Abstract
Background
A large number of stroke survivors worldwide suffer from moderate to severe disability. In Malaysia, long-term uncontrolled stroke risk factors lead to unforeseen rates of recurrent stroke and a growing incidence of stroke occurrence across ages, predominantly among the elderly population. This situation has motivated research efforts focused on tapping into patient education, especially related to patient self-efficacy of understanding and taking medication appropriately. Video narratives integrated with health belief model constructs have demonstrated potential impacts as an aide to patient education efforts.
Objective
The aim of this study was to investigate the feasibility and acceptability of study procedures based on a randomized controlled trial protocol of a video narratives intervention among poststroke patients. We also aimed to obtain preliminary findings of video narratives related to medication understanding and use self-efficacy (MUSE) and blood pressure control.
Methods
A parallel group randomized controlled trial including a control group (without video viewing) and an intervention group (with video viewing) was conducted by researchers at a neurology outpatient clinic on poststroke patients (N=54). Baseline data included patients’ sociodemographic characteristics, medical information, and all outcome measures. Measurements of MUSE and blood pressure following the trial were taken during a 3-month follow-up period. Feasibility of the trial was assessed based on recruitment and study completion rates along with patients’ feedback on the burden of the study procedures and outcome measures. Acceptability of the trial was analyzed qualitatively. Statistical analysis was applied to ascertain the preliminary results of video narratives.
Results
The recruitment rate was 60 out of 117 patients (51.3%). Nevertheless, the dropout rate of 10% was within the acceptable range. Patients were aged between 21 and 74 years. Nearly 50 of the patients (>85%) had adequate health literacy and exposure to stroke education. Most of the patients (>80%) were diagnosed with ischemic stroke, whereby the majority had primary hypertension. The technicalities of randomization and patient approach were carried out with minimal challenge and adequate patient satisfaction. The video contents received good responses with respect to comprehension and simplicity. Moreover, an in-depth phone interview with 8 patients indicated that the video narratives were considered to be useful and inspiring. These findings paralleled the preliminary findings of significant improvement within groups in MUSE (P=.001) and systolic blood pressure control (P=.04).
Conclusions
The queries and feedback from each phase in this study have been acknowledged and will be taken forward in the full trial.
Trial Registration
Australian New Zealand Clinical Trials Registry ACTRN 12618000174280; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373554
Keywords: feasibility and acceptability, medication understanding, use self-efficacy, stroke, video narratives
Introduction
Background
Establishing a patient narrative is a common method for analyzing how individuals with illnesses express themselves to best recognize and reflect the values and teachings that are most important to them and how they react toward their actions [1]. Personal and interpersonal factors such as coping strength and family or social support form the basis of these narratives [2]. Thus, a narrative can influence changes in health behaviors toward achieving appropriate health outcomes [3,4]. Narratives incorporated in multimedia format can effectively deliver patients’ stories to viewers who can then become “carried away” by their peers’ experiences and help them to learn from others [5]. A high percentage of the delivered information in patient education offers engagement via the visual and hearing senses. Therefore, the use of video narratives offers a great chance of proper comprehension and reflection among patients [6].
Video narratives have long been explored and developed for various patient education purposes in chronic disease management [7-9]. However, there are limited studies on video narrative-based interventions in poststroke patients, and their outcome measures varied in terms of the severity of the disease and psychosocial challenges [10,11]. In Malaysia, the ischemic stroke incidence has shown an increase of approximately 30% annually, with an increase of approximately 19% for hemorrhagic stroke, which is a more prominent condition among the aging community [12,13]. The majority of poststroke patients experience physical disability, learning, and speech impairment, which also lead to emotional problems [14,15]. Hence, an individual who experienced stroke will benefit from resilience, which requires self-efficacy [16,17]. Medication nonadherence had been associated with a lack of self-efficacy in poststroke patients, especially with regard to understanding and taking medication [18]. Thus, patient education efforts that focus on enhancing medication understanding and use self-efficacy (MUSE) are warranted. Indeed, sustaining medication adherence is crucial to achieve optimal recurrent stroke treatment effects [19]. Despite the advancement of stroke prevention treatment, medication nonadherence prevalence remains notable among patients with high stroke risk factors such as hypertension and cardiac disease [20,21]. Social learning theory explains that a person’s behavior depends on adaptation of their thoughts and beliefs, which are influenced by the environment and in turn control the individual’s actions [22]. This theory proposes that medication adherence relates to an individual’s perception of health issues, which influences self-efficacy toward prescribed medication [23,24].
There are limited studies aimed at understanding the use of video narratives with the integration of health belief constructs and motivational cues. In addition, little is known about the effect of video narratives on poststroke patients in particular. Stroke survivors require motivational support, which could help them to enhance their effort in understanding prescribed medication and taking it appropriately [25]. We believe that video narratives offer an opportunity to facilitate the existing stroke patient education effort of the medication therapeutic adherence clinic (MTAC) [26]. Moreover, the outpatient clinic waiting time and area offer a potential period and venue for patients to receive these inputs [27]. Thus, we aimed to evaluate the feasibility and acceptability of a video narratives randomized controlled trial (RCT) among poststroke patients in Malaysia.
Objectives
This study was an a priori phase of a powered RCT [28] focused on determining the recruitment, retention, and completion rate of the trial. Patients’ qualitative feedback and views were collected with respect to the acceptability of the videos. We also analyzed the preliminary changes of MUSE over the course of the intervention and compared the findings with a control group.
Methods
Ethical Considerations
The study received ethics approval from the Malaysian Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-15-851-24737) and the Monash University Human Research Ethics Committee (ID 9640).
Sample Size, Eligibility, and Randomization
Given the lack of similar studies, there was no referral for appropriate effect sizes. Moreover, a feasibility study without inferential results does not necessarily require a power analysis [29]. Therefore, we estimated the sample size based on practical considerations and experience of the researchers [30,31]. This pretest and posttest design, two-arm RCT was conducted from March 2018 to June 2018 among informed and consenting stroke survivors who had clinic appointments at the Neurology Outpatient Department of Hospital Kuala Lumpur (HKL), Malaysia. We aimed to recruit a minimum of 25 patients per group. Eligible and consenting patients were adults diagnosed with their first stroke within 6 months of the recruitment period, and were prescribed stroke risk preventative medications from HKL. Those excluded were diagnosed with depression (Patient Health Questionnaire score≥1) and cognitive impairment (Montreal Cognitive Assessment score<26). We only included patients who could comprehend the English or Malay language.
Randomization was performed via the block method between 2, 4, and 6 lengths placed in opaque envelopes. The allocations of each block were also randomized. Patients were either allocated to the standard care (control) or intervention (with video viewing) group. The full description of the study’s methodology is available in our protocol trial report [28].
Video Narratives
Based on research interest and considering the motivational need of poststroke patients, we developed a set of video narratives incorporated with health belief model constructs. The validation procedures and narrative contents in the English and Malay languages have been described in detail in our previous paper [32]. The video narratives provided messages (culturally appropriate for the local context), which served as triggers to motivate patients to be resilient in attaining self-efficacy skills as per their perceived needs. To reflect the purpose of role models, a neurologist and a stroke survivor volunteered to narrate their story in a video to render their honest emotion while stressing the need to adhere to stroke preventative medication (see Multimedia Appendix 1 and Multimedia Appendix 2). Short quotes and subtitles were incorporated to increase attentiveness toward the comprehension of their messages [33].
Intervention Design and Study Procedures
The groups in this RCT received treatment with ongoing patient education and counseling as per HKL neurologists’ recommendations. The treatment compliance practice included MTAC appointments, self-monitoring checks, and outpatient clinic attendance. Both groups received pamphlets on stroke awareness and its preventative medication information, and the “teach-back method” was used to help reduce discrepancies between the two groups [34,35]. The “teach-back” queries were related to medication dose, frequency, indication, and time as recommended by the MTAC. In addition to this standard care, only the intervention group received face-to-face video narratives. Figure 1 illustrates the CONsolidated Standards Of Reporting Trials (CONSORT) flowchart showing patients’ participation throughout the study at data collection time points. We collected the quantitative data at baseline (T0) and 3 months postrandomization (T1), and collected qualitative data via a semistructured interview upon completion of the study. Similar data collection and follow-up procedures as applied in the main study protocol were followed [28]. Blinding was impossible for the patients. This also includes the researchers who conducted the assessment of the questionnaire, except for the treating neurologists.
Figure 1.
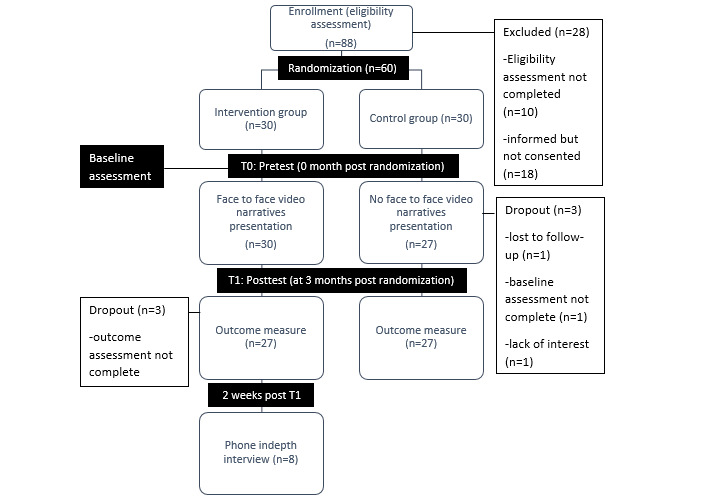
CONsolidated Standards Of Reporting Trials (CONSORT) flowchart of poststroke patients throughout the study.
Outcome Measures
Main Outcome Measure
The main outcome measure assessed at pretest (T0) and posttest (T1) referred to MUSE. The MUSE is assessed on an 8-item Likert-type scale with total scores ranging from 0 to 32, which measures the perceived self-efficacy in understanding and taking prescribed medication. It has good internal consistency (Cronbach α >.70) with adequate construct and predictive validity [36]. We repeated MUSE for each prescribed stroke preventative medication.
Understanding and taking medication is also associated with other factors such as knowledge, perception, or belief [37]. Therefore, we aimed to observe changes in these factors as well. Hence, the feasibility study assessed the baseline of the following secondary outcome measures.
Stroke Knowledge Test
The stroke knowledge test of 20 items is a measure of stroke knowledge, which is vital in evaluating the quality of stroke education modules. The stroke knowledge test has received acceptable and favorable ratings from health and educational experts, which reflected its excellent reliability and construct validity [38].
Brief Illness Perception Questionnaire
The brief illness perception questionnaire (BIPQ) is assessed on a 9-item Likert scale with a 0 to 10 scoring range that intends to evaluate the perceptive and emotive illustrations of the effect of an illness on a patient. This measure demonstrated good test-retest reliability and high concurrent and discriminant validity [39].
Belief About Medicine Questionnaire
Belief About Medicine Questionnaire (BMQ) is an 18-item questionnaire with two constructs: BMQ-General and BMQ-Specific. Both constructs are further divided into the subscales of overuse harm and necessity concerns. The scales of both constructs have acceptable internal consistency, discriminant validity, and reliability [40]. This measure assesses general and specific medication beliefs and perceptions. Nevertheless, only the BMQ-Specific construct measure was repeated for each prescribed stroke preventative medication in this study.
Short Form (36) Health Survey
Short Form (36) Health Survey (SF-36) comprises a 36-item questionnaire with scoring between 0 and 100. The SF-36 is able to measure perceived physical and mental constraints. It has been translated, validated with acceptable psychometric properties, and used widely in various clinical settings to measure the overall health state [41].
Other Measures
Other measures included systolic and diastolic blood pressure, fasting blood glucose, total cholesterol, and international normalized ratio.
We received the “permission to use” from the authors of the original (English) and the translated (Malay language) versions of the questionnaires, deemed to be locally appropriate for this study [42-45]. The researchers also conducted a face and content validity analysis with 5 patients and experts prior to this study to confirm the understandability and the content validity index>0.80 of MUSE, BMQ-specific, and BIPQ for the named medication(s), for which the word “illness” was replaced with “stroke” in the measures.
Data Collection Procedures
We obtained the patients’ sociodemographic and health information using a data collection form that recorded gender, age, ethnicity, educational attainment, and health literacy status using the Newest Vital Sign format [46]. Concurrently, we retrieved clinical health data on the type of stroke and stroke risk factors from the hospital’s patient medical records. All consenting patients from the intervention group had the option to volunteer to participate in a 10-minute phone or face-to-face interview within 2 weeks of follow up from the data collection point (T1). This was conducted to obtain feedback on the burden of outcome measures (questionnaires) and the acceptability of viewing the video as an intervention. The researchers maintained data confidentiality and patients’ safety as per the protocol [28].
Data Analysis
Quantitative Data
Statistical analyses were performed using IBM SPSS Statistics V.24.0 with P<.05 as the threshold significance level. Descriptive statistics (eg, means and percentages) were used to describe the characteristics of both the control and intervention groups, along with the study and intervention’s feasibility and acceptability. Chi square tests were applied to explore dissimilarities in patient characteristics between both groups at baseline. Data differences over time between the two time points (T0 and T1), at intergroup and intragroup levels, were also analyzed for the outcome measures using the Mann-Whitney U test and the Wilcoxon rank-sum test. The results for outcome measures and mean differences were calculated as means (SD), range, or 95% CI as appropriate. Multiple imputation was applied for missing data.
Qualitative Data
The phone interview recordings were transcribed and translated verbatim. Two researchers reviewed the transcripts, wherein they occasionally met to discuss the developed themes. The themes were then verified by another researcher to assure uniformity and quality. The transcripts and written feedback were analyzed using thematic analysis [47]. We applied the software NVivo 11 (qualitative data analysis software; QSR International Pty Ltd, Version 11, 2015) to identify the themes and to help in organizing the codes.
Results
Participant Characteristics
Table 1 presents the sociodemographic and health information of patients who participated in and completed the trial over the full study period. Both groups comprised more men than women with a dominance of more than 50%, and most patients were predominantly of Malay ethnicity in both groups (>80%). Patients were between 21 and 74 years old, with a mean age of 56 years (SD 13.1) for the control group and 53 years (SD 11.6) for the intervention group. Nearly 50 participants (>85%) had secondary education, which included tertiary attainment, with adequate health literacy and exposure to stroke education. More than half of the patients were unemployed. The majority of patients (>80%) had experienced ischemic stroke and had several underlying stroke risk factors inclusive of hypertension, but not all controllable risk factors were documented, such as diet, obesity, and physical inactivity, due to lack of data in medical records. Among them, approximately 25 patients (50%) had diabetes, and more than 50 patients (about 90%) were taking at least three types of stroke preventative medication. There were no significant differences in sociodemographic characteristics and measures between the two groups, except for gender.
Table 1.
Sociodemographic and health data of patients at 3-month follow up (N=54).
| Characteristic | Control (n=27) | Intervention (n=27) | |
| Gender, n (%) |
|
|
|
|
|
Male | 18 (67) | 15 (56) |
|
|
Female | 9 (33) | 12 (44) |
| Age (years), n (%) |
|
|
|
|
|
≥60 | 10 (37) | 8 (30) |
|
|
40-59 | 13 (48) | 16 (59) |
|
|
≤39 | 4 (15) | 3 (11) |
| Age (years), mean (SD) | 56 (13.1) | 53 (11.6) | |
| Ethnicity, n (%) |
|
|
|
|
|
Malay | 22 (82) | 23 (85) |
|
|
Chinese | 1 (4) | 1 (4) |
|
|
Indian | 4 (15) | 3 (11) |
| Education attainment, n (%) |
|
|
|
|
|
Primary | 4 (15) | 2 (7) |
|
|
Secondary | 15 (56) | 18 (67) |
|
|
Tertiary | 8 (30) | 7 (26) |
| Health literacy level, n (%) |
|
|
|
|
|
Adequate | 23 (85) | 24 (89) |
|
|
Limited | 4 (15) | 3 (11) |
| Employment status, n (%) |
|
|
|
|
|
Employed | 11 (41) | 7 (26) |
|
|
Unemployed | 16 (59) | 20 (74) |
| Type of strokea, n(%) |
|
|
|
|
|
Ischemic | 22 (82) | 25 (93) |
|
|
Hemorrhagic | 0 (0) | 0 (0) |
|
|
TIAb | 5 (18) | 2 (7) |
| Stroke risk factors (comorbidities), n (%) |
|
|
|
|
|
Hypertension and other risksc | 24 (89) | 26 (96) |
|
|
Diabetes only | 2 (7) | 1 (4) |
|
|
Other risks only | 1 (4) | 0 (0) |
| Varieties of prescribed medication, n (%) |
|
|
|
|
|
≤2 types | 2 (7) | 3 (11) |
|
|
≥3 types | 25 (93) | 24 (89) |
| Received formal or informal information about stroke prevention, n (%) |
|
||
|
|
Yes | 23 (85) | 24 (89) |
|
|
No | 4 (15) | 3 (11) |
aInclusive of modifiable stroke risk factors other than hypertension (eg, diabetes, heart diseases, hyperlipidemia, current smoking/alcohol).
bTIA: transient ischemic attack.
cOther risks include nonspecific International Classification of Diseases stroke codes.
The following sections are presented as per study objectives and with subdivisions to the feasibility and acceptability of (1) the RCT procedures, (2) video narratives intervention, and (3) preliminary findings of the effect of the video narratives on MUSE and blood pressure as a stroke risk factor control.
Feasibility and Acceptability of the RCT Procedures
The randomizing method, administration, and questionnaire retrieval at the outpatient waiting zone were effectively carried out. We experienced minimal challenges and uninterrupted flow at ushering patients individually to an allocated quiet room for video viewing. We received written feedback from 12 patients. Overall, the patients were satisfied with the study procedures, including the usage of a 5.3-inch-wide screen tablet and headphones, but commented on the burden of the self-administered questionnaires (for assessing the outcome measures). A few remarks were related to the exhaustive repetition of the MUSE and BMQ for each type of medication and the extensive length of the SF-36. Furthermore, there were suggestions to receive a token of appreciation for sustaining their participation.
Recruitment Rate
A total of 117 poststroke patients were screened from clinical records within 1 month for recruitment of trial participation, but only 88 patients were eligible according to the inclusion and exclusion criteria, resulting in an eligibility rate of 75.2%. Among all 88 patients, 70 patients provided consent to participate, but only 60 of them completed the baseline assessment. Hence, the recruitment rate was 51.3%. The most common reasons that patients declined enrollment were a language barrier, afraid of increased stress, and refusal.
Dropout and Study Completion Rates
During the baseline assessment (T0) and the 3-month follow-up assessment (T1), the number of patients completing the study dropped to 54 from 60 (90%), which reflected a dropout rate of 10%. The most common reason for not completing the study was an inability to be contacted, which we considered to indicate refusal for further participation.
Feasibility and Acceptability of the Video Narratives Intervention
We sought to gain in-depth information on technical issues and views on the video narratives’ usefulness as a motivational trigger to improve MUSE. The results of several subthemes identified are presented in Textbox 1.
Themes and quotes associated with the feasibility and acceptability of the video narratives intervention.
Feasibility of the video narratives
Main theme: Engagement and comprehension
- Messages were short, transparent, and easily understoodYou must not make the video too long. Like this one, that is just nice…not boring….What you see on video, the doctor was very goodP2
- Patients had the option to view it in their preferred language; either in English or in MalayThings related to stroke should be explained by patients themselves… not just knowledge but experience… so that others will be awareP6
- The narratives were suitable for the elderlyMost elderly patients are very stubborn about taking medicine. Show the video especially to the elderly patientsP7
- Appropriate video viewing frequencyWatching the video once in a while like this is good….P3
Main theme: Generalizability
- There were suggestions to share the video among friends via other media platforms such as WhatsApp or to continuously play it on air in the hospital.I can share this video with my friends in WhatsAppP1I want to send the video for my friends to watch!P5Maybe what they can do probably is over some TV set… what do you call that…program? Put the show, I mean like this type of video, what’ll happen when you have a stroke and all that… So that patients can listen instead of giving TV1 all the time you know?P2
Acceptability of video narratives
Main theme: Informative and reminder
- The videos narratives were a “trigger” toward proactivity and enhanced patients’ awareness about stroke and its preventative treatment.They remind us of important medicine… They remind us of the danger of the second stroke… to take medicine well and to have a healthy lifestyleP7Helpful….more understanding about strokeP6Awareness… before that we were not really concerned about our health. Now, after the advice it’s different... like a guideP4Patients can recover from stroke and (it) won’t recur if we take the medicine prescribed by doctors according to the right schedule on timeP2
Main theme: Emotional consolation
Viewing the video narratives provided some hope and less fear to overcome stroke challenges.
It’s a bit of both worrying and confidence… There is always a worry about what can happen, but it also gives you an idea (on) what to do, and what to be careful, and what to be aware
P3
- The video was an aid to their plight that there was life after stroke.Because you are a stroke patient, you have to look at the guidelines… you want to know (more)… you have to take care of yourself, right? You’ll be confident when you have such thing (to guide you) … Before this… you don’t know anything… fear about getting another attack… right?P1Others must know that people who got stroke, just like us, but they can recover. Sometimes, for stroke, people can’t really help, except for the patients themselvesP4
Main theme: Perception and confidence
- The motivational cues inspired the patients and raised confidence among themselves.… (sharing) someone’s experience to change others’ mind. Sometimes, we need to listen to their stories for us to make a changeP4
- They had a positive outlook towards stroke recovery and were willing to do better to improve their health condition.I feel that I have to follow the advice, for example, taking medicine, doing blood test… that have been mentioned… (The videos) seem to inspire us to take care of health so that we won’t get sick. Perhaps to give encouragement makes me feel that I can recover from stroke if follow all the adviceP5Usually, if you never had a stroke before, you don’t really care about watching the videos. Once you had (a stroke), you’ll realize that… health is important… you have to take care of it… watch their story… that’s it!P1Now I ask my doctor more questions if I don’t understand….P2
Preliminary Findings
Table 2 presents the results as per the trial protocol of outcome measures at T1 for MUSE and blood pressure control. There were no significant differences in outcome measures at baseline (T0) between the two groups (P<.001). All patients were on antiplatelet therapy, and the majority of patients diagnosed with hypertension were on antihypertensive medication (control group, n=24; intervention group, n=26). Therefore, only the general MUSE and specific MUSE for antithrombotic and antihypertensive medications were applied.
Table 2.
Outcome measurement of both groups at baseline (T0a) and posttest (T1b) assessments.
| Measure | Control group, mean (SD), range | Intervention group, mean (SD), range | |||||||
|
|
|
T0 | T1 | T0 | T1 | ||||
| MUSEc |
|
|
|
|
|||||
|
|
All medications | 27.0 (4.71), 16-32 | 27.6 (3.76) 20-32 | 26.3 (5.81), 16-32 | 30.1 (3.62), 20-32 | ||||
|
|
Hypertensived | 36.2 (4.62), 17-32 | 27.8 (35.7), 22-32 | 26.3 (5.59), 16-32 | 30.1 (3.51), 20-32 | ||||
|
|
Antithrombotice | 27.0 (4.70), 16-32 | 24.4 (3.73), 20-32 | 27.3 (5.43), 16-32 | 30.0 (3.57), 20-32 | ||||
| Systolic blood pressured (mmHg) | 138.7 (7.84), 127-162 | 137.9 (10.78), 124-60 | 147.0 (16.8), 121-186 | 137.8 (12.74), 117-165 | |||||
| Diastolic blood pressured (mmHg) | 79.6 (11.62), 54-107 | 80.0 (10.93), 60-105 | 85.7 (11.59), 58-109 | 85.0 (9.07), 68-100 | |||||
aT0: baseline (control group n=27, intervention group n=30).
bT1: 3 months postrandomization (control group n=27, intervention group n=27).
cMUSE: medication understanding and use self-efficacy.
dPrescribed with antihypertensive medication and diagnosed with hypertension as a primary factor (control group n=24, intervention group n=26).
ePrescribed antithrombotic medication as a prerequisite preventative treatment for stroke (control group n=27, intervention group n=27).
Both groups showed improvement in MUSE scores, but the intervention group presented greater differences from baseline compared to the control group. Similar trends were found for blood pressure control, whereby the intervention group had better systolic pressure regulation compared to the control group (Table 3). The MUSE outcomes of the intervention group were significantly different for the between-group and within-group analysis (Table 4).
Table 3.
Comparison of outcome measurement within groups.
| Measure | Control group | Intervention group | ||||||
|
|
|
T1a – T0b (95%CI) | Z value | P valuec | T1 – T0 | Z value | P valuec | |
| MUSEd |
|
|
|
|
|
|
||
|
|
All medications | 0.52 (–1.61-2.65) | –0.85 | .39 | 4.57 (1.94-7.19) | –3.63 | .001 | |
|
|
Hypertensivee | 1.26 (–0.36-2.89) | 1.79 | .07 | 4.35 (2.18-6.52) | –3.60 | <.001 | |
|
|
Antithromboticf | –0.91 (–1.29-3.11) | –0.37 | .71 | 3.70 (1.62-5.77) | –3.18 | .001 | |
| Systolic blood pressuree (mmHg) | –1.87 (–6.70 to –2.96) | 0.54 | .59 | –13.04 (–22.22 to –3.87) | –2.03 | .04 | ||
| Diastolic blood pressuree (mmHg) | 0.48 (–6.17-7.13) | –0.59 | .56 | –0.87 (–6.58-4.84) | –0.144 | .89 | ||
aT1: baseline (control group n=27, intervention group n=30).
bT0: baseline (control group n=27, intervention group n=30).
cWilcoxan signed-rank test
dMUSE: medication understanding and use self-efficacy.
ePrescribed with antihypertensive medication and diagnosed with hypertension as a primary factor (control group n=24, intervention group n=26).
fPrescribed antithrombotic as a prerequisite preventative treatment for stroke (control group n=27, intervention group n=27).
Table 4.
Comparison of outcome measurement between the control and intervention groups.
| Measure | Difference in T1a (95%CI) | Z value | P valueb | |
| MUSEc |
|
|
|
|
|
|
All medications | 2.74 (1.29-4.19) | –3.14 | .002 |
|
|
Hypertensived | 2.35 (0.87-3.81) | –2.65 | .008 |
|
|
Antithrombotice | 2.78 (1.28-4.29) | –3.14 | .002 |
| Systolic blood pressured (mmHg) | 0.96 (–6.58-8.49) | –0.17 | .86 | |
| Diastolic blood pressured (mmHg) | 6.04 (0.94-11.14) | –1.84 | .07 | |
aT1: mean score/measurement differences between intervention and control groups at 3 months postrandomization.
bMann-Whitney U test.
cMUSE: medication understanding and use self-efficacy.
dPrescribed with antihypertensive medication and diagnosed with hypertension as a primary factor (control group n=24, intervention group n=26).
ePrescribed antithrombotic as a prerequisite preventative treatment for stroke (control group n=27, intervention group n=27).
Discussion
Principal Findings
The aim of this study was to assess the feasibility and acceptability of a planned intervention in an actual clinical setting. We successfully tested the intervention processes as per the trial protocol from the initial stage of recruitment, randomization, baseline assessment, and at the first outcome phase.
Feasibility and Acceptability of Study Procedures and Outcome Measures
The recruitment period of 1 month was found to be appropriate as we were able to enroll more than the minimum planned sample size. The recruitment rate of 51.3% was comparable to the average trend of stroke trials conducted from 1990 to 2014, whereby there were no substantial increase or decline rates over the past 25 years [48]. At 3 months, the attrition rate was below the a priori projection of 15%. The positive recruitment rate might reflect concerns and interest to enhance stroke recovery. Nonetheless, we believe that more effort would be needed to sustain the dropout rate expected for the full 12-month trial, as reflected by the desire for monetary compensation indicated by a few patients. Despite this, we found that poststroke patients were able to cope with the study flow, and the extent of participation persuasion was not coercive, which was within the trial ethics jurisdiction and funding capacity [49].
The repeated MUSE on each stroke preventative medication group was necessary to elicit a significant association of self-efficacy with medication categories. However, there were concerns that the questionnaire administering process was time-consuming and created a feeling of redundancy among the patients. As the majority of poststroke patients were primarily hypertensive [50], it was crucial to obtain responses from patients within the three categories in the full trial. Owing to the inability to recruit more samples with other primary diagnosed stroke factors such as diabetes and hyperlipidemia, it remains to be investigated whether an influx of broader inclusion criteria would change the patient sample proportion. Similarly, comments related to the SF-36 received were similar with respect to the burden of its lengthiness. Nonetheless, it was not possible to substitute this questionnaire with other versions [51] due to the constricted contract for the Malay language version. Therefore, with all these issues taken into consideration, the full trial protocol was carried out as planned without significant changes in its outcome measures and study procedures.
Feasibility and Acceptability of Video Narratives
The video contents were comprehensible (layman terms) and had a sensible touch of emotion suitable for the local culture and language with a clear benefit for aging poststroke patients. There was a rising awareness of how audio-visual technology can influence different age groups and social environments. This feedback was comparable to similar trials with positive outcomes [52-54]. Nevertheless, face-to-face video viewing was maintained in this trial to prevent restriction of sample inclusion and exclusion criteria.
It was not surprising that the videos were perceived to be motivational for the poststroke patients. The qualitative results showed positive responses, which increased our anticipation that the videos incorporated with health belief constructs could facilitate standardized ongoing patient educational efforts in a clinical setting. The concise keywords used as cues added with authentic emotions triggered awareness and inspiration among patients toward being more self-efficacious in understanding and taking medication appropriately. Recent studies in different settings and samples have reported similar findings [55,56]. Other than that, educational video narratives could also improve the doctor-patient relationship. Paralleling previous studies, the combination of both personal views of the doctor and patient in this study potentially caused small positive perception changes in MUSE or initiated regular health monitoring [57,58]. Therefore, the preliminary outcomes in the intervention effectiveness analysis corresponded with our justification.
Preliminary Findings
In an associated review, it was clear that the presentation of “real people” has a motivating effect for peers with similar underlying illness [26] (stroke in this case). Hence, the initial impact on MUSE and systolic blood pressure provides insights toward a purposeful trial. The 3-month gap of video viewing to moderate the burden and the dropout rate was also appropriate, as indicated in a previous study [55]. Thus, we concluded that the measurement of self-efficacy among poststroke patients at the per allocated period could be assessed effectively [59]. Furthermore, these results were consistent with studies indicating a significant improvement in MUSE, which paralleled improved stroke risk factors control, such as systolic blood pressure [60,61]. Nevertheless, as we observed variation (coefficient of variation>1) for all variable differences with inconsistent confidence intervals, a bigger sample size would confirm its significance; otherwise, these positive results would have to be interpreted judiciously.
Strengths and Limitations
In designing the study, we spared no effort at not disrupting the workflow of a real-life outpatient clinic environment. However, there were unavoidable circumstances. For example, the blood pressure measurement was to be carried out by the physician or neurologists in their clinic only. We also foresee an issue since individual follow-up of neurology outpatient clinic appointment dates varied from 2 to 5 months and coincided with other clinical appointments (eg, diabetes clinic, heart disease clinic, rehabilitation, physiotherapy, and MTAC). Therefore, it was appropriate to consider documenting blood parameters at T0, T2, and T4 for the intervention effectiveness analysis. Hence, it was a challenge to ensure patients to view the videos within the 3-month gap from baseline. Nevertheless, we overcame these issues with transport reimbursement provided to the patients so as to maintain the retention rate and self-posted questionnaires to avoid further loss of data.
Several other limitations are the exclusion of patients who were unable to comprehend the English and Malay languages, which would have increased bias and limited the generalizability of the preliminary results. In addition, as a cost-effective approach, we were not able to carry out this study for more than 3 months. Despite all these limitations and challenges, the study procedures and outcome measures strategy were considered to be robust to inform the design of a successful 1-year RCT [28]. This study demonstrated versatile and helpful methods in achieving unanimous consensus.
Conclusion
Preliminary studies are crucial in assessing the success of a novel intervention [62]. This innovative method has been applied in various clinical settings in developed countries [52,63]. However, it has not yet been investigated for the poststroke patient population in Malaysia. This study successfully assessed the feasibility and acceptability of the video narrative intervention. The feedback and lessons learned from the baseline until the first follow-up assessment increased the awareness of both foreseen and unforeseen challenges. More importantly, we tested the initial requirement for full RCT accomplishments such as patient recruitment, feasibility, and acceptability of all outcome measures. Future research on the effectiveness of using culturally appropriate video narratives for a more extended period is warranted.
Acknowledgments
The authors would like to acknowledge the Jeffrey Cheah School of Medicine and Health Sciences, Monash University, Malaysia, for their facilities support. The authors also wish to acknowledge the contributions of the patients, staff nurses, and doctors from the Neurology Clinic, Hospital Kuala Lumpur. With utmost importance, we would like to thank the Director General of Health Malaysia for his permission to publish this article.
Abbreviations
- BIPQ
Brief illness perception questionnaire
- BMQ
Belief about medicine questionnaire
- CONSORT
CONsolidated Standards Of Reporting Trials
- HKL
Hospital Kuala Lumpur
- MTAC
medication therapy adherence clinic
- MUSE
medication understanding and use self-efficacy
- RCT
randomized controlled trial
- SF-36
Short Form (36) Health Survey
- T0
baseline
- T1
3 months postrandomization
Appendix
Screenshot of video narratives of neurologist.
Screenshot of video narratives of patient.
CONSORT checklist.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Brown C, Augusta-Scott T. Narrative Therapy: Making Meaning, Making Lives. Los Angeles: SAGE Publishing; 2006. Aug, [Google Scholar]
- 2.Liburd LC, Namageyo-Funa A, Jack L, Gregg E. Views From Within and Beyond: Illness Narratives of African-American Men With Type 2 Diabetes. Diabetes Spectr. 2004 Oct 01;17(4):219–224. doi: 10.2337/diaspect.17.4.219. [DOI] [Google Scholar]
- 3.Green MC. Narratives and Cancer Communication. J Commun. 2006 Aug 04;56(Suppl 1):S163–S183. doi: 10.1111/j.1460-2466.2006.00288.x. [DOI] [Google Scholar]
- 4.Baldick C. The Oxford Dictionary of Literary Terms. Oxford: Oxford University Press; 2015. Jan 01, [Google Scholar]
- 5.Larkey LK, Hecht M. A model of effects of narrative as culture-centric health promotion. J Health Commun. 2010 Mar;15(2):114–135. doi: 10.1080/10810730903528017. [DOI] [PubMed] [Google Scholar]
- 6.Lopez EJ. The art of using visual aids. Nurse Pract. 2005;30 Suppl Sourcebook:15–16. doi: 10.1097/00006205-200500001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Jeon Y, Kraus SG, Jowsey T, Glasgow NJ. The experience of living with chronic heart failure: a narrative review of qualitative studies. BMC Health Serv Res. 2010 Mar 24;10:77. doi: 10.1186/1472-6963-10-77. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbene R. Patients' narratives of chronic illnesses and the notion of biographical disruption. Commun Med. 2011 Nov 08;8(1):17–27. doi: 10.1558/cam.v8i1.17. [DOI] [PubMed] [Google Scholar]
- 9.Briant KJ, Halter A, Marchello N, Escareño M, Thompson B. The Power of Digital Storytelling as a Culturally Relevant Health Promotion Tool. Health Promot Pract. 2016 Nov 10;17(6):793–801. doi: 10.1177/1524839916658023. http://europepmc.org/abstract/MED/27402721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr N, Mawson S, Wright P, Parker J, Mountain G. Exploring the Experiences of Living With Stroke Through Narrative: Stroke Survivors' Perspectives. Glob Qual Nurs Res. 2016 May 05;3:2333393616646518. doi: 10.1177/2333393616646518. http://europepmc.org/abstract/MED/28462337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinckley JJ. Telling the Story of Stroke When Itʼs Hard to Talk. Top Lang Disord. 2015;35(3):258–266. doi: 10.1097/tld.0000000000000066. [DOI] [Google Scholar]
- 12.Aziz ZA, Lee YYL, Ngah BA, Sidek NN, Looi I, Hanip MR, Basri HB. Acute Stroke Registry Malaysia, 2010-2014: Results from the National Neurology Registry. J Stroke Cerebrovasc Dis. 2015 Dec;24(12):2701–2709. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Tan KS, Tan CT, Churilov L, MacKay MT, Donnan GA. Risk factors and aetiology of cerebral infarction in young adults: a comparative study between Malaysia and Australia. Int J Stroke. 2010 Oct;5(5):428–430. doi: 10.1111/j.1747-4949.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 14.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011 May;377(9778):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 15.Appalasamy JR, Subramanian P, Tan KM, Seeta Ramaiah S, Joseph JP, Chua SS. The Needs and Barriers of Medication-Taking Self-Efficacy Among Poststroke Patients: Qualitative Study. JMIR Nursing. 2019 Jul 22;2(1):e14399. doi: 10.2196/14399. https://nursing.jmir.org/2019/1/e14399/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987 Jul;57(3):316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 17.Warner LM, Ziegelmann JP, Schüz B, Wurm S, Tesch-Römer C, Schwarzer R. Maintaining autonomy despite multimorbidity: self-efficacy and the two faces of social support. Eur J Ageing. 2011 Mar 10;8(1):3–12. doi: 10.1007/s10433-011-0176-6. http://europepmc.org/abstract/MED/28798638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamison J, Sutton S, Mant J, De Simoni A. Barriers and facilitators to adherence to secondary stroke prevention medications after stroke: analysis of survivors and caregivers views from an online stroke forum. BMJ Open. 2017 Jul 16;7(7):e016814. doi: 10.1136/bmjopen-2017-016814. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=28713074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011 Apr;86(4):304–314. doi: 10.4065/mcp.2010.0575. http://europepmc.org/abstract/MED/21389250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naing C, Yeoh PN, Wai VN, Win NN, Kuan LP, Aung K. Hypertension in Malaysia: An analysis of trends from the national surveys 1996 to 2011. Medicine. 2016;95(2):e2417. doi: 10.1097/md.0000000000002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganasegeran K, Rashid A. The prevalence of medication nonadherence in post-myocardial infarction survivors and its perceived barriers and psychological correlates: a cross-sectional study in a cardiac health facility in Malaysia. Patient Prefer Adherence. 2017;11:1975–1985. doi: 10.2147/PPA.S151053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. pp. 384–385. [Google Scholar]
- 23.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004 Apr;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988 Sep 04;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 25.Kreps GL, Villagran MM, Zhao X, McHorney CA, Ledford C, Weathers M, Keefe B. Development and validation of motivational messages to improve prescription medication adherence for patients with chronic health problems. Patient Educ Couns. 2011 Jun;83(3):375–381. doi: 10.1016/j.pec.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Abu Abed M, Himmel W, Vormfelde S, Koschack J. Video-assisted patient education to modify behavior: a systematic review. Patient Educ Couns. 2014 Oct;97(1):16–22. doi: 10.1016/j.pec.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Berkhout C, Willefert-Bouche A, Chazard E, Zgorska-Maynard-Moussa S, Favre J, Peremans L, Ficheur G, Van Royen P. Randomized controlled trial on promoting influenza vaccination in general practice waiting rooms. PLoS One. 2018 Feb 9;13(2):e0192155. doi: 10.1371/journal.pone.0192155. http://dx.plos.org/10.1371/journal.pone.0192155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appalasamy JR, Tha KK, Quek KF, Ramaiah SS, Joseph JP, Md Zain AZ. The effectiveness of culturally tailored video narratives on medication understanding and use self-efficacy among stroke patients. Medicine. 2018;97(22):e10876. doi: 10.1097/md.0000000000010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011 May;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. http://europepmc.org/abstract/MED/21035130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johanson GA, Brooks GP. Initial Scale Development: Sample Size for Pilot Studies. Educ Psychol Meas. 2009 Dec 18;70(3):394–400. doi: 10.1177/0013164409355692. [DOI] [Google Scholar]
- 31.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008 Apr;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 32.Appalasamy JR, Joseph JP, Seeta Ramaiah S, Quek KF, Md Zain AZ, Tha KK. An Intervention to Promote Medication Understanding and Use Self-Efficacy: Design of Video Narratives for Aging Patients at Risk of Recurrent Stroke. JMIR Aging. 2019 Mar 21;2(1):e11539. doi: 10.2196/11539. https://aging.jmir.org/2019/1/e11539/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kincaid J, Cottrell L, Aagard J, Risley P. Implementing the Computer Readability Editing System (CRES) Institute For Simulation and Training. 1981 Jan 01;218 doi: 10.21236/ada098530. [DOI] [Google Scholar]
- 34.Porter K, Chen Y, Estabrooks P, Noel L, Bailey A, Zoellner J. Using Teach-Back to Understand Participant Behavioral Self-Monitoring Skills Across Health Literacy Level and Behavioral Condition. J Nutr Educ Behav. 2016 Jan;48(1):20–26. doi: 10.1016/j.jneb.2015.08.012. http://europepmc.org/abstract/MED/26453368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis TC, Federman AD, Bass PF, Jackson RH, Middlebrooks M, Parker RM, Wolf MS. Improving patient understanding of prescription drug label instructions. J Gen Intern Med. 2009 Jan;24(1):57–62. doi: 10.1007/s11606-008-0833-4. http://europepmc.org/abstract/MED/18979142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron K, Ross E, Clayman M, Bergeron A, Federman A, Bailey S, Davis T, Wolf M. Measuring patients' self-efficacy in understanding and using prescription medication. Patient Educ Couns. 2010 Sep;80(3):372–376. doi: 10.1016/j.pec.2010.06.029. http://europepmc.org/abstract/MED/20650594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long E, Ponder M, Bernard S. Knowledge, attitudes, and beliefs related to hypertension and hyperlipidemia self-management among African-American men living in the southeastern United States. Patient Educ Couns. 2017 May;100(5):1000–1006. doi: 10.1016/j.pec.2016.12.011. http://europepmc.org/abstract/MED/28012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan K, Dunton N. Development and validation of the stroke knowledge test. Top Stroke Rehabil. 2004;11(3):19–28. doi: 10.1310/RED5-V47T-8MJN-JY9H. [DOI] [PubMed] [Google Scholar]
- 39.Broadbent E, Petrie K, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006 Jun;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999 Dec;47(6):555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 41.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992 Jul 18;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. http://europepmc.org/abstract/MED/1285753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowtali SN, Yusoff DM, Harith S, Mohamed M. Translation and validation of the Malay version of the Stroke Knowledge Test. J Arrhythm. 2016 Apr;32(2):112–118. doi: 10.1016/j.joa.2015.10.003. https://linkinghub.elsevier.com/retrieve/pii/S1880-4276(15)00141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norfazilah A, Samuel A, Law P, Ainaa A, Nurul A, Syahnaz MH, Azmawati MN. Illness perception among hypertensive patients in primary care centre UKMMC. Malays Fam Physician. 2013;8(3):19–25. http://europepmc.org/abstract/MED/25893053. [PMC free article] [PubMed] [Google Scholar]
- 44.Tan C, Hassali M, Neoh C, Saleem F, Horne R. Cultural Adaptation and Linguistic Validation of the Beliefs about Medicines Questionnaire in Malaysia. Value Health Reg Issues. 2018 May;15:161–168. doi: 10.1016/j.vhri.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Sararaks S, Azman AB, Low LL, Rugayah B, Aziah AM, Hooi LN, Abdul Razak M, Norhaya MR, Lim KB, Azian AA, Geeta S. Validity and reliability of the SF-36: the Malaysian context. Med J Malaysia. 2005 Jun;60(2):163–179. http://www.e-mjm.org/2005/v60n2/Validity_Reliability_SF_36.pdf. [PubMed] [Google Scholar]
- 46.Powers BJ, Trinh JV, Bosworth HB. Can this patient read and understand written health information? JAMA. 2010 Jul 07;304(1):76–84. doi: 10.1001/jama.2010.896. [DOI] [PubMed] [Google Scholar]
- 47.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006 Jan;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 48.Feldman WB, Kim AS, Chiong W. Trends in Recruitment Rates for Acute Stroke Trials, 1990-2014. Stroke. 2017 Mar;48(3):799–801. doi: 10.1161/STROKEAHA.116.014458. http://europepmc.org/abstract/MED/28104835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander W. The uphill path to successful clinical trials: keeping patients enrolled. Pharm Therapeut. 2013 Apr;38(4):225–227. http://europepmc.org/abstract/MED/23785228. [PMC free article] [PubMed] [Google Scholar]
- 50.Gaciong Z, Siński M, Lewandowski J. Blood pressure control and primary prevention of stroke: summary of the recent clinical trial data and meta-analyses. Curr Hypertens Rep. 2013 Dec 25;15(6):559–574. doi: 10.1007/s11906-013-0401-0. http://europepmc.org/abstract/MED/24158454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998 Nov;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 52.Merchant RC, Clark MA, Mayer KH, Seage Iii GR, DeGruttola VG, Becker BM. Video as an effective method to deliver pretest information for rapid human immunodeficiency testing. Acad Emerg Med. 2009 Feb;16(2):124–135. doi: 10.1111/j.1553-2712.2008.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desta BF, Mohammed H, Barry D, Frew AH, Hepburn K, Claypoole C. Use of mobile video show for community behavior change on maternal and newborn health in rural Ethiopia. J Midwifery Womens Health. 2014 Jan;59(Suppl 1):S65–S72. doi: 10.1111/jmwh.12111. [DOI] [PubMed] [Google Scholar]
- 54.Briant KJ, Halter A, Marchello N, Escareño M, Thompson B. The Power of Digital Storytelling as a Culturally Relevant Health Promotion Tool. Health Promot Pract. 2016 Nov 10;17(6):793–801. doi: 10.1177/1524839916658023. http://europepmc.org/abstract/MED/27402721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houston TK, Allison JJ, Sussman M, Horn W, Holt CL, Trobaugh J, Salas M, Pisu M, Cuffee YL, Larkin D, Person SD, Barton B, Kiefe CI, Hullett S. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med. 2011 Jan 18;154(2):77–84. doi: 10.7326/0003-4819-154-2-201101180-00004. [DOI] [PubMed] [Google Scholar]
- 56.Denny MC, Vahidy F, Vu KYT, Sharrief AZ, Savitz SI. Video-based educational intervention associated with improved stroke literacy, self-efficacy, and patient satisfaction. PLoS One. 2017;12(3):e0171952. doi: 10.1371/journal.pone.0171952. http://dx.plos.org/10.1371/journal.pone.0171952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bessette L, Davison KS, Jean S, Roy S, Ste-Marie LG, Brown JP. The impact of two educational interventions on osteoporosis diagnosis and treatment after fragility fracture: a population-based randomized controlled trial. Osteoporos Int. 2011 Dec;22(12):2963–2972. doi: 10.1007/s00198-011-1533-1. [DOI] [PubMed] [Google Scholar]
- 58.Mazor KM, Baril J, Dugan E, Spencer F, Burgwinkle P, Gurwitz JH. Patient education about anticoagulant medication: is narrative evidence or statistical evidence more effective? Patient Educ Couns. 2007 Dec;69(1-3):145–157. doi: 10.1016/j.pec.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Brouwer-Goossensen D, van Genugten L, Lingsma HF, Dippel DWJ, Koudstaal PJ, den Hertog HM. Self-efficacy for health-related behaviour change in patients with TIA or minor ischemic stroke. Psychol Health. 2018 Dec 30;33(12):1490–1501. doi: 10.1080/08870446.2018.1508686. [DOI] [PubMed] [Google Scholar]
- 60.Breaux-Shropshire TL, Brown KC, Pryor ER, Maples EH. Relationship of Blood Pressure Self-Monitoring, Medication Adherence, Self-Efficacy, Stage of Change, and Blood Pressure Control Among Municipal Workers With Hypertension. Workplace Health Saf. 2012 Jul 01;60(7):303–311. doi: 10.3928/21650799-20120625-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, Schwartz C, Shackleford H, Shovelton C, Varghese J, Williams B, Hobbs FDR, Gooding T, Morrey I, Fisher C, Buckley D. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014 Aug 27;312(8):799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 62.Hassan ZA, Schattner P, Mazza D. Doing A Pilot Study: Why Is It Essential? Malays Fam Physician. 2006;1(2-3):70–73. http://europepmc.org/abstract/MED/27570591. [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell T, Dunt D, Fitzgerald JL, Gordon I. The impact of patient narratives on self-efficacy and self-care in Australians with type 2 diabetes: stage 1 results of a randomized trial. Health Promot Int. 2015 Sep 28;30(3):438–448. doi: 10.1093/heapro/dat058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Screenshot of video narratives of neurologist.
Screenshot of video narratives of patient.
CONSORT checklist.


