Abstract
Subunit vaccines can have excellent safety profiles, but their ability to give rise to robust immune responses is often compromised. For glycan-based vaccines, insufficient understanding of B and T cell epitope combinations that yield optimal immune activation hinders optimization. To determine which antigen features promote desired IgG responses, we synthesized epitope-functionalized polymers using ring-opening metathesis polymerization (ROMP) and assessed the effect of B and T cell epitope loading. The most robust responses were induced by polymers with a high valency of B and T cell epitopes. Additionally, IgG responses were greater for polymers with T cell epitopes that are readily liberated upon endosomal processing. Combining these criteria, we used ROMP to generate a non-toxic, polymeric antigen that elicited stronger antibody responses than a comparable protein conjugate. These findings highlight principles for designing synthetic antigens that elicit strong IgG responses against inherently weak immune targets such as glycans.
Keywords: synthetic antigen, ring-opening metathesis polymerization (ROMP), humoral immune response, B cell activation, carbohydrate vaccine
INTRODUCTION
Subunit vaccines are powerful agents for combatting infectious disease. They overcome many limitations of traditional whole-cell vaccines by incorporating select antigens that produce a protective response. As a result, these simplified subunit scaffolds have improved safety profiles, particularly for immune-compromised individuals.1, 2 Additionally, they afford substantial freedom in the choice of antigen, enabling the development of vaccines against diverse targets including cancers,3 autoimmune diseases4, drug addiction5, and pathogens for which traditional constructs have been ineffective. Despite these benefits, subunit vaccines are often less immunogenic, especially when they target inherently weak antigens such as carbohydrates.6 Diseased cells and pathogenic microbes typically display unique glycans that are rarely present or completely absent from human cells, making them ideal immune targets for vaccination.7, 8 Insight into the optimization of subunit vaccines would reap multiple benefits.
Some subunit vaccines that incorporate carbohydrate antigens can elicit neutralizing antibodies that protect against cancer or infectious disease. For example, vaccines containing capsular polysaccharides from Streptococcus pneumoniae, Neisseria meningitidis, or Haemophilus influenzae have been highly effective in eradicating infections caused by these pathogens.9-11 Additionally, glycoconjugates incorporating tumor-associated carbohydrate antigens have shown early promise as therapeutic cancer vaccines in clinical trials.3, 12 Despite these successes, glycans remain challenging vaccine targets. Polysaccharide and oligosaccharide antigens are often inherently poor immunogens because they lack peptide epitopes that can recruit CD4 T cells to the immune response. The most widely used strategy to address this issue is to conjugate glycans to protein carriers such as diphtheria toxoid or keyhole limpet hemocyanin to generate strong T cell responses.13, 14 However, proteins suffer limitations as vaccine platforms. They are prone to off-target antibody responses and can be challenging to functionalize efficiently and in a homogenous manner.15-19 Additionally, the choice of carrier protein can affect glycoconjugate efficacy which adds variability and ambiguity to the design of protein conjugate vaccines.20
A promising alternative to protein carriers is to link the essential elements required for a strong IgG antibody response, namely a carbohydrate epitope and a CD4 T cell epitope. One strategy is to use chemical synthesis to link immune epitopes directly.21-25 Such component vaccines can generate robust anti-carbohydrate IgG responses, yet they are challenging to synthesize which complicates the optimization of their activities. For example, increasing the carbohydrate epitope valency of a component vaccine could improve immunogenicity but is synthetically difficult to accomplish. Glycan epitopes can also be attached to synthetic scaffolds such as linear polymers,26 dendrimers,27 or nanoparticles.28-32 In this way, many B and T cell epitopes can be displayed from such platforms. Given the many permutations by which epitopes can be attached, defining which parameters influence the activation of a B cell response is advantageous. We reasoned that polymeric scaffolds would be ideal for probing how epitope quantity, density, and mode of linkage influences outcomes. Such information is critical for the rational design of vaccine constructs that will induce potent antibody responses.
B cell activation is sensitive to the structural features of the antigen. For antigens lacking T cell epitopes, the valency of the B cell epitope is one parameter that can influence the magnitude of the antibody response.33-36 A mechanism for this influence is that high valency antigens can induce B cell receptor (BCR) clustering and the signals that promote a B cell response, while low valency antigens fail to trigger signals that surpass the threshold for activation of an antibody response.35 However, few studies have been performed to determine whether responses to antigens bearing B and T cell epitopes require multivalency. In some instances, monovalent protein-based antigens such as hen egg lysozyme have been reported to stimulate B cell activation37, 38 and induce antibody responses.39 By contrast, vaccine platforms for immunization against HIV40, influenza32, and malaria41 exploit multivalency to induce potent antibody responses. Thus, how B cell epitope valency influences the magnitude of the antibody response for antigens bearing both B and T cell epitopes remains unclear.
A related antigen feature that has not been explored is the quantity of T cell epitopes available for antigen presentation. Following antigen engagement, the BCR facilitates antigen uptake and trafficking to compartments containing major histocompatibility complex type II (MHCII) molecules.42-44 Antigen peptides are loaded onto MHCII complexes and shuttled to the cell surface for presentation to T cells. Successful recruitment of T cells results in an immunological synapse wherein the T cells provide CD40 stimulation and cytokine signals that activate B cell proliferation and differentiation.45, 46 T cell recruitment and synapse formation are essential for antigen-specific B cells to initiate the events required for high-affinity IgG production.47 The presentation of even a single peptide can activate T cells.48 However, the likelihood of B cells engaging T cell help is dependent upon the level of antigen presentation.48, 49 Thus, antigens that increase the quantity of presented peptide-MHCII (pMHCII) complexes should be more effective at recruiting T cell participation. We hypothesized two factors could critically alter the level of B cell pMHCII presentation. First, the concentration of peptide epitope in MHCII-loading compartments should affect the number of pMHCII complexes. Second, antigen presentation requires antigen-bearing peptide epitope that is sensitive to endosomal processing.50 Thus, we predicted that antigens with cleavage-sensitive T cell epitopes would be more effective at inducing T cell activation and robust IgG responses.
To establish criteria for the design of synthetic vaccine constructs, we sought to define the effect of B and T cell epitope loading on the immunogenicity of multivalent antigen constructs. We developed a polymer scaffold that can be equipped with B and T cell epitopes with control over epitope loading.50 Polymers are versatile epitope carriers for evaluating antigen structural properties.35, 51, 52 Using ring-opening metathesis polymerization (ROMP),53, 54 we can generate polymers of defined length that are functionalized with multivalent B and T cell epitopes. Altering either the length of the polymer or the level of substitution of attached epitopes affords polymers bearing different levels of epitope functionalization. We previously used this approach to examine the effect of B cell epitope valency on B cell signaling and antigen presentation. We found that long polymers with high B cell epitope valency are more effective at clustering the BCR and triggering signaling than low valency polymers.35 Moreover, long polymers are more effective at inducing BCR internalization and presentation of attached peptides to T cells.50
For the present study, we generated a library of polymer constructs that varied in length, level of epitope substitution, and mode of epitope attachment. We compared the ability of these polymers to induce IgG antibody responses in vivo. We found that polymers with T cell epitopes tethered by a linker amenable to endosomal processing afforded optimal responses. We also determined that the magnitude of the antibody response positively correlates with both the valency of the B cell epitope and the abundance of the T cell epitope present on the polymer scaffold. To establish a molecular mechanism for this latter effect, we examined the impact of T cell epitope loading on antigen presentation and found that polymers with high T cell epitope density are more effective at inducing T cell activation. Our results provide a blueprint for optimizing the immunogenicity of synthetic antigens for the design of effective subunit vaccines.
MATERIALS AND METHODS
Synthesis and characterization of ROMP polymers and DNP-ovalbumin.
A complete description of polymer and DNP-functionalized ovalbumin synthesis and characterization is provided in the supporting information.
BCR and polymer internalization measure by flow cytometry.
A20HL cells were suspended in cell assay buffer at 4×106 cells mL-1. Cells were incubated at 37 °C for 5 min before stimulation. Alexa Fluor 488-labeled antigens were diluted at 10 μM DNP in cell assay buffer and pre-warmed at 37 °C for 20 min. Equal volume aliquots of antigen and cell suspension were combined and incubated at 37 °C, then placed on ice at indicated time points. The A20HL aliquots were then labeled on ice with 2.1 μg mL−1 anti-IgM μ-chain specific Fab fragment Dylight 649 conjugate (Jackson Immunoresearch) for 30 min. Cells were then rinsed twice using ice-cold 1% (w/v) BSA/PBS and resuspended at a final concentration of 1×106 cells mL-1. The extent of BCR labeling and antigen uptake was measured by flow cytometry using a FACScaliber (BD). For each sample, the geometric mean of Dylight 649 and AF488 fluorescence was calculated using the FlowJo software suite. The relative level of surface BCR and the extent of antigen was determined using aliquots of unstimulated cells as a reference and plotted versus time.
T cell IL-2 production.
A20HL cells were plated at 2×105 cells per well in A20HL medium onto tissue culture-treated 96-well plates (BD). Antigen dilutions were prepared in A20HL medium, and plated cells were incubated in the presence or absence of antigen for 3 h. Culture medium was removed by aspiration, and the cells were fixed at a final concentration of 2% (v/v) paraformaldehyde/PBS for 20 min at rt. Wells were quenched with 1% (w/v) BSA/PBS pH 7.4 containing 0.7 M lysine, then washed twice with DO-11.10 medium. Unstimulated, fixed A20HL cells were also treated with antigens at a final concentration of 1 μM Ova323. The wells were plated with DO-11.10 cells at 105 cells per well, and the co-culture was incubated for 20-24 h at 37 °C. The plate was centrifuged, and the co-culture medium, along with IL-2 standards (R&D Systems) prepared in DO-11.10 medium (two-fold dilution series from 1 ng mL−1 to 7 pg mL−1), were transferred to an ELISA plate that had been pre-coated with 4 μg mL−1 anti-IL-2 capture antibody (R&D Systems) and blocked with 0.05% (v/v) Tween-20/PBS (PBS-T) containing 1% (w/v) BSA. The plate was incubated at 4°C overnight and then washed three times with PBS-T for 5 min. Biotinylated IL-2 antibody (R&D Systems) was added to each well at 0.4 μg mL−1 and allowed to incubate 2 h at rt. The washing procedure was repeated, and streptavidin-HRP (Jackson Immunoresearch) was added at 0.5 μg mL−1 for 30 min at rt. The washing procedure was repeated, and TMB substrate (Pierce) was added; the reaction was then quenched with the addition of 1 M aqueous sulfuric acid. The absorbance at 450 nm was measured using a plate reader (Bio-Tek).
Immunizations.
Animals were maintained in accordance with the University of Wisconsin-Madison Research Animal Resources Center and with the MIT Committee for Animal Care (CAC) and Institutional Care and Use Committee (IACUC), which adhere to both national and local guidelines. Groups of 4-6 weeks old female BALB/c mice (Jackson Laboratories) received s.c. immunizations on day 0 and 34 with indicated doses of antigen mixed with 5 μg vaccigrade MPLA (Invivogen) in 100 μL PBS. Serum samples were collected weekly post-injection.
Serum anti-DNP IgG detection.
Serum anti-DNP total IgG was assessed using an ELISA. 96-well polysorp plates were coated for 1 h at rt (21°C) with 5 μg mL−1 DNP8-BSA (DNP-conjugated BSA; Biosearch Technologies) in PBS. Washing steps were performed 3 times with 0.05% Tween-20 in PBS (PBS-T) and the plate was blocked for one hour at RT with 1% BSA in PBS-T (BSA/PBS-T). The plates were washed, and dilutions of serum in BSA/PBS-T were added and incubated for 2 h at rt. Again, the plates were washed, and horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) (SouthernBioTech) diluted 4000:1 in BSA/PBS-T was added. Following a 2 h incubation at rt, the plates were washed. Wells were then incubated with 50 μL Ultra TMB (ThermoFisher Scientific) for 5 min then quenched with 50 μL 1 aqueous sulfuric acid. Plates were read at 450 nm. To measure titers for each IgG antiserum, absorbance curves were fitted, and the titer was assigned as the dilution giving an absorbance of 0.3 O.D. This cutoff value was determined as the mean plus ≥3 times the standard deviation of the negative control group (untreated mice). If no dilution gave an absorbance at or above the cutoff, the titer was considered to be 102. Negative control mice did not raise detectable IgG.
Statistical analysis.
Comparison of serum anti-DNP IgG was made using one-way or two-way analysis of variance (ANOVA), followed by a Tukey-Kramer test for multiple comparisons with Prism 5.0 (GraphPad software). P values less than 0.05 were considered statistically significant and marked with a single asterisk. P values less than 0.01 were marked with two asterisks. P values less than 0.001 were denoted with three asterisks. All values are reported as the mean ± SD.
RESULTS
Polymers with controlled epitope functionalization
To evaluate antigen properties that influence antibody responses, we required defined antigens with control over the quantity of attached B and T cell epitopes. We exploited ring-opening metathesis polymerization (ROMP) to derive polymers amenable to functionalization with immune epitopes. This polymer synthesis method allows control over the number of epitopes present on the polymer by variation of either polymer length or the level of epitope substitution (Figure 1A). To alter polymer length, we employed a ruthenium initiator, which allows access to polymers with narrow dispersity and length.55 The monomer-to-initiator ratio controls the length (Scheme S1).53, 54 To vary the number of epitopes on a polymer, we used a norbornene monomer bearing an N-hydroxysuccinimidyl (NHS) ester.56 The ester is susceptible to attack by amine-bearing epitopes to yield functionalized polymers. By modifying the stoichiometry of polymer to an epitope that is used in the coupling reaction, we controlled the level of substitution.56, 57
Figure 1.
Control of epitope loading (A) A ROMP-derived polymer bearing reactive esters can be substituted with B and T cell epitopes. Control of polymer length or degree of substitution allows variation of the number of displayed epitopes. (B) Polymer B cell epitopes enable B cell receptor (BCR) engagement and activation, while a protease-sensitive T cell epitope can undergo endosomal processing for antigen presentation and T cell activation through the T cell receptor (TCR).
Polymers bearing succinimidyl esters can be functionalized with B and T cell epitopes (Scheme S3A).50 We used the dinitrophenyl (DNP) group as a model of non-peptide haptens such as carbohydrates. As a T cell epitope, we employed a peptide derived from ovalbumin (Ova323-339 denoted Ova323). To facilitate the endosomal release of T cell epitope following polymer internalization by B cells, we attached the peptide to the polymer using a linker sensitive to cleavage by the protease cathepsin D (catD) (Scheme S3A and Figure 2A).50 CatD is an aspartyl protease implicated in antigen processing,58, 59 and we postulated that engineered release of T cell epitopes would yield more effective T cell epitope presentation. We quantified the level of B and T cell epitopes attached to the polymer backbone using NMR spectroscopy (Scheme S3B).50 With this overall approach, we accessed a set of polymers that varied systematically, and we used these agents to evaluate the effect of epitope loading on antibody responses in vivo (Figure 2).
Figure 2.
A series of polymer antigens that vary systematically in B and T cell epitope loading. (A) We prepared polymer variants bearing distinct combinations of B cell epitope DNP (R1) and T cell epitope Ova323. The latter was appended through either a cathepsin D (catD)-sensitive (R2) or –insensitive (R3) linker. The rest of the polymer units contained inert functionality (R5) with the exception of a few that carried a fluorescent dye (AF488) (R4). n = degree of polymerization. (B) The schematic depicts the relative percentage of epitopes per polymer molecule for the polymers detailed in the chart. B cell epitope loading was varied by extending polymer length (polymers 3 vs. 5) or increasing substitution (polymers 3 vs. 6). T cell epitope loading was varied by increasing substitution (polymers 1 – 4).
Peptide-functionalized polymers promote IgG antibody response with a dependence on a cleavable linker
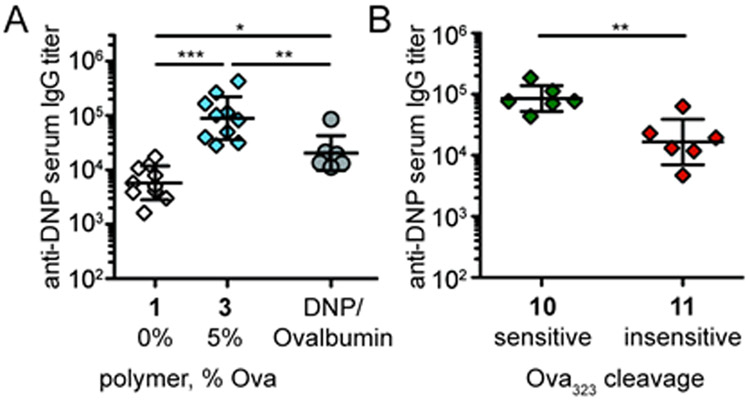
We first determined whether polymers bearing only B cell epitopes could elicit a T cell-dependent (TD) IgG response. Polymers presenting many copies of B cell epitopes are T cell-independent type II (TI-II) antigens—they can induce antibody responses because they engage the BCR in multivalent interactions that trigger signaling.33, 35 This response lacks T cell help and typically affords low IgG titers. To test whether dual-functionalized DNP/Ova323 polymers recruit T cell help, we formulated polymers with monophosphoryl Lipid A (MPLA), a TLR-based adjuvant, and compared the anti-DNP IgG titers for DNP-only polymer 1 and DNP/Ova323 polymer 3. Mice were injected with polymer 1 or 3 or ovalbumin functionalized with DNP (DNP10/Ova). The anti-DNP IgG responses were significantly higher for DNP/Ova323 polymer 3 compared to DNP-only polymer 1, suggesting that treatment with the peptide-functionalized polymer recruits T cell help (Figure 3A). Interestingly, responses for a single antigen dose were significantly stronger for DNP/Ova323 polymer than those from the DNP10/Ova protein conjugate. The haptenated protein required a second dose administration to reach similar titers as DNP/Ova323 polymer 3 (Figure S1A).
Figure 3.
IgG responses from polymers with B and T cell epitopes compared to haptenated protein. BALB/c mice were immunized s.c. with antigen mixed with 5 μg MPLA on day 0 and 34. Anti-DNP IgG sera titers were assessed on day 21 by ELISA. (A) Animals were immunized with 0.2 nmol of DNP polymer 1, DNP/Ova323 polymer 3 or DNP10/Ovalbumin. (B) Animals were immunized with 0.02 nmol of DNP polymer bearing either catD-sensitive Ova323 (10) or catD-insensitive Ova323 (11). Shown are day 21 anti-DNP IgG sera titers with mean ± SD of n=6-10 per group.
We previously demonstrated that peptides attached to the polymer backbone through a linker susceptible to catD protease result in more effective antigen presentation and T cell activation.50 We hypothesized that a polymer with the protease-sensitive linker would elicit superior IgG responses than those with linkers that lack specific cleavage sites. We compared 300mer polymers bearing equivalent substitution levels of DNP and Ova323 but varying in whether the peptide was attached through a catD-sensitive or catD-resistant linker (Figure 2B). DNP/Ova323 polymer with the protease-sensitive linker yielded higher levels of anti-DNP IgG (Figure 3B, Figure S1B). Given that treatment of B cells with the cleavage-sensitive polymer results in more T cell activation,50 the elevated response in vivo is likely due to better recruitment of T cell help.
Higher valency results in greater IgG responses
We varied polymer length and found that the longer polymers (e.g., 30mers versus 300mers) cluster the BCR more effectively.35 Longer polymers promote enhanced signaling35 and uptake of antigen50 by B cells. Because they induce stronger B cell activation, long polymers should afford a more robust IgG response than short polymers. We tested this hypothesis by immunizing mice with DNP/Ova323 polymers of different length. (Figure 2B). Mice were vaccinated with 0.2 nmol of polymer on day 0 and 34, and serum anti-DNP IgG titers were determined. Titers were significantly higher for the long polymer 3 on each day tested (Figure 4A). Additionally, we investigated the biodistribution and toxicity profiles for long and short polymer scaffolds (Fig. S2-3). Both 30mer and 300mer polymers were cleared through the liver, and no visible toxicity was detected. We anticipate the titer difference between lengths is due to the potency with which high valency polymer can stimulate B cell activation. Still, the longer polymer delivers larger doses of the B cell epitope DNP because it has more copies.
Figure 4.
Increasing B cell epitope valency and T cell epitope substitution drive a stronger IgG response. BALB/c mice were immunized s.c. with 0.2 nmol of polymer mixed with 5 μg MPLA on day 0 and 34. Anti-DNP IgG sera titers were assessed over time by ELISA. (A) IgG responses to 300mer polymer 3 and 30mer polymer 5 over time (left) and on day 21 (right). (B) IgG responses to DNP 300mer 1 and DNP/Ova323 300mer with varied Ova323 loading (2, 3, and 4) over time (left) and on day 21 (right). (C) IgG responses to DNP/Ova323 30mer 5 and DNP/Ova323 300mer 6 over time (left) and on day 21 and 41 (right). IgG sera titers are shown as the mean ± SD of two independent experiments with n = 3 – 6 per group.
To determine whether the short polymer could induce this level of activation at higher doses, we immunized with this agent at higher concentrations. Specifically, we compared titers for mice immunized with 30mer 5 at a high dose (2 nmol polymer, 30 nmol DNP) and a low dose (0.2 nmol polymer, 3 nmol DNP) relative to 300mer 3 (0.2 nmol polymer, 30 nmol DNP). In this way, we compared the 30mer 5 and 300mer 3 at either the same polymer concentration (0.2 nmol polymer) or the same DNP concentration (approximately 30 nmol DNP). The responses to the short polymer were always modest (Figure S4). These data indicate that the ability of the polymers to induce immune cell activation depends on how effectively they can engage in multivalent binding.
High peptide loading affords a robust IgG antibody response
Robust B cell activation requires B cell signaling but also effective antigen presentation and T cell help. We tested whether antigens with high B cell epitope valency but different T cell epitope loadings would vary in their ability to recruit T cell help. Polymers are ideal vehicles to address this question as the mole fraction of T cell epitopes can be controlled. Thus, we synthesized polymers of the same length (300mer) with comparable DNP substitution (36-40%), but variable levels of substitution of the catD-sensitive linked Ova323 epitope: polymers 2 (0.5%), 3 (5%), and 4 (12%) (Figure 2B). The lowest substitution we tested had a molar ratio of peptide-to-polymer of about 1:1, while the highest was approximately 38:1. To ensure a homogenous level of peptide substitution, we compared polymers by gel permeation chromatography and determined that DNP/Ova323 polymer retained narrow dispersity following functionalization (data not shown). Mice were immunized with polymers 1, 2, 3, or 4 (0.2 nmol polymer) on day 0 and 34. As anticipated, the inclusion of a single Ova323 epitope resulted in elevated serum anti-DNP IgG titers for polymer 2 compared to DNP-only polymer 1 (Figure 4B). Still, titers were significantly more robust for polymers 3 and 4, each of which had higher levels of Ova323 functionalization, indicating that antigens bearing more T cell epitopes were superior immunogens (Figure 4B).
Given that increased peptide loading generates more IgG production, we assessed whether that response remained sensitive to B cell epitope valency. We, therefore, prepared 300mer 6 with high Ova323 substitution (5%), but a low level of DNP substitution (4%) (Figure 2B). If higher peptide loading can compensate for low B cell epitope valency, we anticipated that the response to 300mer 6 would be stronger than for 30mer 5. Instead, we found the responses to be comparable, both immediately after immune priming on day 0 and following a boost injection on day 34 (Figure 4C). These data suggest multivalent clustering of the B cell receptor is a critical contributor to IgG responses and that T cell activation does not compensate for BCR signaling.
High peptide loading increases antigen presentation
We hypothesized that the augmentation observed with polymers displaying many peptide copies resulted from increased antigen presentation that leads to effective T cell recruitment. To test this hypothesis, we compared the extent of antigen presentation elicited by 300mer polymers with different Ova323 substitution levels. We employed a B cell line expressing a DNP-specific BCR (A20.2J HLTNP denoted as A20HL)60, 61 and a T cell line expressing an Ova323-specific TCR (DO-11.10).62, 63 The polymers had the same DNP valency; however, polymers with different levels of Ova323 T cell epitope substitution might adopt different conformations. We, therefore, measured the rate and extent of internalization to ensure similar levels of antigen uptake by B cells regardless of T cell epitope loading. Polymers 7, 8, and 9 were prepared with DNP and Ova323 epitopes as well as a fluorescent dye (Alexa Fluor 488 or AF488), and the level of cell-surface BCR following exposure to polymer (5 μM DNP) was compared (Figure 5A). Regardless of peptide loading, the polymers induced internalization of 50-60% of cell-surface BCR within 30 minutes. Additionally, we saw that the fluorescence signal for the internalized polymer was comparable (Figure 5B). These data indicate that there is no difference in the rate or extent of antigen internalization for polymers that differ in T cell epitope loading.
Figure 5.
Polymers with different T cell epitope density are internalized to the same extent, but high density is required for antigen presentation. (A & B) A20HL B cells were treated with DNP/Ova323/AF488 polymers with varied Ova323 loading at 5 μM DNP. After polymer treatment for the indicated duration, mean fluorescence intensity was measured by flow cytometry for (A) cell surface BCR labeled with fluorescent anti-BCR Fab and (B) fluorescent polymers. The data were normalized to A20HL cells at t=0 min (A) or unstimulated A20HL cells (B). Error bars represent ±1 standard deviation from the mean of three independent experiments. (C) IL-2 produced by DO-11.10 T cells in response to B cell exposure to DNP/Ova323 polymers for 3 h. The response is indicated as a function of Ova323 concentration. Error bars represent ±1 standard deviation from the mean (n = 3). Data are representative of at least three independent experiments.
Because the polymers are internalized to a similar extent, we tested whether polymers with high Ova323 substitution levels afforded more efficient antigen presentation. We quantified T cell IL-2 production in response to polymer-treated B cells as a measure of antigen presentation. Low substitution polymer 2 elicited IL-2 production, but the levels were reduced by 4-fold relative to those obtained with polymer 3 or 4 substituted with 5 or 12% Ova323 (Figure 5C). Thus, polymers with high peptide loading boost antigen presentation, consistent with their ability to elicit more IgG production.
DISCUSSION
Vaccine safety is a critical public health issue, and subunit vaccines are advantageous in that they can be used in individuals with weakened immune systems.1 Subunit vaccines that target epitopes such as carbohydrates can be prepared by attaching the epitopes to carrier proteins. However, proteins have drawbacks including off-target responses and inefficient antigen functionalizations15-19, and so researchers are seeking alternative scaffolds. To design immunogenic synthetic antigens, structural properties that allow immune recognition and activation must be defined. Using polymers generated by ROMP, our goal has been to determine the parameters that give rise to strong antibody responses.
ROMP is a polymerization reaction that produces polymers with a high degree of control53, 54, so distinct parameters (length, substitution level) can be varied systematically. Thus, we have generated polymers bearing B and T cell epitopes with well-defined and tunable epitope functionalization. We can control the average number of epitopes per antigen by altering either polymer length or the degree of epitope substitution (Figure 1A). We used this approach to assess the effect of changing B or T cell epitope quantity on the antibody response. First, we validated that equipping the polymer with T cell epitope affords a T-cell dependent antigen that can induce robust IgG responses. We found that while polymer bearing only B cell epitope can yield class-switched IgG antibody, the levels are low; however, attachment of a T cell epitope dramatically increases the response (Figure 3A).
Our results also reveal that the IgG response can be augmented by using a peptide linker sensitive to endosomal protease catD (Figure 3B). We found this linker was necessary for in vitro antigen presentation to T cells.50 To explain this observation, we suggest that the catD-sensitive linker facilitates peptide release during a stage in the endolysosomal pathway that augments MHCII loading. MHCI presentation in dendritic cells has been shown to depend upon antigen processing kinetics where antigen release must occur within a narrow window of time following uptake for successful presentation to occur.64 A similar dependence is likely for B cell MHCII presentation given that MHCII-loading is tightly coordinated with BCR signaling and antigen uptake.65 In this scenario, the sensitive linker enables processing when the antigen-loaded endosome reaches MHCII enriched compartments. In the absence of the linker, processing appears to be too slow to release the epitope within the requisite time frame. Polymer lacking the cleavage site likely fails to release the antigen to induce productive B-T cell interactions that promote B cell proliferation and differentiation. Several material-based strategies have been developed to allow epitope release in endolysosomal compartments for MHCII presentation, including the use of linkers that exploit the acidic66 and reducing67-69 environment of the endosome. However, protease-sensitive linkers are advantageous because they are readily incorporated and are stable during the synthesis of the T cell epitope. The specificity of the site can also be tuned to allow release at a stage during the endosomal passage that is ideal for antigen presentation.64 Given these advantages and our results showing that the linker benefits the immune response, we anticipate that protease linkers can be exploited to improve immunogen design.
For antigens lacking T cell epitopes, high BCR epitope valency is a critical parameter for engendering antibody responses.33, 35 We compared IgG responses to long and short polymers to define whether valency is important when antigens have both B and T cell epitopes. The data show that long polymers with high BCR epitope valency yield substantially stronger responses than short polymers with low valency (Figure 4A). One explanation for this effect is a difference in BCR signaling. We previously determined that long polymers induce extensive BCR clustering and stronger signaling than short polymers.35 Superior BCR signaling likely yields to potent B cell activation, thereby affecting the outcome of the antibody response. Additionally, we have shown that long polymers are better internalized than short polymers, resulting in elevated antigen presentation.50 Thus, the long polymer may induce both stronger BCR signaling and more effective antigen presentation, thereby delivering signals that drive a robust IgG response. Observations that multivalent particle-based vaccines bearing pathogen-derived epitopes give strong antibody responses70 suggest a crucial role for valency in responses to antigens bearing B and T cell epitopes. Still, the immunogenicity of these scaffolds could be due to other features, such as physical dimensions that allow effective trafficking to lymphoid tissues. By comparing antigens with different valency, we have validated an essential role for this parameter in generating strong IgG responses.
We also investigated the impact of the number of T cell epitopes delivered on the antibody response. This parameter cannot be readily studied with protein carriers, but polymers provide the means to vary the number of T cell epitopes delivered. Although polymers bearing a single peptide give rise to increased responses relative to those lacking T cell epitopes, a 15-fold increase in peptide substitution affords increased IgG titers by about two orders of magnitude (Figure 4B). We postulated that polymers with high peptide functionalization give rise to higher peptide concentration in MHCII-loading compartments, thereby leading to more antigen presentation. We compared polymers with increasing peptide substitution and found that highly substituted polymers give rise to stronger T cell activation (Figure 5C). This finding is corroborated by recent evidence that during B cell division, the cell that retains higher antigen concentration is capable of more effective antigen presentation.71. Thus, delivery of increased intracellular stores of peptide should drive presentation and T cell activation. However, we found that high peptide functionalization does not compensate for minimal BCR epitope valency. Polymer 6 with high levels of peptide substitution but low BCR epitope valency failed to induce a potent response (Figure 4C). Our finding that low valency antigens are not internalized effectively suggests that such antigens will not afford the intracellular concentration of peptide necessary for effective antigen presentation, regardless of the level of peptide functionalization.
Together, our findings suggest a set of parameters that affect the magnitude of the antibody responses. Antigen valency determines whether BCR signals exceed a minimum threshold for B cell activation and whether an antigen is effectively internalized. A secondary parameter is the concentration of intracellular peptide available for presentation and recruitment of T cell help. Including a protease cleavage site to efficiently release antigen in the endolysomal compartments can lead to more efficient MHCII complex formation. Adding additional copies of T cell epitopes can also augment responses. In designing vaccine antigens, BCR epitope valency and loading and release of T cell epitopes are critical considerations for maximizing the magnitude of the antibody response. These findings can be broadly applied to classical carbohydrate-helper T cell paired constructs as well as the recently reported constructs combining glycans and the invariant natural killer T cell epitope α-GalCer.72, 73 Moreover, our findings are also relevant for the design of constructs that seek to elicit enhanced antibody responses against other epitopes like peptides or small molecules.
Finally, we determined that a single dose of polymer with optimized epitope loading produced a stronger IgG response than a haptenated protein (Figure 3A). Our findings indicate that this difference arises from higher B and T cell epitope quantities for the polymer relative to the protein antigen (>100 DNP and >10 Ova323 for a 300mer polymer versus 10 DNP and 1 Ova323 epitopes for DNP/Ova protein). Our data demonstrate that access to antigens with high epitope functionalization offers a distinct advantage for enhancing the antibody response. Synthetic scaffolds, such as ROMP-derived polymers, can be highly substituted, while functionalization of protein carriers is limited. Additionally, with synthetic scaffolds, the level of substitution is quantifiable and uniform, which is a challenge when generating haptenated protein antigens. These features of polymer scaffolds highlight compelling advantages for creating vaccine antigens that can elicit antibody responses against pathogen- or tumor-derived carbohydrate epitopes.
CONCLUSIONS
The design of subunit vaccines can be improved through better understanding of the combinatorial effects of B and T cell epitope display. We used defined ROMP polymers to reveal key criteria for epitope attachment to enhance antigen immunogenicity. A highly valent display of B cell epitope alone induced IgG class switching. These IgG titers greatly increased upon addition of optimally presented T cell epitope. Specifically, we showed that multivalent display and controlled endosomal release of T cell epitopes yield robust IgG responses. These criteria for T cell attachment influence the level of B cell antigen presentation, a requirement for strong IgG responses. Together, our results demonstrate that antigens with high B cell epitope valency and abundant cleavable T cell epitopes function as robust immunogens. Considering these parameters in the design of vaccine constructs could optimize immune responses against weakly immunogenic carbohydrate antigens.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health (AI055258). NRB was supported by a NIH Biotechnology Training Grant (T32 GM08349) and NSF (DGE-1256259). The Bruker Avance III 500s and Bruker Reflex II instrumentation used in the Paul Bender Chemical Instrumentation Center was supported by a generous gift from Paul J. and Margaret M. Bender. The A20.2J HLTNP line was a generous gift of A. Ochi (University Health Network, Toronto, ON, Canada) and the DO.11-10 cell line was provided by the Marrack and Kappler Group (National Jewish Health, Denver, CO, USA).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publication website.
General procedures and materials; synthesis and characterization of ROMP polymers; preparation of DNP-functionalized Ovalbumin; cell culture conditions; endotoxin measurement; biodistribution and toxicity experimental information; Table S1-S2; Schemes S1-S4; Figures S1-S4
REFERENCES
- (1).Pirofski L.-a.; Casadevall A, Use of Licensed Vaccines for Active Immunization of the Immunocompromised Host. Clin Microbiol Rev 1998, 11, (1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Medical Advisory Committee of the Immune Deficiency, F.; Shearer WT; Fleisher TA; Buckley RH; Ballas Z; Ballow M; Blaese RM; Bonilla FA; Conley ME; Cunningham-Rundles C; Filipovich AH; Fuleihan R; Gelfand EW; Hernandez-Trujillo V; Holland SM; Hong R; Lederman HM; Malech HL; Miles S; Notarangelo LD; Ochs HD; Orange JS; Puck JM; Routes JM; Stiehm ER; Sullivan K; Torgerson T; Winkelstein J, Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol 2014, 133, (4), 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jin K-T; Lan H-R; Chen X-Y; Wang S-B; Ying X-J; Lin Y; Mou X-Z, Recent advances in carbohydrate-based cancer vaccines. Biotechnol Lett 2019, 41, 641–650. [DOI] [PubMed] [Google Scholar]
- (4).Larché M; Wraith DC, Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med 2005, 11, S69. [DOI] [PubMed] [Google Scholar]
- (5).Bremer PT; Janda KD, Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol Rev 2017, 69, (3), 298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pulendran B; Ahmed R, Immunological mechanisms of vaccination. Nat Immunol 2011, 12, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Astronomo RD; Burton DR, Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov 2010, 9, (4), 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Heimburg-Molinaro J; Lum M; Vijay G; Jain M; Almogren A; Rittenhouse-Olson K, Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, (48), 8802–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Stanworth SJ; Estcourt LJ; Powter G; Kahan BC; Dyer C; Choo L; Bakrania L; Llewelyn C; Littlewood T; Soutar R; Norfolk D; Copplestone A; Smith N; Kerr P; Jones G; Raj K; Westerman DA; Szer J; Jackson N; Bardy PG; Plews D; Lyons S; Bielby L; Wood EM; Murphy MF, A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med 2013, 368, (19), 1771–1780. [DOI] [PubMed] [Google Scholar]
- (10).Rosenstein NE, Perkins, Bradley A, Stephens David. S., Popovic, Tanja Hughes, James M, Meningococcal Disease. N Engl J Med 2001, 344, (18), 1378–1388. [DOI] [PubMed] [Google Scholar]
- (11).Kelly DF; Moxon ER; Pollard AJ, Haemophilus influenzae type b conjugate vaccines. Immunology 2004, 113, (2), 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Danishefsky SJ; Shue YK; Chang MN; Wong CH, Development of Globo-H cancer vaccine. Acc Chem Res 2015, 48, (3), 643–652. [DOI] [PubMed] [Google Scholar]
- (13).Wilson RM; Danishefsky SJ, A vision for vaccines built from fully synthetic tumor-associated antigens: from the laboratory to the clinic. J Am Chem Soc 10//, 2013, pp 14462–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pichichero ME, Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 2013, 9, (12), 2505–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schutze MP; Leclerc C; Jolivet M; Audibert F; Chedid L, Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol 1985, 135, (4), 2319–2322. [PubMed] [Google Scholar]
- (16).Buskas T; Li Y; Boons GJ, The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry 2004, 10, (14), 3517–3524. [DOI] [PubMed] [Google Scholar]
- (17).Herzenberg LA; Tokuhisa T; Herzenberg LA, Carrier-priming leads to hapten-specific suppression. Nature 1980, 285, (5767), 664–667. [DOI] [PubMed] [Google Scholar]
- (18).Xu QH; Zhao XN; Cheng JP; Wei CH; Zhang QH; Rong KT, Influence of carrier proteins on the immunologic response to haptenic antitetrodotoxin vaccine. Bioconjugate Chem 2006, 17, (6), 1508–1513. [DOI] [PubMed] [Google Scholar]
- (19).Purcell AW; Zeng W; Mifsud NA; Ely LK; Macdonald WA; Jackson DC, Dissecting the role of peptides in the immune response: theory, practice and the application to vaccine design. J Pept Sci 2003, 9, (5), 255–281. [DOI] [PubMed] [Google Scholar]
- (20).Khatun F; Stephenson RJ; Toth I, An Overview of Structural Features of Antibacterial Glycoconjugate Vaccines That Influence Their Immunogenicity. Chem - Eur J 2017, 23, (18), 4233–4254. [DOI] [PubMed] [Google Scholar]
- (21).Wilkinson BL; Day S; Malins LR; Apostolopoulos V; Payne RJ, Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew Chem Int Ed 2011, 50, (7), 1635–1639. [DOI] [PubMed] [Google Scholar]
- (22).Wilkinson BL; Day S; Chapman R; Perrier S; Apostolopoulos V; Payne RJ, Synthesis and immunological evaluation of self-assembling and self-adjuvanting tricomponent glycopeptide cancer-vaccine candidates. Chemistry 2012, 18, (51), 16540–16548. [DOI] [PubMed] [Google Scholar]
- (23).Sarkar S; Lombardo SA; Herner DN; Talan RS; Wall KA; Sucheck SJ, Synthesis of a single-molecule L-rhamnose-containing three-component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies. J Am Chem Soc 2010, 132, (48), 17236–17246. [DOI] [PubMed] [Google Scholar]
- (24).Lakshminarayanan V; Thompson P; Wolfert MA; Buskas T; Bradley JM; Pathangey LB; Madsen CS; Cohen PA; Gendler SJ; Boons GJ, Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci U S A 2012, 109, (1), 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ingale S; Wolfert MA; Gaekwad J; Buskas T; Boons GJ, Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol 2007, 3, (10), 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Nuhn L; Hartmann S; Palitzsch B; Gerlitzki B; Schmitt E; Zentel R; Kunz H, Water-soluble polymers coupled with glycopeptide antigens and T-cell epitopes as potential antitumor vaccines. Angew Chem Int Ed 2013, 52, (40), 10652–10656. [DOI] [PubMed] [Google Scholar]
- (27).Lo-Man R; Vichier-Guerre S; Perraut R; Deriaud E; Huteau V; BenMohamed L; Diop OM; Livingston PO; Bay S; Leclerc C, A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res 2004, 64, (14), 4987–4994. [DOI] [PubMed] [Google Scholar]
- (28).Yin Z; Nguyen HG; Chowdhury S; Bentley P; Bruckman MA; Miermont A; Gildersleeve JC; Wang Q; Huang X, Tobacco mosaic virus as a new carrier for tumor associated carbohydrate antigens. Bioconjugate Chem 2012, 23, (8), 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yin Z; Comellas-Aragones M; Chowdhury S; Bentley P; Kaczanowska K; Benmohamed L; Gildersleeve JC; Finn MG; Huang X, Boosting immunity to small tumor-associated carbohydrates with bacteriophage qbeta capsids. ACS Chem Biol 2013, 8, (6), 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Parry AL; Clemson NA; Ellis J; Bernhard SS; Davis BG; Cameron NR, ‘Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J Am Chem Soc 2013, 135, (25), 9362–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Miermont A; Barnhill H; Strable E; Lu X; Wall KA; Wang Q; Finn MG; Huang X, Cowpea mosaic virus capsid: a promising carrier for the development of carbohydrate based antitumor vaccines. Chemistry 2008, 14, (16), 4939–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kanekiyo M; Wei CJ; Yassine HM; McTamney PM; Boyington JC; Whittle JR; Rao SS; Kong WP; Wang L; Nabel GJ, Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, (7456), 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dintzis HM; Dintzis RZ; Vogelstein B, Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci U S A 1976, 73, (10), 3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Qin Q; Yin Z; Wu X; Haas KM; Huang X, Valency and density matter: Deciphering impacts of immunogen structures on immune responses against a tumor associated carbohydrate antigen using synthetic glycopolymers. Biomaterials 2016, 101, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Puffer EB; Pontrello JK; Hollenbeck JJ; Kink JA; Kiessling LL, Activating B cell signaling with defined multivalent ligands. ACS Chem Biol 2007, 2, (4), 252–262. [DOI] [PubMed] [Google Scholar]
- (36).Minguet S; Dopfer EP; Schamel WW, Low-valency, but not monovalent, antigens trigger the B-cell antigen receptor (BCR). Int Immunol 2010, 22, (3), 205–212. [DOI] [PubMed] [Google Scholar]
- (37).Kim YM; Pan JY; Korbel GA; Peperzak V; Boes M; Ploegh HL, Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci U S A 2006, 103, (9), 3327–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Avalos AM; Bilate AM; Witte MD; Tai AK; He J; Frushicheva MP; Thill PD; Meyer-Wentrup F; Theile CS; Chakraborty AK; Zhuang X; Ploegh HL, Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J Exp Med 2014, 211, (2), 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Jegerlehner A; Storni T; Lipowsky G; Schmid M; Pumpens P; Bachmann MF, Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol 2002, 32, (11), 3305–3314. [DOI] [PubMed] [Google Scholar]
- (40).Jardine J; Julien JP; Menis S; Ota T; Kalyuzhniy O; McGuire A; Sok D; Huang PS; MacPherson S; Jones M; Nieusma T; Mathison J; Baker D; Ward AB; Burton DR; Stamatatos L; Nemazee D; Wilson IA; Schief WR, Rational HIV immunogen design to target specific germline B cell receptors. Science 2013, 340, (6133), 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Moon JJ; Suh H; Li AV; Ockenhouse CF; Yadava A; Irvine DJ, Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA 2012, 109, (4), 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Aluvihare VR; Khamlichi AA; Williams GT; Adorini L; Neuberger MS, Acceleration of intracellular targeting of antigen by the B-cell antigen receptor: importance depends on the nature of the antigen-antibody interaction. EMBO J 1997, 16, (12), 3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Siemasko K; Eisfelder BJ; Williamson E; Kabak S; Clark MR, Cutting edge: signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J Immunol 1998, 160, (11), 5203–5208. [PubMed] [Google Scholar]
- (44).Lanzavecchia A, Antigen-Specific Interaction between T-Cells and B-Cells. Nature 1985, 314, (6011), 537–539. [DOI] [PubMed] [Google Scholar]
- (45).Elgueta R; Benson MJ; de Vries VC; Wasiuk A; Guo Y; Noelle RJ, Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009, 229, (1), 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bishop GA; Hostager BS, B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr Opin Immunol 2001, 13, (3), 278–285. [DOI] [PubMed] [Google Scholar]
- (47).MacLennan ICM; GulbransonJudge A; Toellner KM; CasamayorPalleja M; Chan E; Sze DMY; Luther SA; Orbea HA, The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev 1997, 156, 53–66. [DOI] [PubMed] [Google Scholar]
- (48).Huang J; Brameshuber M; Zeng X; Xie J; Li QJ; Chien YH; Valitutti S; Davis MM, A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 2013, 39, (5), 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Schwickert TA; Victora GD; Fooksman DR; Kamphorst AO; Mugnier MR; Gitlin AD; Dustin ML; Nussenzweig MC, A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med 2011, 208, (6), 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bennett NR; Zwick DB; Courtney AH; Kiessling LL, Multivalent Antigens for Promoting B and T Cell Activation. ACS Chem Biol 2015, 10, (8), 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Courtney AH; Bennett NR; Zwick DB; Hudon J; Kiessling LL, Synthetic antigens reveal dynamics of BCR endocytosis during inhibitory signaling. ACS Chem Biol 2014, 9, (1), 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Courtney AH; Puffer EB; Pontrello JK; Yang ZQ; Kiessling LL, Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A 2009, 106, (8), 2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Bielawski CW; Grubbs RH, Living ring-opening metathesis polymerization. Prog Polym Sci 2007, 32, (1), 1–29. [Google Scholar]
- (54).Choi TL; Grubbs RH, Controlled living ring-opening-metathesis polymerization by a fast-initiating ruthenium catalyst. Angew Chem Int Ed 2003, 42, (15), 1743–1746. [DOI] [PubMed] [Google Scholar]
- (55).Love JA; Morgan JP; Trnka TM; Grubbs RH, A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile. Angew Chem, Int Ed 2002, 41, (21), 4035–4037. [DOI] [PubMed] [Google Scholar]
- (56).Strong LE; Kiessling LL, A general synthetic route to defined, biologically active multivalent arrays. J Am Chem Soc 1999, 121, (26), 6193–6196. [Google Scholar]
- (57).Kolonko EM; Pontrello JK; Mangold SL; Kiessling LL, General synthetic route to cell-permeable block copolymers via ROMP. J Am Chem Soc 2009, 131, (21), 7327–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Riese RJ; Chapman HA, Cathepsins and compartmentalization in antigen presentation. Curr Opin Immunol 2000, 12, (1), 107–113. [DOI] [PubMed] [Google Scholar]
- (59).Rodriguez GM; Diment S, Role of cathepsin D in antigen presentation of ovalbumin. J Immunol 1992, 149, (9), 2894–2898. [PubMed] [Google Scholar]
- (60).Sato K; Ochi A, Inhibition of B-cell receptor-antigen complex internalization by FcγRIIB1 signals. Immunol Lett 1998, 61, (2–3), 135–143. [DOI] [PubMed] [Google Scholar]
- (61).Watanabe M; Wegmann DR; Ochi A; Hozumi N, Antigen presentation by a B-cell line transfected with cloned immunoglobulin heavy- and light-chain genes specific for a defined hapten. Proc Natl Acad Sci U S A 1986, 83, (14), 5247–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kappler JW; Skidmore B; White J; Marrack P, Antigen-inducible H -2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med 1981, 153, (5), 1198–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Shimonkevitz R; Kappler J; Marrack P; Grey H, Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med 1983, 158, (2), 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Howland SW; Wittrup KD, Antigen release kinetics in the phagosome are critical to cross-presentation efficiency. J Immunol 2008, 180, (3), 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Lankar D; Vincent-Schneider H; Briken V; Yokozeki T; Raposo G; Bonnerot C, Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J Exp Med 2002, 195, (4), 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Heffernan MJ; Kasturi SP; Yang SC; Pulendran B; Murthy N, The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid). Biomaterials 2009, 30, (5), 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Li P; Luo Z; Liu P; Gao N; Zhang Y; Pan H; Liu L; Wang C; Cai L; Ma Y, Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Control Release 2013, 168, (3), 271–279. [DOI] [PubMed] [Google Scholar]
- (68).Hirosue S; Kourtis IC; van der Vlies AJ; Hubbell JA; Swartz MA, Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine 2010, 28, (50), 7897–7906. [DOI] [PubMed] [Google Scholar]
- (69).Wilson DS; Hirosue S; Raczy MM; Bonilla-Ramirez L; Jeanbart L; Wang R; Kwissa M; Franetich J-F; Broggi MAS; Diaceri G; Quaglia-Thermes X; Mazier D; Swartz MA; Hubbell JA, Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat Mater 2019, 18, (2), 175–185. [DOI] [PubMed] [Google Scholar]
- (70).Kanekiyo M; Ellis D; King NP, New Vaccine Design and Delivery Technologies. J Infect Dis 2019, 219, (Supplement_1), S88–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Thaunat O; Granja AG; Barral P; Filby A; Montaner B; Collinson L; Martinez-Martin N; Harwood NE; Bruckbauer A; Batista FD, Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science 2012, 335, (6067), 475–479. [DOI] [PubMed] [Google Scholar]
- (72).Cavallari M; Stallforth P; Kalinichenko A; Rathwell DCK; Gronewold TMA; Adibekian A; Mori L; Landmann R; Seeberger PH; De Libero G, A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat Chem Biol 2014, 10, 950. [DOI] [PubMed] [Google Scholar]
- (73).Yin X-G; Chen X-Z; Sun W-M; Geng X-S; Zhang X-K; Wang J; Ji P-P; Zhou Z-Y; Baek DJ; Yang G-F; Liu Z; Guo J, IgG Antibody Response Elicited by a Fully Synthetic Two-Component Carbohydrate-Based Cancer Vaccine Candidate with α-Galactosylceramide as Built-in Adjuvant. Org Lett 2017, 19, (3), 456–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.