Abstract
Background
In recent years, there is growing literature on the prognostic significance of programmed death-ligand 1 (PD-L1) in cholangiocarcinoma (CCA); however, data have been conflicting. Therefore, the objective of this study was to assess the correlation between PD-L1 and prognosis in CCA through meta-analysis.
Methods
Published studies were retrieved from the Web of Science, PubMed, Embase, and Cochrane Library up to April 17, 2020. The relationships between PD-L1 expression and survival outcomes were assessed using hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Eighteen studies consisting of 2012 patients were included. Overexpression of PD-L1 was significantly associated with worse overall survival (OS) (HR = 1.58, 95%CI = 1.30 − 1.92, p < 0.001) but not with poor disease-free survival (DFS) (HR = 1.03, 95%CI = 0.68 − 1.55, p = 0.895) in CCA. Moreover, PD-L1 was associated with low differentiation (OR = 1.43, 95%CI = 1.09 − 1.87, p = 0.010) and higher pN stage (OR = 1.45, 95%CI = 1.10 − 1.92, p = 0.009) but not with sex, TNM stage, vascular invasion, perineural invasion, age, or tumor size.
Conclusion
High PD-L1 expression was associated with worse OS, poor differentiation, and higher pN stage in patients with CCA. PD-L1 could be a potential prognostic marker in CCA.
1. Introduction
Cholangiocarcinoma (CCA) is the second most frequent type of primary liver cancer, with aggressive nature and a high mortality rate, accounting for 20% of liver-related deaths [1]. The incidence of CCA is increasing during the past decades in Western countries, and the 5-year survival rate is approximately 10% [2, 3]. Surgical resection is the definitive treatment option for CCA; however, recurrence remains high and maintains a poor prognosis [4, 5]. Emerging treatment options, including targeted therapies and immunotherapy with checkpoint inhibitors, are in clinical trials and provide personalized therapeutic strategies for patients with CCA [5]. Efficient prognostic biomarkers are still lacking for CCA; therefore, a reliable prognostic marker is needed for optimal therapeutic strategy selection [6].
In recent years, the tumor microenvironment and immune milieu have attracted much attention [7]. The immune checkpoint molecules, programmed cell death-1 (PD-1) and its ligand programmed death-ligand-1 (PD-L1), regulate immune responses in cancer development [8]. Activation of the PD-1/PD-L1 axis results in immune suppression by inhibition of immune cells and secretion of certain cytokines [9]. Recent evidence also showed the prognostic value of PD-L1 in different types of cancers [10]. The prognostic role of PD-L1 in CCA has also been investigated; however, data were inconsistent [11–28]. Therefore, we conducted a meta-analysis to explore the prognostic and clinicopathologic roles of PD-L1 in patients with CCA.
2. Materials and Methods
This meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [29]. Ethical approval and patient consent were not performed because all data collected were from previously published studies.
2.1. Literature Search
PubMed, Web of Science, Cochrane Library, and Embase were reviewed till April 17, 2020. The search terms used were “PD-L1” or “programmed death ligand 1” or “PDL1” or “B7-H1” or “CD274”, and “bile duct neoplasms” or “cholangiocarcinoma” or “bile duct cancer”. The reference lists in relevant studies were also examined for potential inclusions.
2.2. Inclusion and Exclusion Criteria
The criteria for inclusion were (1) patients histologically diagnosed with CCA; (2) PD-L1 expression detected by immunohistochemistry (IHC); (3) studies reporting the relationship between PD-L1 and survival outcomes including overall survival (OS) and disease-free survival (DFS); (4) sufficient data available for the calculation of hazard ratios (HRs), odds ratios (ORs), and 95% confidence intervals (CIs); and (5) studies published in English.
The exclusion criteria were (1) conference abstracts, case reports, reviews, or letters; (2) studies with insufficient data for analysis; (3) animal studies; and (4) studies recruited overlapping patients.
2.3. Data Extraction
Two independent investigators (Q.X. and L.W.) collected data from the included studies and any discrepancies were settled by discussion with a senior investigator (S.Z.). The following baseline information was extracted: author, year, study country, study design, sample size, treatment method, follow-up, survival outcomes, positive rate of PD-L1 expression, and detection method. Detailed information on PD-L1 antibodies used for IHC (specie, clone, dilution, source, and cutoff value) was also extracted. The HR and 95% CIs of OS and DFS were obtained directly if reported or were calculated by Tierney's method [30].
2.4. Quality Assessment
The Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of eligible studies [31]. The NOS evaluated each study in three aspects. The score ranges from 0-9, and studies with NOS scores of ≥6 are considered high-quality studies.
2.5. Statistical Analysis
The relationships between PD-L1, OS, and DFS were assessed by combining HRs and 95% CIs. Chi-squared tests and inconsistency index (I2) statistics were used to examine heterogeneity. In the presence of significant heterogeneity (I2 > 50%), a random-effect (REM) model was used; otherwise, a fixed-effect model (FEM) was applied. ORs and 95% CIs were used as effective sizes to assess the association between PD-L1 and clinicopathological features. Publication bias was tested using Begg's and Egger's tests. A p < 0.05 was considered to be statistically significant. All statistical analyses were conducted using Stata version 12.0 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.).
3. Results
3.1. Study Characteristics
The initial literature search identified 259 studies. According to the selection criteria, a total of 18 studies [11–28] with 2012 patients were eventually included in the meta-analysis (Figure 1). The basic characteristics of the eligible studies are shown in Table 1. Seven studies were conducted in China [13, 15, 16, 21, 22, 27, 28], three in Japan [18, 24, 25], two in Korea [11, 20], two in Germany [19, 26], two in Germany [14, 17], and one in Thailand [23] and UK [12]. One study was of prospective design [12], and 17 were retrospective cohort studies [11, 13–28]. The sample size ranged from 26 to 320. All included studies had a NOS score of ≥6. Detailed information on the primary antibody used for PD-L1 is summarized in Table 2. All included studies used IHC to detect PD-L1 expression. The cutoff values to stratify high- and low expression of PD-L1 were different, including 1%, 5%, H-score 5, score 3, and 2+.
Figure 1.
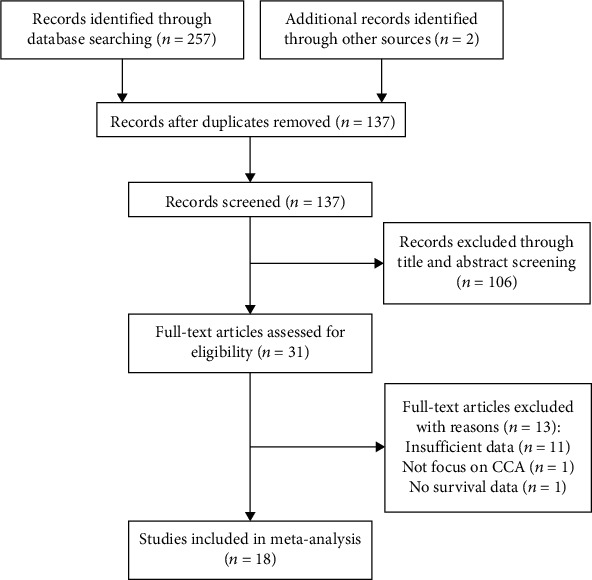
Flow chart of literature search and study selection.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author | Year | Country | No. of patients | Ethnicity | Sex (M/F) | Tumor location | Follow-up (months) Median (range) |
Study design | Treatment | Survival outcome | PD-L1 (+) n (%) |
NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn, S. | 2019 | Korea | 183 | Asian | 122/61 | eCCA | 27.2 | Retrospective | Surgery | OS, DFS | 31 (16.9) | 7 |
| Arkenau, H. T. | 2018 | UK | 26 | Caucasian | 8/18 | CCA | 6.4 (4.1-13.2) | Prospective | Targeted therapy + immunotherapy | OS, DFS | 12 (46.2) | 8 |
| Dong, Z. T. | 2020 | China | 125 | Asian | 58/77 | iCCA | 16 (5-63) | Retrospective | Surgery | OS, DFS | 52 (41.6) | 7 |
| Gani, F. | 2016 | USA | 54 | Caucasian | 17/37 | iCCA | NR | Retrospective | Surgery | OS | 39 (72.2) | 6 |
| Gou, M. M. | 2019 | China | 30 | Asian | 18/12 | CCA | To Sep 2018 | Retrospective | Immunotherapy | DFS | 11 (36.6) | 7 |
| Jing, C. Y. | 2019 | China | 153 | Asian | NR | iCCA | 47.5 (1-88.4) | Retrospective | Surgery | OS | 43 (28.1) | 7 |
| Kim, R. | 2018 | USA | 44 | Caucasian | 23/21 | eCCA | NR | Retrospective | Surgery | OS | 10 (22.7) | 6 |
| Kitano, Y. | 2020 | Japan | 177 | Asian | 115/62 | CCA | 78.7 | Retrospective | Surgery | OS | 54 (30.5) | 6 |
| Kriegsmann, M. | 2019 | Germany | 170 | Caucasian | 109/61 | CCA | NR | Retrospective | Surgery | OS | 19 (11.1) | 6 |
| Lim, Y. J. | 2015 | Korea | 83 | Asian | 61/22 | eCCA | 27 | Retrospective | Surgery | OS, DFS | 56 (83) | 7 |
| Lu, J. C. | 2019 | China | 320 | Asian | 19/129 | iCCA | To Oct 2016 | Retrospective | Surgery | OS, DFS | 99 (30.9) | 7 |
| Ma, K. | 2017 | China | 70 | Asian | 38/32 | eCCA | To Mar 2015 | Retrospective | Surgery | OS | 30 (42.9) | 7 |
| Sangkhamanon, S. | 2017 | Thailand | 46 | Asian | 33/13 | CCA | NR | Retrospective | Surgery | OS | 18 (39.1) | 6 |
| Tamai, K. | 2014 | Japan | 91 | Asian | 62/29 | eCCA | NR | Retrospective | Surgery | OS | 77 (84.6) | 6 |
| Ueno, T. | 2018 | Japan | 117 | Asian | 93/24 | eCCA | 27 (0-189) | Retrospective | Surgery | OS | 10 (8.5) | 7 |
| Walter, D. | 2017 | Germany | 69 | Caucasian | 50/19 | eCCA | 23 (0-100) | Retrospective | Surgery | OS | 8 (11.6) | 7 |
| Yu, F. | 2019 | China | 62 | Asian | 41/21 | eCCA | NR | Retrospective | Surgery | OS, DFS | 20 (32.3) | 6 |
| Zhu, Y. | 2018 | China | 192 | Asian | 115/77 | iCCA | 24 (0.4-85) | Retrospective | Surgery | OS, DFS | 34 (17.7) | 7 |
CCA: cholangiocarcinoma; eCCA: extrahepatic cholangiocarcinoma; iCCA: intrahepatic cholangiocarcinoma; NR: not reported; OS: overall survival; DFS: disease-free survival; NOS: Newcastle-Ottawa scale. CCA includes iCCA and eCCA.
Table 2.
Immunohistochemical technique used in the studies included in the meta-analysis.
| Author | Year | Detection method | Primary antibody | Source | Cut-off value | |||
|---|---|---|---|---|---|---|---|---|
| Antibody | Specie | Clone | Dilution | |||||
| Ahn, S. | 2019 | IHC | Anti-PD-L1 | Mouse, MAB | 22C3 | 1 : 100 | Dako, Carpinteria, CA, USA | 1% |
| Arkenau, H. T. | 2018 | IHC | Anti-PD-L1 | Mouse, MAB | 22C3 | NR | Agilent, Carpinteria, CA | 1% |
| Dong, Z. T. | 2020 | IHC | Anti-PD-L1 | MAB | NR | NR | Cell Signaling Technology, Inc. Danvers, MA, USA | 5% |
| Gani, F. | 2016 | IHC | Anti-PD-L1 | Mouse, MAB | 5H1 | NR | NR | 5% |
| Gou, M. M. | 2019 | IHC | Anti-PD-L1 | NR | NR | NR | NR | 1% |
| Jing, C. Y. | 2019 | IHC | Anti-PD-L1 | Rabbit, MAB | E1L3N | 1 : 200 | Cell Signaling Technology, MA, USA | 5% |
| Kim, R. | 2018 | IHC | Anti-PD-L1 | Mouse, MAB | 5H1 | NR | NR | 1% |
| Kitano, Y. | 2020 | IHC | Anti-PD-L1 | Rabbit, MAB | E1L3N | 1 : 200 | Cell Signaling Technology, Tokyo, Japan | 5% |
| Kriegsmann, M. | 2019 | IHC | Anti-PD-L1 | NR | SP263 | NR | Roche AG, Rotkreuz, Switzerland | 1% |
| Lim, Y. J. | 2015 | IHC | Anti-PD-L1 | Rabbit, MAB | E1L3N | 1 : 100 | Cell Signaling Technology, Danvers, MA, USA | H-score 5 |
| Lu, J. C. | 2019 | IHC | Anti-PD-L1 | Rabbit, MAB | SP142 | 1 : 100 | GeneTech Co. Ltd., Shanghai, China | 5% |
| Ma, K. | 2017 | IHC | Anti-PD-L1 | Rabbit, MAB | NR | 1 : 250 | Abcam, Cambridge, MA, USA | 5% |
| Sangkhamanon, S. | 2017 | IHC | Anti-PD-L1 | NR | NR | 1 : 1000 | Roche Diagnostic GmbH, USA | 1% |
| Tamai, K. | 2014 | IHC | Anti-CD274 | Rabbit, PAB | NR | NR | Abcam, Cambridge, MA, USA | ++ |
| Ueno, T. | 2018 | IHC | Anti-PD-L1 | NR | NR | NR | NR | 5% |
| Walter, D. | 2017 | IHC | Anti-PD-L1 | Rabbit, MAB | E1L3N | 1 : 50 | Cell Signaling Technology, Danvers, MA, USA | Score 3 |
| Yu, F. | 2019 | IHC | Anti-PD-L1 | Rabbit, MAB | E1L3N | 1 : 200 | Cell Signaling Technology, Danvers, MA, USA | Score 3 |
| Zhu, Y. | 2018 | IHC | Anti-PD-L1 | Rabbit, MAB | SP142 | 1 : 50 | Spring Bioscience, Inc., CA, USA | 5% |
MAB: monoclonal antibody; IHC: immunohistochemistry; NR: not reported; PAB: polyclonal antibody.
3.2. Prognostic Value of PD-L1 in OS, DFS, and Subgroup Analysis
Seventeen studies [11–14, 16–28] with a total of 1982 patients reported a correlation between PD-L1 and OS. The pooled HR and 95% CI suggested that overexpression of PD-L1 was significantly correlated with worse OS (HR = 1.58, 95%CI = 1.30 − 1.92, p < 0.001) in patients with CCA (Figure 2; Table 3). For DFS, a total of 7 studies [11, 12, 15, 20, 21, 27, 28] with 896 patients provided the relevant data. The forest plot (Figure 3) showed that PD-L1 expression was not significantly correlated with DFS (HR = 1.03, 95%CI = 0.68 − 1.55, p = 0.895) (Table 3). To further investigate the source of heterogeneity, subgroup analysis stratified by ethnicity (Caucasian and Asian), tumor location (iCCA, eCCA, and CCA), and treatment (surgery and nonsurgery) was performed for OS and DFS. For OS, PD-L1 overexpression remained a prognostic factor for patients of Caucasian (HR = 2.14, 95%CI = 1.52 − 3.02, p < 0.001) and Asian (HR = 1.49, 95%CI = 1.20 − 1.84, p < 0.001) ethnicity; and for those receiving surgery (HR = 1.61, 95%CI = 1.32 − 1.95, p < 0.001) (Table 3). Notably, regarding tumor location, high PD-L1 expression was a prognostic factor for patients with eCCA (HR = 1.71, 95%CI = 1.25 − 2.36, p < 0.001) and CCA including iCCA and eCCA (HR = 1.98, 95%CI = 1.47 − 2.65, p < 0.001). However, elevated PD-L1 expression did not correlate with worse OS in patients with iCCA (HR = 1.31, 95%CI = 0.88 − 1.95, p = 0.180) (Table 3). For DFS, the subgroup analysis indicated that PD-L1 overexpression had no significant prognostic value regardless of ethnicity, treatment method, or tumor location (Table 3).
Figure 2.
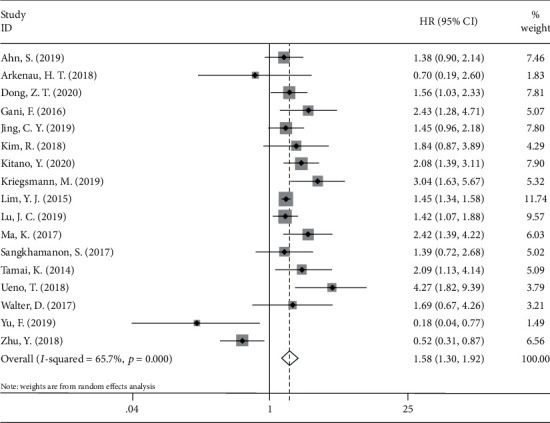
Forest plots for the association between PD-L1 expression and overall survival.
Table 3.
Subgroup analysis of the prognostic value of PD-L1 in OS and DFS in CCA.
| Subgroup factors | No. of studies | No. of patients | HR (95% CI) | p | Effects model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I 2 (%) | p | ||||||
| Overall survival | |||||||
| Total | 17 | 1982 | 1.58 (1.30-1.92) | <0.001 | REM | 65.7 | <0.001 |
| Ethnicity | |||||||
| Caucasian | 5 | 363 | 2.14 (1.52-3.02) | <0.001 | FEM | 11.8 | 0.338 |
| Asian | 12 | 1619 | 1.49 (1.20-1.84) | <0.001 | REM | 70.7 | <0.001 |
| Treatment | |||||||
| Surgery | 16 | 1956 | 1.61 (1.32-1.95) | <0.001 | REM | 67.0 | <0.001 |
| Nonsurgery | 1 | 26 | 0.70 (0.19-2.60) | 0.595 | — | — | — |
| Tumor location | |||||||
| iCCA | 5 | 844 | 1.31 (0.88-1.95) | 0.180 | REM | 76.9 | 0.002 |
| eCCA | 8 | 719 | 1.71 (1.25-2.36) | <0.001 | REM | 63.1 | 0.008 |
| CCA | 4 | 419 | 1.98 (1.47-2.65) | <0.001 | FEM | 44.2 | 0.146 |
| Disease-free survival | |||||||
| Total | 7 | 896 | 1.03 (0.68-1.55) | 0.895 | REM | 72.6 | 0.001 |
| Ethnicity | |||||||
| Caucasian | 1 | 26 | 1.13 (0.55-2.34) | 0.742 | — | — | — |
| Asian | 6 | 870 | 1.00 (0.62-1.61) | 0.991 | REM | 77.2 | 0.001 |
| Treatment | |||||||
| Surgery | 5 | 840 | 0.97 (0.56-1.69) | 0.911 | REM | 81.7 | <0.001 |
| Non-surgery | 2 | 56 | 1.12 (0.67-1.85) | 0.667 | FEM | 0 | 0.965 |
| Tumor location | |||||||
| iCCA | 2 | 512 | 1.02 (0.36-2.92) | 0.972 | REM | 92.2 | <0.001 |
| eCCA | 3 | 328 | 0. (0.40-2.03) | 0.800 | REM | 77.1 | 0.013 |
| CCA | 2 | 56 | 1.12 (0.67-1.85) | 0.667 | FEM | 0 | 0.965 |
FEM: fixed-effects model; REM: random-effects model.
Figure 3.
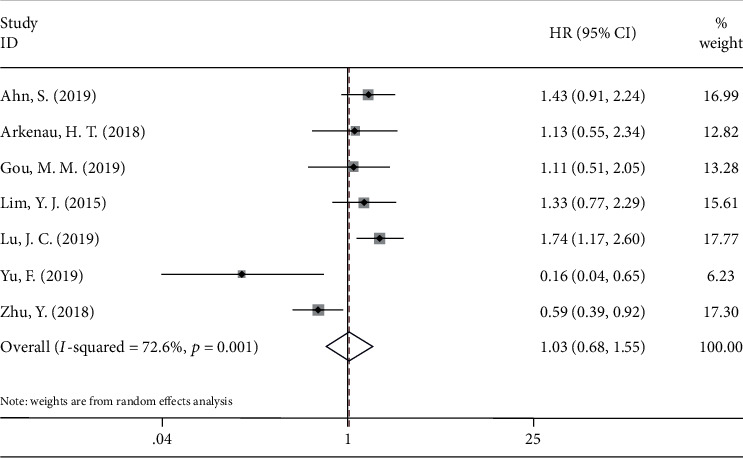
Forest plots for the association between PD-L1 expression and disease-free survival.
3.3. PD-L1 and Clinicopathological Characteristics of CCA
Thirteen studies [11, 13, 16, 18, 19, 21–28] investigated the relationship between PD-L1 and the following eight clinicopathological factors: sex (male vs. female), tumor differentiation (poor vs. well/moderate), pN stage (III+IV vs. I+II), TNM stage (III+IV vs. I+II), vascular invasion (yes vs. no), perineural invasion (yes vs. no), age (>60 vs. ≤60), and tumor size (>5 cm vs. ≤5 cm). As shown in Figure 4, high PD-L1 expression was correlated with poor differentiation (OR = 1.43, 95%CI = 1.09 − 1.87, p = 0.010) and higher pN stage (OR = 1.45, 95%CI = 1.10 − 1.92, p = 0.009). However, no significant correlation was found between PD-L1 and sex (OR = 1.23, 95%CI = 0.95 − 1.58, p = 0.114), TNM stage (OR = 1.42, 95%CI = 0.83 − 2.45, p = 0.204), vascular invasion (OR = 1.28, 95%CI = 0.69 − 2.38, p = 0.431), perineural invasion (OR = 1.00, 95%CI = 0.59 − 1.68, p = 0.994), age (OR = 0.90, 95%CI = 0.61 − 1.33, p = 0.609), and tumor size (OR = 0.97, 95%CI = 0.70 − 1.33, p = 0.828).
Figure 4.
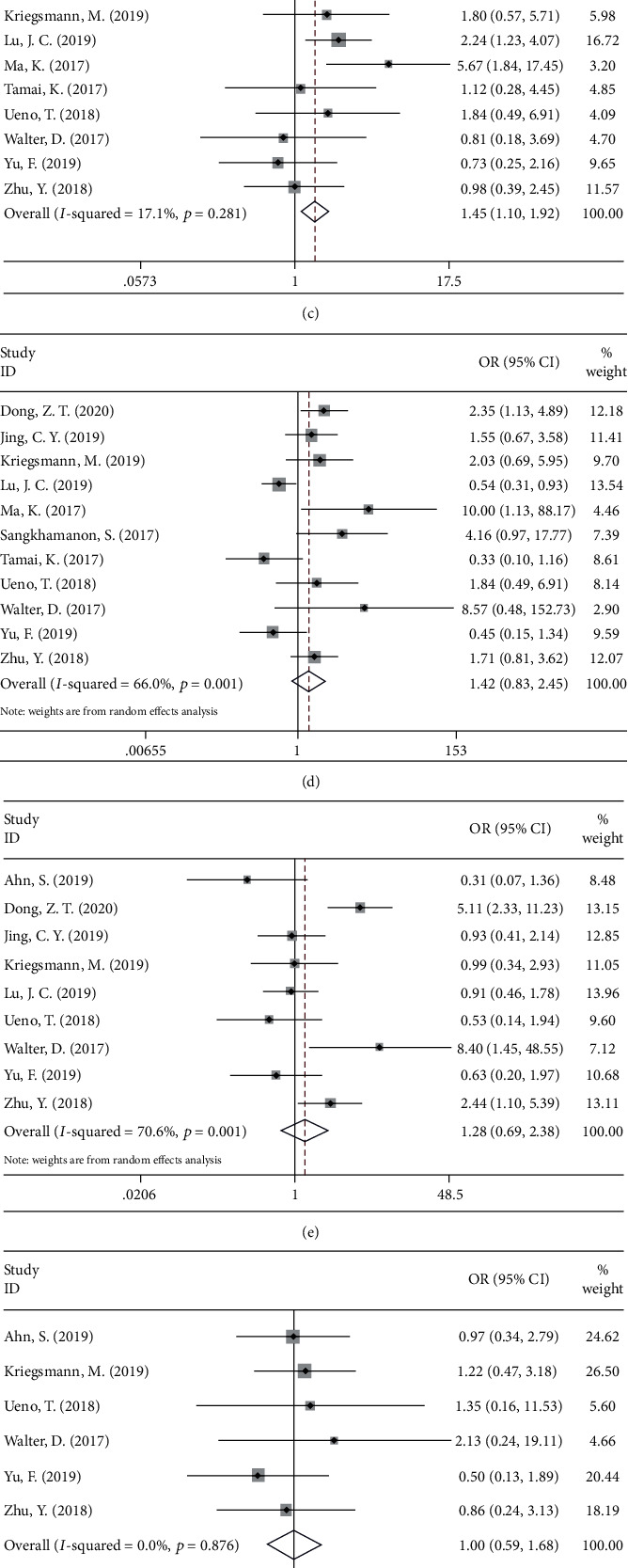
Forest plots of ORs for the association between PD-L1 expression and (a) sex (male vs. female), (b) tumor differentiation (poor vs. well/moderate), (c) pN stage (III+IV vs. I+II), (d) TNM stage (III+IV vs. I+II), (e) vascular invasion (yes vs. no), (f) perineural invasion (yes vs. no), (g) age (>60 vs. ≤60), and (h) tumor size (>5 cm vs. ≤5 cm).
3.4. Publication Bias
Begg's funnel plot and Egger's tests were applied to evaluate the publication bias in this meta-analysis. There was no obvious publication bias for OS (Begg's test of p = 0.902, Egger's test of p = 0.670) or DFS (Begg's test of p = 0.230, Egger's test of p = 0.266).
4. Discussion
CCA is an aggressive cancer, and most patients present at an advanced stage at the time of diagnosis [32, 33]. The current meta-analysis containing 18 studies with 2012 patients showed that high PD-L1 expression was a significant prognostic factor for low OS (HR = 1.58). Particularly, the mortality risk of patients with CCA with high PD-L1 expression increased by 58% compared with that of patients with low PD-L1 expression. PD-L1 expression was not significantly correlated with DFS. In addition, we found that PD-L1 was positively associated with poor differentiation and higher pN stage in CCA. Generally, these results demonstrated that PD-L1 overexpression was associated with invasive clinical features and suggested poorer prognosis of CCA.
The tumor microenvironment in CCA consists of cancer cells, stromal cells, and various immune cells including CCA cells, cancer-associated fibroblasts, tumor-associated macrophages, tumor-infiltrating lymphocytes, and CD8+ cytotoxic T lymphocytes [34]. PD-1 is expressed on B cells, activated CD4+ and CD8+ T cells, and dendritic cells [35]. PD-L1 is a ligand of PD-1 and is expressed on different cell types [36]. Targeting PD-1/PD-L1 is a new strategy for cancer immunotherapy [37]. Recent studies showed that nivolumab (a PD-1 inhibitor) showed considerable safety in patients with metastatic CCA [15]. PD-L1 is mainly expressed by intertumoral immune cells in CCA [38]. Thus, the overexpression of PD-L1 may lead to immune tolerance in the tumor environment and result in tumor progression. This could be a possible mechanism for the correlation between PD-L1 elevation and poor differentiation in CCA.
Recent studies have demonstrated that PD-L1 overexpression is associated with unfavorable prognosis in various types of cancer [39, 40]. A recent meta-analysis showed that high expression of PD-L1 was significantly associated with a poor OS (HR = 1.22, 95%CI = 1.01 − 1.48, p = 0.04) in colorectal cancer [41]. Another meta-analysis including 13 studies also demonstrated that tumor cell PD-L1 expression was correlated with poor OS (HR = 2.128, 95%CI : 1.341–3.378, p = 0.001) in patients with diffuse large B-cell lymphoma [42]. These findings were consistent with the results of this study. Notably, in the current meta-analysis, we included studies using the IHC method to detect PD-L1. The antibodies used for PD-L1 and cutoff values vary among the included studies, which may result in heterogeneity. However, we failed to observe a significant prognostic role of PD-L1 in DFS in patients with CCA. This negative result may be due to the limited sample size, wherein only 7 studies with 896 patients were included for DFS analysis.
Notably, several limitations of this study should be acknowledged. First, the cutoff values of PD-L1 varied in the included studies (Table 2), which may introduce heterogeneity. Further investigations used uniform antibody and a cut-off value of PD-L1 are needed. Second, only one included study was a prospective trial, and the remaining were retrospective studies. Therefore, high-quality prospective studies are still needed. Third, some HRs and 95% CIs were calculated according to survival curves, which may not be as precise as the original data. Fourth, the sample was relatively small. Only 2012 patients were enrolled and most patients were of Asian ethnicity. More studies recruiting patients of diverse ethnicities were needed to verify the results of this meta-analysis. Because of these limitations, well-designed large cohort studies or randomized controlled trials may be recommended to confirm our findings.
5. Conclusions
Our study indicates that PD-L1 was associated with worse OS, poor differentiation, and higher pN stage in patients with CCA. PD-L1 could be a potential prognostic marker for CCA.
Acknowledgments
This study was supported by the Medical and Health Science and Technology Program of Zhejiang Province (2019RC076).
Contributor Information
Qinfen Xie, Email: qinfen.xie@shulan.com.
Shusen Zheng, Email: zyzss@zju.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Q.X. and L.W. collected and analyzed the data and wrote the paper; S.Z. analyzed the data; Q.X. and L.W. conceived and designed this study, analyzed the data, and wrote the paper; and all authors reviewed the paper. All authors read and approved the final manuscript.
References
- 1.Lendvai G., Szekerczés T., Illyés I., et al. Cholangiocarcinoma: classification, histopathology and molecular carcinogenesis. Pathology & Oncology Research. 2020;26(1):3–15. doi: 10.1007/s12253-018-0491-8. [DOI] [PubMed] [Google Scholar]
- 2.Kirstein M. M., Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visceral Medicine. 2016;32(6):395–400. doi: 10.1159/000453013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist A., von Seth E. Epidemiology of cholangiocarcinoma. Best Practice & Research Clinical Gastroenterology. 2015;29(2):221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Mavros M. N., Economopoulos K. P., Alexiou V. G., Pawlik T. M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma systematic review and meta-analysis. JAMA Surgery. 2014;149(6):565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 5.Squires M. H., Woelfel I., Cloyd J. M., Pawlik T. M. Emerging treatment options for cholangiocarcinoma. Expert Opinion on Orphan Drugs. 2018;6(9):527–536. doi: 10.1080/21678707.2018.1476235. [DOI] [Google Scholar]
- 6.Saengboonmee C., Sawanyawisuth K., Chamgramol Y., Wongkham S. Prognostic biomarkers for cholangiocarcinoma and their clinical implications. Expert Review of Anticancer Therapy. 2018;18(6):579–592. doi: 10.1080/14737140.2018.1467760. [DOI] [PubMed] [Google Scholar]
- 7.Paillet J., Kroemer G., Pol J. G. Immune contexture of cholangiocarcinoma. Current Opinion in Gastroenterology. 2020;36(2):70–76. doi: 10.1097/MOG.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. International Immunology. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 9.Li B., VanRoey M., Wang C., Chen T. H. T., Korman A., Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor-secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clinical Cancer Research. 2009;15(5):1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X., Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–97682. doi: 10.18632/oncotarget.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn S., Lee Y., Kim J. W., et al. Programmed cell death ligand-1 (PD-L1) expression in extrahepatic biliary tract cancers: a comparative study using 22C3, SP263 and E1L3N anti-PD-L1 antibodies. Histopathology. 2019;75(4):526–536. doi: 10.1111/his.13901. [DOI] [PubMed] [Google Scholar]
- 12.Arkenau H. T., Martin-Liberal J., Calvo E., et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF) The Oncologist. 2018;23(12, article e136):p. 1407. doi: 10.1634/theoncologist.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Z., Liao B., Shen W., Sui C., Yang J. Expression of programmed death ligand 1 is associated with the prognosis of intrahepatic cholangiocarcinoma. Digestive Diseases and Sciences. 2020;65(2):480–488. doi: 10.1007/s10620-019-05787-0. [DOI] [PubMed] [Google Scholar]
- 14.Gani F., Nagarajan N., Kim Y., et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Annals of Surgical Oncology. 2016;23(8):2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 15.Gou M., Zhang Y., Si H., Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. OncoTargets and Therapy. 2019;Volume 12:861–867. doi: 10.2147/OTT.S195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing C. Y., Fu Y. P., Yi Y., et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. Journal for Immunotherapy of Cancer. 2019;7(1):p. 77. doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R., Coppola D., Wang E., et al. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(34):23366–23372. doi: 10.18632/oncotarget.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitano Y., Yamashita Y. I., Nakao Y., et al. Clinical significance of PD-L1 expression in both cancer and stroma cells of cholangiocarcinoma patients. Annals of Surgical Oncology. 2020;27(2):599–607. doi: 10.1245/s10434-019-07701-4. [DOI] [PubMed] [Google Scholar]
- 19.Kriegsmann M., Roessler S., Kriegsmann K., et al. Programmed cell death ligand 1 (PD-L1, CD274) in cholangiocarcinoma - correlation with clinicopathological data and comparison of antibodies. BMC Cancer. 2019;19(1):p. 72. doi: 10.1186/s12885-018-5254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim Y. J., Koh J., Kim K., et al. High ratio of programmed cell death protein 1 (PD-1)+/CD8+ tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiotherapy and Oncology. 2015;117(1):165–170. doi: 10.1016/j.radonc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Lu J. C., Zeng H. Y., Sun Q. M., et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics. 2019;9(16):4678–4687. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma K., Wei X., Dong D., Wu Y., Geng Q., Li E. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncology Letters. 2017;14(1):250–256. doi: 10.3892/ol.2017.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangkhamanon S., Jongpairat P., Sookprasert A., et al. Programmed death-ligand 1 (PD-L1) expression associated with a high neutrophil/lymphocyte ratio in cholangiocarcinoma. Asian Pacific Journal of Cancer Prevention. 2017;18(6):1671–1674. doi: 10.22034/APJCP.2017.18.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamai K., Nakamura M., Mizuma M., et al. Suppressive expression of CD274 increases tumorigenesis and cancer stem cell phenotypes in cholangiocarcinoma. Cancer Science. 2014;105(6):667–674. doi: 10.1111/cas.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno T., Tsuchikawa T., Hatanaka K. C., et al. Prognostic impact of programmed cell death ligand 1 (PD-L1) expression and its association with epithelial-mesenchymal transition in extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(28):20034–20047. doi: 10.18632/oncotarget.25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter D., Herrmann E., Schnitzbauer A. A., et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71(3):383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 27.Yu F., Gong L., Mo Z., et al. Programmed death ligand-1, tumor infiltrating lymphocytes and HLA expression in Chinese extrahepatic cholangiocarcinoma patients: possible immunotherapy implications. Bioscience Trends. 2019;13(1):58–69. doi: 10.5582/bst.2019.01003. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y., Wang X. Y., Zhang Y., et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. Cancer Management and Research. 2018;Volume 10:4113–4123. doi: 10.2147/CMAR.S172719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1) doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells G. A., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 32.Jarnagin W. R., Fong Y., DeMatteo R. P., et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of Surgery. 2001;234(4):507–519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi S., Khan S. A., Hallemeier C. L., Kelley R. K., Gores G. J. Cholangiocarcinoma -- evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabris L., Perugorria M. J., Mertens J., et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver International. 2019;39(S1):63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber D. L., Wherry E. J., Masopust D., et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 36.Dong H., Strome S. E., Salomao D. R., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Medicine. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 37.Constantinidou A., Alifieris C., Trafalis D. T. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacology & Therapeutics. 2019;194:84–106. doi: 10.1016/j.pharmthera.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Fontugne J., Augustin J., Pujals A., et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(15):24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y., Wen X., Cho N. Y., Kang G. H. Intratumoral immune cells expressing PD-1/PD-L1 and their prognostic implications in cancer: a meta-analysis. International Journal of Biological Markers. 2018;33(4):467–474. doi: 10.1177/1724600818770941. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Liu F., Liu L. Prognostic significance of PD-L1 in solid tumor an updated meta-analysis. Medicine. 2017;96(18):p. e6369. doi: 10.1097/md.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Z., Gu L., Mao D., Chen M., Jin R. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. World Journal of Surgical Oncology. 2019;17(1):p. 4. doi: 10.1186/s12957-018-1544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu L., Zheng H., Zhao X. The prognostic and clinicopathological significance of PD-L1 expression in patients with diffuse large B-cell lymphoma: a meta-analysis. BMC Cancer. 2019;19(1):p. 273. doi: 10.1186/s12885-019-5466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


