Abstract
Coronavirus disease-2019 (COVID-19) has caused a pandemic that has affected millions of people worldwide. COVID-19 is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and is spread by close contact and by respiratory droplets. It has also impacted different aspects of caring for people with kidney disease, including those with acute kidney injury (AKI), chronic kidney disease (CKD), those requiring kidney replacement therapy (KRT), and those with a kidney transplant. All of these patients are considered high risk. The lessons learned from the COVID-19 pandemic will hopefully serve to protect patients with kidney disease in a similar situation in the future.
Introduction
Coronavirus disease-2019 (COVID-19) has caused a pandemic that has affected millions of people worldwide. COVID-19 is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and spread by close contact and by respiratory droplets1. Though SARS-CoV-2 is considered a respiratory virus with the most fearful complication being respiratory failure, it does affect multiple organ systems including the kidneys. In this article we review how COVID-19 involves the kidney, causing acute kidney injury (AKI), and how it affects patients on kidney replacement therapy (KRT) with hemodialysis (HD) and peritoneal dialysis (PD), and those with a kidney transplant. The information in this review reflects the understanding as of the date of writing, which is still evolving, and regular updates are being posted on the NephJC blog (www.nephjc.com/covid19).
Acute kidney injury
Early studies coming primarily from China reported a relatively low incidence of AKI in COVID-19 (CoV-AKI), ranging between 3 and 9% depending on the population studied2, 3, 4. Subsequently, in the initial report from the United States of America (USA) in individuals with COVID-19 admitted to the intensive care unit (ICU), 19% (4/21 patients) acquired AKI5. However, more recent and larger reports (see Table 1 ) from the US and United Kingdom described a higher incidence of CoV-AKI, of 28 to 43% among hospitalized patients and 61 to 76% among those who are critically ill6, 7, 8, 9. Furthermore, rates of KRT have ranged between 15 and 20% of patients with CoV-AKI, and even higher rates of 34 to 73% for those admitted to the ICU. CoV-AKI is also associated with a higher mortality rate8.
Table 1.
Summary of 5 largest reports of COVID-19 and AKI (CoV-AKI) reporting the incidence of AKI and need for kidney replacement therapy. AKI: Acute Kidney Injury; Coronavirus Disease-2019: COVID-19; ICU: Intensive Care Unit; ICNARC: Intensive Care National Audit and Research center; Kidney Replacement Therapy: (KRT).
| Center | Description | Number of patients admitted with COVID-19 | AKI incidence – all hospitalized patients | Requiring KRT – hospitalized patients | AKI incidence – ICU patients | Requiring KRT – of total ICU patients | Mortality rate with AKI | |
|---|---|---|---|---|---|---|---|---|
| Guan et al10 | Mainland China |
Multi-center in multiple health systems | 1099 | 6 (0.5%) | 9 (0.8%) | n/a | n/a | n/a |
| Hirsch et al.7 | Northwell, NY | Multi-center in single health system | 5449 | 1993 (37)%) | 285 (14.3%) | 1060 (76%) | 276 (19.7%) | n/a |
| Mohamed et al.6 | Ochsner, LA | Single Center | 575 | 161 (28%) | 89 (55%) | 105 (61%) | 76 (44%) | 50% |
| Chan et al.8 | Mt Sinai, NY | Multi-center in single health system | 3235 | 1406 (43%) | 280 (20%) | 553 (68%) | 277 (34%) | 52% |
| Intensive Care National Audit & Research center (ICNARC)9(Date accessed June 19, 2020) | Database of ICUs in England, Wales and Northern Ireland | Multi-center in multiple health systems | 9949 | n/a | n/a | n/a | 2393 (24%) | n/a |
CoV-AKI is associated with proteinuria and hematuria, which is not uncommon with acute tubular injury (ATI), however the degree of each is not known as there has been sparse reporting of quantitative data11.
A granular description of the AKI phenotype suggests that the predominant etiology of CoV-AKI, which is largely associated with hemodynamic instability, is indeed acute tubular injury (ATI)6. A smaller subset of patients presented with features suggestive of toxic ATI from rhabdomyolysis, whereas pre-renal azotemia represented only 9% of the causes. An ancillary report from the same study from Mohamed et al. from New Orleans, USA reported that abundant granular and waxy casts indicative of ATI were the predominant finding on urine microscopy12. In agreement with those clinical observations, a postmortem case series by Su et al. reported findings of ATI on autopsies of 9 patients who died from COVID-1913. Thus far, there is a paucity of data from pre-mortem tissue diagnosis. From the same group, 3 patients, all African American (AA), out of 161 patients with CoV-AKI with findings suggestive of glomerular disease underwent kidney biopsy which revealed collapsing glomerulopathy in each biopsy6. This is in line with emerging reports of collapsing glomerulopathy in AA individuals who are carriers of the Apolipoprotien L1 (APOL1) high risk genotype and acquire COVID-1914, 15. Thus, collapsing glomerulopathy should be suspected in AA individuals presenting with AKI and new onset nephrotic range proteinuria, where the COVID-19 potentially serves as a second hit. Nevertheless, this entity does not account for the bulk of the AKI cases. Lastly, even thrombotic microangiopathy has been reported, which is probably rare, but might be underreported given the paucity of kidney biopsy data16.
Despite the notion that ischemic ATI is the predominant etiology of AKI in patients with COVID-19, AKI without a clear cause has been observed. As a result, speculation regarding direct viral entrance of the SARS-CoV-2 coronavirus into the kidneys has arisen. The speculation has been driven by the fact that the membrane-bound peptidase angiotensin converting enzyme 2 (ACE2) acts as a receptor for SARS-CoV-2 and is expressed in the kidney tissue, both in tubules and glomerular podocytes17, 18, 19. The aforementioned postmortem study by Su et al. reported enhanced expression of ACE2 in parietal epithelial cells and presence of viral nucleoproteins in tubular cells. Another report of viral detection in kidney tissue has added ammunition to that contention20. However, there is still no definite demonstration that viral presence in the kidneys is a causative phenomenon in CoV-AKI.
With the sudden surge in COVID-19 cases in the USA, healthcare resources were quickly saturated in states such as New York, California, New Jersey, and Louisiana. There was national coverage in the media regarding the shortage of ventilators due to respiratory failure in COVID-19. However, there was little mention of the stretched dialysis resources. Among the options used as a contingency include shortening the time on dialysis, even for continuous Kidney Replacement Therapy (KRT), by making it less continuous, decreasing dialysis frequency, and decreasing the need for dialysis, by being more judicious in fluid resuscitation, and using potassium binding exchange resins21, 22. Centers throughout the world with similar issues reported initiation of an urgent peritoneal dialysis (PD) program, which is unprecedented in the Western Hemisphere for AKI to be able to provide KRT to patients (further discussed in the following section on PD).
Kidney replacement therapy: chronic hemodialysis
Patients on long term KRT are on either hemodialysis (HD) or peritoneal dialysis (PD) on a regular basis. These patients on long term dialysis have chronic health conditions and immunomodulating factors that make them especially vulnerable to COVID-19. This increased vulnerability to nosocomial and community spread infections requires several steps by caregivers and healthcare systems to continue dialysis treatments in a safe environment.
We will first discuss long term KRT in terms of HD then PD. Patients on chronic HD routinely receive three treatments weekly at an outpatient dialysis unit. During these dialysis sessions, the patients are in continual contact and close proximity with other patients and caregivers. Thus, patients on HD cannot self-isolate, and have repeated and ongoing potential exposure. Dialysis centers implemented strategies to keep the staff and patients from contracting COVID-19 through the known mechanisms of spread23. These strategies included screening at the entrance of the dialysis facilities with a health and symptom questionnaire, and a rapid temperature check for fever24. Patients with symptoms or an elevated temperature were advised to not enter the facility and go for COVID-19 testing. Strategies used to avoid nosocomial spread including cohorting patients with suspected or confirmed COVID in separate units, the last shift of dialysis, and/or physically isolate them as much as possible, All patients and staff were encouraged to wear Centers for Disease Control (CDC) endorsed personal protective equipment (PPE) including facial masks at a minimum, with staff wearing gowns and gloves25.
If a patient who is on dialysis therapy contracted COVID-19, the disease course and severity has ranged widely. As with AKI, the initial data from China has been overshadowed by more grim numbers from larger studies. A small preprint study from a Chinese dialysis unit suggested the course may be milder in dialysis patients compared to other patient cohorts26, 27. Of the 230 patients at this one unit, only 37 developed COVID-19 and 27 of those patients were asymptomatic. Subsequent data is much worse, with mortality rates in the range of 20 - 30% (Table 2 )
Table 2.
Incidence and outcomes with COVID-19 in chronic hemodialysis populations.
| Study | Setting | Incidence of COVID-19 | Mortality rate with COVID-19 |
|---|---|---|---|
| UK Renal Registry | UK | 2211 cases (~4.9%) | 498 (22.5%) |
| ERA-EDTA registry | European registry | 278 cases | 20% |
| Xiong et al. | 65 centres in Wuhan, China | 154 cases of 7154 (2.2%) | 41 (26.6%) |
| Alberici et al. | Four dialysis centers of the Brescia Renal COVID Task Force, Lombardy, Italy | 94 cases of 643 (15%) | 24 (25.5%) |
UK: United Kingdom; ERA-EDTA: European Renal Association and European Dialysis and Transplantation Association.
Dialysis access patency is fundamental to achieving life saving treatments. At the outbreak of the pandemic in the United States in March 2020, all non-emergent surgeries were encouraged to be delayed to preserve healthcare resources. However, patients with dialysis access that had clotting or non-function status cannot wait days and weeks to re-establish patency, since lack of dialysis access means no dialysis. For these patients, vascular access is a lifeline. Hence, the American Society of Diagnostic and Interventional Nephrology (ASDIN) and Vascular Access Society of the Americas (VASA) issued a joint statement urging healthcare centers and hospitals to prioritize dialysis access procedures as life threatening necessary surgeries28.
Peritoneal dialysis
The COVID-19 pandemic brought the benefits of home dialysis with peritoneal dialysis (PD) into sharp focus. The ability to dialyze and stay quarantined in the patient's own home is ideal compared to travelling three times a week to an in-center dialysis unit. However, the other aspect of PD that had an important role was the use of urgent start PD (see Fig. 1 ).
Fig. 1.
Sample protocol for peritoneal dialysis (PD) in acute kidney injury. ICU: Intensive Care unit.32.
The ability to initiate urgent PD has been crucial since during the COVID-19 pandemic many health care systems have been stretched thin including to the point that there was a shortage of continuous KRT machines and supplies21, 22, 23, 24, 25, 26, 27, 28, 29. In some centers, even the availability of HD to accommodate AKI patients needing dialysis became a problem. PD provides a viable option in these situations. Acute PD protocols are clearly outlined in the International Society for Peritoneal Dialysis (ISPD) guidelines30, 31. Specific concerns relate to arranging the insertion of these catheters (typically surgeons, or interventional radiologists or nephrologists), and the specific protocols that need to be used to ensure metabolic and volume control which can be challenging in the critically ill patient21 , 29, 30, 31.
The majority of elective surgeries were initially canceled during the COVID-19 crisis, however many institutes and professional organizations including the American Society of Nephrology classified PD catheter insertion as an essential service32. Avoiding the start of PD would result in a greater pressure on HD services, which are under stress, as discussed before. Even for chronic kidney disease (CKD) patients in need of urgent dialysis (e.g. in cases where the kidney function has worsened rapidly, or were previously not recognized to have CKD ‘crash starts’) an urgent PD start can be considered. Urgent PD can mostly be started within 24–48 h of catheter insertion, using low volume exchanges in the supine position, especially using cyclers33, 34. Peritoneal dialysis in a patient with acute respiratory distress syndrome (ARDS) requiring prone ventilation presents several challenges. PD in prone patients has been previously described, one recent protocol provides some suggestions on managing patients with PD in prone position35, 36. PD catheter exit site could be placed more laterally than usual to accommodate the prone position. In addition, there is some anecdotal experience in using pillows to prop up the chest and pelvis to reduce the intra-abdominal pressure in a prone patient on PD. We have listed the possible advantages and disadvantages of PD in Table 3 .
Table 3.
Advantages and Disadvantages in urgent start PD. ICU: intensive care unit; IR: interventional radiology.
| Peritoneal Dialysis In Acute Kidney Injury in ICU during COVID19 Pandemic | |
|---|---|
| Advantages | Disadvantages |
| Relatively simple to insert bedside PD catheters for ICU patients by IR/Surgeons or Interventional Nephrologists | Precise ultrafiltration is slightly more difficult |
| Avoid vascular access and need for anticoagulation | May need innovative exit sites and dialysis scheduling to accommodate prone ventilation. |
| Intra-abdominal pressure may be affected by prone positioning | |
With regards to home dialysis in general and PD specifically, routine outpatient follow up is adapted to keep patients at home and care is provided virtually by various telemedicine platforms that have come to prominence during the pandemic. Timely CMS waivers provided flexibility to accommodate virtual visits for home dialysis37. Many programs have adapted to this new reality and have used telehealth platforms like Zoom to provide patient care, assessment and education for home dialysis38.
PD depends heavily on supplies provided by the companies that make and supply the consumables required for PD. In addition to the PD bags and cyclers, PD patients require large daily quantities of masks and hand sanitizer supplies to perform the procedure using aseptic precautions. With the broad disruption of supply chains during the pandemic, it is advisable to keep a minimum of 2 weeks of rolling stock at hand to ensure safe PD.
COVID-19 and kidney transplantation
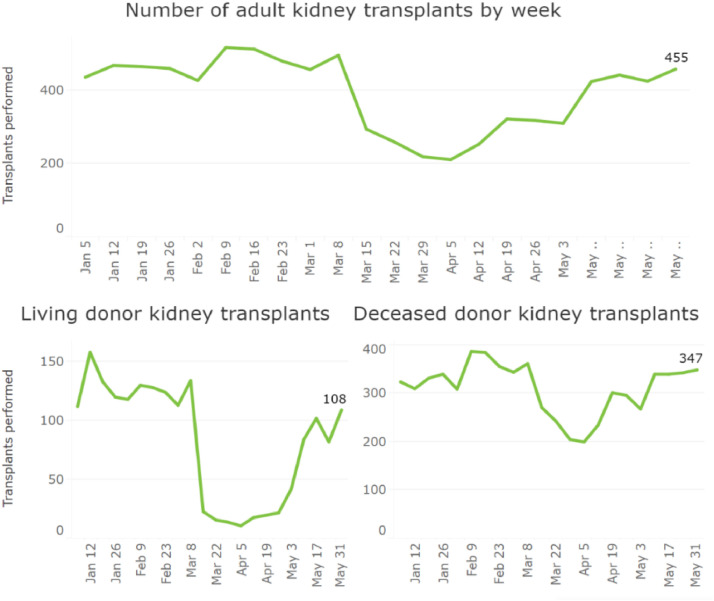
The COVID-19 pandemic has adversely affected multiple aspects of kidney transplantation. Kidney transplant rates dropped (see Fig. 2 ) at the onset of the pandemic with the suspension of 72% of living donor programs and limitations on the operations of 84% of deceased donor programs39. This decline ensued despite the Centers for Medicare and Medicaid Services (CMS) identifying kidney transplantation as a Tier 3b procedure that should not be postponed if possible, reflecting concerns about risk of COVID-19 transmission, limited healthcare resources, and limited knowledge of this novel disease40. As all regions have seen some recovery in transplantation rates in May of 2020, transplant programs continue to grapple with various issues of transplant care during the COVID-19 pandemic41.
Fig. 2.
Transplant Rates during COVID-19 Pandemic. Reproduced with permission by the United Network for Organ Sharing (UNOS). Sourced at https://unos.org/covid.
Initially, transplantation evaluations decreased during the early period of COVID-1942. This occurred due to drops in actual transplantation rates, as well as concern for increasing exposure to COVID-19 during evaluations. In response, the increased use of telemedicine pre- and post-transplant has allowed patients to continue their evaluation while minimizing risk of exposure to COVID-1943. The Organ Procurement and Transplant Network (OPTN) also recognized the decreased access to transplantation during the pandemic. They have allowed for a program to apply for a waiting time modification that back dates listing times to the start of the pandemic44. Additionally, concerns for donor-derived infection have led to the implementation of routine screening of living and deceased donors for COVID-19 with test results available within hours45. Presently, if a donor tests positive for COVID-19 they likely will not be used though this could change in the future46.
Kidney transplant patients are considered a high risk patient population due to immunosuppression and their increased risk of infection47. Risk factors associated with increased morbidity and mortality with COVID-19 infection include increased age, obesity, and history of chronic heart, lung, or kidney disease. All these risk factors are commonly found in patients with CKD and hence in kidney transplant patients.
Kidney transplant recipients infected with COVID-19 appear to have similar clinical presentations as the general population, though morbidity, including need for mechanical ventilation, and mortality vary among reports. In a case series of 15 kidney transplant recipients hospitalized with COVID-19 in New York, patients most often presented with symptoms of fever and/or cough, chest x-ray findings of bilateral infiltrates, and other elevated inflammatory markers. Mechanical ventilation was required in about 30% of these patients48. In another case series of 35 kidney transplant recipients with COVID-19 from both the inpatient and outpatient settings in New York, 39% of patients needed mechanical ventilation, 21% needed KRT, and the mortality rate was 28% at 3 weeks, significantly higher than the general population in the United States whose mortality rate is reported to be 1 to 5%49. This contrasts with a large, international European study, which reported the mortality rate in patients with kidney transplant was 18%, comparable to the 20% mortality rate of patients on dialysis50. For those transplant recipients with mild disease, few comorbidities, and ability to communicate closely with their transplant team, outpatient management may be considered51.
Management of immunosuppression for patients with kidney transplant and COVID-19 primarily consists of discontinuation of the anti-metabolite, usually mycophenolate mofetil or mycophenolate acid, and, with severe infection, also holding the calcineurin inhibitor52. Calcineurin inhibitors in vitro have been shown to inhibit the replication of coronaviruses, though it is unclear if this effect will occur in vivo 53. Corticosteroids have unclear benefit at this time as they have been associated with a longer duration of viral shedding and higher viral loads, but have also been explored as an experimental therapy with variable success54, 55, 56, 57, 58, 59, 60.
A wide range of experimental therapies (see table 3) have been used, including hydroxychloroquine, azithromycin, tocilizumab, and remdesivir39 , 61 , 62. Careful consideration of drug dosing and interactions need to be always considered in the kidney transplant patient. Table 4 shows potential treatments and interactions when considering COVID-19 treatments61, 62.
Table 4.
List of potential COVID-19 treatments with drug interactions with commonly used immunosuppressants (from Nephjc http://www.nephjc.com/news/covidtx).
| Drug | Dose reported in COVID-19 trials | Dose adjustment in CKD? | Drug Interactions in Transplant | Other considerations |
|---|---|---|---|---|
| Remdesivir | 200 mg x 1 then 100 mg daily | ? None1 | None reported | CrCl < 30 exclusion in most trials1 |
|
Lopinavir/Ritonavir Kaletra |
400 mg / 100 mg twice daily | None | ↑Cyclosporine, tacrolimus levels ↑↑ Sirolimus levels ? Effect on mycophenolate |
Both highly protein bound |
|
Chloroquine/ Hydroxychloroquine Plaquenil |
CQ: 500 – 1000 mg a day (or 10 mg/kg) HCQ: 400 mg x1 then 200 mg two or three times a day |
None3 | ↑Cyclosporine, tacrolimus, sirolimus levels | ~ 50–70% protein bound; high volume of distribution |
|
DarunavirPrezista / RitonavirNorvir |
800 mg / 100 mg daily | None | ↑Cyclosporine, tacrolimus levels ↑↑ Sirolimus levels ? Effect on mycophenolate |
Both highly protein bound |
| Favipiravir | 1200 mg twice daily for 2 days then 600 mg 2–3 times a day | ?Yes | None reported | ~50% protein bound |
| Tocilizumab | 8 mg/kg (up to max 800 mg) | None | ↓Cyclosporine, tacrolimus, sirolimus levels | |
| Colchicine | 0.5 mg twice daily2 | Yes4 | ↑Cyclosporine, tacrolimus levels ? Sirolimus levels Avoid with azathioprine |
High volume of distribution; ~40% protein bound |
Names in italics refer to brand names.
This is a common reported exclusion, due to the potential toxicity of the excipient.
Dose in gout is usually 0.6 mg, this dose is reported for the COLCORONA trial.
These drugs do have some clearance by kidneys, so accumulation with occur with chronic dosing, not relevant in the COVID-19 setting.
CrCl < 30 an exclusion in the COLCORONA trial
CQ: chloroquine; HCQ: hydroxychloroquine; CrCl: creatinine clearance.
Telehealth
We have already discussed the rapid adaptation of telehealth in kidney transplantation. However, telehealth or telenephrology was also adapted during the pandemic for use for CKD patients in the outpatient clinic setting. Recently a review by Koraishy et al. discussed telenephrology in detail63. Although telehealth carries limitations in the ability to thoroughly assess patients, nephrology is well-suited for it due to its nature of being primarily a "numerical specialty". CKD is known as a "silent killer" highlighting the fact that most CKD patients are not typically symptomatic until the late stages of CKD. Periodical assessment of changes if kidney function, degree of proteinuria, anemia etc.,is fully accomplished with lab review and addresses the most relevant aspects of CKD care. Patients are required to undergo blood draws at a lab facility and though this is an exposure risk many private and in-hospital clinics have taken precautions to limit exposure. In regard to physical findings, edema, the most salient in this patient population, can be assessed with the use of video or photography. Thus, telehealth proved to be a viable tool to manage outpatient CKD clinics during the pandemic.
Conclusion
COVID-19 has had a tremendous effect on the world and causes very high morbidity and mortality. In the critically ill patient, COVID-19 also causes AKI, with 10 to 30% needing KRT.
We have discussed how in the dialysis population there had to be changes to protect patients and staff. That the stress on the healthcare systems caused at times a lack of availability of certain kidney replacement modalities and that the innovative use of urgent PD became a more widely used option. Finally, the effects on kidney transplantation included a drop in transplant evaluations and transplant surgeries. We noted that while transplant patients are considered high risk for infection with COVID-19, their presentations have been similar to the general population, but with higher mortality rates comparable to that of patients on dialysis. Much of this information, especially the role of specific therapeutic management, is still evolving as trials are ongoing. As we move forward with preventative measures, it is important to remember the unique characteristics of patients with chronic kidney disease, end stage kidney disease, or those with a transplant and how they might be affected by a future situation similar to the COVID-19 epidemic.
References
- 1.Centers for disease control and prevention. What you should know about COVID-19 to protect yourself and others. Accessed June 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/2019-ncov-factsheet.pdf
- 2.Naicker S., Yang C.-.W., Hwang S.-.J., Liu B.-.C., Chen J.-.H., Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ILL Patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed M.M., Lukitsch I., Torres-Ortiz A.E., et al. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360. 2020 doi: 10.34067/KID.000265202010.34067/KID.0002652020. Published online May 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 doi: 10.1016/j.kint.2020.05.006. Published online MayS0085253820305329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan L., Chaudhary K., Saha A., et al. Acute kidney injury in hospitalized patients with COVID-19. Nephrology. 2020 doi: 10.1101/2020.05.04.20090944. [DOI] [Google Scholar]
- 9.ICNARC – Reports. Accessed June 19, 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- 10.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Arroyo C.F., Varghese V., Mohamed M.M.B., Velez J.C.Q. Urinary sediment microscopy in acute kidney injury associated with COVID-19. Kidney360. Published online June 2, 2020:10.34067/KID.0003352020. doi:10.34067/KID.0003352020 [DOI] [PMC free article] [PubMed]
- 13.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. Published online AprilS0085253820303690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H., Larsen C.P., Hernandez-Arroyo C.F., et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020;31(6) doi: 10.1681/ASN.2020050558. ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhaveri K.D., Meir L.R., Flores Chang B.S., et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020 doi: 10.1016/j.kint.2020.05.025. Published online June 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 19.Velez J.C.Q., Bland A.M., Arthur J.M., Raymond J.R., Janech M.G. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol-Ren Physiol. 2007;293(1):F398–F407. doi: 10.1152/ajprenal.00050.2007. [DOI] [PubMed] [Google Scholar]
- 20.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. N Engl J Med. 2020. Multiorgan and Renal Tropism of SARS-CoV-2. Published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldfarb D.S., Benstein J.A., Zhdanova O., et al. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15(6):880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbert G.B., Patel D., Lerma E.V. Patiromer for the treatment of hyperkalemia. Expert Rev Clin Pharmacol. 2020;0(0):1–8. doi: 10.1080/17512433.2020.1774363. [DOI] [PubMed] [Google Scholar]
- 23.American Society of Nephrology. Information for screening and management of COVID-19 in the outpatient dialysis facility. Published March 13, 2020. Accessed June 12, 2020.https://www.asn-nline.org/g/blast/files/DIALYSIS_COVID_2019_Update_03.13.2020_FINAL.pdf
- 24.Kliger A.S., Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15(5):707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Frequently asked questions and answers: Coronavirus disease-2019 (COVID-19) and Children. Accessed June 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html
- 26.Ma Y., Diao B., Lv X., et al. Nephrology; 2020. COVID-19 in Hemodialysis (HD) Patients: Report from One HD Center in Wuhan, China. [DOI] [Google Scholar]
- 27.Wang H. Maintenance hemodialysis and COVID-19: saving lives with caution, care, and courage. Kidney Med. 2020;2(3):365–366. doi: 10.1016/j.xkme.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Society of Diagnostic and Interventional Radiology. Maintaining lifelines for ESKD patients- ASDIN and VASA joint statement. Accessed June 12, 2020. https://cdn.ymaws.com/www.asdin.org/resource/resmgr/covid_19/Maintaining_lifelines_ASDIN_.pdf
- 29.Chua H.-.R., Laren G.M., Choong L.H.-.L., et al. Ensuring sustainability of continuous kidney replacement therapy in the face of extraordinary demand: lessons from the COVID-19 pandemic. Am J Kidney Dis Off J Natl Kidney Found. 2020 doi: 10.1053/j.ajkd.2020.05.008. Published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullis B., Abdelraheem M., Abrahams G., et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int J Int Soc Perit Dial. 2014;34(5):494–517. doi: 10.3747/pdi.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkie M., Davies S. Peritoneal Dialysis in the time of COVID-19. Perit Dial Int J Int Soc Perit Dial. 2020 doi: 10.1177/0896860820921657. Published online April 21. [DOI] [PubMed] [Google Scholar]
- 32.Critical clarification from CMS: PD catheter and vascular access placement is essential | Kidney News. Published June 18, 2020. Accessed June 18, 2020. https://www.kidneynews.org/policy-advocacy/leading-edge/critical-clarification-from-cms-pd-catheter-and-vascular-access-placement-is-essential
- 33.Arramreddy R., Zheng S., Saxena A.B., Liebman S.E., Wong L. Urgent-start peritoneal dialysis: a chance for a new beginning. Am J Kidney Dis Off J Natl Kidney Found. 2014;63(3):390–395. doi: 10.1053/j.ajkd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Povlsen J.V., Sørensen A.B., Ivarsen P. Unplanned start on peritoneal dialysis right after PD catheter implantation for older people with end-stage renal disease. Perit Dial Int J Int Soc Perit Dial. 2015;35(6):622–624. doi: 10.3747/pdi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klisnick A., Souweine B., Filaire M., et al. Peritoneal dialysis in a patient receiving mechanical ventilation in prone position. Perit Dial Int J Int Soc Perit Dial. 1998;18(5):536–538. [PubMed] [Google Scholar]
- 36.KCH-Renal-Covid-Acute-PD-on-ICU-protocol-final.pdf. Accessed June 20, 2020. https://renal.org/wp-content/uploads/2020/04/KCH-Renal-Covid-Acute-PD-on-ICU-protocol-final.pdf
- 37.Sweeping CMS Waivers Give Flexibility to Nephrologists during COVID-19 Crisis| Kidney News. Accessed June 20, 2020. https://www.kidneynews.org/policy-advocacy/leading-edge/cms-issues-sweeping-interim-final-rule-providing-multiple-waivers
- 38.Srivatana V., Liu F., Levine D.M., Kalloo S.D. Early use of telehealth in home dialysis during the COVID-19 pandemic in New York city. Kidney360. 2020 doi: 10.34067/KID.000166202010.34067/KID.0001662020. Published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyarsky B.J., Po‐Yu Chiang T., Werbel W.A., et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020 doi: 10.1111/ajt.15915. Published online May 10ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United Network for Organ Sharing. New adult elective surgery and procedure guidelines prioritize transplant surgeries as essential care. Published March 19, 2020. https://unos.org/news/cms-issues-covid-19-guidance-to-health-care-providers/
- 41.United Network for Organ Sharing. COVID-19 and Solid Organ Transplant. Published June 13, 2020. https://unos.org/covid/
- 42.Ahn C., Amer H., Anglicheau D., et al. Global transplantation COVID report March 2020. Transplantation. 2020 doi: 10.1097/TP.0000000000003258. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Concepcion B.P., Forbes R.C. The role of telemedicine in kidney transplantation: opportunities and challenges. Kidney360. 2020;1(5):420–423. doi: 10.34067/KID.0000332020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Organ Procurement and Transplantation Network. COVID-19 Emergency Policy Package. Published April 3, 2020. https://optn.transplant.hrsa.gov/media/3716/covid-19_emergency_policypackage_and_minibrief.pdf
- 45.The Transplantation Society - Transplant Infectious Disease. Guidance on Coronavirus Disease 2019 (COVID-19) for Transplant Clinicians. Published June 8, 2020. Accessed June 13,2020. https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians
- 46.American Society of Transplantation. 2019-nCoV (Coronavirus): Recommendations and Guidance for Organ Donor Testing. Published May 14, 2020. Accessed June 14, 2020.https://www.myast.org/sites/default/files/internal/COVID19%20FAQ%20Donor%20Testing%2005.14.2020.pdf
- 47.Centers for Disease Control and Prevention. Groups at Higher Risk for Severe Illness. Published May 14, 2020. Accessed June 14, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html
- 48.The Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akalin E., Azzi Y., Bartash R., et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Renal Association- European Dialysis and Transplant Association. The ERA-EDTA COVID-19 database for patients on kidney replacement therapy - fifth ERACODA study report. Published May 6, 2020. https://www.era-edta.org/en/wp-content/uploads/2020/05/ERACODA-Study-Report-2020-05-06.pdf
- 51.Gleeson S.E., Formica R.N., Marin E.P. Outpatient management of the kidney transplant recipient during the SARS-CoV-2 virus pandemic. Clin J Am Soc Nephrol. 2020;15(6):892–895. doi: 10.2215/CJN.04510420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kronbichler A., Gauckler P., Windpessl M., et al. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020 doi: 10.1038/s41581-020-0305-6. Published online May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willicombe M., Thomas D., McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm. J Am Soc Nephrol. 2020;31(6):1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogimi C., Greninger A.L., Waghmare A.A., et al. Prolonged shedding of human coronavirus in hematopoietic cell transplant recipients: risk factors and viral genome evolution. J Infect Dis. 2017;216(2):203–209. doi: 10.1093/infdis/jix264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X., Alekseev K., Jung K., Vlasova A., Hadya N., Saif L.J. Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome. J Virol. 2008;82(9):4420–4428. doi: 10.1128/JVI.02190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee N., Allen Chan K.C., Hui D.S., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 60.University of Oxford. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. Published June 16, 2020. https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf
- 61.University of Liverpool. Interactions with experimental COVID-19 immune therapies. Published June 4, 2020. Accessed June 15, 2020. https://www.covid19-druginteractions.org/prescribing-resources
- 62.University of Liverpool. Interactions with experimental COVID-19 viral therapies. Published June 4, 2020. Accessed June 15, 2020. https://www.covid19-druginteractions.org/prescribing-resources
- 63.Koraishy F.M., Rohatgi R. Telenephrology: an emerging platform for delivering renal health care. Am J Kidney Dis. 2020;0(0) doi: 10.1053/j.ajkd.2020.02.442. [DOI] [PubMed] [Google Scholar]