Abstract
Outbreak of Coronavirus disease 2019 (COVID-19) started in mid of December 2019 and spread very rapidly across the globe within a month of its outbreak. Researchers all across the globe started working to find out its possible treatments. However, most of initiatives taken were based on various hypotheses and till date no successful treatments have been achieved. Some strategies adopted by China where existing antiviral therapy was initially used to treat COVID-19 have not given very successful results. Researchers from Thailand explored the use of combination of anti-influenza drugs such as Oseltamivir, Lopinavir and Ritonavir to treat it. In some cases, combination therapy of antiviral drugs with chloroquine showed better action against COVID-19. Some of the clinical studies showed very good effect of chloroquine and hydroxychloroquine against COVID-19, however, they were not recommended due to serious clinical toxicity. In some cases, use of rho kinase inhibitor, fasudil was found very effective. In some of the countries, antibody-based therapies have proved fairly successful. The use of BCG vaccines came in light; however, they were not found successful due to lack of full-proof mechanistic studies. In Israel as well as in other developed countries, pluristems allogeneic placental expanded cell therapy has been found successful. Some phytochemicals and nutraceuticals have also been explored to treat it. In a recent report, the use of dexamethasone was found very effective in patients suffering from COVID-19. Its effect was most striking among patients on ventilator. The research for vaccines that can prevent the disease is still going on. In light of the dynamic trends, present review focuses on etiopathogenesis, factors associated with spreading of the virus, and possible strategies to treat this deadly infection. In addition, it attempts to compile the recent updates on development of drugs and vaccines for the dreaded disease.
Keywords: COVID-19, Diagnostic kits, Plasma therapy, Hydroxychloroquine, Antiviral drugs
Highlights
-
•
CT scan and RT-PCR are very helpful in diagnosis of COVID-19 infection.
-
•
Antiviral, antimalarial, antimicrobial and ROCK inhibitors were found very effective.
-
•
Dietary supplements such as Glucosamine and Vitamin-C were found effective.
-
•
Vaccines such as Canine Corona vaccine and BCG have been found promising.
-
•
Cell based, Convalescent plasma and Pluristems allogeneic placental expanded cell therapies are being tested.
Abbreviations
- CoVs
Coronavirus
- COVID-19
Coronavirus Disease 2019
- E
Envelope protein
- hDPP-4
Human Dipeptidyl Pentidase-4
- HIV
Human Immunodeficiency Virus
- LPV
Lopinavir
- M
Membrane protein;MAVS,O-mitochondrial antiviral signalling protein
- MERS-CoVs
Middle East Respiratory syndrome coronavirus
- N
Nucleoprotein
- NOX2
nicotinamide adenine dinucleotide phosphate hydrogen oxidase 2
- nsp-3
Non-structural protein-3
- RBD
Receptor Binding Domain
- RNA
Ribonucleic Acid
- S
Spike protein
- SARS
Severe Acute Respiratory Syndrome
- URT
Upper Respiratory Tract
- VLPs
Virions Like Particles
- WHO
World Health Organization
- RT-PCR
Reverse transcription polymerase chain reaction
1. Introduction
Though research on coronavirus disease 2019 (COVID-19) was going on at global level since last two decades, highly virulent transmission of COVID-19 came into existence as highly fatal human pathogen during June 2012 in Arabian Peninsula (https://www.business-standard.com/article/international/china-suspends-public-transport-in-wuhan-confirms-571-cases-of-coronavirus-120012300122_1.html). At that time, it was christened as Middle East Respiratory Syndrome Coronavirus (MERS-CoVs). Corona virus is an enveloped, positive sense ribonucleic acid (RNA) virus found in various species mainly in mammals and birds (Lee, 2015). The World Health Organization (WHO) named the coronavirus (CoVs) as severe acute respiratory syndrome coronavirus-2(SARS-CoV-2) recently.
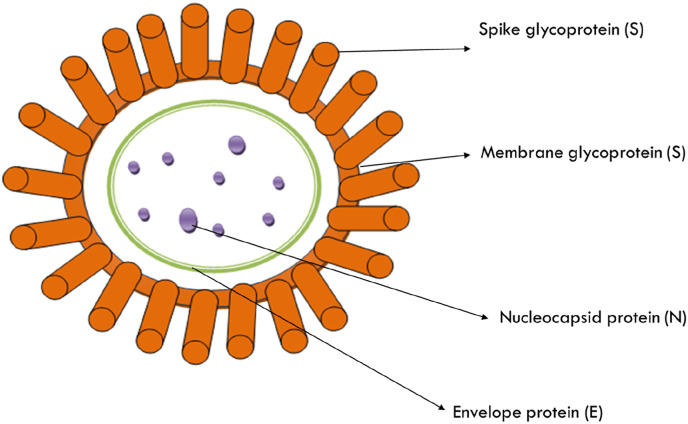
The components of SARS-CoV-2 are spike glycoprotein (S), membrane protein (M), nucleocapsid protein (N) and envelope protein (E). The spikes present on the virus consist of a single-pass trans membrane anchor, a large ectodomain and short intracellular tail. The ecto domain contains two subunits S1 and S2 (Li, 2016). The homo trimers of S-protein help in building of spikes on the viral surface which play a key role in its attachment with host receptors (Beniac et al., 2006; Delmas and Laude, 1990). The M glycoprotein performs three major functions i.e. it provides shape to the virions, aids in promoting curvature of membrane, and facilitates binding to the nucleocapsid (Nal et al., 2005; Neuman et al., 2011). The E-glycoprotein plays a key role in the assembly and pathogenesis of virus (DeDiego et al., 2007; Nieto-Torres et al., 2014). The N- glycoprotein consists of two domains which bind to the RNA genome of the virion. It is also believed that N-glycoprotein bind stonon-structural protein 3 (nsp-3) which, in turn helps in tying the genome to replication-transcription complexes (RTCs) and helps in packaging of enfolded genome into virions (Chang et al., 2006; Fehr and Perlman, 2015; Hurst et al., 2009). The structure of SARS-CoV-2 is shown in Fig. 1 .
Fig. 1.
Structure of SARS-CoV-2. SARS-CoV-2 binds with ACE-2 receptor and influxes host cells through receptor-mediated endocytosis. Inside the cell, SARS-CoV-2 forms a layer called capsid vesicle. Microtubules transport viruses to the cytoplasm. Viral RNA is involved in transcription process in the cytoplasm and conversion into viral genome mRNA. It also affects translation processes and develops viral protein. Viral RNA and viral proteins form new viruses. Thus virus multiplies and forms capsulated nucleoprotein. The multiplied viruses cause apoptosis in the cell and affect other cells also (Bleibtreu et al., 2019).
2. Symptomatic features of COVID-19
The main symptoms of COVID-19 include runny nose, sneezing, common cold, cough, confusion, myalgia, diarrhea, vomiting, shortness of breath, wheezing and fever (Nassar et al., 2018; Tyrrell and Myint, 1996).The virus enters the respiratory tract through nose and stays there for three days. Afterwards, it starts infecting upper respiratory tract (URT) with above-mentioned symptoms. COVID-19 causes URT illness including acute exacerbation of chronic obstructive pulmonary disease, bronchitis and pneumonia, with co-morbidities in digestive, cardiac (Zheng et al., 2020), renal (Cheng et al., 2020) and circulatory systems as well (Huang et al., 2020).
The risk factor for COVID-19 infection is higher in both infants as well as in geriatric age group. Also, diabetic patients are more prone to COVID-19 with higher rate of mortality and co-morbidity. The risk factors associated with the COVID-19 are obesity, smoking, low blood pressure, impaired gas exchange, leukopenia, anemia, disturbance in liver and kidney functions etc.
3. Life cycle of SARS-CoV-2 in host cell
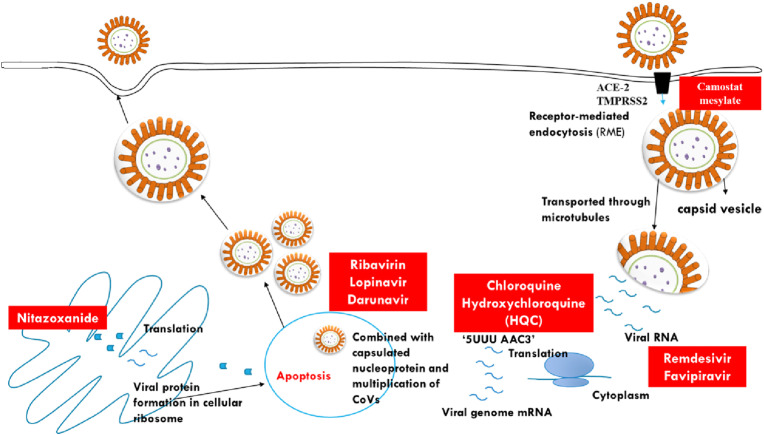
The life cycle of COVID-19 begins when virion enters the host and gets attached to the host cells by interaction between glycoprotein “S” and host receptors human angiotensin converting enzyme-2 (ACE-2) leading to tropism of virus. The virion enters the cytosol with the help of capthesin and transmembrane protease, serine 2 (TMPRSS2) which results in cleavage of “S” protein from two sites (Hoffmann et al., 2020). The first cleavage helps in separation of receptor binding domains (RBD) and fusion domain of “S” protein while second cleavage exposes fusion peptides to endosomes. Six helical bundles are formed, which release virions into the cytoplasm. After attachment, the replication cycle begins, during which, translation of replica gene in virion genomic RNA takes place. This is encoded by two large open reading frames ORF1a and ORF1b that give rise to poly protein 1a and 1b (pp1a and pp1b) and are expressed by slippery sequence ‘5UUU AAC-3'and RNA pseudo-knot.This results in ribosomal shifting and stops the ribosomal elongation. It also acts as mRNA for expression of structural and accessory proteins. Finally, virus spreads to different parts of the body. During this phase, “S1”, “M” and “E” are translated, which help the virion to enter into endoplasmic reticulum and endoplasmic reticulum-golgi intermediate compartments. “M” leads to protein-protein interaction, which further combines with “E” to activate virus-like particles (VLPs), which in turn, lead to formation of corona envelope. Then “N” protein promotes formation of VLPs and fusion of encapsidate with endoplasmic reticulum golgi intermediate compartment (ERGIC) (Shereen et al., 2020).This gives rise to assembly of COVID-19. The exocytosis of virions takes place as a result of interaction between normal and affected cells. Finally the giant cells are formed and virus spreads to other cells (https://www.antibodies-online.com/resources/18/5410/sars-cov-2-life-cycle-stages-and-inhibition-targets/) (Fig. 2 ).
Fig. 2.
Viral cycle of SARS-CoV-2 in target host cell and target drug.
4. Diagnosis of COVID-19 infection
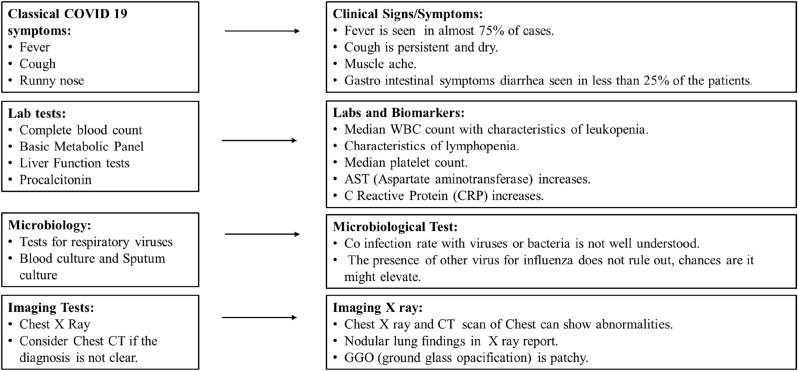
The detection of SARS-CoV-2 is done by performing antibody testing such as enzyme-linked immune sorbent assay (ELISA) for Immunoglobulin G(IgG), and Immunoglobulin A (IgA) or plaque reduction neutralization test (PRNT) in blood or airways fluid (Fig. 3 ). Distal lung sampling results in its satisfactory diagnosis (Bleibtreu et al., 2019; Oh, 2016). SARS-CoV-2 kits are very important tools for easy and fast diagnosis in coronavirus pandemic. Various biopharmaceutical companies are working on virus kit research and development. Various kits made by different countries are mentioned in Table 1 .
Fig. 3.
Tests used for diagnosis of SARS-CoV-2 infection.
Table 1.
Diagnostic kits used to detect SARS-CoV-2 infection.
| Sr. No. | Pathokit | Country | Reference |
|---|---|---|---|
| 1. | GenMarkDx – Multiplex Diagnostics | Canada | (https://www.startus-insights.com/innovators-guide/5-top-diagnostic-test-kits-to-use-during-the-coronavirus-pandemic/) |
| 2. | XCR Diagnostics – Quantitative Polymerase Chain Reaction (qPCR | United states of America | |
| 3. | SensDx – Ultrasensitive Electrodes | Poland | |
| 4. | Aperiomics – Deep Metagenomic Sequencing | United states of America | |
| 5. | MiRXES – MicroRNA Diagnostics | Singapore | |
| 6. | Truenat Beta CoV test | India | (https://www..gov.in/team-india-blogs/truenat-beta-test-covid-19-detection-indiainvestindia) |
4.1. Real time reverse transcription polymer chain reaction (RT-PCR)
It is the most widely used nuclear derived method to detect the genetic material present in the pathogen. In this technique, radioactive isotope markers were conventionally used to identify the desired target genetic material. But now a days these radioactive isotopes are being replaced by fluorescent dyes. In RT-PCR, the sample is taken mainly from the patient's throat and nose. The collected sample is treated with solvents to remove fats and proteins and extract only RNA. The obtained RNA consists of mixture of infected person's genetic material as well as coronavirus RNA. Then the RNA is reverse transcribed to DNA with help of enzymes. Additional short fragments of DNA that are complementary to specific parts of the transcribed viral DNA are then added. The added fragments attach to the target site of viral DNA if sample consists of virus. The added genetic material acts as a builder for DNA strands during amplification, while the labels added help in detecting the virus. The mixture is then placed under the RT-PCR machine. The machine cycles through heating and cooling of sample so that chemical reaction takes place and forms new copies of viral DNA. These cycles take place 35 times, so that at the end of the cycle, about 35 billion identical copies of viral DNA formed. The fluorescence emission by sample is measured by machine and helps in assessing presence or absence of virus (https://www.iaea.org/newscenter/news/how-is-the-covid-19-virus-detected-using-real-timert-pcr).
4.2. Computed tomography (CT) imaging
It has been found that chest CT imaging is more steady, practical and expeditious technique to diagnose and assess COVID-19. In some of the studies, it has been found that computed tomography is more sensitive tool than RT-PCR to assess COVID-19. The sensitivity of CT and RT-PCR was reported to be 98% and 71% respectively (https://www.itnonline.com/content/ct-provides-best-diagnosis-novel-coronavirus-covid-19). Ai et. al., 2020, studied the comparison between RT-PCR and chest CT for the diagnosis of COVID-19. In the study total 1014 patients underwent RT-PCR and chest CT. The results revealed that out of 1014 patients, 601 (59%) showed positive results with RT-PCR while 888 (88%) patients showed positive CT scans results. So, it was concluded that chest CT is more reliable technique for the diagnosis of COVID-19 (Ai et al., 2020; An et al., 2020).
5. Treatment strategies
With the prevalence of COVID-19 reaching a new hight every day, there is an immediate need to find safe and efficacious measures to diagnose, treat, mitigate and combat the disease. Looking at the alarming dimensions that the disease is acquiring, treatment strategies among various systems of medicines are being investigated. Based on the treatments offered so far and clinical findings, the treatment strategies can be categorized into three classes.
5.1. Synthetic drugs
Antibiotics, for obvious reasons, are not expected be effective in the treatment and a combination of antiviral drugs is being used. Studies also confirm that flu shots are not efficient in the fight against COVID-19 as the patients continue to suffer despite the treatment (https://www.com/articles/gilead-sciences-offers-experimental-drug-for-coronavirus-treatments-testing-11580511519wsj). In the meantime, Thai health officials claimed to have successfully handled the infection with acocktail of antiviral drugs that include lopinavir and ritonavir under the name “Kaetra” along with flu medication oseltamivir. However, a lot more studies need to be conducted to declare this combination as a treatment for COVID-19. Randomized clinical trials using combination of antiviral drugs are already being conducted (Hung et al., 2020). Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19 has been tried. The possibility of successful treatment exists because the same combination was successfully used to treat SARS outbreak in 2002 and SARS-CoV-2 is reported to be a strain similar to the earlier one with the genomic sequence of COVID-19 being about 75–80% similar to that of SARS. Lopinavir was used as major antiviral drug to treat during the SARS outbreak. Lopinavir has been reported to treat COVID-19 (https://www.antibodies-online.com/resources/18/5410/sars-cov-2-life-cycle-stages-and-inhibition-targets/),(https://clinicaltrials.gov/ct2/show/NCT03301090?term=NCT03301090#wrapper). However, its efficiency to mitigate COVID-19 is yet to be fully established. To analyze antiviral effect of inferferon-α2b (IFN- α2b) and ribavirin, SARS-CoV-2 was isolated from hCoV-Emc/2012 replication process by Vero and LLC-MK2 cells. Combination of IFN- α2b and ribavirin achieved comparable remission in low concentration. As per Falzarano et al. (2013), combination of IFN- α2b and ribavirin may also prove to be useful (Falzarano et al., 2013). Anti-ebola and anti-HIV drugs combination (remdesivir + galidesivir) showed significant action against the enzymes responsible for virus replication. These drugs have been able to alleviate the symptoms of COVID-19 similar to that in SARS and MERS (Li and De Clercq, 2020). In an invitro cell line study, this drug also showed very good antiviral effect (Wang et al., 2020).
The Gilead biotechnology company, USA reported preclinical trials of Remdesivir (a nucleotide analogue) which led to remission in animal models (Sheahan et al., 2020). Later, it was reported to be effective in the treatment of COVID-19 patients also (Holshue et al., 2020). Another study, conducted on 760 patients in placebo-controlled trials also proved the effectiveness of remdesivir. This drug has now received emergency use authorization by USFDA on 1st May 2020 (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment).
Frequently used antimalarial drug, Chloroquine (CQ) and Hydroxychloroquine (HCQ) have also been explored and found to be quite effective against COVID-19 (Wang et al., 2020). CQ and HCQ increase endosomal pH and interfere with the glycosylation of cellular receptor of SARS-CoV-2. Thereby they have the potential to block viral infection (Wang et al., 2020). Moreover, they change the pH of lysosomes and likely inhibit cathepsins, that leads to the formation of the autophagosome which cleaves SARS-CoV-2 spike protein. It is also reported that CQ and HCQ through the inhibition of MAP-kinase interfere with SARS-CoV-2 molecular crosstalk, besides altering the virion assembly, budding and interfering with the proteolytic processing of the M protein. It is reported that they interfere with ACE-2 receptor's glycosylation. Since, SARS-CoV-2 utilizes the similar surface receptor ACE-2, it is believed that CQ and HCQ can also thus prevent SARS-CoV-2 attachment to the target cells (Zhou et al., 2016). Some studies have also been initiated and showed very good effect of CQ and HCQ against SARS-CoV-2 (Gautret et al., 2020; Singh et al., 2020).; Gao et al. (2020); (Millán-Oñate et al., 2020). However, due to reported potential clinical toxicity issues such as retinal toxicity, the use of CQ and HCQ is not recommended by WHO.
An antiviral drug favipiravir (Avigan), got approval in Japan in 2014. In 2016, this drug was used as an emergency aid for the Ebola virus outbreak. A clinical trial involving 80 participants (in Shenzhen city) demonstrated chest symptoms improvement in patients of COVID-19 treated with favipiravir. The drug was able to shorten the recovery time from 11 days to 4 days in mild and regular cases. Another trial showed that the drug shortened fever duration from an average of 4.2 days–2.5 days. Favipiravir has been reported to be effective, without any obvious side-effects, in helping coronavirus patients recovery. In another study carried out in China, two mild and two severe COVID-19 associated pneumonia patients were treated with combined Western and Chinese medicine treatment (Lopinavir/ritonavir/arbidol/ShufengJiedu Capsule). Three of the four patients showed significant improvement in pneumonia associated symptoms. The remaining patient with severe pneumonia showed signs of improvement; however, the efficacy of this combination treatment warrants further investigation (https://www..nlm.nih.gov/pubmed/32037389ncbi).
In a recent study, Rho kinase (ROCK) inhibitor, Fasudil has been explored to treat COVID-19. In patients with COVID-19, activation of ROCK causes burst in inflammatory features, immune cell migration, apoptosis, coagulation, contraction, and cell adhesion in pulmonary endothelial cells, leading to endothelium barrier dysfunction and edema as hallmarks of lung injury. Fasudil attenuates this effect due to its excellent anti fibrotic activity (Abedi et al., 2020). It has been found that angiotensin-converting enzyme-2 (ACE-2) is the receptor required for the cellular entry of SARS-CoV-2 (Hoffmann et al., 2020). Envelope spike protein of SARS-CoV-2 mediates its attachment and fusion into the human cells through binding ACE-2 with super-affinity and efficiency. ACE-2 is widely expressed in alveolar epithelial cells and converts angiotensin 2 to angiotensin (1–7). Angiotensin 2 triggers a number of adverse effects like interstitial fibrosis, increased coagulation, interference with adaptive immunity by activating macrophages and other cells of the immune system, with consequent increased production of IL-6, TNFα and other inflammatory cytokines. ROCK inhibitors upregulate the axis of ACE-2, and are thereby found effective in treating COVID-19.
In one of the studies, a 53-year-old woman suffering from COVID-19 was treated with a combination of moxifloxacin and oseltamivir. She was treated with moxifloxacin 400 mg intravenously once a day for eight days and antiviral drug oseltamivir 75 mg orally twice a day for 5 days. After seven days treatment, patient's symptoms got reduced and she tested negative (Ding et al., 2020).
In recent clinical studies the use of steroidal drug Dexamethasone has been very effective to treat patients suffering from COVID-19. It easily diffuses through the host cell membranes and bind to the glucocorticoid receptor in the cell cytoplasm. This receptor binding triggers a cascade of reactions that end up suppressing pro-inflammatory cytokines IL-1, IL-2, IL-6, IL-8, TNF, and IFN-gamma and reduce the severity of Covid-19. Dexamethasone also inhibits the overaction of macrophages in patients suffering from COVID-19. A British research team found that Dexamethasone's (2 mg tablet) effect was most striking among patients on ventilators. Those who were receiving oxygen therapy but were not on ventilators also saw improvement: their risk of dying was reduced by 20%. The steroid had no effect on people with less severe cases of COVID-19 — those not receiving oxygen or ventilation (Ledford, 2020).
Table 2 summarizes various antiviral drugs which may have potential to treat COVID-19 with their mechanism of action. Table 3 summarizes possible drugs with dose to mitigate COVID -19 and Table 4 provides update about recent clinical trials on COVID-19 with possible targets.
Table 2.
Various antiviral drugs and their mechanism of action.
| Virus | Drug | Mechanism of action | Reference |
|---|---|---|---|
| Ebola | mAb114 | Rojas et al. (2019) | |
| HIV | Zidovudine | Nucleoside reverse transcriptase Inhibitor | Justice et al. (2004) |
| Nevirapine | Nonnucleoside reverse transcriptase Inhibitor | Jiang et al. (2014) | |
| Ritonavir | Protease inhibitor | Chen et al. (2005) | |
| Enfuvirtide | Entry (Fusion) inhibitor | Wnuk (2008) | |
| Raltegravir | Integrase inhibitor | Taramasso et al. (2015) | |
| Maraviroc | CCR5 receptor inhibitor | Armstrong-James et al. (2010) | |
| SARS- CoVs | Ribavirin | Decrease intracellular guanosine triphosphate which results in inhibition of caps of viral transcripts, suppress cellular and humoral immune response | (Cameron and Castro, 2001; Peiris et al., 2003) |
| Lopinavir (LPV) + Ritonavir (R) | R helps in inhibiting CYP3A4 metabolism of LPV and increased LPV serum conc. | Chu et al. (2004) | |
| Methylprednisolone | Help in decreasing cytokine storm (ILs, and TNF) | Smego and Ahmed (2003) | |
| ZIKA | Chloroquine | Inhibitory effect against early stages of ZIKA Virus in mice |
Li et al. (2017) Devaux et al. (2020) |
Table 3.
List of drugs that have been used to mitigate COVID -19.
| S.N. | Drugs | Brand Name | Manufacturer | Dose | Duration of treatment | References |
|---|---|---|---|---|---|---|
| 1. | Lopinavir/ritonavir | Aluvia® | ABBOTT health care Pvt. Ltd. | 50 mg/20 0 mg in tablet form | Not more than 10 days | Dong et al. (2020) |
| 2. | Ribavirin | Rebetol, Ribasphere, Copegus and Virazole | Valeant Pharmaceuticals | 500 mg thrice a day or given in combination with ritonavir/Lopinavir and INF-α through Intravenous infusion | Not more than 10 days | Dong et al. (2020) |
| 3. | Chloroquine phosphate | Aralen | Novartis, Mylan and Teva | 500 mg, 250 mg twice a day given orally | Not more than 10 days | Dong et al. (2020) |
| 4. | Umifenovir | Arbidol | Pharmastandart | 50 mg, 200 mg thrice a day in oral form | Not more than 10 days | Dong et al. (2020) |
| 5. | Oseltamivir | ANTIFLU cap | Cipla | 75 mg twice in a day in oral form | 3–14 days | (file:///C:/Users/hp/Downloads/Tamiflu_GL-019936.pdf) |
| 6. | Umifenovir | Arbidol | Pharmastandart | 0.2 g three times a day | Not more than 14 days | Zhu et al. (2020) |
| 7. | Baricitinib | Olumiant | Eli Lilly | 2 mg once daily | Not more than 14 days | (Richardson et al., 2020a, 2020b; Stebbing et al., 2020) |
| 8. | Bromhexine | BROLYT | Alco Pharma Ltd. | 4 mg and 8 mg three times a day | – | Rosa and Santos (2020) |
| 9. | Fingolimod | Gilenya | Novartis | 0.5 mg once in a day | Not more than 3 days | (https://clinicaltrials.gov/ct2/show/NCT04280588) |
| 10. | Bevacizumab | Avastin | Aspar Pharmaceuticals | 500 mg | – | (https://clinicaltrials.gov/ct2/show/NCT04275414) |
| 11. | Pirfenidone | Esbriet | Glenmark Pharmaceuticals | 267 mg three times a day | – | (https://clinicaltrials.gov/ct2/show/NCT04282902) |
| 12. | Thalidomide | Thalomid | Grunenthal | 100 mg | Not more than 14 days | (https://clinicaltrials.gov/ct2/show/NCT04273529) |
Table 4.
Clinical trials on COVID-19.
| Sr. No. | Drug | No. of Patients | Mechanism | Clinical Trials | Outcomes | References |
|---|---|---|---|---|---|---|
| 1. | Chloroquine phosphate | 100 | Increase endosomal pH which is required for fusion of virus and cells, also interfere with the glycosylation of cellular receptors of SARS-CoVs | . ChiCTR2000029939 . ChiCTR2000029760 . C hiCTR2000029609 . C hiCTR2000029761 .ChiCTR2000029837 . C h iCTR2000029 9 . Chi CTR2000029826 . ChiCTR2000029803 |
In-vitro studies reveal that drug block the virus at micro molar concentration with half-cytotoxic concentration (CC50) greater than 100 μM and half-maximal effective concentration (EC50) of 1.13 μM. Drug also improved symptoms of pneumonia in COVID-19 patients | Gao et al. (2020) |
| 2. | Shuanghuanglian oral liquid (SHL) | 3 | Mast cells stabilization by activation of mitochondrial calcium uniporter | ChiCTR2000029605 | Treated patients with COVID-19 but further clinical trials are required to evaluate its efficacy against COVID-19 | (Gao et al., 2017; Ni et al., 2020) |
| 3. | Hydroxychloroquine + Azithromycin | 36 | Hydroxyl chloroquine inhibit toll like receptors and stops dentric cell activation and result in antiinflammatory response while azithromycin inhibits protein synthesis (50S) and inhibits translation process in Mrna | Open-label non-randomized clinical trial | Hydroxychloroquine (600 mg) helped in combating COVID-19 and addition of azithromycin synergesis the effect | Gautret et al. (2020) |
| 4 | CamostatMesilate + Hydroxychloroquine | 334 | CamostatMesilate inhibits serine protease TMPRSS2 while Hydroxychloroquine interrupt the viral entry and replication through glycation of ACE2 receptotors | Randomized | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04338906) |
| 5 | Favipiravir | 100 | inhibits RNA polymerase | Randomized | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04336904?cond=COVID-19&draw=2&rank=2) |
| 6. | Clevudine | 60 | Inhibiting the replication of viral genetic materials | Randomized | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04347915?cond = COVID-19&draw = 2&rank = 6) |
| 7. | Desferal | 50 | Inhibits human cytomegalovirus replication | Randomized | Ongoing trials | (Cinatl et al., 1994; Shakiba, 2020) |
| 8. | Losartan | 50 | decreases activation of nuclear factor kappa B and mitogen-activated protein kinases | Interventional | Ongoing trials | (Fedson et al., 2020; Salathe, 2020) |
| 9. | Ruxolitinib | 80 | Lower the hyperinflammation caused by the virus | Interventional | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04348071?cond = COVID-19&draw = 2&rank = 31) |
| 10. | Baricitinib | 80 | Lower the hyperinflammation caused by the virus | Interventional | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04340232?cond=COVID-19&draw=2&rank=32) |
| 11. | Dapagliflozin | 900 | SGLT-2 Inhibitors | Randomized | Ongoing trials | Kosiboro (2020) |
| 12. | Tocilizumab | 400 | IL-6 inhibitor | Interventional | Ongoing trials | Perrone (2020) |
| 13. | Ciclesonide | 141 | blocks coronavirus RNA replication by targeting viral NSP15 | Randomized | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04330586?cond=COVID-19&draw=2&rank=42) |
| Others | ||||||
| 1. | Convalescent Plasma | 55 | – | Interventional | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04343755?cond=COVID-19&draw=2&rank=19) |
| 2. | BCG Vaccine | 700 | – | Interventional | Ongoing trials | (https://clinicaltrials.gov/ct2/show/NCT04348370?cond=COVID-19&draw=4&rank=614) |
5.2. Phytochemicals
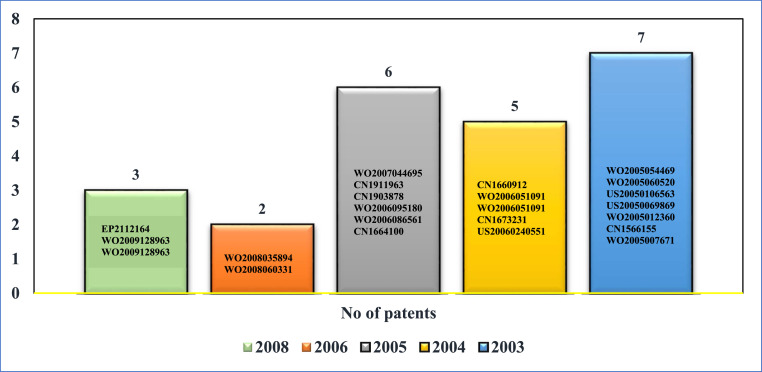
Saikosaponins are triterpene glycosides isolated from medicinal plants like Bupleurum spp, Heteromorpha spp and Scrophularia scorodonia which possess potent antiviral activity (Li et al., 2005). Extracts belonging to Lindera aggregata, Lycoris radiata, Artemisia annua and Pyrrosia lingua have been recorded to show antiviral activity especially against SARS. An Amentoflavone isolated from Torrey a nucifera (belonging to the family Taxaceae), a native of southern Japan and South Korea has been shown to inhibit SARS-CoV 3 CL protease. Isatis tinctoria, known as Asp of Jerusalem belonging to the family Brassicaceae, has been shown to have SARS-CoV 3CL protease inhibition acivity (Lin et al., 2014). With 99% of small molecules failing to be effective, it remains to be seen what can be used in the fight against COVID-19. Fig. 4 exhibits the patents granted on drugs and small molecules which might be effective against COVID-19.
Fig. 4.
Number of patents granted to drugs and small molecules having potential to treat COVID-2019.
Lianhuaqingwen (LH) is a traditional Chinese medicine that has been used previously to combat SARS, influenza virus and enhance immunomodulatory effects. Runfeng et. al. (2020), studied the antiviral and anti-inflammatory effect of LH against SARS-CoV-2. In this study, African green monkey's kidney epithelial cell (Vero E6 cells) was used as an in vitro cell line. The cytopathic effect (CPE) and plaque reduction assay was used to assess the antiviral activity of LH in Vero E6 cells. The results revealed that the LH helped in inhibition of SARS-CoV-2 replication. It has been also found that LH showed significant reduction in pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrotic factor-α (TNF- α) and chemokine (C–C motif) ligand 2/monocyte chemo attractant protein 1 (CCL2/MCP-1).
5.3. Vaccines
In 1986, US patent (US 4567043A) was granted for Canine Corona vaccine which on parenteral administration provided humoral protection from virulent canine corona virus that mostly affect the intestinal tract of dogs (Acree et al., 1986). A novel vaccine was developed using cDNA that is encoded with structural antigens such as spike (S) protein, membrane (M) protein, envelope (E) protein and nucleocapsid (N) protein for SARS caused by COVID-19. Among the developed vaccines, (M) and (N) DNA vaccines showed cytotoxic T Lymphocytes (CTL) activity and human T-cell proliferation in SCID-PBL/hu mice and in vivo human model (Okada et al., 2007). Recently, a new strategy has been developed based on the immunogenetics and immunogenomics. Molecular docking technique was employed for predicting the combination effect of B- and T-cell epitope on the nonstructural protein 4 coronavirus. To target the virus, two peptide sequences from the nonstructural protein 4 of beta coronavirus (IRNTTNPSAR and PTDTYTSVYLGKFRG) were selected and were found to be potent T-cell epitopes. They were found to interact perfectly with epitope grooves of major histocompatibility complex (MHC) allelic protein (HLA-A*01:01 and HLA-DRB5*01:01) that formed a stable MHC complex. It can, therefore, be considered as potential peptide for development of peptide based corona virus vaccine (Basu et al., 2020). In another study, computational approach along with bioinformatics tools was adopted for vaccine design. Based on the docking score as well as antigencity scores, natural inhibitors such as tanshinoneIia and methyl tanshinonate were identified as effective drugs and FVFLVLLPL (MHC class-I allele) and FVFLVLLPL(MHC class-II allele) were selected as best antigenic epitope respectively for vaccine design (Kumar, 2020). Recently, US developed a vaccine and started first human trial (Phase 1 trial) in healthy human volunteers to monitor the desired responses in human immune system along with safety profile. The trial has enrolled 45 healthy volunteers for a period of 6 weeks. The vaccine is based on “messenger RNA vaccine platform technology”. The experience on previous MERS vaccine development for targeting the surface protein of virus led to the idea of this vaccine (Roberts, 2020). Out of the two vaccines, REGN 3048 and REGN 3051, manufactured by Regeneron, USA, were able to more keenly bind to viruses resulting in possibility of developing antibodies. Vaccine REGN 3048 was found to be ineffective against COVID-19, however the company is retesting it for the possible effects against COVID-19 (https://clinicaltrials.gov/ct2/show/NCT03301090?term=NCT03301090#wrapper). The traditional immunization technique of using live attenuated or inactivated viruses has been reported with drawback of leaving the recipient sick again. The use of genetic coding technology in the development of new vaccine development can assure safety over the traditional immunization technique.
Bacille Calmette-Guérin (BCG) is a live attenuated strain of mycobacterium bovis used against tuberculosis and leprosy (https://www..int/biologicals/areas/vaccines/bcg/en/who). Vaccine repositioning indicated its potential use against multiple sclerosis, reducing blood sugar levels in type-1 diabetes and also in various types of cancer like non-Hodgkin's lymphoma and bladder cancer. It demonstrates a non-specific effect and thus its use may prove to be favorable against viral pathogens as well (Moorlag et al., 2019). A study reported that oral zinc sulphate being an effective immunomodulator can be combined with BCG vaccine to provide protection against COVID-19 (Sharquie, 2020). The outcomes of the ongoing randomized trials (phase III) of BCG vaccine are eagerly anticipated (https://clinicaltrials.gov/ct2/show/NCT04328441?term=BCG+vaccine&cond=COVID-19&draw=2&rank=1);(https://clinicaltrials.gov/ct2/show/NCT04327206?term=BCG+vaccine&cond=COVID-19&draw=1&rank=2).
Table 5 compiles the patents on vaccines having therapeutic potential to treat COVID-19 while Table 6 summarizes the trials going on across the globe for the development of drugs and vaccines for the treatment of COVID-19.
Table 5.
Existing patented vaccines that have been repurposed to treat COVID-19.
| Sr. No. | Vaccine | Publication | Patent Number | Patent Date | Targeted site | Observation | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Live attenuated corona virus vaccines | United State Patent Application | US20060039926 | Feb 23, 2006 | Orf1a/polyprotein (p59/nsp14/ExoN) | MHV virus showed reduction of replication in mice at 5th day with intracerebral inoculation | (https://docs.google.com/viewer?url=patentimages.storage.googleapis.com/pdfs/US20060039926.pdf) |
| 2. | DNA based vaccines | International | WO2005081716 | 9 Sept 2005 | calreticulin−nucleocapsid fusion | Resulted in potent nucleocapsid-specific humoral and T cell-mediated immune responses | (Wu et al., 2005) |
| 3. | Protein-Based Vaccines. | International | WO2010063685 | June 10, 2010 | S-Trimer subunit, ACE2 Receptor |
O/W emulsion helped to treat SARS-CoVs by neutralizing antibody responses in animal models | Baras et al. (2012) |
| 4. | Virus-like Particle Vaccines | International | WO2015042373 | 19 Sept 2014 | S protein | The Sera (SAB-300 or SAB-301) were injected into Ad5-hDPP4 transduced BALB/c mice, which protected mice against MERS-COVs | Smith et al. (2015) |
| 5. | mRNA-Based Vaccines. | International | WO2017070626 | April 27, 2017 | mRNA-1273 | Intradermal administration of a lipid nanoparticle (LNP)-encapsulated mRNA mixture encoding MERS-CoVs S proteins into mice resulted in translation in vivo and induction of humoral immune responses |
Ciaramella and Himansu (2017) |
Table 6.
Summary of the research projects currently ongoing for the development of drugs and vaccine against COVID-19.
5.4. Role of nutraceuticals in RNA virus infection
Certain nutraceuticals have also shown efficacy in combating COVID-19. Their mechanisms are discussed here. Viral toll-like receptor (TLR7) of COVID-19 is responsible to trigger hydrogen peroxide generation within the alveolar macrophages via nicotinamide adenine dinucleotide phosphate hydrogen oxidase 2 (NOX2) activation which oxidizes Cys98 on TLR7. Such oxidation blocks receptor potential to conduct signals for type 1 interferon production. Nutraceuticals having potential to inhibit NOX2, superoxide generation or preventing oxidation of Cys98 in TLR7 have potential to evoke TLR7 mediated type 1 interferon production towards RNA virus infections including COVID-19. RNA virus infections have been shown to induce O-GlcNacylation of mitochondrial antiviral signalling protein (MAVS) at multiple sites which prohibits its susceptibity for K63-linked ubiquitination and further interferon regulatory factor 3 (IRF3) activation. Glucosamine supplementation has been investigated to upregulate MAVS to activate IRF3 in response to viral infections. Currently, vitamin C infusion is under clinical trial for the treatment of severe COVID-19 virus infected pneumonia as it plays important role in reducing inflammatory response and has antioxidant property. Vitamin C has earlier been reported to prevent neutrophil accumulation, alveolar fluid and cytokine surge caused by sepsis (Peng, 2020).
The US-FDA has recently issued certain guidelines pertaining to the ongoing clinical trials for the development of medicinal products. Impact on the conduct of clinical trials of medical products is anticipated since challenges may arise from quarantines, site closures, travel limitations, interruptions to the supply chain for the investigational product, or other considerations if site personnel or trial subjects become infected with COVID-19 (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-pandemic).
5.5. Convalescent plasma therapy
Plasma therapy can reduce the mortality rate of COVID-19 (da Silva, 2020). In this therapy, convalescent plasma or immunoglobulins administrated to the patients who are suffering with COVID-19. This therapy can enhance the immunity of the patients (Bloch et al., 2020). In one of the studies in 2014, convalescent plasma therapy was used for the treatment of Ebola virus. It was recommended by WHO (Chen et al., 2020). The Indian Council of Medical Research (ICMR) has approved first clinical trials for convalescent plasma therapy in SVP Hospital, Ahmedabad, India. (theweek.in/news/india/2020/04/19/gujarat-to-start-clinical-trial-with-convalescent-plasma-therapy-for-covid-19.html). FDA is also working on development of convalescent plasma therapy. They provided guidance in mentions about patient eligibility, investigation pathways of convalescent plasma, labelling and records keeping for donated convalescent plasma and regarding treatments (https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma).
5.6. Pluristems allogeneic placental expanded cell therapy
Placenta expanded cells are obtained from the placenta and are designed in such a way that theycan be administered to the patients without tissue or genetic matching. The main function of these cells is to release biomolecules such as growth factors, cytokines and chemokines. These cells act in a endocrine and paracrine manner and help the body to stimulate its defense mechanism and promote healing (https://www.pluristem.com/placental-expanded-plx-products/). The Israel biotech company, Pluristem Therapeutics Inc. reported that the patients who had SARS-CoV-2 and were at a higher risk of death due to respiratory collapse and multiorgan failiure such as kidney and heart failure recovered after receiving this therapy. This happens due to the immunomodulatory effect of the pluripotent plasma cells (PLX). During treatment the 15 mL doses of PLX cells is administered to patients by intramuscular route (https://.com.au/israel-has-found-a-possible-100-cure-for-coronavirus/theindiantelegraph).
5.7. Miscellaneous treatment strategies
Melatonin is a N-acetyl-5-methoxytryptamine and having many health benefits such as remission from sleeping disorders, viral infections, delirium, respiratory disease and atherosclerosis (Reiter et al., 2020). Recent studies on COVID-19 revealed that the main cause of COVID-19 pathology is exaggerated immune response, oxidation and inflammation. All these factors lead to cytokine storm and give rise to Acute Respiratory Distress Syndrome (ARDS) and often death. Zhang et al., (2020), described the anti-inflammatory, immunomodulatory and adjuvant effects of melatonin against SARS-CoV-2. Melatonin showed anti-inflammatory action by acting on sirtuin-1 (SIRT1) and nuclear factor kappa-B (NF-κB) pathways. Melatonin resulted in inhibition of high mobility group boxechromosomal protein1 (HMGB1) and led to downregulation of the polarization of macrophages. Melatonin's action on NF-κB resulted in inhibition of pro-oxidative and pro-inflammatory response. The immunomodulatory action of melatonin is due to maturation and proliferation of natural killer cells, B and T lymphocytes, monocytes and granulocytes in bone marrow and other tissues (Miller et al., 2006; Zhang et al., 2020).
Miscellaneous antibodies, cell-based therapies and RNA based therapies which are beingused now a days to mitigate effect of COVID-19 and are under preclinical and clinical trials are depicted in Table 7 .
Table 7.
List of various treatments currently used against COVID-19.
6. Conclusion and future perspective
The worldwide spread of COVID-19 has become a big challenge to control. It has already been declared as pandemic with more than 10 922 324 peoples affected across 195 countries till July 4th 2020. An aggressive approach is required to take care of critically compromised patients in addition to sincere efforts to stop the transmission of disease. Currently many government agencies and pharmaceutical companies are working towards development of effective medicines and vaccines. Also, the available treatment strategies have been adopted to benefit the affected people, however, major step still remains to stop the transmission and alleviate the symptoms of affected people. Use of hydroxychloroquine as well as antiviral drugs are found effective against COVID-19, however, detailed clinical studies are required. Some biotechnology-basedtechniques such as antibodies, cell and RNA based therapies have also been found to be very effective. It is expected that dexamethasone could bring some hope to treat this disease.However, it will be too early to give conclusive remarks on the currently available treatments since more evidence-based data is required to be generated. Government agencies are working on their part; however, a coordinated effort is needed globally to help prepare the healthcare framework cope up with the unprecedented challenge of COVID-19.
Declaration of competing interest
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors declare no conflict of interest.
References
- https://www.aa.com.tr/en/health/pfizer-biontech-jointly-work-on-coronavirus-vaccine/1769465 Accessed date: 20 May 2020.
- Abedi F., Rezaee R., Karimi G. Plausibility of therapeutic effects of rho kinase inhibitors against severe acute respiratory syndrome coronavirus 2 (COVID-19) Pharmacol. Res. 2020;156:104808. doi: 10.1016/j.phrs.2020.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acree W.M., Edwards B., Black J.W. 1986. Canine Corona Virus Vaccine. PMC7131519. [Google Scholar]
- https://adnas.com/coronoavirus-applied-dna-linearx-takis-biotech-vaccine/ Accessed date: 21 May 2020.
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://ir.alexion.com/news-releases/news-release-details/alexion-statement-solirisr-eculizumab-and-covid-19 Accessed date: 18 May 2020.
- An P., Song P., Lian K., Wang Y. CT manifestations of novel coronavirus pneumonia: a case report. Balkan Med. J. 2020;37:163–165. doi: 10.4274/balkanmedj.galenos.2020.2020.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.antibodies-online.com/resources/18/5410/sars-cov-2-life-cycle-stages-and-inhibition-targets Accessed date: 5 May 2020.
- Armstrong-James D., Stebbing J., Scourfield A., Smit E., Ferns B., Pillay D., Nelson M. Clinical outcome in resistant HIV-2 infection treated with raltegravir and maraviroc. Antivir. Res. 2010;86:224–226. doi: 10.1016/j.antiviral.2010.02.324. [DOI] [PubMed] [Google Scholar]
- Baras B., Callendret B., Escriou N., Lorin V., Marianneau P., van der Werf S., Wettendorff M.A.C. 2012. Vaccine. CA2704283A1. [Google Scholar]
- Basu A., Sarkar A., Maulik U. Strategies for vaccine design for corona virus using Immunoinformatics techniques. bioRxiv. 2020 doi: 10.1101/2020.02.27.967422. [DOI] [Google Scholar]
- Beniac D.R., Andonov A., Grudeski E., Booth T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006;13:751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://biobuzz.io/altimmune-becomes-the-second-maryland-based-biotech-advance-potential-coronavirus-covid-19-vaccine/ Accessed date: 20 May 2020.
- https://www.biocentury.com/article/304658 Accessed date: 13 May 2020.
- https://www.biospace.com/article/releases/innovation-pharmaceuticals-brilacidin-to-be-researched-as-possible-novel-coronavirus-covid-19-vaccine-brilacidin-now-being-tested-as-drug-and-vaccine-at-different-institutions/ Accessed date: 5 April 2020.
- https://www.biospace.com/article/barda-says-athersys-cell-therapy-highly-relevant-for-covid-19/ Accessed date: 10 March 2020.
- https://www.biospace.com/article/releases/celltex-autologous-stem-cell-case-study-published-by-gavin-publishers-demonstrates-potential-cure-for-rheumatoid-arthritis/?keywords=Autologous+Adipose-Tissue+Derived+Mesenchymal+Stem+Cells+(ADMSCs) Accessed date: 10 May 2020.
- Bleibtreu A., Bertine M., Bertin C., Houhou-Fidouh N., Visseaux B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV) Med. Maladies Infect. 2019 doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.business-standard.com/article/international/china-suspends-public-transport-in-wuhan-confirms-571-cases-of-coronavirus-120012300122_1.html Accessed date: 10 April 2020.
- https://www.businesswire.com/news/home/20200402005167/en/Vir-Alnylam-Expand-Collaboration-Advance-Investigational-RNAi Accessed date: 10 April 2020.
- https://www.businesswire.com/news/home/20200413005160/en/FDA-Authorizes-Athersys-Initiate-Pivotal-Clinical-Trial Accessed date: 14 April 2020.
- https://www.businesswire.com/news/home/20200414005353/en/NantKwest-ImmunityBio-Announce-Therapeutics-Vaccines-Combatting-COVID-19 Accessed date: 15 April 2020.
- https://www.businesswire.com/news/home/20200416005144/en/New-COVID-19-Clinical-Trial-Supported-Octapharma-USA Accessed date: 10 June 2020.
- https://www.businesswire.com/news/home/20200420005221/en/Alexion-Announces-Plans-Initiate-Phase-3-Study Accessed date: 12 April 2020.
- Cameron C.E., Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr. Opin. Infect. Dis. 2001;14:757–764. doi: 10.1097/00001432-200112000-00015. [DOI] [PubMed] [Google Scholar]
- Chang C.-k., Sue S.-C., Yu T.-h., Hsieh C.-M., Tsai C.-K., Chiang Y.-C., Lee S.-j., Hsiao H.-h., Wu W.-J., Chang W.-L. Modular organization of SARS coronavirus nucleocapsid protein. J. Biomed. Sci. 2006;13:59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Lu X.-H., Yan S., Chai H., Yao Q. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem. Biophys. Res. Commun. 2005;335:874–882. doi: 10.1016/j.bbrc.2005.07.155. [DOI] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., Kao R., Poon L., Wong C., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramella G., Himansu S. 2017. Respiratory Virus Vaccines. WO2017070626 (2017) [Google Scholar]
- Cinatl J., Jr., Cinatl J., Rabenau H., Gümbel H., Kornhuber B., Doerr H. In vitro inhibition of human cytomegalovirus replication by desferrioxamine. Antivir. Res. 1994;25:73–77. doi: 10.1016/0166-3542(94)90095-7. [DOI] [PubMed] [Google Scholar]
- https://www.clinicaltrialsarena.com/news/ubc-apeiron-biologics-covid-19-trial/ Accessed date: 10 May 2020.
- https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/ Accessed date: 10 May 2020.
- https://www.cytodyn.com/newsroom/press-releases/detail/392/cytodyn-files-ind-and-protocol-for-phase-2-clinical-trial Accessed date: 10 May 2020.
- da Silva J.A.T. Convalescent plasma: a possible treatment of COVID-19 in India. Med. J. Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M.L., Álvarez E., Almazán F., Rejas M.T., Lamirande E., Roberts A., Shieh W.-J., Zaki S.R., Subbarao K., Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81:1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Zhu C., Yao W. A cured patient with 2019-nCoV pneumonia. Am. J. Med. 2020 doi: 10.1016/j.amjmed.2020.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries & Therapeutics. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- https://www.eusapharma.com/news/eusa-pharma-and-the-papagiovanni-xxiii-hospital/ Accessed date: 13 March 2020.
- Falzarano D., De Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. 10.1038%2Fsrep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment Accessed date: 13 March 2020.
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-pandemic Accessed date: 15 April 2020.
- https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma Accessed date: 10 March 2020.
- Fedson D.S., Opal S.M., Rordam O.M. Hiding in plain sight: an approach to treating patients with severe covid-19 infection. mBio. 2020;11 doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: an Overview of Their Replication and Pathogenesis, Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.fiercebiotech.com/biotech/gigagen-jumps-into-covid-19-arena-polyclonal-antibodies Accessed date: 10 June 2020.
- https://www.fiercebiotech.com/research/melatonin-stem-cells-researchers-step-up-unconventional-approaches-to-covid-19?mkt_tok=eyJpIjoiTlRKak1UZzROV1l5TWpnMSIsInQiOiJ6K0xXSlwvcFZhcmpWenhOS1pxOExUTnlsdzlPM080bVRiQ2phcG1mQmowQWQ2S24yZXZ2alBqVHNJaVUzZGRNQjM5aFQySkg4cEFvdUZrQ0lBWmluTkl1a3NVK3dFUEFRTTUwSkhEMnIwTHR1QUt4VEs4ZGsrc1pZZWF6bWdNTEZ2YXRsc2RlT0haMGV6VWE1bkNVUHB3PT0ifQ%3D%3D&mrkid=72869502 Accessed date: 10 April 2020.
- https://www.fool.com/investing/2020/03/12/biogen-and-vir-biotechnology-to-collaborate-to-fin.aspx Accessed date: 10 May 2020.
- Gao Y., Hou R., Fei Q., Fang L., Han Y., Cai R., Peng C., Qi Y. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep38736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1101/2020.03.16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- file:///C:/Users/hp/Downloads/Tamiflu_GL-019936.pdf Accessed date: 18 May 2020.
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst K.R., Koetzner C.A., Masters P.S. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J. Virol. 2009;83:7221–7234. doi: 10.1128/JVI.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.investindia.gov.in/team-india-blogs/truenat-beta-test-covid-19-detection-india Accessed date: 10 April 2020.
- https://investors.vir.bio/news-releases/news-release-details/gsk-and-vir-biotechnology-enter-collaboration-find-coronavirus Accessed date: 19 June 2020.
- http://www.irdirect.net/prviewer/release/id/4280782 Accessed date: 15 May 2020.
- https://www.itnonline.com/content/ct-provides-best-diagnosis-novel-coronavirus-covid-19 Accessed date: 18 May 2020.
- J Jielun Zhu A.D. Cision; 2020. I-mab Biopharma Receives IND Approval from NMPA to InitiateClinical Trials for its Anti-GM-CSF Monoclonal Antibody TJM2 in China.https://www.prnewswire.com/news-releases/i-mab-biopharma-receives-ind-approval-fromnmpa-to-initiate-clinical-trials-for-its-anti-gm-csf-monoclonal-antibody-tjm2-in-china-300958517.html (Accessed date: April 26, 2020) [Google Scholar]
- Jawerth N. How is the COVID-19 virus detected using real time RT-PCR? 2020. https://www.iaea.org/newscenter/news/how-is-the-covid-19-virus-detected-using-real-timert-pcr (Accessed April 23, 2020)
- Jiang H.-Y., Zhang M.-N., Chen H.-J., Yang Y., Deng M., Ruan B. Nevirapine versus efavirenz for patients co-infected with HIV and tuberculosis: a systematic review and meta-analysis. Int. J. Infect. Dis. 2014;25:130–135. doi: 10.1016/j.ijid.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Justice A.C., Stein D.S., Fusco G.P., Sherrill B.H., Fusco J.S., Danehower S.C., Becker S.L., Hansen N.I., Graham N.M. Disease progression in HIV-infected patients treated with stavudine vs. zidovudine. J. Clin. Epidemiol. 2004;57:89–97. doi: 10.1016/S0895-4356(03)00245-2. [DOI] [PubMed] [Google Scholar]
- Kosiboro M. Dapagliflozin in respiratory failure in patients with COVID-19 (DARE-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04350593?cond=COVID- 19&draw=2&rank=16 (Accessed date: April 17, 2020)
- Kumar S. 2020. Drug and Vaccine Design against Novel Coronavirus (2019-nCoV) Spike Protein through Computational Approach. [DOI] [Google Scholar]
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582 doi: 10.1038/d41586-020-01824-5. 469-469. [DOI] [PubMed] [Google Scholar]
- Lee J. Better understanding on MERS corona virus outbreak in Korea. J. Kor. Med. Sci. 2015;30:835–836. doi: 10.3346/jkms.2015.30.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annual review of virology. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Nature Publishing Group; 2020. Therapeutic Options for the 2019 Novel Coronavirus (2019-nCoV) [DOI] [PubMed] [Google Scholar]
- Li S.-y., Chen C., Zhang H.-q., Guo H.-y., Wang H., Wang L., Zhang X., Hua S.-n., Yu J., Xiao P.-g. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhu X., Ji X., Quanquin N., Deng Y.-Q., Tian M., Aliyari R., Zuo X., Yuan L., Afridi S.K. Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine. 2017;24:189–194. doi: 10.1016/j.ebiom.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral natural products and herbal medicines. Journal of traditional and complementary medicine. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.marketwatch.com/story/uk-biotech-tiziana-life-sciences-says-its-tzls-501-may-be-of-use-in-treating-coronavirus-2020-03-11 Accessed date: 10 May 2020.
- https://www.medicago.com/en/pipeline/ Accessed date: 15 April 2020.
- http://www.migal.org.il/Migal.covid Accessed date: 07 March 2020.
- Millán-Oñate J., Millan W., Mendoza L.A., Sánchez C.G., Fernandez-Suarez H., Bonilla-Aldana D.K., Rodríguez-Morales A.J. Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann. Clin. Microbiol. Antimicrob. 2020;19:1–9. doi: 10.1186/s12941-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.C., Pandi P.S., Esquifino A.I., Cardinali D.P., Maestroni G.J. The role of melatonin in immuno‐enhancement: potential application in cancer. Int. J. Exp. Pathol. 2006;87:81–87. doi: 10.1111/j.0959-9673.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S., Arts R.J., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- Nal B., Chan C., Kien F., Siu L., Tse J., Chu K., Kam J., Staropoli I., Crescenzo-Chaigne B., Escriou N. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J. Gen. Virol. 2005;86:1423–1434. doi: 10.1099/vir.0.80671-0. [DOI] [PubMed] [Google Scholar]
- Nassar M., Bakhrebah M., Meo S., Alsuabeyl M., Zaher W. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4956–4961. doi: 10.26355/eurrev_201808_15635. [DOI] [PubMed] [Google Scholar]
- https://www.ncbi.nlm.nih.gov/pubmed/32037389 Accessed date: 20 April 2020.
- https://clinicaltrials.gov/ct2/show/NCT03301090?term=NCT03301090#wrapper Accessed date: 25 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04273529 Accessed date: 20 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04275414 Accessed date: 20 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04280588 Accessed date: 20 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04282902 Accessed date: 20 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04327206?term=BCG+vaccine&cond=COVID-19&draw=1&rank=2 Accessed date: 20 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04328441?term=BCG+vaccine&cond=COVID-19&draw=2&rank=1 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04330586?cond=COVID-19&draw=2&rank=42 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04336904?cond=COVID-19&draw=2&rank=2 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04338347?type=Expn&cond=COVID-19&draw=2 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04338906 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04340232?cond=COVID-19&draw=2&rank=32 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04343755?cond=COVID-19&draw=2&rank=19 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04347915?cond=COVID-19&draw=2&rank=6 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04348071?cond=COVID-19&draw=2&rank=31https://clinicaltrials.gov/ct2/show/NCT04348071?cond=COVID-19&draw=2&rank=31 Accessed date: 21 May 2020.
- https://clinicaltrials.gov/ct2/show/NCT04348370?cond=COVID-19&draw=4&rank=614 Accessed date: 21 May 2020.
- Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Zhou L., Zhou M., Zhao J., Wang D.W. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front. Med. 2020:1–5. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Castano-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://ir.novavax.com/news-releases/news-release-details/novavax-advances-development-novel-covid-19-vaccine Accessed date: 10 April 2020.
- https://www.npr.org/sections/health-shots/2020/02/19/807338329/hunt-for-new-coronavirus-treatments-includes-gene-silencing-and-monoclonal-antib Accessed date: 10 April 2020.
- Oh M.-d. The Korean Middle East Respiratory Syndrome Coronavirus outbreak and our responsibility to the global scientific community. Infection & chemotherapy. 2016;48:145–146. doi: 10.3947/ic.2016.48.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Okuno Y., Hashimoto S., Kita Y., Kanamaru N., Nishida Y., Tsunai Y., Inoue R., Nakatani H., Fukamizu R. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine. 2007;25:3038–3040. doi: 10.1016/j.vaccine.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.-M., Cheng V.C.-C., Chan K., Hung I., Poon L.L., Law K.-I., Tang B., Hon T., Chan C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. 2020. Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia. (Accessed March 10, 2020) [Google Scholar]
- Perrone F. Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID-19) 2020. https://clinicaltrials.gov/ct2/show/NCT04317092 (Accessed June 28, 2020)
- https://www.pharmaceutical-technology.com/news/bioxytran-coronavirus-patients-treatment/ Accessed date: 11 April 2020.
- https://www.pharmaceutical-technology.com/news/can-fite-lattice-biologics-covid-19-treatments/ Accessed date: 30 April 2020.
- https://www.pharmaceutical-technology.com/news/covid-19-aj-vaccines-algernon-drug/ Accessed date: 20 April 2020.
- https://www.pharmaceutical-technology.com/news/roche-actemra-coronavirus-complications/ Accessed date: 18 June 2020.
- https://www.pharmaceutical-technology.com/news/tonix-pharmaceuticals-covid-19-vaccine/ Accessed date: 20 May 2020.
- https://pink.pharmaintelligence.informa.com/PS141926/US-FDA-To-Exercise-Maximum-Regulatory-Flexibility-For-COVID19-PlasmaDerived-Therapeutics Accessed date: 20 May 2020.
- http://www.pharmatimes.com/news/gsk_recruits_clover_biopharmaceuticals_in_latest_coronavirus_effort_1326917 Accessed date: 10 March 2020.
- https://phrma.org/coronavirus Accessed date: 13 April 2020.
- https://pipelinereview.com/index.php/2020020273689/Vaccines/Vaxart-Announces-Initiation-of-Coronavirus-Vaccine-Program.htm Accessed date: 20 May 2020.
- https://www.prnewswire.co.uk/news-releases/airway-therapeutics-announces-filing-with-nih-to-evaluate-at-100-as-a-therapy-for-novel-coronavirus-821624689.html Accessed date: 20 April 2020.
- https://www.prnewswire.com/news-releases/celularity-announces-fda-clearance-of-ind-application-for-cynk-001-in-coronavirus-first-in-cellular-therapy-301034141.html Accessed date: 20 April 2020.
- Reiter R.J., Ma Q., Sharma R. Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Research. 2020;3:43–57. [Google Scholar]
- https://www.reuters.com/article/health-coronavirus-novartis/novartis-ceo-malaria-drug-is-biggest-hope-against-coronavirus-sonntagszeitung-idUSL8N2BM02W Accessed date: 10 June 2020.
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395:30. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P.J., Corbellino M., Stebbing J. Baricitinib for COVID-19: a suitable treatment?–Authors' reply. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. 2020. Coronavirus: US Volunteers Test First Vaccine. [DOI] [Google Scholar]
- https://roivant.com/roivant-announces-development-of-anti-gm-csf-monoclonal-antibody-to-prevent-and-treat-acute-respiratory-distress-syndrome-ards-in-patients-with-covid-19/ Accessed date: 18 May 2020.
- Rojas M., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Ansari A.A., Gershwin M.E., Anaya J.-M. Ebola virus disease: an emerging and re-emerging viral threat. J. Autoimmun. 2019:102375. doi: 10.1016/j.jaut.2019.102375. [DOI] [PubMed] [Google Scholar]
- Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Públic. 2020;44 doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacological research. 2020 Mar 20 doi: 10.1016/j.phrs.2020.104761. 104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://sabbiotherapeutics.com/2020/04/13/hhs-facilitatesdevelopment-ofimmunotherapies-for-covid-19-patients/ Accessed date: 10 April 2020.
- Salathe M. Study of open label losartan in COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04335123?cond=COVID-19&draw=2&rank=15 (Accessed April 17, 2020)
- https://www.sciencedaily.com/releases/2020/03/200317150116.htm Accessed date: 20 April 2020.
- https://seekingalpha.com/article/4331827-biocryst-pharmaceuticals-multiple-value-drivers-coronavirus-kicker Accessed date: 19 April 2020.
- Shakiba D.Y. Application of desferal to treat COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04333550 (Accessed April 17, 2020)
- Sharquie I. BCG is a good immunotherapeutic agent for viral and autoimmune diseases: is it a new weapon against coronavirus (COVID-19)? Electronic journal of general medicine. 2020;17:2516–3507. 2020. [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes & Metabolic Syndrome: Clin. Res. Rev. 2020:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smego R., Ahmed N. A systematic review of the adjunctive use of systemic corticosteroids for pulmonary tuberculosis. Int. J. Tubercul. Lung Dis. 2003;7:208–213. [PubMed] [Google Scholar]
- Smith G., Liu Y., Massare M. 2015. Immunogenic Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Compositions and Methods. WO2015042373A1. [Google Scholar]
- https://spectrumlocalnews.com/nys/rochester/coronavirus/2020/03/12/oyagen-lab-discovers-compound-that-they-believe-could-help-with-the-covid-19- Accessed date: 20 April 2020.
- https://www.startus-insights.com/innovators-guide/5-top-diagnostic-test-kits-to-use-during-the-coronavirus-pandemic/ Accessed date: 25 April 2020.
- https://www.statnews.com/2020/03/16/remdesivir-surges-ahead-against-coronavirus/ Accessed date: 10 May 2020.
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramasso L., Madeddu G., Ricci E., De Socio G., Menzaghi B., Orofino G., Passerini S., Franzetti M., Maggi P., Dentone C. Raltegravir-based therapy in a cohort of HIV/HCV co-infected individuals. Biomed. Pharmacother. 2015;69:233–236. doi: 10.1016/j.biopha.2014.12.006. [DOI] [PubMed] [Google Scholar]
- https://theindiantelegraph.com.au/israel-has-found-a-possible-100-cure-for-coronavirus/ Accessed date: 25 April 2020.
- https://www.thepharmaletter.com/article/india-s-zydus-joins-fight-against-novel-coronavirus Accessed date: 10 April 2020.
- https://www.thestreet.com/markets/mergers-and-acquisitions/beyondspring-looks-to-future-after-ipo-pipe-launch-14308176 Accessed date: 13 April 2020.
- theweek.in/news/india/2020/04/19/gujarat-to-start-clinical-trial-with-convalescent-plasma-therapy-for-covid-19.html Accessed date: 10 June 2020.
- Tyrrell D.A., Myint Steven H. fourth ed. University of Texas Medical Branch at Galveston; 1996. Coronaviruses, Medical Microbiology; pp. 10–15. [PubMed] [Google Scholar]
- https://docs.google.com/viewer?url=patentimages.storage.googleapis.com/pdfs/US20060039926.pdf Accessed date: 20 June 2020.
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.who.int/biologicals/areas/vaccines/bcg/en Accessed date: 18 May 2020.
- Wnuk A. Enfuvirtide–new clinical data of the management of HIV-infected patients. HIV & AIDS Review. 2008;7:10–16. [Google Scholar]
- https://www.wsj.com/articles/drugmaker-takeda-is-working-on-coronavirus-drug-11583301660?mod=article_inline Accessed date: 12 April 2020.
- https://www.wsj.com/articles/gilead-sciences-offers-experimental-drug-for-coronavirus-treatments-testing-11580511519 Accessed date: 19 June 2020.
- Wu T.-C.U., Hung Chien-Fu, Us K.I.M., Tae Woo K.R. 2005. A VACCINES TARGETING ANTIGENS OF THE SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS (SARS-COV) WO/2005/081716 (2005) [Google Scholar]
- Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]