Significance
The liver plays a central role in systemic metabolism. Hepatocytes rarely proliferate in normal liver, but these cells readily enter the cell cycle after injuries that reduce functional hepatic mass. Prior studies have documented that metabolic functions of the liver are transiently downregulated during liver regeneration, but the underlying mechanisms are poorly understood. Here, we demonstrate that cyclin D1 and HNF4α, the best-characterized intracellular mediators of hepatocyte proliferation and differentiation (respectively), are reciprocal negative regulatory partners that control both cell cycle progression and metabolism in the liver. Repression of HNF4α transcriptional activity by cyclin D1 provides a mechanism by which liver metabolic function is broadly attenuated during regeneration and potentially other contexts.
Keywords: liver regeneration, partial hepatectomy, glycogen, cell cycle, pyruvate carboxylase
Abstract
Hepatocyte nuclear factor 4α (HNF4α) is a master regulator of liver function and a tumor suppressor in hepatocellular carcinoma (HCC). In this study, we explore the reciprocal negative regulation of HNF4α and cyclin D1, a key cell cycle protein in the liver. Transcriptomic analysis of cultured hepatocyte and HCC cells found that cyclin D1 knockdown induced the expression of a large network of HNF4α-regulated genes. Chromatin immunoprecipitation-sequencing (ChIP-seq) demonstrated that cyclin D1 inhibits the binding of HNF4α to thousands of targets in the liver, thereby diminishing the expression of associated genes that regulate diverse metabolic activities. Conversely, acute HNF4α deletion in the liver induces cyclin D1 and hepatocyte cell cycle progression; concurrent cyclin D1 ablation blocked this proliferation, suggesting that HNF4α maintains proliferative quiescence in the liver, at least, in part, via repression of cyclin D1. Acute cyclin D1 deletion in the regenerating liver markedly inhibited hepatocyte proliferation after partial hepatectomy, confirming its pivotal role in cell cycle progression in this in vivo model, and enhanced the expression of HNF4α target proteins. Hepatocyte cyclin D1 gene ablation caused markedly increased postprandial liver glycogen levels (in a HNF4α-dependent fashion), indicating that the cyclin D1-HNF4α axis regulates glucose metabolism in response to feeding. In AML12 hepatocytes, cyclin D1 depletion led to increased glucose uptake, which was negated if HNF4α was depleted simultaneously, and markedly elevated glycogen synthesis. To summarize, mutual repression by cyclin D1 and HNF4α coordinately controls the cell cycle machinery and metabolism in the liver.
The liver performs many essential metabolic functions, and the nuclear receptor transcription factor HNF4α plays a critical role in promoting liver development and hepatocyte-specific gene expression (1–4). HNF4α also acts as a tumor suppressor in liver cancer and represses hepatocyte proliferation in normal liver (5). HNF4α forms a homodimer that regulates the expression of a plethora of hepatic genes involved in diverse aspects of metabolism. Its transcriptional activity is modulated through numerous mechanisms including binding to coregulatory proteins, interaction with other transcription factors, and posttranslational modifications. However, the full spectrum of factors that regulate HNF4α in the liver remain to be identified.
Unlike most other organs, the liver has a remarkable capacity to “regenerate” after injuries that reduce functional hepatic mass (6, 7). In normal liver, hepatocytes proliferate infrequently, but these cells can rapidly divide and restore liver mass following hepatic resection or other acute injuries. In patients with acute and chronic liver diseases, the extent of hepatocyte proliferation correlates with survival (8, 9). In the most widely used model of liver regeneration, two-thirds partial hepatectomy (PH) in rodents, a large population of hepatocytes enters the cell cycle in a relatively synchronous manner, and liver mass and function are restored within 1 to 2 wk.
In injuries such as PH that result in loss of large number of hepatocytes, numerous hepatic functions are impaired, and this metabolic insufficiency may help to trigger the regenerative response (7, 10). Furthermore, robust cell proliferation and tissue growth require significant alterations in cellular metabolism to provide for the synthesis of new cell components (11, 12), yet the liver must also continue to perform sufficient metabolic functions to sustain homeostasis. The mechanisms by which hepatocytes balance the competing metabolic needs of growth and systemic homeostasis during liver regeneration have not been well characterized.
The cell cycle is regulated by protein kinase complexes consisting of cyclins and cyclin-dependent kinases (Cdks). In hepatocytes and many other cells, cyclin D1 is markedly up-regulated beginning in the G1 phase and activates Cdk4, which then promotes expression and activation of cyclin/cdk complexes that regulate progression through subsequent phases of the cell cycle. In addition to activation of the canonical cell cycle apparatus, cyclin D1 regulates transcription via Cdk-dependent and Cdk-independent mechanisms (13). Our prior studies have demonstrated that expression of cyclin D1 alone is sufficient to promote hepatocyte proliferation and liver growth in the absence of other mitogenic signals (14–18). Furthermore, knockdown of cyclin D1 inhibits proliferation of hepatocytes and hepatic cell lines in culture (19–22). Interestingly, cyclin D1 appears to regulate the expression of genes involved in diverse metabolic functions during hepatocyte proliferation in vivo (23). For example, cyclin D1 represses de novo lipogenesis as well as lipid catabolism in hepatocytes (19–21) and regulates sex steroid metabolism in the liver (22). We have previously shown that it decreases binding of HNF4α and peroxisome proliferator-activated receptor α (PPARα) to several target genes involved in lipid metabolism (19, 20). Importantly, aberrant cyclin D1 expression is oncogenic, and it is one of the most commonly overexpressed genes in human cancers including HCC (24, 25) where it is considered to be a driver oncogene (26).
Given the large number of metabolic genes that it regulates (23), we hypothesized that cyclin D1 may repress HNF4α as a general mechanism to affect diverse aspects of hepatic function during cell cycle progression. In theory, if cyclin D1 transiently diminishes HNF4α-mediated gene expression during liver regeneration, this could allow more cellular resources to be directed to biosynthesis and other needs of growth. Our results suggest a robust interaction between cyclin D1, a pivotal mediator cell cycle progression, and HNF4α, the master regulator of hepatic metabolism.
Results
Cyclin D1 Represses HNF4α-Mediated Transcriptional Activity in Hepatic Cell Lines and the Liver.
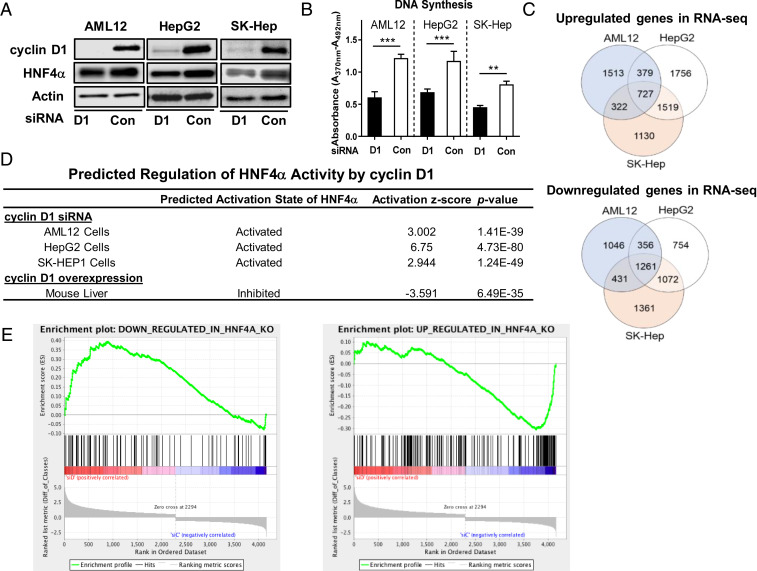
To gain insight into the role of cyclin D1 in overall hepatic cell function, we performed RNA-sequencing (RNA-seq) analysis in three different lines after small interfering RNA (siRNA) -mediated knockdown of cyclin D1. We used the mouse hepatocyte AML12 cell line as well as human liver cancer lines HepG2 and Sk-Hep1, each of which express HNF4α (19, 27–30). As is shown in Fig. 1 A and B, cyclin D1 siRNA effectively eliminated expression of this protein and significantly inhibited cell cycle progression as measured by DNA synthesis. RNA-seq analysis was performed using 6–10 replicates per condition (control vs. cyclin D1 siRNA), providing substantial statistical power. Although many genes were uniquely regulated in each cell line, a substantial portion was up- and down-regulated similarly in all three lines (Fig. 1C).
Fig. 1.
Cyclin D1 represses HNF4α target gene expression in AML12 hepatocytes, human liver cancer cell lines, and the liver. Mouse AML12 hepatocytes and human liver cancer cell lines HepG2 and Sk-Hep1 were treated with cyclin D1 and control siRNA. (A) Western blot of cyclin D1 and HNF4α expressions in the three cell lines. (B) DNA synthesis as determined by BrdU uptake. (C) Venn diagrams of genes up-regulated and down-regulated by cyclin D1 depletion in the three cell lines. (D) Predicted activity of HNF4α as determined by IPA upstream regulatory analysis of RNA-seq following cyclin D1 knockdown in the three cells lines and following transient cyclin D1 overexpression in mouse liver. (E) GSEA of AML12 cells treated with cyclin D1 siRNA for genes that were down-regulated (Left) or up-regulated (Right) following acute HNF4α KO in mouse liver.
The transcriptomic data were analyzed using Ingenuity Pathway Analysis (IPA), and the most highly affected upstream regulatory pathways are shown in Dataset S1. HNF4α activity was found to be among the most highly activated transcription regulators following cyclin D1 depletion in each of these cell lines (Fig. 1D). We also reevaluated older transcriptomic data from mouse liver with acute cyclin D1 expression (23), and HNF4α activity was found to be the most highly inhibited transcriptional regulator in the IPA analysis. For comparison, estrogen receptor 1 (ESR1) activity was substantially inhibited in the IPA analyses by cyclin D1 knockdown (and induced by cyclin D1 overexpression, Dataset S1), compatible with prior literature showing that this protein induces estrogenic signaling in the liver and the activity of ESR1 in other cell types (13, 22). These data indicate that cyclin D1 affects a broad array of HNF4α target genes in hepatic cell lines and the liver and thus may regulate diverse aspects of hepatic function through this mechanism.
In a second approach, we used differentially expressed genes in a previously published RNA-seq dataset from mouse liver after acute knockout (KO) of HNF4α (31, 32) to perform gene set enrichment analysis (GSEA). Enrichment plots of the RNA-seq data from AML12 cells demonstrated that genes down-regulated by acute hepatic HNF4α KO were mostly up-regulated by cyclin D1 depletion, whereas genes that were induced by HNF4α Ko were down-regulated (Fig. 1E). These data further support the hypothesis that cyclin D1 represses HNF4α transcriptional activity.
Cyclin D1 Inhibits Binding of HNF4α to Thousands of Targets in the Liver.
The above data indicate that cyclin D1 broadly represses HNF4α-mediated gene expression. In a prior study (19), we found that cyclin D1 bound to HNF4α and inhibited its binding to two target genes in hepatocytes that promote de novo lipogenesis. A truncation mutant of cyclin D1, called the repressor domain ([RD], consisting of amino acids 143–255, ref. 33), also bound to and inhibited HNF4α occupancy on these two genes (19). Cyclin D1-RD, which does not bind or activate Cdks, has also been shown to bind and inhibit the activity of another nuclear receptor transcription factor, the androgen receptor (33). To gain a detailed understanding of how cyclin D1 affected HNF4α target gene occupancy in the liver, we transduced mice with recombinant adenoviruses (ADVs) encoding full-length cyclin D1 or the cyclin D1-RD truncation mutant and harvested the livers 1 d later to perform HNF4α ChIP-seq. Recombinant ADVs efficiently target hepatocytes in vivo and have been widely used to study the effect of single-gene expression in the liver. After 1 d, cyclin D1, but not cyclin D1-RD, induces robust hepatocyte proliferation in the liver (16) as evidenced by increased hepatocyte Ki-67 immunostaining (Fig. 2A) and induction of Cdk1, a marker of hepatocyte proliferation, by Western blot (Fig. 2B). Expression of cyclins D1 and D1-RD did not significantly affect HNF4α expression (Fig. 2C).
Fig. 2.
Cyclin D1 regulates HNF4α gene occupancy in the liver. Male BALB/c mice were transduced with ADVs encoding full-length cyclin D1 or a truncation mutant and cyclin D1-RD, and livers were harvested 1 d later. Both the cyclin D1 and the D1-RD constructs contained a FLAG peptide tag. Control livers were transduced with ADV-green fluorescent protein (GFP). (A) The Ki-67 labeling index as determined by counting the percentage of hepatocyte nuclei staining positive for this marker. (B) Western blot analysis of liver lysates. The cyclin D1 antibody is to the C terminus of this protein, which is absent in cyclin D1-RD. (C) Western blot of liver nuclear extracts for HNF4α and TATA binding protein ([TBP], used as a loading control). The relative abundance of HNF4α (normalized to TBP) is shown at the right. (D) Regulation of HNF4α binding sites by cyclin D1. Representation of ChIP-seq data showing the log2 fold change in HNF4α binding to distinct genomic sites in mouse liver induced by cyclin D1 and D1-RD. The number of unique binding sites in each quadrant is shown. (E) The most highly regulated canonical [athways by IPA analysis of genes down-regulated by cyclin D1 in the liver that had a parallel decrease in HNF4α binding by ChIP-seq.
To gain an understanding of how cyclin D1 regulates HNF4α function on a global level, we performed HNF4α ChIP-seq on livers transduced with cyclins D1 or D1-RD. This analysis showed that both full-length cyclin D1 and the cyclin D1-RD truncation mutant regulated HNF4α binding to thousands of unique sites in the liver with the predominant effect being reduced gene occupancy (Fig. 2D and Datasets S2 and S3); more than 91% of the affected binding sites were decreased by both cyclin D1 and D1-RD. The characteristics of HNF4α binding motifs that were regulated by cyclin D1 are shown in SI Appendix, Fig. S1.
We used our older messenger RNA (mRNA) gene array data from the mouse liver 1 d after transduction with ADV-D1 (23) to gain insight into how HNF4α gene occupancy correlated with gene expression in this model. This older transcriptomic data are limited by the fact that the array represented fewer genes than would be analyzed by RNA-seq, but it nonetheless provides insight into the effects of cyclin D1 at this time point. Notably, 33% (152 out of 456) of the mRNAs down-regulated by ADV-D1 in the liver on the gene array had a corresponding decrease in HNF4α occupancy in nearby gene regions (SI Appendix, Fig. S1D). Representative peak tracing of HNF4α binding to down-regulated genes are shown in SI Appendix, Fig. S1E. We performed IPA analysis of the mRNA down-regulated by cyclin D1 in the liver that had a parallel reduction in adjacent HNF4α gene occupancy by ChIP-seq; as is shown in Fig. 2E, the top 20 regulated canonical pathways showed regulation of diverse metabolic functions. Interestingly, among the two most down-regulated pathways were triacylglycerol degradation and fatty acid β-oxidation, which we have recently shown to be inhibited by cyclin D1 using distinct approaches (20, 21). These data support the hypothesis that cyclin D1 regulates hepatic function by reducing HNF4α binding to and expression of genes involved in a range of metabolic pathways.
Acute HNF4α Deletion Promotes Hepatocyte Proliferation via Induction of Cyclin D1.
Prior studies have demonstrated that acute deletion of HNF4α in the liver induces hepatocyte proliferation and the expression of cell cycle proteins including cyclin D1 (34–36). Based on these data, it has been presumed that HNF4α represses cyclin D1 expression in a normal liver (5, 37). To examine this further, we used HNF4αfl/fl (38), cyclin D1fl/fl (39), or compound cyclin D1/HNF4αfl/fl mice for experiments to acutely delete these proteins from hepatocytes in vivo using an established vector that specifically targets the Cre recombinase to these cells [AAV8-TBG-Cre (37, 40)].
As previously reported, acute HNF4α KO led to induction of cyclin D1 mRNA and protein expression and promoted hepatocyte proliferation (as evidenced by Cdk1 expression and hepatocyte proliferating cell nuclear antigen [PCNA] staining on immunohistochemistry) (Fig. 3 A–C). The induction of cyclin D1 was mostly in zone 2 of the liver lobule (41), and hepatocytes adjacent to portal tracts and central veins did not express this protein (SI Appendix, Fig. S2A). Concurrent deletion of cyclin D1 along with HNF4α led to substantially decreased hepatocyte proliferation. These studies suggest that inhibition of cyclin D1 by HNF4α in the resting liver prevents hepatocyte proliferation and support a model in which reciprocal negative regulation of these two proteins plays a role in balancing hepatocyte proliferation and differentiated function in vivo.
Fig. 3.
Acute deletion of HNF4α, cyclin D1, or both in hepatocytes in vivo. Male HNF4αfl/fl, cyclin D1fl/fl, or cyclin D1/HNF4αfl/fl mice were injected with AAV8-TBG-CRE (or a GFP control vector) 1 wk before liver harvest to acutely delete these proteins from hepatocytes. Serum-stimulated AML12 cells were treated with siRNA to cyclin D1, HNF4α, or both. (A) Western blot of liver lysates. (B) Hepatocyte PCNA staining by immunohistochemistry. The percentage hepatocytes with PNCA-positive nuclei is shown. (C) mRNA expression of cyclin D1 and HNF4α in liver. (D) Western blot of AML12 lysates treated with siRNA to cyclin D1 and/or HNF4α. Densitometry for cyclin D1 expression is also shown. (E) DNA synthesis in AML12 cells as determined by BrdU uptake.
We also examined the reciprocal regulation of HNF4α and cyclin D1 in mitogen-stimulated AML12 cells, in which HNF4α activity is already repressed (as shown in Fig. 1 and ref. 19). siRNA-mediated depletion of HNF4α further increased cyclin D1 expression and cell proliferation as measured by DNA synthesis (Fig. 3 D and E). Thus, even in the setting of mitogen stimulation and active proliferation, depletion of HNF4α further induces cyclin D1 and cell cycle progression.
Acute Deletion of Cyclin D1 Markedly Inhibits Hepatocyte Cell Cycle Progression and Promotes the Expression of HNF4α Target Proteins in the Regenerating Liver.
We have previously shown that expression of cyclin D1 is sufficient to promote hepatocyte proliferation in culture and in vivo and that cyclin D1 siRNA inhibits hepatocyte proliferation in culture (14–22). However, prior studies have not documented whether cyclin D1 KO inhibits hepatocyte proliferation in the regenerating liver. To address this, we acutely deleted cyclin D1 from hepatocytes in vivo as in Fig. 3 and then performed PH, which is the most commonly used model of liver regeneration (6, 42, 43). In this model, most of the remaining hepatocytes enter the cell cycle in a relatively synchronous manner with peak DNA synthesis occurring at about 42 h after surgery.
As previously shown (44, 45), cyclin D1 was induced after PH at 24 h and persisted at 7 d in control mice (Fig. 4A), and this was associated with the expected robust hepatocyte proliferation peaking at 42 h (Fig. 4 B and C). Immunohistochemical studies at this time point showed that cyclin D1 was strongly induced in both nucleus and cytoplasm of hepatocytes in zone 2 of the hepatic lobule (SI Appendix, Fig. S2B). In stark contrast, mice with acute hepatic cyclin D1 KO had markedly reduced hepatocyte proliferation as evidenced by Cdk1 expression, PCNA immunostaining, and mitotic index (Fig. 4 A and B). Interestingly, in mice with chronic hepatocyte cyclin D1 KO beginning early in life (via the Albumin-Cre transgene), hepatocyte proliferation was not impaired at 42 h (SI Appendix, Fig. S3A). This suggests that compensatory changes during development in the chronic cyclin D1 KO model allow for hepatocyte proliferation in the absence of this protein, which is compatible with the observation that the liver has multiple redundant pathways to promote regeneration (6). However, in wild-type (WT) cyclin D1fl/fl mice with normal liver development, KO of cyclin D1 1 wk prior to PH markedly inhibits proliferation, confirming its pivotal role in normal hepatocyte cell cycle progression in vivo.
Fig. 4.
Acute deletion of cyclin D1 in regenerating liver. Female Cyclin D1fl/fl mice were treated with AAV8-TBG-CRE (or a GFP control vector) 1 wk prior to experiments to create acute cyclin D1 KO mice. Mice then underwent PH and were harvested at the indicated time points. (A) Western blot of liver lysates. Mice that underwent no treatment (0 h) or were harvested 42 h after sham surgery are show at the left from the same Western blot. (B) Hepatocyte PCNA expression by immunohistochemistry. (C) Hepatocyte mitotic index. (D) The most highly up- and down-regulated proteins in acute hepatocyte-specific cyclin D1 KO livers at 42 h after PH by proteomic analysis. The gene name for these proteins is shown. (E) The top two upstream regulatory pathways in cyclin D1 KO livers after PH in IPA analysis of the proteomics results. (F) The most highly affected canonical pathways in IPA analysis of the proteomics results.
Neither acute or chronic KO of cyclin D1 led to a significant increase in D3 expression, but cyclin D2 expression was modestly increased in the chronic KO model (SI Appendix, Fig. S3B). There was mild induction of these other D-type cyclins in the cell lines treated with cyclin D1 siRNA (SI Appendix, Fig. S3C). Prior studies have shown that the expressions of cyclins D2 and D3 are significantly less than that of cyclin D1 in regenerating liver (44, 46). Thus, although the D-type cyclins can have some similar effects, induction of cyclins D2 and D3 do not appear to markedly compensate for cyclin D1 depletion in our models.
A significant number of metabolic genes, including HNF4α targets, are down-regulated in the regenerating liver (7, 10, 37). To investigate the role of cyclin D1 in global protein expression, we isolated proteins and performed tandem mass spectrometry (MS) on livers harvested 42 h after PH (five control mice and five with acute cyclin D1 KO, each analyzed separately). Using a P value of ≤0.05, more than 950 proteins were differentially regulated in the KO liver relative to the control liver after PH with the majority being down-regulated (Dataset S4). The most highly up- and down-regulated proteins induced by cyclin D1 KO at 42 h after PH (compared to control mice at this time point) are listed in Fig. 4C. Not surprisingly, the five most down-regulated proteins are associated with cell proliferation. On the other hand, the five most up-regulated proteins in the setting of D1 KO were related to metabolic control. The first three (Gjb1, Ivd, and Bhmt2) are potential HNF4α targets (31, 32, 47), and HNF4α binding to the regions of these genes was reduced by cyclin D1 in the ChIP-seq (Dataset S2). The next two most-up-regulated proteins (Cyp4a10 and Cyp4a14) are PPARα targets, which is consistent with our prior study showing that cyclin D1 represses this transcription factor (20), and the fact that PPARα expression in the liver is dependent on HNF4α (34).
When we analyzed the proteins that were differentially regulated by acute cyclin D1 KO at 42 h after PH in IPA, the two most highly affected upstream regulatory mediators were PPARα and HNF4α, which were predicted to be markedly activated (Fig. 4D). The most highly regulated canonical pathways are listed in Fig. 4E. These show that acute cyclin D1 KO induced proteins involved in numerous metabolic functions; the most up-regulated pathway was fatty acid oxidation, supporting our recent studies indicating that cyclin D1 represses lipid catabolism in proliferating hepatocytes (20, 21). Cumulatively, the proteomic data indicate that in addition to promoting hepatocyte cell cycle progression the induction of cyclin D1 in the regenerating liver down-regulates the expression of HNF4α targets involved in diverse metabolic functions.
Cyclin D1 Inhibits Hepatic Glycogen Accumulation in Response to Feeding.
The liver plays a central role in whole-body glucose metabolism and is a major site of glycogen storage, which is dependent on HNF4α (18, 21, 37). Two previous studies have shown that cyclin D1 can be induced by refeeding mice after a period of fasting (48, 49). To examine whether the cyclin D1–HNF4α axis regulates hepatic glycogen accumulation in response to feeding, we fasted mice for 20 h and then refed them with a high-carbohydrate diet for 1 d as previously described (9). Refeeding induced cyclin D1 expression in WT mice (Fig. 5A), which was limited to zone 2 of the hepatic lobule (SI Appendix, Fig. S2C). Compared to WT mice, mice with chronic hepatic cyclin D1 KO had 25% more glycogen in the postprandial state (Fig. 5B). The effect was even more pronounced in the setting of acute cyclin D1 KO, which had threefold more hepatic glycogen than control animals. Importantly, acute deletion of both cyclin D1 and HNF4α reduced glycogen levels to that seen in mice with normal cyclin D1 levels, suggesting that the effect of cyclin D1 KO on glycogen storage was entirely dependent on HNF4α. Refeeding induced phosphorylation of Akt, glycogen synthase kinase 3β, and 4E-BP1 in each strain of mice, suggesting that insulin signaling and TORC1 activation were intact (Fig. 5C). In refed mice, acute cyclin D1 KO did not affect blood glucose levels, but liver weight was increased in these mice, likely reflecting increased glycogen content (SI Appendix, Fig. S4).
Fig. 5.
Cyclin D1 inhibits hepatic glycogen accumulation in the liver in a HNF4α-dependent fashion. Male mice with chronic or acute cyclin D1 KO (and the corresponding control mice) were fasted for 20 h and fed a high carbohydrate diet for 1 d. Additionally, refed mice with acute deletion of both cyclin D1 and HNF4α were examined. (A) Western blot of cyclin D1 induction with fasting or refeeding. (B) Liver glycogen levels with refeeding (C) Western blot analysis of livers in the acute KO model after refeeding. (D) RT-PCR Gys2 and Gck in refed mice. (E) Glucose uptake in AML12 cells with treated with siRNA to cyclin D1 and/or HNF4α (F) Gck mRNA expression in AML12 cells. (G) AML12 cells treated with [U-13C]-glucose for 24 h prior to harvest and followed by extraction and analysis by gas chromatography–MS (GC–MS) and NMR. The abundance of glucose-6-phosphate isotopologues by GC–MS is shown at the left, and abundance of 12C- and 13C-glycogen by NMR is shown at the right.
Glycogen synthesis and degradation are regulated by numerous enzymes, the best characterized of which are glycogen synthase (Gys2 in the liver) and glycogen phosphorylase (Pgyl in the liver), which promote glycogen synthesis and degradation, respectively (50). HNF4α transcriptionally activates Gys2, the rate-limiting enzyme in glycogen synthesis (31, 32, 51). The Gys2 gene and protein expression after refeeding were induced in the setting of acute cyclin D1 KO, which was prevented if HNF4α was also deleted (Fig. 5 C and D). Glycogen synthesis is also influenced by the rate of glucose flux into hepatocytes; a key modulator of this is glucokinase (gene name Gck) that catalyzes the synthesis of glucose-6-phosphate, which traps glucose in the cell (52). In refed mice, hepatic Gck expression was increased in mice with acute cyclin D1 KO, but the expression of this gene was blunted in mice with combined deletion of cyclin D1 and HNF4α.
To examine whether the cyclin D1-HNF4α interaction regulates glucose uptake, we examined AML12 cells treated with siRNA to cyclin D1 and HNF4α. This showed that cyclin D1 depletion increased glucose uptake (Fig. 5E) but not when HNF4α was also depleted, suggesting that cyclin D1-mediated inhibition of glucose uptake in these cells was dependent on HNF4α. Similarly, Gck was induced by cyclin D1 siRNA in a HNF4α-dependent manner (Fig. 5F). To further examine the effect of cyclin D1 on glucose incorporation into glycogen, we incubated AML12 cells with [U-13C]-glucose in the setting of cyclin D1 knockdown. This showed that cyclin D1 siRNA led to increased abundance of 13C-labeled glucose-6-phosphate and a 2.9-fold increase in labeled glycogen (Fig. 5G). Thus, under fixed media conditions, cyclin D1 inhibits glucose incorporation and glycogen synthesis in hepatocytes in a cell-automatous fashion.
The cumulative data in Fig. 5 demonstrate that physiologically expressed cyclin D1 functions to repress glycogen accumulation in response to feeding via mechanisms that include decreased glycogen synthase expression and reduced glucose uptake. Although further studies are required to dissect the mechanism(s) involved, these studies indicate that cyclin D1 acts in a negative feedback mechanism during feeding and that the cyclin D1- HNF4α axis regulates hepatic metabolism in states other than liver regeneration.
Example of a Metabolic Enzyme Regulated by the Cyclin D1- HNF4α Axis: Pyruvate Carboxylase.
Fig. 6 shows an example of a metabolic protein that we found to be down-regulated by cyclin D1 in each of the models described here. Pyruvate carboxylase (PC), a highly expressed protein in the liver, catalyzes the conversion of pyruvate into oxaloacetate, and plays a role in gluconeogenesis, lipogenesis, insulin signaling, and other metabolic processes (53, 54). Prior studies have shown that mouse PC (mouse gene name Pcx) is regulated by HNF4α (28) and that its mRNA expression is markedly reduced in the liver after acute HNF4α KO (31, 32). Cyclin D1 transduction (using ADV-D1) decreased PC mRNA and protein levels (Fig. 6A). It also significantly reduced HNF4α binding to several sites in the region of the PC gene in the ChIP-seq (Dataset S2 and Fig. 6B), including a region adjacent to the transcriptional start site that corresponds to the previously identified HNF4α binding site in this gene (28). In the resting liver, which has low levels of cyclin D1 expression, acute cyclin D1 KO mildly increased PC mRNA and protein expression, which was prevented if HNF4α was also deleted (Fig. 6C). After PH, PC expression was decreased, but this was ameliorated if cyclin D1 was acutely deleted (Fig. 6D). In refed mice, PC expression was increased in the setting of acute cyclin D1 KO in a HNF4α-dependent manner (Fig. 6E). In AML12 cells, cyclin D1 siRNA led to increased PC expression, but concurrent HNF4α knockdown prevented this induction (Fig. 6F). Thus, in multiple models, the key metabolic enzyme PC is inhibited by cyclin D1, and the data support a model in which cyclin D1 represses hepatic metabolic function via decreased HNF4α activity.
Fig. 6.
Cyclin D1 and HNF4α reciprocally regulate pyruvate carboxylase expression. (A) PC protein and mRNA expression in the livers of BALB/c mice 1 d after cyclin D1 transduction as in Fig. 2. (B) Tracings of HNF4α binding to regions of the PC gene in the mouse liver from the integrated genomics viewer genome browser. Three independent animals are shown for each group. (C) PC protein and mRNA expression in livers with acute deletion of cyclin D1, HNF4α, or both as in Fig. 3. (D) PC protein and mRNA in regenerating liver 42 h after PH in control and acute hepatocyte cyclin D1 KO livers as in Fig. 4. (E) PC mRNA and protein expression at 42 h after PH in control mice and mice with acute cyclin D1 KO as in Fig. 4. (F) PC mRNA in AML12 cells after deletion of cyclin D1, HNF4α, or both.
Discussion
The data presented here demonstrate that cyclin D1 and HNF4α, the best-characterized intracellular mediators of hepatocyte proliferation and differentiation (respectively), are reciprocal negative regulatory partners that control both cell cycle progression and metabolism in the liver. The negative regulation of HNF4α activity by cyclin D1 supports a model in which basal hepatic metabolic function is broadly diminished by the cell cycle machinery, allowing for cellular resources to be redirected to the demands of growth and proliferation. Furthermore, our data indicate that HNF4α-mediated repression of hepatocyte proliferation is based, at least, in part, on its inhibition of cyclin D1. Cyclin D1 is induced by feeding and inhibits glucose uptake and glycogen synthesis in a HNF4α-dependent manner, indicating that this cell cycle protein plays a role in hepatic physiology beyond liver regeneration. While the regulation of hepatic regeneration and metabolism involves a plethora of mediators and pathways, the cyclin D1-HNF4α regulatory axis provides a potential mechanistic framework for understanding the balance between these two important aspects of liver biology.
The studies described here used several “-omics” approaches to discover novel effects of cyclin D1 on gene expression—e.g., RNA-seq, ChIP-seq, and proteomics—and the data demonstrate that cyclin D1 regulates binding of HNF4α to a wide range of targets thereby modulating diverse metabolic genes in the liver. Notably, analysis of both transcriptomic and proteomic data using an unbiased platform (IPA) provided strong evidence that cyclin D1 represses the expression of HNF4α target genes. We used several in vivo models, such as hepatocyte-specific cyclin D1 and HNF4α KO, cyclin D1 overexpression, liver regeneration, and fasting–refeeding, each of which suggested a significant regulatory interaction between these two proteins.
Although cyclin D1 has been shown to modulate numerous transcriptional regulators (13), including other nuclear receptors, the use of ChIP-seq in this study provides a more complete picture of how it regulates gene occupancy of another transcription factor. We were surprised to find that cyclin D1 inhibits HNF4α binding to thousands of genomic sites in the liver, suggesting that this inhibitory interaction has the potential to regulate a wide range of hepatic functions. The ChIP-seq data indicate that the effect of cyclin D1 is largely regulated by the RD domain (which does not bind Cdk4). Prior studies have shown that cyclin D1 represses the activity of other nuclear receptor proteins (PPARα and the androgen receptor) via the RD domain (20, 33). In prior work, we showed that full-length cyclin D1 and cyclin D1-RD bound to HNF4α in the liver by immunoprecipitation, whereas a mutant of cyclin D1 lacking the RD domain did not (19). We speculate that binding of cyclin D1 (via the RD domain) to HNF4α inhibits its association with genomic targets, although further studies will be required to clarify the precise mechanism(s) involved. It will be of interest to determine whether cyclin D1 regulates additional nuclear receptor transcription factors involved in metabolic function in a similar manner.
HNF4α function is regulated by diverse mechanisms, including posttranslation modifications, interactions with transcriptional coregulatory proteins, crosstalk with other nuclear receptors, and the circadian rhythm (4, 36, 55). The expression of HNF4α itself is also regulated by inflammatory mediators, components of the insulin signaling pathway, and microRNAs (4). In addition, isoforms of HNF4α driven by different promoters (P1 and P2) are regulated distinctly; the P1 isoform is the primary form in the adult liver. A recent study demonstrated that HNF4α protein expression in the liver is transiently down-regulated in the early hours after PH in mice via unknown posttranscriptional mechanisms, but returns to near-normal levels by 2 d (37). The early decline in HNF4α expression was associated with decreased hepatic expression of selected HNF4α target genes at 6 and 12 h after PH (37). Similar findings were reported at 4 h after PH in mice (56). At these early time points, there is minimal induction of cyclin D1 (45); in the current study, we examined a later time point (42 h) that manifests markedly increased cyclin D1 levels and found that it inhibited the expression of numerous HNF4α target proteins. The combined results from these studies indicate that distinct mechanisms regulate HNF4α activity at different stages of regeneration. The data presented here demonstrate that cyclin D1 (via the RD domain) inhibits binding of HNF4α to target genes without affecting expression of this protein. Interestingly, cyclin D1 expression increased binding of HNF4α to a small portion of genes in the ChIP-seq, suggesting that additional levels of regulation occur.
A key finding in this study is that acute hepatocyte-specific deletion of cyclin D1 in the adult liver markedly inhibits proliferation of these cells as documented by PCNA immunohistochemistry, mitotic rate, and Cdk1 expression. Coupled with prior work (14–22), the current data provide strong support to the concept that cyclin D1 plays a central role in normal hepatocyte cell cycle progression (6, 7). The differential proteomic analysis in cyclin D1 KO mice after PH indicates that cyclin D1 modulates hundreds of proteins involved in diverse cellular processes; similarly, the RNA-seq results demonstrate that acute cyclin D1 knockdown in hepatic cell lines regulates a large number of transcripts representing many functional pathways. The combined results further indicate that cyclin D1 represents an important regulatory node in proliferating hepatocytes that modulates a broad range of cellular functions.
Prior studies have shown that acute KO of HNF4α in the resting liver triggers hepatocyte proliferation and induction of cell cycle genes including cyclin D1 (34–36), which we confirmed here. Notably, deletion of cyclin D1 along with HNF4α markedly reduced cell cycle progression, suggesting that HNF4α-mediated repression of cyclin D1 plays a role in maintaining proliferative quiescence. The mechanisms by which HNF4α represses cyclin D1 expression in hepatocytes remain to be determined, but the ChIP-seq demonstrated that HNF4α binds several sites in the region of the cyclin D1 gene (Datasets S2 and S3). The data presented here demonstrate that the cyclin D1-HNF4α negative regulatory interaction is bilateral and functionally significant in vivo.
We also examined the effect of hepatocyte-specific cyclin D1 KO in the hepatic response to feeding. Two groups have shown that feeding after a period of fasting induces cyclin D1 in the liver and that chronic cyclin D1 KO in the liver results in increased gluconeogenesis (48, 49). These authors showed that cyclin D1/cdk4 represses the activity of transfected proliferator-activated receptor γ coactivator 1-α (PGC-1α), a coactivator that enhances expression of glucogenic genes, but did not study the effect(s) on native PGC-1α and did not examine HNF4α. Although PGC-1α is known to coactivate HNF4α on gluconeogenic genes (e.g., Pck1 or G6pc), it does not regulate HNF4α activity on most other genes (4). Thus, inhibition of PGC-1α activity would not explain the broader effect of cyclin D1 on HNF4α-mediated gene expression (or binding of HNF4α to genomic targets) reported here.
In the refeeding model used in this study, hepatocyte-specific cyclin D1 KO led to substantially increased glycogen storage but did not alter blood glucose levels. We present data that cyclin D1 depletion increased glucose uptake as well as Gck and Gys2 expressions, each in a HNF4α-dependent manner. In cultured AML12 hepatocytes, cyclin D1 knockdown markedly increased 13C-glucose incorporation into glycogen, indicating that cyclin D1 inhibits glycogen synthesis in a cell-autonomous manner even when the extracellular milieu is held constant. Coupled with the prior data suggesting that cyclin D1 KO increase gluconeogenesis (48, 49), these findings suggest that physiologically induced cyclin D1 plays a negative feedback role in both glucose production and glucose uptake and storage in the liver after feeding. Further study will be required to unravel the details of how cyclin D1 regulates glucose metabolism, but these studies demonstrate that it is a bona fide glucoregulatory protein in the liver.
While the overall pattern of cyclin D1 inhibiting HNF4α function is well supported by the data, there is a high degree of complexity in these biological models. As noted above, cyclin D1 regulates numerous other transcriptional regulators (including the canonical Rb-E2F pathway) (13), and crosstalk between HNF4α and these other factors is likely to be significant. In addition, cyclin D1 enhanced binding of HNF4α to a small portion of sites in the liver, suggesting that the interaction between these proteins is context dependent. It is also likely that the cyclin D1 modulates HNF4α-mediated gene expression through indirect mechanisms. For example, our ChIP-seq data indicate that cyclin D1 repressed HNF4α binding to the genomic region of other important hepatic transcription factors (e.g., Rara, Ppara, Nr1i2, and Hnf1a) and microRNAs (e.g., MiR-122 and Let7) that are known to play an important role in regulating liver function (Datasets S2 and S3). Interestingly, in three models examined here—acute HNF4α deletion, PH, and refeeding—cyclin D1 was predominantly expressed in zone 2 of the hepatic lobule, suggesting that it may be expressed preferentially in hepatocyte subpopulations. Further studies, such as single-cell sequencing, may provide insight into the role that cyclin D1 plays within the complex functional zonation architecture of the liver (41, 57, 58).
Our studies support a model in which induction of cyclin D1 during hepatocyte proliferation broadly down-regulates HNF4α activity, providing a mechanism by which the cell cycle machinery can reprogram metabolism during liver growth. This mechanism also appears to be utilized after feeding, indicating that cyclin D1 functions as a metabolic regulator in situations other than hepatocyte proliferation. Future studies will address the mechanisms by which this protein regulates glucose utilization and other aspects of hepatic metabolism.
Materials and Methods
All of the methods in this study are detailed in SI Appendix, Materials and Methods, including cell culture, mouse studies, RNA, and protein analysis, RNA-seq, histology studies, ChIP-seq, analysis of sequencing data, proteomics studies, and metabolomics studies. Animal studies were approved by the institutional animal use and care committee at the Minneapolis VA Health Care System.
Data Availability.
The RNA-seq and ChIP-seq data used in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/ (accession no. GSE146053).
Supplementary Material
Acknowledgments
We thank LeeAnn Higgins and Todd Markowski for help with proteomics analysis, the University of Pennsylvania Diabetes Research Center for the use of the Functional Genomics Core (P30 DK19525), Wendy S. Larson, and Susan K. Dachel from MVAHCS Pathology for performing immunostains. This work was supported by the NIH Grants R01DK54921 (to J.H.A.), R01DK102667 (to K.H.K.), R01DK98414, and R56112768 (to U.A.) and R01CA202634 (to P.S.). N.M.R. and M.S. were recorded using the Metabolism Shared Resources supported, in part, by NIH Grants P30CA177558 (to B. M. Evers) and 1U24DK097215-01A1 (to R. M. Higashi, T.W.M.F., and A.N.L.).
Footnotes
Competing interest statement: P.S. has been a consultant at Novartis, Genovis, Guidepoint, The Planning Shop, ORIC Pharmaceuticals, and Exo Therapeutics; his laboratory receives research funding from Novartis.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq and ChIP-seq data used in this paper have been deposited in Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/ (accession no. GSE146053).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002898117/-/DCSupplemental.
References
- 1.Watt A. J., Garrison W. D., Duncan S. A., HNF4: A central regulator of hepatocyte differentiation and function. Hepatology 37, 1249–1253 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez F. J., Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab. Pharmacokinet. 23, 2–7 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Kaestner K. H., Making the liver what it is: The many targets of the transcriptional regulator HNF4alpha. Hepatology 51, 376–377 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Lu H., Crosstalk of HNF4α with extracellular and intracellular signaling pathways in the regulation of hepatic metabolism of drugs and lipids. Acta Pharm. Sin. B 6, 393–408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walesky C., Apte U., Role of hepatocyte nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene Expr. 16, 101–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalopoulos G. K., Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. Am. J. Pathol. 176, 2–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fausto N., Campbell J. S., Riehle K. J., Liver regeneration. Hepatology 43 (2, suppl. 1), S45–S53 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Delhaye M. et al., Relationship between hepatocyte proliferative activity and liver functional reserve in human cirrhosis. Hepatology 23, 1003–1011 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Fang J. W., Bird G. L., Nakamura T., Davis G. L., Lau J. Y., Hepatocyte proliferation as an indicator of outcome in acute alcoholic hepatitis. [published erratum appears in Lancet 1994 May 7;343(8906):1170] [see comments]. Lancet 343, 820–823 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Rudnick D. A., Elucidating the metabolic regulation of liver regeneration. Am. J. Pathol. 184, 309–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J., Thompson C. B., Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 20, 436–450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosios A. M., Vander Heiden M. G., The redox requirements of proliferating mammalian cells. J. Biol. Chem. 293, 7490–7498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hydbring P., Malumbres M., Sicinski P., Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 17, 280–292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrecht J. H., Hansen L. K., Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 10, 397–404 (1999). [PubMed] [Google Scholar]
- 15.Hansen L. K., Albrecht J. H., Regulation of the hepatocyte cell cycle by type I collagen matrix: Role of cyclin D1. J. Cell Sci. 112, 2971–2981 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Nelsen C. J., Rickheim D. G., Timchenko N. A., Stanley M. W., Albrecht J. H., Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 61, 8564–8568 (2001). [PubMed] [Google Scholar]
- 17.Nelsen C. J., Rickheim D. G., Tucker M. M., Hansen L. K., Albrecht J. H., Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J. Biol. Chem. 278, 3656–3663 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Nelsen C. J. et al., Amino acids regulate hepatocyte proliferation through modulation of cyclin D1 expression. J. Biol. Chem. 278, 25853–25858 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Hanse E. A. et al., Cyclin D1 inhibits hepatic lipogenesis via repression of carbohydrate response element binding protein and hepatocyte nuclear factor 4α. Cell Cycle 11, 2681–2690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamarajugadda S. et al., Cyclin D1 represses peroxisome proliferator-activated receptor alpha and inhibits fatty acid oxidation. Oncotarget 7, 47674–47686 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H. et al., Evidence for a novel regulatory interaction involving cyclin D1, lipid droplets, lipolysis, and cell cycle progression in hepatocytes. Hepatol. Commun. 3, 406–422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullany L. K. et al., Cyclin D1 regulates hepatic estrogen and androgen metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G884–G895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullany L. K. et al., Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle 7, 2215–2224 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beroukhim R. et al., The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zack T. I. et al., Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B. et al., Harnessing big “omics” data and AI for drug discovery in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 17, 238–251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolotin E. et al., Integrated approach for the identification of human hepatocyte nuclear factor 4α target genes using protein binding microarrays. Hepatology 51, 642–653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavalit T., Rojvirat P., Muangsawat S., Jitrapakdee S., Hepatocyte nuclear factor 4α regulates the expression of the murine pyruvate carboxylase gene through the HNF4-specific binding motif in its proximal promoter. Biochim. Biophys. Acta 1829, 987–999 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Yao D., Peng S., Dai C., The role of hepatocyte nuclear factor 4alpha in metastatic tumor formation of hepatocellular carcinoma and its close relationship with the mesenchymal-epithelial transition markers. BMC Cancer 13, 432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M. J., Lee W., Park E. J., Park S. Y., Role of hepatocyte nuclear factor-4alpha in transcriptional regulation of C1qTNF-related protein 5 in the liver. FEBS Lett. 584, 3080–3084 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Gunewardena S., Walesky C., Apte U., Global gene expression changes in liver following hepatocyte nuclear factor 4 alpha deletion in adult mice. Genom. Data 5, 126–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walesky C. et al., Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology 57, 2480–2490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petre-Draviam C. E. et al., A central domain of cyclin D1 mediates nuclear receptor corepressor activity. Oncogene 24, 431–444 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Bonzo J. A., Ferry C. H., Matsubara T., Kim J.-H., Gonzalez F. J., Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4α in adult mice. J. Biol. Chem. 287, 7345–7356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walesky C. et al., Hepatocyte-specific deletion of hepatocyte nuclear factor-4α in adult mice results in increased hepatocyte proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G26–G37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fekry B. et al., Incompatibility of the circadian protein BMAL1 and HNF4α in hepatocellular carcinoma. Nat. Commun. 9, 4349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huck I., Gunewardena S., Espanol-Suner R., Willenbring H., Apte U., Hepatocyte nuclear factor 4 alpha activation is essential for termination of liver regeneration in mice. Hepatology 70, 666–681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J., Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y. J. et al., The requirement for cyclin D function in tumor maintenance. Cancer Cell 22, 438–451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanger K. et al., Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kietzmann T., Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 11, 622–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell C., Willenbring H., A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 3, 1167–1170 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Mitchell C., Willenbring H., Addendum: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 9 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Rickheim D. G. et al., Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology 36, 30–38 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Albrecht J. H., Hu M. Y., Cerra F. B., Distinct patterns of cyclin D1 regulation in models of liver regeneration and human liver. Biochem. Biophys. Res. Commun. 209, 648–655 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Boylan J. M., Gruppuso P. A., D-type cyclins and G1 progression during liver development in the rat. Biochem. Biophys. Res. Commun. 330, 722–730 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Battle M. A. et al., Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc. Natl. Acad. Sci. U.S.A. 103, 8419–8424 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhalla K. et al., Cyclin D1 represses gluconeogenesis via inhibition of the transcriptional coactivator PGC1α. Diabetes 63, 3266–3278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y. et al., Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature 510, 547–551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adeva-Andany M. M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E., Glycogen metabolism in humans. BBA Clin. 5, 85–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandard S. et al., Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cell. Mol. Life Sci. 64, 1145–1157 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karim S., Adams D. H., Lalor P. F., Hepatic expression and cellular distribution of the glucose transporter family. World J. Gastroenterol. 18, 6771–6781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuel V. T., Shulman G. I., Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 27, 22–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valle M., “Pyruvate carboxylase, structure and function”. Subcell. Biochem. 83, 291–322 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Qu M., Duffy T., Hirota T., Kay S. A., Nuclear receptor HNF4A transrepresses CLOCK:BMAL1 and modulates tissue-specific circadian networks. Proc. Natl. Acad. Sci. U.S.A. 115, E12305–E12312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiao H., Zhu Y., Lu S., Zheng Y., Chen H., An integrated approach for the identification of HNF4alpha-centered transcriptional regulatory networks during early liver regeneration. Cell. Physiol. Biochem. 36, 2317–2326 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Halpern K. B. et al., Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 542, 352–356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aizarani N. et al., A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 572, 199–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq and ChIP-seq data used in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/ (accession no. GSE146053).