53.41±5.34 | 52.18±3.94 | 0.272 |
| Sex (female/male, n) | 16/19 | 14/22 | 0.561 |
| BMI (kg·m–2) | 23.56±2.03 | 22.69±1.78 | 0.059 |
| ASA I to II (n) | 17/18 | 19/17 | 0.723 |
| FEV1/FVC (%) | 87.83±2.80 | 89.45±3.62 | 0.083 |
| Left/Right (n) | 26/9 | 25/11 | 0.650 |
| Comorbidity, n (%) Hypertension Diabetes mellitus Coronary heart disease |
10 (28.57%) 6 (17.14%) 4 (11.43%) |
9 (25.00%) 6 (16.67%) 5 (13.89%) |
1.000 |
| Type of surgery, n (%) Lobectomy Segmentectomy Wedge resection |
22 (62.86%) 8 (22.86%) 5 (14.29%) |
26 (72.22%) 6 (16.67%) 4 (11.11%) |
0.715 |
Notes: Variables presented as mean ± SD or number of patients n (%).
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiology; FEV1/FVC, forced vital capacity rate of one second/forced vital capacity.
Intraoperative Variables
Compared with the D1 group, the mean arterial pressure was significantly decreased from T2 to T8 and the heart rate was significantly decreased from T2 to T7 in the D2 group (P<0.05; Figure 2). However, all readings were within the clinically accepted ranges. Consumption of sevoflurane, remifentanil, and DEX were significantly reduced in the D2 group (P <0.05; Table 2). There were no significant differences between the two groups with respect to the dermatomal level of analgesia, surgery and anesthesia duration, number of patients using vasoactive agents, or Riker Sedation–Agitation Scale (P>0.05; Table 2). However, the recovery time was significantly shorter in the D2 group (P<0.01; Table 2).
Figure 2.
Comparison of the intraoperative hemodynamic changes between the two groups. *P<0.05 vs the D1 group.
Table 2.
Intraoperative Variables Between the Two Groups
| Group D1 (n=35) | Group D2 (n=36) | P-values | |
|---|---|---|---|
| Duration of surgery (min) | 118.45±23.83 | 125.74±33.22 | 0.293 |
| Duration of anaesthesia (min) | 165.29±32.44 | 168.33±35.02 | 0.706 |
| Intraoperative bleeding (mL) | 120.23±31.03 | 111.83±29.28 | 0.245 |
| Fluids (mL) | 1829.53±158.73 | 1725.81±129.88 | 0.056 |
| Urine output (mL) | 637.92±63.42 | 629.63±73.09 | 0.612 |
| Dexmedetomidine (μg·kg–1·h–1) | 0.38±0.33 | 0.25±0.24* | 0.043 |
| Remifentanil (μg·kg–1·min–1) | 0.15±0.04 | 0.11±0.06** | 0.002 |
| Sevoflurane (%) | 1.99±0.55 | 1.70±0.65* | 0.047 |
| Cisatracurium dosage (mg·kg–1) | 0.22±0.06 | 0.23±0.05 | 0.448 |
| Dermatomal analgesia span, n (%) 4 dermatomes 5 dermatomes 6 dermatomes 7 dermatomes |
5 (14.29%) 10 (28.57%) 12 (34.29%) 8 (22.86%) |
5 (13.89%) 9 (25.00%) 13 (36.11%) 9 (25.00%) |
1.000 |
| Recovery time (min) | 18.34±5.31 | 14.53±3.45** | 0.001 |
| Riker Sedation-Agitation Scale | 4.43±0.43 | 4.31±0.21 | 0.138 |
| Number of using vasoactive agent n (%) | 12 (34.29%) | 18 (50.00%) | 0.180 |
Notes: Variables presented as mean ± SD or number of patients n (%). *P < 0.05 vs Group D1; **P < 0.01 vs Group D1.
Postoperative Variables
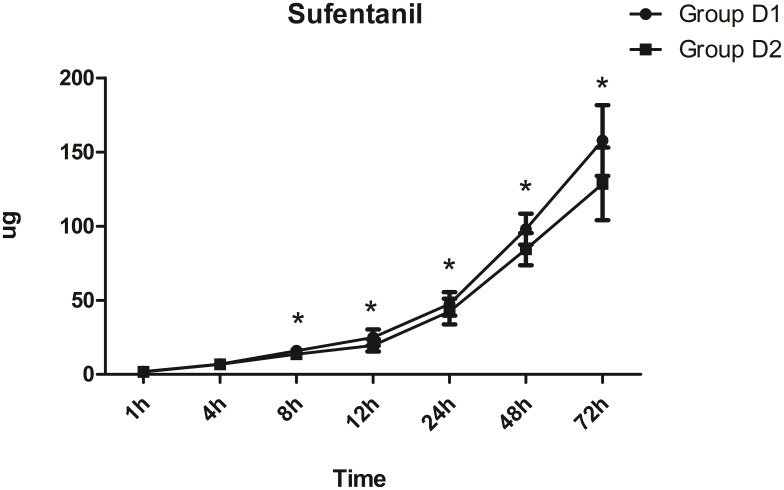
VASr during the first 24 h after surgery was significantly lower in the D2 group than the D1 group (P<0.05; Figure 3). However, VASc during the first 48 h after surgery was significantly lower in the D2 group (P<0.05; Figure 3). The consumption of sufentanil at 8–72 h postoperatively was significantly lower in the D2 group (P<0.05; Figure 4).
Figure 3.
Comparison of postoperative pain intensity (at rest and with coughing) between the two groups. *P<0.05 vs the D1 group.
Figure 4.
Comparison of postoperative sufentanil consumption between the two groups. *P<0.05 vs the D1 group.
Compared with the D1 group, the number of patients who required rescue analgesia, the time to the first dose of rescue analgesia, and the total dose of rescue analgesia were significantly lower in the D2 group (P<0.05; Table 3). However, the time to chest tube removal and length of hospital stay were comparable between the two groups, as were the satisfaction scores of patients and surgeons (P>0.05; Table 3). Both groups were also comparable regarding ADLS, QOLS, and the prevalence of chronic pain at 3 months after surgery (P>0.05; Table 3).
Table 3.
Postoperative Variables Between the Two Groups
| Group D1 (n=35) | Group D2 (n=36) | P-values | |
|---|---|---|---|
| Patients required rescue analgesia, n (%) | 14 (40.00%) | 6 (16.67%)* | 0.029 |
| Time to first dose of rescue analgesia (h) | 4.26 (2.73–7.83) | 6.34 (3.56–9.27)* | 0.025 |
| Total dose of rescue analgesia (µg) | 35.76±6.07 | 21.66±3.47** | 0.001 |
| Patient satisfaction score | 4.00 (3.00–5.00) | 4.25 (3.25–5.00) | 0.065 |
| Surgeon satisfaction score | 4.50 (4.00–5.00) | 4.75 (4.25–5.00) | 0.085 |
| Chest tube removed (d) | 2.13±0.56 | 1.91±0.62 | 0.122 |
| Length of hospital (d) | 6.73±1.47 | 6.98±1.62 | 0.499 |
| Quality of Life Scale | 72.34±14.92 | 65.24±18.24 | 0.077 |
| Activity of daily living score | 14.55±4.83 | 16.23±4.77 | 0.145 |
| The prevalence of chronic pain | 10 (28.57%) | 8 (22.22%) | 0.539 |
Notes: Variables presented as mean±SD, number of patients n (%) or median (interquartile range). *P <0.05 vs Group D1, **P <0.01 vs Group D1.
The most common postoperative complication in both groups was nausea, but it was less frequent in the D2 group (34.29% in the D1 group vs 13.89% in the D2 group, P=0.044; Table 4). Pneumonia was also less frequent in the D2 group than that in the D1 group (20.00% vs 5.56%, P=0.085; Table 4). No patients exhibited cardiovascular events or block-related complications, such as local anesthetic toxicity, hematoma, infection, and pneumothorax.
Table 4.
Postoperative Adverse Effects in the Two Groups
| Group D1 (n=35) | Group D2 (n=36) | P-values | |
|---|---|---|---|
| Nausea | 12 (34.29%) | 5 (13.89%)* | 0.044 |
| Urinary retention | 8 (22.86%) | 5 (13.89%) | 0.329 |
| Itching | 4 (11.43%) | 3 (8.33%) | 0.710 |
| Hypotension | 2 (5.71%) | 5 (13.89%) | 0.429 |
| Pneumonia | 7 (20.00%) | 2 (5.56%)* | 0.085 |
| Cardiovascular events | 0 | 0 | 1.000 |
| SAPB related complications | 0 | 0 | 1.000 |
Notes: Variables presented as number of patients n (%). *P < 0.05 vs Group D1.
Discussion
The results of this study show that 1 µg/kg DEX (as an adjuvant to ropivacaine) for SAPB is more effective than 0.5 µg/kg DEX. The analgesic effect of 1 µg/kg DEX significantly reduced both the consumption of sufentanil and pain intensity, while stable hemodynamics were maintained. Additionally, the time to the first dose of rescue analgesia, number of patients who required rescue analgesia, total dose of rescue analgesia, and postoperative nausea were all reduced in the group involving 1 µg/kg DEX.
VATS has been widely used as an alternative to thoracotomy in the last 20 years due to lower postoperative pain, fewer complications, and improved quality of life.18 However, 30–50% of patients undergoing VATS still have moderate-to-severe pain.2 This may be due to local damage caused by the skin incision, retraction, dislocation of the costovertebral joints, injury of the intercostal nerves, irritation of the pleura by chest tubes, and visceral pain.19 As a result, improved postoperative analgesia is a particularly important component in fast-track VATS applications, as mentioned in the latest guidelines of the European Society of Thoracic Surgeons (ESTS).20 Opioids, nonsteroidal anti‐inflammatory drugs (NSAIDs), paracetamol, ketamine, gabapentin, pregabalin, selective serotonin reuptake inhibitors (SSRIs), and duloxetine are commonly used systemic drugs for postoperative pain. However, opioids remain the cornerstone treatment for acute postoperative pain.21 Dale et al report that reducing opioid consumption is very important because of their side effects, such as urinary retention, pruritus, oversedation, opioid-induced hyperalgesia, respiratory depression, nausea, and vomiting.22
Albrecht et al have shown that regional analgesia may be a crucial component of multimodal postoperative pain management to reduce perioperative opioid consumption.23 TEB, PVB, INB, and interpleural blocks all have their own adverse effects and limitations. TEB can cause epidural hematoma and dural puncture. Besides, it has a high failure rate and involves hypotension due to unnecessary bilateral block.24 The major potential complications associated with PVB are total spinal block, pneumothorax, and neuronal injury, and the rate of technical failure of is about 6–12%.25 INB is time consuming, requires multiple injections, and is associated with a relatively high incidence of pneumothorax.26 Additionally, both TEB and PVB have special requirements regarding the posture of patients.25
SAPB was first described by Blanco et al and assessed by anatomical and radiological investigation in fresh cadavers.15 SAPB involving the superficial plane leads to improved drug spread and longer-lasting paraesthesia (750–840 min) compared to SAPB involving the deep plane, though the area of sensory loss to pinpricks is the same for superficial and deep injections.15 Additionally, preemptive analgesia prevents sensitization of the peripheral and central nervous systems and chronic neuropathic pain by blocking the introduction of noxious stimuli.27 As a result, postoperative sufentanil consumption was considerably reduced in this study compared to Chu et al reported in a previous study.28
Ropivacaine has been widely used in regional anesthesia due to its long-acting local anesthetic effects and lower systemic toxicity and neurotoxicity.29 Huang et al showed that SAPB with 0.5% ropivacaine was beneficial for postoperative analgesia during the first 24 h after breast surgery.30 Kunigo et al reported that the number of affected dermatomes (assessed by both cold and pinprick test) was significantly greater for patients receiving 40 mL of 0.375% ropivacaine than for patients receiving 20 mL; however, the time to the first dose of rescue analgesia was similar between the two groups.31 Because of this finding and for safety reasons, we injected 20 mL of 0.5% ropivacaine combined with different doses of DEX in the superficial plane before surgery.12,13 Time to the first dose of rescue analgesia in the D2 group in our study was considerably longer than that in the previous study, which may be because we added 1 µg/kg DEX to ropivacaine during SAPB in the D2 group. Although we injected the drugs in the superficial plane, Blanco et al found that the analgesic effect is equally effective when the drug is injected in the deep plane, though the difference between these methods has not yet been explained.15
An advantage of SAPB over INB and PVB is that a single injection (into the superficial or deep plane of the serratus muscle) is sufficient in the former.32 The dermatomal level of analgesia associated with SAPB was similar to that reported in a previous study, though we did observe some interindividual variability in our study.33 Hanley et al have shown that SAPB is a non-inferior regional anesthesia technique in terms of analgesic efficacy compared to PVB when incorporated within a multimodal analgesia regimen.16 In our study, patients in the D2 group had a significantly lower VASr score (during the first 24 h after surgery) and opioid requirement after surgery; however, the prevalence of chronic pain and functional measures were similar between the two groups. Khalil et al reported that SAPB maintained hemodynamic stability compared with TEB and led to lower pain scores and less morphine consumption in the early postoperative period after thoracotomy, with no noteworthy complications. As a result, SAPB appeared to be a good alternative to TEB for achieving paresthesia of a hemithorax.34 Compared to local anesthetic infiltration, SAPB led to better pain relief and lower opioid consumption after VATS.35
Kim et al reported that SAPB can be used as the main component of multimodal analgesia for patients undergoing VATS rather than as a direct alternative to PVB or TEB due to the lower pain intensity and the shorter time of analgesia.36 Abdallah et al confirmed that ultrasound-guided SAPB with 1 µg/kg DEX was safe and effective for patients undergoing VATS; however, only one dose of DEX was assessed.37 Our results showed that 1 µg/kg DEX is a more beneficial adjuvant when used with ropivacaine for SAPB compared to 0.5 µg/kg DEX. Compared with a single-shot block, a continuous ropivacaine infusion may increase the risk of catheter-related infections and delay early postoperative activity, especially for patients with cachexia (involving low muscle mass) who need a subcutaneous tunneling approach to maintain the placement of the catheter.9 As a result, we only added the two doses of DEX during the SAPB. Although there is lack of direct evidence of α2-adrenoceptors on the axons of normal peripheral nerves, the opioid-sparing mechanisms of DEX are thought to include centrally mediated analgesia, action on peripheral nerve α2B-adrenoceptors, and attenuation of the inflammatory response.38
Gomez-Brouchet et al reported that sufentanil consumption was significantly increased within 12–24 h after conducting a regional nerve block. This may be related to the following factors: the preserved nociceptive signal memory was amplified when the blocking effect disappeared; and the intrinsic proinflammatory properties of local anesthetics may lead to hyperalgesia after regional block.39 However, this phenomenon did not occur in this study because of the use of preemptive analgesia. Rescue analgesia was required by 14 patients (40.00%) in the D1 group compared with only 6 patients (16.67%) in the D2 group. The reason may be partly because SAPB might miss both the anterior cutaneous branches of the intercostal nerve and the supraclavicular nerves.34
In this study, we found that the patients in the D2 group had a lower incidence of postoperative nausea than those in the D1 group, which may be due to the opioid-sparing effect of DEX. Because of both the superficial injection point and the ultrasound guidance of SAPB adopted in this study, complications such as pneumothorax and hemothorax were rare. The potential complications of SAPB also include nerve injury and local anesthetic toxicity,10 which did not occur in this study. We assessed post‐thoracotomy pain syndrome (PTPS) 3 months after surgery, as the International Association for the Study of Pain (IASP) reported that thoracotomy impaired daily activity up to 50% and sleep patterns up to 25% even at 2 months after surgery.40 Unlike the incidence of PTPS, Hetmann et al reported, the incidence was about 25% in our study. This may be because SAPB can relieve intercostal nerve injury, which is key to the development of PTPS.41
Our study has several limitations. First, we did not measure the ropivacaine level in the plasma because no studies have reported any local anesthetic-related complications associated with SAPB. Second, we only recorded postoperative pain up to 3 months after VATS and failed to follow-up further. Third, we only used two doses of DEX (0.5 and 1.0 μg/kg) in addition to 20 mL of 0.5% ropivacaine. A dose–response study should be performed to confirm the optimum DEX– ropivacaine regimen. Finally, the relatively small sample size and the restriction to healthy younger patients (with ASA I and II) aged 40–60 years limit the generalizability of our findings.
In conclusion, 1 µg/kg DEX as a beneficial adjuvant to ropivacaine for single-injection superficial SAPB in patients undergoing VATS decreased the consumption of sufentanil, pain intensity, number of patients who required rescue analgesia, time to the first dose of rescue analgesia, total dose of rescue analgesia, and rate of postoperative nausea, while stable hemodynamics were maintained.
Funding Statement
There is no funding to report.
Data Sharing Statement
The authors intend to share the participants’ data collected during the trial and the documents associated with the trial (including the study protocol, statistical analysis plan, informed consent forms, and clinical study report). Information will be available (to investigators whose proposed use of the information has been approved by an independent review committee) on request to the corresponding author by email.
Ethical Approval
All procedures performed on the patients were in accordance with the 1964 Helsinki declaration and its later amendments. The study was approved by the Institutional Review Boards of both Liaocheng People’s Hospital and The First People’s Hospital of Tianmen.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
- 1.Moyse DW, Kaye AD, Diaz JH, et al. Perioperative ketamine administration for thoracotomy pain. Pain Physician. 2017;20(3):173–184. [PubMed] [Google Scholar]
- 2.Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X [DOI] [PubMed] [Google Scholar]
- 3.Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: state of the art and perspectives. Lung Cancer. 2016;101:28–34. doi: 10.1016/j.lungcan.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 4.Wu CN, Wu XH, Yu DN, et al. A single-dose of stellate ganglion block for the prevention of postoperative dysrhythmias in patients undergoing thoracoscopic surgery for cancer: a randomized controlled double-blind trial. Eur J Anaesthesiol. 2020;37(4):323–331. doi: 10.1097/EJA.0000000000001137 [DOI] [PubMed] [Google Scholar]
- 5.Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med. 2019;8:10–15. [DOI] [PubMed] [Google Scholar]
- 6.Okajima H, Tanaka O, Ushio M, et al. Ultrasound-guided continuous thoracic paravertebral block provides comparable analgesia and fewer episodes of hypotension than continuous epidural block after lung surgery. J Anesth. 2015;29(3):373–378. doi: 10.1007/s00540-014-1947-y [DOI] [PubMed] [Google Scholar]
- 7.Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg. 2017;154(2):652–659.e1. doi: 10.1016/j.jtcvs.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Madabushi R, Tewari S, Gautam SK, et al. Serratus anterior plane block: a new analgesic technique for post-thoracotomy pain. Pain Physician. 2015;18(3):E421–424. [PubMed] [Google Scholar]
- 9.Vig S, Bhan S, Ahuja D, et al. serratus anterior plane block for post-thoracotomy analgesia: a novel technique for the surgeon and anaesthetist. Indian J Surg Oncol. 2019;10(3):535–539. doi: 10.1007/s13193-019-00937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park MH, Kim JA, Ahn HJ, et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. 2018;73(10):1260–1264. doi: 10.1111/anae.14424 [DOI] [PubMed] [Google Scholar]
- 11.Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a randomised trial. Eur J Anaesthesiol. 2020;19. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Yu Y, Zhang W, et al. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia: a randomized controlled trial. Drug Des Devel Ther. 2019;13:1171–1175. doi: 10.2147/DDDT.S197431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abd-Elshafy SK, Abdallal F, Kamel EZ, et al. Paravertebral dexmedetomidine in video-assisted thoracic surgeries for acute and chronic pain prevention. Pain Physician. 2019;22(3):271–280. [PubMed] [Google Scholar]
- 14.Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181. doi: 10.1093/bja/aew411 [DOI] [PubMed] [Google Scholar]
- 15.Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi: 10.1111/anae.12344 [DOI] [PubMed] [Google Scholar]
- 16.Hanley C, Wall T, Bukowska I, et al. Ultrasound-guided continuous deep serratus anterior plane block versus continuous thoracic paravertebral block for perioperative analgesia in videoscopic-assisted thoracic surgery. Eur J Pain. 2020;24(4):828–838. doi: 10.1002/ejp.1533 [DOI] [PubMed] [Google Scholar]
- 17.Mier JM, Chavarin A, Izquierdo-Vidal C, et al. A prospective study comparing three-port video-assisted thoracoscopy with the single-incision laparoscopic surgery (SILS) port and instruments for the video thoracoscopic approach: a pilot study. Surg Endosc. 2013;27(7):2557–2560. doi: 10.1007/s00464-012-2782-6 [DOI] [PubMed] [Google Scholar]
- 18.Medbery RL, Gillespie TW, Liu Y, et al. Nodal upstaging is more common with thoracotomy than with vats during lobectomy for early-stage lung cancer: an analysis from the national cancer data base. J Thorac Oncol. 2016;11(2):222–233. doi: 10.1016/j.jtho.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg. 2014;45(6):959–966. doi: 10.1093/ejcts/ezt525 [DOI] [PubMed] [Google Scholar]
- 20.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115. doi: 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 21.Mitra S, Carlyle D, Kodumudi G, et al. New advances in acute postoperative pain management. Curr Pain Headache Rep. 2018;22(5):35. doi: 10.1007/s11916-018-0690-8 [DOI] [PubMed] [Google Scholar]
- 22.Dale O, Moksnes K, Kaasa S. European palliative care research collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects: a systematic review. Palliat Med. 2011;25(5):494–503. doi: 10.1177/0269216310384902 [DOI] [PubMed] [Google Scholar]
- 23.Albrecht E, Chin KJ. Advances in regional anaesthesia and acute pain management: a narrative review. Anaesthesia. 2020;75(Suppl 1):e101– e110. doi: 10.1111/anae.14868 [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama Y, Nakagomi T, Shikata D, et al. Combined analgesic treatment of epidural and paravertebral block after thoracic surgery. J Thorac Dis. 2017;9(6):1651–1657. doi: 10.21037/jtd.2017.05.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD004158.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheets NW, Davis JW, Dirks RC, et al. Intercostal nerve block with liposomal bupivacaine vs epidural analgesia for the treatment of traumatic rib fractures. J Am Coll Surg. 2020;17. [DOI] [PubMed] [Google Scholar]
- 27.Lönnqvist PA. Pre-emptive analgesia with thoracic paravertebral blockade? Br J Anaesth. 2005;95(6):727–728. doi: 10.1093/bja/aei268 [DOI] [PubMed] [Google Scholar]
- 28.Chu L, Zhang X, Lu Y, et al. Improved analgesic effect of paravertebral blocks before and after video-assisted thoracic surgery: a prospective, double-blinded, randomized controlled trial. Pain Res Manag. 2019;2019:9158653. doi: 10.1155/2019/9158653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun N, Wang S, Ma P, et al. Postoperative analgesia by a transversus abdominis plane block using different concentrations of ropivacaine for abdominal surgery: a meta-analysis. Clin J Pain. 2017;33(9):853–863. doi: 10.1097/AJP.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Zheng L, Wu B, et al. Effects of ropivacaine concentration on analgesia after ultrasound-guided serratus anterior plane block: a randomized double-blind trial. J Pain Res. 2020;13:57–64. doi: 10.2147/JPR.S229523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunigo T, Murouchi T, Yamamoto S, et al. Injection volume and anesthetic effect in serratus plane block. Reg Anesth Pain Med. 2017;42(6):737–740. doi: 10.1097/AAP.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 32.Jack JM, McLellan E, Versyck B, et al. The role of serratus anterior plane and pectoral nerves blocks in cardiac surgery, thoracic surgery and trauma: a qualitative systematic review. Anaesthesia. 2020;16. [DOI] [PubMed] [Google Scholar]
- 33.Reyad RM, Shaker EH, Ghobrial HZ, et al. The impact of ultrasound-guided continuous serratus anterior plane block versus intravenous patient-controlled analgesia on the incidence and severity of post-thoracotomy pain syndrome: a randomized, controlled study. Eur J Pain. 2020;24(1):159–170. doi: 10.1002/ejp.1473 [DOI] [PubMed] [Google Scholar]
- 34.Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31(1):152–158. doi: 10.1053/j.jvca.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Li Y, Zhang Y, et al. Effects of serratus anterior plane block for postoperative analgesia after thoracoscopic surgery compared with local anesthetic infiltration: a randomized clinical trial. J Pain Res. 2019;12:2411–2417. doi: 10.2147/JPR.S207116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DH, Oh YJ, Lee JG, et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. 2018;126(4):1353–1361. doi: 10.1213/ANE.0000000000002779 [DOI] [PubMed] [Google Scholar]
- 37.Abdallah NM, Bakeer AH, Youssef RB, et al. Ultrasound-guided continuous serratus anterior plane block: dexmedetomidine as an adjunctive analgesic with levobupivacaine for post-thoracotomy pain. A prospective randomized controlled study. J Pain Res. 2019;12:1425–1431. doi: 10.2147/JPR.S195431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chabot-Doré AJ, Schuster DJ, Stone LS, et al. Analgesic synergy between opioid and α2-adrenoceptors. Br J Pharmacol. 2015;172(2):388–402. doi: 10.1111/bph.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Brouchet A, Blaes N, Mouledous L, et al. Beneficial effects of levobupivacaine regional anaesthesia on postoperative opioid induced hyperalgesia in diabetic mice. J Transl Med. 2015;13:208. doi: 10.1186/s12967-015-0575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Brouchet A, Blaes N, Mouledous L, et al. Reconsidering the International Association for the Study of Pain definition of pain. Pain Rep. 2018;3(2):e634. doi: 10.1097/PR9.0000000000000634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetmann F, Kongsgaard UE, Sandvik L, et al. Post-thoracotomy pain syndrome and sensory disturbances following thoracotomy at 6- and 12-month follow-ups. J Pain Res. 2017;10:663–668. doi: 10.2147/JPR.S126639 [DOI] [PMC free article] [PubMed] [Google Scholar]