Significance
L-type Ca2+ channel (Cav1.2) blockers (LCCBs) represent a large family of drugs widely used in the clinic for over 70 y to treat hypertension, angina, and cardiac arrhythmias. Using genetically modified cells, animal models, and human studies, we demonstrate that all the three major classes of LCCBs activate STIM proteins by acting on a 10-amino acid N-terminal region located in the endoplasmic reticulum lumen. The activation of STIM triggers store-operated Ca2+ entry and promotes vascular remodeling. These results provide unique mechanistic insights into how widely used drugs activate a Ca2+ signaling pathway and suggest that the use of LCCBs in patients with chronic hypertension, where levels of STIM proteins and vascular remodeling are already enhanced, should be avoided.
Keywords: Cav1.2, STIM1, calcium signaling, vascular remodeling, hypertension
Abstract
Voltage-gated L-type Ca2+ channel (Cav1.2) blockers (LCCBs) are major drugs for treating hypertension, the preeminent risk factor for heart failure. Vascular smooth muscle cell (VSMC) remodeling is a pathological hallmark of chronic hypertension. VSMC remodeling is characterized by molecular rewiring of the cellular Ca2+ signaling machinery, including down-regulation of Cav1.2 channels and up-regulation of the endoplasmic reticulum (ER) stromal-interacting molecule (STIM) Ca2+ sensor proteins and the plasma membrane ORAI Ca2+ channels. STIM/ORAI proteins mediate store-operated Ca2+ entry (SOCE) and drive fibro-proliferative gene programs during cardiovascular remodeling. SOCE is activated by agonists that induce depletion of ER Ca2+, causing STIM to activate ORAI. Here, we show that the three major classes of LCCBs activate STIM/ORAI-mediated Ca2+ entry in VSMCs. LCCBs act on the STIM N terminus to cause STIM relocalization to junctions and subsequent ORAI activation in a Cav1.2-independent and store depletion-independent manner. LCCB-induced promotion of VSMC remodeling requires STIM1, which is up-regulated in VSMCs from hypertensive rats. Epidemiology showed that LCCBs are more associated with heart failure than other antihypertensive drugs in patients. Our findings unravel a mechanism of LCCBs action on Ca2+ signaling and demonstrate that LCCBs promote vascular remodeling through STIM-mediated activation of ORAI. Our data indicate caution against the use of LCCBs in elderly patients or patients with advanced hypertension and/or onset of cardiovascular remodeling, where levels of STIM and ORAI are elevated.
Globally, one in four adults is diagnosed with systemic arterial hypertension, which remains the highest risk factor for cardiovascular disease and mortality (1). Clinical complications include retinopathy, chronic kidney disease, ischemic stroke, peripheral artery remodeling, ischemic heart disease, and heart failure. Clinical treatment of hypertension uses a spectrum of antihypertensive drugs, including α-blockers, β-blockers, angiotensin receptor blockers (ARBs), diuretics, angiotensin-converting enzyme (ACE) inhibitors, and Cav1.2 voltage-gated L-type Ca2+ channel blockers (LCCBs). However, treated patients exhibit multiple complications, suggesting that the molecular mechanisms and cellular side effects of antihypertensive drugs are still elusive (2).
In healthy vessels, medial vascular smooth muscle cells (VSMCs) are quiescent and rarely proliferate and migrate. Their main physiological role is to contract and relax to regulate vascular tone (3). In addition to enhanced arterial tone, a significant pathological hallmark of hypertension is the structural remodeling of arteries, which contributes to increased peripheral resistance. Vascular remodeling is the thickening of the medial layer of arteries from enhanced VSMC proliferation and migration, ultimately decreasing the luminal area of the vessel and enhancing arterial resistance (4). Emerging evidence reveals that Ca2+ signaling is a significant contributor to VSMC remodeling (5–8). VSMC remodeling is characterized by reprogramming of the Ca2+ signaling machinery (6–8). The Ca2+ channels essential for excitation–contraction coupling, ryanodine receptors (RyRs), and Cav1.2 L-type voltage-gated Ca2+ channels are down-regulated (6, 9–12). Reciprocally, receptor-activated Ca2+ channels such as stromal-interacting molecule (STIM)/ORAI channels, which mediate store-operated Ca2+ entry (SOCE), are up-regulated (6, 13–17).
Mammals express two STIM homologs, STIM1 and STIM2, which span the endoplasmic reticulum (ER) membrane (18, 19). In VSMCs, stimulation by agonists to phospholipase C (PLC)-coupled receptors triggers the formation of inositol-1,4,5-trisphosphate (IP3) (20). Diffusible IP3 induces ER Ca2+ release through IP3 receptors, which depletes ER Ca2+ and causes Ca2+ dissociation from the low-affinity luminal EF-hand domains of STIM proteins (21–24). This causes STIM to undergo a conformational switch, migrate toward ER–plasma membrane (PM) junctions, and expose their C-terminal STIM-ORAI activating region (SOAR) (25, 26). SOAR is able to physically trap and activate the Ca2+-selective family of PM ORAI channels. The three mammalian ORAI homologs, ORAI1, ORAI2, and ORAI3, mediate SOCE from the extracellular milieu into the cytosol (22). STIM/ORAI-mediated SOCE drives transcriptional gene programs, including nuclear factor for activated T cells (NFAT) transcription factors, that promote vascular remodeling in vitro and in animal models of restenosis and hypertension (7, 8, 14, 16, 22, 27–31).
The three major classes of LCCB drugs used to treat hypertension are as follows: dihydropyridines (e.g., amlodipine), phenylakylamines (e.g., verapamil), and benzothiazepines (e.g., diltiazem); all block Cav1.2 channels to cause vasorelaxation (32). However, during chronic hypertension, VSMCs dedifferentiate from a contractile quiescent phenotype into a proliferative and migratory phenotype (termed synthetic). Synthetic VSMCs have reduced contractile responses due to the decrease in expression of contractile proteins, including the expression of Cav1.2 channels. In contrast, synthetic VSMCs up-regulate proproliferative and promigratory signaling pathways, including STIM/ORAI channels activated by receptors to growth and vasoactive agonists. Here, we reveal that all of the three major classes of LCCBs, well-described as specific Cav1.2 channel blockers (33), unexpectedly also target the N terminus of STIM proteins to induce their relocalization to junctions and subsequent activation of ORAI channels. We show that this LCCB-mediated activation of STIM/ORAI-dependent Ca2+ entry occurs independently of store depletion and Cav1.2. We further reveal that clinically relevant concentrations of LCCBs found in the plasma of hypertensive patients treated with these drugs (34) stimulate VSMC proliferation and migration in a STIM1-dependent manner. We further show that STIM1 is up-regulated and SOCE is enhanced in VSMC isolated from chronically hypertensive rats but not in VSMC from normotensive rats. Intriguingly, epidemiological evidence suggests that LCCBs are associated with heart failure, a clinical consequence of prolonged vascular remodeling, more than other antihypertensive drugs. Our results reveal that LCCBs promote vascular remodeling through STIM-mediated activation of ORAI and indicate caution in the use of LCCBs in patients with advanced hypertension or vascular remodeling.
Results
LCCBs Increase Cytosolic Ca2+ and Induce VSMC Migration and Proliferation.
When contractile VSMCs are isolated from healthy vessels and cultured in vitro, they dedifferentiate into a “synthetic” phenotype, which recapitulates many of the characteristics of dedifferentiated VSMCs found in remodeled vessels during restenosis, atherosclerosis, and chronic hypertension (7, 15, 35). These synthetic VSMCs represent an excellent in vitro model for remodeled VSMCs (36). We isolated VSMC from aortas of normotensive rats using enzymatic dispersion as described earlier (13). To ensure VSMC purity, we used immunofluorescence of the smooth muscle marker α-smooth muscle actin (α-SMA) (Fig. 1A). We recently showed that 20 μM of the dihydropyridine LCCB amlodipine activated a Ca2+ entry route in glioblastoma cells that bears the pharmacological features of SOCE (37). We and others have noted that LCCBs interfere with the signal of the Ca2+ dye Fura2 and strongly blunt its fluorescence, thus underestimating the magnitude of LCCBs-activated Ca2+ signals (37, 38). Therefore, we used Fura2 (being ratiometric) with 10 mM extracellular Ca2+ to enhance the driving force and amplify Ca2+ signals. We show that 20 μM amlodipine stimulates a robust and sustained cytosolic Ca2+ signal in synthetic VSMCs (Fig. 1 B and C). Similar results were obtained with 20 μM of the benzothiazepine LCCB, diltiazem (Fig. 1 D and E). To ensure that low concentrations of amlodipine (0.5 µM), which are reminiscent of physiological concentrations found in the plasma of hypertensive patients treated by LCCBs (34), can activate a Ca2+ signal in VSMCs, we performed a dose–response curve using the nonratiometric Ca2+ dye Fluo4 and show that 0.5 µM amlodipine activated a small but statistically significant Ca2+ entry in VSMCs (Fig. 1 F and G). Unless stated otherwise, in subsequent experiments, cells were loaded with Fura2 and stimulated with 20 μM of LCCBs in 2 mM extracellular Ca2+.
Fig. 1.
LCCBs increase intracellular Ca2+ and induce VSMC remodeling. (A) Synthetic VSMCs immune-stained with α-SMA antibody and Hoechst. (Scale bar: 100 μm.) (B) Cytosolic Ca2+ measurements in VSMCs (Fura2) stimulated with 20 μM amlodipine or vehicle. (C) Quantification of maximal Ca2+ entry from B. (D) Cytosolic Ca2+ in VSMCs stimulated with 20 μM diltiazem or vehicle. (E) Quantification of maximal Ca2+ entry from D. (F) Cytosolic Ca2+ in VSMCs (Fluo-4) stimulated with 0.5 to 20 μM amlodipine. (G) Quantification of maximal Ca2+ entry from F. (H and I) Quantification of migration in VSMC stimulated with vehicle, amlodipine (0.5 μM), PDGF (0.5 ng/mL), amlodipine (0.5 μM) + PDGF (0.5 ng/mL), or PDGF (10 ng/mL) at 12 to 24 h (H and I). (J) Bright field images of VSMC migration. (Scale bar: 500 μm.) (K) VSMC proliferation over 72 h with vehicle, amlodipine (0.5 μM), PDGF (0.5 ng/mL), amlodipine (0.5 μM) + PDGF (0.5 ng/mL), or PDGF (10 ng/mL). Ca2+ imaging: ****P < 0.0001. VSMC migration and proliferation: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 when compared to vehicle. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 compared with 0.5 ng/mL PDGF. Unpaired Student’s t test for two comparisons and ANOVA with Dunnett’s test for multiple comparisons.
Increased cytosolic Ca2+ in synthetic VSMCs has been shown to induce VSMC proliferation and migration (6). Since the expression of Ca2+ channels crucial for contraction (Cav1.2 and RyR) is decreased while that of proproliferative promigratory Ca2+ channels (STIM/ORAI) is augmented in “synthetic” VSMCs (6, 7, 13, 27), we determined whether concentrations of LCCBs that are reminiscent of those found in the plasma of hypertensive patients treated by LCCBs (0.5 µM) (34) can increase VSMC proliferation and migration. The potent mitogen, platelet-derived growth factor (PDGF), which is secreted in pathological vascular remodeling and induces smooth muscle cells to proliferate and migrate (39), was used as a control throughout. VSMC migration was measured using the gap closure assay. VSMCs are treated in low-serum media (0.4% fetal bovine serum [FBS]) with either 1) vehicle, 2) submaximal concentration (0.5 μM) of amlodipine, 3) submaximal concentration (0.5 ng/mL) of PDGF, 4) 0.5 μM amlodipine + 0.5 ng/mL PDGF, or 5) maximal concentration (10 ng/mL) of PDGF. To rule out contributions from VSMC proliferation, 0.4% FBS culture media was supplemented with 10 μg/mL mitomycin C. VSMCs migrated minimally in vehicle and only slightly more when either 0.5 μM amlodipine or 0.5 ng/mL PDGF were added. However, VSMCs migrated significantly more when treated with 10 ng/mL PDGF (Fig. 1 H–J). Interestingly, VSMCs treated with the combination of 0.5 μM amlodipine + 0.5 ng/mL PDGF showed a synergetic effect and migrated significantly more than 0.5 μM amlodipine or 0.5 ng/mL PDGF alone (Fig. 1 H–J). This increased migration was similar to that induced by maximal concentrations of PDGF (10 ng/mL). Similarly, VSMC proliferation was measured over 72 h with the dye CyQUANT. The combination of 0.5 μM amlodipine + 0.5 ng/mL PDGF caused significantly higher proliferation than 0.5 μM amlodipine or 0.5 ng/mL PDGF alone (Fig. 1K). These data suggest that low concentrations of amlodipine, reminiscent of circulating concentrations in hypertensive patients, synergize with submaximal concentrations of PDGF to further enhance VSMC proliferation and migration and promote vascular remodeling.
LCCBs Activate a Pathway Bearing the Biophysical Signature of SOCE.
To determine the molecular identity of the Ca2+ signal activated by LCCBs, we used HEK293 cells in which we can relatively easily perform gene knockout; 20 μM amlodipine induced a robust cytosolic Ca2+ signal in HEK293 cells, exhibiting similar kinetics and amplitude to the Ca2+ signal induced by amlodipine in VSMCs (Fig. 2 A and B). To rule out nonspecific effects on membrane potential, we used whole-cell patch clamp to measure the current elicited by LCCBs. When Ca2+ in the pipette was buffered to 150 nM, 20 μM amlodipine in the bath elicited a small (∼0.5 pA/pF at −100 mV) Ca2+ current in HEK293 cells (Fig. 2 C, D, and G), reminiscent of the current mediated by SOCE, the Ca2+ release-activated Ca2+ current (ICRAC) that is encoded by STIM/ORAI and triggered by ER Ca2+ store depletion (40). When we coexpressed STIM1 and ORAI1 in HEK293 cells, 20 μM amlodipine activated a large (∼40 pA/pF at −100 mV) inwardly rectifying Ca2+-selective current with positive reversal potential (∼ +60 mV) (Fig. 2 E, F, and H).
Fig. 2.
LCCBs activate ICRAC which require STIM and ORAI. (A) Cytosolic Ca2+ measurements in HEK293 cells stimulated with amlodipine or vehicle. (B) Quantification of maximal Ca2+ entry from A. (C) Whole-cell patch-clamp recording of native ICRAC in HEK293 cells stimulated first with vehicle (dimethyl sulfoxide [DMSO]) and then 20 μM amlodipine. (D) Ca2+ current/voltage (I/V) curves from C, taken where indicated by “1” and “2”. (E) Whole-cell patch-clamp recording from HEK293 cells expressing eYFP-STIM1 and mCherry-ORAI1 stimulated first with DMSO and then amlodipine. (F) Ca2+ I/V curves of E, taken where indicated by “3” and “4”. (G) Quantification of peak current density activated by amlodipine. (H) Quantification of peak current density activated by amlodipine in HEK293 cells coexpressing eYFP-STIM1 and mCherry-ORAI1. (I) Cytosolic Ca2+ measurement in ORAI1/2/3-TKO cells, STIM1/2-DKO cells, and HEK293 cells stimulated with amlodipine. (J) Quantification of maximal Ca2+ entry from I. (K) STIM1/2-DKO cells expressing 1 μg of either an empty plasmid, or CFP-ORAI1, or coexpressing STIM1-eYFP (2 μg) and CFP-ORAI1 (1 μg) and stimulated with amlodipine. (L) Quantification of maximal Ca2+ entry from K along with that of similar experiments performed on STIM1/2-DKO cells transfected with CFP-ORAI2 (1 μg), or CFP-ORAI3 (1 μg) either alone or cotransfected with STIM1-eYFP (2 μg) and stimulated with amlodipine. Also quantified experiments using cells coexpressing STIM2-eYFP (instead of STIM1-eYFP) and CFP-ORAI1 and stimulated with amlodipine (traces in SI Appendix, Fig. S2). (M–R) Cytosolic Ca2+ traces and quantifications of maximal Ca2+ entry from STIM1/2-DKO cells with STIM/ORAI expression combinations in K and L, except now cells are stimulated with other LCCBs. Cells are stimulated with either nifedipine (M and N), verapamil (O and P), or diltiazem (Q and R). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Unpaired Student’s t test for two comparisons and ANOVA with Dunnett’s test for multiple comparisons.
STIM and ORAI Are Required for LCCB-Activated Ca2+ Signaling.
Mammals express two STIM and three ORAI proteins, with STIM1 and ORAI1 contributing the dominant proportion of SOCE in most cells (21, 24). To determine if STIM and ORAI mediate the increase in cytosolic Ca2+ in response to amlodipine, we generated STIM1 and STIM2 double knockout (STIM1/2-DKO) and ORAI1/2/3 triple knockout (ORAI1/2/3-TKO) HEK293 cells using CRISPR/Cas9 technology and confirmed STIM1/2 and ORAI1 knockout by Western blots (41–43) (SI Appendix, Fig. S1 A–E). Since there are currently no reliable ORAI2/3 antibodies, we utilized two guide RNAs (gRNAs) flanking the target gene to completely excise the whole gene. Hence, mRNA measurements are a reliable means to document knockout. ORAI1/2/3-TKO cells have no detectable ORAI2 and ORAI3 mRNA compared to parental HEK293 cells (SI Appendix, Fig. S1 F and G). Genomic sequencing further confirmed knockout. Thapsigargin (2 μM), a pharmacological inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) added to cells in nominally Ca2+-free solution, causes passive ER Ca2+ depletion. When 2 mM Ca2+ was restored to the bath, robust SOCE was measurable only in wild-type HEK293 cells (SI Appendix, Fig. S1 H and I), providing functional evidence that STIM/ORAI proteins have been knocked out.
The significant increase in cytosolic Ca2+ with 20 μM amlodipine was absent in ORAI1/2/3-TKO and STIM1/2-DKO cells (Fig. 2 I and J). To provide evidence that these results are not due to off-target effects from CRISPR/Cas9, we overexpressed ORAI1 in the STIM1/2-DKO cells either with or without rescuing STIM1 expression. As a control, STIM1/2-DKO cells were transfected with an empty GFPN-1 construct (Fig. 2 K, black trace and L, black data), which did not support Ca2+ entry in response to 20 μM amlodipine (Fig. 2 K and L). Similarly, 20 μM amlodipine did not stimulate Ca2+ entry in STIM1/2-DKO cells expressing ORAI1 alone (Fig. 2 K, light blue trace and L, light blue data). However, expression of both STIM1 and ORAI1 caused 20 μM amlodipine to stimulate Ca2+ entry (Fig. 2 K, dark blue trace and L, dark blue data). Similar results were obtained when either ORAI2 or ORAI3 was coexpressed with STIM1 in STIM1/2-DKO cells (traces in SI Appendix, Fig. S2 A and E; statistics in Fig. 2L). Expression of ORAI2 or ORAI3 alone in STIM1/2-DKO cells did not rescue Ca2+ entry in response to 20 μM amlodipine (traces in SI Appendix, Fig. S2 A and E; statistics in Fig. 2L). Further, coexpression of STIM2 (instead of STIM1) with ORAI1 in STIM1/2-DKO cells was sufficient to support amlodipine-mediated Ca2+ entry (traces in SI Appendix, Fig. S2I; statistics in Fig. 2L) although this Ca2+ entry was less robust than that supported by STIM1, consistent with the known weaker activity of STIM2 relative to STIM1. These results suggest that the combination of one STIM and one ORAI isoform is necessary for amlodipine to activate a cytosolic Ca2+ signal.
We next sought to determine whether the activation of STIM/ORAI-dependent Ca2+ signal can be triggered by other LCCBs belonging to the two other major families, namely phenylalkylamines and benzothiazepines. We showed that 20 μM of another dihydropyridine, nifedipine, as well as the benzothiazepine, diltiazem, and the phenylalkylamine, verapamil, were equally capable of stimulating an increase in cytosolic Ca2+ when either ORAI1, ORAI2, or ORAI3 was coexpressed with either STIM1 or STIM2 in STIM1/2-DKO cells (Fig. 2 M–R and SI Appendix, Fig. S2 B–L). Expression of the empty GFPN-1 vector or expression of either ORAI1, ORAI2, or ORAI3 alone (without STIM) did not support LCCB-mediated increase in cytosolic Ca2+. The T-type voltage-gated Ca2+ channel blocker Mibefradil also blocks L-type Ca2+ channels (44). Mibefradil stimulated a Ca2+ signal in STIM1/2-DKO cells coexpressing STIM1 and ORAI1 (SI Appendix, Fig. S3 A and B). Furthermore, stimulation with 20 μM of the (+) enantiomer of Bay K 8644, which is a blocker of Cav1.2 (45), stimulated an increase in cytosolic Ca2+ in STIM1/2-DKO cells coexpressing STIM1 and ORAI1 (SI Appendix, Fig. S3 C and D) while the (−) enantiomer of Bay K 8644, which activates Cav1.2, was less efficient (SI Appendix, Fig. S3 C and D).
We also tested drugs inhibiting other voltage-gated Ca2+ channels. We tested whether ω-conotoxin, a neurotoxic peptide that blocks N-type voltage-gated Ca2+ channels (46), can activate a STIM/ORAI-dependent cytosolic Ca2+ signal; 20 μM ω-conotoxin did not activate a Ca2+ signal in STIM1/2-DKO cells coexpressing STIM1 and ORAI1 (SI Appendix, Fig. S3 E and F). We also explored the possibility that voltage-gated Na+ channel blockers, such as local anesthetics, tetracaine, and benzocaine (47), can activate STIM/ORAI-dependent cytosolic Ca2+ signal. Stimulation with 20 to 200 μM tetracaine or 20 μM to 2 mM benzocaine did not activate Ca2+ entry in STIM1/2-DKO cells coexpressing STIM1 and ORAI1 (SI Appendix, Fig. S3 G–J). These data suggest that the broad chemical class of LCCBs, which have diverse structures and binding mechanisms to Cav1.2, increase cytosolic Ca2+ by activating STIM and ORAI proteins.
LCCBs Cause STIM and ORAI Puncta and STIM–ORAI Interaction.
The activation of SOCE requires STIM proteins to migrate to the ER–PM junctions where they form puncta. At rest, the SOAR domain of STIM which activates ORAI (25, 26) is engaged in an intramolecular clamp with the adjacent coiled coil-1 (CC1) domain. On store depletion, STIM gains an extended conformation, opening the CC1-SOAR clamp and exposing SOAR, which binds the C terminus of PM ORAI channels to cause their activation (48). We used STIM1/2-DKO cells coexpressing C-terminal fluorescently tagged STIM1 (STIM1-YFP) and the N-terminal fluorescently tagged ORAI1 (CFP-ORAI1). Using a confocal microscope, we show that, at rest, STIM1 proteins have the typical reticular organization while ORAI1 proteins are diffusely localized in the PM with little colocalization (Fig. 3 A–C). Upon stimulation with either amlodipine or diltiazem, both STIM1 and ORAI1 formed puncta and showed enhanced colocalization at 8 min (Fig. 3 A–C). To document STIM1–ORAI1 close interactions, we measured Förster resonance energy transfer (FRET) between STIM1-YFP and CFP-ORAI1. Diltiazem induced an increase in STIM1-ORAI1 FRET (Fig. 3 D–F). The maximal FRET signal induced by diltiazem was of similar magnitude to that induced by thapsigargin (Fig. 3 G–I). Because amlodipine emits some fluorescence within the range of emission of CFP (49), this precluded reliable FRET measurements with amlodipine.
Fig. 3.
LCCBs cause STIM1/ORAI1 interaction and puncta formation. (A) Confocal images of localization of CFP-ORAI1 (1 μg) and STIM1-eYFP (2 μg) and the merged distribution in STIM1/2-DKO cells. Images of cells before (0) and after treatment (8 min) with vehicle. (B and C) Cells before (0) and after (8 min) stimulation with either amlodipine or diltiazem. (Scale bar: 10 μm.) (D) Sensitized emission Förster resonance energy transfer (seFRET) between STIM1-eYFP (2 μg) and CFP-ORAI1 (1 μg) expressed in STIM1/2-DKO cells and stimulated with diltiazem or vehicle. (E) Quantification of maximum seFRET. (F) YFP/CFP ratios from E. (G) seFRET between STIM1-eYFP (2 μg) and CFP-ORAI1 (1 μg) expressed in STIM1/2-DKO cells and stimulated with either thapsigargin or vehicle. (H) Quantification of maximum seFRET from G. (I) YFP/CFP ratios from G. ****P < 0.0001; ns, not significant (unpaired Student’s t test).
LCCBs Activate STIM without Depleting ER Ca2+ Stores.
The movement of STIM proteins, their interaction with ORAI, and activation of SOCE are triggered physiologically through depletion of ER Ca2+ stores (21, 24). Therefore, we reasoned that LCCBs-activated Ca2+ entry through STIM-ORAI was likely the result of LCCBs causing Ca2+ store depletion. We first used Fura2 to perform cytosolic Ca2+ measurements in HEK293 cells stimulated with 20 μM amlodipine in a nominally Ca2+-free extracellular solution. Under these conditions, amlodipine failed to cause an increase in cytosolic Ca2+ (Fig. 4 A and B). However, when extracellular Ca2+ was restored to 2 mM, now amlodipine caused an increase in cytosolic Ca2+ (Fig. 4 A and B), suggesting that the action of amlodipine on STIM-ORAI is independent of store depletion. To provide stronger evidence that the ER Ca2+ content is not affected by amlodipine, we performed direct ER Ca2+ measurements in HEK293 cells using the ER-targeted Ca2+ sensor, GCaMP6-150 (50). We first documented that stimulation of cells with either the agonist carbachol (Cch) or the reversible SERCA inhibitor cyclopiazonic acid (CPA) caused a decrease in ER Ca2+ (Fig. 4 F–I). When CPA was washed out, we could readily observe the refilling of ER Ca2+ stores. However, the addition of either amlodipine or diltiazem did not cause any detectable decrease in ER Ca2+ (Fig. 4 C–E and I). Yet, the subsequent addition of 1 μM ionomycin to the same cells caused complete depletion of ER Ca2+ stores identical to that of vehicle-treated cells, arguing that activation of STIM-ORAI proteins by LCCBs is store-independent.
Fig. 4.
LCCBs do not deplete ER Ca2+ stores. (A) Cytosolic Ca2+ measurements and (B) quantification of maximal cytosolic Ca2+ in HEK293 cells stimulated with either amlodipine or vehicle. Amlodipine was first added to cells in nominally Ca2+-free, and when 2 mM Ca2+ was restored to the bath. (C) ER Ca2+ measurements in HEK293 cells transfected with the ER Ca2+ sensor GCaMP6-150 after addition of either amlodipine or vehicle. At the end of each recording, 1 μM ionomycin was added to fully empty Ca2+ stores. (D) Similar to C, diltiazem or vehicle was added. (E) At 14 min, normalized GCaMP6-150 fluorescence between vehicle, amlodipine, and diltiazem was quantified. (F) ER Ca2+ measurements in HEK293 cells after stimulation with either CPA or (G) carbachol. (H) Quantification of GCaMP6-150 fluorescence after carbachol or CPA stimulation at 14 min. (I) Images of HEK293 cells expressing GCaMP6-150. (Scale bar: 10 μm.) ****P < 0.0001, ns, not significant (ANOVA with Dunnett’s multiple comparison test).
So far, we showed that LCCBs can activate Ca2+ entry only when one STIM and one ORAI isoform are present in cells, but not when an ORAI isoform is expressed alone (Fig. 2 A–D), suggesting that LCCBs cannot directly activate ORAI channels in a STIM-independent manner. Although unlikely, it is however possible that LCCBs could first act on ORAI causing their aggregation, which would then trap STIM leading to ORAI gating and activation. To rule out this “outside-in” mode of LCCBs action, we used STIM1/2-DKO cells expressing CFP-ORAI1 (without STIM) to determine whether amlodipine or diltiazem can cause ORAI1 puncta formation. In these cells, amlodipine and diltiazem did not cause ORAI1 puncta (Fig. 5 A and C). However, both drugs caused significant STIM1 puncta formation in ORAI1/2/3-TKO cells expressing STIM1-YFP (Fig. 5 B and C), strongly arguing that the target of LCCBs is STIM.
Fig. 5.
The target of LCCBs is STIM. (A) Confocal images of distribution of CFP-ORAI1 (1 μg) expressed in STIM1/2-DKO cells before and after treatment for 8 min with either vehicle, diltiazem, or amlodipine. (Scale bar: 10 μm.) (B) Confocal images show distribution of STIM1-eYFP (1 μg) in ORAI1/2/3-TKO cells before and after treatment for 8 min with either vehicle, diltiazem, or amlodipine. (Scale bar: 10 μm.) (A and B) Zoom-in Insets. (Scale bar: 2.5 μm.) (C) Quantification of ORAI1 and STIM1 puncta upon stimulation with vehicle, diltiazem, and amlodipine. (D) Truncated STIM1 constructs (STIM11–310-CFP) and YFP-tagged SOAR1 (YFP-SOAR1) with the denoted domains: signal peptide (SP), EF hand, sterile α motif (SAM), transmembrane (TM), and coiled-coil 1 (CC1). (E) Epifluorescence images of STIM1/2-DKO cells coexpressing STIM11–310-CFP (1 μg), YFP-SOAR1 (1 μg), colocalization, and seFRET. (Scale bar: 10 μm.) (F) seFRET between STIM11–310-CFP and YFP-SOAR1-SOAR1 expressed in STIM1/2-DKO cells stimulated with either diltiazem or vehicle. At the end, cells were stimulated with 1 μM ionomycin (Iono) for maximal dissociation of SOAR1 from STIM11–310. (G) Quantification of seFRET at 8 min. (H) Quantification of YFP/CFP ratios from F. (I) Western blots of HeLa cell membranes expressing STIM1 (A230C). Prior to isolating membranes, cells were incubated with vehicle, amlodipine, or diltiazem. Isolated membranes are then incubated with ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (0 µM Ca2+) or Ca2+ at final concentrations ranging from 0.3 to 2,000 μM during iodine-induced cross-linking. (J) The percentage of STIM1 dimer formation at each indicated Ca2+ concentration for cells treated with vehicle, amlodipine, or diltiazem. *P < 0.05, ****P < 0.0001, ns, not significant (unpaired Student’s t test for FRET experiments and ANOVA with Dunnett’s test for puncta experiments).
Additional evidence that that the target of LCCBs is STIM was gleaned from experiments utilizing a two-component FRET system of STIM1 (51), which consists of 1) a construct encoding the N terminus, the transmembrane domain, and CC1 domain of STIM1 (STIM11–310) tagged with CFP on the C terminus; and 2) a construct encoding the SOAR domain of STIM1 (SOAR1) tagged with YFP on the N terminus (Fig. 5D). These two-tagged constructs were coexpressed in STIM1/2-DKO cells where they were shown to associate through CC1-SOAR1 interactions to recapitulate full-length STIM1 in the ER (51). Indeed, at rest, STIM11–310 and SOAR1 were highly colocalized at the ER and displayed high FRET values due to CC1 tethering to SOAR1 (Fig. 5 E and F). Diltiazem induced SOAR1 to dissociate from STIM11–310 manifested by a dramatic decrease in FRET (Fig. 5 E and F). The subsequent addition of 1 μM ionomycin caused only a marginal additional decrease in FRET (red trace, Fig. 5F). However, the addition of ionomycin to cells expressing STIM11–310 and SOAR1 and treated first with vehicle (no diltiazem) caused a robust decrease in FRET (black trace, Fig. 5F).
Further evidence that STIM proteins are the target of LCCBs were obtained from in vitro disulfide cross-linking assays that probe STIM1 activation in isolated ER membranes (52). HeLa cells expressing an engineered STIM1 (A230C) protein (SI Appendix, Fig. S4A) were treated with 20 μM amlodipine, or 20 μM diltiazem, or with vehicle. Cells were lysed, and isolated membranes—still in the presence of the compounds—were subjected to cross-linking at concentrations of Ca2+ ranging from 0 to 2 mM. The principle of the assay is that low concentrations of Ca2+, mimicking store depletion, favor a STIM1 conformational change that brings the transmembrane helices together and allows disulfide cross-linking via the engineered cysteine residues. STIM1 cross-linking was analyzed by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and Western blotting with STIM1-specific antibody. As expected, decreasing the concentration of Ca2+ increased STIM1-STIM1 cross-linking in all samples (Fig. 5 I and J). Importantly, pretreatment of cells with amlodipine or diltiazem resulted in significantly higher proportions of STIM1 dimers at Ca2+ concentrations in the range 300 μM to 1 mM, compared to cells exposed only to vehicle (Fig. 5 I and J). Neither STIM1 from cells treated with LCCBs nor STIM1 from cells treated with 1 μM thapsigargin exhibited appreciable cross-linking when the reaction was conducted in vitro in the presence of 2 mM Ca2+ (Fig. 5 I and J and SI Appendix, Fig. S4B), indicating that STIM1 in isolated ER membranes does not retain a conformational memory of its activation and oligomerization in vivo prior to isolation. These results establish that LCCBs render STIM1 susceptible to activation even at resting ER Ca2+ concentrations, thus accounting for STIM-ORAI pathway activation by LCCBs independent of ER Ca2+ store depletion.
A STIM1 N-Terminal Region Is Required for SOCE Activation by LCCBs.
We explored the possibility that LCCBs directly bind to STIM1 to induce a conformational change and subsequent activation. To identify the potential domain of STIM1 required for activation by LCCBs, we used a variety of truncated STIM1-YFP constructs expressed in HEK293 cells (SI Appendix, Fig. S5A). Cells were then treated with diltiazem, followed by the SOCE modifier 2-aminodiphenylborate (2-APB), which has been shown to unfold the CC1-SOAR clamp of the C-terminal region of STIM proteins and activate SOCE independently of store depletion (42, 53). We generated four different C-terminally YFP-tagged STIM1 truncated constructs: 1) STIM1235–685, lacking both the N-terminal and the transmembrane domain; 2) STIM1215–685, lacking the N-terminal domain; 3) STIM1120–685; and 4) STIM175–685 (SI Appendix, Fig. S5A).
We then coexpressed theses STIM1 constructs with CFP-ORAI1 in STIM1/2-DKO cells. When the YFP C-terminally tagged STIM1235–685 construct, which consists of STIM1 C-terminal domain (STIM1-Ct) containing the CC1-SOAR1 clamp, is expressed in HEK293 cells, it mostly fails to support significant SOCE activity (25) (however, see ref. 54). Indeed, we show that the STIM1235–685 construct diffusely localizes in the cytosol and fails to support significant SOCE upon thapsigargin stimulation (SI Appendix, Fig. S5 B and C). However, subsequent addition of 50 μM 2-APB induced a robust and transient Ca2+ entry, consistent with previous studies showing that 2-APB can transiently cause STIM1-Ct to activate ORAI1 (53). Diltiazem failed to induce significant STIM-Ct–mediated Ca2+ entry (SI Appendix, Fig. S5 D and E). However, 2-APB caused a significant cytosolic Ca2+ signal in the same cells (SI Appendix, Fig. S5D). These data argue that STIM1-Ct alone is incapable of supporting Ca2+ entry in response to LCCBs. The other three STIM1 constructs (SI Appendix, Fig. S5A) showed ER localization (SI Appendix, Fig. S5 B, F, H, and J) but failed to support Ca2+ entry in response to 20 to 50 µM diltiazem (SI Appendix, Fig. S5 D, E, G, I, and K). These results suggest that the LCCBs action on STIM is distinct from the direct mechanism of action of 2-APB on STIM1-Ct, and that LCCBs action requires the full-length STIM protein.
To gain insights into the STIM domain mediating LCCBs action, we tested whether LCCBs activation of STIM was conserved in other vertebrates. Current measurements demonstrated that LCCBs do not activate native SOCE in Xenopus oocytes (SI Appendix, Fig. S6). We then coexpressed Xenopus STIM1 and ORAI1 (xSTIM1-YFP and xORAI1-CFP) in STIM1/2-DKO cells. Confocal microscopy confirmed that, under resting conditions, the tagged xSTIM1 and xORAI1 are located in the ER and PM, respectively (Fig. 6A). The addition of thapsigargin (+TG) for 8 min induced xSTIM1 and xORAI1 to form puncta and colocalize (Fig. 6A). However, xSTIM1 and xORAI1 failed to form significant puncta and to colocalize in response to 20 μM amlodipine (Fig. 6B). Side-by-side comparisons between xSTIM1 and human STIM1 (hSTIM1) showed significant hSTIM1 puncta formation upon amlodipine stimulation (Fig. 6C). Furthermore, SOCE induced by thapsigargin was rescued in STIM1/2-DKO by coexpression of xSTIM1 and xORAI1 (Fig. 6 D and E). Interestingly, amlodipine failed to cause significant Ca2+ entry in STIM1/2-DKO cells coexpressing xSTIM1 and xORAI1, while inducing a significant Ca2+ signal in cells coexpressing hSTIM1 and hORAI1 (Fig. 6 F and G). These results suggest that LCCBs-mediated activation of SOCE is specific to human STIM and is not supported by xSTIM1 and xORAI1.
Fig. 6.
LCCBs effect requires a small N-terminal region of STIM1. (A) Confocal images of Xenopus STIM1-YFP (xSTIM1, 4 μg) and Xenopus ORAI1-CFP (xORAI1, 2 μg) in STIM1/2-DKO cells before and after treatment for 8 min with thapsigargin (TG). (B) Similar images showing localization of xSTIM1-YFP and xORAI1-CFP before and after treatment with amlodipine. (C) Quantification of xSTIM1or hSTIM1 puncta after stimulation with amlodipine. (D) SOCE triggered by thapsigargin in STIM1/2-DKO cells cotransfected with either xSTIM1-YFP and xORAI1-CFP or an empty plasmid. (E) Quantification of maximal Ca2+ entry from D. (F) Cytosolic Ca2+ measurements on STIM1/2-DKO cells cotransfected with either xSTIM1 + xORAI1 or with hSTIM1 + hORAI1. Cells were stimulated with amlodipine. (G) Quantification of maximal Ca2+ signals from F. (H) SOCE was induced with thapsigargin in STIM1/2-DKO cells expressing ORAI1-CFP (1 µg) with either STIM1 (2 µg), or STIM1-Δ29–46, or STIM1-Δ31–40. (I) Quantification of maximal Ca2+ entry from H. (J) Confocal images of STIM1, STIM1-Δ29–46, and STIM1-Δ31–40 expressed in STIM1/2-DKO cells before and after thapsigargin. (K) Amlodipine was used to stimulate Ca2+ entry in STIM1/2-DKO cells expressing ORAI1-CFP (1 µg) with 2 µg plasmid of either full-length STIM1, or STIM1-Δ29–46, or STIM1-Δ31–40. (L) Quantification of maximal Ca2+ entry from K. (M) Western blots of HeLa cell membranes expressing STIM1 (A230C). For “Intact Cells,” cells were incubated with either amlodipine or vehicle and then lysed, and membranes were isolated. For the “Membranes” condition, cells were lysed first, and the isolated membranes were incubated with either amlodipine or vehicle. For both conditions, the isolated membranes were then incubated with EGTA (0 µM Ca2+) or Ca2+ at final concentrations ranging from 0.3 to 2,000 μM, cross-linked and analyzed by nonreducing SDS/PAGE. (N) Quantification of the percentage of STIM1 dimer after treatment with either amlodipine or vehicle at each Ca2+ concentration. (A, B, and J) Scale bars: 10 μm and 2.5 μm for Zoom in Insets. **P < 0.01, ****P < 0.0001 (unpaired Student’s t test for two comparisons and ANOVA with Dunnett’s test for multiple comparisons).
We performed sequence alignments of hSTIM1 and xSTIM1 and identified 10 to 18 amino acids within the N terminus of hSTIM1 that are either absent or not conserved in xSTIM1 (SI Appendix, Fig. S6H). We then created two truncations within this N-terminal region of hSTIM1, namely STIM1Δ29–46 and STIM1Δ31–40 (SI Appendix, Fig. S6H). Importantly, these truncated hSTIM1 constructs were fully functional and mediated SOCE identical to that of full-length STIM1 (Fig. 6 H and I). Furthermore, STIM1Δ29–46 and STIM1Δ31–40 distributed into puncta in response to stimulation with thapsigargin in a manner similar to full-length STIM1 (Fig. 6J). However, these two truncated constructs failed to support Ca2+ entry in response to amlodipine (Fig. 6 K and L), suggesting that this N-terminal region of hSTIM1 is necessary for LCCB-mediated activation of SOCE.
An Intermediate Pathway Is Required for LCCBs to Activate STIM.
To determine whether LCCBs directly activate STIM1, we used the cross-linking described earlier. Instead of treating cells with LCCBs before lysis, we first lysed the cells expressing the engineered STIM1-A230C (SI Appendix, Fig. S4A), isolated the membranes, and incubated these membranes with LCCBs in the presence of varying concentrations of Ca2+. The original protocol used in Fig. 5 where intact cells are treated with LCCBs was performed side-by-side. Unlike intact cells treated with LCCBs, when we treated isolated membranes with amlodipine, we failed to see increases in the proportion of STIM1 dimers at higher concentrations of Ca2+ (Fig. 6 M and N). These findings suggest that STIM1 activation by LCCBs requires intact cells, presumably because a cellular protein or factor required for modifying or binding to STIM1 is missing from isolated membrane preparations.
We considered the possibility that the protein required for LCCBs action on STIM is Cav1.2. While the Cav1.2 channel is only functional in excitable cells, the α1C subunit of Cav1.2 is still expressed HEK293 cells (33). Previous studies showed that Cav1.2 is negatively regulated by STIM1 through direct binding of SOAR1 to the C terminus of the α1C subunit of Cav1.2, causing α1C internalization (55, 56). We reasoned that perhaps LCCBs disrupt resting Cav1.2-STIM1 interactions, unlocking this pool of STIM1 in an extended conformation to activate ORAI.
To generate functional Cav1.2 channels in HEK293 cells, we coexpressed the YFP-tagged Cav1.2 α1C along with its auxiliary subunits β1A and α2δ1 (57), which allowed trafficking of the channel to the membrane (SI Appendix, Fig. S7A). An antibody specific to α1C detected the native Cav1.2 α1C and the YFP-tagged Cav1.2 α1C (SI Appendix, Fig. S7B). Depolarization with 134 mM KCl caused a robust Ca2+ entry only in cells expressing Cav1.2 subunits (SI Appendix, Fig. S7 C and D). As expected, this Cav1.2-mediated Ca2+ signal was blocked by 0.5 μM amlodipine (SI Appendix, Fig. S7 C and D). Interestingly, both native SOCE induced by thapsigargin (SI Appendix, Fig. S7 E and F) and Ca2+ entry induced by amlodipine (SI Appendix, Fig. S7 G and H) were not enhanced as would be expected if Cav1.2 is mediating the effects of LCCBs on STIM proteins. Quite the opposite, SOCE and amlodipine-activated Ca2+ entry were slightly reduced when Cav1.2 was expressed in HEK293 cells, suggesting that Cav1.2 is likely sequestering native STIM proteins and reducing their accessibility to ORAI.
Next, we knocked down the α1C subunit of Cav1.2 in HEK293 cells using short hairpin RNA (shRNA). Western blots confirmed ∼50% knockdown (SI Appendix, Fig. S7 I and J). Native SOCE activated by thapsigargin (SI Appendix, Fig. S7 K and L) and Ca2+ entry induced by amlodipine (SI Appendix, Fig. S7 M and N) were slightly augmented in shCav1.2-expressing cells, in agreement with previous studies showing that Cav1.2 knockdown liberates a STIM1 pool that can now activate ORAI channels (55, 56). These results suggest that LCCBs action on the STIM1 N terminus is Cav1.2-independent and mediated by an intermediary mechanism.
LCCBs Activate VSMC Remodeling through STIM1.
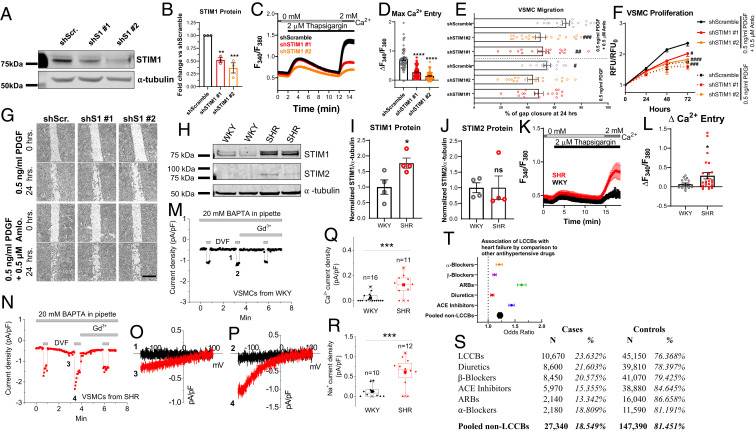
To determine whether STIM1 is required for low doses (0.5 μM) of amlodipine to mediate enhanced VSMC proliferation and migration, we generated VSMCs with stable STIM1 knockdown using two different shRNAs. The shSTIM1#1- and shSTIM1#2-transfected VSMC showed reduced STIM1 protein (Fig. 7 A and B) and reduced SOCE (Fig. 7 C and D) compared to the shScramble-transfected VSMCs. Importantly, the synergistic effect of 0.5 μM amlodipine +0.5 ng/mL PDGF on VSMC migration (Fig. 7 E and G and SI Appendix, Fig. S8 A and B) and proliferation (Fig. 7F and SI Appendix, Fig. S8C) was significantly reduced in shSTIM1#1- and shSTIM1#2-transfected VSMCs, suggesting that the effects of amlodipine on VSMC proliferation and migration is STIM1-dependent.
Fig. 7.
LCCBs promote vascular remodeling through STIM1. (A) STIM1 Western blots of VSMCs transfected with Scrambled shRNA (shScr.) and two STIM1 shRNA (shSTIM1#1 [shS1 #1] and shSTIM1#2 [shS1 #2]). (B) STIM1 protein quantification from A using densitometry normalized to tubulin. (C) SOCE triggered by thapsigargin in VSMC stably expressing either shScramble, or shSTIM1#1, or shSTIM1#2. (D) Quantification of maximal Ca2+ entry from C. (E) Quantification of VSMC migration at 24 h in ShScramble and shSTIM1-expressing VSMCs treated with either 0.5 ng/mL PDGF or 0.5 μM amlodipine + 0.5 ng/mL PDGF. (F) Quantification in normalized relative fluorescence units (RFU) of proliferation in ShScramble- and shSTIM1-expressing VSMCs treated with either 0.5 ng/mL PDGF or 0.5 μM amlodipine + 0.5 ng/mL PDGF. (G) Bright field images of VSMC migration from E. (Scale bar: 500 μm.) (H) STIM1 and STIM2 Western blots in VSMCs acutely isolated from endothelial-denuded aortic rings of SHR and WKY rats. (I and J) Quantification of STIM1 (I) and STIM2 (J) in WKY and SHR normalized to tubulin. (K) SOCE measurements from SHR and WKY VSMCs. (L) Quantification of maximal Ca2+ entry from K. (M and N) Whole-cell ICRAC induced by 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) in VSMCs from WKY (M) and SHR (N). Divalent free (DVF) bath solution was used to potentiate a Na+ current. (O) Ca2+ ICRAC and (P) Na+ ICRAC I/V relationships obtained from M and N where indicated by numbers (1 to 4). (Q) Peak Ca2+ ICRAC and (R) peak Na+ ICRAC densities from SHR and WKY VSMCs. (S) Data from Penn State University Hospital with reported cases of heart failure when patients are treated with LCCBs or other antihypertensive drugs. Exposed control did not develop heart failure to date. In italics is the percentage of patients in that exposed group. The last row reports pooled patients exposed to antihypertensive drugs that are not LCCBs. (T) Odds ratio of tabulated results in S. Error bars are 95% CIs (*P < 0.05, ***P < 0.001; unpaired Student’s t test). ANOVA and Dunnett’s multiple comparison test were used for B and D, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (E and G) #P < 0.05, ##P < 0.01, ###P < 0.001 when compared to 0.5 ng/mL PDGF.
VSMCs from Chronically Hypertensive Rats Have Up-Regulated STIM1 and Enhanced SOCE.
An excellent model for chronic hypertension associated with VSMC remodeling is the spontaneously hypertensive rats (SHRs) and respective control Wistar-Kyoto rats (WKYs). SHRs have systolic blood pressures over 150 mm Hg after 1 mo of age (58). After 12-wk, the aortas of SHRs undergo considerable remodeling, with VSMC hyperplasia and vessel thickening (59). Using 12-wk-old male rats, endothelial denuded aortic rings were dissected, and VSMCs were isolated from SHR and WKY rats. VSMCs acutely isolated from SHR showed enhanced STIM1 protein expression (Fig. 7 H and I) but only a marginal increase in STIM2 protein expression (Fig. 7 H and J). SOCE activated by thapsigargin and ICRAC were significantly increased in SHR VSMCs compared to WKY VSMCs (Fig. 7 K–N). Both peak current densities at −100 mV of Ca2+ currents (Fig. 7 O and Q) and Na+ ICRAC recorded in divalent free bath solutions (Fig. 7 P and R) were increased in SHR VSMCs. Thus, in vivo VSMC remodeling in chronic hypertension is associated with enhanced STIM1 expression and increased SOCE and ICRAC activities. It is established that isolating VSMC and culturing them causes their dedifferentiation into a synthetic phenotype (7, 15, 35). We show that, whether VSMCs are isolated from SHR or WKY, once they are placed into cell culture for 3 d, both SHR and WKY VSMCs show equally enhanced SOCE in response to thapsigargin (SI Appendix, Fig. S8 D and E), strongly arguing that, regardless of the origin of VSMCs, once in culture, they equally dedifferentiate into a synthetic phenotype.
LCCBs Are Associated with Increased Incidence of Heart Failure in Patients.
The pathological VSMC remodeling in chronic hypertension is accompanied with decreased luminal diameter, increased systemic peripheral resistance (4), and heart failure (60). Therefore, we considered the possibility that amlodipine and other LCCBs could be associated with enhanced incidence of heart failure in hypertensive patients treated with those drugs. Using the Penn State clinical database known as i2b2, we compared the incidence of heart failure in hypertensive patients treated exclusively with LCCBs (exposed cases) to those treated with none-LCCBs antihypertensive medications (unexposed cases). Our analysis showed that hypertensive patients treated with LCCBs had a significantly higher incidence of heart failure (23.632%) compared to patients treated with other antihypertensive drugs (18.549%) (Fig. 7S). Statistically, patients treated with LCCBs were more prone to heart failure when compared to pooled patients treated with other antihypertensive drugs (Fig. 7T) (95% CI for odds ratio = [1.222,1.252]). The demographics including sex, race, and age among the cohorts were similar (SI Appendix, Fig. S9 A–C). After stratification based on sex, LCCBs were still more associated with heart failure than other antihypertensive drugs (SI Appendix, Fig. S9D) (95% CI for odds ratio = [1.100, 1.138] and [1.369, 1.417] for males and females, respectively), with the exception of diuretics and β-blockers in male patients.
We also examined other smooth muscle proliferative diseases and certain types of cancers that are associated with enhanced STIM expression and increased SOCE activity (61). The smooth muscle neoplasms, leiomyomas of the uterus (fibroids; 95% CI for odds ratio = [0.900, 1.001]), leiomyomas (1.000, 1.201), or leiomyosarcomas (0.777, 1.081) were no more and no less associated with LCCBs than other antihypertensive drugs (SI Appendix, Fig. S10 A–F). However, LCCBS were more associated with prostate (1.245, 1.381), breast (1.057, 1.176), and bladder cancer (1.156, 1.240) (SI Appendix, Fig. S11 A–F).
Discussion
Here, we unravel a mechanism whereby LCCBs activate STIM to trigger SOCE and smooth muscle proliferation and migration. These findings were epidemiologically associated with increased heart failure in hypertensive patients on LCCBs. In previous studies, we showed that STIM1-ORAI1 proteins, SOCE and ICRAC are increased in neointimal VSMCs from rat and mice models of vascular remodeling (14, 28). Knockdown or knockout of STIM1 or ORAI1 in rat and mice inhibited neointima formation and hypertension (14, 29). In the present study, we show that STIM1 proteins, SOCE and ICRAC are up-regulated in VSMCs isolated from vessels of 12-wk-old spontaneously hypertensive rats, suggesting that enhanced SOCE is a common theme in VSMC remodeling. It is established that sub-µM concentrations of LCCBs inhibit Cav1.2 channels and induce vasorelaxation (62). Thus, LCCBs have been widely prescribed for treatments of hypertension for over 70 y. However, during chronic hypertension, Cav1.2 channels are down-regulated in remodeled VSMCs, and many Ca2+ signaling proteins like STIM-ORAI are up-regulated (6, 7). Since Cav1.2, which represents the target of LCCBs, is decreased during vascular remodeling, the efficacy of LCCBs during long-term chronic hypertension is uncertain.
Our unbiased screen of ∼1,700 FDA-approved drugs in glioblastoma discovered that LCCBs activated a Ca2+ entry pathway that was inhibited by pharmacological blockers of SOCE (37). Here, we determined the molecular target of LCCBs and their impact on VSMC function. LCCBs activate SOCE and ICRAC mediated by STIM-ORAI. Using gene knockout in HEK293 cells, we determined that one STIM and one ORAI isoform are necessary to support LCCB-activated Ca2+ entry. The target for LCCBs in VSMCs is STIM1, and STIM1 activation by LCCBs requires an N-terminal portion that is absent from Xenopus STIM1. LCCBs activation of STIM1 occurs independently of ER Ca2+ depletion. Interestingly, all major classes of LCCBs with different chemical structures, including dihydropyridines, benzothiazepines, and phenylalkylamines, activate STIM-ORAI. Each of these three classes of LCCBs binds to distinct sites on Cav1.2 (63, 64). Since STIM1/2 were activated by all classes of LCCBs, this offers further support that the mechanism of STIM activation by LCCBs is indirect. This and the failure of LCCBs to activate STIM1 dimer formation in isolated membranes are compatible with the requirement of an intermediary factor or process. Because Cav1.2 has been shown to interact with STIM1 (55, 56), and is a target common to all LCCBs, we excluded Cav1.2 as this intermediary protein. Although the identification of this intermediary mechanism requires further investigations, this mechanism might involve a soluble factor, a protein–protein interaction, or a posttranslational modification. This includes modification by phosphorylation or reactive oxygen species, which have been shown to activate STIM1 independently of store depletion (65–67).
STIM1-mediated SOCE enhances VSMC proliferation and migration (14, 28, 68). During vascular remodeling, local PDGF secreted from endothelial and immune cells induces VSMC proliferation and migration (39). STIM1 is required for PDGF-induced smooth muscle migration and proliferation (27). Here, we show that low concentrations of amlodipine (reminiscent of those circulating in patients treated with those drugs) (34) synergize with submaximal concentrations of PDGF to enhance STIM1-dependent VSMC proliferation and migration. Interestingly, this effect seems unique to LCCBs since other antihypertensive medications like ACE inhibitors inhibit vascular remodeling (69, 70). SHR rats have exacerbated vascular remodeling during the chronic phase of hypertension. We show that acutely isolated VSMCs from 12-wk-old SHRs have increased STIM1 protein, and enhanced SOCE and ICRAC, consistent with previous studies (71). We previously showed that acutely isolated VSMCs have little ORAI1 protein, marginal SOCE activity, and undetectable ICRAC (13, 14, 27). We also demonstrated that smooth muscle-specific STIM1 knockout mice were protected against hypertension induced by angiotensin II infusion (29). These findings and the established role of STIM1 in driving VSMC neointimal hyperplasia during balloon angioplasty (14) strongly argue that STIM1 and SOCE play an active role in exacerbating hypertension.
Heart failure is a clinical consequence of chronic hypertension and vascular remodeling (4, 60). Epidemiological evidence suggests that hypertensive patients on LCCBs are more likely to develop heart failure compared to hypertensive patients on other antihypertensive medications. This may be due to the mechanisms described above and summarized herein. Although larger epidemiological studies and drug interventional studies in animals are warranted, our data suggest that the management of chronic hypertension with LCCBs should be reevaluated in elderly patients or in patients with chronic hypertension or a history of cardiovascular remodeling. Female hypertensive patients treated with amlodipine for 2 wk show a greater decrease in blood pressure than males (72); and acutely isolated VSMCs from normotensive female mice have larger Cav1.2 channel clusters, activity, and myogenic tone than male VSMCs (73), suggesting that Cav1.2 function is more prominent in females than males. However, our study showed female patients on LCCBs have stronger association with heart failure than males. This is likely because VSMCs from the chronically hypertensive patients in our study are remodeled with down-regulated Cav1.2 and enhanced STIM1 expression.
STIM1 protein has been reported to be enhanced in certain neoplasms and necessary for their development (61). We found that LCCBs administration is no more associated with smooth muscle neoplasms than other antihypertensive medications. This might be because STIM1 does not provide the oncogenic trigger to transform the relatively oncogenic-resistant smooth muscle cells (74). However, prostate, breast, and bladder cancers are more associated with LCCBs use than other antihypertensive medications. Although STIM1 has not been studied in bladder cancer, it was shown to drive breast (75) and prostate (76) cancer growth. Interestingly, studies have also shown a similar association of LCCBs with breast cancer (77). Our results identify LCCBs as activators of STIM proteins. This causes enhancement of SOCE and stimulation of VSMC remodeling. Treatment with LCCBs is clinically associated with elevated incidence of heart failure, which prompts a careful examination of the use of LCCBs during chronic hypertension where vascular remodeling is evident.
Methods
Details of VSMC isolation from rat aortas and maintenance, complementary DNA construct cloning, generation of CRISPR/Cas9 knockout cell lines for STIM and ORAI isoforms, VSMC and HEK293 cell culture, stable transfections, patch-clamp recordings of CRAC currents, ER and cytosolic Ca2+ measurements with chemical and genetically encoded indicators, colocalization of STIM/ORAI by confocal imaging, STIM1 cross-linking assays, FRET analysis, Western blot analysis, animal and human patient studies, and statistics are provided in SI Appendix.
Data Availability.
All materials and experimental protocols; all raw data and raw unprocessed gels and Western blots; and the source and reference number of reagents, recombinant DNA, and animals necessary for replication are included in SI Appendix. The exact P values for all statistical comparisons are also included in SI Appendix.
Supplementary Material
Acknowledgments
We thank the following for kindly providing constructs: Dr. Kurt Beam (University of Colorado) for CMV-YFP-Cav1.2-⍺1c, CMV-Cav1.2-β1A, and CMV-Cav1.2-⍺2△; and Dr. Yubin Zhou (Texas A&M University) for CMV-STIM1(1-310)-CFP. This work was supported by NIH/National Heart, Lung, and Blood Institute Grant R35-HL150778 (to M.T.), Grant F30-HL147489-01A1 (to M.T.J.), NIH/National Institute of General Medical Sciences Grant 1R35 GM131916 (to D.L.G.), NIH/National Institute of Allergy and Infectious Diseases Grant R01-AI084167 (to P.G.H.) and Grant R01-AI040127 (to Anjana Rao and P.G.H.), NIH Grant TL1TR002016 (to M.T.J.), Department of Defense Grant CDMRP W81XWH1810209 (to W.L.), Qatar National Research Fund Grants NPRP7-542-3-145 and NPRP8-110-3-021 (to M.T. and K.M.), and funds from Weill Cornell Medicine-Qatar of the Qatar Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007598117/-/DCSupplemental.
References
- 1.Oparil S. et al., Hypertension. Nat. Rev. Dis. Primers 4, 18014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull F. et al.; Blood Pressure Lowering Treatment Trialists’ Collaboration , Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 336, 1121–1123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halayko A. J., Solway J., Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J. Appl. Physiol. (1985) 90, 358–368 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Brown I. A. M. et al., Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 38, 1969–1985 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge M. J., Calcium signalling remodelling and disease. Biochem. Soc. Trans. 40, 297–309 (2012). [DOI] [PubMed] [Google Scholar]
- 6.House S. J., Potier M., Bisaillon J., Singer H. A., Trebak M., The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 456, 769–785 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trebak M., STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol. 590, 4201–4208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M., Trebak M., ORAI channels in cellular remodeling of cardiorespiratory disease. Cell Calcium 79, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gollasch M. et al., L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. 12, 593–601 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Ihara E. et al., Mechanism of down-regulation of L-type Ca(2+) channel in the proliferating smooth muscle cells of rat aorta. J. Cell. Biochem. 87, 242–251 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Quignard J. F. et al., Transient down-regulation of L-type Ca(2+) channel and dystrophin expression after balloon injury in rat aortic cells. Cardiovasc. Res. 49, 177–188 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Vallot O. et al., Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler. Thromb. Vasc. Biol. 20, 1225–1235 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Potier M. et al., Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: Role in proliferation and migration. FASEB J. 23, 2425–2437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W. et al., Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ. Res. 109, 534–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berra-Romani R., Mazzocco-Spezzia A., Pulina M. V., Golovina V. A., Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am. J. Physiol. Cell Physiol. 295, C779–C790 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubart F. C. et al., RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol. Ther. 17, 455–462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trebak M. et al., What role for store-operated Ca2+ entry in muscle? Microcirculation 20, 330–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou J. et al., STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos J. et al., STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streb H., Irvine R. F., Berridge M. J., Schulz I., Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 (1983). [DOI] [PubMed] [Google Scholar]
- 21.Trebak M., Kinet J. P., Calcium signalling in T cells. Nat. Rev. Immunol. 19, 154–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trebak M., Putney J. W. Jr., ORAI calcium channels. Physiology (Bethesda) 32, 332–342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soboloff J., Rothberg B. S., Madesh M., Gill D. L., STIM proteins: Dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 13, 549–565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakriya M., Lewis R. S., Store-operated calcium channels. Physiol. Rev. 95, 1383–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C. Y. et al., STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan J. P. et al., SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 11, 337–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisaillon J. M. et al., Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am. J. Physiol. Cell Physiol. 298, C993–C1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancarella S. et al., Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 27, 893–906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassan M. et al., Essential role of smooth muscle STIM1 in hypertension and cardiovascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 36, 1900–1909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Trebak M., Vascular balloon injury and intraluminal administration in rat carotid artery. J. Vis. Exp., 52045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Cobos J. C. et al., Store-independent Orai1/3 channels activated by intracrine leukotriene C4: Role in neointimal hyperplasia. Circ. Res. 112, 1013–1025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright J. M., Musini V. M., Gill R., First-line drugs for hypertension. Cochrane Database Syst. Rev. 4, CD001841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catterall W. A., Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3, a003947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abernethy D. R., Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology 80 (suppl. 1), 31–36 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Beamish J. A., He P., Kottke-Marchant K., Marchant R. E., Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue Eng. Part B Rev. 16, 467–491 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rensen S. S., Doevendans P. A., van Eys G. J., Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 15, 100–108 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z. et al., Induction of store-operated calcium entry (SOCE) suppresses glioblastoma growth by inhibiting the Hippo pathway transcriptional coactivators YAP/TAZ. Oncogene 38, 120–139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asai M. et al., Misinterpretation of the effect of amlodipine on cytosolic calcium concentration with fura-2 fluorospectrometry. Naunyn Schmiedebergs Arch. Pharmacol. 377, 423–427 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Qi Y. X. et al., PDGF-BB and TGF-beta1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc. Natl. Acad. Sci. U.S.A. 108, 1908–1913 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penner R., Matthews G., Neher E., Regulation of calcium influx by second messengers in rat mast cells. Nature 334, 499–504 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Zhang X. et al., A calcium/cAMP signaling loop at the ORAI1 mouth drives channel inactivation to shape NFAT induction. Nat. Commun. 10, 1971 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emrich S. M. et al., Cross-talk between N-terminal and C-terminal domains in stromal interaction molecule 2 (STIM2) determines enhanced STIM2 sensitivity. J. Biol. Chem. 294, 6318–6332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoast R. E. et al., The native ORAI channel trio underlies the diversity of Ca2+ signaling events. Nat. Commun. 11, 2444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezprozvanny I., Tsien R. W., Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol. Pharmacol. 48, 540–549 (1995). [PubMed] [Google Scholar]
- 45.Franckowiak G., Bechem M., Schramm M., Thomas G., The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur. J. Pharmacol. 114, 223–226 (1985). [DOI] [PubMed] [Google Scholar]
- 46.Nielsen K. J., Schroeder T., Lewis R., Structure-activity relationships of omega-conotoxins at N-type voltage-sensitive calcium channels. J. Mol. Recognit. 13, 55–70 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Strichartz G., Molecular mechanisms of nerve block by local anesthetics. Anesthesiology 45, 421–441 (1976). [DOI] [PubMed] [Google Scholar]
- 48.Luik R. M., Wu M. M., Buchanan J., Lewis R. S., The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moussa B. A., El-Zaher A. A., Mahrouse M. A., Ahmed M. S., Simultaneous determination of amlodipine besylate and atorvastatin calcium in binary mixture by spectrofluorimetry and HPLC coupled with fluorescence detection. Anal. Chem. Insights 8, 107–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Juan-Sanz J. et al., Axonal endoplasmic reticulum Ca(2+) content controls release probability in CNS nerve terminals. Neuron 93, 867–881.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma G. et al., Inside-out Ca(2+) signalling prompted by STIM1 conformational switch. Nat. Commun. 6, 7826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirve N., Rajanikanth V., Hogan P. G., Gudlur A., Coiled-coil formation conveys a STIM1 signal from ER lumen to cytoplasm. Cell Rep. 22, 72–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y. et al., STIM protein coupling in the activation of Orai channels. Proc. Natl. Acad. Sci. U.S.A. 106, 7391–7396 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y. et al., STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol. 17, 112–116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park C. Y., Shcheglovitov A., Dolmetsch R., The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 330, 101–105 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Wang Y. et al., The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polster A., Perni S., Bichraoui H., Beam K. G., Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 112, 602–606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doggrell S. A., Brown L., Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 39, 89–105 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Qu Y. Y. et al., Reduced expression of the extracellular calcium-sensing receptor (CaSR) is associated with activation of the renin-angiotensin system (RAS) to promote vascular remodeling in the pathogenesis of essential hypertension. PLoS One 11, e0157456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drazner M. H., The progression of hypertensive heart disease. Circulation 123, 327–334 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Vashisht A., Trebak M., Motiani R. K., STIM and Orai proteins as novel targets for cancer therapy. A review in the theme: Cell and molecular processes in cancer metastasis. Am. J. Physiol. Cell Physiol. 309, C457–C469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zamponi G. W., Striessnig J., Koschak A., Dolphin A. C., The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang L. et al., Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature 537, 117–121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang L., Gamal El-Din T. M., Lenaeus M. J., Zheng N., Catterall W. A., Structural basis for diltiazem block of a voltage-gated Ca2+ channel. Mol. Pharmacol. 96, 485–492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawkins B. J. et al., S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 190, 391–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grupe M., Myers G., Penner R., Fleig A., Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 48, 1–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yazbeck P. et al., STIM1 phosphorylation at Y361 recruits Orai1 to STIM1 puncta and induces Ca2+ entry. Sci. Rep. 7, 42758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodríguez-Moyano M. et al., Urotensin-II promotes vascular smooth muscle cell proliferation through store-operated calcium entry and EGFR transactivation. Cardiovasc. Res. 100, 297–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong J. et al., Angiotensin-converting enzyme inhibition abolishes medial smooth muscle PDGF-AB biosynthesis and attenuates cell proliferation in injured carotid arteries: Relationships to neointima formation. Circulation 96, 1631–1640 (1997). [DOI] [PubMed] [Google Scholar]
- 70.Tang L., Both K., Taylor R., ACE inhibition and fibroblast growth factor in cultured human vascular smooth muscle. Vasc. Med. 4, 129–134 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Giachini F. R. et al., Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: A novel insight into vascular dysfunction. Hypertension 53, 409–416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kloner R. A., Sowers J. R., DiBona G. F., Gaffney M., Wein M., Sex- and age-related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Am. J. Cardiol. 77, 713–722 (1996). [DOI] [PubMed] [Google Scholar]
- 73.O’Dwyer S. C. et al., Kv2.1 channels play opposing roles in regulating membrane potential, Ca2+ channel function, and myogenic tone in arterial smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 117, 3858–3866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Momin E. N., Vela G., Zaidi H. A., Quiñones-Hinojosa A., The oncogenic potential of mesenchymal stem cells in the treatment of cancer: Directions for future research. Curr. Immunol. Rev. 6, 137–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulkarni R. P. et al., miRNA-dependent regulation of STIM1 expression in breast cancer. Sci. Rep. 9, 13076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y. et al., Suppression of STIM1 inhibits the migration and invasion of human prostate cancer cells and is associated with PI3K/Akt signaling inactivation. Oncol. Rep. 38, 2629–2636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jick H. et al., Calcium-channel blockers and risk of cancer. Lancet 349, 525–528 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials and experimental protocols; all raw data and raw unprocessed gels and Western blots; and the source and reference number of reagents, recombinant DNA, and animals necessary for replication are included in SI Appendix. The exact P values for all statistical comparisons are also included in SI Appendix.