Significance
The prefrontal cortex (PFC) has long been recognized as a crucial region for suppressing impulsive behavior, but the specific neurons mediating the effect remained elusive. Using projection-specific labeling of PFC neurons for imaging and optogenetic manipulation in mice performing a go/no-go task, we showed that the subset of layer 5 pyramidal neurons projecting to the subthalamic nucleus, but not those projecting to the lateral hypothalamus or visual cortex, play a critical role in inhibiting impulsive responses. Our demonstration of a distinct functional property of a projection-defined PFC pyramidal neuron population provides an important entry point for delineating the PFC circuit underlying impulse control.
Keywords: prefrontal cortex, impulsive behavior, subthalamic nucleus, lateral hypothalamus, two-photon calcium imaging
Abstract
The prefrontal cortex (PFC) plays a critical role in curbing impulsive behavior, but the underlying circuit mechanism remains incompletely understood. Here we show that a subset of dorsomedial PFC (dmPFC) layer 5 pyramidal neurons, which project to the subthalamic nucleus (STN) of the basal ganglia, play a key role in inhibiting impulsive responses in a go/no-go task. Projection-specific labeling and calcium imaging showed that the great majority of STN-projecting neurons were preferentially active in no-go trials when the mouse successfully withheld licking responses, but lateral hypothalamus (LH)-projecting neurons were more active in go trials with licking; visual cortex (V1)-projecting neurons showed only weak task-related activity. Optogenetic activation and inactivation of STN-projecting neurons reduced and increased inappropriate licking, respectively, partly through their direct innervation of the STN, but manipulating LH-projecting neurons had the opposite effects. These results identify a projection-defined subtype of PFC pyramidal neurons as key mediators of impulse control.
Impulse control is an essential cognitive process that allows the animal to inhibit inappropriate habitual responses and facilitate behaviors consistent with the internal goal (1–3). A simple behavioral paradigm well suited for measuring response inhibition is the go/no-go task, in which the subject is required to respond to the go stimulus but withhold response to the no-go stimulus. Previous studies in multiple species have shown that lesions in the prefrontal cortex (PFC) cause marked increases in false-alarm errors (inappropriate responses in no-go trials) (e.g., refs. 4 and 5), and optogenetic inactivation of the mouse dorsomedial PFC (dmPFC) demonstrated the importance of its activity on a trial-by-trial basis (6). In tasks that require waiting for a sensory cue before making a motor response, lesion or inactivation of the rat dmPFC also increased premature response errors (7–10). Together, these findings indicate a critical role of the rodent dmPFC in inhibiting impulsive responses.
A variety of task-related signals have been observed in PFC neuron activity, including those associated with sensory cues, motor actions, and task outcomes (reward and punishment) (9, 11–14). Within a given PFC subregion, different subtypes of inhibitory interneurons—labeled by distinct molecular markers—are preferentially activated by different task-related events, but neurons of the same subtype exhibit similar activity (15). PFC pyramidal neurons also exhibit a high degree of functional heterogeneity, but its relationship with molecularly defined cell types is much less understood. During a delayed go/no-go task, the delay-period activity of some pyramidal neurons in mouse dmPFC is higher in no-go trials (NG-preferring), signaling the intention to withhold the motor response, but other neurons are selectively active in go trials (go-preferring) (6), similar to that found in the motor cortex (16). Although the NG-preferring neurons are outnumbered by go-preferring neurons, nonselective inhibition of pyramidal neuron activity caused much more false alarms (FAs) than misses (failure to respond in go trials), suggesting that the NG-preferring neurons are crucial for response inhibition. Calcium imaging showed that NG-preferring neurons are more abundant in deep than superficial cortical layers, but even in deep layers, there are many go-preferring neurons intermingled with NG-preferring neurons (6). This makes it difficult to target the NG-preferring neurons selectively to examine the cause and consequence of their activation in go/no-go behavior.
Previous studies in sensory (17–20), motor (21, 22), and prefrontal (23–25) cortical areas have shown that the functional properties of neurons are correlated with their projection targets. For response inhibition, a growing number of studies have indicated the importance of the subthalamic nucleus (STN) in the basal ganglia (26–33), which receives direct input from the PFC through the hyperdirect pathway (34). This raises the possibility that response inhibition in no-go trials is partly mediated by STN-projecting dmPFC neurons. To test this hypothesis, we selectively labeled the dmPFC pyramidal neurons projecting to the STN, visual cortex (V1), or lateral hypothalamus (LH) using a retrograde virus. Calcium imaging showed that the great majority of STN-projecting neurons are NG-preferring, whereas most of the LH-projecting neurons are go-preferring; V1-projecting neurons showed only weak go or no-go preference. Optogenetic activation of STN-projecting dmPFC neurons or their axons in the STN markedly improved task performance mainly by reducing the FA rate, whereas inactivating these neurons had the opposite effect. In contrast, bidirectional manipulation of LH-projecting neurons showed that their activity impairs performance by increasing the FA rate. Together, these results show that STN-projecting dmPFC pyramidal neurons play a critical role in impulse control through the hyperdirect pathway.
Results
Different dmPFC Layer 5 Neurons Project to STN, V1, and LH.
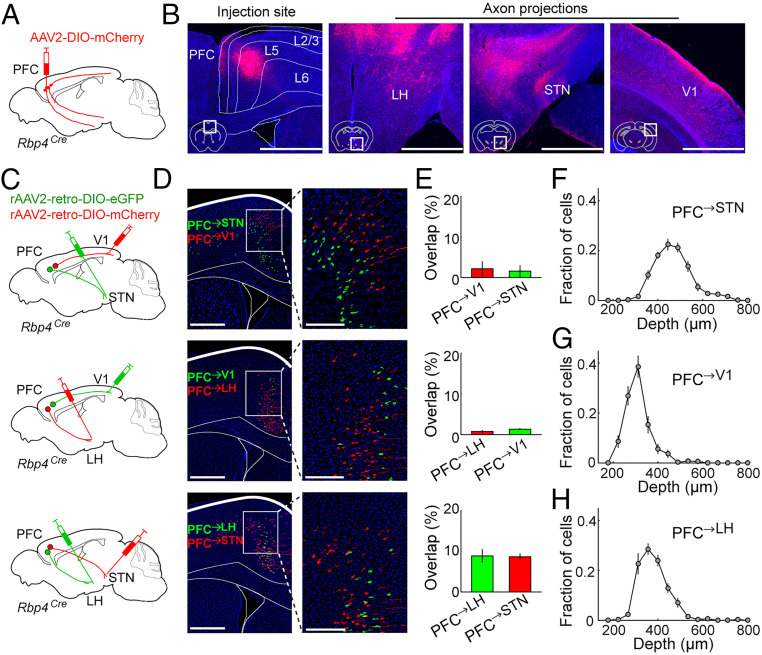
The projection from the PFC to the STN originates primarily from pyramidal tract neurons in layer 5 (35, 36). To compare the STN-projecting and other neuronal populations in the same layer, we used Rbp4Cre mice, which express Cre-recombinase specifically in layer 5 pyramidal neurons (37). Injection of Cre-inducible adeno-associated virus expressing mCherry (AAV2-EF1α-DIO-mCherry) into the dmPFC of Rbp4Cre mice revealed multiple projection targets. In addition to the ipsilateral STN, layer 5 neurons also project bilaterally to the LH and V1 among other brain areas (Fig. 1 A and B and SI Appendix, Fig. S1).
Fig. 1.
Different dmPFC layer 5 neurons project to STN, V1, and LH. (A) Virus injection for tracing axonal projections from dmPFC layer 5 neurons. (B) Fluorescence images of the injection site (white box in coronal diagram) and several ipsilateral projection targets. Red, mCherry; blue, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). (Scale bar, 1 mm.) (C) Injection procedure for dual retrograde tracing. (D) Fluorescence images of retrogradely labeled PFC neurons. (Right) Enlarged view of the region in white box (Left). Red, mCherry; green, eGFP; blue, DAPI. (Scale bar, Left, 500 µm, and Right, 100 µm.) (E) Quantification of overlap between each pair of projection-defined populations (n = 3 mice each). Spatial distribution of (F) PFC→STN (n = 6 mice), (G) PFC→V1 (n = 6 mice), and (H) PFC→LH (n = 6 mice) neurons. The three distributions are significantly different (PFC→STN vs. PFC→V1, P = 7.7 × 10−6; PFC→LH vs. PFC→V1, P = 0.03; PFC→STN vs. PFC→LH, P = 0.006; Kolmogorov–Smirnov test, Bonferroni–Holm corrected). All error bars indicate ±SEM.
We then performed dual retrograde labeling to identify dmPFC neurons that are separate from those projecting to the STN (referred to as “PFC→STN neurons”). Previous studies showed that PFC→STN neurons send axon collaterals to multiple brain regions but not sensory cortices such as V1 (35). Indeed, Cre-inducible retrograde AAVs (38) expressing mCherry (rAAV2-retro-EF1α-DIO-mCherry) and eGFP (rAAV2-retro-EF1α-DIO-eGFP) injected into the STN and V1, respectively, labeled distinct layer 5 populations (Fig. 1 C–E), although the incomplete retrograde labeling of each population is likely to result in underestimation of the overlap (Discussion). The different spatial distributions of mCherry- and eGFP-labeled neurons (Fig. 1 F and G) (P = 7.7 × 10−6, Kolmogorov–Smirnov test) are consistent with the notion that PFC→V1 neurons are intratelencephalic, separate from PFC→STN pyramidal tract neurons (36, 39). Another major projection target of the dmPFC is the LH (Fig. 1B), in which GABAergic neurons strongly promote reward-seeking behaviors (40). Dual retrograde tracing showed that PFC→LH layer 5 neurons are largely distinct from both PFC→V1 and PFC→STN populations (Fig. 1 C–H). We thus compared the functional roles of these three dmPFC layer 5 populations in response inhibition.
Activity of PFC→STN, PFC→V1, and PFC→LH Neurons during Go/No-Go Task.
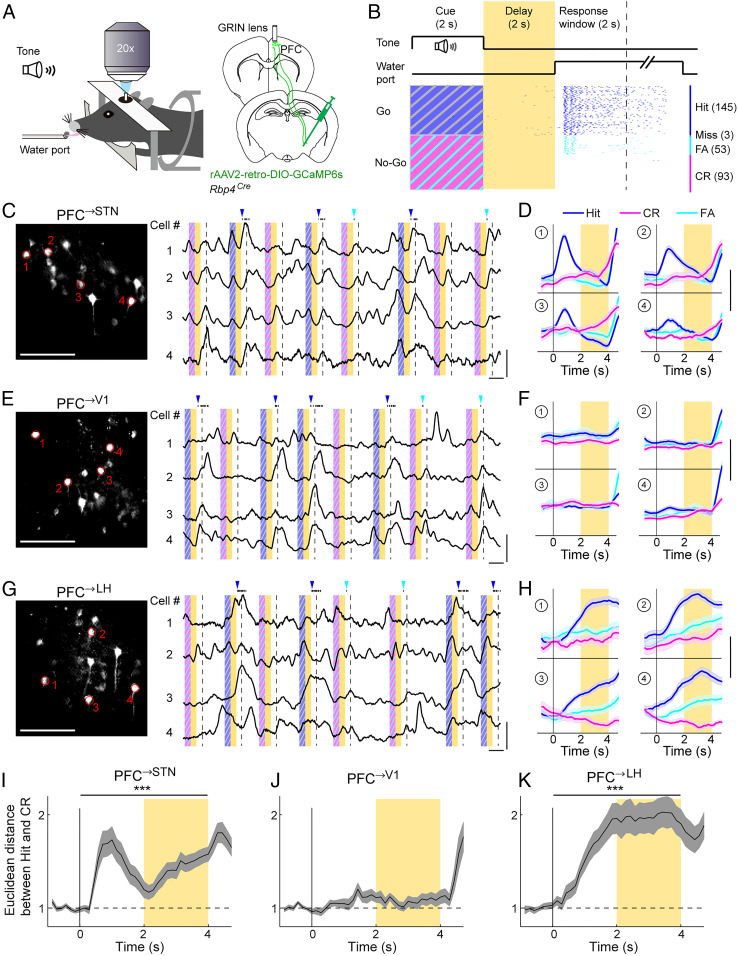
We trained head-fixed mice on a delayed go/no-go task (Fig. 2 A and B). During each trial, either a go (17 kHz) or no-go (9 kHz) auditory cue was presented, and the response period began when a water port was presented. Licking in go trials within the response window (hit) was rewarded, but licking in no-go trials (FA) was punished; licking outside of the response window was ignored. A 2-s delay period was inserted between the cue and response periods, allowing us to examine the temporal dynamics of dmPFC activity separate from the cue-evoked and reward/punishment-evoked responses. Mice learned this task within several weeks, licking mostly during the response window (SI Appendix, Fig. S2A) and performing at ∼80% correct rate (SI Appendix, Fig. S2B). Similar to previous studies (6, 15), most of the errors were FAs rather than misses, indicating that the performance depends mainly on successful inhibition of inappropriate responses.
Fig. 2.
Activity of PFC→STN, PFC→V1, and PFC→LH neurons during go/no-go task. (A) Schematic of calcium imaging during task. (B) Schematic for the task structure (Top) and an example behavioral session (Bottom). Each tick indicates one lick. Yellow shading indicates delay period. Dashed line indicates end of response window. Numbers in brackets indicate the number of trials of each type. (C) (Left) Field of view in an example imaging session for PFC→STN neurons. Red outlines indicate example cells shown on the right. (Scale bar, 100 µm.) (C) (Right) Raw fluorescence traces of the example ROIs. Yellow shading indicates delay period; blue/magenta stripes indicate cue periods with target/nontarget tones, respectively. Dashed line indicates end of response window. Black ticks on top are lick responses. Blue/cyan arrowheads indicate delivery of reward/punishment. (Vertical scale bar, 3 Z scores; horizontal scale bar, 5 s.) (D) Trial-averaged activity of the example ROIs. Shading indicates ±SEM. (Scale bar, 1 Z score.) (E and F) Similar to C and D but for PFC→V1 neurons. (G and H) Similar to C and D but for PFC→LH neurons. (I) Euclidean distance between the population activity in the correct go (hit) and no-go (CR) trials for PFC→STN neurons. The distance during the cue and delay periods was significantly higher than baseline (P = 1.9 × 10−7, Wilcoxon rank sum test, n = 6 mice, ***P < 0.001). (J) Similar to I but for PFC→V1 neurons (P = 0.69, n = 6 mice). (K) Similar to I but for PFC→LH neurons (P = 1.2 × 10−5, n = 6 mice).
To measure the activity of each layer 5 neuronal population, we injected Cre-inducible rAAV2-retro expressing GCaMP6s (38, 41) into the STN, V1, or LH of Rbp4Cre mice (Fig. 2A). Two-photon calcium imaging was performed in the dmPFC through a gradient refractive index (GRIN) lens. All three retrogradely labeled populations exhibited robust activity during the response window (Fig. 2 C, E, and G), largely reflecting widespread responses to reward and punishment (15). To understand the circuit mechanism for response inhibition, we focused on activity before the response window for the remainder of the study. During the cue and delay periods, the percentage of neurons with task-modulated activity (P < 0.01, two-way ANOVA; Materials and Methods) was significantly higher among PFC→STN (79.8%) and PFC→LH (69.1%) than PFC→V1 (54.4%) neurons (P = 5.7 × 10−4, χ2 test). Whereas large fractions of PFC→STN and PFC→LH neurons showed different activity in go and no-go trials, most PFC→V1 neurons were indifferent to the trial type (Fig. 2 D, F, and H), as quantified by the Euclidean distance between the population activity in the correct go (hit) and no-go (CR) trials (Fig. 2 I–K).
Go vs. No-Go Preference of PFC→STN and PFC→LH Neurons.
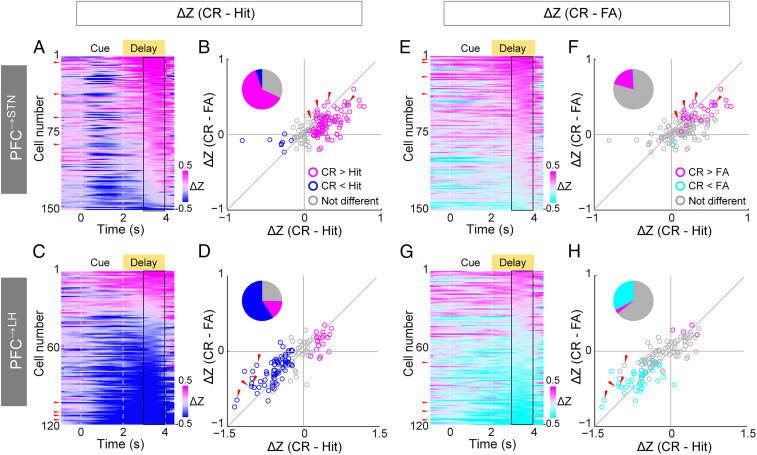
Although both PFC→STN and PFC→LH neurons encode the upcoming motor response, they exhibit distinct temporal dynamics and go vs. no-go preference. For most PFC→STN neurons, the go cue elicited a transient excitation followed by decreasing activity throughout the delay period, whereas the no-go cue evoked slow but persistent upward ramping activity (Fig. 2D). In contrast, the majority of PFC→LH neurons showed strong and sustained excitation in go but not no-go trials (Fig. 2H). To quantify the go vs. no-go preference of each neuron, we computed the difference between the trial-averaged activity in hit and CR trials, focusing on the late delay period that is most relevant to the motor response (Fig. 3 A–D). Of the 150 task-modulated PFC→STN neurons, 94 showed significantly higher activity in CR than hit trials (P < 0.05, two-sided t test, CR-preferring), and only 8 were hit-preferring (Fig. 3B). In contrast, in the PFC→LH population, hit-preferring neurons (73/123) greatly outnumbered CR-preferring neurons (19/123) (Fig. 3D).
Fig. 3.
Go vs. no-go preference of PFC→STN and PFC→LH neurons. (A) Color-coded difference between averaged Z-scored activity in CR and hit trials (CR − hit) for each PFC→STN neuron. Neurons are sorted based on the averaged difference during the late delay period (3 to 4 s). Black rectangle indicates the late delay period quantified in B. Red arrows indicate the neurons shown in Fig. 2D. (B) CR – FA vs. CR – hit activity difference during the late delay period for PFC→STN neurons. Magenta/blue circles indicate neurons with higher/lower activity in CR than hit trials (P < 0.05, two-sided t test). Red arrows indicate the neurons shown in Fig. 2D. (C and D) Similar to A and B but for PFC→LH neurons. Red arrows indicate the neurons shown in Fig. 2H. (E and F) Similar to A and B but for the difference between CR and FA trials. (G and H) Similar to E and F but for PFC→LH neurons.
We also compared trial-averaged activity between FA and CR trials and found 30 CR-preferring and only one FA-preferring PFC→STN neurons (Fig. 3 E and F). In contrast, there were much fewer CR-preferring (4/123) than FA-preferring (40/123) PFC→LH neurons (Fig. 3 G and H). Since FA and CR trials have the same auditory cue and differ only in the behavioral response, this indicates that PFC→STN and PFC→LH neurons have the opposite motor rather than sensory preferences.
Inputs to PFC→STN, PFC→V1, and PFC→LH Neurons.
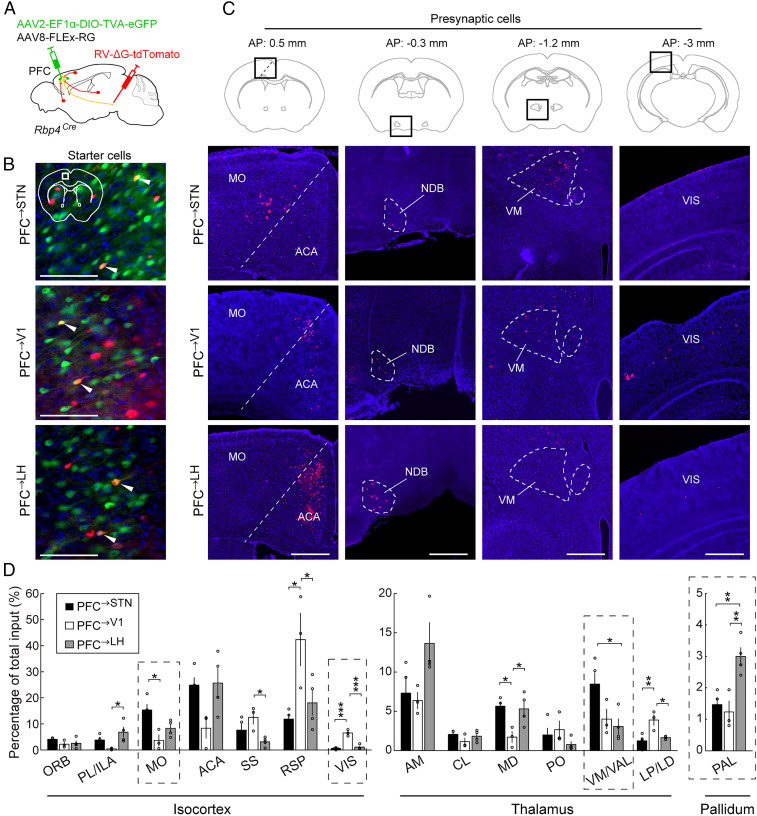
To understand the circuit mechanism underlying the task-related activity of different dmPFC layer 5 neurons, we mapped the monosynaptic inputs to each population. AAV2-EF1α-DIO-TVA-eGFP and AAV8-FLEx-RG were injected into the dmPFC of Rbp4Cre mice, and 3 wk later, rabies virus (RV) expressing tdTomato was injected into the STN, V1 or LH (Fig. 4A). This allowed RV to be taken up by the axon terminals in each region, expressed in the PFC→STN, PFC→V1, or PFC→LH layer 5 neurons (Fig. 4B), and transsynaptically label their presynaptic inputs.
Fig. 4.
Inputs to PFC→STN, PFC→V1, and PFC→LH neurons. (A) Schematic for RV-mediated transsynaptic retrograde tracing. (B) Fluorescence images of starter cells in dmPFC (white box in coronal diagram). Red, RV-tdTomato; green, TVA-eGFP; blue, DAPI. White arrowheads indicate starter cells expressing both RV-tdTomato and TVA-eGFP. (Scale bar, 100 µm.) (C) Fluorescence images of presynaptic cells in several brain regions (black boxes in coronal diagrams). Red, RV-tdTomato; blue, DAPI. MO, somatomotor areas; ACA, anterior cingulate area; NDB, diagonal band nucleus; VM, ventral medial nucleus of the thalamus; VIS, visual cortical area. (Scale bar, 500 µm.) (D) Percentages of total presynaptic inputs (PFC→STN, n = 4 mice; PFC→V1, n = 3 mice; PFC→LH, n = 4 mice; *P < 0.05; **P < 0.01, ***P < 0.001, one-way ANOVA, Tukey’s post hoc test). Only regions with >2% inputs to at least one of the three populations are shown. Dashed boxes indicate regions shown in C. ORB, orbital area; PL, prelimbic area; ILA, infralimbic area; SS, somatosensory areas; RSP, retrosplenial area; AM, anteromedial nucleus of the thalamus; CL, central lateral nucleus of the thalamus; MD, mediodorsal nucleus of the thalamus; PO, posterior complex of the thalamus; VAL, ventral anterior-lateral complex of the thalamus; LP, lateral posterior nucleus of the thalamus; LD, lateral dorsal nucleus of the thalamus. Error bars indicate ±SEM.
Although the three dmPFC populations receive inputs from largely overlapping brain areas (Fig. 4D), there are quantitative differences (Fig. 4 C and D). For example, compared to PFC→V1 and PFC→LH neurons, PFC→STN neurons receive stronger inputs from the motor cortex (MO) and ventral anterior lateral complex (VAL) of the thalamus, both of which are essential areas for motor control (42). PFC→V1, but not PFC→LH and PFC→STN neurons, receive substantial inputs from V1 (43), consistent with reciprocity of corticocortical connections (44). PFC→LH neurons, on the other hand, receive stronger inputs from the pallidum (PAL) (43), whose involvement in reward and motivation (45) may contribute to the strong activation of PFC→LH neurons in go trials.
Causal Functions of PFC→STN and PFC→LH Neurons.
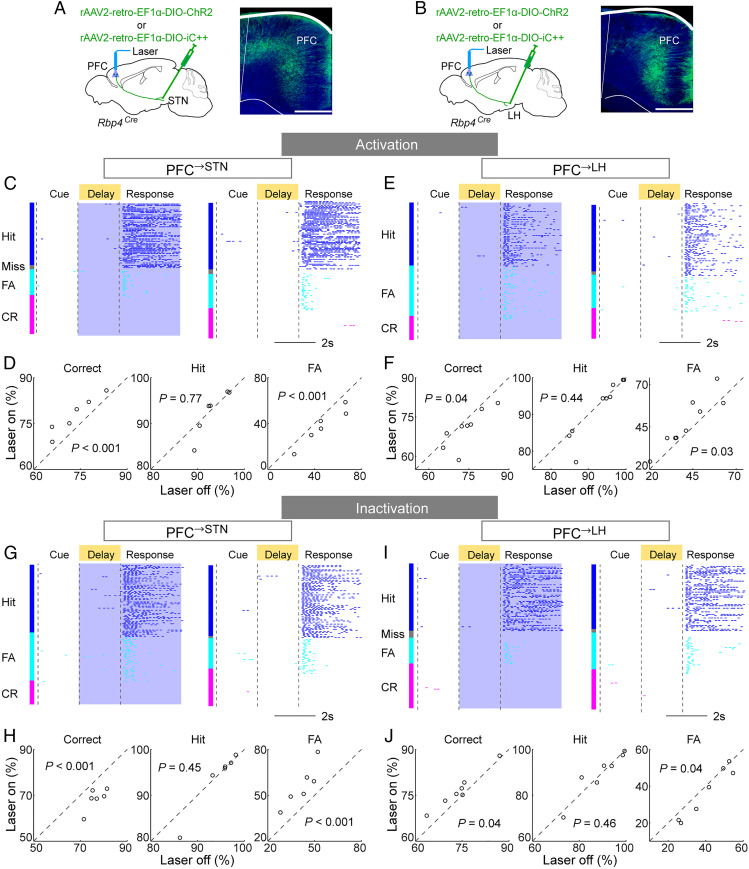
To test the causal role of each dmPFC population in the go/no-go task, we injected rAAV2-retro-EF1α-DIO-ChR2 (41) into the STN, V1, or LH of Rbp4Cre mice (Fig. 5 A and B and SI Appendix, Fig. S3A). Laser stimulation was applied only during the delay and response periods of each trial to minimize the potential effect on cue perception. Activation of PFC→STN neurons caused a marked improvement in task performance measured by correct rate (P < 0.001, bootstrap; Materials and Methods) by reducing the FA (P < 0.001) but not hit rate (P = 0.77; Fig. 5 C and D). In contrast, activating PFC→LH neurons caused a significant behavioral impairment (P = 0.04) due to increased FA rate (P = 0.03; Fig. 5 E and F); activating PFC→V1 neurons had no significant effect (SI Appendix, Fig. S3B; correct rate, P = 0.18; hit rate, P = 0.12; FA rate, P = 0.58).
Fig. 5.
Causal functions of PFC→STN and PFC→LH neurons. (A) Schematic of optogenetic activation/inactivation of PFC→STN neurons (Left) and fluorescence image of dmPFC expressing ChR2-eYFP (Right). Green, ChR2-eYFP; blue, DAPI. (Scale bar, 400 µm.) (B) Similar to A but for PFC→ LH neurons. (C) An example session with PFC→STN neuron activation. (Left) Laser on trials and (Right) laser off trials. Blue shading indicates laser stimulation. Each tick indicates one lick. (D) Activation of PFC→STN neurons caused a marked improvement in task performance by reducing the FA rate (correct rate, P < 0.001; hit rate, P = 0.77; FA rate, P < 0.001; bootstrap). Each circle represents one mouse (n = 6 mice). (E and F) Similar to C and D but for behavioral impairment caused by PFC→LH neuron activation (correct rate, P = 0.04; hit rate, P = 0.44; FA rate, P = 0.03; n = 9 mice). (G and H) Similar to C and D but for PFC→STN neuron inactivation (correct rate, P < 0.001; hit rate, P = 0.45; FA rate, P < 0.001; n = 6 mice). (I and J) Similar to G and H but for PFC→LH neuron inactivation (correct rate, P = 0.04; hit rate, P = 0.46; FA rate, P = 0.04; n = 7 mice).
We then tested the effect of silencing each population through the light-activated chloride channel iC++ (46). Optogenetic inactivation of PFC→STN neurons severely impaired the task performance (Fig. 5 G and H; correct rate, P < 0.001) by increasing the FA rate (P < 0.001), whereas inactivating PFC→LH neurons improved the performance (Fig. 5 I and J; correct rate, P = 0.04) by decreasing the FA rate (P = 0.04). Interestingly, although PFC→STN and PFC→LH neurons strongly regulated the percentage of FA trials, their activation or inactivation had no effect on the motor execution of licking within hit or FA trials (SI Appendix, Fig. S4).
Function of the Hyperdirect Pathway.
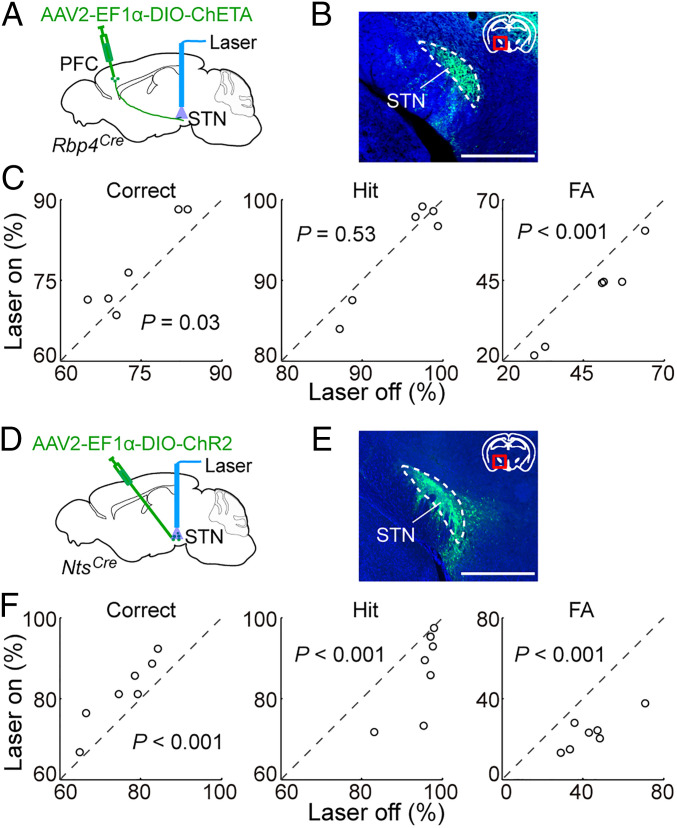
Since PFC→STN neurons send axon collaterals to multiple brain regions, we tested the effect of activating their axon terminals in the STN. In Rbp4Cre mice injected with Cre-inducible AAV expressing the ChR2 variant ChETA (AAV2-EF1α-DIO-ChETA) (47) in the dmPFC (Fig. 6 A and B), 50-Hz laser stimulation at the STN caused a significant behavioral improvement (Fig. 6C; correct rate, P = 0.03; FA rate, P < 0.001), although no significant effect was observed with 10-Hz stimulation (SI Appendix, Fig. S5 A and B; correct rate, P = 0.28; FA rate, P = 0.72). In contrast, axon stimulation in V1 had no significant effect (SI Appendix, Fig. S3 C and D; correct rate, P = 0.52), and stimulation in the LH significantly impaired the performance (SI Appendix, Fig. S5 C and D; correct rate, P = 0.006; FA rate, P = 0.01), consistent with the effects of activating PFC→V1 and PFC→LH cell bodies.
Fig. 6.
Effects of activating PFC→STN axons or STN neurons. (A) Schematic of optogenetic activation of dmPFC axon terminals in STN. (B) Fluorescence image showing ChETA-eYFP expressing dmPFC axons in STN. Green, ChETA-eYFP; blue, DAPI. (Scale bar, 1 mm.) (C) Activation of dmPFC axon terminals in STN (50 Hz) caused a significant improvement in task performance by reducing the FA rate (correct rate, P = 0.03; hit rate, P = 0.53; FA rate, P < 0.001; bootstrap, n = 6 mice). (D) Schematic of optogenetic activation of STN cells. (E) Fluorescence image showing ChR2-eYFP expression in STN neurons. Green, ChR2-eYFP; blue, DAPI. (Scale bar, 1 mm.) (F) Activation of STN cell (10 Hz) caused a significant improvement in task performance (correct, hit, and FA rates are all P < 0.001; bootstrap, n = 7 mice).
Stimulation of dmPFC axons in the STN could induce antidromic spiking and therefore activation of other collateral projections. To further examine the function of the PFC→STN pathway, we tested the effect of direct STN neuron activation. Since the peptide neurotensin (NTS) labels STN glutamatergic neurons (but not those in nearby structures) with a high specificity (SI Appendix, Fig. S6), we injected AAV2-EF1α-DIO-ChR2 into the STN of NtsCre mice (48) (Fig. 6 D and E). Laser stimulation at 10 Hz significantly improved the task performance (P < 0.001) by inducing a strong reduction of FA rate (−47.3 ± 4.6%, SEM) but a much weaker reduction of hit rate (−8.8 ± 3.0%; Fig. 6F and SI Appendix, Fig. S7 A and B). This supports the hypothesis that inhibition of inappropriate responses by PFC→STN neurons is at least partly mediated by their excitation of STN neurons through the hyperdirect pathway.
Discussion
Using projection-specific labeling and calcium imaging, we have shown that STN- but not V1- or LH-projecting dmPFC layer 5 neurons are preferentially active when the animal withholds a prepotent licking response. Optogenetic activation and inactivation showed that activity of PFC→STN neurons improves response inhibition, but PFC→LH neuron activity is detrimental to the task performance. The effect of PFC→STN neurons is mediated at least in part by the hyperdirect pathway.
Unlike the entire layer 5 population, which contains go-preferring, NG-preferring, and weakly modulated neurons (Fig. 2), an overwhelming majority of the PFC→STN subpopulation are NG-preferring (Fig. 3), suggesting a specialized role in inhibiting habitual responses, a function known to depend on the medial frontal cortex (49, 50). In contrast, PFC→LH neurons are more heterogeneous; although their activation causes an impairment of response inhibition (Fig. 5), a small but significant fraction of the neurons are NG-preferring. Given the ∼10% overlap between PFC→LH and PFC→STN neurons (Fig. 1E, which is likely an underestimate due to incomplete retrograde labeling of each population), some of the NG-preferring PFC→LH neurons may also project to the STN. In addition to the PFC→STN layer 5 population, dmPFC neurons projecting to the basal ganglia indirect pathway (i.e., innervating striatal neurons expressing D2 dopamine receptors) may also contribute to response inhibition (51).
The weak task-dependent modulation of PFC→V1 neurons (Fig. 2F) and the lack of effect of their activation on behavioral performance (SI Appendix, Fig. S3B) are not surprising, because the task used in this study does not require visual processing. However, the behavioral effects of manipulating PFC→LH neurons (Fig. 5 E, F, I, and J) were unexpected. The improvement induced by their inactivation suggests that the nonoptimal task performance is partly due to overactivation or insufficient inhibition of PFC→LH neurons, which may drive inappropriate reward-seeking by activating LH GABAergic neurons (40). PFC→LH neuron activity could be modulated by long-range inputs from numerous brain regions (Fig. 4); given the opposing functions of PFC→STN and PFC→LH neurons observed in this study, it is also possible that the two populations inhibit each other through local GABAergic interneurons.
The STN is well known to be important for response inhibition (26–33). In the current task it is likely to receive stronger excitation from PFC→STN neurons in no-go than go trials (Fig. 3 A and B). Notably, optogenetic activation of STN neurons with 10-Hz laser stimulation improved task performance even when applied in both go and no-go trials (Fig. 6F). However, a much stronger activation at 50 Hz caused a severe impairment, due to a near-complete suppression of licking in all trials (SI Appendix, Fig. S7 C–E; P < 0.001 for correct, hit, and FA rates). Such a general suppression is consistent with a recent study (52), presumably mediated by a strong excitation of the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNr), which broadly inhibit multiple motor circuits (33, 42, 51). With moderate STN activation, however, the excitation of GPi/SNr may be overridden by a strong inhibition elicited by the go cue, resulting in selective sparing of hit rate (SI Appendix, Fig. S7 A and B).
A prominent feature of the PFC is functional diversity among neighboring pyramidal neurons (11–14). In addition to laminar differences (6, 15), a high degree of heterogeneity in go vs. no-go preference was observed within layer 5 of the dmPFC, similar to that in the motor cortex (16). Here we show that a subset of pyramidal neurons—defined by both their layer 5 laminar identity and projection to the STN—exhibit strong functional homogeneity regarding response inhibition. This provides an important entry point for delineating the PFC circuit underlying impulse control.
Materials and Methods
Animals.
Rbp4Cre (GENSAT, 031125-UCD) was obtained from GENSAT and NtsCre (Jackson, 017525) was obtained from Jackson Laboratory. Experiments were performed in adult mice (2-6 mo old) of both genders. Mice were group-housed before surgery and single-housed after they were implanted with GRIN lenses or optical fibers. Mice were housed in 12-h light–dark cycle (lights on at 07:00 AM and off at 07:00 PM) with free access to food and water outside of the training and testing period. All procedures were approved by the Animal Care and Use Committees of the University of California, Berkeley.
Virus Preparation.
AAV2-EF1α-DIO-ChR2-eYFP, AAV2-EF1α-DIO-mCherry, AAV2-EF1α-DIO-ChETA-eYFP, and AAV8-FLEx-RG (RG, rabies glycoprotein) were obtained from the University of North Carolina (∼5 × 1012 genome copies [gc]/mL) vector core. AAV2-EF1α-DIO-TVA-eGFP (∼5 × 1012 gc/mL), rAAV2-retro-EF1α-DIO-GCaMP6s (∼1013 gc/mL, but diluted 5 times before injection), rAAV2-retro-EF1α-DIO-ChR2-eYFP (∼5 × 1012 gc/mL), rAAV2-retro-EF1α-DIO-iC++-eYFP (∼5 × 1012gc/mL), rAAV2-retro-EF1α-DIO-mCherry (∼5 × 1012 gc/mL), and rAAV2-retro-EF1α-DIO-eGFP (∼5 × 1012 gc/mL) were packaged in house according to previously described protocols (43, 53). RG-deleted, tdTomato-expressing rabies virus (RV-∆G-tdTomato, ∼1.5 × 109 IU/mL) was amplified in B7GG cells, pseudotyped with BHK-EnvA cells, and titered with HEK293-TVA cells (54). RV-ΔG-tdTomato was a gift from B. Lim at University of California San Diego, La Jolla, CA. B7GG cells, BHK-EnvA, cells and HEK293-TVA cells were gifts from E. Callaway at the Salk Institute for Biological Studies, La Jolla, CA.
Surgical Procedures.
Adult mice (2 to 6 mo old) were anesthetized with isoflurane (5% induction and 1.5% maintenance) and placed on a stereotaxic frame (David Kopf Instruments). Body temperature was kept stable throughout the procedure using a heating pad. Eye ointment was applied to keep the eyes from drying. Mice of specific genotype were randomly assigned to experimental groups. Investigators were not blinded to animal identity and outcome assessment.
For anterograde tracing and dual retrograde labeling experiments, a craniotomy was made on top of the target regions (see below for coordinates), 0.1 to 0.3 µL virus was injected into the target regions using Nanoject II (Drummond Scientific), and mice were sacrificed 3 wk later for histology. For rabies-mediated retrograde transsynaptic tracing experiments, a mixture of AAV2-EF1α-DIO-TVA-eGFP and AAV8-FLEx-RG (∼0.3 µL) was injected into dmPFC. Three weeks later, RV-∆G-tdTomato was injected into STN, V1, or LH, and mice were sacrificed 7 d later for histology.
For imaging experiments, 0.3 to 0.5 µL virus was injected into each target region. A stainless steel headplate was then affixed to the skull. The region surrounding the craniotomy was protected with a silicone elastomer (Kwik-Cast; World Precision Instruments). After a week of recovery, the mice underwent the initial stages of behavioral training (habituation and conditioning) for ∼2 wk, and a second surgical procedure was performed to implant the GRIN lens (diameter, 1 mm; length, 4.2 mm; pitch, 0.5; numerical aperture, 0.5; Inscopix) to target the dmPFC (6, 15).
For optogenetic activation/inactivation experiments, 0.3 to 0.5 µL virus was injected into the target region. Two weeks after viral injection, optic fibers (0.2 mm diameter; Thorlabs) were implanted with the tip 0.2 mm above the target region. A stainless steel headplate was affixed to the skull, and dental cement was applied to cover the exposed skull completely and to secure the implants for behavioral training.
Stereotaxic coordinates for virus injection, GRIN lens and optic fiber implantation are as follows: dmPFC, anteroposterior (AP) 1 mm, mediolateral (ML) 0.5 mm, dorsoventral (DV) 0.8 mm; STN, AP −1.8 mm, ML 1.6 mm, DV 4.7 mm; LH, AP −1 mm, ML 0.6 mm, DV 5.1 mm; and V1, AP −3 mm, ML 2.5 mm, DV 0.5 mm.
Behavioral Procedures.
We trained head-fixed mice on a delayed go/no-go auditory task, including habituation, conditioning, and discrimination phases (6). In the habituation phase, the mouse was given free water rewards (∼4 µL) for each lick. In the conditioning phase, the mouse was trained to lick in response to a target tone stimulus (17 kHz, 65 dB), which lasted for 2 s in each trial. The mouse was advanced to the next phase once the number of licks exceeded 150 within 30 min. In the discrimination phase, each trial started either with the target or nontarget (9 kHz, 65 dB) tone (in go or no-go trials, respectively). The auditory stimulus (cue period) lasted for 2 s, followed by a delay period (which increased gradually from 0 to 2 s over multiple training sessions; see below). A water port was presented after the delay. Licking in response to a target tone (hit) within the first 2 s after the water port presentation (response window) was rewarded with a drop of water delivered through the water port, while licking in response to a nontarget tone (FA) triggered a combination of electric shock at the tip of the tongue (less than 1 mA), airpuff to the cheek (15 to 20 psi, 200 ms), and 7-s timeout period. No licking in response to a target or nontarget tone within the response window was regarded as a miss or correct rejection (CR) trial, respectively. Licking outside of the response window had no consequence. The water port was retracted after 3 s from the first lick in hit and FA trials or 2 s after the end of the response window in miss and CR trials. The intertrial interval was 7.5 s. All licks were detected by an infrared beam-break lickometer. The delay period was gradually increased from 0 to 2 s in 500-ms steps when behavioral performance reached ∼70% correct in a 50-trial block. Mice were trained daily (one session per day, ∼300 trials per session, except for a ∼4-d break for GRIN lens implantation) until reaching criterion performance, defined as >70% correct trials for at least 3 consecutive days or >75% correct for 2 consecutive days. These criteria were chosen to avoid overtraining prior to the experimental manipulations, while ensuring above-chance performance. Mice were water restricted and ordinarily had access to water only during training. However, additional water was given if necessary to ensure that their body weight (monitored daily) did not drop below 85% of the starting value. Once the mice reached these criteria, we started performing calcium imaging experiments or optogenetic manipulation experiments.
Two-Photon Calcium Imaging.
Two-photon calcium imaging was performed using a custom two-photon microscope that has been described previously (55). In brief, the microscope (Movable Objective Microscope; Sutter Instrument) was controlled by the ScanImage software (56) and the objective was a 20× water immersion lens (XLUMPlanFI, 0.95 NA; Olympus). Excitation intensity from a tunable femtosecond laser (Wideband, Tsunami Mode-Locked Ti:Sapphire Laser; Spectra-Physics) was controlled by a Pockels cell (350-80-LA-02; Conoptics). GCaMP6s was excited at 920 nm, and fluorescence emission was collected using a GaAsP PMT (H10770PA-40; Hamamatsu). Images (128 × 128 pixels) were acquired at ∼6.73 Hz.
Optogenetic Manipulation.
Optic fibers (200 µm diameter; ThorLabs) were attached to a 473-nm blue laser diode (Shanghai laser), and light pulses were generated under the control of a Master 8 (A.M.P.I.). Optogenetic activation experiments were performed unilaterally, and optogenetic inhibition experiments were performed bilaterally. Laser stimulation started at the delay period onset and continued for 5 s (covering the delay period, the response window, and 1 s after response window) in each trial. Light pulses (5 ms per pulse, 10 or 50 Hz) were applied for optogenetic activation experiments. The laser power was 1 to 2 mW or ∼10 mW at fiber tip for cell body activation or terminal activation experiments, respectively. For optogenetic inactivation experiments, constant light (∼5 mW) was used. Unless otherwise stated, laser stimulation was applied in 50% of pseudorandomly selected trials, but the same condition never occurred in more than five consecutive trials. In STN cell body activation experiments with 50-Hz pulse train, laser stimulation was applied in 25% of trials to reduce negative effect on the motivation to perform the task. Each experimental session lasted for ∼1 h, and each animal was tested for five to eight sessions.
Calcium Imaging Data Analyses.
The brain motion was corrected by a custom MATLAB script using an open source toolkit ANTs (57–59) (picsl.upenn.edu/software/ants/). Regions of interest (ROIs) corresponding to identifiable cell bodies were selected using the SD projection of the motion-corrected video using ImageJ (23) and a pixel-wise activity map (15, 60). The pixels within each ROI were averaged to create a fluorescence time series. The baseline fluorescence of the ROI, F0, was estimated as the eighth percentile (61) of the fluorescence in a ±60-s window and used to calculate ΔF/F0 = ((F − F0)/F0). Unless otherwise stated, ΔF/F0 traces were Z scored.
To assess whether a neuron was significantly modulated by the task, we performed a two-way ANOVA with factors trial type (Hit, CR, and FA) and epoch (1 s before cue, cue, and delay). A neuron was deemed significantly modulated if P < 0.01 for at least one of the factors or interaction terms. Unless otherwise stated, trials were excluded if lick happened during the delay period. Miss trials were not considered as the numbers of miss trials were very small in most cases.
To quantify the separability between the population activity in the correct go (hit) and no-go (CR) trials, we computed the Euclidean distance for trial-averaged Z-score traces of a population of neurons. Ten neurons were randomly selected in each imaging session. The Euclidean distances in these neurons were computed for each time point between hit trials and CR trials and then normalized by the mean distance during the baseline period (1 s before cue onset). We repeated this procedure 1,000 times to get the averaged distance. Statistical significance was determined using Wilcoxon rank sum test (P < 0.05 as the criterion).
Response preference was quantified during the late delay period (3 to 4 s after cue onset) based on Z-scored traces averaged within each trial type for each neuron. A neuron with higher/lower activity in CR trials than in hit trials (P < 0.05, two-sided t test) was defined as a CR/hit-preferring neuron. Similar comparison was performed between FA and CR trials.
Behavioral Data Analyses.
Correct rate, hit rate, and FA rate were quantified as follows:
Significance of the behavioral effects caused by optogenetic manipulation was determined using bootstrap (22, 62–64). Briefly, in each iteration we resampled the original data with replacement from 1) animals, 2) sessions performed by each animal, and 3) the trials within each session. We then computed the performance change on the resampled dataset. This procedure was repeated 10,000 times to obtain a distribution of performance changes. The P value was computed as the fraction of times the bootstrapping produced an inconsistent performance change.
Immunohistochemistry and Fluorescence In Situ Hybridization.
Mice were deeply anesthetized and transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde in PBS. Brains were kept overnight in 4% paraformaldehyde for fixation and then in 30% sucrose in PBS solution for 36 to 48 h for cryoprotection. After embedding and freezing, brains were sectioned into 30-µm (for fluorescence in situ hybridization [FISH]) or 50-µm coronal slices using a cryostat. For immunohistochemistry, brain slices were washed using PBS three times, permeabilized using PBST (0.3% Triton X-100 in PBS) for 30 min, and then incubated with blocking solution (5% normal goat serum or normal donkey serum in PBST) for 1 h, followed by primary antibody incubation overnight at 4 °C using anti-RPF antibody (600-401-379, Rockland; 1:1,000). The next day, slices were washed three times with PBS, incubated with the secondary antibody (Donkey anti-Rabbit IgG, Alexa Fluor 594, A-21207, Invitrogen; 1:1,000) for 2 h. To determine overlap between Nts and Slc17a6, dual FISH was done using RNAscope assays according to the manufacturer’s instructions (Advanced Cell Diagnostics). Fluorescence images were acquired using a high-throughput slide scanner (Nanozoomer-2.0RS; Hamamatsu) and a fluorescence microscope (Keyence, BZ-X710).
Statistics.
All statistical analysis was performed using MATLAB. Unless otherwise stated, data are presented as mean ± SEM. Statistical method, statistic, and corresponding P values are reported in the figure legends.
Data Availability Statement.
All relevant data are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank Zhe Zhang, Danqian Liu, and Kai-Siang Chen for sharing the virus for the retrograde tracing and rabies virus tracing experiments; Lucas Pinto and Tsukasa Kamigaki for providing programs for data analysis; and Yaxuan Zhou, Jing Zhang, and Fei Hu for help with behavioral training. This work was supported by the Howard Hughes Medical Institute.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000523117/-/DCSupplemental.
References
- 1.Fuster J. M., The Prefrontal Cortex, (Academic, Amsterdam, ed. 4, 2008). [Google Scholar]
- 2.Bari A., Robbins T. W., Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Sebastian A. et al., Frontal dysfunctions of impulse control—A systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front. Hum. Neurosci. 8, 698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iversen S. D., Mishkin M., Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp. Brain Res. 11, 376–386 (1970). [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H., Clegg R. A., Feeley A., Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp. Neurol. 79, 434–451 (1983). [DOI] [PubMed] [Google Scholar]
- 6.Kamigaki T., Dan Y., Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat. Neurosci. 20, 854–863 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broersen L. M., Uylings H. B., Visual attention task performance in Wistar and Lister hooded rats: Response inhibition deficits after medial prefrontal cortex lesions. Neuroscience 94, 47–57 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Risterucci C., Terramorsi D., Nieoullon A., Amalric M., Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur. J. Neurosci. 17, 1498–1508 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Narayanan N. S., Laubach M., Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52, 921–931 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardung S. et al., A functional gradient in the rodent prefrontal cortex supports behavioral inhibition. Curr. Biol. 27, 549–555 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Euston D. R., Gruber A. J., McNaughton B. L., The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller E. K., Cohen J. D., An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Machens C. K., Romo R., Brody C. D., Functional, but not anatomical, separation of “what” and “when” in prefrontal cortex. J. Neurosci. 30, 350–360 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigotti M. et al., The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto L., Dan Y., Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron 87, 437–450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zagha E., Ge X., McCormick D. A., Competing neural ensembles in motor cortex gate goal-directed motor output. Neuron 88, 565–577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T. et al., Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 80, 1477–1490 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Glickfeld L. L., Andermann M. L., Bonin V., Reid R. C., Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci. 16, 219–226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J. L., Carta S., Soldado-Magraner J., Schneider B. L., Helmchen F., Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499, 336–340 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Jarosiewicz B., Schummers J., Malik W. Q., Brown E. N., Sur M., Functional biases in visual cortex neurons with identified projections to higher cortical targets. Curr. Biol. 22, 269–277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Economo M. N. et al., Distinct descending motor cortex pathways and their roles in movement. Nature 563, 79–84 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Li N., Chen T. W., Guo Z. V., Gerfen C. R., Svoboda K., A motor cortex circuit for motor planning and movement. Nature 519, 51–56 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Otis J. M. et al., Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murugan M. et al., Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C. K. et al., Molecular and circuit-dynamical identification of top-down neural mechanisms for restraint of reward seeking. Cell 170, 1013–1027.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank M. J., Samanta J., Moustafa A. A., Sherman S. J., Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318, 1309–1312 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Eagle D. M. et al., Stop-signal reaction-time task performance: Role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex 18, 178–188 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Cavanagh J. F. et al., Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 14, 1462–1467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baunez C., Robbins T. W., Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur. J. Neurosci. 9, 2086–2099 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Isoda M., Hikosaka O., Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J. Neurosci. 28, 7209–7218 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron A. R., Poldrack R. A., Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aron A. R., Herz D. M., Brown P., Forstmann B. U., Zaghloul K., Frontosubthalamic circuits for control of action and cognition. J. Neurosci. 36, 11489–11495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt R., Leventhal D. K., Mallet N., Chen F., Berke J. D., Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci. 16, 1118–1124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nambu A., Tokuno H., Takada M., Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci. Res. 43, 111–117 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Kita T., Kita H., The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: A single-axon tracing study in the rat. J. Neurosci. 32, 5990–5999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd G. M., Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerfen C. R., Paletzki R., Heintz N., GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tervo D. G. R. et al., A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S. et al., Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennings J. H. et al., Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K. S. et al., A hypothalamic switch for REM and non-REM sleep. Neuron 97, 1168–1176.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Parent A., Hazrati L. N., Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 20, 91–127 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Zhang S. et al., Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat. Neurosci. 19, 1733–1742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh S. W. et al., A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith K. S., Tindell A. J., Aldridge J. W., Berridge K. C., Ventral pallidum roles in reward and motivation. Behav. Brain Res. 196, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berndt A. et al., Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc. Natl. Acad. Sci. U.S.A. 113, 822–829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunaydin L. A. et al., Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Leinninger G. M. et al., Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isoda M., Hikosaka O., Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 10, 240–248 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Duan C. A., Erlich J. C., Brody C. D., Requirement of prefrontal and midbrain regions for rapid executive control of behavior in the rat. Neuron 86, 1491–1503 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Gerfen C. R., Surmeier D. J., Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fife K. H. et al., Causal role for the subthalamic nucleus in interrupting behavior. eLife 6, e27689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauswirth W. W., Lewin A. S., Zolotukhin S., Muzyczka N., “Production and purification of recombinant adeno-associated virus” in Methods in Enzymology, K. Palczewski, Ed. (Academic Press, 2000), Vol. 316, pp. 743–761. [DOI] [PubMed] [Google Scholar]
- 54.Osakada F., Callaway E. M., Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y. et al., Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 486, 118–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pologruto T. A., Sabatini B. L., Svoboda K., ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avants B. B., Epstein C. L., Grossman M., Gee J. C., Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Betley J. N. et al., Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox J., Pinto L., Dan Y., Calcium imaging of sleep-wake related neuronal activity in the dorsal pons. Nat. Commun. 7, 10763 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahrens M. B. et al., Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvey C. D., Coen P., Tank D. W., Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484, 62–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Z. et al., A cortico-cerebellar loop for motor planning. Nature 563, 113–116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Z. V. et al., Flow of cortical activity underlying a tactile decision in mice. Neuron 81, 179–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Efron B., Tibshirani R., An Introduction to the Bootstrap, (Chapman & Hall, New York, 1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript and SI Appendix.