Abstract
Objectives
Existing findings regarding the relationship between comorbidities and the severity of coronavirus disease 2019 (COVID-19) are inconsistent and insufficient. The aim of this study was to evaluate the association between different comorbidities and the severity of COVID-19.
Methods
The PubMed, Embase, and Cochrane Library databases were searched to identify studies reporting the rates of comorbidities in COVID-19 patients with severe/fatal outcomes. Subgroup analyses were conducted according to disease severity and the country of residence. Odds ratios (OR) with 95% confidence intervals (CI) were pooled using random-effects models.
Results
A total of 34 eligible studies were identified. In patients with severe/fatal COVID-19, the most prevalent chronic comorbidities were obesity (42%, 95% CI 34–49%) and hypertension (40%, 95% CI 35–45%), followed by diabetes (17%, 95% CI 15–20%), cardiovascular disease (13%, 95% CI 11–15%), respiratory disease (8%, 95% CI 6–10%), cerebrovascular disease (6%, 95% CI 4–8%), malignancy (4%, 95% CI 3–6%), kidney disease (3%, 95% CI 2–4%), and liver disease (2%, 95% CI 1–3%). In order of the prediction, the pooled ORs of the comorbidities in patients with severe or fatal COVID-19 when compared to patients with non-severe/fatal COVID-19 were as follows: chronic respiratory disease, OR 3.56 (95% CI 2.87–4.41); hypertension, OR 3.17 (95% CI 2.46–4.08); cardiovascular disease, OR 3.13 (95% CI 2.65–3.70); kidney disease, OR 3.02 (95% CI 2.23–4.08); cerebrovascular disease, OR 2.74 (95% CI 1.59–4.74); malignancy, OR 2.73 (95% CI 1.73–4.21); diabetes, OR 2.63 (95% CI 2.08–3.33); and obesity, OR 1.72 (95% CI 1.04–2.85). No correlation was observed between liver disease and COVID-19 aggravation (OR 1.54, 95% CI 0.95–2.49).
Conclusions
Chronic comorbidities, including obesity, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, respiratory disease, kidney disease, and malignancy are clinical risk factors for a severe or fatal outcome associated with COVID-19, with obesity being the most prevalent and respiratory disease being the most strongly predictive. Knowledge of these risk factors could help clinicians better identify and manage the high-risk populations.
Keywords: Comorbidity, Severe, ICU admission, Fatality, COVID-19
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), caused by a novel enveloped RNA betacoronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lu et al., 2020), has become a public health emergency of international concern. The manifestations of COVID-19 pneumonia run the spectrum from asymptomatic disease to severe acute respiratory infection (Chen et al., 2020a, Deng and Peng, 2020, Huang et al., 2020a, Wang et al., 2020a), and the recognized human-to-human transmission significantly increases the risk of much wider spread of the disease (Li et al., 2020a, Phan et al., 2020, Rothe et al., 2020). As of June 11, 2020, a total of 7 273 958 confirmed COVID-19 cases and 413 372 deaths had been reported globally. At present, the number of COVID-19 cases is still growing around the world, and there are increasing global concerns about this outbreak (Wang et al., 2020b). Given this, the identification of clinical risk factors for poor outcomes associated with COVID-9 are desperately needed to distinguish those populations at increased risk and thus needing more aggressive management to prevent severe or fatal outcomes.
A number of literature reports have documented the increased risks of poorer clinical outcomes in patients infected with avian influenza (Martínez et al., 2019, Mauskopf et al., 2013, Placzek and Madoff, 2014), severe acute respiratory syndrome coronavirus (SARS-CoV) (Booth et al., 2003), and Middle East respiratory syndrome coronavirus (MERS-CoV) (Alanazi et al., 2020, Alqahtani et al., 2018, Badawi and Ryoo, 2016a, Matsuyama et al., 2016, Yang et al., 2017), and have shown that comorbidities including diabetes mellitus (DM), hypertension, cardiovascular disease (CVD), chronic respiratory diseases, chronic kidney disease (CKD), and malignancy are significantly associated with a poor prognosis or even death. Some clinical risk factors for a poor prognosis associated with COVID-19 have also been reported, including older age, male sex, and the presence of comorbidities (Deng and Peng, 2020, Yang et al., 2020a), with hypertension being the most common, followed by DM and CVD (Huang et al., 2020b, Wang et al., 2020c). Given the virtually unstoppable global trend of SARS-CoV-2, together with the high prevalence of comorbidities worldwide, the combination of these two conditions will pose greater clinical, societal, and economic burdens to humanity (Emanuel et al., 2020).
To date, only a limited number of meta-analyses (Borges do Nascimento et al., 2020, Li et al., 2020b, Lippi et al., 2020, Wang et al., 2020d, Yang et al., 2020b) have been published regarding the prevalence of comorbidities in COVID-19 infection, all of which have indicated that hypertension and CVD are risk factors for COVID-19 patients progressing to severe or critical disease. However, with the limited datasets comparing the proportions of comorbidities between severe and non-severe cases, there are uncertainties or controversies regarding the relationship between particular comorbidities, including DM, obesity, CKD, liver disease, and malignancy, and the severity of COVID-19. For instance, the meta-analyses conducted by Yang et al. (Yang et al., 2020c) and Li et al. (Li et al., 2020c) showed no significant association between DM and the severe outcomes associated with COVID-19, and the meta-analysis conducted by Wang et al. (Wang et al., 2020e) showed no correlation between CKD, liver disease, or malignancy and the aggravation of disease in COVID-19 patients. To date, no meta-analysis has been conducted to evaluate the relationship between obesity and the severity of COVID-19. Besides, the criteria used to categorize severe and non-severe disease were not uniform in those previous meta-analyses, which used a variety of outcomes including severe clinical symptoms, intensive care unit (ICU) admission, and/or mortality. With the lack of the separate analyses according to disease severity, whether and how much the comorbidities impact the risk of ICU admission or mortality has not been clearly established. In addition, studies included in those meta-analyses were confined to China, mainly Wuhan, making their conclusions somewhat unrepresentative.
With the epidemiological and clinical features of COVID-19 cases increasingly reported around the world, the aim of this study was to integrate recent advances and present an updated meta-analysis of the relationships between comorbidities and severe or fatal outcomes associated with COVID-19.
Materials and methods
This meta-analysis was performed based on a pre-specified protocol. The methods and results are reported according to the PRISMA guidelines (Stewart et al., 2015).
Search strategy and selection criteria
A systematic search was conducted to identify published articles in the PubMed, Embase, and Cochrane Library databases from inception to April 25, 2020. According to the indices of the various databases, the following search terms were used: “2019 novel coronavirus”, “COVID-19”, “novel coronavirus pneumonia”, “2019-Cnov”, “SARS-CoV-2”, “Betacoronavirus”, “bat coronavirus”, “comorbidities”, “pre-existing disease”, “underlying disease”, “clinical characteristics”, “epidemiological”, “severe”, “critical”, “intensive care unit”, “ICU”, “death”, “fatality”, and “mortality” in English. The reference list of each selected paper was checked to identify any missing studies. The detailed search strategy is available in Supplementary Material Table S1.
The primary outcome measure was to evaluate the overall prevalence of comorbidities in severe or fatal COVID-19 and to stratify the estimates by disease severity (severe disease based on clinical symptoms, ICU admission, and death) and country of residence. In particular, a clinically validated definition of ‘severe disease’ was used, i.e., patients experiencing severe respiratory distress, or needing mechanical ventilation, vital life support, or ICU admission. The following inclusion criteria were applied: (1) published studies reporting the relationship between comorbidities and patients with COVID-19 written in English; and (2) the inclusion of data on the prevalence of comorbidities in COVID-19 patients who had severe manifestations, or were admitted to an ICU, or died. Exclusion criteria were as follows: (1) duplicate publications, (2) non-human studies, (3) reviews, editorials, correspondence/letters, case reports, and family-based studies, (4) studies on pregnant women or children, and (5) studies without adequate information.
Data extraction
Two independent authors (Y. Zhou, Q. Yang) screened the search results and selected studies in accordance with the inclusion and exclusion criteria. Two authors (J.W. Chi, B.Z. Dong) used predefined forms to extract the data from each included study: trial characteristics (first author, publication year, study design, sample size, definition of severity) and patient baseline data, i.e., age (from reported mean or median age) and the prevalence of comorbidities including obesity (body mass index (BMI) >30 kg/m2), hypertension, DM (both type I and type II, if mentioned separately), CVD, cerebrovascular disease, chronic respiratory disease, CKD, liver disease, and malignancy. The prevalence of comorbidities was determined by extracting the proportions of coexisting medical conditions from confirmed COVID-19 cases with versus without severe manifestations, ICU versus non-ICU cases, and survivors versus non-survivors. In articles including the same or duplicate patients, only the most complete data were included to avoid overlap. If any overlap was suspected, the corresponding authors were contacted to clarify the discrepancy. Any resulting disagreements were resolved by discussion with a third author (W. Lv). Disagreements between authors were resolved by consensus.
Data synthesis and statistical analysis
A pooled analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (95% CI) of comorbidities in COVID-19 patients with versus without severe clinical symptoms, ICU versus non-ICU patients, and survivors versus non-survivors. Considering the heterogeneity within and between studies, a random-effects model was used to estimate the average effect and its precision, which would give a more conservative estimate of the 95% CI. A p-value <0.05 for any test or model was considered statistically significant. The degree of between-study variability attributable to heterogeneity beyond chance was calculated using the I 2 statistic and Q statistic (Higgins and Thompson, 2002). Outcomes with I 2 levels from 0% to 40% were considered minimally heterogeneous, while I 2 >50% was considered an indication of statistically significant heterogeneity among included studies.
Perspective subgroup analyses were further conducted according to the severity of disease (severe disease based on clinical symptoms, ICU admission, or death) and country of residence, to address the clinical heterogeneity of included studies. Forest plots were used for graphical representation of the data. Funnel plots and the Egger test were used to assess publication bias. The meta-analyses were performed using Stata software version 12.0 (StataCorp, College Station, TX, USA).
Results
Search results and study characteristics
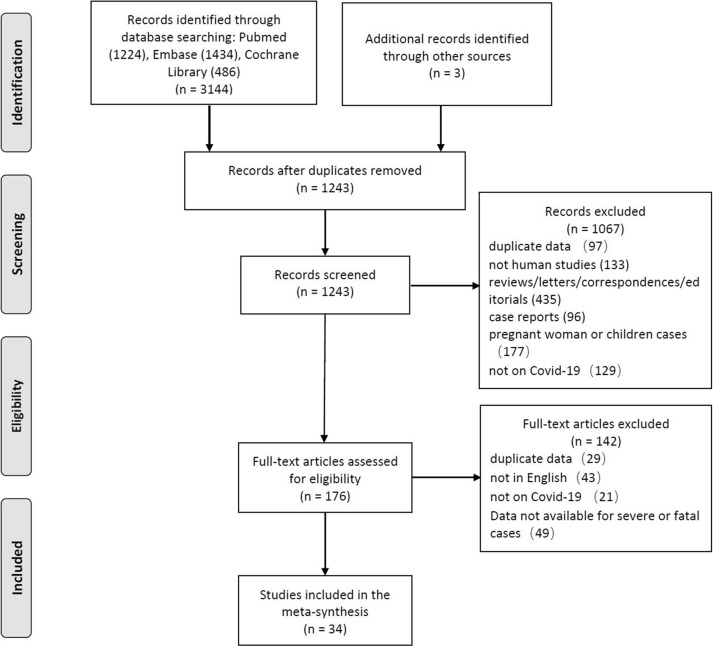
The combined search of the PubMed, Embase, and Cochrane Library databases identified 3144 citations, from which 34 articles met all inclusion criteria (COVID-19 National Emergency Response Center, 2020, COVID-19 National Incident Room Surveillance Team, 2020, CDC COVID-19 Response Team, 2020; Barrasa et al., 2020, Cai et al., 2020, Chen et al., 2020b; Chen et al., 2020; Deng et al., 2020, Feng et al., 2020; Gao et al., 2020; Goyal et al., 2020, Grasselli et al., 2020; Guan et al., 2020a, Guan et al., 2020b; Huang et al., 2020a, Li et al., 2020a, Liu et al., 2020, McLaughlin et al., 2014, Piva et al., 2020, Qin et al., 2020, Shen et al., 2020; Simonnet et al., 2020; Wan et al., 2020; Wang et al., 2020e, Wang et al., 2020f, Wu et al., 2020a, Yang et al., 2020a, Yao et al., 2020, Young et al., 2020, Zhang et al., 2020a, Zheng et al., 2020a, Zheng et al., 2020b, Zheng et al., 2020c, Zhou et al., 2020a, Zhou et al., 2020b). After the removal of duplicates, the title and abstract were screened for relevance. The full texts of remaining results were assessed independently in duplicate by two authors (J.W. Chi and W.S. Lv) for inclusion based on the predetermined criteria. The process used to select the studies for the meta-analysis is given in Figure 1 .
Figure 1.
Flowchart of the search and selection process (PRISMA).
The characteristics of the included studies are reported in Supplementary Material Table S1. Of the 34 articles, 23 reported retrospective cohort studies (Barrasa et al., 2020, Cai et al., 2020, Chen et al., 2020a, Chen et al., 2020b, Deng et al., 2020, Feng et al., 2020; Gao et al., 2020; Guan et al., 2020a, Guan et al., 2020b; Huang et al., 2020b, Li et al., 2020b, Liu et al., 2020, Piva et al., 2020, Qin et al., 2020, Shen et al., 2020; Simonnet et al., 2020; Wu et al., 2020b, Yang et al., 2020b, Yao et al., 2020, Zhang et al., 2020b, Zheng et al., 2020a, Zheng et al., 2020b, Zhou et al., 2020a, Zhou et al., 2020b), seven reported retrospective case series (Goyal et al., 2020, Grasselli et al., 2020; Wan et al., 2020; Wang et al., 2020a, Wang et al., 2020b, Young et al., 2020, Zheng et al., 2020c), and four reported surveillance studies (studies extracting data from a database of cases, systematically gathering epidemiological data, as coordinated by a country or the World Health Organization, 2020a, World Health Organization, 2020b), (COVID-19 National Emergency Response Center, 2020; COVID-19 National Incident Room Surveillance Team, 2020, CDC COVID-19 Response Team, 2020; McLaughlin et al., 2014). These studies included a total of 16 110 patients from nine different countries including China, the USA, the UK, Italy, France, Spain, Australia, Singapore, and Korea. These studies reported the prevalence of comorbidities including obesity, hypertension, DM, CVD, cerebrovascular disease, chronic respiratory disease, CKD, chronic liver disease, and malignancy. In particular, 18 studies (Cai et al., 2020, Chen et al., 2020c, Feng et al., 2020; Gao et al., 2020; Goyal et al., 2020; Guan et al., 2020a, Guan et al., 2020b; Li et al., 2020c, Liu et al., 2020, Qin et al., 2020; Simonnet et al., 2020; Wan et al., 2020; Wan et al., 2020, Wu et al., 2020a, Young et al., 2020, Zhang et al., 2020a, Zheng et al., 2020a, Zheng et al., 2020b, Zheng et al., 2020c) compared the rates of comorbidities in severe versus non-severe cases based on clinical symptoms, with a sample of 4001 patients, 1272 (31.8%) of whom were classified as having developed severe disease. Four studies, (CDC COVID-19 Response Team, 2020; Huang et al., 2020; Shen et al., 2020, Wang et al., 2020a) compared the rates of comorbidities in ICU versus non-ICU cases, with a sample of 6652 patients, 1138 (17.1%) of whom were classified as admitted to the ICU. Five studies (Chen et al., 2020c, Deng et al., 2020, McLaughlin et al., 2014, Yao et al., 2020, Zhou et al., 2020a) compared the rates of comorbidities in survivors versus non-survivors, with a sample of 3436 patients, 1624 (47.3%) of whom died. Another seven studies (COVID-19 National Emergency Response Center, 2020; COVID-19 National Incident Room Surveillance Team, 2020; Barrasa et al., 2020, Grasselli et al., 2020, Piva et al., 2020, Yang et al., 2020a, Zhou et al., 2020b) only reported the proportions of comorbidities in severe/ICU/fatal populations, without comparison to their non-severe/ICU/fatal counterparts.
Proportions of comorbidities in COVID-19 patients with severe or fatal outcomes
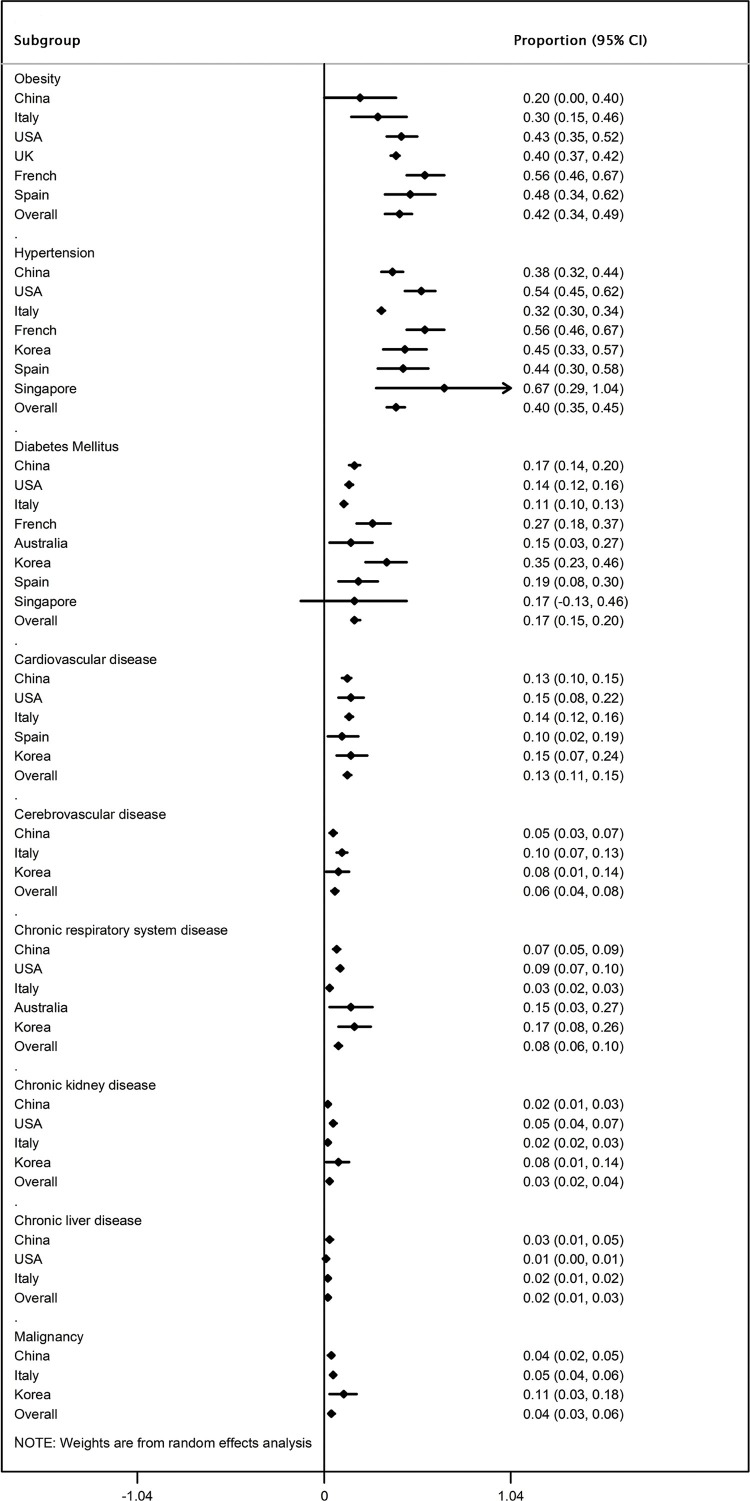
As shown in Figure 2, in COVID-19 patients with severe or fatal outcomes, the most prevalent comorbidities were obesity (42%, 95% CI 34–49%) and hypertension (40%, 95% CI 35–45%), followed by DM (17%, 95% CI 15–20%), CVD (13%, 95% CI 11–15%), respiratory disease (8%, 95% CI 6–10%), cerebrovascular disease (6%, 95% CI 4–8%), malignancy (4%, 95% CI 3–6%), and CKD (3%, 95% CI 2–4%); the least prevalent comorbidity was liver disease (2%, 95% CI 1–3%). Subgroup analyses according to country of residence of the study participants further showed the ethnic differences in the prevalence of comorbidities among patients with severe or fatal COVID-19.
Figure 2.
Meta-analysis of the proportions of comorbidities in severe or fatal COVID-19 cases stratified by the country of residence. 95% CI, 95% confidence interval.
Risk of comorbidities in severe/ICU/fatal patients compared to non-severe/ICU/fatal patients
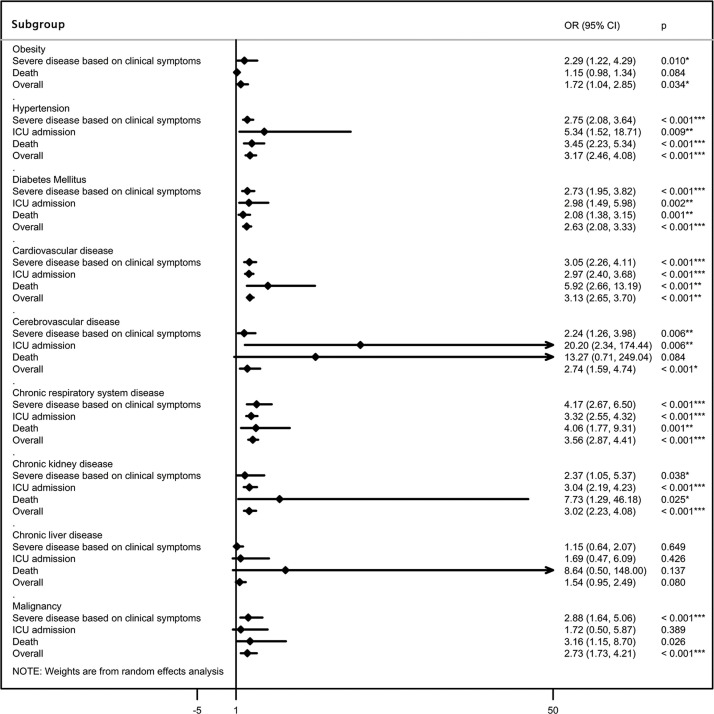
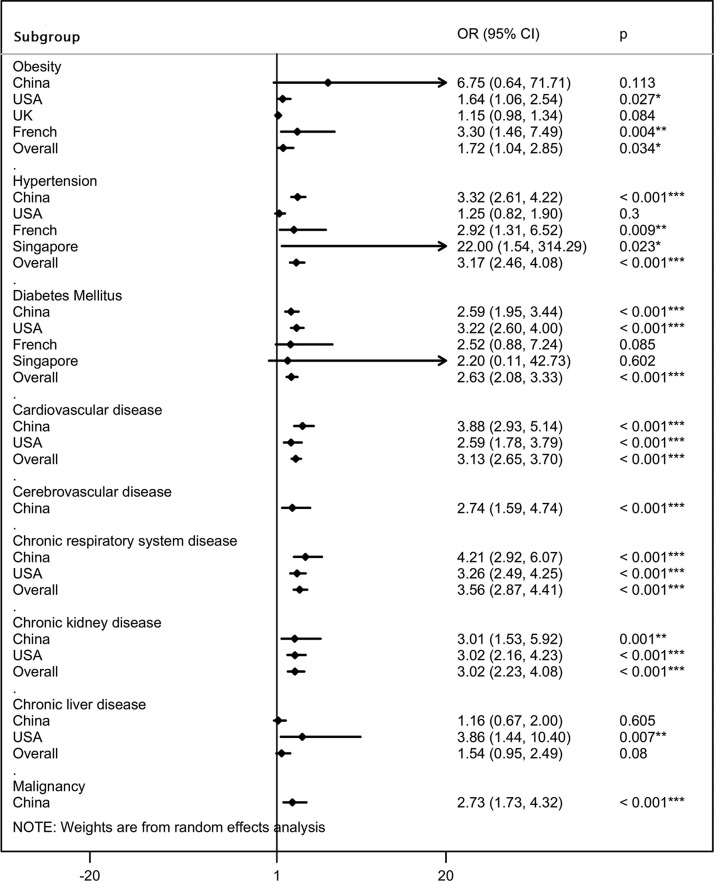
The difference in prevalence of comorbidities was then compared between severe and non-severe patients, ICU and non-ICU patients, and survivors and non-survivors, stratifying the estimates by disease severity (Table 1; Figure 3) and country of residence ( Figure 4 ). The pooled summary estimates from included studies indicated that comorbidities including obesity, hypertension, DM, CVD, cerebrovascular disease, respiratory disease, CKD, and malignancy were clinical risk factors for severe or fatal COVID-19.
Table 1.
Results of the subgroup analysis based on severe clinical outcomes associated with COVID-19.
| Comorbidities | Number of studies | OR (95% CI) | p-Value | Heterogeneity |
|
|---|---|---|---|---|---|
| I2 | ph | ||||
| Obesity | 4 | 1.72 (1.04, 2.85) | 0.034 | 69.8 | 0.019 |
| Clinical symptoms | 3 | 2.29 (1.22, 4.29) | 0.010 | 38.5 | 0.197 |
| Death | 1 | 1.15 (0.98, 1.34) | 0.084 | – | – |
| Hypertension | 24 | 3.17 (2.46, 4.08) | <0.001 | 55.6 | 0.001 |
| Clinical symptoms | 17 | 2.75 (2.08, 3.64) | <0.001 | 48.7 | 0.013 |
| ICU admission | 3 | 5.34 (1.52, 18.71) | 0.009 | 69.7 | 0.037 |
| Death | 4 | 3.45 (2.23, 5.34) | <0.001 | 35.6 | 0.199 |
| Diabetes mellitus | 25 | 2.63 (2.08, 3.33) | <0.001 | 32.6 | 0.060 |
| Clinical symptoms | 17 | 2.73 (1.95, 3.82) | <0.001 | 33.5 | 0.088 |
| ICU admission | 4 | 2.98 (1.49, 5.98) | 0.002 | 48.0 | 0.124 |
| Death | 4 | 2.08 (1.38, 3.15) | 0.001 | 0.0 | 0.728 |
| Cardiovascular disease | 22 | 3.13 (2.65, 3.70) | <0.001 | 0.0 | 0.476 |
| Clinical symptoms | 14 | 3.05 (2.26, 4.11) | <0.001 | 0.0 | 0.570 |
| ICU admission | 4 | 2.97 (2.40, 3.68) | <0.001 | 0.0 | 0.667 |
| Death | 4 | 5.92 (2.66, 13.19) | <0.001 | 32.9 | 0.215 |
| Cerebrovascular disease | 8 | 2.74 (1.59, 4.74) | <0.001 | 0.0 | 0.511 |
| Clinical symptoms | 6 | 2.24 (1.26, 3.98) | 0.006 | 0.0 | 0.937 |
| ICU admission | 1 | 20.20 (2.34, 174.44) | 0.006 | – | – |
| Death | 1 | 13.27 (0.71, 249.04) | 0.084 | – | – |
| Chronic respiratory disease | 20 | 3.56 (2.87, 4.41) | <0.001 | 0.0 | 0.900 |
| Clinical symptoms | 13 | 4.17 (2.67, 6.50) | <0.001 | 0.0 | 0.918 |
| ICU admission | 3 | 3.32 (2.55, 4.32) | <0.001 | 0.0 | 0.618 |
| Death | 4 | 4.06 (1.77, 9.31) | 0.001 | 23.2 | 0.272 |
| Chronic kidney disease | 10 | 3.02 (2.23, 4.08) | <0.001 | 0.0 | 0.958 |
| Clinical symptoms | 5 | 2.37 (1.05, 5.37) | 0.038 | 0.0 | 0.837 |
| ICU admission | 3 | 3.04 (2.19, 4.23) | <0.001 | 0.0 | 0.933 |
| Death | 2 | 7.73 (1.29, 46.18) | 0.025 | 0.0 | 0.676 |
| Chronic liver disease | 13 | 1.54 (0.95, 2.49) | 0.080 | 0.0 | 0.451 |
| Clinical symptoms | 8 | 1.15 (0.64, 2.07) | 0.649 | 0.0 | 0.723 |
| ICU admission | 4 | 1.69 (0.47, 6.09) | 0.426 | 25.4 | 0.259 |
| Death | 1 | 8.64 (0.50, 148.0) | 0.137 | – | – |
| Malignancy | 15 | 2.73 (1.73, 4.32) | <0.001 | 0.0 | 0.920 |
| Clinical symptoms | 9 | 2.88 (1.64, 5.06) | <0.001 | 0.0 | 0.820 |
| ICU admission | 2 | 1.72 (0.50, 5.87) | 0.389 | 0.0 | 0.547 |
| Death | 4 | 3.16 (1.15, 8.70) | 0.026 | 0.0 | 0.580 |
Abbreviations: CIconfidence interval; ICUintensive care unit; ORodds ratio.
Figure 3.
Association between chronic comorbidities and severe or fatal COVID-19, according to severe clinical outcomes (severe disease based on clinical symptoms, ICU admission, and death). ICU, intensive care unit; OR, odds ratio; 95% CI, 95% confidence interval.
Figure 4.
Association between chronic comorbidities and severe or fatal COVID-19 according to country of residence. OR, odds ratio; 95% CI, 95% confidence interval.
In particular,despite being the most common comorbidity, obesity was not a strong predictor for COVID-19 severity. Subgroup analysis showed that obesity was significantly related to an increased risk of mechanical ventilation during the ICU admission (OR 2.29, 95% CI 1.22–4.29), but was not associated with excess mortality (OR 1.15, 95% CI 0.98–1.34; Supplementary Material Figure S1A). Additionally, patients with coexisting hypertension were 2.75 (95% CI 2.08–3.64), 5.34 (95% CI 1.52–18.71), and 3.45 (95% CI 2.23–5.34) times more likely to develop severe manifestations, be admitted to the ICU, and die, respectively, compared to those without hypertension (Supplementary Material Figure S1B). Patients with diabetes were 2.73 (95% CI 1.95–3.82), 2.98 (95% CI 1.49–5.98), and 2.08 (95% CI 1.38–3.15) times more likely to progress to severe manifestations, ICU admission, and death, respectively, compared to non-diabetic patients (Supplementary Material Figure S1C). Meanwhile, patients with CVD were more likely to progress to severe manifestations, ICU admission, and death when compared to those without CVD, with ORs of 3.05 (95% CI 2.26–4.11), 2.97 (95% CI 2.40–3.68), and 5.92 (95% CI 2.66–13.19), respectively (Supplementary Material Figure S1D). Having cerebrovascular disease also significantly increased the risk of severe manifestations, ICU admission, and death, with ORs of 2.24 (95% CI 1.26–3.98), 20.20 (95% CI 2.34–174.44), and 13.27 (95% CI 0.71–249.04), respectively (Supplementary Material Figure S1E). Of note, although respiratory disease was relatively uncommon among the comorbidities, it was by far the most strongly predictive comorbidity for the composite adverse outcomes associated with COVID-19, with ORs of 4.17 (95% CI 2.67–6.50), 3.32 (95% CI 2.55–4.32), and 4.06 (95% CI 1.77–9.31) for severe clinical manifestations, ICU admission, and death, respectively (Supplementary Material Figure S1F). Additionally, comorbid CKD significantly increased the risk of severe manifestations, ICU admission, and death, with ORs of 2.37 (95% CI 1.05–5.37), 3.04 (95% CI 2.19–4.23), and 7.73 (95% CI 1.29–46.18), respectively (Supplementary Material Figure S1G). Pre-existing malignancy also significantly increased the risk of severe manifestations and death, with ORs of 2.88 (95% CI 1.64–5.06) and 3.16 (95% CI 1.15–8.70), respectively, and insignificantly increased the risk of ICU admission (1.72 (95% CI 0.50–5.87);Supplementary Material Figure S1I). The lack of significance for the association between ICU admission and malignancy might be attributed to an insufficient number of studies analyzing this subgroup. Among the comorbidities, liver disease was the only one that was not significantly associated with severe or fatal COVID-19 (OR 1.54, 95% CI 0.95–2.49;Supplementary Material Figure S1H). In addition, subgroup analyses according to the country of residence showed ethnic differences in the association between comorbidities and severe or fatal COVID-19 ( Figure 4).
Assessment of publication bias
Funnel plots did not reveal any obvious asymmetry and Egger’s test did not show any significant publication bias (Egger’s test: p > 0.05), indicating that the results are relatively robust. The funnel plots are presented in Supplementary Material Figure S2A–H.
Discussion
This study systematically evaluated the association between chronic comorbidities and poor clinical outcomes associated with COVID-19, with a large sample size and extensive coverage of the regions around the world. The findings showed that, among patients with severe or fatal COVID-19, the most prevalent comorbidities were obesity (42%) and hypertension (40%), followed by diabetes (17%), CVD (13%), respiratory disease (8%), cerebrovascular disease (6%), malignancy (4%), CKD (3%), and liver disease (2%). Comorbid respiratory disease was identified as the strongest risk factor for COVID-19 severity, with a nearly 3.6-fold higher risk of a poor outcome, followed by hypertension, CVD, CKD, cerebrovascular disease, malignancy, diabetes, and obesity. The meta-analysis did not present a significant correlation between liver disease and COVID-19 aggravation. Knowledge of these factors could help clinicians better identify those populations at high risk of COVID-19 and provide more specific approaches to prevent severe or fatal outcomes.
Similar comorbidity effects have been noted in other severe acute respiratory outbreaks, such as influenza illness (Mertz et al., 2013), MERS (Badawi and Ryoo, 2016b), and SARS (Chan et al., 2003). However, the strength of association between particular comorbidities and the prognosis of these respiratory diseases has been less consistent when reviewing the literature reports (Booth et al., 2003, Garbati et al., 2016, Matsuyama et al., 2016, Mertz et al., 2013). Recent pathology and virology studies have indicated that, compared with influenza virus and respiratory syncytial virus, SARS-CoV-2 can lead to a muted response that lacks robust induction of a subset of cytokines, including the type I and type III interferons, as well as numerous chemokines (Blanco-Melo et al., 2020). Moreover, the growth rate of COVID-19 is much greater than that of SARS and MERS, determining its special prevalence in the early stage (Liang, 2020). To date, only a limited number of meta-analyses have been published to report the prevalence of comorbidities in COVID-19 infection (Borges do Nascimento et al., 2020, Li et al., 2020c, Lippi et al., 2020, Wang et al., 2020b, Yang et al., 2020b), all of which have indicated that hypertension and CVD are risk factors for patients with COVID-19 progressing to severe disease. However, with limited data comparing the proportions of comorbidities between severe and non-severe cases, conclusions regarding the associations between obesity, diabetes, CKD, malignancy, and the serious adverse outcomes associated with COVID-19 are inconsistent or lacking. In addition, the studies included in these previous meta-analyses were confined to China, mainly Wuhan, and the judgment criteria for severe and non-severe disease were not uniform, making their conclusions confusing and unconvincing.
This study, with a large sample size and extensive coverage of the regions worldwide, provides further evidence of the relationship between different comorbidities and the severity of COVID-19. The findings reported here are in keeping with current knowledge that circulatory and endocrine diseases (including hypertension, cardiovascular diseases, obesity, and diabetes) represent the most common category of comorbidity (Guan et al., 2020a, Guan et al., 2020b). Meanwhile, the findings add new evidence that obesity, with a proportion of up to 42%, is the most prevalent comorbidity among patients with severe or fatal COVID-19. Of note, it was found that obesity led to a higher risk of mechanical ventilation than of death, among patients admitted to the ICU. In fact, obesity has been considered a risk factor for increased morbidity among critically ill patients, but not consistent with mortality (Aldawood et al., 2006, Fezeu et al., 2011, Goulenok et al., 2004, Peake et al., 2006, Sakr et al., 2008). Specifically, some researchers have indicated that a low BMI, but not a high BMI, is a significant and independent predictor of mortality (Galanos et al., 1997, Tremblay and Bandi, 2003). In contrast, despite being uncommon in the study population, chronic respiratory disease was identified as the strongest risk factor for severe or fatal COVID-19. Except chronic liver disease, patients with any comorbidity (e.g., obesity, hypertension, diabetes, CVD, cerebrovascular disease, respiratory disease, CKD, and malignancy) had poorer clinical outcomes associated with COVID-19. This finding differs from the results of the previous meta-analyses conducted by Yang et al. (Yang et al., 2020c) and Li et al. (Li et al., 2020c), which found that the association between diabetes and severe COVID-19 was non-significant, and the meta-analysis conducted by Wang et al. (Wang et al., 2020c), which found that there was no correlation between CKD or malignancy and disease aggravation in COVID-19 patients. Regardless, the present study findings suggest that all of the comorbidities mentioned above should be taken into account when predicting the prognosis in patients with COVID-19, and better protection should be given to the high-risk patients upon diagnosis.
Chronic comorbidities share several standard features with infectious disorders, such as a prolonged proinflammatory state and dysfunction of innate and adaptive immunity (Brownlee, 2001, Hameed et al., 2015), which may be the key drivers of the worse clinical outcomes in patients infected with SARS-CoV-2. In particular, angiotensin-converting enzyme 2 (ACE2), which is highly expressed in the lung and heart tissues (Turner et al., 2004), has been identified as an important functional receptor for SARS-CoV-2 invasion (Wu et al., 2020b). In patients with obesity, diabetes, or CVD, the expression of ACE2 has been found to be upregulated, thus increasing the susceptibility to SARS-CoV-2 infection and the risk of disease aggravation (Jia et al., 2020, Roca-Ho et al., 2017, Zheng and Ma, 2020). In addition, pulmonary physiological abnormalities (Ashburn et al., 2010, Kida et al., 1983) and microangiopathy (Teeter and Riese, 2008, Williams et al., 1984) associated with obesity and diabetes have been shown to increase viral diversity and titers (Domingo, 1997, Lauring and Andino, 2010) and to prolong viral shedding (Ryan and Caplice, 2020), which might also increase the risk of disease aggravation. On the other hand, SARS-CoV-2 infection could in turn worsen pre-existing comorbidities, or even cause new-onset endocrine and metabolic disorders, which would further result in a vicious circle effect [Au?1]. Researchers have observed that insulin doses increase and blood glucose becomes difficult to control when diabetic patients become infected with SARS-CoV-2 (Guo et al., 2020, Zhou and Tan, 2020), suggesting that the virus has an impact on glucose metabolism. In fact, it has been proposed that SARS-CoV-2 could bind to ACE2 in liver and pancreatic islet, with a potential role in the development of insulin resistance and impaired insulin secretion, further causing acute diabetes or worsening the diabetes prognosis (Wang et al., 2020e, Yang et al., 2010). Pre-existing CVDs also become unstable, with the increased incidence of acute coronary syndromes, heart failure, and arrhythmias in the setting of SARS-CoV-2 infection, probably resulting from the imbalance between the increased metabolic demand and reduced cardiac reserve (Kochi et al., 2020), concurrent with an accentuated inflammatory response and myocardial damage (Wang et al., 2020f). In addition to the lung and heart tissues, the expression of ACE2 has also been found in kidneys, testes, bladder, liver, stomach, intestinal epithelium, and vascular endothelium (Hamming et al., 2004, Yang et al., 2010, Zhang et al., 2020b), providing a mechanism for the multi-organ dysfunction that can be seen in SARS-CoV-2 infection.
The findings of this study add to the existing literature on the spectrum of comorbidities in patients with COVID-19, based on a large sample size and with a population representative of the whole patient population around the world. However, several limitations of this study are acknowledged. First, there was heterogeneity among the eligible studies in some meta-analyses, which was probably the result of differences in the baseline characteristics of the participants, and there were large variations in sample size among the studies. For this reason, a random-effects model was used for all analyses, and subgroup analyses were performed to identify the sources of heterogeneity. Second, as an inherent limitation of all meta-analyses of observational data, we cannot exclude the possibility that the associations were affected by certain confounding factors, such as age, BMI, and baseline respiratory function. Taking into account these confounding risk factors would only have been relevant to the extent that comorbidities were considered as a causative factor. Next, although we divided disease severity based on clinical symptoms, ICU care, and death, the included studies still varied in their differentiation of patient disease severity in clinical definition, with classifications of ‘mild, moderate, severe, and critical’, ‘ordinary and severe/critical’, ‘common and severe’, and ‘non-severe and severe’ disease.
In conclusion, chronic comorbidities including obesity, hypertension, diabetes, CVD, cerebrovascular disease, respiratory disease, kidney disease, and malignancy, are clinical risk factors for severe or fatal outcomes associated with COVID-19, with obesity being the most prevalent and respiratory disease being the most strongly predictive. Knowledge of these risk factors could lead to the better identification of high-risk COVID-19 patients and thus allow a more specific approach to prevent severe or fatal outcomes. Further investigations are needed to clarify the pathophysiological mechanisms underlying the associations found in this meta-analysis.
Author contributions
Y.G. Wang and L.Y. Shen designed the study. Y. Zhou and Q. Yang performed the literature search. J.W. Chi and B.Z. Dong contributed to the data acquisition. Y. Zhou, Q. Yang, and B.Z. Dong contributed to the data interpretation and statistical analysis. L.Y. Shen and W.S. Lv contributed to supervision or mentorship. Y. Zhou, Q. Yang, and J.W. Chi wrote the first draft of the report. Y.G. Wang and L.Y. Shen edited the report and all authors contributed to the revision of the report. All authors reviewed the manuscript, approved the final draft, and agreed to submit it for publication.
Funding
Y. Wang received funding support from MOST (Ministry of Science and Technology of the People’s Republic of China) with grant number 2020ZX09201−018. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
This meta-analysis study was exempted from ethics approval, as the data collected and synthesized were from previous clinical trials for which informed consent had already been obtained by the trial investigators.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.07.029.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- World Health Organization . 2020. Coronavirus disease (COVID-19) outbreak.t Available online: https://wwwwhoin. [Google Scholar]
- World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports. Available online: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [Google Scholar]
- Alanazi K.H., Abedi G.R., Midgley C.M., Alkhamis A., Alsaqer T., Almoaddi A., et al. Diabetes Mellitus, Hypertension, and Death among 32 Patients with MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis. 2020;26(1):166–168. doi: 10.3201/eid2601.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldawood A., Arabi Y., Dabbagh O. Association of obesity with increased mortality in the critically ill patient. Anaesth Intensive Care. 2006;34(5):629–633. doi: 10.1177/0310057X0603400501. [DOI] [PubMed] [Google Scholar]
- Alqahtani F.Y., Aleanizy F.S., Ali El Hadi Mohamed R., Alanazi M.S., Mohamed N., Alrasheed M.M., et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;147:1–5. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn D.D., DeAntonio A., Reed M.J. Pulmonary system and obesity. Crit Care Clin. 2010;26(4):597–602. doi: 10.1016/j.ccc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa H., Rello J., Tejada S., Martín A., Balziskueta G., Vinuesa C., et al. SARS-Cov-2 in Spanish Intensive Care: Early Experience with 15-day Survival In Vitoria. Anaesth Critical Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Møller R., Panis M., Sachs D., et al. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv. 2020;2020 03.24.004655. [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I., et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clinical Med. 2020;9(4):941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z., et al. 2020. COVID-19 in a Designated Infectious Diseases Hospital Outside Hubei Province, China. Allergy 2020;n/a(n/a) [DOI] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions among Patients with Coronavirus Disease 2019 - United States, February 12-March 28, 2020. MMWR. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.W., Ng C.K., Chan Y.H., Mok T.Y., Lee S., Chu S.Y., et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020;2020 doi: 10.1093/cid/ciaa449. 02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 National Emergency Response Center Coronavirus Disease-19: The First 7,755 Cases in the Republic of Korea. Osong public health and research perspectives. 2020;11(2):85–90. doi: 10.24171/j.phrp.2020.11.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 National Incident Room Surveillance Team . Commun Dis Intell; 2020. Australia: Epidemiology Report 10 (Reporting week to 23:59 AEST 5 April 2020) [DOI] [PubMed] [Google Scholar]
- Deng S.-Q., Peng H.-J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J Clinical Med. 2020;9(2):575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E. RNA virus evolution, population dynamics, and nutritional status. Biolog Trace Element Res. 1997;56(1):23–30. doi: 10.1007/BF02778981. [DOI] [PubMed] [Google Scholar]
- Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. New England J Med. 2020 doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. Am J Respir Critical Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E., et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obesity Reviews. 2011;12(8):653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- Galanos A.N., Pieper C.F., Kussin P.S., Winchell M.T., Fulkerson W.J., Harrell F.E., Jr., et al. Relationship of body mass index to subsequent mortality among seriously ill hospitalized patients. SUPPORT Investigators. The Study to Understand Prognoses and Preferences for Outcome and Risks of Treatments. Critical Care Med. 1997;25(12):1962–1968. doi: 10.1097/00003246-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utiliT.y of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbati M.A., Fagbo S.F., Fang V.J., Skakni L., Joseph M., Wani T.A., et al. A Comparative Study of Clinical Presentation and Risk Factors for Adverse Outcome in Patients Hospitalised with Acute Respiratory Disease Due to MERS Coronavirus or Other Causes. PloS one. 2016;11(11) doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulenok C., Monchi M., Chiche J.-D., Mira J.-P., Dhainaut J.-F., Cariou A. Influence of Overweight on ICU Mortality: A Prospective Study. Chest. 2004;125(4):1441–1445. doi: 10.1378/chest.125.4.1441. [DOI] [PubMed] [Google Scholar]
- Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-j, Ni Z.-y, Hu Y., Liang W.-h, Ou C.-q, He J.-x, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. 2020. Diabetes is a Risk Factor for the Progression and Prognosis of COVID-19; p. e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed I., Masoodi S.R., Mir S.A., Nabi M., Ghazanfar K., Ganai B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598–612. doi: 10.4239/wjd.v6.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T. Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Statistics Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Yin C., Lu S., Chen Y., Liu Q., Bai J., et al. Preprints; 2020. Two Things about COVID-19 Might Need Attention. 2020020315. [Google Scholar]
- Kida K., Utsuyama M., Takizawa T., Thurlbeck W.M. Changes in lung morphologic features and elasticity caused by streptozotocin-induced diabetes mellitus in growing rats. Am Respir Dis. 1983;128(1):125. doi: 10.1164/arrd.1983.128.1.125. [DOI] [PubMed] [Google Scholar]
- Kochi A.N., Tagliari A.P., Forleo G.B. 2020. Cardiac and Arrhythmic Complications in Covid-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauring A.S., Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6(7) doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., et al. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Eng J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K. Mathematical model of infection kinetics and its analysis for COVID-19, SARS and MERS. Infect Genetics Evol. 2020;82 doi: 10.1016/j.meegid.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Wong J., Henry B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Polish Archives Int Med. 2020 doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- Liu W., Tao Z.W., Lei W., Ming-Li Y., Kui L., Ling Z., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A., Soldevila N., Romero-Tamarit A. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. Plos One. 2019;14(1) doi: 10.1371/journal.pone.0210353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama R., Nishiura H., Kutsuna S., Hayakawa K., Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health. 2016;16(1):1203. doi: 10.1186/s12889-016-3881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauskopf J., Klesse M., Lee S., Herrera-Taracena G. The burden of influenza complications in different high-risk groups: a targeted literature review. J Med Eco. 2013;16(2):264–277. doi: 10.3111/13696998.2012.752376. [DOI] [PubMed] [Google Scholar]
- McLaughlin T., Liu L.-F., Lamendola C., Shen L., Morton J., Rivas H., et al. T-Cell Profile in Adipose Tissue Is Associated With Insulin Resistance and Systemic Inflammation in Humans. Arteriosclerosis Thrombosis Vascular Bio. 2014;34(12):2637–2643. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz D., Kim T.H., Johnstone J., Lam P.P., Science M., Kuster S.P., et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake S.L., Moran J.L., Ghelani D.R., Lloyd A.J., Walker M.J. The effect of obesity on 12-month survival following admission to intensive care: a prospective study. Critical Care Med. 2006;34(12):2929–2939. doi: 10.1097/01.CCM.0000248726.75699.B1. [DOI] [PubMed] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., et al. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Eng J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva S., Filippini M., Turla F., Cattaneo S., Margola A., De Fulviis S., et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Critical Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek H.E., Madoff L.C. Association of age and comorbidity on 2009 influenza A pandemic H1N1-related intensive care unit stay in Massachusetts. Am J Public Health. 2014;104(11):e118–25. doi: 10.2105/AJPH.2014.302197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2020. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int J Mol Sci. 2017;18(3):563. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Eng J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P.M., Caplice N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation and Cytokine Amplification in COVID-19. Obesity (Silver Spring) 2020;28(7):1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr Y., Madl C., Filipescu D., Moreno R., Groeneveld J., Artigas A., et al. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34(11):1999–2009. doi: 10.1007/s00134-008-1243-0. [DOI] [PubMed] [Google Scholar]
- Shen L., Li S., Zhu Y., Zhao J., Tang X., Li H., et al. Clinical and laboratory-derived parameters of 119 hospitalized patients with coronavirus disease 2019 in Xiangyang, Hubei Province, China. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L.A., Clarke M., Rovers M., Riley R.D., Simmonds M., Stewart G., et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD Statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- Teeter J.G., Riese R.J. Cross-Sectional and Prospective Study of Lung Function in Adults with Type 2 Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Response to Yeh et al. 2008;31(10) doi: 10.2337/dc08-1090. e82-e. [DOI] [PubMed] [Google Scholar]
- Tremblay A., Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202–1207. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacolog Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12 doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clinical Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.G., Morris A.I., Hayter R.C., Ogilvie C.M. Respiratory responses of diabetics to hypoxia, hypercapnia, and exercise. Thorax. 1984;39(7):529–534. doi: 10.1136/thx.39.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Ja Xia, Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Int Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Ja Xia, Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.M., Hsu C.Y., Lai C.C., Yen M.F., Wikramaratna P.S., Chen H.H., et al. Impact of Comorbidity on Fatality Rate of Patients with Middle East Respiratory Syndrome. Scientific Reports. 2017;7(1):11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X., et al. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Polish Archives Internal Med. 2020 doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Xu H., Yang M., Zeng Y., Chen H., Liu R., et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clinical Virol. 2020;127 doi: 10.1016/j.jcv.2020.104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T. COVID-19 and the cardiovascular system. Net Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism: Clinical Experimental. 2020;107 doi: 10.1016/j.metabol.2020.154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Han T., Chen J., Hou C., Hua L., He S., et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases with COVID-19. Clinical Translational Sci. 2020 doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.