Significance
The molecular mechanisms by which gut microbes modulate host longevity remain elusive. Using genome-wide lifespan screens and extensive interspecies genetic analysis, we identified that the gut microbe-derived metabolite methylglyoxal (MG) modulated host longevity. MG is a reactive carbonyl species involved in the formation of advanced glycation end products, which are implicated in various human pathologies. We identified that Escherichia coli producing reduced levels of MG increased the lifespan of Caenorhabditis elegans due to inhibition of TORC2/SGK-1 and activation of DAF-16. These findings challenge the current paradigm that MG is toxic due to the formation of glycation adducts on biomolecules. Instead, our results highlight the importance of gut microbe-derived MG in regulating the host TORC2/SGK-1/DAF-16 signaling pathway in the interspecies context.
Keywords: gut microbe, longevity, methylglyoxal, DAF-16
Abstract
Gut microbes play diverse roles in modulating host fitness, including longevity; however, the molecular mechanisms underlying their mediation of longevity remain poorly understood. We performed genome-wide screens using 3,792 Escherichia coli mutants and identified 44 E. coli mutants that modulated Caenorhabditis elegans longevity. Three of these mutants modulated C. elegans longevity via the bacterial metabolite methylglyoxal (MG). Importantly, we found that low MG-producing E. coli mutants, Δhns E. coli, extended the lifespan of C. elegans through activation of the DAF-16/FOXO family transcription factor and the mitochondrial unfolded protein response (UPRmt). Interestingly, the lifespan modulation by Δhns did not require insulin/insulin-like growth factor 1 signaling (IIS) but did require TORC2/SGK-1 signaling. Transcriptome analysis revealed that Δhns E. coli activated novel class 3 DAF-16 target genes that were distinct from those regulated by IIS. Taken together, our data suggest that bacteria-derived MG modulates host longevity through regulation of the host signaling pathways rather than through nonspecific damage on biomolecules known as advanced glycation end products. Finally, we demonstrate that MG enhances the phosphorylation of hSGK1 and accelerates cellular senescence in human dermal fibroblasts, suggesting the conserved role of MG in controlling longevity across species. Together, our studies demonstrate that bacteria-derived MG is a novel therapeutic target for aging and aging-associated pathophysiology.
In the human body, symbiotic microbes outnumber human somatic cells by 10-fold. The majority of these microbes (95%) reside within the gastrointestinal tract and are known as gut microbiota (1). Gut microbiota have important roles in host fitness, including protection against pathogenic bacteria, maturation of the host immune system, digestion of nonmetabolizable sugars, and production of micronutrients (1, 2). Recent studies also indicate that gut microbes play a role in the modulation of host longevity (3, 4); however, the molecular mechanisms underlying this process remain poorly understood.
Caenorhabditis elegans is a widely used model organism for aging research (5). Many genes and signaling pathways that are involved in the regulation of longevity are conserved in C. elegans, including insulin/insulin-like growth factor 1 (IIS) signaling, target of rapamycin (TOR) signaling, dietary restriction (DR), immunity pathways, mitochondria pathways, and stress response pathways. The IIS pathway is an evolutionarily conserved pathway to regulate longevity (6). In C. elegans, activation of the insulin-like peptide receptor DAF-2 leads to serial phosphorylation of AGE-1/PI3-kinase, PDK1, and Thr350 in AKT kinases. In parallel, target of rapamycin complex 2 (TORC2), also known as PDK2, phosphorylates AKT and SGK1 at the hydrophobic motif site Ser517, resulting in full activation (7). Activated AKT phosphorylates DAF-16/Forkhead Box O (FOXO) transcription factors at three conserved sites, RXRXXS/T (8), limiting their localization in the cytosol (9).
C. elegans is a useful model for gut microbe-host aging studies (10–12). Nitric oxide and quorum sensing pentapeptide produced by bacilli have been shown to increase the lifespan of C. elegans through HSF-1 and DAF-16 (13, 14). More recently, bacterial colanic acid (CA), an extracellular polysaccharide, was shown to extend the lifespan of C. elegans through activation of the mitochondrial unfolded protein response (UPRmt) (15).
Gut microbes produce a variety of metabolites, such as short-chain fatty acids and trimethylamine, which are known to have both positive and negative effects on host physiology (16, 17). Methylglyoxal (MG) is a highly reactive dicarbonyl metabolite generated in the metabolism of almost all organisms, including human gut microbes (18, 19). MG induces formation of advanced glycation end products (AGEs) through nonenzymatic reactions with proteins, lipids, and DNA (20). AGEs have been implicated in diabetes and neurodegenerative diseases, such as Parkinson’s and Alzheimer’s disease (21, 22), and also has been reported to activate TORC2 in yeast and mammalian cells (23). The effects of MG derived from gut bacteria on the physiology of the host remain unknown, however.
In this study, we demonstrated that bacteria-derived MG play a critical role in the modulation of host longevity. Bacteria that produced less MG, such as Δhns E. coli and E. coli that overproduced MG-degrading enzymes, extended the lifespan of C. elegans through activation of DAF-16. TORC2/SGK-1, but not IIS-associated kinases, were involved in this lifespan regulation. Interestingly, we found that Δhns E. coli/host DAF-16 activated a transcriptional program that differed from that of IIS. Moreover, MG also enhanced the phosphorylation of hSGK1 and accelerated cellular senescence in human dermal fibroblasts (HDFs), suggesting its conserved role across species. Our findings demonstrate that the bacteria-derived metabolite MG modulates host longevity, acting through the TORC2/SGK-1/DAF-16 pathway.
Results
Interspecies Genetic Regulation of Longevity.
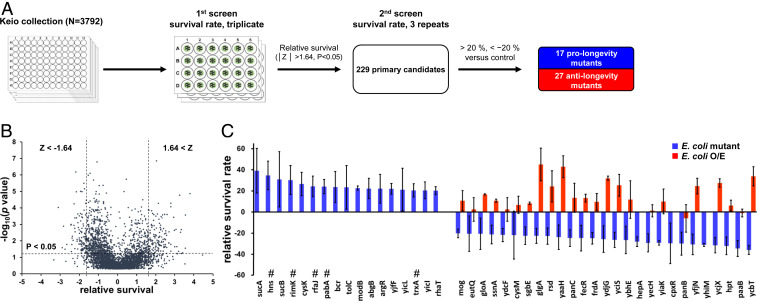
To systemically study the role of gut microbes in the regulation of host longevity, we performed genome-wide survival screens using C. elegans grown on each of the 3,792 E. coli K12 single gene mutants from the Keio Collection (24) (Fig. 1A). The total standard-normalized survivals of C. elegans in each plate were plotted against the P values (Fig. 1B). Primarily, 229 E. coli mutants that potentially enhanced or reduced C. elegans survival were selected (/Z/ > 1.64; P < 0.05). Standard normalization led to false-positive results, particularly where standard deviations were small. Therefore, to select true-positive candidates, secondary survival screens were performed with three biological replicates, and the survival rates were compared with those on control E. coli K12 strains. Finally, we isolated 17 prolongevity E. coli mutants and 27 anti-longevity E. coli mutants that resulted in alterations in the survival rate by ± ≥20% (Fig. 1C and SI Appendix, Table S1).
Fig. 1.
Genome-wide lifespan screen. (A) Schematic of the genome-wide lifespan screens. (B) A total of 3,792 relative survival rates were standard-normalized and plotted against the P values (|Z|> 1.64, P < 0.05, where Z = 1.64 indicates ∼95% of the normal distribution). (C) The genetic background of the E. coli affects the longevity of C. elegans. Error bars represent SD. #Indicates prolongevity mutations that were also isolated in independent screens (15, 49).
Overexpression of genes that shorten the lifespan when mutated have been reported to extend the lifespan in a single organism context (25, 26). Therefore, we aimed to determine whether this also applies in an interspecies context. We generated 24 different E. coli mutants overexpressing a gene associated with anti-longevity when mutated. Interestingly, these bacteria generally enhanced the survival of C. elegans, with survival on eight of these bacteria >20% greater than that on control (Fig. 1C and SI Appendix, Table S1). Taken together, these findings suggest that the genetic background of gut microbes is a bona fide regulator of host longevity.
Δhns E. coli Mutants Promote Longevity in C. elegans through Activation of DAF-16.
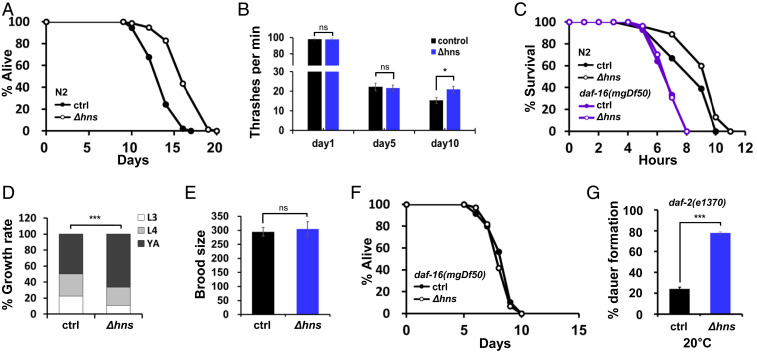
Of the 17 prolongevity mutations isolated, the E. coli hns gene, encoding H-NS, is of particular interest. H-NS is a transcriptional regulator that is highly conserved in enterobacteria (27). It regulates ∼5% of the entire E. coli genome that is involved in metabolism, stress response, and bacterial surface protein synthesis (28, 29). Consistent with our screening data, Δhns E. coli significantly extended the lifespan of C. elegans compared with the E. coli BW25113 parental strain (Fig. 2A and SI Appendix, Table S2). Both Δhns E. coli and BW25113 are of the E. coli K12 background; hereinafter, they are referred to as Δhns and control, respectively, unless noted otherwise. In addition, C. elegans grown on Δhns maintained healthy conditions with aging, as determined using movement and heat stress resistance assays (Fig. 2 B and C and SI Appendix, Table S2). This was not likely due to dietary restriction, as C. elegans grew faster and reproduction capacity was unaffected on Δhns (Fig. 2 D and E and SI Appendix, Table S2). These data indicate that Δhns E. coli not only extended the lifespan, but also improved the health span of C. elegans.
Fig. 2.
Δhns E. coli promotes longevity in C. elegans in a daf-16–dependent manner. (A) Δhns E. coli mutants extended the lifespan of wild-type N2 worms. Lifespans were determined at least in three experiments unless noted otherwise; results from a single representative experiment are shown. All lifespans were assayed at 25 °C unless noted otherwise. Δhns E. coli were from the E. coli Keio Collection K12 mutant library. E. coli K12 BW25113 parental strains were used as the wild-type control unless noted otherwise. (B) The thrashing rate was enhanced by Δhns E. coli on day 10 compared with control. *P < 0.05, Student’s t test. Error bars indicate SEM. Results from one of two independent experiments are shown. (C) Δhns E. coli mutants enhanced the thermotolerance of C. elegans in a daf-16–dependent manner. P < 0.05, Student’s t test. Results from one of three independent experiments are shown. (D) Growth rates of N2 on the indicated E. coli strains. C. elegans grew faster on Δhns than on control E. coli. ***P < 0.001, χ2 test. Results from one of three independent experiments are shown. (E) Brood size was not significantly impacted by Δhns E. coli. P > 0.05, Student’s t test. Results from one of three independent experiments are shown. (F) A daf-16 null mutation completely abolished lifespan extension by Δhns. (G) Dauer formation on indicated E. coli. The daf-2(e1370) mutants formed no dauers at 15 °C and 100% dauers at 25 °C, irrespective of the E. coli strain used. At the permissive temperature of 20 °C, Δhns E. coli significantly enhanced dauer formation. ***P < 0.001, Student’s t test. Results from one of three independent experiments are shown.
Next, to gain insight into its prolongevity mechanisms, we aimed to determine whether Δhns extended the lifespan through well-known longevity pathways. To this end, we used the following C. elegans mutants: IIS, daf-16(mgDf50); TOR, raga-1(ok386), rsks-1(ok1255); DR, eat-2(ad465), sir-2.1(ok434), aak-2(ok524); immunity, sek-1(km4), pmk-1(km25), tol-1(nr2013); mitochondria, isp-1(qm150), nuo-6(qm200), atfs-1(gk3094), drp-1(tm1108); and stress response, skn-1(zu135), hsf-1(sy441), jnk-1(gk7) (Fig. 2F and SI Appendix, Fig. S1 A–J and Table S3). Interestingly, the lifespan extension by Δhns was completely abolished only in the daf-16(mgDf50) mutants (Fig. 2F), suggesting that Δhns E. coli activated C. elegans DAF-16 to extend its lifespan.
In adverse environments, C. elegans enters the dauer diapause, which is dependent on DAF-16 (30). Therefore, to confirm that Δhns E. coli activates DAF-16, dauer formation was assessed in IIS-compromised daf-2(e1370) mutants. The daf-2(e1370) mutants grown on Δhns formed ∼78% dauers at a semipermissive temperature of 20 °C, compared with ∼24% dauers when grown on control E. coli (P < 0.0001; Fig. 2G and SI Appendix, Table S2). Moreover, the enhanced heat resistance by Δhns E. coli was also daf-16–dependent (Fig. 2C). Taken together, these data suggest that Δhns E. coli activates C. elegans DAF-16 to enhance longevity, dauer formation, and stress resistance.
Δhns E. coli Extends the Lifespan in a DAF-16– and UPRmt-Dependent Manner.
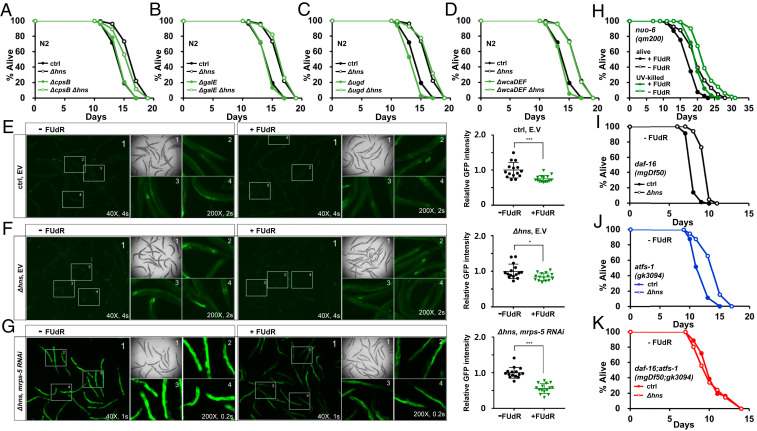
Five E. coli mutants—Δhns, ΔhyfR, ΔuidC, Δlon, and ΔsapD—have been previously reported to produce high levels of the extracellular polysaccharide CA and extend the lifespan of C. elegans, through activation of the mitochondrial longevity pathway (15). Notably, unlike the other four mutants, the contribution of CA to the lifespan extension by Δhns E. coli was only partial (15). This suggests that Δhns E. coli extends the lifespan via both CA-dependent and CA-independent mechanisms. To confirm that Δhns E. coli mutants can prolong the longevity of C. elegans in a CA-independent manner, we knocked out the cpsB, galE, and ugd genes, which encode the biosynthetic enzymes for the CA precursors GDP-fucose, UDP-galactose, and UDP-glucuronic acid, respectively, in the Δhns mutant background (31) (SI Appendix, Fig. S2A). As expected, none of these mutations led to complete suppression of lifespan extension by Δhns E. coli mutants (Fig. 3 A–C and SI Appendix, Table S4). We further introduced mutations in the CA biosynthetic enzymes in the Δhns mutant background, including mutations in wcaDEF, which encode the CA repeating unit polymerase, glycosyl transferase, and acetyltransferase (32). Although the ΔwcaD mutation was shown to completely suppress lifespan extension by Δlon E. coli mutants (15), the Δhns;ΔwcaDEF quadruple E. coli mutant extended the lifespan similarly to the Δhns E. coli mutant (Fig. 3D and SI Appendix, Table S4). In contrast to the lack of lifespan extension by CA in C. elegans mitochondrial longevity mutants such as nuo-6(qm200), isp-1(qm150), drp-1(tm1108), and atfs-1(gk3094) (15), we found that Δhns E. coli mutants extended the lifespan of all of the mitochondrial longevity mutants tested, including the nuo-6(qm200), isp-1(qm150), drp-1(tm1108), and atfs-1(gk3094) mutants (SI Appendix, Fig. S1 F and G and Table S3). Therefore, our data clearly demonstrate that Δhns E. coli mutants are able to extend the lifespan of C. elegans via E. coli CA/C. elegans UPRmt-independent mechanisms.
Fig. 3.
Δhns E. coli mutants activate both DAF-16 and UPRmt in C. elegans. (A–D) Mutations leading to deficiencies in CA synthesis did not fully suppress the lifespan extension by Δhns E. coli mutants. The results shown are from one of three independent experiments. (E–G) FUdR reduced the GFP intensity induced by UPRmt. The expression of the hsp-6p::gfp, UPRmt reporter was compared with that of the non–FUdR-treated control. Δhns E. coli synergistically enhanced hsp-6p::gfp expression with mrps-5 RNAi, a mild UPRmt inducer (G). GFP intensity was quantified using ImageJ. *P < 0.05; ***P < 0.001, Student’s t test. Ctrl and Δhns represent HT115 and Δhns HT115 E. coli, respectively. One of three independent experiments is shown. (H) FUdR reduced the lifespan of long-lived nuo-6 (qm200) mutants. This suggests that FUdR acts directly on the mitochondrial longevity pathway in C. elegans, rather than affecting E. coli physiology. One of two independent experiments is shown. (I–K) Lifespan assays of daf-16(mgDf50) (I), atfs-1(gk3094) (J), and daf-16(mgDf50); atfs-1(gk3094) mutants (K) were performed in the absence of FUdR. Neither daf-16(mgDf50) nor atfs-1(gk3094) mutants suppressed the lifespan extension by Δhns E. coli mutants, while the daf-16(mgDf50); atfs-1(gk3094) double mutation fully suppressed lifespan extension. One of at least two independent experiments is shown.
In contrast to our data showing that DAF-16 is required for lifespan extension by Δhns E. coli mutants, Han et al. (15) reported that Δhns E. coli mutants could increase the lifespan of daf-16 mutants, although this effect appeared to be less pronounced compared with wild-type N2. Because in that study the lifespan assays were performed without FUdR, an inhibitor of DNA synthesis, we asked whether FUdR might suppress the activation of UPRmt by Δhns mutants, thereby resulting in no lifespan extension in daf-16(mgDf50) mutants (Fig. 2F). To our surprise, FUdR treatment reduced the expression of hsp-6p::gfp, a UPRmt reporter, on wild-type E. coli and Δhns mutants compared with non-FUdR-treated controls (Fig. 3 E and F, Right). Because CA has been reported to increase the expression of hsp-6::gfp (15), Δhns E. coli mutants synergistically enhanced hsp-6p::gfp expression in combination with the mild UPRmt inducer mrps-5 RNAi (33) (Fig. 3 G, Left). Again, FUdR significantly reduced hsp-6p::gfp expression on Δhns HT115 E. coli harboring mrps-5 RNAi (Fig. 3G, Right). These results suggest that FUdR, at least in part, suppresses UPRmt.
Next, we asked whether FUdR might affect the lifespan of the long-lived mitochondrial mutants. As expected, FUdR significantly reduced the lifespan of the long-lived mitochondrial mutants, such as nuo-6(qm200) and isp-1(qm150), without affecting the lifespan of the N2 wild-type worms (Fig. 3H and SI Appendix, Fig. S2 B–D). Of note, FUdR was unable to fully suppress longevity of the mitochondrial mutants, as FUdR-treated nuo-6 (qm200) mutants still lived longer than the N2 control. To exclude the possibility that FUdR might affect E. coli physiology and in turn reduce the lifespan of the C. elegans mitochondrial mutants, we measured the lifespan of nuo-6 (qm200) mutants on UV-killed E. coli with or without FUdR. We found that FUdR reduced the lifespan of nuo-6 (qm200) mutants on both live and UV-killed E. coli to a similar extent (Fig. 3H and SI Appendix, Table S4). These data suggest that FUdR, at least in part, inhibits the C. elegans mitochondrial longevity pathways, such as UPRmt, resulting in a reduction in the lifespan of long-lived mitochondrial mutants.
Accordingly, without FUdR, we found that Δhns E. coli mutants extended the lifespan of daf-16(mgDf50) mutants, likely due to intact UPRmt activation (Fig. 3I and SI Appendix, Table S4). In addition, under the same conditions as in the previous study (15), we found that Δhns E. coli mutants extended the lifespan of mitochondrial mutants, such as nuo-6(qm200) and atfs-1(gk3094), with no FUdR at 20 °C (SI Appendix, Fig. S2 E and F). Finally, to confirm that Δhns E. coli mutants activated both DAF-16 and UPRmt in parallel to prolong longevity, we generated daf-16(mgDf50); atfs-1(gk3094) mutants in which both DAF-16 and UPRmt were impaired. Whereas the Δhns E. coli mutants extended the lifespan of daf-16(mgDf50) mutants and atfs-1(gk3094) mutants (Fig. 3 I and J and SI Appendix, Table S4), the lifespan extension by the Δhns mutation was completely abolished in daf-16(mgDf50); atfs-1(gk3094) double mutants in the absence of FUdR (Fig. 3K and SI Appendix, Table S4). Taken together, these data suggest that Δhns mutants modulate the lifespan via CA/UPRmt-dependent mechanisms as well as CA-independent/DAF-16-dependent mechanisms (SI Appendix, Fig. S2G).
Δhns E. coli Activates DAF-16 through TORC2/SGK-1.
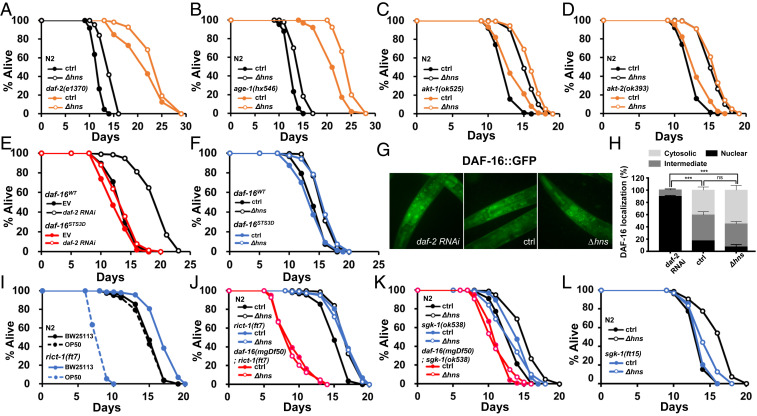
To identify the molecular mechanisms underlying Δhns E. coli-mediated activation of DAF-16 in C. elegans, we first aimed to determine whether the IIS pathway was involved. We measured the lifespan of IIS component mutants or by RNAi, including daf-2(e1370), pdk-1(RNAi), age-1(hx546), akt-1(ok525), and akt-2(ok393). RNAi was used to knock down pdk-1. Since AKT-1 and AKT-2 played redundant functions (9), akt-2 was knocked down in akt-1(ok525) mutants using RNAi. Surprisingly, Δhns further increased the lifespan of all tested IIS-compromised C. elegans (Fig. 4 A–D and SI Appendix, Fig. S3 A and B and Table S5), demonstrating that IIS was not involved. IIS has been shown to inhibit DAF-16 function through phosphorylation of three consensus sites (RXRXXS/T) (34). To further confirm that Δhns E. coli modulates DAF-16 activity independently of IIS, DAF-16STS3D transgenic C. elegans was generated, in which all three Ser/Thr were mutated to Asp. The DAF-16d/f isoform was used, as this isoform is the most abundant isoform in the intestine, the major tissue in lifespan regulation (26, 35, 36). As expected, lifespan extension by daf-2 RNAi was significantly impaired in daf-16(mgDf50); DAF-16STS3D worms compared with daf-16(mgDf50); DAF-16WT animals (Fig. 4E and SI Appendix, Table S5). Surprisingly, Δhns still prolonged the longevity of daf-16(mgDf50); daf-16STS3D animals compared with controls (Fig. 4F and SI Appendix, Table S5). Furthermore, in contrast to the enhanced nuclear translocation of DAF-16 by daf-2 RNAi, Δhns E. coli did not induce DAF-16 nuclear translocation (Fig. 4 G and H). These findings suggest that Δhns E. coli enhances DAF-16 transcriptional activity in the nucleus, rather than affecting its nuclear translocation. Notably, the known regulators that activate DAF-16, including AAK-2, JNK-1, and SMK-1 (37–39), were dispensable for Δhns-mediated lifespan extension (SI Appendix, Fig. S1 D, J, and K and Table S3), suggesting that a different signaling pathway was involved.
Fig. 4.
Δhns E. coli modulate longevity through TORC2/SGK-1. (A–D) IIS is not involved in lifespan regulation in Δhns E. coli. Δhns E. coli significantly extended lifespan in daf-2(e1370) (A), age-1(hx546) (B), akt-1(ok525) (C), and akt-2(ok393) (D) mutants. (E and F) The daf-16STS3D transgene did not rescue the lifespan extension by daf-2 RNAi (E) but fully rescued the lifespan extension by Δhns E. coli (F). (G and H) Compared with control, nuclear localization of DAF-16 was enhanced by daf-2 RNAi (***P < 0.001, Fisher’s exact test; n = 23), but not by Δhns E. coli (P = 0.6151, Fisher’s exact test; n = 23). Error bars indicate SEM. Results from one of three independent experiments are shown. (I) Lifespan of C. elegans on standard E. coli B strain OP50 and E. coli K12 strain BW25113. C. elegans rict-1(ft7) mutants lived significantly longer on the BW25113 strain compared with OP50. Wild-type N2 exhibited comparable lifespans irrespective of E. coli strain. (J and K) TORC2/SGK-1 is critical for lifespan modulation by Δhns E. coli. Δhns E. coli did not further extend the lifespan of rict-1(ft7) (J) or sgk-1(ok538) (K) mutants. daf-16(mgDf50) suppressed the long lifespan of rict-1(ft7) (J) or sgk-1(ok538) mutants (K). (L) Lifespan extension was attenuated in sgk-1 gain-of-function mutant sgk-1(ft15).
We next asked whether TORC2, also known as PDK2, was involved in this lifespan regulation. RICT-1 is an essential component of TORC2 (40). As reported previously (36, 41), rict-1(ft7) mutants had a shorter lifespan than wild-type N2 worms on E. coli B strain OP50, whereas E. coli K12 strain significantly extended the rict-1(ft7) lifespan compared with N2 (Fig. 4I and SI Appendix, Table S5). Of note, the lifespans of N2 were comparable in the two different E. coli strains (Fig. 4I). Strikingly, Δhns E. coli did not further extend the lifespan of rict-1(ft7) mutants compared with control, and the long lifespans of rict-1(ft7) were dependent on DAF-16 (Fig. 4J and SI Appendix, Table S5). These data suggest that DAF-16 is already activated in rict-1(ft7) mutants, leading to no further lifespan extension by Δhns E. coli.
TORC2 is known to phosphorylate the serine on the hydrophobic motifs (HMs) of AKT-1, AKT-2, and SGK-1 (7, 36, 41). Since AKTs were not implicated in the lifespan extension by Δhns, we asked whether SGK-1 could act downstream of TORC2 in response to Δhns. Similar to rict-1(ft7) mutants, sgk-1(ok538) mutants lived longer than N2 animals on control E. coli K12, but the lifespan of sgk-1(ok538) mutants was not further extended by Δhns (Fig. 4K and SI Appendix, Table S5). In addition, similar to rict-1(ft7) mutants, daf-16(mgDf50) suppressed the lifespan extension by sgk-1(ok538) (Fig. 4K and SI Appendix, Table S5).
To further confirm the involvement of TORC2/SGK-1 in the lifespan extension by Δhns E. coli, we asked whether Δhns could modulate sgk-1 gain-of-function mutant sgk-1(ft15). Consistent with mutant lifespan data, the lifespan modulation by Δhns E. coli was significantly attenuated in the sgk-1(ft15) background (Fig. 4L and SI Appendix, Table S5). Taken together, these findings demonstrate that Δhns E. coli extends the lifespan of C. elegans through TORC2/SGK-1 signaling rather than by IIS.
The Bacteria-Derived Metabolite MG Modulates Host Longevity.
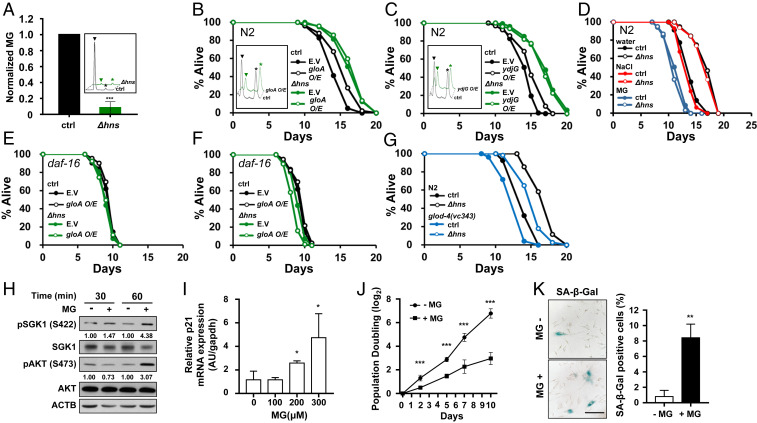
MG is a ubiquitous metabolite produced from bacteria to humans (18). Previous studies have shown that MG activates TORC2 (23), which is involved in lifespan modulation by Δhns E. coli. In addition, our screens identified two E. coli mutants of MG degrading enzymes as antilongevity mutants, gloA and ydjG, encoding glyoxalase I and methylglyoxal reductase, respectively (Fig. 1C). Thus, we hypothesized that MG levels might be lower in Δhns, which could inhibit TORC2/SGK-1, activate DAF-16, and thereby extend the lifespan in C. elegans. To test this, MG levels were measured in Δhns using high-performance liquid chromatography (HPLC). Surprisingly, MG was barely detected in Δhns E. coli (Fig. 5A). To further confirm that bacteria-derived MG could modulate host longevity, we generated GloA- or YdjG-overexpressing E. coli. As expected, these bacteria produced less MG and extended the lifespan of C. elegans compared with control E. coli harboring control vector (Fig. 5 B and C, black lines and SI Appendix, Table S6).
Fig. 5.
Bacteria-derived metabolite MG modulates host longevity. (A) MG was measured using HPLC. MG was barely detected in Δhns E. coli. Error bars indicate SEM from three independent experiments. Inside the box, black represents control E. coli and green represents Δhns. ▼ indicates MG; * indicates the internal standard, 5-methylquinoxaline (5-MQ). (B and C) Overexpression of MG-degrading enzymes gloA (B) or ydjG (C) extended the C. elegans lifespan in wild-type control E. coli but not in Δhns E. coli. Inside the box, black represents control and green represents overexpression of E. coli. MG level was reduced by 25% on overexpression of gloA (B) and by 51% on overexpression of ydjG (C). One of two independent assays is shown. (D) MG supplementation (20 mM) abolished the lifespan extension by Δhns E. coli. NaCl (20 mM) was used as an equivalent osmolarity control in the lifespan assays. One of three independent lifespan assays is shown. (E and F) The daf-16(mgDf50) mutation abolished the lifespan extension by the overexpression of gloA (E) and ydjG (F). (G) The Δhns mutation did not fully restore the shortened lifespan of glod-4(gk189) mutants (blue open circle) to that of N2 (black open circle). (H) MG enhanced the phosphorylation of SGK1 and AKT at HM. Immunoblot analysis was performed to detect the indicated proteins in HDF cells treated with (+) or without (−) MG (300 μM). The pSGK1/SGK1 and pAKT/AKT ratios are presented to allow for quantitative comparisons. Expression levels were quantified using ImageJ software with ACTB as a loading control. (I) Relative mRNA expression of p21 in HDF cells treated with MG (100 to 300 μM). p21 expression was normalized to expression of Gapdh. (J) The average rate of population doubling was determined by plating 5 × 105 cells in duplicate and counting the number of HDF cells every 3–4 d. (K, Left) Representative differential interference contrast images of HDF cells stained for SA-β-Gal. (K, Right) quantification of SA-β-Gal–positive cells. Results represent the average of three independent staining experiments. HDF cells were treated with (+) or without (−) MG (300 μM). (Scale bar: 200 μm.) *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test.
We next aimed to determine whether the effects on lifespan by Δhns and MG degrading enzymes are additive. Compared with control, GloA or YdjG overexpression in the Δhns background did not further extend the lifespan of C. elegans (Fig. 5 B and C, green lines and SI Appendix, Table S6), demonstrating that the Δhns mutation and MG degrading enzymes acted via a common mechanism. Finally, we found that the lifespan extension by Δhns was completely suppressed by MG supplementation compared with an equivalent osmolarity control (Fig. 5D). Taken together, these data show that bacteria-derived MG is critical for lifespan modulation by Δhns E. coli.
The fact that MG modulates lifespan through regulation of the host’s signaling pathways appears to be contradictory to the general idea that MG induces nonspecific damage to biomolecules (20). As with Δhns E. coli, overexpression of GloA or YdjG in E. coli did not extend the lifespan in daf-16(mgDf50) mutants (Fig. 5 E and F and SI Appendix, Table S6). Moreover, high MG-producing bacteria, such as ΔgloA and ΔydjG mutants (42), shortened the lifespan of N2 worms (Fig. 1C and SI Appendix, Fig. S4 A and B and Table S6), but not of daf-16(mgDf50) mutants (SI Appendix, Fig. S4 C and D and Table S6). These findings further support the hypothesis that bacteria-derived MG modulates host longevity mainly through the TORC2/DAF-16 pathway rather than through induction of nonspecific damage to biomolecules.
Recent reports have suggested that C. elegans glyoxalase mutant, glod-4, has reduced lifespan (43, 44). This suggests that protection against MG is critical for the normal aging processes. However, no previous studies have investigated the role of MG from different sources, that is, MG generated in the host vs. MG derived from gut bacteria. To assess the role of bacteria-derived MG on the modulation of host longevity, we grew glod-4(gk189) mutants on Δhns E. coli, which had very low levels of MG production. If bacteria-derived MG were more critical for lifespan reduction than MG generated in the host, we would expect that Δhns E. coli could recover the short lifespan of glod-4(gk189) mutants close to that of N2. However, glod-4(gk189) still exhibited reduced longevity compared with N2 on Δhns E. coli (Fig. 5G and SI Appendix, Table S6). These findings suggest that both bacterial MG and endogenous MG modulate host longevity.
Recent reports have suggested that endogenous MG activates SKN-1/NRF1 through TRPA-1 (44). However, we found that Δhns extended the lifespan of trpa-1(ok999) mutants to a similar extent as wild-type N2 (SI Appendix, Fig. S1L and Table S3), showing that TRPA-1 was not involved in lifespan modulation by bacteria-derived MG. Taken together, these data demonstrate that bacteria-derived MG modulates host longevity through regulation of DAF-16 in C. elegans.
MG Induces Cellular Senescence in HDF Cells.
As human gut microbes produce large amounts of MG (19), we aimed to determine whether MG can modulate the aging process in mammalian cells, using HDFs. MG has been reported to phosphorylate AKT at the HM through TORC2 (23), but whether SGK1 phosphorylation is also induced by MG is not known. Since SGK-1 is involved in lifespan regulation by bacterial MG in C. elegans, we first asked whether MG phosphorylates SGK1 in HDF cells. Remarkably, MG enhanced the phosphorylation of both AKT and SGK1 at their HMs (Fig. 5H), suggesting that MG activated both AKT and SGK1 and in turn could inhibit FOXO in mammalian cells. MG also induced expression of a senescence-related gene, p21, in a dose-dependent manner (Fig. 5I). Cellular senescence was consistently induced on MG treatment, as shown by reduced cell proliferation (Fig. 5J) and the high levels of senescence-associated β-galactosidase in HDF cells (Fig. 5K). Given that inhibition of FOXO has been shown to accelerate cellular senescence (45), our data suggest that MG modulates the aging processes via conserved pathways across species.
Δhns E. coli Mutants Regulate the Novel Class 3 DAF-16 Targets.
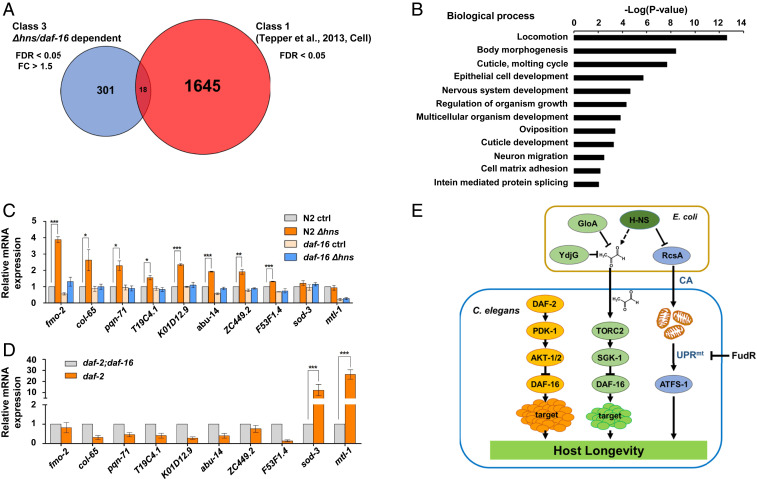
DAF-16/FOXO is the major transcription factor regulating hundreds target genes downstream of IIS (46). As Δhns E. coli activated DAF-16 through TORC2/SGK-1 rather than IIS, we asked whether the respective downstream targets overlapped or were distinct. Using RNA-seq analysis of N2 control and daf-16(mgDf50) mutants grown on control E. coli or Δhns mutants, we identified 319 C. elegans genes that were up-regulated by Δhns E. coli in a DAF-16–dependent manner (fold change >1.5; false discovery rate [FDR] <0.05) (Fig. 6A and Dataset S1).
Fig. 6.
Δhns E. coli regulate novel DAF-16 targets. (A) Venn diagram representing the number of DAF-16 targets that are differentially regulated by Δhns E. coli and IIS. The red circle indicates DAF-16 target genes that are up-regulated by IIS (FDR <0.05); the blue circle shows DAF-16 target genes up-regulated by Δhns E. coli (FDR <0.05, fold change >1.5). A complete list of genes is presented in Dataset S1. (B) Gene Ontology analysis of 301 DAF-16 target genes that are up-regulated by Δhns E. coli. (C and D) mRNA levels of DAF-16 targets quantified by qRT-PCR. Data are from three independent experiments. Error bars represent SEM. Δhns E. coli mutants significantly increased mRNA levels of fmo-2, col-65, pqn-71, T19C4.1, K01D12.9, abu-14, ZC449.2, and F53F1.4, while no significant changes were observed for sod-3 and mtl-1 mRNA. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test (C). Compromised IIS did not increase mRNA levels of fmo-2, col-65, pqn-71, T19C4.1, K01D12.9, abu-14, ZC449.2, and F53F1.4, but significantly increased mRNA levels of sod-3 and mtl-1. ***P < 0.001, Student’s t test (D). (E) Proposed model for interspecies genetic regulation of longevity by bacteria-derived MG. Yellow represents the canonical IIS pathway regulating DAF-16; green, MG-mediated regulation of DAF-16; blue, UPRmt activation by CA.
DAF-16 targets have been classified into two classes: daf-2(−)-induced class 1 and daf-2(−)-repressed class 2 (46, 47). To our surprise, the majority of our targets (>94%) were distinct from known class 1 targets (46) (Fig. 6A). Gene Ontology analyses revealed that our targets were enriched in genes associated with locomotion, body morphogenesis, and molting cycle and were depleted for previously identified aging-associated genes (Fig. 6B). These findings suggest that Δhns E. coli activate the novel DAF-16 targets to modulate longevity, hereinafter referred to as class 3 targets. A large number of genes (class 2) have been previously reported to be down-regulated by the daf-2 mutation (46); however, only a few genes were down-regulated in our analysis (10 genes; fold change >1.5, FDR <0.05; Dataset S1). This suggests that Δhns/DAF-16 activates mainly target genes, while IIS/DAF-16 regulates targets in both positive and negative ways. In addition, lifespan extension by Δhns was independent of PQM-1 (SI Appendix, Fig. S5A and Table S7), a transcription factor responsible for class 2 DAF-16 target regulation (46).
To confirm the data obtained via RNA-seq, mRNA levels were examined for eight of the class 3 DAF-16 targets using qRT-PCR. All eight class 3 DAF-16 targets were up-regulated by Δhns E. coli in a daf-16–dependent manner (Fig. 6C); however, these targets were not up-regulated in IIS-compromised daf-2(e1370) mutants (Fig. 6D). Conversely, the class 1 targets sod-3 and mtl-1 were up-regulated only in the daf-2(e1370) background, and not by Δhns E. coli (Fig. 6 C and D). Remarkably, MG supplementation abolished the Δhns-induced up-regulation of these class 3 genes. These findings support the hypothesis that Δhns E. coli modulate longevity through MG (SI Appendix, Fig. S6A).
Finally, we asked whether the expression of these new DAF-16 targets could be regulated by TORC2/SGK-1 signaling. Consistent with their requirement for lifespan regulation by Δhns E. coli, the class 3 target genes were significantly activated in rict-1(ft7) and sgk-1(ok538) mutants (SI Appendix, Fig. S6 B and C), further supporting our model (Fig. 6E). One of these eight targets, fmo-2, when overexpressed, has been previously shown to increase the lifespan of C. elegans (48). However, we found that Δhns E. coli still prolonged the longevity of fmo-2(ok2147) mutants (SI Appendix, Fig. S5B and Table S7). The remaining seven target genes were knocked down by RNAi, but no single RNAi suppressed lifespan extension by Δhns E. coli (SI Appendix, Fig. S5 C–F and Table S7). It is possible that multiple genes may act in a cumulative manner to extend the lifespan. Taken altogether, our results suggest that Δhns E. coli activates the novel class 3 DAF-16 targets to prolong the longevity of C. elegans in an interspecies context (Fig. 6E).
Discussion
In this study, genome-wide lifespan screens identified 44 E. coli mutants that regulate the lifespan of C. elegans. Through extensive interspecies genetic analysis, we demonstrated that low-MG-producing E. coli mutants, Δhns E. coli, extends host longevity in a RICT-1/SGK-1/DAF-16–dependent manner. Once activated by Δhns E. coli, DAF-16 up-regulates the novel class 3 target genes, which are distinct from previously identified class 1 and 2 targets. Interestingly, chromatin immunoprecipitation data showed that DAF-16 bound to the <1-kb upstream region in seven of eight of the class 3 targets, as confirmed by qRT-PCR (https://wormbase.org//#012-34-5). This strongly suggests that DAF-16 regulates the distinct sets of target genes in response to different upstream signals, such as IIS and bacteria-derived MG.
Two genome-wide screens were recently reported using C. elegans grown on E. coli Keio Collection mutants. One screen was performed to assess prolongevity mutants (15), and the other was performed to identify dauer-enhancing mutants (49). Common and distinct prolongevity mutants were isolated from the three independent screens, including those two previously conducted screens and the screen performed in the present study (Fig. 1C and SI Appendix, Fig. S7A).
Han et al. (15) reported that five E. coli mutants producing high levels of CA, including Δhns E. coli, extended the lifespan of C. elegans through activation of the mitochondrial longevity pathway. Unlike the other four E. coli mutants, which directly control CA production through the RcsA transcriptional regulator (Fig. 6E), lifespan extension by Δhns E. coli is not completely dependent on CA production (15). Consistent with these findings, we found that mutations in the CA biosynthetic pathway did not fully suppress the lifespan extension induced by Δhns E. coli. We also found that FUdR, which was normally used to prevent progeny in C. elegans lifespan assays, suppressed Δhns-induced UPRmt activation. Of note, FUdR did not affect Δhns E. coli-induced up-regulation of class 3 DAF-16 targets (SI Appendix, Fig. S7B). This allowed us to identify the bacterial CA/host UPRmt-independent mechanisms required for lifespan extension by the Δhns E. coli mutants. In experiments conducted without FUdR, we demonstrated that the lifespan extension by Δhns E. coli was completely abolished only in daf-16(mgDf50); atfs-1(gk3094) double mutants, in which both DAF-16 and UPRmt signaling were blocked, but not in daf-16(mgDf50) or atfs-1(gk3094) single-mutant backgrounds.
MG is a membrane-permeable metabolite (50) that is inevitably produced during metabolism in both bacteria and humans. MG induces the formation of AGEs, which are implicated in such human pathologies as diabetes and neurodegenerative diseases. Notably, our studies demonstrate that bacteria-derived MG modulates C. elegans longevity through the host signaling pathway rather than via nonspecific damages to biomolecules. Using HDF cells, we found that MG enhances the phosphorylation of SGK1 at the HM and accelerates cellular senescence. Interestingly, previous reports suggested that RICT-1 and SGK-1 regulated lifespan in a parallel pathway to DAF-2 for the regulation of the UPRmt (51). Although Δhns E. coli mutants modulate longevity via both the MG/RICT-1/SGK-1 pathway and the CA/UPRmt, it is of interest to identify whether these two seemingly parallel pathways share, if any, common factors. Further analysis is needed to identify the molecular mechanisms through which MG activates TORC2. Taken together, our data suggest that reducing the bacteria-derived MG levels may be a useful therapeutic intervention for MG-associated pathophysiology, such as diabetes, neurodegenerative disease, and aging.
Materials and Methods
More detailed information is provided in SI Appendix, Materials and Methods. The C. elegans strains were maintained at 20 °C using standard cultivation techniques unless noted otherwise. C. elegans strains were purchased from the Genetic Genome Center or were gifts from other laboratories.
Data Availability.
All data are included in the main text and SI Appendix. RNA-seq data have been deposited to the NCBI Gene Expression Omnibus (GEO) under accession no. GSE114160.
Supplementary Material
Acknowledgments
We thank Seung-Min Lee and Young Hoon Son for advice and critical comments on the manuscript and Choong-Min Ryu and Keith Blackwell for the E. coli and C. elegans strains. Some strains were kindly provided by the Caenorhabditis Genetics Center, which is funded by the NIH’s National Center for Research Resources. This research was granted by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Korean Government (Ministry of Science and ICT) (2013M3A9B6076434 and 2017M3A9D8048709) and the Korea Research Institute of Bioscience and Biotechnology Initiative Program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE114160).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915719117/-/DCSupplemental.
References
- 1.Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I., Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Maynard C. L., Elson C. O., Hatton R. D., Weaver C. T., Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagi E. et al., The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 165, 180–184 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Smith P. et al., Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife 6, e27014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenyon C. J., The genetics of ageing. Nature 464, 504–512 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Blüher M., Kahn B. B., Kahn C. R., Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M., Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Yen K., Narasimhan S. D., Tissenbaum H. A., DAF-16/Forkhead box O transcription factor: Many paths to a single fork(head) in the road. Antioxid. Redox Signal. 14, 623–634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paradis S., Ruvkun G., Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12, 2488–2498 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portal-Celhay C., Bradley E. R., Blaser M. J., Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 12, 49 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen T. P., Clarke C. F., Folate status of gut microbiome affects Caenorhabditis elegans lifespan. BMC Biol. 10, 66 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virk B. et al., Folate acts in E. coli to accelerate C. elegans aging independently of bacterial biosynthesis. Cell Rep. 14, 1611–1620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusarov I. et al., Bacterial nitric oxide extends the lifespan of C. elegans. Cell 152, 818–830 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Donato V. et al., Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signalling pathway. Nat. Commun. 8, 14332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han B. et al., Microbial genetic composition tunes host longevity. Cell 169, 1249–1262.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy M., Thaiss C. A., Elinav E., Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 30, 1589–1597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postler T. S., Ghosh S., Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 26, 110–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalapos M. P., Methylglyoxal in living organisms: Chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 110, 145–175 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Baskaran S., Rajan D. P., Balasubramanian K. A., Formation of methylglyoxal by bacteria isolated from human faeces. J. Med. Microbiol. 28, 211–215 (1989). [DOI] [PubMed] [Google Scholar]
- 20.Thornalley P. J., Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 27, 565–573 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Wautier J. L., Guillausseau P. J., Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab. 27, 535–542 (2001). [PubMed] [Google Scholar]
- 22.Münch G., Westcott B., Menini T., Gugliucci A., Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids 42, 1221–1236 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Nomura W., Inoue Y., Methylglyoxal activates the target of rapamycin complex 2-protein kinase C signaling pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 35, 1269–1280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba T. et al., Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin K., Hsin H., Libina N., Kenyon C., Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28, 139–145 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Kwon E. S., Narasimhan S. D., Yen K., Tissenbaum H. A., A new DAF-16 isoform regulates longevity. Nature 466, 498–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atlung T., Ingmer H., H-NS: A modulator of environmentally regulated gene expression. Mol. Microbiol. 24, 7–17 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Kline K. A., Fälker S., Dahlberg S., Normark S., Henriques-Normark B., Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5, 580–592 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Nell S., Suerbaum S., Josenhans C., The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat. Rev. Microbiol. 8, 564–577 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb S., Ruvkun G., daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137, 107–120 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland I. W., Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem. J. 115, 935–945 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson G., Andrianopoulos K., Hobbs M., Reeves P. R., Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccharide colanic acid. J. Bacteriol. 178, 4885–4893 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett C. F. et al., Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun. 5, 3483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rena G., Guo S., Cichy S. C., Unterman T. G., Cohen P., Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274, 17179–17183 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Bansal A. et al., Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longev. Healthspan 3, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soukas A. A., Kane E. A., Carr C. E., Melo J. A., Ruvkun G., Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 23, 496–511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greer E. L. et al., The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 282, 30107–30119 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Oh S. W. et al., JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. U.S.A. 102, 4494–4499 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff S. et al., SMK-1, an essential regulator of DAF-16-mediated longevity. Cell 124, 1039–1053 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov D. D. et al., Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Jones K. T., Greer E. R., Pearce D., Ashrafi K., Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 7, e60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subedi K. P., Choi D., Kim I., Min B., Park C., Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol. Microbiol. 81, 926–936 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Morcos M. et al., Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 7, 260–269 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Chaudhuri J. et al., A Caenorhabditis elegans model elucidates a conserved role for TRPA1-Nrf signaling in reactive α-Dicarbonyl detoxification. Curr. Biol. 26, 3014–3025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyoung Kim H. et al., Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 60, 4–9 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Tepper R. G. et al., PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154, 676–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy C. T. et al., Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Leiser S. F. et al., Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science 350, 1375–1378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khanna A. et al., A genome-wide screen of bacterial mutants that enhance dauer formation in C. elegans. Sci. Rep. 6, 38764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thornalley P. J., Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 254, 751–755 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gatsi R. et al., Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF, and mTORC2 in C. elegans. PLoS One 9, e107671 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the main text and SI Appendix. RNA-seq data have been deposited to the NCBI Gene Expression Omnibus (GEO) under accession no. GSE114160.