Abstract
We experienced a 72-year-old man who developed laboratory-confirmed human coronavirus HKU1 pneumonia. PCR testing for SARS-CoV-2 from a nasopharyngeal specimen was negative twice, and rapid immunochromatographic antibody test (RIAT) using a commercially available kit for IgM and IgG against SARS-CoV-2 showed him turning positive for IgG against SARS-CoV-2. We then performed RIAT in stored serum samples from other patients who suffered laboratory-confirmed human common cold coronaviruses (n = 6) and viruses other than coronavirus (influenza virus, n = 3; rhinovirus, n = 3; metapneumovirus, n = 1; adenovirus, n = 1) admitted until January 2019. Including the present case, four of 7 (57%) showed false-positive RIAT results due to human common cold coronaviruses infection. Two of the 4 patients showed initial negative to subsequent positive RIAT results, indicating seroconversion. RIAT was positive for IgG and IgM in viruses other than coronavirus in 2 (25.0%) and 1 (12.5%) patient. Because of high incidence of false positive RIAT results, cross antigenicity between human common cold coronaviruses and SARS-CoV-2 can be considered. Results of RIAT should be interpreted in light of epidemics of human common cold coronaviruses infection. Prevalence of past SARS-CoV-2 infection may be overestimated due to high incidence of false-positive RIAT results.
Keywords: Severe acute respiratory syndrome coronavirus 2, Coronavirus disease 2019, Rapid immunochromatographic antibody testing, Immunity passport, Prevalence
Abbreviations: Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2; Coronavirus disease 2019, COVID-19; Rapid immunochromatographic antibody testing, RIAT
1. Introduction
Novel coronavirus SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) is causing a worldwide pandemic of coronavirus disease 2019 (COVID-19) that has resulted in many deaths. Some governments are suggesting an ‘immunity passport’ or ‘risk-free certificate’ based on SARS-CoV-2 antibody detection that could allow people to travel or go to work under the assumption that they cannot be re-infected; however, there is scant evidence on antibody-mediated immunity to SARS-CoV-2 to validate such an ‘immunity passport’. Further, epidemiological surveillance by serological approaches are also have been started. However, scientists are afraid of the possibility of false-positive results due to cross-reaction of antibodies between SARS-CoV-2 and human common cold coronaviruses [1]. World Health Organization suggests that rapid immunochromatographic antibody testing (RIAT) must also accurately discriminate between past SARS-CoV-2 infections and infection from human common cold coronaviruses [2]. However, no studies have clarified whether human common cold coronaviruses infections cause false-positive results of RIAT for SARS-CoV-2.
2. Case report
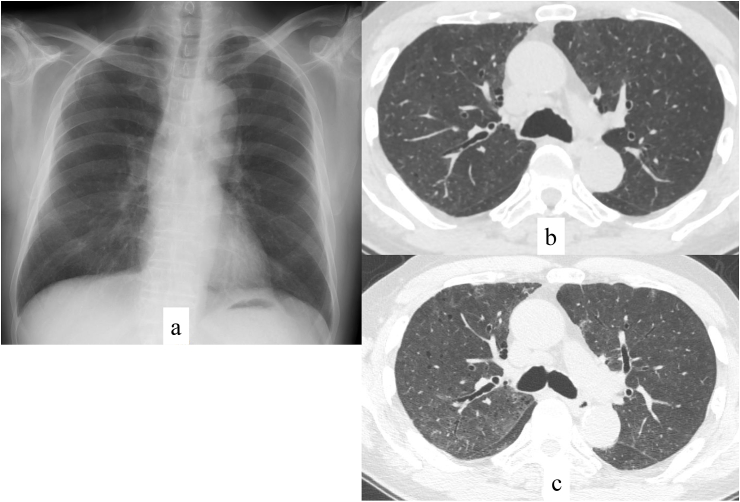
A 72-year-old man who had undergone aortic stenting for abdominal aneurysm at 70 years of age developed dyspnea and low-grade fever in March 2020 (day 1). He developed cough and fever >38 °C from day 7. He presented to our hospital on day 12 for regular follow up by his cardiologist, and chest X-ray (Fig. 1a) and computed tomography (CT) scan (Fig. 1b) showed bilateral ground-glass opacities, for which he was referred to a pulmonary physician. He had a history of smoking 20 cigarettes per day from 20 to 70 years old. He had had no contact with sick people or a recent travel history.
Fig. 1.
Chest imaging. Chest X-ray on admission showed bilateral ground-glass opacities (a). Chest computed tomography (CT) on admission showed mild and bilateral ground-glass opacities (b). There were no pleural effusions or significant lymphadenopathy. Chest CT performed on HD 5 showed worsening of ground-glass opacities and consolidation (c).
His vital signs included a blood pressure of 116/53 mmHg, heart rate of 73 bpm, respiratory rate of 21/min, and SpO2 under ambient air of 93%. He was afebrile because he had taken an antipyretic. Chest auscultation revealed no murmurs or bilateral fine crackles. Blood gas analysis under ambient air showed pH of 7.44, PaCO2 of 33.3 Torr, PaO2 of 84.9 Torr, and bicarbonate of 22.1 mmol/L. Laboratory testing showed white blood cell count of 8000/mm3 (neutrophils, 64.4%; lymphocytes, 16.4%; monocytes, 8.4%; eosinophils, 8.3%; basophils, 0.5%), hemoglobin of 12.1 g/dL, platelets of 25.1 × 104/mm3, normal transaminase value (<40 IU/L), blood urea nitrogen of 15 mg/dL, creatinine of 1.12 mg/dL, C-reactive protein of 5.70 mg/dL, and procalcitonin of 0.06 ng/dL. His beta-D glucan value was <6.0 pg/mL, and KL-6 value was slightly elevated at 508 U/mL.
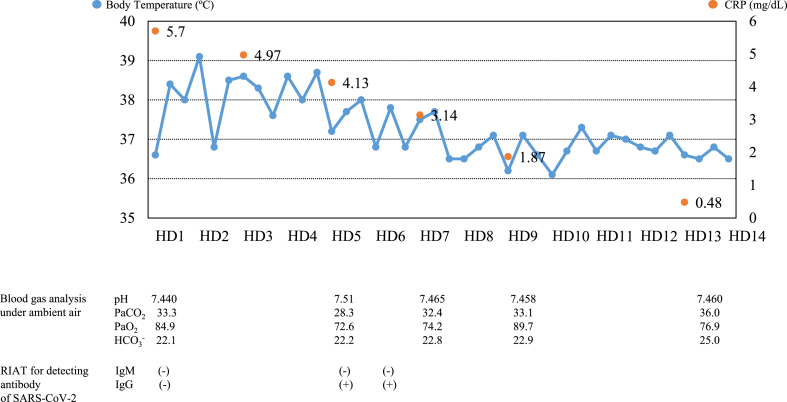
We considered COVID-19 and performed a PCR test for SARS-CoV-2, which was negative, but human coronavirus HKU1 was detected by multiplex PCR test via a commercially available kit (FTD Resp 21 Kit; Fast Track Diagnostics, Silema, Malta). Rapid test for urinary Streptococcus pneumoniae antigen, Legionella antigen, and nasopharyngeal influenza virus and Mycoplasma pneumoniae were all negative. Serum antibodies against HIV and Trichosporon asahii, which is the most frequent antigen of hypersensitivity pneumonitis in Japan, were negative He was admitted to our hospital on day 17 (hospital day [HD] 1) and was followed up without antibiotics. However, his fever continued (Fig. 2), and general fatigue increased after admission. Blood gas analysis under ambient air on HD 5 showed a PaO2 of 72.6 Torr. Chest CT performed on HD 5 showed worsening of ground-glass opacities and consolidation (Fig. 1c). We performed RIAT using a commercially available kit (RF-NC0001, RF-NC0002 with lateral flow style, KURABO Ltd., Osaka, Japan) for IgM and IgG against SARS-CoV-2, which was positive for IgG. We repeated RIAT on HD 6 and received the same result. We repeated both PCR testing for SARS-CoV-2 and multiplex PCR using nasopharyngeal swab specimens, which were negative for SARS-CoV-2 but again positive for human coronavirus HKU1. We performed RIAT using preserved frozen serum obtained on admission, which showed negative results for both IgM and IgG, indicating seroconversion. His body temperature gradually improved, and his PaO2 on HD 9 had risen to 89.7 Torr. Pulmonary shadows on CT also improved, and he was discharged on HD 14. After returning to home, his symptoms have never relapsed. Serum antibodies against influenza virus, Mycoplasma pneumoniae, Chlamydophila pneumoniae, C. psittaci, respiratory syncytial virus, adenovirus, and parainfluenza virus did not increase in the convalescent phase, and we ultimately diagnosed the patient as having primary human coronavirus HKU1 pneumonia.
Fig. 2.
Clinical course of the patient. Body temperature decreased to <37 °C on hospital day 8. C-reactive protein gradually decreased. Blood gas analysis worsened after admission and then improved. IgG antibody against SARS-CoV-2 was negative on admission but turned positive. IgM antibody against SARS-CoV-2 was negative throughout the clinical course.
RIAT, rapid immunochromatographic antibody test.
HD, hospital day.
3. Discussion
We experienced a patient suffering human coronavirus HKU1 pneumonia who showed false-positive results for IgG against SARS-CoV-2 using an RIAT.
An excellent sensitivity of RIAT for SARS-CoV-2 has been reported. We performed RIAT using a commercially available kit for IgM and IgG against SARS-CoV-2 in serum samples of 24 patients with laboratory-confirmed COVID-19 (admitted from February to April 2020), 7 patients with human common cold coronavirus pneumonia (Table 1), and 8 patients with viral pneumonia due to other than coronavirus (admitted from January 2015 to January 2019) admitted to our institution, all of whom showed fever and bilateral ground-glass opacities and consolidation on computed tomography. For RIAT in patients with human common cold coronavirus infection and non-coronavirus infection, serum samples stored at −80 °C were used. Respiratory pathogens were detected on a Rotor-Gene Q instrument (Qiagen, Hilden, Germany) with a multiplex, real-time PCR (RT-PCR) using an FTD Resp 21 Kit (Fast Track Diagnostics, Silema, Malta). RIAT was performed according to manufacturer's instructions.
Table 1.
Results of rapid immunochromatographic test for detecting SARS-CoV-2 antibody.
| Case | Onset (month year) | Age, sex | Underlying disease | Coronavirus subtype | Specimen in which virus was detected | Laboratory results |
Serum antibody |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC,/mm3 | Lym,/mm3 | CRP, mg/dL | D-dimer, μg/mL | PCT, ng/mL | IgG | Day of illness sample obtained | IgM | Day of illness sample obtained | ||||||
| 1 | Feb 2017 | 71, F | HT | OC43 | BALF | 14,300 | 500 | 14.91 | 2.04 | 0.19 | – | 23 | – | 23 |
| 2 | Oct 2015 | 49, M | None | 229E | BALF | 8900 | 1500 | 4.82 | 0.34 | N.E | – | 5 | – | 5 |
| 3 | Jun 2016 | 65, F | None | 229E | BALF | 9200 | 2400 | 0.40 | 0.76 | N.E | + | 31 | + | 31 |
| 4 | Aug 2017 | 81, M | HT | 229E | BALF | 9600 | 1400 | 17.49 | 1.88 | 0.09 | – | 8 | – | 8 |
| 5 | Oct 2017 | 76, M | DM | 229E | BALF | 4600 | 600 | 9.21 | 6.04 | 0.08 | – | 12 | – | 12 |
| + | 95 | – | ||||||||||||
| 6 | Oct 2019 | 65, M | HT, AF | 229E | Sputum | 12,000 | 1500 | 9.57 | 0.39 | 0.04 | + | 6 | – | 6 |
| 7 | Apr 2020 | 72, M | Gout | HKU1 | Nasopharyngeal swab | 8000 | 1300 | 5.70 | 3.15 | 0.06 | – | 16 | – | 16 |
| + | 22 | – | 22 | |||||||||||
| + | 23 | – | 23 | |||||||||||
M denotes male; F, female; DM, diabetes mellitus; HT, hypertension; AF, atrial fibrillation; BALF, bronchoalveolar lavage fluid; WBC, white blood cell; Lym, lymphocytes; CRP, C-reactive protein; PCT, procalcitonin; and N.E, not evaluated.
As a result, all patients with laboratory-confirmed COVID-19 showed positive RIAT results for IgG and IgM with median 12 days from illness onset. Our result that all patients with laboratory-confirmed COVID-19 showed positive RIAT results for IgG and IgM with median 12 days from illness onset was compatible with previous reports [3,4].
On the other hand, four (57%) of the 7 patients with human common cold coronavirus pneumonia showed positive IgG against SARS-CoV-2, and all but one showed negative IgM (Table 1). One patient (Case 7, the present case) repeatedly showed negative nasopharyngeal PCR results for SARS-CoV-2 but positive results for human coronavirus HKU1 PCR. Two patients showed seroconversion of IgG against SARS-CoV-2. We speculated that IgG antibody against human common cold coronavirus infection had cross-reacted to SARS-CoV-2 antibody and showed seroconversion. Because of sequence identity between human common cold coronaviruses (HKU1, OC43, NL63, 229E) and SARS-CoV-2 [5], cross antigenicity can be considered. Recent study suggests cross-reactive T-cell recognition between circulating human common cold coronaviruses and SARS-CoV-2 [6]. Another study also showed that antibodies to human common cold coronavirus are cross-reactive among coronavirus strains [7]. Three of the 7 patients showed negative RIAT results, 2 of whom were tested within 2 weeks of illness onset. Poor positive rates of IgM and IgG against SARS-CoV-2 have been reported in the early phase improved in >2 weeks [3,4]. This may have affected our RIAT test results showing negative in 3 of our 7 patients, 2 of whom were tested within 2 weeks of illness onset.
Further, 8 patients with viral pneumonia due to H1N1 influenza (n = 3), adenovirus (n = 1), rhinovirus (n = 3), and human metapneumovirus (n = 1) showed positive IgG in 2 patients (25.0%) (those with H1N1 influenza and rhinovirus) and positive IgM in the one patient with H1N1 influenza (12.5%). We considered these patients to have had a previous human common cold coronavirus infection. It has not fully known how long antibodies against human common cold coronavirus last, whereas IgG antibodies against SARS-CoV last for 3 years [8].
Cross-reactivity is potentially a limitation of immunoassays as it impacts the reliability of the test. Our results indicated that test specificity may decrease when patients with human common cold coronavirus infections are included in a control group. From this high incidence of false-positive RIAT results due to human common cold coronavirus infection found in our study, our concern is that people not infected by SARS-CoV-2 but infected by human common cold coronavirus can be falsely labelled as positive. They may then receive an ‘immunity-passport’ and be exposed to COVID-19 patients that causes subsequent spread of infection. Moreover, the prevalence of past SARS-CoV-2 infection may be overestimated when RIAT is used for epidemiological investigation because human common cold coronavirus infections are common. Results of RIAT should be interpreted in light of previous epidemics of conventional human coronavirus infection.
To date, development of commercial antibody tests is accelerating in numerous companies. Because the number of cases reported here is limited, further studies are needed to determine the accuracy, reliability, and performance of RIAT.
In conclusion, we treated a patient with human coronavirus HKU1 pneumonia who showed false-positive IgG results from a RIAT. We found a high incidence of false-positive RIAT results in patients with human common coronavirus pneumonia. Physicians and epidemiologists should consider the possibility that patients infected with conventional coronavirus can potentially be falsely labelled as being positive for SARS-CoV-2.
Ethical statement
This study was approved by ethical committee of Saitama Cardiovascular and Respiratory Center (approval no. 2019055).
Funding
A part of this study was supported by a Research Fund from Saitama Cardiovascular and Respiratory Center under Grant Nos. 16ES, 17ES, and 18ES, 19ES.
Authors’ contributions
T. I is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. S. S and N. T aggregated the data, created the figures, and helped draft the discussion of the manuscript. Y. K and Y S performed PCR. M K and T N performed RIAT.
Declaration of competing interest
The authors report no potential conflicts of interest exist with any companies/organisations whose products or services may be discussed in this letter.
Contributor Information
Shun Shibata, Email: lyonparkmarines@yahoo.co.jp.
Takashi Ishiguro, Email: ishiguro.takashi@pref.saitama.lg.jp.
Yasuhito Kobayashi, Email: kobayashiyasuhito@yahoo.co.jp.
Mayumi Koike, Email: koike.mayumi@pref.saitama.lg.jp.
Tsuyoshi Numano, Email: numano.tsuyoshi@pref.saitama.lg.jp.
Yoshihiko Shimizu, Email: shimizu.yoshihiko@pref.saitama.lg.jp.
Noboru Takayanagi, Email: takayanagi.noboru@pref.saitama.lg.jp.
References
- 1.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395(10230):1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva, Switzerland: April 2020. “Immunity Passports'’ in the Context of COVID-19.https://www.who.int/news-room/commentaries/detail/immunity-passports-in-the-context-of-covid-19 Date last accessed: April 30 2020. [Google Scholar]
- 3.Liu Y., Liu Y., Diao B. 2020. Diagnostic Indexes of a Rapid IgG/IgM Combined Antibody Test for SARS-CoV-2. medRxiv. [DOI] [Google Scholar]
- 4.Jin Y., Wang M., Zuo Z. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int. J. Infect. Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen A., David J.K., Maden S. Human leukocyte antigen susceptibility map for SARS-CoV-2. J. Virol. 2020 Apr 17 doi: 10.1128/JVI.00510-20. pii: JVI.00510-20. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorse G.J., Donovan M.M., Patel G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020;92:512–517. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L.P., Wang N.C., Chang Y.H. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007;13 doi: 10.3201/eid1310.070576. 1562–15. [DOI] [PMC free article] [PubMed] [Google Scholar]