Significance
In the United States, 13% of households depend on an unregulated private well for their water. Compared with children in houses served by a regulated water utility, children in these homes have a 25% increased risk of elevated blood lead. Because lead is a neurotoxin, these children are at greater risk of experiencing irreversible cognitive damage, which can decrease their performance in school and increase their risks of behavioral problems. This study assesses associations between children’s blood lead and dependence on an unregulated private well for drinking water. It highlights the need for interventions to control lead corrosion from plumbing and well components (such as drop pipes, pump parts, and valves and fittings) in households depending on private wells.
Keywords: drinking water, private well, children’s health, lead exposure, blood lead
Abstract
Although the Flint, Michigan, water crisis renewed concerns about lead (Pb) in city drinking water, little attention has been paid to Pb in private wells, which provide drinking water for 13% of the US population. This study evaluates the risk of Pb exposure in children in households relying on private wells. It is based on a curated dataset of blood Pb records from 59,483 North Carolina children matched with household water source information. We analyze the dataset for statistical associations between children’s blood Pb and household drinking water source. The analysis shows that children in homes relying on private wells have 25% increased odds (95% CI 6.2 to 48%, P < 0.01) of elevated blood Pb, compared with children in houses served by a community water system that is regulated under the Safe Drinking Water Act. This increased Pb exposure is likely a result of corrosion of household plumbing and well components, because homes relying on private wells rarely treat their water to prevent corrosion. In contrast, corrosion control is required in regulated community water systems. These findings highlight the need for targeted outreach to prevent Pb exposure for the 42.5 million Americans depending on private wells for their drinking water.
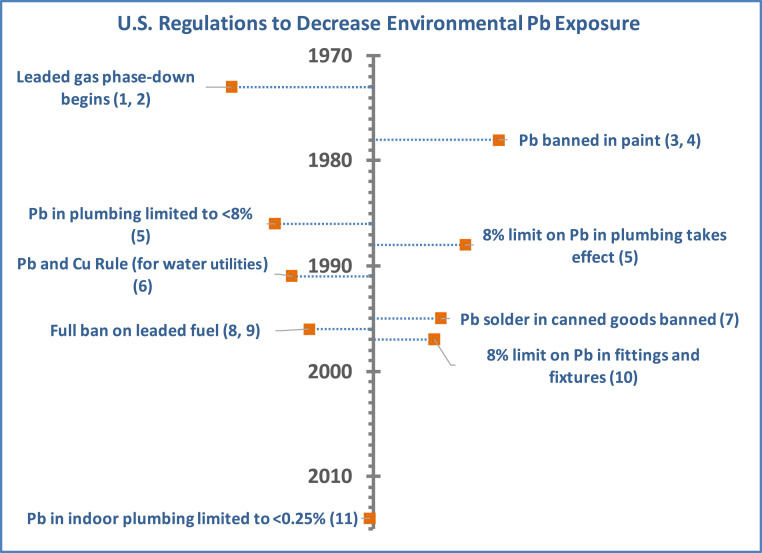
Lead (Pb) is a neurotoxin that contributes to irreversible cognitive and developmental impairment in exposed children. Pb exposure before age 7 has been associated with decreased IQ (1–5), poor performance in school (6–8), and increased risks of behavioral problems such as attention-deficit/hyperactivity disorder (9) and juvenile delinquency (10, 11). Regulations on Pb in gasoline, paint, and food cans enacted during the 1970s through the 1990s (Fig. 1) interrupted many environmental sources of Pb exposure. In response, average US blood Pb concentrations declined from 12.8 µg/dL in 1976 to 0.84 µg/dL by 2014 (12). Nonetheless, Pb exposure remains a serious health concern for some US children (13). The US Centers for Disease Control and Prevention (CDC) has established 5 µg/dL as a reference level for elevated blood Pb based on the upper tail of the current population distribution of blood Pb (14). As of 2016, 4% of children in the United States had blood Pb above this reference level, and the state-level prevalence ranged as high as 9% (in Pennsylvania) (14). However, this threshold is not health-based; the Agency for Toxic Substances and Disease Registry has stated that even “the lowest blood lead levels are associated with serious adverse effects (e.g. declining cognitive function in children)” (15). Nationwide, there are persistent socioeconomic and racial disparities in children’s Pb exposure. Blood Pb above 5 µg/dL occurs nearly four times as often among low-income as among high-income children (16). It is twice as frequent among African Americans as among children of other racial and ethnic groups (16). The latter disparity may be a legacy of racial residential segregation and contribute to racial inequalities in the intergenerational transfer of poverty through its negative impact on early childhood educational achievement (8, 17).
Fig. 1.
US regulations designed to decrease exposure to lead in the environment. No regulations specifically apply to water from private wells. 1) Clean Air Act Amendments of 1970. Pub. L. 91-604. 81 Stat. 486. 31 December 1970. 2) US Environmental Protection Agency. Regulation of Fuels and Fuel Additives. 40 CFR 80 (1973). 3) Lead-Based Paint Poisoning Prevention Act. Pub. L. 91-695. 84 Stat. 2078. 13 January 1971. 4) US Consumer Product Safety Commission. Lead-Containing Paint and Certain Consumer Products Bearing Lead-Containing Paint, 16 CFR 1303 (1977). 5) Safe Drinking Water Act Amendments of 1986. Pub. L. 99-339. 100 Stat. 666. 8 December 1986. 6) US Environmental Protection Agency. Drinking Water Regulations: Maximum Contaminant Level Goals and National Drinking Water Regulations for Lead and Copper. 40 CFR 141 (1991). 7) US Food and Drug Administration. Lead-Soldered Food Cans. 21 CFR 189 (1995). 8) Clean Air Act Amendments of 1990. Pub. L. 101-549. 104 Stat. 2399. 15 November 1990. 9) US Environmental Protection Agency. Prohibition on Gasoline Containing Lead or Lead Additives for Highway Use. 40 CFR 80 (1996). 10) Safe Drinking Water Act Amendments of 1996. Pub. L. 104-182. 6 August 1996. 11) Water Infrastructure Improvements for the Nation Act. Pub. L. 114-322. 130 Stat. 1628. 16 December 2016.
Research suggests that as Pb concentrations have declined in other environmental media, the relative importance of water as an exposure source has increased (18, 19). Pb in drinking water usually originates from corrosion of water distribution systems and/or indoor plumbing (20). (Indeed, the word “plumbing” and chemical symbol “Pb” derive from the Latin “plumbum,” meaning lead.) It can be effectively controlled by adding corrosion inhibitors, such as phosphates or silicates, to water supplies and by replacing older, Pb-bearing water service lines and plumbing (21). Since 1991, the Lead and Copper Rule provision of the Safe Drinking Water Act has required all community water utilities to monitor Pb in water at selected consumer taps. Utilities must replace Pb service lines and/or optimize their corrosion control if more than 10% of samples exceed 15 µg/L (22). Although the 2015 Flint, Michigan, water crisis highlighted the consequences of noncompliance with the Lead and Copper Rule (23, 24), multiple studies have shown major declines in Pb at consumer taps in community water systems in response to this rule (25). For example, a study comparing Pb in 166 water utilities nationwide with a history of Pb problems found that the median of the 90th percentile Pb concentration at consumer taps decreased from nearly 30 µg/L before the Lead and Copper Rule to ∼5 µg/L during 2000 to 2005 (26).
A largely overlooked route of Pb exposure in water is unregulated private water systems, on which 42.5 million US residents (13% of the population) rely for their drinking water (27). The vast majority of private water systems are wells serving a single household. The Safe Drinking Water Act’s requirements for Pb monitoring and control do not cover private wells (28). Households relying on private wells must be stewards of their own water quality, monitoring for Pb on their own and, when necessary, installing and managing their own corrosion-control systems or replacing Pb-containing well components and plumbing materials (29–31). Prior studies have found a high prevalence of elevated Pb in private well water (32, 33). A recent study in Wake County, North Carolina (NC), found that Pb occurrence in kitchen tap water in homes with private wells was similar to that in Flint during the water crisis (33). Research also has shown that very few private well owners follow recommended guidelines for water quality monitoring and are largely unaware of Pb exposure risks (34–36). For example, a survey of NC private well owners reported that only 16% follow public health guidelines for well water testing (29).
Despite the evidence that private well water can pose high risks of Pb exposure and that awareness of this risk is low, the extent to which private well water contributes to the persistent burden of elevated blood Pb in some children is unknown. One prior study estimated that elevated water Pb could trigger elevated blood Pb in 10% of children in predominantly African American communities in North Carolina relying on private wells (33). However, this estimate was based on blood Pb predictions from a biokinetic model, not on Pb measured from blood tests. To our knowledge, no prior studies have investigated whether measured blood Pb concentrations in US children obtaining their water from private wells are higher than those of children whose homes are served by community water systems that are regulated by the Safe Drinking Water Act. Determining whether children getting their water from private wells are at increased risk is critical to ongoing initiatives to ensure that all US children are sufficiently protected from the lifelong adverse consequences of exposure to environmental Pb.
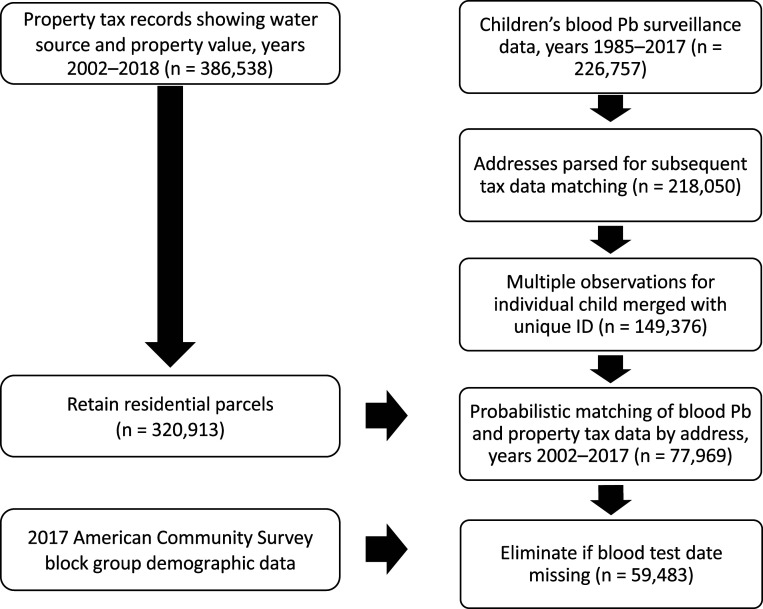
Using a curated dataset built by merging 59,483 routinely collected blood Pb measurements from children in Wake County, NC (Fig. 2), with drinking water source (Fig. 3) and demographic data (Fig. 2), this study assesses whether children in households relying on unregulated private wells have higher blood Pb levels and are at increased risk of elevated blood Pb, compared with children served by regulated water utilities. To our knowledge, this is the first study to use biomonitoring data to assess whether children in households with private wells are at increased risk of Pb exposure from their water. The results could help to inform the ongoing campaign of the US Department of Health and Human Services, through its Healthy People 2020 strategic plan, to decrease the 97.5th percentile of children’s blood Pb from 5.8 to 5.2 µg/dL and to eliminate disparities in blood Pb levels by race and ethnicity (37).
Fig. 2.
Steps used to build the curated dataset linking children’s blood Pb, water source, household attributes, and demographic information.
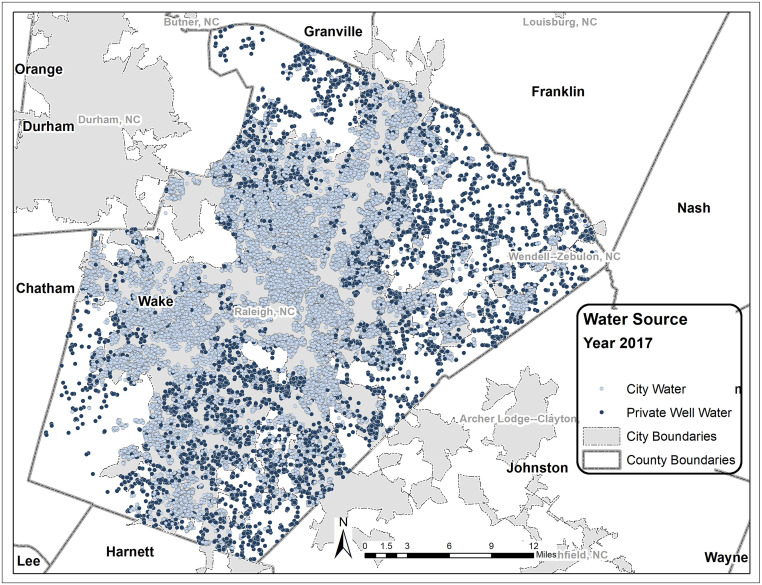
Fig. 3.
Water sources for children whose blood Pb was tested in 2017. In 2017, n = 725 children in homes relying on private wells were tested, and n = 4,007 with community water service were tested.
Results
Influence of Private Well Water on Children’s Blood Pb.
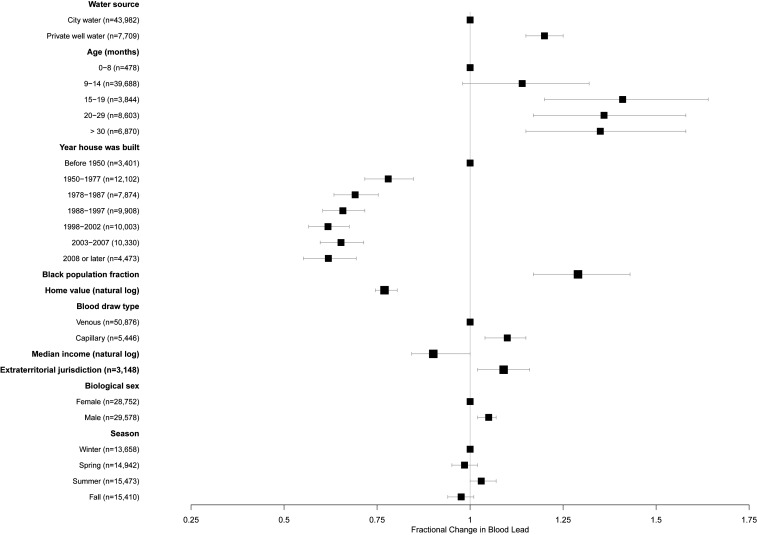
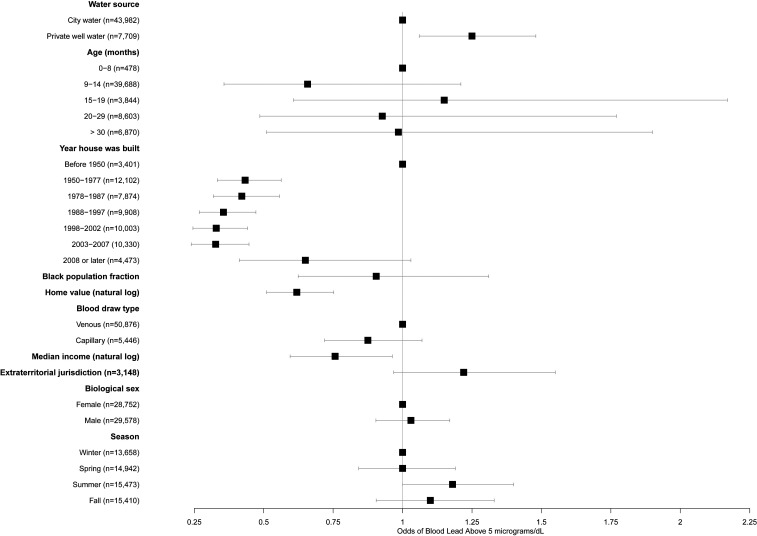
Blood Pb concentrations were significantly higher, on average, among children relying on private well water, compared with those served by a regulated water utility (Table 1; 1.75 versus 1.59 µg/dL, P < 0.001). When controlling for the child’s age and biological sex, construction year and value of their home, year of their blood test, and racial and socioeconomic characteristics of their neighborhood, blood Pb concentrations among children in houses with private wells were 20% higher than among other children [Fig. 4 and SI Appendix, Table S2; exp(β) = 1.20, P < 0.001]. Interactions between private well water use and year of home construction were not significant (SI Appendix, Table S1, legend). This latter result suggests that the 1986 legislation to limit Pb in indoor plumbing to 8% or less by mass was not effective in decreasing Pb exposure from private well water.
Table 1.
Summary statistics for the curated dataset used in this analysis
| Variable | n (%) | Arithmetic mean blood lead, µg/dL |
| Total | 59,483 (100) | 1.73 |
| Water source | ||
| Private well | 7,709 (14.9) | 1.75 |
| Regulated water utility | 43,982 (85.1) | 1.59 |
| Age (mo) | ||
| 0–8 | 478 (0.8) | 1.71 |
| 9–14 | 39,688 (66.7) | 1.63 |
| 15–19 | 3,844 (6.5) | 1.99 |
| 20–29 | 8,603 (14.5) | 1.80 |
| ≥30 | 6,870 (11.6) | 2.07 |
| Biological sex | ||
| Female | 28,752 (49.3) | 1.72 |
| Male | 29,578 (50.7) | 1.74 |
| Year tested | ||
| 2002–2006 | 15,307 (25.7) | 2.46 |
| 2007–2012 | 24,149 (40.6) | 1.70 |
| 2013–2017 | 20,027 (33.7) | 1.20 |
| Year house was built | ||
| Before 1950 | 3,401 (5.9) | 2.14 |
| 1950–1977 | 12,102 (20.8) | 1.89 |
| 1978–1987 | 7,874 (13.6) | 1.79 |
| 1988–1997 | 9,908 (17.1) | 1.74 |
| 1998–2002 | 10,003 (17.2) | 1.71 |
| 2003–2007 | 10,330 (17.8) | 1.48 |
| 2008 or later | 4,473 (7.7) | 1.30 |
| Home value | ||
| Quartile 1 (<$132,880) | 13,854 (25.0) | 2.00 |
| Quartile 2 ($132,881–188,281) | 13,855 (25.0) | 1.73 |
| Quartile 3 ($188,282–292,135) | 13,853 (25.0) | 1.56 |
| Quartile 4 (>$292,135) | 13,854 (25.0) | 1.52 |
| Black population proportion in census block group | ||
| Quartile 1 (<0.095) | 14,927 (25.0) | 1.58 |
| Quartile 2 (0.095–0.211) | 14,827 (25.2) | 1.64 |
| Quartile 3 (0.212–0.370) | 14,918 (25.0) | 1.78 |
| Quartile 4 (>0.370) | 14,794 (24.9) | 1.91 |
| Median income in census block group | ||
| Quartile 1 (<$49,012) | 14,867 (25.0) | 1.96 |
| Quartile 2 ($49,012–62,925) | 14,905 (25.1) | 1.78 |
| Quartile 3 ($62,926–83,985) | 15,291 (25.7) | 1.64 |
| Quartile 4 (>$83,985) | 14,398 (24.2) | 1.53 |
| Blood draw type | ||
| Capillary | 5,446 (9.7) | 1.76 |
| Venous | 50,876 (90.3) | 1.70 |
| Season | ||
| Winter | 13,658 (23.0) | 1.73 |
| Spring | 14,942 (25.1) | 1.70 |
| Summer | 15,473 (26.0) | 1.77 |
| Fall | 15,410 (25.9) | 1.73 |
Fig. 4.
Fractional change in child’s blood lead concentration with changes in risk factors for elevated blood lead. Blood lead increases to the right of the line at x = 1 and decreases to the left of this line. Values reflect adjustment for all variables shown and for year of blood test. Error bars represent 95% confidence intervals. Note: “African American population” indicates African American population fraction in child’s census block group; “median income” indicates log-transformed median income in child’s census block group; “extraterritorial jurisdiction” indicates whether child lives in an unincorporated area under the zoning authority of a nearby city or town.
Influence of Private Well Water on Risk of Elevated Blood Pb.
The odds of elevated blood Pb (5 µg/dL or greater) were 25% higher among children relying on private wells, compared with children served by a regulated water utility (Fig. 5 and SI Appendix, Table S3; odds ratio [OR] = 1.25, P = 0.008). As was the case when estimating the influence of water source on blood Pb concentration, this increased risk held when controlling for neighborhood demographic and socioeconomic characteristics known to be associated with elevated blood Pb (Fig. 5 and SI Appendix, Table S3). Interactions between age of the home and water source were not significant, again suggesting that limits on Pb in plumbing have been insufficient to decrease Pb exposure risks from private well water. Adjusting for all other variables, the risk of elevated blood Pb among children with private wells was 2.1%, compared with 1.7% in children with community water service (marginal effects were calculated from SI Appendix, Table S3).
Fig. 5.
Odds of a child’s blood lead exceeding the Centers for Disease Control and Prevention 5 µg/dL threshold for elevated blood lead for different risk factors. Risk increases to the right of the line at x = 1 and decreases to the left of this line. Values reflect adjustment for all variables shown and for year of blood test. Error bars represent 95% confidence intervals.
Influence of Private Well Water on the Probability of Blood Pb above the Population Median.
The relationships between water source and blood Pb held when considering the risk of a child’s blood Pb exceeding the median for this population (1 µg/dL) (SI Appendix, Table S4). Compared with children served by a regulated water utility, those served by a private well had 47% higher odds of blood Pb above the median (OR = 1.47, P < 0.0001).
Demographic and Socioeconomic Factors Associated with Children’s Blood Pb.
Demographic and socioeconomic characteristics of the child, his or her home, and the child’s neighborhood also were significantly associated with blood Pb (Fig. 4 and SI Appendix, Table S2). These characteristics included the child’s age and biological sex, age and value of the home, and neighborhood racial and economic composition.
Compared with children under 9 mo, older children had higher blood Pb, with the highest concentrations in those aged 16 to 20 mo [Fig. 4 and SI Appendix, Table S2; exp(β) = 1.41, P < 0.001]. However, the child’s age was not significantly associated with the risk of elevated blood Pb (Fig. 5 and SI Appendix, Table S3). Boys, on average, had slightly higher blood Pb concentrations [Fig. 4 and SI Appendix, Table S2; exp(β) = 1.04, P < 0.001]. However, their odds of elevated blood Pb were not higher than for girls (Fig. 5 and SI Appendix, Table S3).
Children in homes built after 1950 had lower blood Pb concentrations than those in older homes (Fig. 4 and SI Appendix, Table S2; all P < 0.001). The significant decline in concentrations among children living in homes built after 1978 highlights the effectiveness of the 1978 lead paint ban [SI Appendix, Table S2; exp(β) ≤ 0.69 for all newer age categories, all P < 0.001, compared with exp(β) = 0.78 for homes built in 1950 to 1977].
Children’s blood Pb concentrations also decreased as the value of the child’s home increased [SI Appendix, Table S2; exp(β) = 0.77, P < 0.001 for the log of home value], and the risk of elevated blood Pb likewise decreased as home value increased [SI Appendix, Table S3; exp(β) = 0.62, P = 0.002 for the log of home value]. As shown in Table 1, children in homes in the highest-value quartile (>$291,295) had mean blood Pb concentrations of 1.52 µg/dL, compared with 2.00 µg/dL for children in homes in the lowest-value quartile (<$132,254). Similarly, children’s blood Pb concentrations and the risk of elevated blood Pb increased as median household income in the block group in which the child lives decreased [SI Appendix, Table S2; exp(β) = 0.90, P = 0.002; SI Appendix, Table S3; OR = 0.76, P = 0.023]. Children living in census block groups in the highest (>$83,842) income quartile had an average blood Pb concentration of 1.53 µg/dL, while those living in block groups in the lowest (<$48,984) quartile had a mean blood Pb concentration of 1.96 µg/dL (Table 1).
Children’s blood Pb concentrations also increased with the proportion of non-Hispanic blacks in their neighborhood (Fig. 4 and SI Appendix, Table S2). Every 10% increase in the non-Hispanic black census block group population proportion was associated with a 29% increase in the blood Pb concentration in children living in that neighborhood [SI Appendix, Table S2; exp(β) = 1.29, P < 0.001]. Children living in block groups with fewer than 4% non-Hispanic black population proportions had mean blood lead levels of 1.3 µg/dL, compared with >1.6 µg/dL in neighborhoods with more than a 64% non-Hispanic black population (SI Appendix, Fig. S2). However, the risk of elevated blood Pb did not vary significantly with the racial composition of the child’s census block group (Fig. 5 and SI Appendix, Table S3).
Association between Municipal Zoning Policies and Children’s Blood Pb.
An important predictor of blood Pb was the child’s residence in a municipal extraterritorial jurisdiction—a neighborhood bordering a city or town but without legally mandated access to municipal services, including community water service. Children in households in extraterritorial jurisdictions had significantly higher blood Pb concentrations than those living within municipal boundaries or rural areas [Fig. 4 and SI Appendix, Table S2; exp(β) = 1.09, P = 0.011], after holding all other variables constant. The risk of elevated blood Pb was also marginally higher among children in these areas (Fig. 5 and SI Appendix, Table S3; OR = 1.2, P = 0.089). Children born in these extraterritorial neighborhoods were significantly more likely to have blood Pb above the population median than other children (SI Appendix, Table S4; OR = 1.18, P = 0.019).
Discussion
This research provides the evidence that children living in US households relying on private wells that are unregulated by the Safe Drinking Water Act have higher blood Pb levels and are at higher risk of elevated blood Pb (>5 µg/dL) than children in homes served by regulated water utilities. Children relying on private wells had blood Pb concentrations that were 20% higher, on average, than children with community water service. These children also had 25% higher odds of having elevated blood Pb. Blood Pb concentrations and risks of elevated blood Pb were also higher among children in older or lower-valued homes, in areas with a higher percentage of non-Hispanic black residents, and in neighborhoods excluded from nearby municipal services.
Although our results establish an association between private well water and children’s blood Pb in the United States, they are consistent with prior research linking children’s blood Pb concentrations to Pb in community water supplies in North America and Europe. In 2016, Ngueta et al. (38) collected blood Pb and tap water samples for 298 children receiving water from Montreal’s municipal water system. They found that for every 1 ppb (part per billion) increase in Pb concentration in the water, children’s blood Pb concentrations increased by 35% after 150 d of exposure. Similarly, Lanphear et al. (39) in 2002 found a significant association between the concentration of Pb in the tap water and blood Pb levels in 249 children served by the water supply for Rochester, New York. A 2015 study of 484 French children reported that tap water Pb was significantly associated with blood Pb in children drinking tap water; an increase in the concentration of Pb in water from the 25th percentile (<1 ppb) to the 99th percentile (6.1 ppb) was associated with a 36% increase in blood Pb (40).
The prior research connecting Pb in community water systems to Pb in children’s blood, along with studies showing relatively high risks of Pb occurrence in private well water, suggests a causal mechanism for our findings. Specifically, tap water in homes relying on private wells is at increased risk of releasing Pb from corrosion of household plumbing and/or components of the wells themselves, such as drop pipes, pump components, or brass valves and fittings (32). Since households with private wells are not required to monitor their water quality, and rarely report doing so on a voluntary basis, they are generally unaware of these risks. In turn, children consume water with higher Pb in homes using private wells than in homes with community-supplied water containing corrosion inhibitors. Increased ingestion of Pb from water increases blood Pb levels, even when other exposure sources (such as old paint and soil containing legacy Pb contamination from polluted air) are controlled.
The decrease in average blood Pb in more recent years and with age of the child’s home suggests that measures to prevent Pb exposure from routes other than private well water were effective in reducing Pb exposure for most children. However, the lack of a statistically significant interaction between home age and private well use suggests that the limit on Pb in household plumbing fixtures to less than 8% by mass, which took effect in 1988 (Fig. 1), was not effective in decreasing Pb exposure in drinking water among children in homes using private wells. If this regulation had reduced Pb release from plumbing in homes with private wells, then the effect of the household’s water source (private well or community system) would have been attenuated in houses built after 1988, implying a negative coefficient on the interaction term between house age and water source. The fact that the estimated coefficient was near 0 suggests, then, that the regulation was ineffective in reducing Pb release in households relying on private wells. This is perhaps unsurprising, since plumbing materials, such as brass and galvanized steel, which have been implicated as sources of Pb in private wells in other studies (32), often contain up to 8% Pb, but would rarely have had Pb contents dramatically higher than 8%. As a result, it is plausible that the 1986 regulations had little effect on the Pb content of many common brass or galvanized steel water system components. Beginning in 2014, the definition of Pb-free plumbing was changed under the 2011 Reduction of Lead in Drinking Water Act to allow no more than 0.25% Pb in plumbing and fixtures intended for drinking water use (41). This latter act may prove to have greater impacts on Pb in drinking water than the 1986 regulations. However, it does not apply to plumbing components (such as pump components and drop pipes) used within private wells (42). In addition, it may still fail to entirely eliminate leaching of Pb from corrosion of brass and galvanized parts, because laboratory studies show that Pb can leach even from fixtures containing less than 0.25% Pb under corrosive water conditions (43). Because only 2% of the homes in our dataset were built after 2014 (when the Reduction of Lead in Drinking Water Act took effect), and only a minority of these had private wells, the effects of the 2011 act could not be robustly evaluated in this study.
The significant decrease in children’s blood Pb as home value increased observed in this study is consistent with prior research. For example, a 2014 study of soil Pb in Toledo, Ohio, found that property value was one of the most important predictors of elevated blood Pb risk associated with children’s exposure to soil (44). The authors suggested that families in lower-valued homes may not be able to afford Pb abatement. In a review of Pb exposure risks from urban gardening, Brown et al. (45) cited evidence that home value is related to food insecurity, which also is associated with increased risks of elevated blood Pb through its effects on nutritional status. Children with poor nutritional status are more likely to be deficient in iron and calcium, increasing their absorption of Pb (46). A study examining the influence of neighborhood demographic variables on children’s blood Pb in Syracuse, New York, found that blood Pb concentrations decreased as the lower quartile of housing unit value increased (47). The authors concluded that “blood Pb levels are at least partially a function of where people can afford to live.” Further work to characterize the mechanisms mediating the strong observed associations between home value and blood Pb is needed and may generate new evidence to inform efforts to target and prevent environmental Pb exposure and reduce disparities.
Our finding that children born in municipal extraterritorial jurisdictions (unincorporated urban areas) have higher blood Pb concentrations may reflect the decreased economic capacity of these areas to afford interventions—such as corrosion control in water supplies and Pb paint abatement—to decrease childhood Pb exposure. While some of these unincorporated urban areas are inhabited by wealthy suburbanites, overall these communities are less well-off than their urban counterparts (48). In our dataset, median household incomes were significantly lower (mean $64,700 vs. $70,300, P < 0.001) in municipal extraterritorial jurisdictions than in other parts of the county (within-city or rural). Home values also were significantly lower in these areas (mean $225,000 vs. $250,000, P < 0.001). Anderson (49) has argued that unincorporated urban areas are less well-equipped than their urban neighbors to provide collective services (such as adequately maintained water infrastructure) due to their reliance on only one tier of government (the county). She contends,
Urban life placed outside the reach of municipal government thus reveals itself as a condition that can...enable the continuation of severe service deficiencies...and inhibit participatory voice for low-income communities. When we leave conditions associated with city life—including housing density, poverty, and crime—within the jurisdiction of an underfunded, weak, or distant local [county] government, we leave low-income residents to bear costs that, in other contexts, a greater collective would assume. (49)
Our prior research has found that African American neighborhoods in these municipal extraterritorial jurisdictions are significantly less likely to be served by a public water utility than majority-white extraterritorial jurisdiction neighborhoods (48, 50), a legacy of racial residential segregation policies designed to exclude African American communities from full participation in municipal governance (51–54). The fact that residence in an extraterritorial jurisdiction was associated with higher blood Pb levels after controlling for water source type, home value, and other socioeconomic and demographic covariates suggests that incorporation and access to municipal water and other services can help reduce environmental Pb exposure and uptake.
Overall, our findings suggest that private well water remains an important source of Pb exposure for many children and that, unlike other environmental pathways, these risks remain significant even in newer homes.
Limitations.
The major limitation of this study is lack of measured household tap water Pb concentrations for the children in the North Carolina Childhood Lead Poisoning Prevention Program database. However, the strength of the association between water source (private well or regulated water utility) and blood Pb concentration is highly significant (Z = 8.13, P < 0.0001) even when controlling for other variables that may indicate other exposure sources (such as household age, an indicator of the risk of exposure from dust and soil containing remnants of Pb paint). In addition, our findings were highly robust against model forms, including inclusion or exclusion of other independent variables, use of alternative thresholds (e.g., 2 µg/dL) as indicators of elevated Pb concentrations, and consideration of different levels of geographic clustering or left censoring. Thus, we have high confidence that reliance on private well water is, indeed, an important risk factor for childhood Pb exposure.
Conclusions
This study highlights the need for interventions to decrease Pb exposure risks in households relying on private wells for their drinking water. Approximately 3.2 million children under age 7 (13% of this age group) live in such households. Our findings suggest that these children are at significantly increased risk of exposure to Pb in water, compared with their peers with community water service. These risks are compounded in African American neighborhoods that remain excluded from access to nearby municipal water service, a legacy of discriminatory zoning practices that could contribute to persistent intergenerational poverty through its impacts on children’s cognitive development.
Multiple types of policy interventions could help prevent exposure to Pb in drinking water from private wells. First, legislation may be needed to ensure that all components in new wells, as well as in plumbing and fixtures connecting private wells to houses, are Pb-free. These components currently are not covered by legislation restricting Pb use in indoor plumbing (42). Recent case studies illustrate the potential risks of Pb exposure that can arise from leaching of well components, even in new wells. For example, Pieper et al. (42) reported that 14% of water samples collected from new private wells between 2008 and 2012 contained Pb above 15 ppb.
Second, questions about water source could be added to Pb risk screening questionnaires. For example, North Carolina recommends the administration of a blood Pb risk screening questionnaire for all children at 12 and 24 mo of age (55). The screening questionnaire asks about multiple potential risk factors for Pb exposure—including whether the child lives in or regularly visits a home built before 1950, lives in or visits a home built before 1978 that has ongoing renovations, lives in a house with vinyl miniblinds, has a sibling who has had a high blood Pb level, lives in a high-risk zip code, or is a refugee—but does not ask about the child’s drinking water source.
Third, resources to support outreach to private well owners by local health departments could be increased. At a minimum, health departments could use this additional support to promote water testing (through media platforms, mailers, pediatricians’ offices, childcare centers, and other venues frequented by families with young children). They could also increase their capacity to provide guidance on selection and implementation of control measures such as replacement of confirmed or suspected Pb-containing water system components, installation and maintenance of corrosion-control systems, and/or point-of-use or point-of-entry water filters capable of removing Pb, in cases where high Pb concentrations in drinking water are detected or suspected. To implement such outreach campaigns, local health departments are likely to need targeted financial support to hire and train personnel.
Even with these measures, some well owners will need financial assistance to control Pb in their water systems. Prior research suggests that costs are a barrier to routine monitoring and maintenance of private well water (29, 36). In North Carolina, typical costs to test well water for Pb are over $100. A basic filter to remove Pb in water at the kitchen tap costs about $200, not including hiring a plumber for installation. Replacement filter cartridges, costing about $50, are needed every 6 mo. For low-income families, these costs may pose an insurmountable barrier to maintaining water quality. The Safe Drinking Water Act provides funding mechanisms, such as the Drinking Water State Revolving Loan Fund (56), to support community water supplies. However, no such support is available to households relying on private wells. Prior research suggests that the economic payback from interventions to decrease Pb exposure risk from air, paint, and community water systems has been high (57). Such benefits also are expected to accrue from decreases in children’s exposure to Pb in private well water. To date, however, this potential exposure route has been largely overlooked, and affected households are left to manage risks on their own.
Materials and Methods
Overview.
This study leverages routinely collected blood Pb surveillance data from Wake County, NC (population 1.1 million). We linked these data to publicly available information on household water sources, construction dates, and property values, along with neighborhood demographic variables. We then explored associations between water source (private well or regulated community system) and blood Pb, controlling for other household and neighborhood characteristics that might influence Pb exposure. The study was reviewed and approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (no. 18-1434).
Database Development.
North Carolina’s Childhood Lead Poisoning Prevention Program provided all blood Pb test results for children screened in Wake County during 1985 to 2017. Data included the child’s address, birth date, biological sex, blood sample date, and sample type (venous or capillary). All NC healthcare providers are required to report children’s blood Pb test results to this program, which compiles the results for surveillance purposes. Pediatricians are encouraged to test all children at ages 12 and 24 mo. Screening is mandatory for children enrolled in federal and state assistance programs (Women, Infants, and Children; Medicaid; Health Choice) and refugees. Children are tested by the collection of a capillary or venous blood sample, which is analyzed at the state public health laboratory. Results (in µg/dL) are rounded to the nearest integer, with a detection limit of 1 µg/dL. Wake County typically has high rates of blood Pb screening; in 2017, 45.6% of children aged 12 to 24 mo were tested (58). In total, we received 226,757 blood test records.
Since there were repeat analyses for some children, our analysis focuses on the first blood Pb measurement for any one child. We used the first measurement because many follow-up blood tests are conducted to assess whether an intervention to decrease Pb exposure has been effective. Therefore, these second tests do not accurately reflect the role of water source, all other factors being equal. Whereas initial Pb screenings are recommended for all children (and, in Wake County, conducted for the majority of them), additional testing is more likely among children whose first measurement shows a higher Pb level. Children who were tested more than once had significantly higher blood Pb on the first test than those tested only once [mean 1.99 µg/dL for the first blood Pb test in children tested at least twice versus mean 1.69 µg/dL for children tested only once, t(41,500 degrees of freedom) = 21.6, P < 0.0001]. The set of second blood Pb measurements therefore reflects a process in which, for some children, a first test has triggered a response to decrease Pb exposure. As a result, incorporating testing beyond the first screening would produce a biased estimate of the effect of well water on blood Pb in the absence of an intervention.
Blood Pb records were merged at the address level with residential property tax records obtained from the Wake County Geographic Information Systems Division. The house’s water source (private well or community system), size (ft2), and tax value were obtained. In addition, an indicator variable was included for houses zoned in municipal extraterritorial jurisdictions. Under North Carolina law, cities and towns can exercise zoning authority over land up to 3 mi from their borders (depending on the town’s population) without providing municipal services (such as municipally supplied water) in those areas, although towns sometimes extend their water services into these areas. This variable was included because our prior research demonstrated racial disparities in access to community water service in such extraterritorial jurisdictions, with access less likely in majority-African American areas (50), and high levels of lead in private well water in these areas (33).
Information on access to community water service for each household was available for 2002 to 2010, 2013, and 2018. For years in which utility data were not available (2011 to 2012; 2014 to 2017), the last available utility data were propagated forward (e.g., if a parcel had municipal water service in 2010, it was assumed to have similar service in 2011). A data check confirmed that <0.1% of parcels changed water service type during the study period (e.g., from private well to community water system or vice versa). Each child’s address also was matched to 2013 to 2017 American Community Survey data on racial composition and median household income at the block group level.
Blood Pb and property data were cleaned and merged in STATA 15.1 (StataCorp) by city, address, and zip code using the DTAlink probabilistic matching function. Briefly, merge parameters were iteratively revised, and results were reviewed until the merge produced results that included only exact matches and plausible inexact matches upon inspection of subsets of 100 matches. Because more than one child lived at some addresses, a many-to-one merge was used. Addresses for each parcel were then mapped to census blocks in ArcMap (version 10.6; ESRI) using a shapefile of Wake County from the Wake County Geographic Information Services Division. Fewer than 1% of addresses were not matched to census blocks, and these were dropped.
The merged dataset contained 77,969 unique records. All records for which blood Pb measurements or blood draw date were missing were dropped. In addition, all records prior to 2002 (the first year in which municipal water access data were available) were dropped. Our final, curated dataset contained 59,483 unique records, corresponding to 41,871 unique addresses (see summary statistics in SI Appendix, Table S5). Fig. 2 summarizes the process used to merge the data.
Data Analysis.
Separate regression analyses were conducted to assess the influence of private well water on 1) the child’s blood lead concentration and 2) the proportion of children for whom blood Pb concentrations exceeded the CDC’s 5 µg/dL action level. While acknowledging that there is no known safe Pb exposure level, the CDC has established 5 µg/dL as a threshold “to identify children with blood Pb levels that are much higher than most children’s levels” (59). In North Carolina and many other states, a household Pb investigation must be offered to the family of any child whose blood Pb concentration is 5 to 9 µg/dL; an investigation is mandatory when the child’s blood Pb exceeds this range (60).
A conceptual model of child lead exposure was developed (SI Appendix, Fig. S2) and relevant variables associated with nodes in the conceptual model were identified. These were then included in univariable (ordinary least-squares) regressions (SI Appendix, Table S1) and in subsequent multivariable models. Child age at the time of blood draw was discretized into bins (0 to 8; 9 to 14; 15 to 19; 20 to 29; ≥30 mo) that were designed to capture the underlying structure of the data (most children were tested near their first or second birthday) and/or previously reported associations with blood lead [levels have been reported to increase between birth and 18 to 24 mo (61)]. Building age was discretized (<1950; 1950 to 1977; 1978 to 1987; 1988 to 1997; 1998 to 2002; 2003 to 2007; ≥2008) into bins based on relevant changes in regulations (e.g., bans on lead in paint and gasoline; SI Appendix, Fig. S1), construction practices, and building codes over time (62).
To control for the effects of multiple observations per residential address and the effects of left censoring on the dataset, a mixed-effects tobit regression of log-transformed blood lead levels was conducted using Stata’s metobit function. Children were clustered on street address with robust SEs clustered on the census block group. The tobit approach accounts for left censoring by representing the different processes generating censored and uncensored observations (63). (In our sample, 62.9% of the observations were left-censored at 1 µg/dL due to the detection limit of standard blood Pb tests.) A sensitivity analysis to test the robustness of any influence of water source on Pb exposure risk was performed by using a Monte Carlo permutation test in which the effects of randomly assigning households across census block groups were simulated with 1,000 repetitions. A mixed-effects logistic regression model clustered on street address with robust SEs clustered on census block group was also used to estimate the influence of independent variables on the risk of a blood Pb level equal to or greater than 5 µg/dL. In both regressions (the mixed-effects tobit and logistic models), interactions between water source type and building age were also tested to assess whether the restriction on Pb content of plumbing to 8% or less by weight enacted under Section 1417 of the Safe Drinking Water Act Amendment of 1986 (SI Appendix, Fig. S1) influenced Pb exposure risk differences between children with and without regulated community water service at home.
Data Availability.
This analysis is based on identifiable medical data. Deidentified data are available at https://scholarworks.iu.edu/dspace/handle/2022/25638. Those wishing to use the full, identifiable dataset would need to sign a data-use agreement with the NC Department of Health and Human Services. Code used for data cleaning and analysis is included in SI Appendix.
Supplementary Material
Acknowledgments
We are grateful to the NC Childhood Lead Poisoning Prevention Program for providing access to the children’s blood Pb data central to this research. In particular, we thank Program Manager Ed Norman, Data Manager Tena Hand, and Public Health Epidemiologist Melanie Napier. This research was funded by the US Environmental Protection Agency Science to Achieve Results Program under Grant 83927901.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. B.L. is a guest editor invited by the Editorial Board.
Data deposition: The deidentified data used in this paper have been deposited at the IUScholarWorks repository, https://scholarworks.iu.edu/dspace/handle/2022/25638.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002729117/-/DCSupplemental.
References
- 1.Needleman H. L., Leviton A., Bellinger D., Lead-associated intellectual deficit. N. Engl. J. Med. 306, 367 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Lanphear B. P. et al., Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 113, 894–899 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazumdar M. et al., Low-level environmental lead exposure in childhood and adult intellectual function: A follow-up study. Environ. Health 10, 24 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Searle A. K. et al., Tracing the long-term legacy of childhood lead exposure: A review of three decades of the Port Pirie Cohort study. Neurotoxicology 43, 46–56 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Needleman H. L., Schell A., Bellinger D., Leviton A., Allred E. N., The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N. Engl. J. Med. 322, 83–88 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Miranda M. L. et al., The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environ. Health Perspect. 115, 1242–1247 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang N. et al., Early childhood lead exposure and academic achievement: Evidence from Detroit public schools, 2008-2010. Am. J. Public Health 103, e72–e77 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen L. C., Fox A. M., Jung H., Martin E. G., Lead exposure and academic achievement: Evidence from childhood lead poisoning prevention efforts. J. Popul. Econ. 32, 179–218 (2019). [Google Scholar]
- 9.Goodlad J. K., Marcus D. K., Fulton J. J., Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clin. Psychol. Rev. 33, 417–425 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Sampson R. J., Winter A. S., Poisoned development: Assessing childhood lead exposure as a cause of crime in a birth cohort followed through adolescence. Criminology 56, 269–301 (2018). [Google Scholar]
- 11.Needleman H. L., McFarland C. E., Ness R., Bone lead levels in adjudicated delinquency: A case-control study. Neurotoxicol. Teratol. 24, 11–17 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Tsoi M. F., Cheung C. L., Cheung T. T., Cheung B. M., Continual decrease in blood lead level in Americans: United States National Health Nutrition and Examination Survey 1999-2014. Am. J. Med. 129, 1213–1218 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Schnur J., John R. M., Childhood lead poisoning and the new Centers for Disease Control and Prevention guidelines for lead exposure. J. Am. Assoc. Nurse Pract. 26, 238–247 (2014). [DOI] [PubMed] [Google Scholar]
- 14.US Centers for Disease Control and Prevention , “U.S. totals blood lead surveillance” (2016). https://www.cdc.gov/nceh/lead/data/Chart_Website_StateConfirmedByYear_1997_2015.pdf. Accessed 9 December 2016.
- 15.Agency for Toxic Substances and Disease Registry , “Toxicological profile for lead” (2019). https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. Accessed 23 June 2020. [PubMed]
- 16.Centers for Disease Control and Prevention (CDC) , Blood lead levels in children aged 1-5 years—United States, 1999-2010. MMWR Morb. Mortal. Wkly. Rep. 62, 245–248 (2013). [PMC free article] [PubMed] [Google Scholar]
- 17.Aizer A., Currie J., Disadvantage and health at birth. Science 344, 856–861 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triantafyllidou S., Edwards M., Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Crit. Rev. Environ. Sci. Technol. 42, 1297–1352 (2012). [Google Scholar]
- 19.Renner R., Exposure on tap: Drinking water as an overlooked source of lead. Environ. Health Perspect. 118, A68–A72 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin R. et al., Lead exposures in U.S. children, 2008: Implications for prevention. Environ. Health Perspect. 116, 1285–1293 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Water Works Association (AWWA) , Internal Corrosion Control in Water Distribution Systems (Manual M58, AWWA, ed. 1, 2011). [Google Scholar]
- 22.US Environmental Protection Agency , “Lead and Copper Rule” (1991). https://www.ecfr.gov/cgi-bin/text-idx?SID=9c5415b2fe8eb76878a169c14454171f&mc=true&node=sp40.25.141.i&rgn=div6. Accessed 10 June 2019.
- 23.Clark A., The Poisoned City: Flint’s Water and the American Urban Tragedy, (Metropolitan Books, 2018). [Google Scholar]
- 24.Hanna-Attisha M., LaChance J., Sadler R. C., Champney Schnepp A., Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 106, 283–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornwell D. A., How have we done in reducing lead in water since the LCR? J. Am. Water Works Assoc. 110, 30–37 (2018). [Google Scholar]
- 26.Brown R. A., McTigue N. E., Cornwell D. A., Strategies for assessing optimized corrosion control treatment of lead and copper. J. Am. Water Works Assoc. 105, 62–75 (2013). [Google Scholar]
- 27.Dieter C. A., Maupin M. A., “Public supply and domestic water use in the United States, 2015” (Rep. No. 2017-1131, US Geological Survey, 2017).
- 28.US Congress , “Safe Drinking Water Act” (1974). Public Law 93-523. https://www.epa.gov/sites/production/files/2020-05/documents/safe_drinking_water_act-title_xiv_of_public_health_service_act.pdf. Accessed 19 June 2020.
- 29.Stillo F. III, Bruine de Bruin W., Zimmer C., Gibson J. M., Well water testing in African-American communities without municipal infrastructure: Beliefs driving decisions. Sci. Total Environ. 686, 1220–1228 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Fizer C., “Barriers to private well and septic management: An analysis of homeowner decision-making,” MSc thesis, University of North Carolina at Chapel Hill, Chapel Hill, NC (2016).
- 31.Malecki K. M. C., Schultz A. A., Severtson D. J., Anderson H. A., VanDerslice J. A., Private-well stewardship among a general population based sample of private well-owners. Sci. Total Environ. 601–602, 1533–1543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieper K. J., Krometis L. A. H., Gallagher D. L., Benham B. L., Edwards M., Incidence of waterborne lead in private drinking water systems in Virginia. J. Water Health 13, 897–908 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Stillo F., MacDonald Gibson J., Racial disparities in access to municipal water supplies in the American South: Impacts on children’s health. Int. Public Health J. 10, 309–323 (2018). [Google Scholar]
- 34.Swistock B. R., Clemens S., Sharpe W. E., Rummel S., Water quality and management of private drinking water wells in Pennsylvania. J. Environ. Health 75, 60–66 (2013). [PubMed] [Google Scholar]
- 35.Knobeloch L., Gorski P., Christenson M., Anderson H., Private drinking water quality in rural Wisconsin. J. Environ. Health 75, 16–20 (2013). [PubMed] [Google Scholar]
- 36.Fizer C., Bruine de Bruin W., Stillo F., Gibson J. M., Barriers to managing private well and septic systems in underserved communities: Mental models of homeowner decision making. J. Environ. Health 81, 8–15 (2018). [Google Scholar]
- 37.US Department of Health and Human Services , “Environmental Health. Healthy People 2020” (2010). https://www.healthypeople.gov/2020/topics-objectives/topic/environmental-health/objectives. Accessed 9 February 2016.
- 38.Ngueta G., Abdous B., Tardif R., St-Laurent J., Levallois P., Use of a cumulative exposure index to estimate the impact of tap water lead concentration on blood lead levels in 1- to 5-year-old children (Montréal, Canada). Environ. Health Perspect. 124, 388–395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanphear B. P., et al. , Environmental lead exposure during early childhood. J. Pediatr. 140, 40–47 (2002). Correction in: J. Pediatr.140, 490 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Etchevers A. et al., Environmental determinants of different blood lead levels in children: A quantile analysis from a nationwide survey. Environ. Int. 74, 152–159 (2015). [DOI] [PubMed] [Google Scholar]
- 41.US Congress , “Reduction of Lead in Drinking Water Act” (2011). Public Law 111-380. https://www.congress.gov/111/plaws/publ380/PLAW-111publ380.pdf. Accessed 19 June 2020.
- 42.Pieper K. J. et al., Elevated lead in water of private wells poses health risks: Case study in Macon County, North Carolina. Environ. Sci. Technol. 52, 4350–4357 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Pieper K. J., Krometis L.-A., Edwards M., Quantifying lead-leaching potential from plumbing exposed to aggressive waters. J. AWWA 108, E458–E466 (2016). [Google Scholar]
- 44.Stewart L. R., Farver J. R., Gorsevski P. V., Miner J. G., Spatial prediction of blood lead levels in children in Toledo, OH using fuzzy sets and the site-specific IEUBK model. Appl. Geochem. 45, 120–129 (2014). [Google Scholar]
- 45.Brown S. L., Chaney R. L., Hettiarachchi G. M., Lead in urban soils: A real or perceived concern for urban agriculture? J. Environ. Qual. 45, 26–36 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Mahaffey K. R., Nutrition and lead: Strategies for public health. Environ. Health Perspect. 103 (suppl. 6), 191–196 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson D. L., McDade K., Griffith D., Seasonal variation in paediatric blood lead levels in Syracuse, NY, USA. Environ. Geochem. Health 18, 81–88 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Leker H. G., MacDonald Gibson J., Relationship between race and community water and sewer service in North Carolina, USA. PLoS One 13, e0193225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson M. W., Cities inside out: Race, poverty, and exclusion at the urban fringe. UCLA Law Rev. 55, 1095–1160 (2008). [Google Scholar]
- 50.MacDonald Gibson J., DeFelice N., Sebastian D., Leker H., Racial disparities in access to community water supply service in Wake County, North Carolina. Front. Public Health Serv. Syst. Res. 3, Article 6 (2014). [Google Scholar]
- 51.Johnson J. H., Parnell A., Joyner A. M., Christman C. J., Marsh B., Racial apartheid in a small North Carolina town. Rev. Black Polit. Econ. 31, 89–107 (2004). [Google Scholar]
- 52.Aiken C. S., Race as a factor in municipal underbounding. Ann. Assoc. Am. Geogr. 77, 564–579 (1987). [Google Scholar]
- 53.Marsh B., Parnell A. M., Joyner A. M., Institutionalization of racial inequality in local political geographies. Urban Geogr. 31, 691–709 (2010). [Google Scholar]
- 54.Joyner A. M., Christman C. J., Segregation in the Modern South: A Case Study of Southern Moore County, (Cedar Grove Institute for Sustainable Communities, Mebane, NC, 2005). [Google Scholar]
- 55.NC Department of Health and Human Services , “Lead risk assessment questionnaire” (2013). EHS 3958. https://ehs.ncpublichealth.com/docs/forms/cehu/2012-0416-46LeadRiskAssessmentQuestionnaire3958.pdf. Accessed 19 June 2020.
- 56.Tiemann M., “Drinking Water State Revolving Loan Fund: Overview issues and legislation” (Report R45304, Congressional Research Service, Washington, DC, 2018). https://fas.org/sgp/crs/misc/R45304.pdf. Accessed 19 June 2020.
- 57.Gould E., Childhood lead poisoning: Conservative estimates of the social and economic benefits of lead hazard control. Environ. Health Perspect. 117, 1162–1167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.North Carolina Division of Public Health , North Carolina childhood blood lead surveillance data (2019). https://ehs.ncpublichealth.com/hhccehb/cehu/lead/docs/BloodLeadTbl2017.pdf. Accessed 19 June 2020.
- 59.US Centers for Disease Control and Prevention , “CDC—Lead—New blood lead level information (2017). https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnceh%2Flead%2Facclpp%2Fblood_lead_levels.htm. Accessed 17 June 2019.
- 60.Norman E., “Revised Childhood Lead Poisoning Prevention Program expansion implementation plan” (Division of Public Health, Raleigh, NC, 2017). Memorandum. https://ehs.ncpublichealth.com/docs/position/RevisedChildhoodLeadExpansionImplementationPlan12212017.pdf. Accessed 17 June 2019.
- 61.Canfield R. L. et al., Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 348, 1517–1526 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown M. J., Margolis S., Lead in drinking water and human blood lead levels in the United States. MMWR Suppl. 61, 1–9 (2012). [PubMed] [Google Scholar]
- 63.McBee M., Modeling outcomes with floor or ceiling effects: An introduction to the tobit model. Gift. Child Q. 54, 314–320 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This analysis is based on identifiable medical data. Deidentified data are available at https://scholarworks.iu.edu/dspace/handle/2022/25638. Those wishing to use the full, identifiable dataset would need to sign a data-use agreement with the NC Department of Health and Human Services. Code used for data cleaning and analysis is included in SI Appendix.