Significance
Excess nitrogen (N) in freshwaters is problematic due to its impacts on eutrophication, biodiversity losses, and harmful algal blooms. Some microbial processes such as denitrification and anammox can remove N from systems while others such as N-fixation can add a usable form of N. However, not enough is known about when and where these competing processes are occurring in lakes, and understanding this can provide insight to management. Here, we determined that nearly all of the 34 lakes that we examined in the upper Midwest had a net loss of N2 that must be compensated by watershed inputs to maintain steady state. These results suggest that N-fixation in these lakes was not enough to offset denitrification and anammox.
Keywords: denitrification, nitrogen fixation, nitrogen cycling, biogeochemistry
Abstract
Little is known about the exchange of gaseous nitrogen (N2) with the atmosphere in freshwater systems. Although the exchange of N2, driven by excess or deficiencies relative to saturation values, has little relevance to the atmospheric N2 pool due to its large size, it does play an important role in freshwater and marine nitrogen (N) cycling. N-fixation converts N2 to ammonia, which can be used by microbes and phytoplankton, while denitrification/anammox effectively removes it by converting oxidized, inorganic N to N2. We examined N2 saturation to infer net biological nitrogen processes in 34 lakes across 5° latitude varying in trophic status, mixing regime, and bathymetry. Here, we report that nearly all lakes examined in the upper Midwest (USA) were supersaturated with N2 (>85% of samples, n = 248), suggesting lakes are continuously releasing nitrogen to the atmosphere. The traditional paradigm is that freshwaters compensate for N-limitation through N-fixation, but these results indicate that lakes were constantly losing N to the atmosphere via denitrification and/or anammox, suggesting that terrestrial N inputs are needed to balance the internal N cycle.
Recent research has shown that freshwaters play a critical role in the global nutrient cycles via mineralization of terrigenous inputs (1, 2), burial of organic matter (3, 4), and the release of the three most significant greenhouse gases—CO2, CH4, and N2O—into the atmosphere before ultimate transfer to the oceans (5 –7). The magnitude and rates of these processes has led to the conclusion that freshwaters are biogeochemical hotspots in terms of their capacity to process carbon (C), nitrogen (N), and phosphorus (P) as water moves from terrestrial soils to the sea (8 –10). Lakes are particularly important along this journey as they enable longer biogeochemical processing of nutrients than rivers and streams due to their longer hydraulic residence times. There are 117 million lakes spanning the globe, covering 3.7% of the land surface, therefore, lakes can have important impacts on local, regional, and global cycling of nutrients (11).
Humans have altered global nutrient cycles through the use of fertilizers, which are increasing N and P availability by over 100% and 800%, respectively (12 –15). These nutrients can be found in aquatic ecosystems in excess and contribute to eutrophication, some manifestations of which are harmful algal blooms, water column anoxia, biodiversity loss, and seepage into subsurface wells, resulting in elevated nitrate in drinking water (12, 16). Work on the C cycle over the last 20+ years has demonstrated that dissolved CO2 and CH4 are typically supersaturated in freshwaters, leading to transfer of these important greenhouse gases to the atmosphere (6, 7, 17, 18). The freshwater N cycle, like the C cycle, has important atmospheric losses, but unlike the freshwater C cycle, the major form of N loss, N2, has no significance to climate. Nonetheless, some N is lost as N2O, which is a greenhouse gas ca. 300 times more potent than CO2 (19). Even so, it is important to quantify N losses to the atmosphere given that those pools of N are no longer available to support growth and eutrophication in the waters that they exit.
In this study, like previous work on C cycling in freshwater systems, we set out to determine if lakes are sources or sinks of N2 to the atmosphere to help understand net nitrogen processing. N2 saturation, like all atmospheric gases, is influenced by temperature, atmospheric mixing, and salinity (20). Biological processes within aquatic systems can alter concentrations of N2 in the water column, providing a flux that makes them either a net source or sink. Microbial processes that should increase N2 saturation relative to atmospheric equilibrium are denitrification and anammox. Denitrification is a dissimilative process performed by heterotrophic microbes, which converts oxidized inorganic N, often as nitrate (NO3 −) to N2 gas, ultimately removing N to the atmosphere, and potentially off-setting or exceeding the removal of N2 in the water column due to the microbial process of N-fixation (21). Anammox is the process of converting ammonium and nitrite into N2 gas performed by chemoautotrophic microbes in anoxic conditions (22). Anammox is not studied widely in freshwater systems and is considered to be a very small fraction of N2 production in most lakes (21, 23), but there are exceptions (24). Although both denitrification and anammox are anaerobic processes, they can occur in aerobic and microaerophilic regions as long as there are microzones of low dissolved oxygen concentrations (25, 26). The main process decreasing N2 saturation, potentially leading to undersaturation relative to atmospheric equilibrium, is N-fixation, as it consumes N2 and converts it into a bioavailable form of inorganic nitrogen, ammonia (NH3) (21, 22). In fact, this process is often invoked as one of the major reasons that N is not limiting in freshwaters (27 –29), but the extent to which it can actually relieve N-limitation has been questioned recently (30). In this study, we set out to quantify N2 saturation in order to help understand net nitrogen processing in lakes.
The goals of this research were to determine if lakes in the Upper Midwest are net sources or sinks of N2 gas and if there was water column or seasonal variation in N2 saturation based on a survey of lakes across Minnesota and northern Iowa. From these results, we were able to make informed predictions of net nitrogen processing and patterns in lakes. We hypothesized that lakes would be net sources of N2 gas, which would imply that lakes are constantly losing nitrogen to the atmosphere via microbial processing of N.
Methods
Site Description.
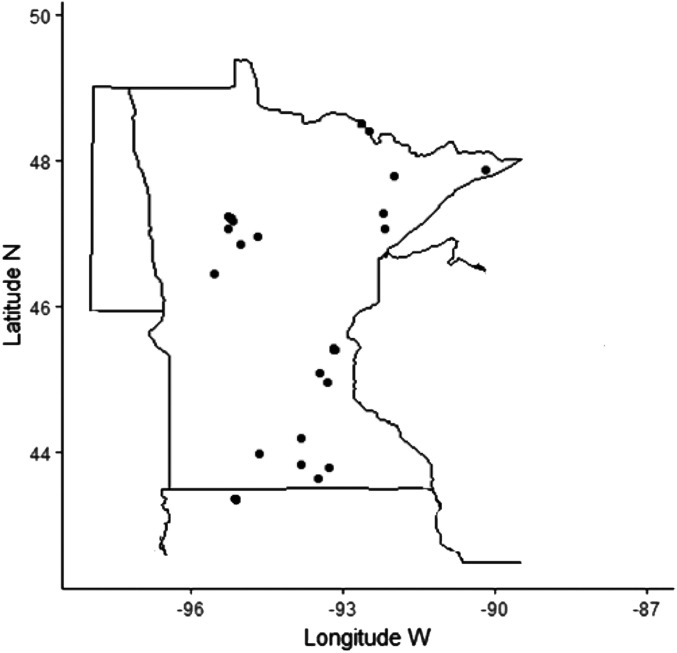
We sampled 34 lakes between October 2016 and September 2017 with 66 total lake visits (Fig. 1). The lakes sampled spanned five degrees latitude (43.36–48.50 °N), four ecoregions, various watershed land uses, diverse bathymetry with varying maximum depths, and trophic status ( SI Appendix, Table S1). The ecoregions, as outlined by Heiskary et al. (31), included the North Central Hardwood Forests, Northern Lakes and Forests, Western Corn Belt Plains, and Red River Valley. We sampled seven lakes in the North Central Hardwood Forests reaching across much of central Minnesota. The lakes in this ecoregion are characterized as eutrophic with most lakes exhibiting summer stratification. These lakes tend to have high levels of phosphorus and low water clarity. We sampled 17 lakes in the Northern Lakes and Forests Region, which covers north-central and northeastern Minnesota. This region is the most forested, and the lakes have the least amount of phosphorus compared to the other ecoregions (31). We sampled 10 lakes in the Western Corn Belt Plains spanning southern Minnesota and northern Iowa (32). The Western Corn Belt Plains are characterized by some of the highest cultivation rates among the ecoregions. The lakes in this region contain high levels of phosphorus and are usually shallow, resulting in mixing throughout the open water period (31). We sampled one lake in the Red River Valley, which covers the western edge of Minnesota from west-central to northern Minnesota. The lakes in the Red River Valley are set on the sediments of glacial Lake Agassiz, resulting in nutrient rich soils and peatlands. The origin of lakes in this area are glacial prairie potholes which are kettle drum-shaped lakes. This region has high levels of cultivation as well (31, 33).
Fig. 1.
Sampling sites from October 2016 to September 2017 represented by the black dots. Some lakes sampled were so close to one another that they may appear as a single dot.
Sample Collection.
Water column samples were taken using a Van Dorn bottle every 2–5 m of depth from 2 m below the surface to 1–2 m from the sediment. Sampling depths were determined based on maximum depth and stratification properties of the lake at the time of sampling. For stratified lakes, water was sampled at least once from each mixing layer, including epilimnion (surface mixed layer), metalimnion (temperature gradient boundary), hypolimnion (bottom layer), and monimolimnion (deep salinity gradient layer, when present). Unstratified lakes, as determined by temperature and oxygen profiles, were sampled every few meters to get an accurate dissolved gas profile. Water samples were collected into 6-mL, air-tight vials, which were filled from the bottom up allowing for 3 volumes to overflow and to minimize air mixing into the water sample. Vials were collected in triplicate at each depth and stored in a 4 °C cold, dark room to inhibit microbial metabolism until analyses, which were performed within 4 d. If samples were unable to be analyzed within 4 d, the water was treated with mercuric chloride to eliminate further microbial metabolism (34, 35). We examined the effects of two preservatives over a 72-h period and found no significant differences between preservation of N2 by HgCl2 (0.604) and ZnCl2 (P = 0.619) and unpreserved samples within our detection limits. Furthermore, cold preservation limited the consumption of O2 to 3–6 µg O2/L over the 4 d, a small enough change that most dissolved oxygen instruments would not be able to detect it (34, 35).
We used a membrane inlet mass spectrometer to analyze dissolved gas concentrations of O2, N2, and Ar, and also from these measurements, we quantified N2/Ar saturation (36). The water sample was drawn through a stainless-steel tube from the bottom of the vial to minimize air mixing with the sample. The water was then pumped through a stainless-steel tube in a water bath at the temperature the sample was collected. Then the water was pumped through a semipermeable microbore silicone membrane. Dissolved gas concentrations were determined using a quadrupole mass spectrometer, and oxygen interference was analyzed by comparing N2 saturation to dissolved oxygen in the samples ( SI Appendix, Fig. S1). Sample gas concentrations were compared to an air-equilibrated standard of deionized water at the same temperature the samples were being analyzed. We compared the N2/Ar ratio in our samples against the standard to determine saturation levels (36). N2/Ar ratios measured in the samples were compared to the N2/Ar ratio expected assuming complete mixing and Henry’s law of gas solubility (20, 36, 37). We used these ratios to give a calibrated N2/Ar signal in the samples using the following equation (36):
We then compared the calibrated N2:Ar in the sample to the N2:Ar expected to determine the saturation ratio using the following equation:
Kana et al. (36) found that normalizing N2 to Ar provided a more stable and accurate estimate of N2 saturation because Ar has similar mixing properties to N2 and is not involved in any biological processes. When we refer to N2 saturation hereafter, it is technically N2/Ar saturation. Samples >1.00 in N2:Ar saturation were considered supersaturated, while samples <1.00 were considered unsaturated.
Data Availability.
The data collected in this experiment are available through the Data Repository for the University of Minnesota (38).
Results and Discussion
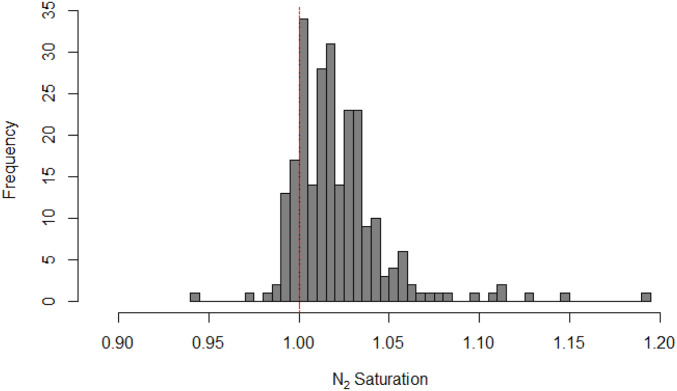
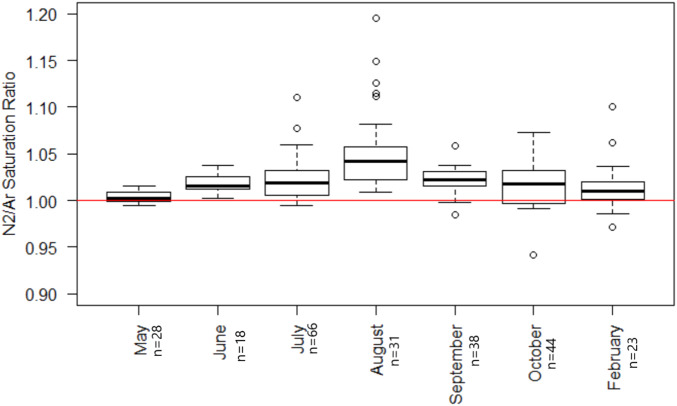
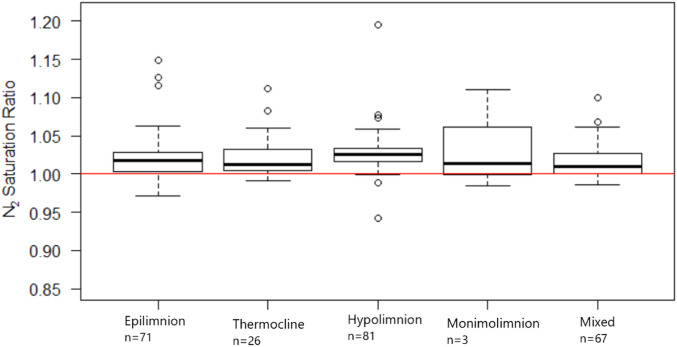
Of the 247 water column samples collected, 85.8% were supersaturated with N2, and all but one lake showed supersaturation at a minimum of one depth (Fig. 2). Saturation values ranged from 0.94 to 1.19 ( SI Appendix, Table S2). Mean levels of N2 saturation increased from February to August and then decreased from September to October with N2 saturation levels in August being significantly higher than in any other month (P < 0.05). Mean levels of N2 saturation among all samples were supersaturated every month that we collected samples (Fig. 3). When comparing the saturation levels across all lakes, all layers of the lakes (unstratified, epilimnion, metalimnion, hypolimnion, and monimolimnion) were supersaturated on average, and the monimolimnion present in one lake sampled three times, had the highest variability (Fig. 4).
Fig. 2.
N2/Ar saturation ratio frequencies from all samples. The histogram was made with 40 breaks, and the red dotted line denotes saturation given physical mixing properties of the gases in water. Values greater than 1 are supersaturated; values less than 1 are unsaturated.
Fig. 3.
N2/Ar saturation ratios by month. The y axis shows N2/Ar saturation ratios. The red line denotes the saturation ratio of the gases given physical mixing properties. The sample sizes for each month are next to the month label. October samples were from 2016; every other month samples were from 2017. Not every lake was sampled every month that is represented on this graph. This graph contains all of the data collected.
Fig. 4.
N2/Ar saturation ratios in mixing layers of lakes across all seasons. The red line denotes saturation of the gases given physical mixing properties. The sample size for each mixing layer are beneath the mixing layer label. This graph contains all of the data collected.
These results suggest that there may be a “leaking” of N from lakes as N2 and that the leaks are not compensated by N-fixation, unless there are high rates of N-fixation at times when these lakes were not sampled. This does not necessarily mean that these lakes will be N-limited as we did not measure other inputs of N to these lakes, such as runoff and groundwater inputs, which can be significant, particularly in the most southern lakes we sampled as they are heavily impacted by agriculture (39). Nonetheless, it does imply that there was a tendency for most of these lakes to remove excess N through denitrification or anammox and may move toward N-limited conditions without sufficient allochthonous inputs, particularly in late summer when external inputs are minimized (40). Furthermore, the fact that we observed the strongest supersaturation in summer (Fig. 3) and in the deepest parts of the lakes (Fig. 4) implies that deep lakes may be the ones that might be most susceptible to N limitation in the absence of significant inputs from the watershed or airshed (N deposition), although trophic status likely also plays a role (41).
The fact that we observed the highest supersaturation in summer is likely due to the fact that lakes had been stratified long enough for organic matter to accumulate from primary production and/or anaerobic conditions to occur, leading to an accumulation of N2 from denitrification and anammox in bottom waters. Lakes in this Midwest region are highly productive in summer, leading to increased organic C availability, which could also stimulate facultative microbes to conduct denitrification in increasingly anoxic waters (42, 43).
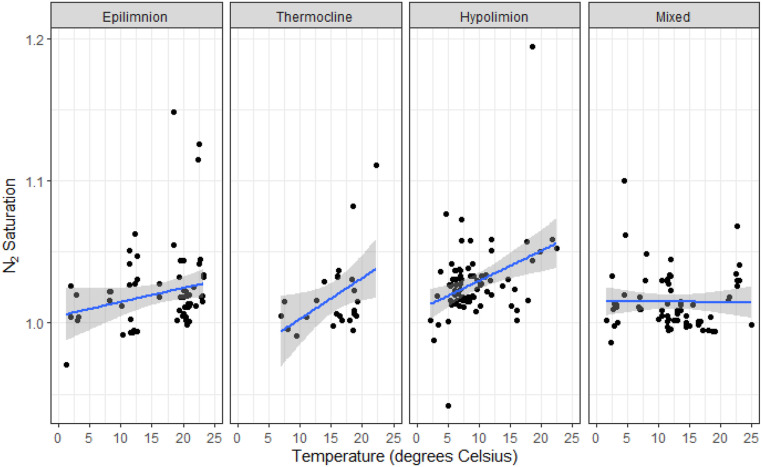
However, perhaps most surprising was the fact that the highest net losses occurred in summer at the same time that N-fixation rates should also be maximized (30, 44). Climate change is predicted to increase lake stratification stability with earlier onset and longer duration (45, 46). Hypolimnetic water temperatures may also increase with increased air temperatures and epilimnetic temperatures, both of which would increase N2 production via denitrification or anammox (45, 47). Interestingly, we observed increasing sensitivity of N2 saturation to temperature in the deepest regions of the lakes, suggesting that even a small change in temperature at depth could lead to large changes in N2 losses from lakes (Fig. 5). These changes could increase the potential size of the N “leak” out of lakes to the atmosphere, especially in late summer when there can be conductive warming of the metalimnion and shallow hypolimnetic waters (47).
Fig. 5.
N2/Ar saturation ratios in relation to temperature and depth. The top axis breaks the data into distinct mixing layers based on stratification patterns. The epilimnion represents the mixing layer at the atmosphere-water interface. The thermocline is a temperature transition area from warm surface waters to cooler bottom waters. The hypolimnion is the lowest layer that interacts with lake sediments. Mixed represents lakes that were unstratified. The lines indicate individual linear regression trends per layer. Temperature was a significant variable in the epilimnion, thermocline, and hypolimnion (P < 0.01) and not significant trend in mixed lakes.
The fact that temperature had the greatest effect on N2 supersaturation in the deepest samples is supported by research where denitrification potential across a latitudinal gradient was examined from tropical to temperate systems, and it was observed that hypolimnetic temperatures were important for determining denitrification potential (48). It seems that the bulk of denitrification occurs in lake sediments, although whether the supersaturation in shallower samples is due to bottom-water mixing up through the water column or denitrification occurring in microhabitats of anoxia is unknown (25). In the future, we will look into important drivers of these saturation trends including trophic status, lake area, percent littoral area, water clarity, dissolved nutrients, and land use.
Although we did not examine the potential for nitrous oxide to exchange with the atmosphere, it also can be an important loss of N, but unlike N2, it is also a greenhouse gas with concentrations increasing at approximately a 0.3% annual rate in the atmosphere (15). Nitrous oxide concentrations often exceed saturation levels, and it was recently shown that Canadian lakes in winter can exceed saturation by a factor of six (49). Both denitrification and nitrification can be important sources of N2O, with denitrification being more important in soils and nitrification playing a more important role in the oceans (50). The important drivers in freshwaters are not known. Nonetheless, N2O release from terrestrial soils is increased by application of agricultural fertilizers, so eutrophication in marine and freshwater systems is also likely increasing N2O concentrations and subsequent release to the atmosphere from freshwaters, with the bulk of N removal occurring in the form of N2 losses (51 –53).
Our data indicate that lakes are supersaturated with N2 at multiple times throughout the year and at multiple depths, suggesting that N losses may be continuous. This is important to our understanding of the future of nutrient limitation in freshwater systems. Because phosphorus does not have a quantitatively significant gaseous process that removes it from lakes, it can exist in sediments as legacy phosphorus, resolubilizing during anoxic periods for decades once it is deposited (54, 55). Conversely, if lakes are constantly losing nitrogen, it may become a more important limiting or colimiting nutrient (56), particularly in the most productive lakes (41, 44). Finlay et al. demonstrated (57) that large, unproductive deep lakes tend toward P rather than N limitation, perhaps due to limited denitrification in these systems (58). Although we did not quantify N-transformation rates, the fact that N2 was supersaturated in almost all instances suggests that denitrification and anammox were greater than N-fixation. This suggests that N-fixation is not likely to alleviate a nitrogen deficiency in upper Midwest lakes and that the traditional narrative of P limitation in freshwater systems should certainly not be the only one and that N limitation/colimitation should be studied further (59, 60). Many systems may be at risk for becoming nitrogen-limited due to net N2 losses, but it is probably most likely in eutrophic systems where temperatures are high, and dissolved oxygen saturation, and allochthonous N inputs are relatively low due to climate change and drought (40, 41, 44, 61, 62).
Supplementary Material
Acknowledgments
We thank the following people for their advice on this project: Sara Winikoff and Jacques Finlay. This project was funded The Itasca Director’s Graduate Research Fellowship through the College of Biological Sciences at the University of Minnesota.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been deposited at Data Repository for U of M. http://hdl.handle.net/11299/214017.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921689117/-/DCSupplemental.
References
- 1. Raymond P. A.et al., Global carbon dioxide emissions from inland waters. Nature 503, 355–359 (2013). [DOI] [PubMed] [Google Scholar]
- 2. Maranger R., Jones S. E., Cotner J. B., Stoichiometry of carbon, nitrogen, and phosphorus through the freshwater pipe. Limnol. Oceanogr. Lett. 3, 89–101 (2018). [Google Scholar]
- 3. Tranvik L. J.et al., Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 54, 2298–2314 (2009). [Google Scholar]
- 4. Mendonça R.et al., Organic carbon burial in global lakes and reservoirs. Nat. Commun. 8, 1694 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang H., Wang W., Yin C., Wang Y., Lu J., Littoral zones as the “hotspots” of nitrous oxide (N2O) emission in a hyper-eutrophic lake in China. Atmos. Environ. 40, 5522–5527 (2006). [Google Scholar]
- 6. Prairie Y. T., Giorgio P. A., Prairie Y. T., Giorgio P. A., A new pathway of freshwater methane emissions and the putative importance of microbubbles. Inland Waters 2041, 311–320 (2017). [Google Scholar]
- 7. Cole J. J.et al., Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems (N. Y.) 10, 171–184 (2007). [Google Scholar]
- 8. Seitzinger S.et al., Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 16, 2064–2090 (2006). [DOI] [PubMed] [Google Scholar]
- 9. Cheng F. Y., Basu N. B., Biogeochemical hotspots: Role of small water bodies in landscape nutrient processing. Water Resour. Res. 53, 1–19 (2017). [Google Scholar]
- 10. Duvert C., Butman D. E., Marx A., Ribolzi O., Hutley L. B., CO2 evasion along streams driven by groundwater inputs and geomorphic controls. Nat. Geosci. 11, 3–7 (2018). [Google Scholar]
- 11. Verpoorter C., Kutser T., Seekell D. A., Tranvik L. J., A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 41, 6396–6402 (2014). [Google Scholar]
- 12. Heathwaite A. L., Multiple stressors on water availability at global to catchment scales: Understanding human impact on nutrient cycles to protect water quality and water availability in the long term. Freshw. Biol. 55, 241–257 (2010). [Google Scholar]
- 13. Falkowski P.et al., The global carbon cycle: A test of our knowledge of earth as a system. Science 290, 291–296 (2000). [DOI] [PubMed] [Google Scholar]
- 14. Carpenter S.et al., Nonpoint pollution of surface waters with phosphorus and nitrogen. Issues Ecol. 4, 1–12 (1998). [Google Scholar]
- 15. Schlesinger W., Bernhardt E. S., Biogeochemistry: An Analysis of Global Change, (Elsevier Inc., ed. 3, 2013). [Google Scholar]
- 16. Minnesota Environmental Quality Board , 2015 EQB Water Policy Report (2015).
- 17. Cole J. J., Caraco N. F., Kling G. W., Kratz T. K., Carbon dioxide supersaturation in the surface waters of lakes. Science 265, 1568–1570 (1994). [DOI] [PubMed] [Google Scholar]
- 18. Walter K. M., Zimov S. A., Chanton J. P., Verbyla D., Chapin F. S. 3rd, Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature 443, 71–75 (2006). [DOI] [PubMed] [Google Scholar]
- 19. Montzka S. A., Dlugokencky E. J., Butler J. H., Non-CO2 greenhouse gases and climate change. Nature 476, 43–50 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Weiss R. F., The solubility of nitrogen, oxygen and argon in water and seawater. Deep. Res. Oceanogr. Abstr. 17, 721–735 (1970). [Google Scholar]
- 21. McCarthy M. J., Gardner W. S., Lehmann M. F., Guindon A., Bird D. F., Benthic nitrogen regeneration, fixation, and denitrification in a temperate, eutrophic lake: Effects on the nitrogen budget and cyanobacteria blooms. Limnol. Oceanogr. 61, 1406–1423 (2016). [Google Scholar]
- 22. Bernhard A., The nitrogen cycle: Processes, players and human impact. Nat. Educ. Knowl. 2, 1–8 (2010). [Google Scholar]
- 23. Davidson E. A., Seitzinger S., The enigma of progress in denitrification research. Ecol. Appl. 16, 2057–2063 (2006). [DOI] [PubMed] [Google Scholar]
- 24. Crowe S. A.et al., Novel anammox bacteria and nitrogen loss from Lake Superior. Sci. Rep. 7, 13757 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reisinger A. J., Tank J. L., Hoellein T. J., Hall R. O., Sediment, water column, and open-channel denitrification in rivers measured using membrane-inlet mass spectrometry. J. Geophys. Res. Biogeosci. 121, 1258–1274 (2016). [Google Scholar]
- 26. Sweeney B. W., Newbold J. D., Streamside forest buffer width needed to protect stream water quality, habitat, and organisms: A literature review. J. Am. Water Resour. Assoc. 50, 560–584 (2014). [Google Scholar]
- 27. Schindler D. W., Carbon, nitrogen, and phosphorus and the eutrophication of freshwater lakes. J. Phycol. 7, 321–329 (1971). [Google Scholar]
- 28. Schindler D. W., Carpenter S. R., Chapra S. C., Hecky R. E., Orihel D. M., Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 50, 8923–8929 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Schindler D. W.et al., Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. U.S.A. 105, 11254–11258 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott J. T., McCarthy M. J., Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 55, 1265–1270 (2010). [Google Scholar]
- 31. Heiskary S. A., Wilson C. B., Larsen D. P., Analysis of regional patterns in lake water quality: Using ecoregions for lake management in Minnesota. Lake Reserv. Manage. 3, 337–344 (1987). [Google Scholar]
- 32. Omernik J. M., Map supplement: Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr. 77, 118–125 (1987). [Google Scholar]
- 33. Stoner J. D., Lorenz D. L., Wiche G. J., Goldstein R. M., Red River of the North basin, Minnesota, North Dakota, and South Dakota. Water Resour. Bull. 29, 575–615 (1994). [Google Scholar]
- 34. Hall E. K., Cotner J. B., Interactive effect of temperature and resources on carbon cycling by freshwater bacterioplankton communities. Aquat. Microb. Ecol. 49, 35–45 (2007). [Google Scholar]
- 35. Carignan R., Planas D., Vis C., Planktonic production and respiration in oligotrophic Shield lakes. Limnol. Oceanogr. 45, 189–199 (2000). [Google Scholar]
- 36. Kana T. M.et al., Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal. Chem. 66, 4166–4170 (1994). [Google Scholar]
- 37. Hamme R. C., Emerson S. R., The solubility of neon, nitrogen and argon in distilled water and seawater. Deep. Res. Part I Oceanogr. Res. Pap. 51, 1517–1528 (2004). [Google Scholar]
- 38. Loeks-Johnson B. M., Cotner J. B., Upper Midwest lakes are supersaturated with N2. Data Repository for U of M. http://hdl.handle.net/11299/214017. Deposited 18 June 2020. [DOI] [PMC free article] [PubMed]
- 39. Minnesota Pollution Control Agency , Nitrogen in Minnesota Surface Waters: Conditions, trends, sources, and reductions, (Nitrogen in Minnesota Surface Waters, 2013). [Google Scholar]
- 40. Hayes N. M., Vanni M. J., Horgan M. J., Renwick W. H., Climate and land use interactively affect lake phytoplankton nutrient limitation status. Ecology 96, 392–402 (2015). [DOI] [PubMed] [Google Scholar]
- 41. Scott J. T., McCarthy M. J., Paerl H. W., Nitrogen transformations differentially affect nutrient‐limited primary production in lakes of varying trophic state. Limnol. Oceanogr. Lett. 4, 96–104 (2019). [Google Scholar]
- 42. Reddy K. R., Patrick W. H., Lindau C. W., Nitrification-denitrification at the plant root-sediment interface in Wetlands. Source Limnol. Oceanogr. Limnol. Ocean. 34, 1004–1013 (1989). [Google Scholar]
- 43. Holmroos H.et al., Dynamics of dissolved nutrients among different macrophyte stands in a shallow lake. Limnology 16, 31–39 (2015). [Google Scholar]
- 44. Howarth R. W., Marino R., Lane J., Cole J. J., Nitrogen fixation in freshwater, estuarine, and marine ecosystems. Limnol. Oceanogr. 33, 669–687 (1988). [Google Scholar]
- 45. Hondzo M., Stefan H. G., Regional water temperature characteristics of lakes subjected to climate change. Clim. Change 24, 187–211 (1993). [Google Scholar]
- 46. Kraemer B. M.et al., Morphometry and average temperature affect lake stratification responses to climate change. Geophys. Res. Lett. 42, 4981–4988 (2015). [Google Scholar]
- 47. Adrian R.et al., Lakes as sentinels of climate change. Limnol. Oceanogr. 54, 2283–2297 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lewis W. M., Causes for high frequency of nitrogen limitation in tropical lakes. Int. Vereinigung für Theor. und Angew. Limnol. Verhandlungen 28, 210–2013 (2002). [Google Scholar]
- 49. Cavaliere E., Baulch H. M., Denitrification under lake ice. Biogeochemistry, 10.1007/s10533-018-0419-0 (2018). [DOI] [Google Scholar]
- 50. Thomson A. J., Giannopoulos G., Pretty J., Baggs E. M., Richardson D. J., Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1157–1168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Griffis T. J.et al., Nitrous oxide emissions are enhanced in a warmer and wetter world. Proc. Natl. Acad. Sci. U.S.A. 114, 12081–12085 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maavara T.et al., Nitrous oxide emissions from inland waters: Are IPCC estimates too high? Glob. Chang. Biol. 25, 473–488 (2019). [DOI] [PubMed] [Google Scholar]
- 53. Harrison J. A.et al., The regional and global significance of nitrogen removal in lakes and reservoirs. Biogeochemistry 93, 143–157 (2009). [Google Scholar]
- 54. Sharpley A.et al., Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 42, 1308–1326 (2013). [DOI] [PubMed] [Google Scholar]
- 55. Holmroos H., Hietanen S., Niemistö J., Horppila J., Sediment resuspension and denitrification affect the nitrogen to phosphorus ratio of shallow lake waters. Fundam. Appl. Limnol. Arch. Hydrobiol. 180, 193–205 (2012). [Google Scholar]
- 56. Grantz E. M., Haggard B. E., Thad Scott J., Stoichiometric imbalance in rates of nitrogen and phosphorus retention, storage, and recycling can perpetuate nitrogen deficiency in highly-productive reservoirs. Limnol. Oceanogr. 59, 2203–2216 (2014). [Google Scholar]
- 57. Finlay J. C., Small G. E., Sterner R. W., Human influences on nitrogen removal in lakes. Science 342, 247–250 (2013). [DOI] [PubMed] [Google Scholar]
- 58. Small G. E., Cotner J. B., Finlay J. C., Stark R. A., Sterner R. W., Nitrogen transformations at the sediment-water interface across redox gradients in the Laurentian Great Lakes. Hydrobiologia 731, 95–108 (2014). [Google Scholar]
- 59. Elser J. J.et al., Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2007). [DOI] [PubMed] [Google Scholar]
- 60. Paerl H. W.et al., It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 50, 10805–10813 (2016). [DOI] [PubMed] [Google Scholar]
- 61. Kolzau S.et al., Seasonal patterns of nitrogen and phosphorus limitation in four German lakes and the predictability of limitation status from ambient nutrient concentrations. PLoS One 9, e96065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen N., Wu J., Chen Z., Lu T., Wang L., Spatial-temporal variation of dissolved N2 and denitrification in an agricultural river network, southeast China. Agric. Ecosyst. Environ. 189, 1–10 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected in this experiment are available through the Data Repository for the University of Minnesota (38).