Abstract
We treated two patients with COVID-19 pneumonia requiring mechanical ventilation. Case 1 was a 73-year-old Japanese man. Computed tomography (CT) revealed ground-glass opacities in both lungs. He had severe respiratory failure with a partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen ratio (P/F ratio) of 203. Electrocardiogram showed a heart rate (HR) of 56 beats/min, slight ST depression in leads II, III, and aVF, and mild saddle-back type ST elevation in leads V1 and V2. High-sensitivity cardiac troponin T (cTnT) level was slightly elevated. Despite a high fever and hypoxemia, his HR remained within 50–70 beats/min. Case 2 was a 52-year-old Japanese woman. CT revealed ground-glass opacities in the lower left lung. Electrocardiogram showed a HR of only 81 beats/min, despite a body temperature of 39.2 °C, slight ST depression in leads V4, V5, V6, and a prominent U wave in multiple leads. She had an elevated cTnT and a P/F ratio of 165. Despite a high fever and hypoxemia, her HR remained within 50–70 beats/min. Both patients had a poor compensatory increase in their HR, despite their critical status. Relative bradycardia could be a cardiovascular complication and is an important clinical finding in patients with COVID-19.
<Learning objective: We report two Japanese cases of COVID-19 pneumonia with relative bradycardia as a condition and no significant compensatory increase in heart rate despite high fever and severe hypoxemia. Relative bradycardia in COVID-19 might be associated with myocardial injury due to not only direct viral involvement but also systemic inflammation. We should carefully observe the occurrence of relative bradycardia because it could potentially be a clinical sign of COVID-19.>
Keywords: Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome, Coronavirus 2 (SARS-CoV-2), Relative bradycardia, Myocardial injury
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China in December 2019 and has spread worldwide, presenting a serious ongoing problem [1], [2]. Recent reports have presented that COVID-19 is related to direct myocardial injury, and patients have a risk of cardiovascular complications including myocardial infarction, myocarditis, heart failure, cardiogenic shock, and cardiac arrythmias [3], [4], [5]. Relative bradycardia is sinus bradycardia as a phenomenon of dissociation between heart rate and body temperature, insufficient increase in pulse despite high fever, and it is a characteristic feature of some particular infections [6], [7]. However, relative bradycardia associated with COVID-19 has not been reported previously. Herein, we report two Japanese cases of COVID-19 with severe pneumonia who demonstrated relative bradycardia despite the severity of their condition and requiring respiratory management. With increasing illness severity, neither patient showed a compensatory increase in heart rate (HR), even with increased body temperature and worsening hypoxemia. In addition, ST depression, saddle-back type ST elevation, and prominent U waves in the electrocardiogram and the elevation of cardiac troponin level were observed. Relative bradycardia may be an important clinical feature and could be a sign of potential myocardial injury in patients with COVID-19.
Case report
Case 1
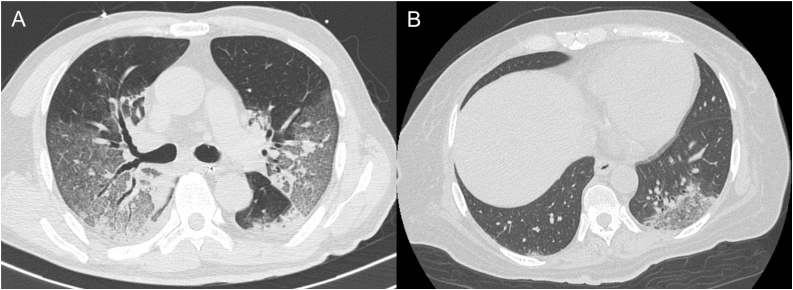
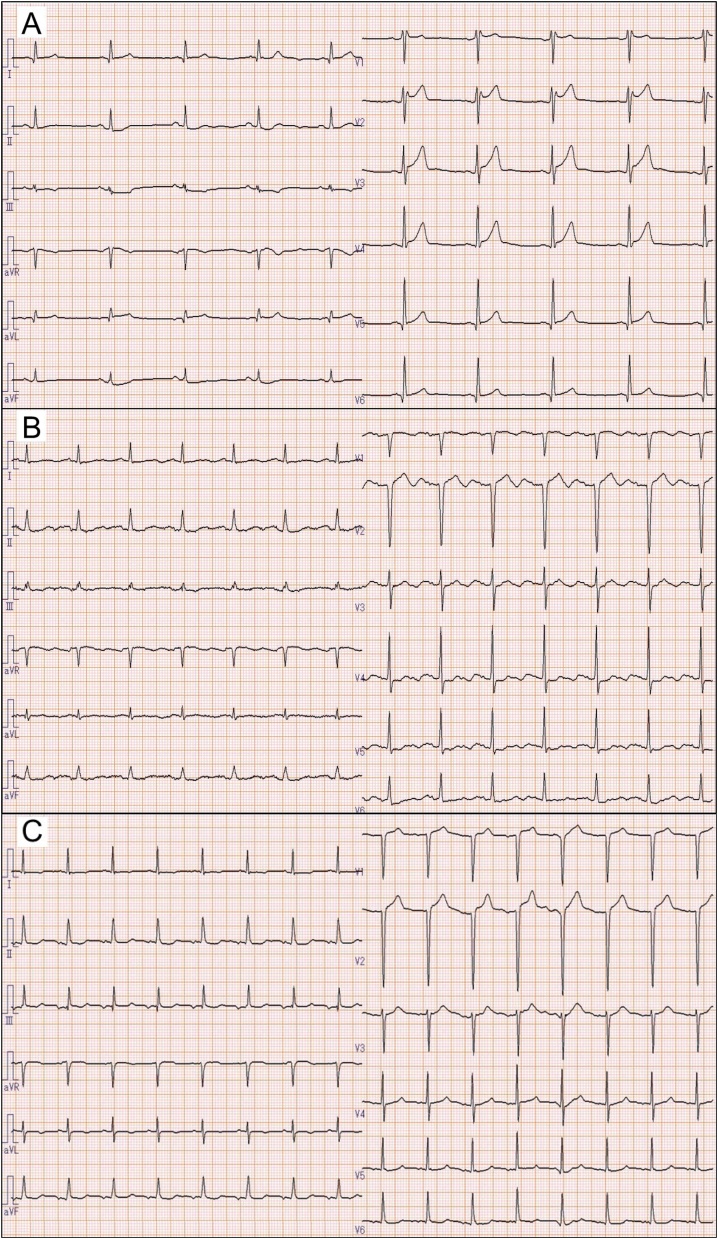
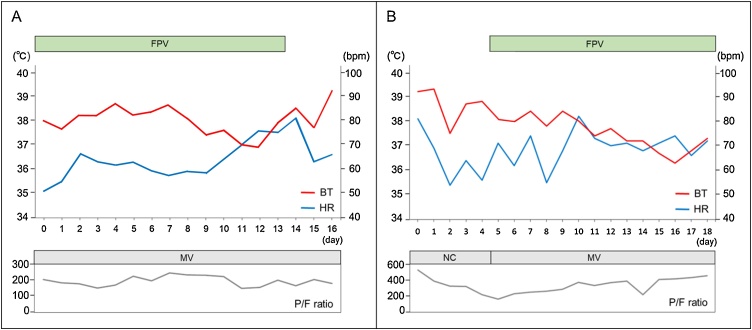
A 73-year-old Japanese man with hypertension and type 2 diabetes mellitus was diagnosed with COVID-19 and transferred to our hospital on mechanical ventilation. He was taking a calcium channel blocker, not an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. His chest computed tomography (CT) revealed ground-glass opacities in both lungs (Fig. 1A). However, his partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen ratio (P/F ratio) was 203 on admission, despite administration of favipiravir. Next day, his electrocardiogram (ECG) showed a HR of 56 beats/min with sinus rhythm, slight ST depression in leads II, III, and aVF, and mild saddle-back type ST elevation in leads V1 and V2 (Fig. 2A). His high-sensitivity cardiac troponin T (cTnT) level was slightly elevated (0.078 ng/mL, normal range: <0.014 ng/mL). The high-sensitivity C-reactive protein level (hs-CRP) was increased to a peak of 23.24 mg/dL on Day 7 (normal range: <0.14 mg/dL). The echocardiogram revealed good left ventricular systolic function, without asynergy through the clinical course. Despite a high fever (>38 °C) and hypoxemia, his HR remained within 50–70 beats/min with sinus rhythm and was not increasing (Fig. 3A). On admission, his blood pressure was 126/55 mmHg using noradrenaline 0.12 μg/kg/min. The dose of noradrenaline was gradually decreased and noradrenaline was stopped on Day 4. Thereafter, his systolic blood pressure remained at 110–140 mmHg. He was sedated with intravenous midazolam (2.5–10.0 mg/h) until Day 17 when a tracheotomy was performed. He was discharged and transferred for rehabilitation on Day 37. After recovery his HR was 99 beats/min with sinus rhythm at a body temperature 36.5 °C and percutaneous oxygen saturation (SpO2) 99% breathing room air. His blood pressure on discharge was 133/76 mmHg.
Fig. 1.
Chest computed tomography images of Case 1 (A) and Case 2 (B).
Fig. 2.
Electrocardiograms of Case 1 (A) and Case 2 (B) on admission. Electrocardiogram of Case 2 on Day 31 (C).
Fig. 3.
The time course of heart rate, fever, oxygenation, treatment in Case 1 (A) and Case 2 (B).
bpm, beats per minute; BT, body temperature; FPV, favipiravir; HR, heart rate; MV, mechanical ventilation; NC, nasal cannula; P/F ratio, partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen ratio.
Case 2
A 52-year-old Japanese woman with chronic renal failure due to diabetic nephropathy was diagnosed with COVID-19. Her chest CT revealed ground-glass opacities in the lower left lung (Fig. 1B). On admission, her ECG showed a HR of only 81 beats/min with sinus rhythm, despite a body temperature of 39.2 °C, and slight ST depression in leads V4, V5, V6 and a prominent U wave in multiple leads (Fig. 2B). Her cTnT level was elevated (0.207 ng/mL). Her blood pressure was 170/65 mmHg on admission and catecholamines were not administered. Her P/F ratio decreased to 165, so mechanical ventilation support and favipiravir were initiated on Day 5. The hs-CRP level peaked at 22.32 mg/dL on Day 9. Echocardiography showed preserved left ventricular contraction without wall motion abnormality. Her HR remained 50–70 beats/min with sinus rhythm, despite a body temperature >38 °C, and poor oxygenation. Her HR increased as her fever decreased and oxygenation improved (Fig. 3B). Her blood pressure gradually stabilized and her systolic blood pressure remained 120–140 mmHg. She was sedated using intravenous midazolam (1.25–5.0 mg/h) from Day 5 to Day 32. The electrocardiogram on Day 31 showed a HR of 93 beats/min with sinus rhythm and no significant ST segment changes and no U wave (Fig. 2C). She had a tracheotomy on Day 32 and was discharged on Day 60. Her HR after recovery was 72 beats/min with sinus rhythm at a body temperature of 36.8 °C and SpO2 of 100% breathing room air. Her blood pressure on discharge was 139/76 mmHg.
Discussion
Relative bradycardia is a condition with no significant compensatory increase in HR despite high fever, and may occur in specific infectious diseases including Legionnaire's disease, Chlamydial pneumonia, typhoid fever, and Ebola hemorrhagic fever [6]. Although detailed mechanisms of relative bradycardia remain unknown, direct pathogenic effects on the heart muscle, the release of inflammatory cytokines (granulocyte colony-stimulating factor, interleukin-6, tumor necrosis factor-α), or systemic autonomic dysregulation have been described [7].
A report described the clinical features of 138 patients with COVID-19 from China [1]. The 36 patients admitted to the intensive care unit had a median HR of 89 beats/min; 22% were diagnosed with acute cardiac damage; and 44% had arrhythmias. However, the changes in HR during the clinical course of their illness, and the types of arrhythmia were not reported. Patients with COVID-19 may experience acute myocardial injury due to direct involvement by SARS-CoV-2 of endothelial cells, intramyocardial smooth muscle cells, and cardiac myocytes via angiotensin-converting enzyme 2 receptor, additionally due to hypoxemia, systemic inflammation, or abnormal immune response [3], [4], [5]. He et al. reported a COVID-19 patient who presented ECG changes including temporary atrioventricular (AV) block [8]. Kir et al. [9] reported a case of COVID-19 with intermittent AV block, despite normal cardiac biomarkers and echocardiography. Peigh et al. [10] reported two cases of new-onset and persisting sinus node dysfunction associated with COVID-19 and the potential mechanisms for sinus node dysfunction include myocardial inflammation or direct viral infiltration.
Our patients had relative sinus bradycardia and did not have a compensatory increase in HR despite severe pneumonia, high fever, and hypoxemia. Patients were sedated properly and no inotropic agents or beta-blockers were used. Therefore, our patients satisfied the definition of relative bradycardia and the bradycardia was not drug-induced. The ST depression in both patients and saddle-back type ST elevation in Case 1 and prominent U wave in Case 2 on ECG and elevated cTnT, suggested possible myocardial damage, although their echocardiographic findings were normal. The myocardial injury may have been localized and reversible because: (1) SR was maintained and no conduction disturbances were observed; and (2) Case 2’s HR, ST level, U wave, and cTnT level were normalized after her general condition improved (Fig. 2C). On the other hand, a very high level of hs-CRP indicated severe systemic inflammation in both patients. Therefore, relative bradycardia might be associated with not only sinoatrial node dysfunction due to direct viral involvement but also myocardial injury due to inflammatory cytokines. In addition, we speculate that relative bradycardia in COVID-19 could be related to its severity. Furthermore, disproportionately mild dyspnea has also been reported in patients with COVID-19 even when they are in a severely hypoxic state. This phenomenon might exacerbate hypoxemia in addition to relative bradycardia. Although the detailed mechanisms of relative bradycardia with COVID-19 remain unclear and may be multifactorial, relative bradycardia could be one of the cardiovascular complications and an important clinical feature of COVID-19.
The clinical implications of relative bradycardia in COVID-19 especially for cardiologists or emergency and intensive care physicians are: (1) it may be useful as a clue for suspecting COVID-19, because the differentiation between COVID-19 pneumonia and acute pulmonary edema is often difficult from CT findings; and (2) it may result in a low cardiac output and exacerbation of systemic oxygen deficiency, especially in patients with acute heart failure. Therefore, it is necessary to pay attention to the occurrence of relative bradycardia. Further studies and more systematic data collection will be needed to explore our hypothesis in a larger population of patients with COVID-19 of various severity. This could lead to a more informed assessment of the incidence of relative bradycardia as a potential clinical sign of COVID-19 also with treatment implications.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This report has not been presented elsewhere. We wish to thank all the co-medical staff involved in the treatment.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 4.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha B.A. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6:633–634. doi: 10.1046/j.1469-0691.2000.0194f.x. [DOI] [PubMed] [Google Scholar]
- 7.Ye F., Hatahet M., Youniss M.A., Toklu H.Z., Mazza J.J., Yale S. The clinical significance of relative bradycardia. WMJ. 2018;117:73–78. [PubMed] [Google Scholar]
- 8.He J., Wu B., Chen Y., Tang J., Liu Q., Zhou S. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020;36 doi: 10.1016/j.cjca.2020.03.028. 966.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kir D., Mohan C., Sancassani R. HEART BRAKE – an unusual cardiac manifestation of coronavirus disease 2019 (COVID-19) JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peigh G., Leya M.V., Baman J.R., Cantey E.P., Knight B.P., Flaherty J.D. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep. 2020 doi: 10.1093/ehjcr/ytaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]