Significance
Basal ganglia (BG) beta oscillations are a hallmark of Parkinson’s disease (PD) and are specifically reported to correlate with akinesia and rigidity during wakefulness. Studying spontaneous sleep in the nonhuman primate MPTP model of PD, we report that beta oscillations persist into NREM sleep. In the setting of the sleeping brain, BG beta oscillations correlate with a reduction of slow oscillations across the cortex and BG, and scale with insomnia severity. We suggest that similarly to the way synchronous cortico-BG beta oscillations are postulated to advance the PD akinetic motor symptoms during wakefulness, they may contribute to slow oscillation destabilization and insomnia during sleep, thereby underlying two seemingly diverging symptoms of PD.
Keywords: sleep, Parkinson’s disease, insomnia, beta oscillations
Abstract
Sleep disorders are among the most debilitating comorbidities of Parkinson’s disease (PD) and affect the majority of patients. Of these, the most common is insomnia, the difficulty to initiate and maintain sleep. The degree of insomnia correlates with PD severity and it responds to treatments that decrease pathological basal ganglia (BG) beta oscillations (10–17 Hz in primates), suggesting that beta activity in the BG may contribute to insomnia. We used multiple electrodes to record BG spiking and field potentials during normal sleep and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism in nonhuman primates. MPTP intoxication resulted in severe insomnia with delayed sleep onset, sleep fragmentation, and increased wakefulness. Insomnia was accompanied by the onset of nonrapid eye movement (NREM) sleep beta oscillations that were synchronized across the BG and cerebral cortex. The BG beta oscillatory activity was associated with a decrease in slow oscillations (0.1–2 Hz) throughout the cortex, and spontaneous awakenings were preceded by an increase in BG beta activity and cortico-BG beta coherence. Finally, the increase in beta oscillations in the basal ganglia during sleep paralleled decreased NREM sleep, increased wakefulness, and more frequent awakenings. These results identify NREM sleep beta oscillation in the BG as a neural correlate of PD insomnia and suggest a mechanism by which this disorder could emerge.
Parkinson’s disease (PD) patients suffer from myriad nonmotor symptoms including severe sleep disorders (1–4). Insomnia, the difficulty to initiate and maintain sleep (1, 5), affects as many as 60% of all PD patients (5, 6). Among its many long-term consequences, insomnia increases the risk of cognitive decline (7) and mental illness (8, 9), and is a significant predictor of poor quality of life (10). Several mechanisms have been proposed to account for insomnia in PD, including the degeneration of brainstem or hypothalamic nuclei governing sleep (11, 12), the persistence of motor symptoms during sleep (5), the medical therapy (3, 4), and even some comorbid situations, such as depression, restless leg syndrome, and autonomic symptoms (e.g., urinary and gastrointestinal dysfunction) (3, 13, 14). Nonetheless, data relating insomnia in PD (or in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [MPTP]-intoxicated primate, its leading animal model) to specific alterations in neuronal activity are not available to date.
Beta oscillations were recorded in the normal brain (15, 16), but they are especially prominent in the basal ganglia (BG) in idiopathic PD (17) and its animal models (18), where they were postulated to represent aberrant information processing in the BG main axis (19). The beta frequency band varies across species. In MPTP-intoxicated primates, it overlaps the low beta band seen in PD patients and spans the 10- to 17-Hz range (20). Beta oscillations are commonly found during wakefulness and are correlated with awake PD motor symptoms (21) but were also recently observed in BG field potentials during sleep in patients (22–24). However, these findings were heterogeneous across individuals, did not extend to BG spiking activity, were limited to the subthalamic nucleus, and notably were not correlated with the severity and the temporal structure of the PD sleep symptoms.
The degree of insomnia correlates with PD severity (25), and the sleep symptoms respond to treatments for PD that decrease BG beta activity and ameliorate waking motor symptoms such as dopamine replacement therapy (26) and deep brain stimulation (DBS) (27, 28). This suggests that BG pathophysiological processes may not only play a mediating role in Parkinson’s motor symptoms during wakefulness, but also contribute to PD insomnia.
Here, we used the nonhuman primate MPTP model of PD (29), which has been shown to replicate the major biochemical, pathological, and clinical signs of PD. We conducted whole-night multisite field potential (FP) and spiking activity recordings from BG structures along with full polysomnography in healthy and Parkinsonian animals to elucidate the neural underpinnings of PD insomnia.
Results
Experimental Parkinsonism Is Associated with Severe Insomnia.
Following a normal sleep recording period, two Vervet monkeys were treated with systemic injections of the neurotoxin MPTP, which rendered them severely Parkinsonian. The neuronal recordings targeted the subthalamic nucleus (STN) and the globus pallidus, external (GPe), and internal (GPi) segments, representing three computational hubs of the BG in which alterations in neuronal activity have been detected in PD during wakefulness (30). The recordings followed the normal light/dark and sleep schedules of the monkeys, lasted throughout the night, and were assisted by full polysomnography (electroencephalogram [EEG], electrooculogram [EOG], and electromyogram [EMG]) and video recordings. The MPTP-intoxicated monkeys were treated with l-DOPA/carbidopa in the morning. Sleep recordings were carried out at least 12 h after the last l-DOPA dose (off stage).
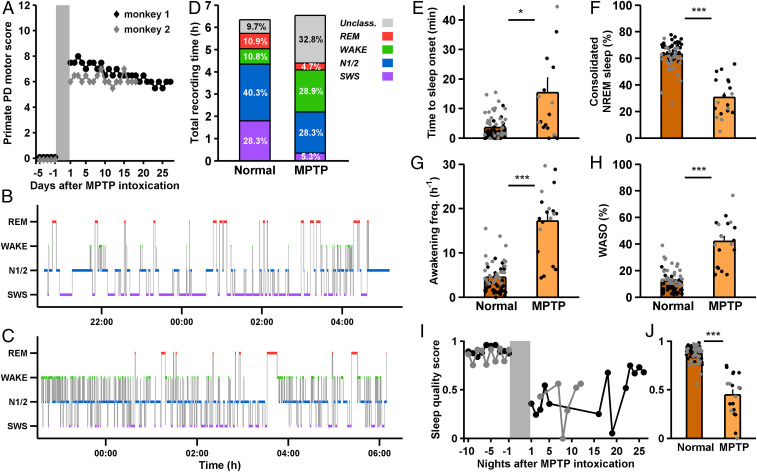
After the MPTP treatment, the animals exhibited the motor symptoms characteristic of PD, including bradykinesia, tremor, rigidity, and a flexed posture during the day (Fig. 1A). The Parkinsonian state was accompanied by severe insomnia, as was previously shown for PD (1, 3) and its MPTP macaque model (31, 32). Representative hypnograms for the normal and MPTP states are provided in Fig. 1 B and C. Parkinsonian nights were characterized by a decrease in NREM sleep (including N1/2 and deeper slow wave sleep, SWS) and an increase in wakefulness and transitional states (Fig. 1D and SI Appendix, Fig. S1). MPTP intoxication resulted in an elongation of the time needed for the monkeys to fall asleep (from an average of 3.52 min to 15.39 min, Fig. 1E) and in a decrease in consolidated NREM sleep (Fig. 1F). A consolidated bout of NREM sleep was defined as an uninterrupted stretch of NREM sleep lasting 40 s or more (SI Appendix). Consolidated SWS also showed a decrease: Normal vs. MPTP, 9.81% vs. 0.99% of all classified epochs, P = 3.92 × 10−9, Mann–Whitney U test). Sleep was not only diminished and harder to initiate, but was also fragmented: The monkeys failed to maintain long sleeping bouts, waking up every ∼3.5 min on average (vs. every ∼13.5 min during normal sleep, Fig. 1G) and experiencing longer waking bouts during the night (Fig. 1H). To arrive at a unified measure of the degree of insomnia that incorporated the extended time to sleep onset, decreased consolidated NREM sleep, increased frequency of awakenings, and protracted waking time after sleep onset, we defined the sleep quality score (ranging from 0, considerable insomnia, to 1, relatively stable sleep; Methods). MPTP intoxication significantly reduced the sleep quality score (0.88 before vs. 0.45 after, Fig. 1 I and J). Sleep quality improved over recording days but never reached pre-MPTP intoxication levels (Fig. 1I). As previously reported for macaque monkeys and cats (31–33), REM sleep was completely abolished for five recording nights after intoxication for monkey 1 and two nights for monkey 2, but then partially recovered. Here, we concentrate on the effects of induced Parkinsonism on NREM sleep.
Fig. 1.
Parkinsonism introduces severe insomnia. (A) Primate PD motor score (range: 0–12. Bradykinesia, tremor, rigidity, and postural deficits were each assigned a score from 0 to 3, where 3 represents severe symptoms) for both monkeys, normal state (mean, 0.00 ± 0.00, n = 38 and n = 29 d for monkey 1 and 2, respectively) vs. after MPTP intoxication (monkey 1: mean ± SEM, 6.67 ± 0.13, n = 27 d. Monkey 2: mean, 6.19 ± 0.09, n = 18 d. P = 2.11 × 10−14 and P = 4.88 × 10−11, respectively, Mann–Whitney U test). (B and C) All-night hypnograms for exemplary normal (B) and MPTP (C) nights, monkey 1. (D) Relative proportions of different sleep stages (unclass., unclassified, corresponding to transitional states), out of the total recording time, over all nights and both monkeys. Color scale as in B and C (Normal monkey, n = 38 recording nights for monkey 1 and n = 29 for monkey 2. MPTP monkey, n = 13 recording nights for monkey 1 and n = 6 for monkey 2. Normal vs. MPTP, change in SWS, P = 6.71 × 10−10, N1/2, P = 0.030, wakefulness, P = 8.29 × 10−8; REM, P = 1.50 × 10−4; Unclassified, P = 7.60 × 10−9; Mann–Whitney U test). (E) Time to consolidated sleep onset (first sleep bout of length > 40 s), normal vs. MPTP, for both monkeys. *P < 0.05, Mann-Whitney U test. Each point represents one night, error bars represent SEM: black, monkey 1; gray, monkey 2. A single point in the MPTP bar (at 86.17 min) is not included in this plot. (F) Percentage of consolidated NREM sleep out of all classified epochs. normal vs. MPTP. ***P < 0.0001, Mann–Whitney U test. (G) Awakening frequency, normal vs. MPTP. ***P < 0.0001, Mann–Whitney U test. (H) WASO, in % of all classified epochs, normal vs. MPTP. ***P < 0.0001, Mann–Whitney U test. (I) Sleep quality score for the last normal recording days and MPTP recording days, for both monkeys. Sleep quality improved in the last five recording days relative to the first 5 d (0.67 vs. 0.37, P = 0.016, but never reached preintoxication levels, 0.67 vs. 0.88, P = 6.67 × 10−4, Mann–Whitney U test). (J) Average sleep quality score, normal vs. MPTP, for both monkeys. ***P < 0.0001, Mann–Whitney U test. Unless otherwise noted, the data are presented as averages in this and the subsequent figures.
Insomnia Is Accompanied by the Onset of BG and Cortical Beta Oscillations during NREM Sleep.
We previously reported (34) that during NREM sleep in the normal monkey, BG neurons maintain a state of high amplitude slow oscillatory activity (0.1–2 Hz, Fig. 2A, dark traces; see also Fig. 5A). In contrast, Parkinsonian NREM sleep was characterized by aberrant beta (10–17 Hz) oscillations throughout the BG, in both the FP and multiunit spiking levels (Fig. 2 C and D, and see the example in Fig. 2B). Power spectral analysis of all FP recording sites in each BG structure revealed that beta activity was more prominent during Parkinsonian sleep than in normal sleep (Fig. 2C), and that a more substantial fraction of FP recording sites during NREM sleep in the Parkinsonian monkey showed beta activity (Fig. 2 C, Inset). As with the FP, beta oscillations were more prominent and prevalent in BG neuronal multiunit spiking during NREM sleep in the MPTP vs. the healthy monkey (Fig. 2D). Accordingly, FP and spiking beta power was higher during NREM sleep in the MPTP monkey than in the normal monkey (Fig. 2 E and F).
Fig. 2.
Parkinsonism is associated with an increase in beta oscillations in the BG during NREM sleep. (A and B) Typical field potential (FP, Upper) and concurrent spiking (Lower) recordings from a GPi electrode during NREM sleep in the normal monkey (A) and after MPTP intoxication (B). Horizontal bar, 0.5 s. Vertical bars, 50 µV. Colored are segments of spiking slow oscillation (A, dark blue) and beta oscillation (B, light blue), and their concurrent FP segments. (C) FP power spectra for GPe (red), GPi (blue), and STN (green) during NREM sleep, normal (dark traces) vs. MPTP (light traces). Shading represents SEM. (Inset) The proportion of FP recording sites exhibiting beta activity, averaged across four different thresholds (Methods). For the entire figure, n = 305, 172, and 94 sites recorded during NREM sleep (FP and spiking) for the normal GPe, GPi, and STN, respectively (of which n = 161, 61, and 63 were obtained from monkey 1, and the rest from monkey 2). n = 211, 51, and 43 for the GPe, GPi, and STN, respectively, during MPTP intoxication (of which n = 154, 15, and 20 were obtained from monkey 1 and the rest from monkey 2). (D) Spiking power spectra for GPe, GPi, and STN, normal vs. MPTP. Shading and Inset as in C. (E) NREM sleep and wakefulness FP beta power (normalized by subtraction of the average power in the flanking frequency ranges), normal (dark boxplots) vs. MPTP (light boxplots), GPe, GPi, and STN, ***P < 0.0001, Mann–Whitney U test. Black line represents the median; boxes represent the 25–75 percentiles, and whiskers denote the 10 and 90 percentiles. n = 271, 160, and 79 sites recorded during wakefulness (FP and spiking) for the normal GPe, GPi, and STN, respectively (of which n = 129, 50, and 53 were obtained from monkey 1 and the rest from monkey 2). n = 209, 50, and 44 for the GPe, GPi, and STN, respectively, during wakefulness following MPTP intoxication (of which n = 152, 13, and 20 were obtained from monkey 1 and the rest from monkey 2). For the GPe and STN, MPTP wakefulness vs. NREM, nonsignificant. (F) NREM sleep and wakefulness spiking beta power, normal vs. MPTP, GPe, GPi, and STN, *P < 0.05, ***P < 0.0001, Mann–Whitney U test. For the GPe, MPTP wakefulness vs. NREM, nonsignificant. (G) NREM sleep FP beta episode probability, beta episode duration (Upper Inset) and beta episode frequency (Lower Inset), normal vs. MPTP, GPe, GPi, and STN, ***P < 0.0001, Mann–Whitney U test. (H) NREM sleep spiking beta episode probability, beta episode duration, and beta episode frequency, normal vs. MPTP, GPe, GPi, and STN, **P < 0.005, ***P < 0.0001, n.s., nonsignificant, Mann–Whitney U test.
Fig. 5.
The increase in beta activity in the BG is correlated with a decrease in BG and cortical slow oscillatory activity. (A) Power spectra of firing rates recorded during NREM sleep in the normal (dark traces) and MPTP monkey (light traces), GPe (red), GPi (blue), and STN (green). Error bars represent SEM. Normal, n = 305, 172, and 94 neurons for GPe, GPi, and STN, respectively. MPTP, n = 211, 51, and 43 for GPe, GPi, and STN, respectively. (B) Average NREM sleep firing rate 0.1–1 Hz relative power, for each BG nucleus, across recording nights, normal vs. MPTP monkey. *P < 0.05, ***P < 0.0001, Mann–Whitney U test. (C) Bilateral frontal, central and occipital EEG power spectra in the normal (dark trace, n = 67 nights) and MPTP monkey (light trace, n = 19 nights). (D) Average EEG slow oscillation (SO, 0.1–2 Hz) relative power during NREM sleep, across recording nights, normal vs. MPTP monkey. *P < 0.05, Mann–Whitney U test. (E and F) Firing rate slow oscillation power for the different nuclei of the BG, divided into four quartiles by the degree of FP (E) or spiking (F) beta power recorded by the same electrode in the same neuronal site. As beta activity became stronger, firing rate slow oscillatory activity decreased. Colored traces represent recording sites from the GPe (red), GPi (blue), and STN (green). Purple bars represent the average across all recording nights from all BG nuclei. Error bars represent SEM. (G) Example scatter plots depicting the correlation between BG FP beta power and EEG SO power during NREM sleep following MPTP intoxication, GPe (red), GPi (blue), and STN (green). Each point represents one 10-s NREM sleep epoch. (H) Average Pearson’s correlation coefficients between BG FP beta power and EEG SO activity, for all of the sites and units exhibiting a significant correlation with the EEG SO (white, percent out of all sites and units exhibiting beta activity), n = 72, 28, and 8 sites for GPe, GPi, and STN, respectively. A single point in the STN bar (at 0.53) is not included in this plot. (I) Same as G, only for the correlation between BG spiking beta power and EEG SO power. (J) Same as H, only for Pearson’s correlation coefficients between BG spiking beta power and EEG SO activity. n = 11, 5, and 2 sites.
The emergence of beta activity in the BG was also manifested in the increase in beta episodes (18, 35) during Parkinsonian NREM sleep. For the field potential as well as the spiking activity, MPTP intoxication increased the probability of beta episodes; i.e., the total duration of beta episodes in a sleep epoch out of the full epoch length (Fig. 2 G and H). This was a result of longer episode duration (Fig. 2 G and H, Upper Inset) and increased episode frequency (Fig. 2 G and H, Lower Inset) during NREM sleep post-MPTP intoxication, relative to the normal monkey. These findings are consistent with results acquired from awake PD patients (35) and monkeys (18).
BG beta activity is widely reported in awake PD patients (19) and MPTP monkeys (18). In our data, BG beta power in the awake monkey was significantly higher than during NREM sleep for FP activity in the GPi and for spiking activity in the GPi and STN (Fig. 2 E and F). Similarly, the probability of FP beta episodes was higher during wakefulness in the GPi (9.89% during NREM sleep vs. 15.85% during wakefulness, P = 1.16 × 10−5, Mann–Whitney U test). Thus, even if elevated during wakefulness, beta activity is prevalent in the BG over the entire vigilance cycle, and in the GPe it is as prominent during NREM sleep as it is during wakefulness.
Beta oscillations were not confined to the BG but were also recorded in the cerebral cortex (see example in Fig. 3A). They were evident in the scalp EEG during Parkinsonian NREM sleep (Fig. 3 B and C, light traces and boxplots) but not during normal sleep (Fig. 3 B and C, dark traces and boxplots). After MPTP intoxication, EEG beta power was as prominent during NREM sleep as it was during wakefulness (Fig. 3 B and C), indicating that cortical beta activity during Parkinsonian sleep may be as clinically significant as it is during wakefulness. Finally, EEG beta episodes during Parkinsonian sleep displayed a similar profile to those in the BG, being more probable, more frequent, and lasting longer than beta episodes during normal sleep (Fig. 3D).
Fig. 3.
Parkinsonism is associated with an increase in beta oscillations in the cerebral cortex during NREM sleep. (A) Examples of central EEG (C1) activity during normal NREM sleep (Upper, notice the predominant slow oscillatory activity) and during Parkinsonian NREM sleep (Lower, where beta activity is more prominent). Horizontal bar, 0.5 s. Vertical bars, 50 µV. (B) Power spectra of scalp EEG (average over frontal, central and occipital electrodes ipsilateral to BG electrodes) during NREM sleep and wakefulness, normal vs. MPTP nights. Pale shading represents SEM. n = 67 and 19 nights for the normal and MPTP recordings, respectively. (C) NREM sleep and wakefulness EEG beta power (normalized as previously) for both conditions and sleep stages, **P < 0.005, ***P < 0.0001, Mann–Whitney U test. During MPTP, NREM beta power was not significantly different from the wakefulness beta power, Wilcoxon signed rank test. (D) NREM sleep EEG beta episode probability, beta episode duration (Upper Inset), and beta episode frequency (Lower Inset), normal vs. MPTP, ***P < 0.0001, Mann–Whitney U test.
Beta Oscillations Are Coherent across the BG and Cortex.
During normal wakefulness, BG spiking activity maintains a state of decorrelated firing (36). BG slow oscillations in firing rate (0.1–2 Hz) remain desynchronized even during normal SWS, one of the most synchronized brain states (34), and during Parkinsonian NREM sleep (SI Appendix, Fig. S2). We examined whether this defining feature of normal BG physiology would persist in Parkinsonian sleep beta activity, given the robust emergence of synchronous beta oscillations in awake Parkinsonian animals (18). To that end, we simultaneously recorded FP and spiking activity in the BG using two to four microelectrodes (located 0.5–2 mm apart). Fig. 4 provides an example of a two-electrode recording exhibiting synchronous beta activity in the GPi FP (Fig. 4A) and spiking (Fig. 4B). At the population level, beta oscillations in both FP (Fig. 4C) and spiking (Fig. 4D) were coherent between recording sites within BG nuclei. Further, the BG also displayed increased FP-spiking coherence in the beta range (Fig. 4E).
Fig. 4.
Beta oscillations during NREM sleep are coherent within and between the BG and cerebral cortex. (A and B) Examples of field potential (FP, A) and spiking activity (B) recorded simultaneously by two microelectrodes in the GPi during Parkinsonian NREM sleep. Horizontal bar, 0.5 s (A) or 0.25 s (B). Vertical bars, 50 µV. Spiking traces represent 1 s. (C and D) Coherence of simultaneously recorded FP (C) and spiking (D) in the BG during NREM sleep in the Parkinsonian monkey. Dashed lines represent the same recording sites, with time-shifted FP/spiking traces. Pale shading represents SEM. For FP and spiking coherence, n = 278, 85, and 26 paired recordings for the GPe, GPi, and STN, respectively. (Inset) Area under the curve (AUC) evaluated for the beta range (10–17 Hz), the baseline being the average 20–40 Hz range coherence, nonshifted (left boxes) vs. shifted data (right boxes). ***P < 0.0001, Mann–Whitney U test. (E) FP-spiking coherence for the MPTP data vs. shifted data, conventions and Inset as in C and D. For the GPe, GPi and STN, respectively, n = 211, 51, and 43 recordings. (F) Coherence between ipsilateral EEG electrodes during Parkinsonian NREM sleep, relative to shifted data (n = 19 nights). Inset, AUC as before. **P < 0.005, ***P < 0.0001, Mann-Whitney U test. (G and H) Coherence between averaged ipsilateral EEG and BG FP (G) or spiking (H) activity during Parkinsonian NREM sleep. Dashed lines represent the coherence spectra between shifted data. (Insets) AUC for the nonshifted and shifted data, as previously.
BG beta oscillations were not only coherent within the BG, but also between EEG contacts (Fig. 4F) and between EEG and simultaneously recorded BG FP (Fig. 4G) and spiking (Fig. 4H) beta oscillations. Finally, FP and EEG beta coherence during normal sleep were similar to the MPTP monkey. This was not true for spiking and FP-spiking beta coherence, which were negligible in the normal monkey (SI Appendix, Fig. S3).
The Increase in BG Beta Activity during NREM Sleep Is Correlated with a Decrease in Slow Oscillations.
The increase in beta activity during NREM sleep in the Parkinsonian monkey, in comparison with the normal monkey, coincided with a decrease in GPe and GPi firing rate slow oscillations (SOs, Fig. 5 A and B). We analyzed the firing rate power spectra (which is based on the firing rate activity extracted from detected single-neuron action potentials) since SOs in firing rate have been shown to characterize normal NREM sleep (34). The firing rate power spectra should not be confused with multiunit spiking power spectra, which are based on the electric fields of multiple neurons around the electrode, and were shown in Fig. 2 to exhibit increased beta activity (Methods). Note that unlike the GPe and GPi, SO showed a tendency to increase in the MPTP monkeys in the STN. A decrease in NREM sleep SOs in the Parkinsonian monkey was also evident in the scalp EEG (Figs. 3A and 5 C and D).
To determine whether the breakdown of slow oscillations and the onset of beta activity were related, we correlated the SO power changes in the EEG and BG firing rate with concurrent BG FP and spiking beta power. Changes in beta oscillation and slow oscillation were correlated: As field potential and spiking beta power increased, firing rate SO power decreased (Fig. 5 E and F). Similarly, the correlation between BG beta power and concurrent cortical EEG SO power (ipsilateral frontal, central, and occipital locations) during Parkinsonian NREM sleep was negative (Fig. 5 G and I). This correlation ranged from −0.27 to −0.39 for the FP, and from −0.33 to −0.40 for the spiking activity (Fig. 5 H and J). Fig. 5 H and J displays only those sites for which the correlation was significant. For the FP, these constituted the majority of cases, whereas for the spiking activity significant correlations were found in fewer than half the sites. Importantly, for both FP and spiking, all of these significant correlations were negative (except for a single FP STN site).
BG Beta Activity Rises before Spontaneous Awakening and Is Associated with Diminished and Fragmented Sleep.
If slow oscillation power could be taken to reflect NREM sleep depth (37, 38), then it is possible that the implication of the correlation between increased beta oscillations and decreased slow oscillations could extend to overall changes in sleep architecture (Fig. 1). We examined whether the measures of FP and spiking beta activity reported thus far would vary with the vigilance cycle, and specifically how these changes might relate to periods of lighter NREM sleep (e.g., falling asleep and awakening) and deeper sleep (SWS). Analysis of the dynamics of FP beta power, beta episode probability, and FP-EEG beta coherence in all BG nodes during NREM sleep revealed that falling asleep (i.e., uninterrupted NREM sleep following an initial waking state) was associated with a gradual decrease in the FP beta activity across the BG (Fig. 6 A and B). SWS was associated with relatively minimal FP beta power and beta episode probability, and beta oscillations became more prominent toward wake-up. Similar dynamics were exhibited by the BG FP to EEG beta coherence that decreased as sleep deepened and increased prior to awakening (Fig. 6C). Spiking beta power and probability displayed similar trends, in that they decreased as NREM sleep deepened and were greater postawakening relative to SWS. However, these trends reached statistical significance only for some BG nodes and were weaker than for the FP.
Fig. 6.
BG beta activity rises before spontaneous awakening and correlates with the severity of insomnia in Parkinsonism. (A) FP beta power in the GPe (red), GPi (blue), and STN (green) during NREM sleep decreases as sleep deepens, is relatively low during SWS, and increases steadily before awakening (dashed red line), when it plateaus. Shading represents SEM. Numbers of recording sites as in Fig. 2. SWS beta power was lower than the beta power in the first NREM epoch after falling asleep for GPe and STN, lower than the average N1/2 beta power (all BG structures), and lower than the beta power 10 s before wake-up for GPe. For STN, the beta power 40 s after falling asleep was lower than 10 s before wake-up. For all structures, the beta power was not significantly different for any one of the wakefulness points, Kruskal–Wallis H test, P > 0.05. For all structures, the average postawakening beta power was greater than the beta power during SWS and greater than the beta power 40 s after falling asleep. Unless stated otherwise, the statistical test used for all comparisons was the Mann–Whitney U test, and all P < 0.05. (B) Same as A, only for FP beta episode probability. All statistical relationships in A also hold for the beta probability, except for the relationship between the first NREM sleep epoch and SWS, which was true for GPe and GPi, and the N1/2 vs. SWS comparison, which was not significant for STN. (C) Same as A and B, only for FP-EEG beta range coherence. In the GPe, SWS beta coherence was lower than beta coherence for the first NREM epoch, lower than beta coherence for the average of the last three epochs before awakening, and lower than N1/2 beta coherence. In the STN, the beta coherence 10 s before awakening was greater than the SWS beta coherence. For all structures, the beta coherence was not significantly different for any one of the wakefulness points, Kruskal–Wallis H test, P > 0.05. For GPi, the average postawakening beta coherence was greater than during SWS and 40 s before awakening. (D) EEG SO power during NREM sleep increases as sleep deepens, is relatively high during SWS and before awakening, and decreases abruptly after awakening when it plateaus. SWS SO power was greater than SO power in the first NREM epoch after falling asleep, N1/2 SO power, and postawakening SO power. EEG SO power was not significantly different for any of the 40-s to 10-s points before awakening and was not significantly different for any one of the wakefulness points, Kruskal–Wallis H test, P > 0.05. Finally, the average EEG SO power 20 s and 30 s after awakening was significantly lower than the average EEG SO power 40 s to 10 s before awakening. (E) Increased FP NREM sleep beta power in the BG correlates with poorer sleep. NREM sleep (% out of all classified epochs) (Left), wakefulness (%) (Center), and hourly awakening frequency (Right). Shading represents SEM. Beta power levels are shown on the x axis as sixths of the total beta range recorded for each night to enable comparisons between nights. Lowest vs. highest normalized beta power group, for the MPTP monkey, NREM sleep percentage was lower for the highest beta group, and both wakefulness percentage and awakening frequency were higher for the highest beta group, for all structures except wakefulness percentage for STN (P < 0.05, Mann–Whitney U test). (F) Same as E, only for spiking beta power. Lowest vs. highest normalized beta power group, for the MPTP monkey, NREM sleep percentage was lower for the highest beta group, and both wakefulness percentage and awakening frequency were higher for the highest beta group for GPe and STN only (P < 0.001, Mann–Whitney U test).
We examined whether the negative correlation between BG beta and cortico-BG SO activity during sleep (Fig. 5) was also reflected in SO power dynamics. EEG SO power increased from NREM sleep onset to culminate during SWS, and remained relatively unchanged until awakening, after which it decreased sharply (Fig. 6D).
STN and GPe firing rate SO power exhibited similar behavior: SO power during deep stages of NREM sleep was significantly increased relative to SO power during wakefulness and during NREM sleep immediately after falling asleep (SI Appendix, Fig. S4).
Next, we examined whether NREM sleep beta oscillation power in the BG was correlated with the overall degree of insomnia. For each NREM sleep epoch, we averaged the beta power across all simultaneously recorded FP or spiking traces in all recording sites in a given BG nucleus. We parceled each night into overlapping periods covering 25% of the total night length (Methods), and then calculated the fraction of NREM sleep and wakefulness (out of the total duration of staged epochs) and the frequency of awakenings for each segment. Next, for each such period, the average per-structure NREM sleep beta power was calculated. Finally, all of the recording periods from a single night were assigned to six groups based on the NREM sleep beta power they exhibited, relative to the total range of beta power that night (the first group had the lowest average beta, and the sixth group had the highest).
Analysis of the relationship between the average FP/spiking beta power and the polysomnographic measures across nights showed that for the Parkinsonian monkey, periods with higher average NREM sleep beta power across the BG were characterized by decreased NREM sleep propensity, increased waking propensity, and elevated awakening frequency (Fig. 6E for FP and Fig. 6F for spiking, solid light traces). Thus, an increase in beta oscillation in the Parkinsonian monkey was associated with a decrease in slow oscillations at the single-second level, preceded spontaneous awakenings at the level of single sleep bouts, and correlated with more profound insomnia at the level of the entire sleep cycle.
Finally, muscle rigidity has been hypothesized to contribute to sleep symptoms in PD (5). To study the possible contribution of rigidity to insomnia, we used trapezius tone (quantified as the root mean square of the EMG signal) as a marker for muscle rigidity (39). We found an increase in trapezius tone in the Parkinsonian monkey (SI Appendix, Fig. S5A) but failed to detect a correlation between the trapezius tone and EEG beta activity (SI Appendix, Fig. S5B) or FP beta activity in the GPe, GPi, or STN (SI Appendix, Fig. S5 C–E) during NREM sleep. There was no association between increased trapezius tone and decreased NREM sleep, increased wakefulness, or increased awakening frequency (SI Appendix, Fig. S5 F–H).
Discussion
BG Beta Oscillations Are Elevated during Parkinsonian Sleep.
Beta oscillations have been studied in the healthy brain, where they were suggested to represent a “status quo” state of the motor system (15) or to play a role in working memory (16). However, beta oscillatory activity is particularly prominent in the BG of patients with idiopathic PD (19), where it correlates primarily with akinesia and rigidity during wakefulness (21). Further, the extent of clinical benefit in akinesia and rigidity is correlated with the decrease in FP beta power in PD patients (40). In our MPTP model, beta activity was consistently more prominent and widespread in the Parkinsonian monkey, also during NREM sleep. The Parkinsonian state was also accompanied by an increase in beta coherence within the BG and cortex, and between them. One finding that challenged this rule was relatively elevated FP and EEG beta coherence during normal sleep. This elevated coherence may be due to volume conductance of lower amplitude beta activity in the cortex and BG, which does not pertain to unit spiking.
Beta oscillations during sleep were reported in three studies that used FP data obtained from the STN of human PD patients. All showed beta during NREM sleep (22–24), but neither showed any relationship between beta oscillations and the severity or temporal structure of sleep disorders. Here, we introduce beta oscillation as a neural correlate for PD insomnia and report the relationship between beta activity and NREM sleep quality in Parkinsonism.
The Differences between MPTP Insomnia and PD Insomnia.
The above human studies reported modest NREM sleep beta activity in the STN, relative to wakefulness (22–24). In our results beta activity was prominent during NREM sleep, and for some cases like the scalp EEG, it was comparable to waking beta. This difference in NREM sleep beta activity may reflect the fact that MPTP does not fully capture the natural history and clinical manifestation of idiopathic PD. Our MPTP paradigm introduces a more fulminant and more severe disability than that found in PD patients undergoing STN DBS surgery. Furthermore, the acuteness of MPTP intoxication raises the concern that its effect might subside over time, thereby not replicating the sleep impairments seen in PD. In our model, beta activity and its relationship with the vigilance cycle were stable across 26 recording days, apart from a decrease in GPe FP beta power to above-normal levels in the last few recording days. Sleep quality improved in the last days of recording but stayed significantly lower than preintoxication levels. Possible contributing factors to this improvement might be partial resolution of the MPTP effect on the dopaminergic system or on other neuromodulatory system over time (41) and our chronic administration of l-DOPA that might have had long-term effects (42).
Concerning the molecular mechanisms eliciting insomnia in our model and in idiopathic PD, we cannot rule out the possibility that MPTP insomnia is caused by a different mechanism than PD insomnia. Our use of MPTP may have resulted in toxic injury to other brain structures orchestrating sleep (43) or the circadian rhythm (44) that are not severely affected in idiopathic PD. Inversely, PD insomnia might be mediated by the degeneration of cell groups that are spared by MPTP intoxication, such as the orexin neurons (45). However, the similarity between the sleep disorders reported here and in other works using MPTP monkeys (31, 32) and the sleep disorders in idiopathic PD (5) does suggest that the process mediating insomnia may be common to PD and MPTP intoxication (i.e., the degeneration of substantia nigra pars compacta dopaminergic neurons (14) and other brain monoaminergic neurons). Further, the fact that the sleep symptoms in the idiopathic disease respond to treatments that decrease BG beta activity (26–28) offers support to the hypothesis that the pathophysiological pathways for PD insomnia and MPTP insomnia are at least partially overlapping.
Beta Oscillations Are Different from Sleep Spindles.
An overlap exists between the frequency ranges of beta oscillations and sleep spindles that are prevalent in normal NREM sleep (46, 47). It is possible that normal or pathological sleep spindle activity was detected by our methods as beta activity. However, beta activity in our data exhibited two features that do not classically characterize sleep spindles. First, both normal and Parkinsonian beta episodes had significantly shorter durations and higher frequencies of occurrence compared to cortical sleep spindles in primates (Figs. 2 G and H and 3D) (47). Second, BG and cortical beta oscillations in the Parkinsonian monkey were similarly prevalent during sleep and during wakefulness. In contrast, in the normal monkey they were relatively weak regardless of the vigilance cycle (Figs. 2 E and F and 3 B and C). This would not be expected from sleep spindles, a hallmark of NREM sleep (46).
Cortico-BG Beta Oscillations May Contribute to Insomnia in Parkinsonism.
Although the mechanism of beta generation and propagation through the cortex and BG in Parkinsonism is still debated (48), evidence shows that the BG are an important hub for the generation of beta oscillations (49). Waking BG beta oscillatory output is hypothesized to disrupt cortical computation in PD (17), possibly contributing to the waking symptoms of the disease (50). The BG output structures (GPi and substantia nigra pars reticulata, SNr) target a number of thalamic nuclei that innervate the frontal cortex where slow oscillations emerge during sleep (38). Thus, similar to wakefulness, synchronized beta oscillations from the BG could be transmitted to cortical areas, disrupting the generation or propagation of cortical slow oscillations during NREM sleep and contributing to insomnia. This putative mechanism is congruent with the observation that increased BG beta power is associated with a decrease in cortical slow oscillation (Fig. 5 G–J), that beta activity increases prior to awakening (Fig. 6 A–C) and correlates with the severity of insomnia (Fig. 6 E and F), and that SO power falls upon awakening (Fig. 6D). Remarkably, STN DBS, which has been shown to reduce beta oscillation (51), is associated with an improvement in subjective sleep quality (27) and reductions in insomnia and sleep fragmentation in PD (27, 28). Finally, a dual BG involvement in sleep homeostasis and motor control was recently supported by a work showing that GAD2 neurons in the SNr promote sleep generation, possibly through their projections to various monoaminergic nuclei orchestrating the vigilance cycle (52).
Additional Possible Factors Contributing to PD Insomnia.
Although this work provides strong correlational links between beta oscillations and insomnia in Parkinsonism, this correlation does not imply causation. Insomnia in idiopathic PD may result from a host of other factors such as an underlying neurodegenerative process affecting brainstem or hypothalamic sleep/wake centers (11, 12), PD medical treatment (3, 4), or comorbid situations including depression (13), restless leg syndrome, and nocturia (14). Even if insomnia does originate from a BG pathology subsequent to dopaminergic denervation, it might not be a direct consequence of beta oscillation. Rather, it could result from PD motor symptoms (5) such as muscle rigidity. This setting, where beta oscillations might only serve as a surrogate marker for disease severity, could possibly be reflected in the correlation found here between the degree of insomnia and beta power. However, in our data, there was no association between muscle rigidity and beta activity in the EEG or in the BG, or between muscle rigidity and sleep quality. Additionally, in a study that assessed the effect of nocturnal DBS on PD patients, the number of body position changes during sleep was similar before and on stimulation (27). These findings suggest that insomnia in our model, and generally in PD, cannot be attributed solely to the persistence of motor symptoms into sleep.
Conclusions
In the MPTP-treated Parkinsonian monkey, NREM sleep beta oscillations were associated with reduced cortical slow oscillations, increased before spontaneous awakening, and correlated with the degree of insomnia. Thus, beta oscillations may be used as a marker for both the waking motor and sleep-related symptoms of PD. Phase-specific brain stimulation was recently shown to either augment or suppress brain oscillations (53, 54). Thus, future closed-loop adaptive DBS therapy for PD aimed at suppressing NREM beta oscillations and augmenting slow oscillations by phase-specific stimulation could be helpful in the control of insomnia in PD.
Methods
All experimental protocols were conducted in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals and with the Hebrew University guidelines for the use and care of laboratory animals in research. The experiments were supervised by the institutional Animal Care and Use Committee of the Faculty of Medicine, the Hebrew University. The Hebrew University is an internationally accredited institute of the Association for Assessment and Accreditation of Laboratory Animal Care.
The following subsections are to be found in the SI Appendix, Methods section: Animals, sleep habituation and surgery; MPTP intoxication; Polysomnography and sleep staging; Electrophysiological Recordings and Data Collection; Synchronization analysis; Beta episode analysis; Coherence analysis and EMG tone analysis.
Analysis of Sleep Measures.
For each recording night, a number of sleep architecture measures were obtained. Sleep onset was defined as the start of the first bout of at least 40 s that the monkey spent in NREM or REM sleep. The time to sleep onset was defined as the time elapsed since the monkey was left in the dark recording chamber to sleep onset. Wakefulness after sleep onset (WASO) was calculated as the total waking time after sleep onset divided by the total length of all classified epochs. A bout of consolidated NREM sleep/SWS was defined as a stretch of at least 40 s of NREM sleep/SWS. Finally, the frequency of awakenings per hour was calculated as the number of transitions from any sleep stage to a period of wakefulness lasting at least 30 s, throughout the night, divided by the total recording time in hours. Using various higher and lower duration thresholds provided similar results.
For each night, each aforementioned sleep measure was normalized to the 0–1 range by dividing the range delineated by the minimal and maximal value of the sleep measure across all normal and MPTP nights (after subtraction of the minimal value). Then, the sleep quality score for that night was obtained in the following manner:
The sleep quality score ranged from 0 to 1, increasing as NREM sleep became more abundant and as the time to sleep onset, awakening frequency, and WASO decreased.
Power Spectrum Analysis.
Each neuronal unit’s recording time was divided into 10-s epochs corresponding to the sleep staging epochs. We conducted a spectral analysis of the FP and the spiking activity recorded in the vicinity of each of the analyzed neurons. The raw signal (hardware filtration, 0.075 Hz–10 kHz, sampling at 44 kHz) was digitally bandpass filtered using a zero-phase Butterworth filter (0.1–300 Hz, bandstop at 0.05 and 350 Hz for FP; 300–6,000 Hz, bandstop at 200 and 6,500 Hz for spiking) and down-sampled to 1,375 samples per s (FP) or to 11,000 samples per s (spiking). Spiking traces were further full-wave rectified, and all of the real-time detected (high-amplitude) spikes, which represent the firing of a single proximal neuron, were removed to obtain a less-biased representation of the multiunit signal. Spike removal was done by automated detection and replacement of spike waveforms with white noise generated as a random vector of numbers with a mean identical to the 10-s epoch average voltage and a range between one-half a SD (of the entire voltage trace from which spikes were removed) below and above the mean. In roughly one-third of the recording sites, across BG structures and recording days, a high-power, narrow band peak (mostly 0.34 Hz at its base) appeared at ∼17.5 Hz in the FP spectrum. Due to the extremely narrow frequency band characterizing it and its resemblance to higher frequency artifactual peaks (i.e., at 50 Hz), these recordings were filtered with a narrow band Butterworth notch filter prior to further analysis. FP and spiking signals, postfiltration, were converted to z-scores.
Power spectra for both FP and spiking signals were obtained using 16,384 (FP) or 131,072 (spiking) FFT point Hamming windows, yielding a frequency resolution of 0.084 Hz. The relative power at each frequency was then calculated by division of the entire spectrum by the overall power in the range of 0.1–40 Hz.
For each FP/spiking recording site, beta power was calculated in relation to the power in two adjacent frequency ranges. First, a five-point Gaussian kernel was used to smooth the average power spectrum. Next, a beta peak was detected in the 11- to 15-Hz range, and the average power 3 Hz around it was calculated. The choice of the 11- to 15-Hz range was motivated by the observation that in the vast majority of cells, pathological beta power was concentrated within these bounds. Further, it guaranteed that the 3-Hz segment around the peak would be within the 10- to 17-Hz range. The power in the left flanking frequency range was the average power 2 Hz around a similarly detected trough in the 6- to 10-Hz range, and the power in the right flanking frequency range was set to be the average power at 20–25 Hz. Beta power was calculated as the average power around the beta peak minus the average power across both flanks (SI Appendix, Fig. S6). A spiking/FP location was considered as exhibiting beta activity if its beta power exceeded a predefined threshold (different for spiking and FP, constant across structures, and between normal and MPTP data).
In addition, for each recorded neuron, we calculated the firing rate power spectrum: for each 10-s epoch, a firing rate vector was calculated based on the detected action potentials from that neuron, in 5-ms nonoverlapping bins. The per-epoch firing rate power spectrum was obtained from the firing rate signal.
Beta to Slow Oscillation Correlation Analysis.
For the analysis of beta and slow oscillations during NREM sleep in the FP/spiking and EEG signals, all signals were filtered and normalized as described above. The only exception was for the power spectrum normalization of the EEG power, in which slow oscillations were detected. Instead of being normalized by division by total power, it was divided by the total power excluding the beta range. This was done to make sure that no artifactual negative correlations were detected due to the increase in EEG beta power that was concurrent with the increase in FP/spiking beta power. The correlation between BG and cortical activity was only calculated for BG sites (FP/spiking) that exhibited beta oscillations. For each site, the correlation coefficient between EEG beta/SO and BG beta was calculated over all 10-s epochs in which FP/spiking activity was recorded from that site. The results were similar across various other normalization methods. The analyses reported in Figs. 3–6 returned similar results when performed separately for each of the different EEG derivations examined. Therefore, data from the different EEG derivations are averaged in these figures.
BG Beta Power and Polysomnographic Events.
To arrive at a measure of the average FP NREM sleep beta power across BG recording sites, the power in the beta range was first calculated for each NREM epoch in each FP recording site, as explained above. Next, the per-BG structure average beta power was calculated across all simultaneously recorded FP traces in the GPe, GPi, or STN. The night was divided into overlapping periods lasting 25% of the total night length (100 s separating the onsets of two consecutive periods). The average NREM sleep BG beta power, as well as the percentage of NREM sleep and wakefulness, and the hourly awakening frequency, were calculated for each period. We chose to parcel the night into relatively long periods to increase the accuracy of the polysomnographic measure estimation. The use of overlapping periods allowed us to detect finer modulations of beta activity throughout the night. The analysis of longer and shorter period lengths resulted in similar results. Finally, all of the recording periods obtained in a single night were assigned to six groups based on the NREM sleep beta power they exhibited, relative to the total range of beta power that night (the first group had the lowest average beta power and the sixth group had the highest). For each group, the average of the polysomnographic measures was calculated. Data from all nights and structures were pooled.
The analysis of spiking activity was identical except that for the FP data; beta power was first averaged across all simultaneously recorded sites and then grouped. For the spiking data, beta power was not averaged across recording sites, rather the grouping was carried out for each recording site individually. The rationale behind this separation was that the FP is a highly correlated signal across the BG (as also shown in Fig. 4), whereas different cells could exhibit different levels of basal spiking beta power. A relatively higher spiking beta power for one recording location could be similar to a lower spiking beta power for a different location, and averaging these different levels together could potentially cancel out trends that would have been meaningful at the single spiking trace level. Thus, for spiking activity, the data were first assigned to beta power groups at the individual spiking trace level and only then averaged over all simultaneously recorded sites.
Statistics.
Analyses were conducted identically on the different neuronal assemblies. The data from the two monkeys were pooled since no significant differences were detected between them. A threshold of 0.05 was used to establish statistical significance. Nonparametric statistical tests were used throughout this manuscript (the Mann–Whitney U test for two nonpaired groups, the Wilcoxon signed rank test for two paired groups, and the Kruskal–Wallis H test for more than two groups). For multiple comparisons, the FDR correction (Benjamini–Hochberg procedure) was utilized. Analyses were performed using Matlab 2013a (Mathworks). Data, code, and other materials are available on Zenodo at https://zenodo.org/record/3928145#.Xv28tZpvaUk.
Supplementary Material
Acknowledgments
We thank Yaron Dagan and Tamar Ravins-Yaish for assistance with animal care; Atira Bick and Adi Payis for assistance with the MRI scan; and Hila Gabbay, Sharon Freeman, Uri Werner-Reiss, and Esther Singer for general assistance. We thank Anatoly Shapochnikov for help in preparing the experimental setup. This work was supported by grants from the European Research Council and the Rosetrees trust (to H.B.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data, code, and other materials are available on Zenodo at https://zenodo.org/record/3928145#.Xv28tZpvaUk.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001560117/-/DCSupplemental.
References
- 1.De Cock V. C., Vidailhet M., Arnulf I., Sleep disturbances in patients with parkinsonism. Nat. Clin. Pract. Neurol. 4, 254–266 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Factor S. A., McAlarney T., Sanchez-Ramos J. R., Weiner W. J., Sleep disorders and sleep effect in Parkinson’s disease. Mov. Disord. 5, 280–285 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Videnovic A., Högl B., “Disorders of sleep and circadian rhythms” in Parkinson’s Disease, Videnovic A., Högl B., Eds. (Springer Vienna, 2015), pp. 79–91. [Google Scholar]
- 4.Chahine L. M., Amara A. W., Videnovic A., A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med. Rev. 35, 33–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kryger M., Roth T., Dement W. C., Principles and Practice of Sleep Medicine, (Elsevier, 2017). [Google Scholar]
- 6.Gjerstad M. D., Wentzel-Larsen T., Aarsland D., Larsen J. P., Insomnia in Parkinson’s disease: Frequency and progression over time. J. Neurol. Neurosurg. Psychiatry 78, 476–479 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stavitsky K., Neargarder S., Bogdanova Y., McNamara P., Cronin-Golomb A., The impact of sleep quality on cognitive functioning in Parkinson’s disease. J. Int. Neuropsychol. Soc. 18, 108–117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K. et al., Correlation between depressive symptoms and nocturnal disturbances in Japanese patients with Parkinson’s disease. Parkinsonism Relat. Disord. 15, 15–19 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Rutten S. et al., The bidirectional longitudinal relationship between insomnia, depression and anxiety in patients with early-stage, medication-naïve Parkinson’s disease. Parkinsonism Relat. Disord. 39, 31–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsen K. H., Larsen J. P., Tandberg E., Maeland J. G., Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 66, 431–435 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H. et al., Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kalaitzakis M. E., Gentleman S. M., Pearce R. K. B., Disturbed sleep in Parkinson’s disease: Anatomical and pathological correlates. Neuropathol. Appl. Neurobiol. 39, 644–653 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Happe S. et al., Sleep disorders and depression in patients with Parkinson’s disease. Acta Neurol. Scand. 104, 275–280 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Monti J. M., Pandi-Perumal S. R., Chokroverty S., “Alteration of Biological Rhythms in Diseases of the Central Dopaminergic System: Focus on Parkinson’s Disease; Parkinson’s Disease and Sleep/Wake Disturbances” in Dopamine and Sleep - Molecular, Functional, and Clinical Aspects, Monti J. M., Pandi-Perumal S. R., Chokroverty S., Nature S., Eds. (Springer, 2016), pp. 91–146. [Google Scholar]
- 15.Engel A. K., Fries P., Beta-band oscillations–Signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R. et al., Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J. Neurosci. 39, 8231–8238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond C., Bergman H., Brown P., Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 30, 357–364 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Deffains M., Bergman H., Bergman H., Parkinsonism-related β oscillations in the primate basal ganglia networks–Recent advances and clinical implications. Parkinsonism Relat. Disord. 59, 2–8 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Brown P., Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. 18, 357–363 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Moran A., Stein E., Tischler H., Bar-Gad I., Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol. Dis. 45, 583–590 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Beudel M. et al., Oscillatory beta power correlates with akinesia-rigidity in the parkinsonian subthalamic nucleus. Mov. Disord. 32, 174–175 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Urrestarazu E. et al., Beta activity in the subthalamic nucleus during sleep in patients with Parkinson’s disease. Mov. Disord. 24, 254–260 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Thompson J. A. et al., Sleep patterns in Parkinson’s disease: Direct recordings from the subthalamic nucleus. J. Neurol. Neurosurg. Psychiatry 89, 95–104 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Christensen E., Abosch A., Thompson J. A., Zylberberg J., Inferring sleep stage from local field potentials recorded in the subthalamic nucleus of Parkinson’s patients. J. Sleep Res. 28, e12806 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Norlinah M. I. et al., Sleep disturbances in Malaysian patients with Parkinson’s disease using polysomnography and PDSS. Parkinsonism Relat. Disord. 15, 670–674 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Kim R., Jeon B., Nonmotor effects of conventional and transdermal dopaminergic therapies in Parkinson’s disease. Int. Rev. Neurobiol. 134, 989–1018 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Baumann-Vogel H. et al., The impact of subthalamic deep brain stimulation on sleep-wake behavior: A prospective electrophysiological study in 50 Parkinson patients. Sleep (Basel) 40, zsx033 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Zuzuárregui J. R. P., Ostrem J. L., The impact of deep brain stimulation on sleep in Parkinson’s disease: An update. J. Parkinsons Dis. 10, 393–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox S. H., Brotchie J. M., The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog. Brain Res. 184, 133–157 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Deffains M. et al., Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. eLife 5, e16443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barraud Q. et al., Sleep disorders in Parkinson’s disease: The contribution of the MPTP non-human primate model. Exp. Neurol. 219, 574–582 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Belaid H., Adrien J., Karachi C., Hirsch E. C., François C., Effect of melatonin on sleep disorders in a monkey model of Parkinson’s disease. Sleep Med. 16, 1245–1251 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Pungor K., Papp M., Kékesi K., Juhász G., A novel effect of MPTP: The selective suppression of paradoxical sleep in cats. Brain Res. 525, 310–314 (1990). [DOI] [PubMed] [Google Scholar]
- 34.Mizrahi-Kliger A. D., Kaplan A., Israel Z., Bergman H., Desynchronization of slow oscillations in the basal ganglia during natural sleep. Proc. Natl. Acad. Sci. U.S.A. 115, E4274–E4283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinkhauser G. et al., The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140, 1053–1067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nini A., Feingold A., Slovin H., Bergman H., Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J. Neurophysiol. 74, 1800–1805 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Iber C., Ancoli-Israel S., Chesson A. L., Quan S. F., The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, (American Association of Sleep Medicine, 2007). [Google Scholar]
- 38.Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G., The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duval C. et al., The effect of Trager therapy on the level of evoked stretch responses in patients with Parkinson’s disease and rigidity. J. Manipulative Physiol. Ther. 25, 455–464 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Kühn A. A. et al., Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp. Neurol. 215, 380–387 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Ballanger B. et al., Imaging dopamine and serotonin systems on MPTP monkeys: A longitudinal PET investigation of compensatory mechanisms. J. Neurosci. 36, 1577–1589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belaid H. et al., Sleep disorders in Parkinsonian macaques: Effects of L-dopa treatment and pedunculopontine nucleus lesion. J. Neurosci. 34, 9124–9133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pifl C., Schingnitz G., Hornykiewicz O., Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 44, 591–605 (1991). [DOI] [PubMed] [Google Scholar]
- 44.Fifel K. et al., Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. PLoS One 9, e86240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensaid M. et al., Sparing of orexin-A and orexin-B neurons in the hypothalamus and of orexin fibers in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated macaques. Eur. J. Neurosci. 41, 129–136 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Andrillon T. et al., Sleep spindles in humans: Insights from intracranial EEG and unit recordings. J. Neurosci. 31, 17821–17834 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi S. et al., Spatiotemporal organization and cross-frequency coupling of sleep spindles in primate cerebral cortex. Sleep (Basel) 39, 1719–1735 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharott A. et al., Spatio-temporal dynamics of cortical drive to human subthalamic nucleus neurons in Parkinson’s disease. Neurobiol. Dis. 112, 49–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevan M. D., Magill P. J., Terman D., Bolam J. P., Wilson C. J., Move to the rhythm: Oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 25, 525–531 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Turner R. S., Desmurget M., Basal ganglia contributions to motor control: A vigorous tutor. Curr. Opin. Neurobiol. 20, 704–716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn E. J. et al., Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov. Disord. 30, 1750–1758 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Liu D. et al., A common hub for sleep and motor control in the substantia nigra. Science 367, 440–445 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Holt A. B. et al., Phase-dependent suppression of beta oscillations in Parkinson’s disease patients. J. Neurosci. 39, 1119–1134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peles O., Werner-Reiss U., Bergman H., Israel Z., Vaadia E., Phase-specific microstimulation differentially modulates beta oscillations and affects behavior. Cell Rep. 30, 2555–2566.e3 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.