Significance
Parkinson’s disease (PD)-linked familial mutations in LRRK2 impact its enzymatic activity by commonly increasing kinase activity, either directly within the kinase domain or indirectly via the GTPase domain by impairing GTP hydrolysis. Familial LRRK2 mutations also commonly promote neuronal toxicity in cultured cells, and for the common G2019S mutation, these effects are kinase dependent. The mechanisms underlying familial LRRK2 mutations in animal models are uncertain, due to the general lack of robust phenotypes. Our study demonstrates important roles for kinase and GTPase activities in mediating the neurodegenerative effects of G2019S LRRK2 in rodents, highlighting both as promising therapeutic targets for PD.
Keywords: LRRK2, kinase, GTPase, Rab, neurodegeneration
Abstract
Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of late-onset, autosomal-dominant familial Parkinson’s disease (PD). LRRK2 functions as both a kinase and GTPase, and PD-linked mutations are known to influence both enzymatic activities. While PD-linked LRRK2 mutations can commonly induce neuronal damage in culture models, the mechanisms underlying these pathogenic effects remain uncertain. Rodent models containing familial LRRK2 mutations often lack robust PD-like neurodegenerative phenotypes. Here, we develop a robust preclinical model of PD in adult rats induced by the brain delivery of recombinant adenoviral vectors with neuronal-specific expression of human LRRK2 harboring the most common G2019S mutation. In this model, G2019S LRRK2 induces the robust degeneration of substantia nigra dopaminergic neurons, a pathological hallmark of PD. Introduction of a stable kinase-inactive mutation or administration of the selective kinase inhibitor, PF-360, attenuates neurodegeneration induced by G2019S LRRK2. Neuroprotection provided by pharmacological kinase inhibition is mediated by an unusual mechanism involving the robust destabilization of human LRRK2 protein in the brain relative to endogenous LRRK2. Our study further demonstrates that G2019S LRRK2-induced dopaminergic neurodegeneration critically requires normal GTPase activity, as hypothesis-testing mutations that increase GTP hydrolysis or impair GTP-binding activity provide neuroprotection although via distinct mechanisms. Taken together, our data demonstrate that G2019S LRRK2 induces neurodegeneration in vivo via a mechanism that is dependent on kinase and GTPase activity. Our study provides a robust rodent preclinical model of LRRK2-linked PD and nominates kinase inhibition and modulation of GTPase activity as promising disease-modifying therapeutic targets.
Parkinson’s disease (PD) typically occurs in a sporadic manner through a complex interaction between genetic risk, aging, and environmental exposure (1). A small proportion of PD cases are inherited and are known to be caused by mutations in at least 14 different genes (2). Among them, mutations in the leucine-rich repeat kinase 2 (LRRK2) gene cause late-onset, autosomal-dominant PD and represent the most common cause of familial PD (3–6). Genome-wide association studies further link common variants at the LRRK2 locus with increased risk for sporadic PD (7, 8). LRRK2 has therefore emerged as an important player and therapeutic target for familial and sporadic PD. In mammals, LRRK2 is ubiquitously expressed with enrichment in kidney, lung, and various peripheral immune cells and is present in multiple cell types throughout the brain, including neurons (9–11). LRRK2 is a large multidomain protein containing two central enzymatic domains, a Ras-of-Complex (Roc) GTPase domain and a tyrosine kinase-like kinase domain, linked by a C-terminal-of-Roc (COR) domain and flanked by four protein interaction repeat domains (12). Familial PD-linked mutations cluster within the Roc-COR tandem (N1437H, R1441C/G/H, R1628P, and Y1699C) and kinase (I2012T, G2019S, and I2020T) domains of LRRK2, suggesting important roles for both enzymatic activities in the pathophysiology of PD.
LRRK2 can function as both a GTPase and kinase in vitro and in cells with an intact GTPase domain, and the capacity for GTP-binding being critically required for kinase activity (13–16). Familial PD-linked mutations in LRRK2 commonly increase its kinase activity in mammalian cells to varying degrees and promote substrate phosphorylation (i.e., a subset of Rab GTPases) and autophosphorylation (i.e., at Ser1292) (17–19). For the common G2019S mutation located within the kinase activation loop, the effect on kinase activity is direct, whereas Roc-COR domain mutations are considered to act indirectly by impairing GTP hydrolysis activity and thereby prolonging the GTP-bound “on” state of LRRK2 (15, 20–24). While GTPase and kinase activities of LRRK2 are clearly altered by familial mutations, it is less clear whether or how these enzymatic activities contribute to neuronal toxicity induced by mutant LRRK2. For example, many studies have routinely employed kinase-inactive mutations at the kinase proton acceptor site (D1994) to block neuronal damage in primary culture models induced by PD-linked mutant LRRK2 (25–30). However, null mutations at the D1994 residue (i.e., D1994A/N/S) selectively destabilize LRRK2 protein in primary neurons versus cell lines (13, 26, 31, 32), thereby making these types of single-cell neuronal assays difficult to adequately control. Similarly, commonly used hypothesis-testing mutations that disrupt GDP/GTP binding within the phosphate-binding loop (P-loop) of the GTPase domain (K1347A or T1348N) tend to markedly impair LRRK2 protein stability in most cell types (13, 23). Due to this adverse impact on LRRK2 protein levels, the neuroprotective effects of genetically inhibiting kinase activity or GTP binding in neuronal models based upon PD-linked LRRK2 mutants have been difficult to robustly demonstrate. Notwithstanding these concerns, it is generally accepted that neuronal toxicity in primary culture models induced by mutant LRRK2 is mediated via a kinase-dependent mechanism, whereas the contribution of GTPase activity is less certain (12).
The molecular mechanisms underlying the pathogenic effects of familial LRRK2 mutations in the mammalian brain are poorly understood, due largely to the lack of robust neurodegenerative phenotypes in most animal models (33). While certain transgenic mouse models with high-level overexpression of mutant LRRK2 can develop a modest yet late-onset loss of substantia nigra dopaminergic neurons (34–36), these models are generally the exception, with most LRRK2 transgenic or knockin models of PD developing only subtle if any neuropathology over their lifespan (12, 33). Viral-mediated gene transfer in the rodent brain using large-capacity vectors such as a herpes simplex virus (HSV) amplicon or human adenovirus serotype 5 (Ad5) has been successful in producing models with robust and progressive dopaminergic neurodegeneration induced by human G2019S LRRK2, occurring over a shorter more feasible timeframe i.e., 3 to 6 wk (29, 32, 37). In the HSV-LRRK2 mouse model, G2019S LRRK2 induces dopaminergic neuronal loss that is kinase dependent, albeit based upon using the unstable D1994A mutation or nonselective LRRK2 kinase inhibitors (29). Similarly, the Ad5-LRRK2 rat model reveals neuropathology induced by G2019S LRRK2 in a kinase-dependent manner, again using the unstable D1994N mutation (32). While these prior studies tend to support an important role for LRRK2 kinase activity in mediating neurodegeneration in PD, there have been few studies in rodent models rigorously evaluating whether or how kinase or GTPase activity meaningfully contributes to neurodegeneration induced by familial PD-linked LRRK2 mutations.
Here, we extend our prior work and have optimized adenoviral production, titer, and delivery site in the Ad5-LRRK2 rat model of PD to provide a robust genetic and pharmacological evaluation of the contribution of kinase, GTP-binding, and GTP hydrolysis activities to nigrostriatal pathway dopaminergic neurodegeneration induced by the common G2019S mutation in LRRK2. Our study highlights the importance of kinase and GTPase activity of LRRK2 in mediating neurodegeneration in vivo and validates both enzymatic activities as key therapeutic targets for PD.
Results
Pilot Study of G2019S LRRK2 Kinase Activity following the Intranigral Delivery of Ad5-LRRK2 Vectors.
To begin to evaluate the enzymatic mechanisms by which G2019S LRRK2 mediates neuronal cell death in the adult rat brain, we developed second generation E1-, E2a-, and E3-deleted recombinant human Ad5 vectors expressing either eGFP or full-length 3xFLAG-tagged human LRRK2 variants from a neuronal-specific synapsin-1 promoter (32, 37). The neuronal-restricted transgene expression produced by this Ad5 vector permits an investigation of the cell-autonomous pathogenic effects of LRRK2 variants in nigrostriatal pathway dopaminergic neurons. The most common PD-linked G2019S mutation was employed either alone or in combination with two distinct kinase-inactive mutations, D1994N (proton acceptor site) or K1906M (ATP-binding site), located in the kinase domain of LRRK2 (12). Prior studies by us and others have revealed that null mutations at the D1994 residue destabilize the LRRK2 protein selectively in primary neurons and brain tissue, whereas mutations at the K1906 residue are generally well tolerated and stable (13, 31, 32). Therefore, we elected to compare the effects of these distinct kinase-inactive mutations on G2019S LRRK2-induced phenotypes. Purified Ad5 vectors are able to infect human SH-SY5Y neural cells in a titer-dependent manner and, most importantly, produce equivalent levels of each FLAG-LRRK2 variant (G2019S, G2019S/D1994N, and G2019S/K1906M), as shown by Western blot analysis (SI Appendix, Fig. S1A). We next conducted pilot studies in the adult rat brain by the stereotactic unilateral delivery of these Ad5 vectors to the substantia nigra pars compacta (SNpc) using a maximal viral titer of ∼4.5 × 109 viral particles (vp), similar to our previous studies (32, 37). We opted to evaluate direct delivery of Ad5 vectors at a single site in the SNpc, since our prior study using a similar viral titer produced inefficient retrograde axonal transport of viral particles from the striatum to the SNpc (32). At 21 d postinjection, numerous neurons labeled with GFP or LRRK2 variants (FLAG) were detected within the ipsilateral nigra largely confined to the region spanning ±80 μm from the injection site, suggesting limited viral transduction throughout the entire substantia nigra at this titer (SI Appendix, Fig. S1B). Consistent with our prior studies (13, 32), we detect qualitatively equivalent levels of G2019S and G2019S/K1906M LRRK2 proteins, whereas G2019S/D1994N LRRK2 protein was detected at markedly lower levels, indicating the reduced stability of this variant in the brain (Fig. 1B). To evaluate the impact of these LRRK2 variants on neurodegeneration, unbiased stereology was used to estimate the number of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the ipsilateral versus contralateral SNpc at 21 d after Ad5 delivery (SI Appendix, Fig. S1C). G2019S LRRK2 induces a marked significant loss of nigral dopaminergic neurons (42.7 ± 12.9% loss) compared to an eGFP control (20.7 ± 12.9% loss). Notably, G2019S/D1994N and G2019S/K1906M LRRK2 both attenuate neuronal loss compared to G2019S LRRK2, producing levels comparable to eGFP (25.9 ± 14.9 and 23.8 ± 10.7% loss, respectively), although these reductions do not reach significance due to the limited number of animals used (SI Appendix, Fig. S1D). Together, these pilot data validate the intranigral delivery of Ad5 vectors as a potential approach for modeling dopaminergic neurodegeneration induced by G2019S LRRK2, and initially suggest that these pathogenic effects are kinase dependent, based upon the stable kinase-inactive K1906M mutation.
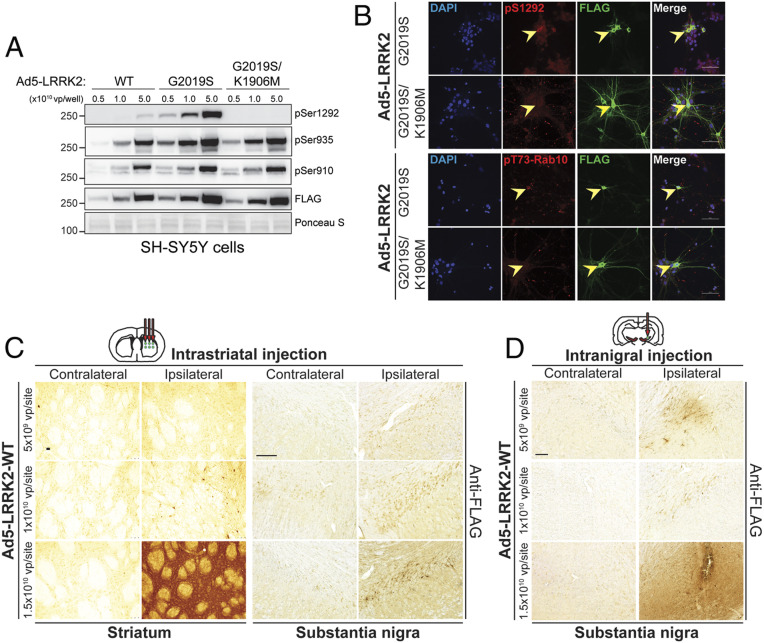
Fig. 1.
Evaluation of Ad5 vector infectivity in rat brain via distinct delivery paradigms. (A) Validation of new high-titer Ad5-LRRK2 (WT, G2019S, and G2019S/K1906M) vectors and LRRK2 phosphorylation in SH-SY5Y cells. Cells were transduced with increasing viral particles of each Ad5-LRRK2 vector (0.5, 1, or 5.0 × 1010 vp/well). Western blot analysis of cell extracts with FLAG antibody (transduced human LRRK2) or antibodies to detect LRRK2 autophosphorylation (pSer1292) or constitutive phosphorylation (pSer935, pSer910) sites to detect a titer-dependent increase in human LRRK2 levels/activity. Ponceau S stain was used as a protein loading control. Molecular mass markers are indicated in kilodaltons. (B) Immunofluorescent colocalization analysis in primary cortical neurons infected with Ad5-LRRK2 vectors (1.5 × 1010 vp/dish) at DIV3 and fixed at DIV6. Arrowheads indicate cortical neurons expressing human LRRK2 (FLAG, green), their corresponding signal for pS1292-LRRK2 or pT73-Rab10 (red), and merged images. (Scale bar: 50 µm.) (C) Evaluation of Ad5-LRRK2 vectors following intrastriatal delivery with increasing viral titer (0.5, 1, or 1.5 × 1010 vp/site, at 2.5 μL/site) at six distinct injection sites. Immunohistochemistry showing dose-dependent expression of human LRRK2-WT (anti-FLAG antibody) in the ipsilateral rat striatum and substantia nigra at 10 d postinjection. (Scale bars: 500 μm.) (D) Evaluation of Ad5-LRRK2 vectors following intranigral delivery with increasing viral titer (0.5, 1, or 1.5 × 1010 vp/site, in 2.5 μL) at a single injection site. Immunohistochemistry showing dose-dependent expression of human LRRK2-WT (anti-FLAG antibody) in rat substantia nigra at 10 d postinjection. (Scale bars: 500 μm.)
Optimization of Ad5-LRRK2 Vector Titer and Route of Delivery in the Rat Brain.
We next sought to optimize and improve Ad5 vector production, titer, and delivery to further enhance the expression levels of human LRRK2 variants (wild type [WT], G2019S, and G2019S/K1906M) in nigral dopaminergic neurons of this rat model. We elected to move ahead with the kinase-inactive K1906M mutant due to its improved protein stability in the brain relative to the D1994N mutant. We decided to compare two Ad5 delivery paradigms (intranigral versus intrastriatal) since both approaches have proven successful in producing human LRRK2 expression in nigral dopaminergic neurons at a viral titer of ∼4.5 × 109 vp/site (SI Appendix, Fig. S1 and ref. 37), albeit with some limitations in transduction efficiency. For example, the limited number of nigral neurons locally transduced following intranigral Ad5 delivery may relate to the low somatic expression in dopaminergic neurons of the coxsackie and adenovirus receptor (CAR) that is critical for efficient viral uptake (38). Alternatively, intrastriatal Ad5 delivery can produce inefficient retrograde transport of viral particles along dopaminergic axons to the SNpc, subsequently minimizing G2019S LRRK2-induced neurodegeneration (32). To overcome these issues, we successfully improved the titer of Ad5 vectors by optimizing different steps in the viral amplification and purification procedures. These improvements led to a three- to fourfold increase in the stock titer of Ad5-LRRK2 vectors (WT, G2019S, and G2019S/K1906M) to 6 to 8 × 109 vp/μL (from ∼2 × 109 vp/µL), which would allow the maximal delivery of up to ∼1.6 × 1010 vp/site (in a 2-μL volume) in both injection paradigms. To first evaluate these improved Ad5-LRRK2 preparations, we demonstrated the titer-dependent and equivalent expression levels of G2019S and G2019S/K1906M FLAG-LRRK2 variants in SH-SY5Y cells by Western blot analysis (Fig. 1A), whereas WT LRRK2 levels were modestly lower. We confirmed the markedly increased kinase activity of G2019S LRRK2 relative to WT LRRK2 in cells by monitoring autophosphorylation at Ser1292, whereas G2019S/K1906M LRRK2 was kinase inactive (Fig. 1A). Constitutive phosphorylation at Ser910 and Ser935 was not altered between these LRRK2 kinase-active and kinase-inactive variants (Fig. 1A), consistent with prior reports (39). We further demonstrated increased immunofluorescence for pSer1292-LRRK2 and pThr73-Rab10, a well-established LRRK2 kinase substrate (18, 40), induced by G2019S LRRK2 in primary cortical neurons transduced with Ad5-LRRK2 vectors that are absent with G2019S/K1906M LRRK2 (Fig. 1B). Our data confirm the kinase activity status (pSer1292 or pRab10) of our Ad5-LRRK2 vectors, indicating that G2019S is hyperactive and G2019S/K1906M is inactive.
To evaluate the transduction efficiency of these new Ad5-LRRK2 preparations in vivo, we injected adult rats using intrastriatal (six sites, 2 μL/site) or intranigral (single site, 2 μL) delivery paradigms with an Ad5-LRRK2 WT vector at three different titers (∼5 × 109, 1 × 1010, and 1.5 × 1010 vp/site) (Fig. 1 C and D). At 10 d postinjection, we observed a titer-dependent expression of FLAG-LRRK2 in neurons and neuritic processes of the ipsilateral striatum and SNpc following intrastriatal Ad5 delivery (Fig. 1C) and increased labeling of FLAG-LRRK2 in the ipsilateral SNpc following intranigral delivery (Fig. 1D). LRRK2-positive neurons or neurites were not generally detected in the contralateral striatum or SNpc using either delivery method or at any viral titer (Fig. 1 C and D). While FLAG-LRRK2-positive nigral dopaminergic neurons can already be detected following intrastriatal delivery of Ad5 at the lowest titer of 5 × 109 vp/site, we observed a titer-dependent increase of LRRK2-positive nigral neuron number, suggesting a marked improvement in retrograde axonal transport of viral particles with increasing amounts of Ad5 (Fig. 1C). Intranigral delivery, using Ad5 at 5 × 109 and 1 × 1010 vp/site, resulted in LRRK2-positive expression that was limited around the injection site in the SNpc (Fig. 1D). However, a substantially greater area of the SNpc containing LRRK2-positive neuronal soma and neurites was transduced by Ad5 at the highest titer of 1.5 × 1010 vp/site, suggesting a broader diffusion of viral particles at this dose (Fig. 1D). Intrastriatal delivery produced abundant LRRK2-positive labeling limited to nigral dopaminergic neuronal soma throughout the entire rostrocaudal axis of the ipsilateral SNpc, whereas intranigral delivery displayed a more limited distribution to neurons and neurites within the SNpc (Fig. 1 C and D). Using a similar approach, we confirmed the selective transduction of nigral dopaminergic neurons by confocal colocalization following delivery of a new high-titer Ad5-eGFP vector to the rat striatum at the maximal viral titer of ∼1.5 × 1010 vp/site (SI Appendix, Fig. S2). Taken together, our pilot studies varying titer and delivery site of Ad5 vectors demonstrate that intranigral and intrastriatal delivery routes in the adult rat brain are sufficient to achieve LRRK2 expression in nigral dopaminergic neurons in a viral titer-dependent manner. Accordingly, we elected to use the maximal Ad5 titer of 1.5 × 1010 vp/site for all subsequent studies to obtain the highest expression of human LRRK2 throughout the nigrostriatal pathway.
Analysis of Protein Levels and Kinase Activity of Ad5-LRRK2 Variants in the Rat Brain.
Prior to exploring the role of kinase activity in driving neurodegeneration, we first sought to confirm the protein levels and kinase activity status of the Ad5-LRRK2 vectors in the rat brain. To obtain the highest expression of human LRRK2 variants, we employed the intrastriatal delivery of Ad5-LRRK2 vectors (WT, G2019S, and G2019S/K1906M) in adult rats (SI Appendix, Fig. S3). At 10 d postinjection, the ipsilateral striatum was subjected to sequential detergent extraction to produce 1% Triton-soluble and Triton-insoluble (2% sodium dodecyl sulfate [SDS]-soluble) fractions. Human LRRK2 variants were detected in both Triton-soluble and -insoluble fractions where the steady-state levels of G2019S and G2019S/K1906M LRRK2 proteins were equivalent by Western blot analysis, whereas the level of WT LRRK2 protein was modestly lower (SI Appendix, Fig. S3A). These data are similar to our observations in SH-SY5Y cells (Fig. 1A), indicating that genetic inactivation of kinase activity by the K1906M mutation does not alter the protein stability of G2019S LRRK2 in neural tissue and is generally well tolerated.
We next sought to monitor LRRK2 kinase activity in brain extracts by evaluating Rab10 phosphorylation (pThr73-Rab10). While endogenous Rab10 is readily detected in Triton-soluble and -insoluble fractions from striatum, we detected minimal levels of pT73-Rab10 that are not altered between extracts expressing WT, G2019S, or G2019S/K1906M LRRK2 (SI Appendix, Fig. S3A). These data suggest that pT73-Rab10 does not serve as a sensitive readout of LRRK2 kinase activity in the brain of this rat model, similar to prior reports in rodent brain (41). Instead, LRRK2 kinase activity was monitored by autophosphorylation at Ser1292 in brain tissue and primary cortical neurons. Following the immunoprecipitation (IP) of FLAG-LRRK2 variants from soluble striatal extracts, we detected a modest yet distinct pSer1292 signal for G2019S LRRK2 compared to WT LRRK2 by Western blot analysis, but a complete absence of pSer1292 signal for the G2019S/K1906M LRRK2 protein (SI Appendix, Fig. S3B). The corresponding levels of constitutive Ser935 phosphorylation were not altered between these LRRK2 variants (SI Appendix, Fig. S3B) as expected (39). A similar yet more obvious increase in pSer1292 signal induced by G2019S LRRK2, but not G2019S/K1906M LRRK2, was obtained using anti-FLAG IPs from primary cortical neurons transduced with Ad5-LRRK2 vectors (SI Appendix, Fig. S3B). These findings confirm our observations in SH-SY5Y cells (Fig. 1A) and indicate that G2019S LRRK2 is kinase hyperactive, whereas G2019S/K1906M LRRK2 is kinase inactive in brain tissue and cultured neurons. We next attempted to validate the alterations in pSer1292-LRRK2 levels by immunofluorescent labeling and confocal microscopy in the striatum and substantia nigra following intrastriatal delivery of Ad5-LRRK2 vectors (SI Appendix, Fig. S3C). At 42 d postinjection, we were able to readily detect small numbers of FLAG-LRRK2-positive neurons in the ipsilateral striatum and SNpc; yet these neurons exhibited minimal labeling for pSer1292-LRRK2. Moreover, the low levels of pSer1292-LRRK2 did not differ between neurons expressing G2019S or G2019S/K1906M LRRK2 (SI Appendix, Fig. S3C), suggesting that the pS1292-LRRK2 antibody is not sufficiently sensitive for immunofluorescent detection in rat tissues compared to cultured primary neurons (Fig. 1B). Overall, our data provide evidence for increased kinase activity of G2019S LRRK2 in the rat brain that is inhibited by addition of the K1906M mutation.
G2019S LRRK2 Induces Dopaminergic Neurodegeneration in Rats via a Kinase-Dependent Mechanism.
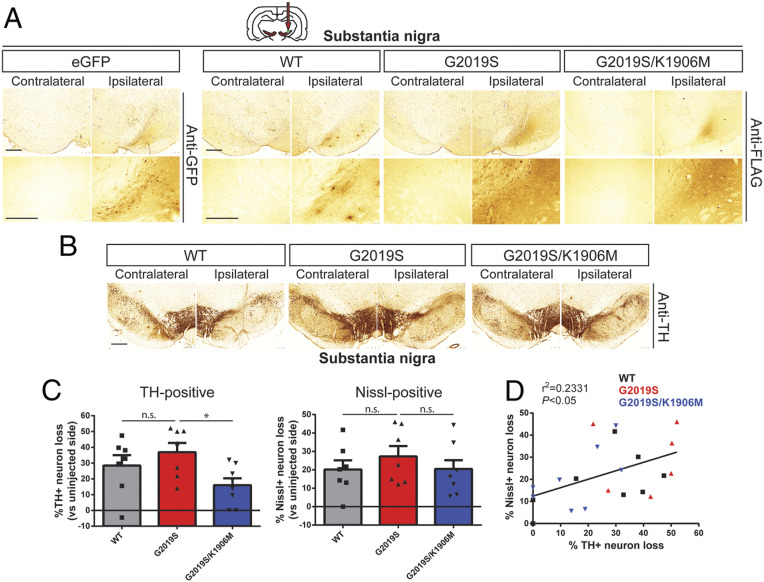
To extend our pilot data (SI Appendix, Fig. S1) and rigorously evaluate the contribution of LRRK2 kinase activity in driving dopaminergic neurodegeneration, we employed our optimized delivery paradigms (intranigral versus intrastriatal, Fig. 1) and increased animal cohort sizes to compare between Ad5-LRRK2 variants (WT, G2019S, and G2019S/K1906M). Adult rats were first subjected to a single intranigral injection of Ad5 vectors (∼1.5 × 1010 vp) and assessed for neurodegeneration after 21 d (Fig. 2). We observe the robust labeling of neurons and neurites with FLAG-LRRK2 variants or eGFP in the SNpc (Fig. 2A) comparable to the levels observed at 10 d postinjection (Fig. 1D). We find equivalent levels of FLAG-LRRK2-positive labeling between animals expressing the G2019S and G2019S/K1906M LRRK2 proteins (Fig. 2A), further confirming the normal protein stability of the G2019S/K1906M variant in brain tissue. Unbiased stereological counting reveals robust dopaminergic neurodegeneration induced by G2019S LRRK2 (36.9 ± 15.6% loss) in the ipsilateral SNpc that is only modestly reduced with WT LRRK2 (28.4 ± 17.0% loss), whereas neuronal loss is significantly attenuated with G2019S/K1906M LRRK2 (16.0 ± 12.4% loss) (Fig. 2 B and C). The corresponding loss of total Nissl-positive neurons in the ipsilateral SNpc for each LRRK2 variant, albeit nonsignificant, suggests that LRRK2 variants induce neuronal cell death rather than altering TH phenotype (Fig. 2C), as previously reported (37). While there is a significant positive correlation between TH-positive and Nissl-positive neuronal loss in general within these animal cohorts (R2 = 0.2331, P < 0.05), for unknown reasons this relationship does not always correlate for individual animals (Fig. 2D). Our data provide further evidence that G2019S LRRK2 induces dopaminergic neurodegeneration in rats via a mechanism that requires kinase activity following the direct intranigral delivery of Ad5 vectors. However, at this maximal Ad5 titer, there is already a high baseline of neuronal loss, and differences between LRRK2 variants are rather modest.
Fig. 2.
Intranigral delivery of Ad5-LRRK2-G2019S induces dopaminergic neurodegeneration in a kinase-dependent manner in adult rat brain. (A) Ad5-LRRK2 (WT, G2019S, or G2019S/K1906M) vectors were unilaterally delivered at a single site (1.5 × 1010 vp/site, in 2.5 μL) in the rat substantia nigra. Immunohistochemistry reveals expression of eGFP (anti-GFP antibody) or human LRRK2 variants (anti-FLAG antibody) that persists up to 21 d postinjection. Images indicate low and high magnification for each vector. (Scale bars: 500 μm.) (B) Immunohistochemistry revealing dopaminergic neurons (anti-TH antibody) in contralateral and ipsilateral substantia nigra at 21 d postdelivery of Ad5 vectors. (Scale bars: 500 μm.) (C) Unbiased stereological analysis of TH-positive dopaminergic and total Nissl-positive neuron number in the substantia nigra at 21 d postinjection. Bars represent % neuronal loss in the injected ipsilateral nigra relative to the contralateral nigra (mean ± SEM, n = 7 to 8 animals/vector), *P < 0.05 by one-way ANOVA with Bonferroni’s multiple comparisons test; n.s., nonsignificant. (D) Linear regression analysis indicating a significant positive correlation between TH-positive and Nissl-positive neuron loss in the substantia nigra. Data points represent individual animals. Pearson’s correlation coefficient, r2 = 0.2331, P < 0.05.
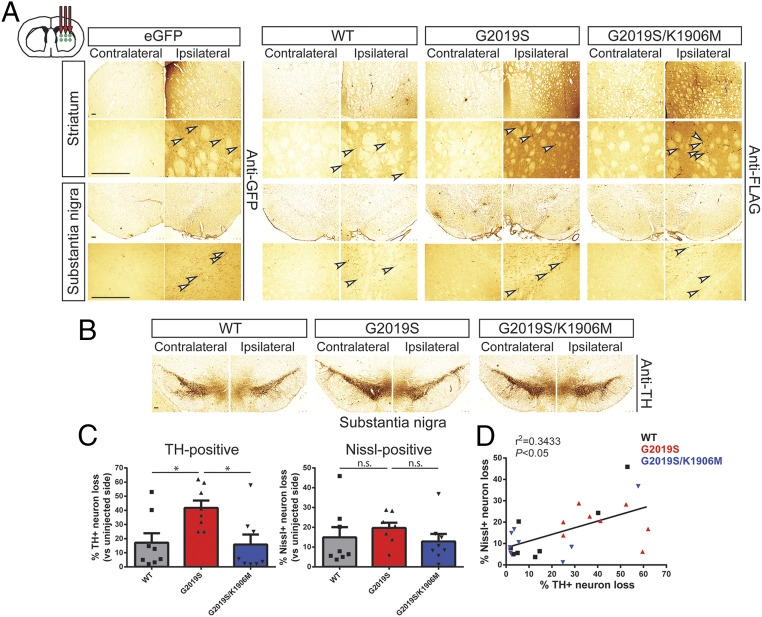
We next conducted similar studies using intrastriatal delivery of Ad5-LRRK2 vectors (∼1.5 × 1010 vp/site at six distinct sites) in adult rats and evaluated neurodegeneration at 42 d postinjection (Fig. 3). Using a hexon protein-specific antibody that detects the capsid protein of Ad5 particles, we confirmed equivalent amounts of each Ad5-LRRK2 vector following delivery to the ipsilateral striatum (SI Appendix, Fig. S4A). FLAG-LRRK2 variants or eGFP were robustly detected in neurons and neurites of the ipsilateral striatum and within dopaminergic neurons of the ipsilateral SNpc at 42 d postinjection, with equivalent levels of G2019S and G2019S/K1906M LRRK2 proteins (Fig. 3A). FLAG-LRRK2-positive staining in the ipsilateral striatum and SNpc at 42 d was qualitatively reduced compared to pilot studies at 10 d postinjection (Fig. 1C), indicating that the pool of adenoviral particles undergoes clearance over this extended period. Unbiased stereology confirmed robust nigral dopaminergic neurodegeneration in this model induced by G2019S LRRK2 (41.7 ± 14.8% neuronal loss) that is significantly and comparably attenuated with WT LRRK2 (17.1 ± 19.1%) or G2019S/K1906M LRRK2 (15.8 ± 20.1%) (Fig. 3 B and C). TH-positive neuronal loss induced by each LRRK2 variant was supported by a parallel loss of total Nissl-positive neurons in the ipsilateral SNpc (Fig. 3C), with a significant positive correlation between these neuronal counts within these animal cohorts (R2 = 0.3433, P < 0.05; Fig. 3D). Our data further demonstrate a key requirement of kinase activity for the robust dopaminergic neurodegeneration induced by G2019S LRRK2 in the rat brain following the intrastriatal delivery of Ad5 vectors. Although the extent of neuronal loss induced by G2019S LRRK2 is comparable between intranigral and intrastriatal Ad5 delivery paradigms, a key difference lies in the more modest impact of WT LRRK2 in the intrastriatal model, whereas G2019S/K1906M LRRK2 exhibits a consistently low level of neuronal loss in both delivery paradigms. The delivery of Ad5-eGFP vectors at this maximal viral titer produced extensive nonspecific neuronal toxicity in the substantia nigra, a problem commonly encountered in other viral-based rodent models (42), indicating that GFP does not serve as a useful negative control in vivo. Instead, we have used kinase-inactive G2019S/K1906M LRRK2 to set our baseline levels of neuronal toxicity in this rat model to control for the nonspecific effects due to LRRK2 overexpression and/or Ad5 infection (Figs. 2 and 3). Taken together, these data indicate that the intrastriatal delivery of Ad5-LRRK2 vectors produces a more robust rodent model of neurodegeneration in which to identify a critical contribution of kinase activity.
Fig. 3.
Intrastriatal delivery of Ad5-LRRK2-G2019S induces kinase-dependent dopaminergic neurodegeneration in adult rat brain. (A) Ad5-LRRK2 (WT, G2019S, and G2019S/K1906M) vectors were unilaterally delivered at six injection sites (1.5 × 1010 vp/site, in 2.5 μL) to the rat striatum. Immunohistochemistry reveals expression of eGFP (anti-GFP antibody) or human LRRK2 variants (anti-FLAG antibody) in the ipsilateral striatum and nigra that persists up to 42 d postinjection. Images indicate low and high magnification for each vector and brain region. (Scale bars: 500 μm.) (B) Immunohistochemistry revealing dopaminergic neurons (anti-TH antibody) in contralateral and ipsilateral substantia nigra at 42 d postdelivery of Ad5 vectors. All sections were counterstained with Cresyl violet. (Scale bars: 500 μm.) (C) Unbiased stereological analysis of TH-positive dopaminergic and total Nissl-positive neuron number in the substantia nigra at 42 d postinjection. Bars represent % neuronal loss in the injected ipsilateral nigra relative to the contralateral nigra (mean ± SEM, n = 8 animals/vector), *P < 0.05 by one-way ANOVA with Bonferroni’s multiple comparisons test; n.s., nonsignificant. (D) Linear regression analysis indicating a significant positive correlation between TH-positive and Nissl-positive neuron loss in the substantia nigra. Data points represent individual animals. Pearson’s correlation coefficient, r2 = 0.3433, P < 0.05.
Evaluation of PD-Relevant Neuropathological Markers in the Ad5-LRRK2 Rat Model.
Our prior study using intrastriatal delivery of Ad5-LRRK2 vectors at a lower viral titer (∼4.5 × 109 vp/site) demonstrated that G2019S LRRK2 could induce striatal pathology in a kinase-dependent manner, including neurite degeneration (Gallyas silver stain), ubiquitin-positive neuronal inclusions, and abnormal phospho-neurofilament distribution (32). To evaluate the extent of neuropathology throughout the nigrostriatal pathway induced by Ad5-LRRK2 vector delivery at a threefold higher titer (∼1.5 × 1010 vp/site) in this study, we assessed a number of markers to monitor for protein inclusions or aggregates, neurite and axonal damage, and microglial activation (SI Appendix, Figs. S4–S7). In rat brains subjected to intrastriatal delivery of Ad5-LRRK2 vectors, we observed an increase in ubiquitin immunoreactivity in the ipsilateral striatum at 42 d, although this is equivalent between WT, G2019S, and G2019S/K1906M LRRK2, suggesting it arises in a manner independent of LRRK2 or its kinase activity (SI Appendix, Fig. S4). In contrast, G2019S LRRK2 specifically increased APP-positive inclusions in the ipsilateral striatum, a sensitive marker of axonal damage (43, 44), that is absent with WT or G2019S/K1906M LRRK2 variants (SI Appendix, Fig. S4). G2019S LRRK2 also increased silver-positive neurites in the striatum, a marker of neurite degeneration (44–46), relative to WT or G2019S/K1906M LRRK2 (SI Appendix, Fig. S4). These data suggest that G2019S LRRK2 induces axonal degeneration via a kinase-dependent mechanism in this rat model, consistent with our prior study (32). This neuropathology was generally absent from the ipsilateral SNpc, suggesting that it is confined to the striatum where human LRRK2 levels are highest. Consistent with our prior study (32), α-synuclein pathology was not induced by G2019S LRRK2 in this rat model following the evaluation of α-synuclein levels, Ser129 phosphorylation, or insoluble species in the striatum and ventral midbrain compared to WT or G2019S/K1906M LRRK2 (SI Appendix, Fig. S5). While we did observe increased immunoreactivity for phosphorylated tau (pSer202/pThr205; AT8) in the ipsilateral SNpc, this is most likely nonspecific, as it occurred equivalently with all LRRK2 variants and did not result in alterations in tau levels, phosphorylation, or detergent solubility in extracts from striatum and ventral midbrain (SI Appendix, Fig. S6). Microglial activation was detected in the ipsilateral striatum and SNpc as indicated by the modest increase of Iba1-positive immunoreactivity induced equivalently by all Ad5-LRRK2 vectors at 42 d following their intrastriatal delivery (SI Appendix, Fig. S7). In comparison, robust microglial activation was evident in the SNpc at 21 d following the intranigral delivery of Ad5-LRRK2 vectors (SI Appendix, Fig. S7), indicating a more pronounced inflammatory response to Ad5 delivery in the SNpc relative to the striatum. Microglial activation occurred largely independently of LRRK2 kinase activity in the rat brain, despite a nonsignificant increase with G2019S LRRK2 (SI Appendix, Fig. S7), and was most likely due to Ad5 infection. Collectively, these data demonstrate that G2019S LRRK2 selectively induces modest axonal pathology (APP and Gallyas silver) in the striatum via a kinase-dependent mechanism, whereas ubiquitin accumulation, tau hyperphosphorylation, and microglial activation in this model are unrelated to LRRK2 kinase activity.
Pharmacological Inhibition of Kinase Activity with PF-360 Protects against G2019S LRRK2 in Rats.
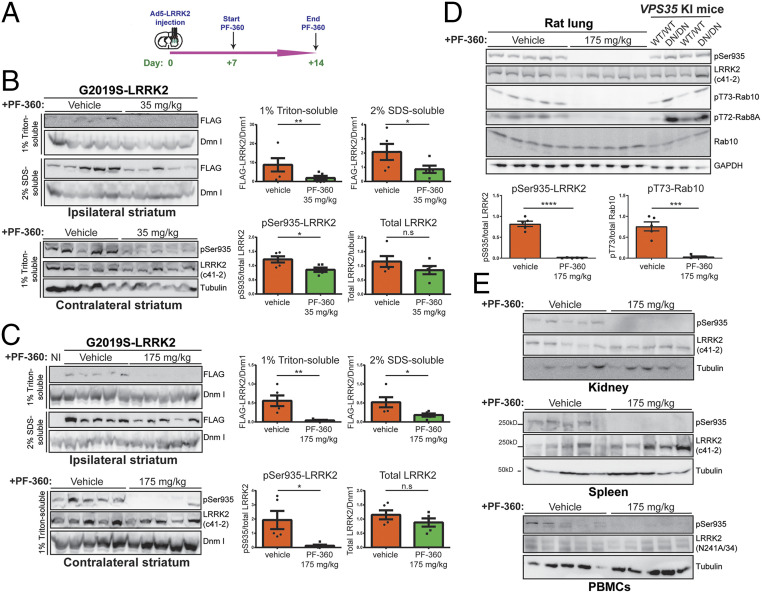
To further evaluate the contribution of kinase activity to the pathogenic effects of G2019S LRRK2 in this rat model, we explored the neuroprotective effects of a third generation, selective, potent, and brain-permeable LRRK2 kinase inhibitor, PF-06685360 (PF-360) (47). Rodent chow was developed containing PF-360 at either 35 or 175 mg/kg of chow, as recently described (41), that was previously shown to be well tolerated and produced complete inhibition of LRRK2 kinase activity in lung and near-complete inhibition in rat brain. Rats were first subjected to the unilateral intrastriatal delivery of Ad5-LRRK2 G2019S vector followed by in-diet administration of PF-360 for 7 d beginning at 7 d postinjection (Fig. 4A). PF-360 administration at 35 mg/kg (equivalent to ∼3.2 mg/kg/day) produces a significant yet modest inhibition of endogenous LRRK2 kinase activity in brain by monitoring pS935-LRRK2 levels, a constitutive phosphorylation site and well-established surrogate pharmacodynamic marker of kinase inhibition (41, 48), in detergent extracts from the contralateral striatum with negligible effects on total LRRK2 levels (Fig. 4B). In contrast, human G2019S LRRK2 levels were dramatically reduced in the ipsilateral striatum by PF-360 at this low dose (Fig. 4B). With PF-360 dosing at 175 mg/kg (equivalent to ∼16 mg/kg/day), endogenous pS935-LRRK2 was almost fully inhibited in the brain; yet total LRRK2 levels remained unaffected, whereas human G2019S LRRK2 levels were further reduced (Fig. 4C). These data suggest that human G2019S LRRK2 is selectively and markedly destabilized in the rat brain by PF-360 administration relative to endogenous LRRK2. Administration of PF-360 (175 mg/kg chow) also resulted in near maximal levels of LRRK2 kinase inhibition in peripheral tissues of rats as indicated by a complete reduction of pS935-LRRK2 and Rab phosphorylation (pT73-Rab10 and pT72-Rab8A) levels in lung relative to the known increase in pRab levels in D620N VPS35 knockin mice (Fig. 4D) (49), as well as in rat kidney, spleen, and peripheral blood mononuclear cells (PBMCs) (Fig. 4E), yet with minimal effects on the total levels of endogenous LRRK2 at this dose. To initially establish whether LRRK2 kinase inhibition influences Ad5 infectivity in the brain, we subjected LRRK2 knockout (KO) and WT littermate adult mice to intrastriatal delivery of Ad5-eGFP vector at four distinct sites (∼4.2 × 109 vp/site). At 10 d postinjection, we observed equivalent levels of GFP in the striatum and SNpc of WT and KO mice, confirming that endogenous LRRK2 is not required for efficient Ad5 infection in the rodent brain (SI Appendix, Fig. S8). To further explore the selective destabilization of human LRRK2 induced by kinase inhibition in rat brain, we compared the effects of PF-360 dosing (175 mg/kg chow) for 7 d on the steady-state levels of human WT, G2019S, and G2019S/K1906M LRRK2 proteins. We found that WT and G2019S LRRK2 levels were dramatically and equivalently reduced in the ipsilateral striatum by PF-360 treatment, whereas G2019S/K1906M LRRK2 was resistant to PF-360-induced destabilization (SI Appendix, Fig. S9). These data suggest that kinase activity and/or the K1906 site in the ATP-binding pocket are required for the destabilizing effects of PF-360 on human LRRK2.
Fig. 4.
In-diet pharmacological kinase inhibition (PF-360) markedly destabilizes human G2019S-LRRK2 protein in rat brain. (A) Ad5-G2019S-LRRK2 vectors were unilaterally delivered at six distinct locations (1.5 × 1010 vp/site, in 2.5 μL) in the rat striatum. Rats were fed with control (vehicle) or PF-360 (35 or 175 mg/kg) chow at day 7 postinjection for 7 consecutive days. Brain, peripheral tissues (lung, kidney, and spleen) and PBMCs were collected at 14 d postinjection and subjected to sequential extraction in buffers containing 1% Triton X-100 (Triton-soluble fraction) and 2% SDS (SDS-soluble fraction). (B and C) Western blot analysis of ipsilateral striatum extracts indicating human G2019S-LRRK2 levels (anti-FLAG antibody), or contralateral striatum extracts indicating phosphorylated (pSer935) and total (MJFF2/c41-2) endogenous LRRK2 levels in rats fed with vehicle or PF-360 chow at (B) 35 or (C) 175 mg/kg chow. Dynamin I (DnmI) or β-tubulin were used as loading controls for normalization. NI, noninjected striatum. Densitometric quantitation is shown for human G2019S-LRRK2 (FLAG) levels in each detergent fraction, and endogenous pSer935-LRRK2 or total LRRK2 levels. Bars represent the mean ± SEM (n = 5 animals/group). (D) Western blot analysis of Triton-soluble lung extracts indicating endogenous pSer935 and total LRRK2 levels (c41-2), or pT73-Rab10, p72-Rab8A, and total Rab10 levels, in rats fed with vehicle or PF-360 (175 mg/kg) chow, or from homozygous D620N VPS35 knockin mice (DN) and their WT littermates (n = 2 mice/genotype). Graphs indicate quantitation of pSer935-LRRK2 or pT73-Rab10 levels in rat lung (mean ± SEM, n = 5 animals/group). (E) Western blot analysis of Triton-soluble kidney, spleen, or PBMC extracts indicating levels of endogenous pSer935 and total (c41-2 or N241A/34) LRRK2 in rats fed with vehicle or PF-360 (175 mg/kg) chow. *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001 by unpaired Student’s t test; n.s., nonsignificant.
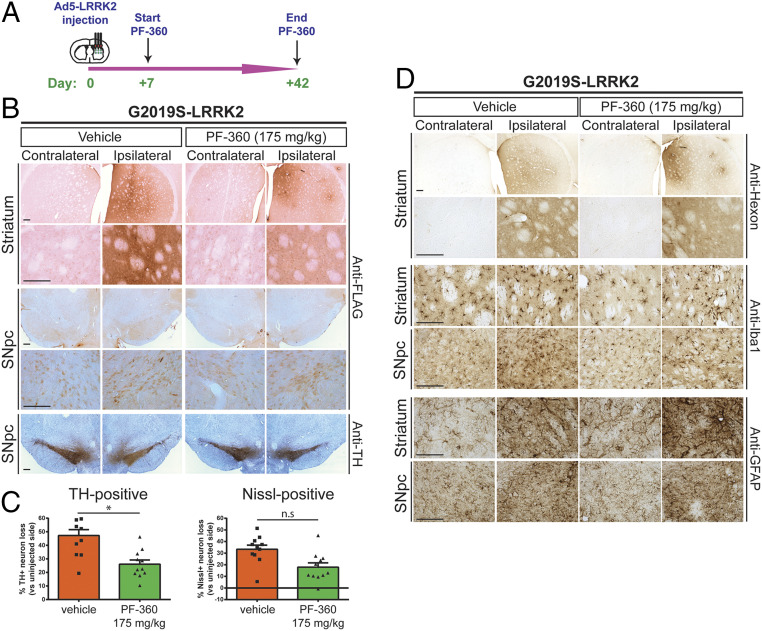
We next subjected rats to similar intrastriatal injections of Ad5-LRRK2 G2019S vector followed by chronic in-diet dosing of PF-360 (175 mg/kg) from 7 to 42 d postinjection to evaluate its neuroprotective effects (Fig. 5A). PF-360 dosing for 35 d resulted in a near-complete inhibition of endogenous LRRK2 kinase activity (pSer935) and the robust destabilization of human G2019S LRRK2 protein in the brain (SI Appendix, Fig. S10 A–C). PF-360 dosing was well tolerated over this period with consistent and equivalent weekly body weight gain and food intake among treated animals (SI Appendix, Fig. S10 D and E), with rats receiving an estimated dose of ∼16 mg/kg/day PF-360. At 42 d postinjection, G2019S LRRK2 was robustly detected in the striatum and SNpc of animals treated with vehicle chow, whereas PF-360 dosing led to a marked reduction of FLAG-LRRK2 immunoreactivity (Fig. 5B). Hexon capsid immunostaining indicated equivalent levels of Ad5-LRRK2 particles in the striatum with vehicle or PF-360 treatment (Fig. 5D), further indicating that LRRK2 kinase inhibition does not compromise Ad5 infectivity. Consistent with this observation, PF-360 treatment did not alter the levels of reactive gliosis (Iba1 and GFAP) in the striatum induced by Ad5-LRRK2 delivery (Fig. 5D). Unbiased stereology revealed robust nigral dopaminergic neurodegeneration induced by G2019S LRRK2 (47.2 ± 4.3% neuronal loss) that is significantly attenuated by PF-360 administration (26.1 ± 3.1% loss) (Fig. 5 B and C). Collectively, these data demonstrate that in-diet dosing with the LRRK2 kinase inhibitor, PF-360, provides neuroprotection against G2019S LRRK2 via an unexpected mechanism involving the selective destabilization of human LRRK2 protein.
Fig. 5.
Pharmacological kinase inhibition (PF-360) protects against dopaminergic neurodegeneration induced by human G2019S LRRK2. (A) Ad5-G2019S-LRRK2 vectors were unilaterally delivered at six distinct locations (1.5 × 1010 vp/site, in 2.5 μL) to the rat striatum. Rats were fed continuously with control (vehicle) or PF-360 (175 mg/kg) chow from days 7 to 42 postinjection. Brain tissues were harvested at 42 d postinjection and subjected to immunohistochemical analysis. (B) Immunohistochemistry reveals expression of human LRRK2 (anti-FLAG antibody) in striatum and nigra, or nigral dopaminergic neurons (anti-TH antibody), of rats at 42 d postinjection. Images indicate low and high magnification for each group and brain region. TH sections were counterstained with Cresyl violet. (Scale bars: 500 μm.) (C) Unbiased stereological analysis of TH-positive dopaminergic and total Nissl-positive neuron number in the substantia nigra at 42 d postinjection. Bars represent % neuronal loss in the injected ipsilateral nigra relative to the contralateral nigra (mean ± SEM, n = 12 animals/group), *P < 0.05 by unpaired Student’s t test; n.s., nonsignificant. (D) Immunohistochemistry at 42 d postinjection showing expression of Ad5 capsid (anti-hexon) in the ipsilateral striatum, or microglia (Iba1) and astrocytes (GFAP) in striatum and substantia nigra of rats expressing human G2019S-LRRK2 treated with vehicle or PF-360 chow. (Scale bars: 500 μm.)
Contribution of GTPase Activity to G2019S LRRK2-Induced Neurodegeneration in Rats.
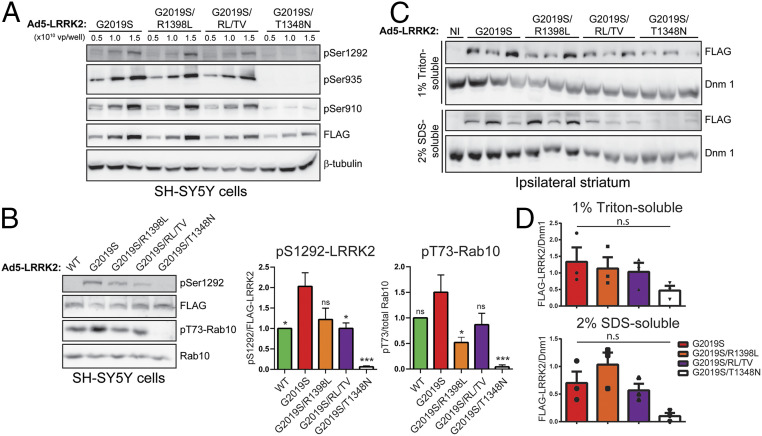
GTP binding via the P-loop can increase the kinase activity of LRRK2 (13, 14, 16), whereas synthetic mutations that disrupt P-loop GTP binding (K1347A or T1348N) markedly impair GTP hydrolysis and kinase activity (13, 14). PD-linked mutations in the Roc-COR tandem domain commonly impair the GTP hydrolysis activity of LRRK2, thereby prolonging the GTP-bound “on” state and indirectly enhancing kinase activity (15). We have previously identified hypothesis-testing mutations in the switch II catalytic motif of the Roc domain that markedly enhance (R1398L) or reduce (R1398L/T1343V) GTP hydrolysis activity in vitro and lead to an increase in the GDP-bound “off” or GTP-bound “on” state of LRRK2, respectively (13, 23). Given the importance of the GTPase domain to LRRK2 activity and the paucity of selective small molecules targeting this domain, we sought to employ these genetic mutations to evaluate the contribution of GTP-binding and GTP hydrolysis activity to neurodegeneration induced by G2019S LRRK2 in the rat brain. Accordingly, new high-titer Ad5-LRRK2 G2019S vectors were developed that additionally harbor distinct functional mutations in the Roc domain (G2019S/T1348N, G2019S/R1398L, and G2019S/R1398L/T1343V). To initially evaluate these Ad5-LRRK2 vectors, we demonstrate the titer-dependent and equivalent expression levels and phosphorylation status (Ser935, Ser910, and Ser1292) of G2019S, G2019S/R1398L, and G2019S/R1398L/T1343V LRRK2 variants in SH-SY5Y cells by Western blot analysis (Fig. 6A), whereas G2019S/T1348N LRRK2 levels and phosphorylation are markedly reduced, indicating the impaired stability and kinase activity of this variant. We next quantified the impact of each GTPase variant on the kinase activity of G2019S LRRK2 in SH-SY5Y cells following their transduction with Ad5-LRRK2 vectors. We find that G2019S/R1398L and G2019S/R1398L/T1343V LRRK2 modestly reduce pS1292-LRRK2 levels compared to G2019S LRRK2, whereas G2019S/R1398L markedly reduces pT73-Rab10 levels (Fig. 6B). G2019S/T1348N LRRK2 completely inhibits pS1292-LRRK2 and pT73-Rab10 levels in cells (Fig. 6B), similar to our prior in vitro studies of LRRK2 kinase activity (13), confirming that GTP-binding capacity is critical for normal kinase activity (13, 14, 16). These data demonstrate that genetic inhibition of GTP binding impairs the protein stability and kinase activity of G2019S LRRK2.
Fig. 6.
Evaluation of Ad5-LRRK2-G2019S vectors with genetic disruption of GTP binding or GTP hydrolysis in cells and rat brain. (A) Validation of high-titer Ad5-LRRK2 (G2019S, G2019S/R1398L, G2019S/R1398L/T1343V, or G2019S/T1348N) vectors and LRRK2 phosphorylation in SH-SY5Y cells. Cells were transduced with increasing viral particles of each Ad5-LRRK2 vector (0.5, 1, or 5.0 × 1010 vp/well). Western blot analysis of cell extracts with FLAG antibody (transduced human LRRK2) or antibodies to detect LRRK2 autophosphorylation (pSer1292) or constitutive phosphorylation (pSer935, pSer910) sites to detect a titer-dependent increase in human LRRK2 levels/activity. β-Tubulin was used as protein loading control. (B) Western blot analysis of SH-SY5Y cells infected with Ad5-LRRK2 vectors (WT, G2019S, G2019S/R1398L, or G2019S/R1398L/T1343V at 1.5 × 1010 vp/dish, or G2019S/T1348N at 3 × 1010 vp/dish) for pS1292-LRRK2 and human LRRK2 (FLAG), or endogenous pT73-Rab10 and total Rab10. Graphs indicate quantitation of pS1292-LRRK2 levels normalized to FLAG-LRRK2, or pT73-Rab10 levels normalized to Rab10 (mean ± SEM, n = 3 experiments). (C) Ipsilateral striatum was harvested at 10 d after unilateral intrastriatal delivery of Ad5-LRRK2 vectors and subjected to sequential detergent extraction in 1% Triton X-100 and 2% SDS. Striatal fractions were analyzed by Western blot analysis (n = 3 animals/vector) indicating human LRRK2 variant levels (anti-FLAG antibody). Expression of dynamin-I (Dnm1) was used as loading control. NI, noninjected striatum. (D) Densitometric analysis of human LRRK2 variant levels (FLAG) normalized to Dnm1 levels in each detergent fraction. Bars represent the mean ± SEM (n = 3 animals/vector). *P < 0.05 or ***P < 0.001 by one-way ANOVA with Dunnett’s multiple comparisons test; n.s., nonsignificant.
To explore the effects of these GTPase mutations in vivo, we delivered Ad5-LRRK2 vectors (G2019S, G2019S/R1398L, G2019S/R1398L/T1343V, and G2019S/T1348N) to the striatum of rats (∼1.25 × 1010 vp/site) and conducted biochemical analysis at 10 d postinjection. Human LRRK2 variants were detected in 1% Triton-soluble and -insoluble extracts from the ipsilateral striatum with equivalent levels of G2019S, G2019S/R1398L, and G2019S/R1398L/T1343V LRRK2 proteins by Western blot and densitometric analyses (Fig. 6 C and D). The levels of G2019S/T1348N LRRK2 are dramatically reduced in the striatum relative to other LRRK2 variants, further supporting the reduced protein stability of this GTPase variant (Fig. 6 C and D). Our data indicate that functional mutations that alter GTP hydrolysis are well tolerated in cells and in vivo and can reduce the kinase activity of G2019S LRRK2, whereas inhibition of GTP binding dramatically compromises LRRK2 protein stability and kinase activity, consistent with prior studies (13, 14, 16).
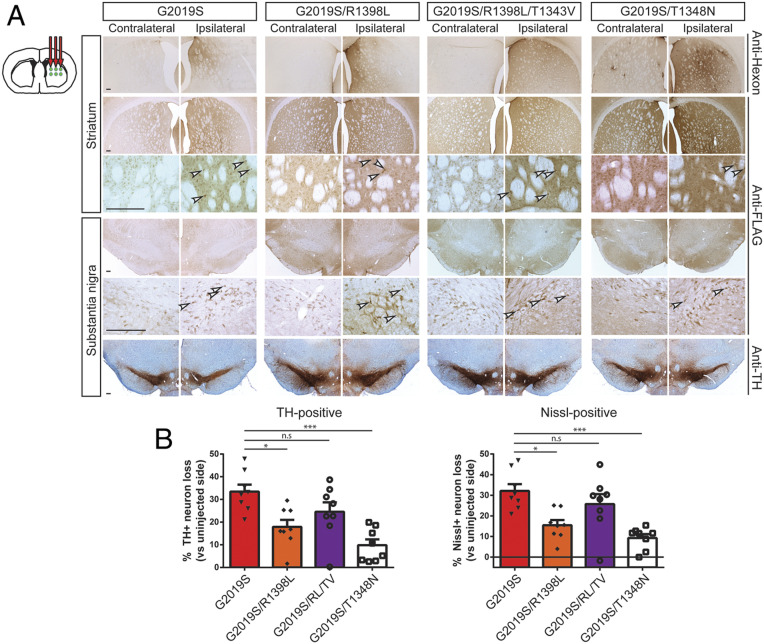
The neuroprotective capacity of these functional GTPase variants was next evaluated at 42 d following the intrastriatal delivery of Ad5-LRRK2 vectors. FLAG-LRRK2 immunoreactivity indicates that G2019S, G2019S/R1398L, and G2019S/R1398L/T1343V LRRK2 proteins are detected in the ipsilateral striatum and SNpc at equivalent levels, whereas G2019S/T1348N LRRK2 levels are markedly reduced (Fig. 7A). Hexon capsid immunostaining indicates equivalent levels of Ad5 particles for each LRRK2 variant in the striatum (Fig. 7A), indicating that G2019S/T1348N LRRK2 exhibits reduced protein stability in the brain. Intriguingly, unbiased stereology reveals robust dopaminergic neuronal loss induced by G2019S LRRK2 (33.4 ± 8.8% loss) that is significantly attenuated with G2019S/R1398L (17.9 ± 8.8%) or G2019S/T1348N (9.8 ± 7.3%) LRRK2 variants (Fig. 7 A and B). Neuronal loss induced by G2019S/R1398L/T1343V LRRK2 (24.6 ± 11.9% loss) is also reduced relative to G2019S LRRK2 although this effect is not significant (Fig. 7 A and B). The loss of total Nissl-positive SNpc neurons parallels the loss of TH-positive SNpc neurons in this rat model thereby confirming neuronal cell death (Fig. 7B). Taken together, these data suggest that genetically enhancing GTP hydrolysis (R1398L) or disrupting GTP-binding (T1348N) are sufficient to protect against G2019S LRRK2 in the rat brain. However, the protective effects elicited by the T1348N variant are likely mediated by destabilizing the LRRK2 protein rather than via diminished GTP-binding per se.
Fig. 7.
Ad5-G2019S-LRRK2 induces dopaminergic neurodegeneration in a GTPase-dependent manner in adult rat brain. (A) Ad5-LRRK2 (G2019S, G2019S/R1398L, G2019S/R1398L/T1343V, G2019S/T1348N) vectors were unilaterally delivered at six injection sites (1.5 × 1010 vp/site, in 2.5 μL) in the rat striatum. Immunohistochemistry indicates human LRRK2 variant (anti-FLAG) expression in the ipsilateral striatum and substantia nigra that persists up to 42 d postinjection. Immunohistochemistry also indicates Ad5 capsid (anti-hexon) in the ipsilateral rat striatum, and nigral dopaminergic neurons (anti-TH antibody) with Cresyl violet counterstain, at 42 d. Images indicate low and high magnification for each vector and brain region. (Scale bars: 500 μm.) (B) Unbiased stereological analysis of TH-positive dopaminergic and total Nissl-positive neuron number in the substantia nigra at 42 d postinjection. Bars represent % neuronal loss in the injected ipsilateral nigra relative to the contralateral nigra (mean ± SEM, n = 8 animals/vector), *P < 0.05 or ***P < 0.001 by one-way ANOVA with Bonferroni’s multiple comparisons test; n.s., nonsignificant.
Discussion
Transgenic and knockin rodent models of LRRK2-linked PD generally exhibit mild phenotypes which has hampered the identification of key mechanisms that drive neurodegeneration due to familial mutations (12, 33). However, prior studies using large-capacity viral vectors, such as HSV or Ad5, delivered to the brains of adult rodents have proven successful in recapitulating the age-dependent degeneration of substantia nigra dopaminergic neurons induced by the common G2019S mutation in LRRK2 (29, 37). Our prior studies using Ad5-LRRK2 vectors suggested that G2019S LRRK2 induces neuropathology in rats via a kinase-dependent mechanism (32). However, these pathological effects were confined to the striatum and relied upon a kinase-inactive version of the LRRK2 protein, D1994N, that is unstable in rat brain and primary neurons (13, 32). In the present study, we further develop and optimize this Ad5-LRRK2 rat model of PD to demonstrate that human G2019S LRRK2 induces robust nigral dopaminergic neurodegeneration over 6 wk following the stereotactic delivery of Ad5 vectors directly to the striatum or substantia nigra. Using a stable kinase-inactive mutation in the ATP-binding pocket, K1906M, we show that G2019S LRRK2 induces neuronal loss through a kinase-dependent mechanism. In addition, we demonstrate that the pharmacological inhibition of LRRK2 kinase activity using the selective and potent inhibitor PF-360 can attenuate dopaminergic neuronal loss induced by G2019S LRRK2 in rat brain. Unexpectedly, PF-360-mediated kinase inhibition selectively destabilizes the exogenous human LRRK2 protein in the rat brain relative to its modest effects on endogenous LRRK2. Our data also reveal a critical role for GTPase activity in mediating the pathogenic actions of G2019S LRRK2. Intriguingly, a mutation in the switch II motif of the Roc domain that enhances GTP hydrolysis activity, R1398L, attenuates neuronal loss induced by G2019S LRRK2 via a mechanism that may involve reduced kinase activity. A second mutation that impairs GDP/GTP binding via the Roc P-loop motif, T1348N, is also neuroprotective, albeit by dramatically destabilizing the LRRK2 protein. Taken together, these data convincingly support a critical role for kinase and GTPase activity in mediating the neurodegenerative effects of the LRRK2 G2019S mutation in vivo.
It has proven difficult to develop relevant and robust preclinical animal models for PD-linked LRRK2 mutations to directly explore the underlying mechanisms of neurodegeneration and evaluate novel therapeutic strategies (12, 33). Viral-mediated gene transfer for human LRRK2 has allowed the identification of robust neuropathology in rodent models, although subsequent mechanistic studies have been lacking. Although HSV vectors were initially used to reveal dopaminergic neuronal loss induced by human G2019S LRRK2 in the mouse brain, a role for kinase activity in this model relied upon an unstable kinase-inactive mutation (D1994A) and the use of generation “0” LRRK2 inhibitors that are not selective for LRRK2 (i.e., GW5074 or indirubin-3′-monoxime) (29, 47). For example, GW5074 is a potent inhibitor of c-Raf1 kinase with an IC50 of ∼9 nM, whereas indirubin-3′-monoxime potently inhibits GSK-3β (IC50 ∼22 nM), making it difficult to assign neuroprotective effects specifically to LRRK2 kinase inhibition in this model. Furthermore, the HSV-LRRK2 model no longer appears to be in active use. Our prior studies with neuronal-expressing Ad5-LRRK2 vectors in rat brain also revealed specific neuronal loss and neuropathology with G2019S LRRK2 but also employed an unstable kinase-inactive D1994N mutation in LRRK2 (32). A similar study using broadly expressing Ad5-LRRK2 vectors in mouse brain compared G2019S and another kinase-inactive mutation, D1994A LRRK2, revealing neuroinflammation and vacuolization in aged mice due to LRRK2 expression in glial cells (50). A recent study in the nonhuman primate, Microcebus murinus, using long-acting canine adenovirus type 2 vectors, revealed an equivalent level of neuronal loss induced by WT and G2019S LRRK2 but without evaluating kinase activity (51). Therefore, the contribution of kinase activity in these or any mutant LRRK2 animal model has not been clearly established. So far, pharmacological LRRK2 kinase inhibition has been evaluated indirectly in rodent models with adeno-associated virus (AAV)-mediated human α-synuclein expression where PF-475 protects against dopaminergic neurodegeneration in rats induced by WT α-synuclein (52) but PF-360 lacked neuroprotection against A53T α-synuclein (53). Despite the paucity of direct evidence in LRRK2 animal models, selective LRRK2 kinase inhibitors (i.e., DNL151 and DNL201) are currently in phase I clinical trials.
Our data extend these prior observations in LRRK2 viral models by first demonstrating robust dopaminergic neurodegeneration induced by G2019S LRRK2 in the rat brain when transgene expression is restricted to neurons using the human synapsin-1 promoter in the Ad5 vector. This finding implies that G2019S LRRK2 is able to induce dopaminergic neuronal loss via a cell-autonomous mechanism but does not exclude a noncell-autonomous role for LRRK2 in astrocytes and/or microglia. Indeed, Ad5 vector delivery in this rat model induces modest and prolonged neuroinflammation which may itself contribute to or be required for the neurotoxic effects of G2019S LRRK2. Secondly, we demonstrate a key role for kinase activity in the Ad5-LRRK2 model using a kinase-inactive mutant (K1906M) that is stably expressed in rat brain relative to kinase-active LRRK2 variants. Importantly, by monitoring LRRK2 autophosphorylation at Ser1292 in cells, primary neurons and brain, and Rab10 phosphorylation in neurons, we show that the K1906M mutation impairs the kinase activity of G2019S LRRK2. Our attempts to use pT73-Rab10 antibody to monitor G2019S LRRK2 activity in rat brain were unsuccessful. This is potentially due to endogenous Rab10 and exogenous human LRRK2 residing in different cell populations in this model, the low abundance of Rab10 in dopaminergic neurons, and/or because the G2019S mutation produces a more modest increase in Rab10 phosphorylation compared to other PD-linked familial mutations (18). Introducing the K1906M mutation robustly rescues nigral neuronal loss induced by G2019S LRRK2 following either intrastriatal or intranigral Ad5 delivery, although intrastriatal delivery produced lower background effects of the Ad5 vectors. Third, the genetic analyses of LRRK2 kinase activity were complemented by pharmacological studies with PF-360, a potent and selective generation “3” LRRK2 inhibitor (47). PF-360 is highly potent with an in vivo IC50 of ∼3 nM for LRRK2, and exhibits an improved selectivity profile, oral bioavailability, and brain permeability compared to earlier generations of LRRK2 inhibitors (41, 47, 54). We demonstrate full inhibition of endogenous LRRK2 kinase activity in peripheral tissues and brain of our rat model with chronic in-diet dosing of PF-360 at 175 mg/kg chow. While PF-360 produces neuroprotection against G2019S LRRK2 in rats at this dose, it most likely achieves this effect via an unexpected mechanism involving the selective and robust destabilization of human LRRK2 protein in brain compared to its mild effects on endogenous rat LRRK2. This destabilizing effect was also prominently observed at lower doses of PF-360 (35 mg/kg chow) and with human WT LRRK2, whereas kinase-inactive G2019S/K1906M LRRK2 was notably resistant. Structurally distinct LRRK2 kinase inhibitors (i.e., MLi-2, PF-475, GSK2578215A, and HG-10-102-01) have been shown to induce LRRK2 protein destabilization via proteasomal degradation in cells and tissues that is dependent on dose and exposure time (55). This effect is consistent with the destabilizing effects of certain kinase-inactive mutations (D1994A/N/S) (13, 26, 31, 32), suggesting an intricate role for kinase activity in maintaining normal stability and limiting the turnover of LRRK2 protein. However, our data imply a strong bias for destabilization either due to species (human over rat LRRK2) and/or due to levels of overexpression and availability (exogenous over endogenous LRRK2). These findings could have ramifications for the treatment of patients with LRRK2 kinase inhibitors, although it is not feasible at present to determine whether kinase inhibition will dramatically destabilize brain LRRK2 in human tissues. In any case, the destabilizing effect of PF-360 treatment on G2019S LRRK2 in our model proves target engagement and indicates that early inhibition and/or reduction of mutant LRRK2 in this model is sufficient for neuroprotection. Our future studies will explore the impact of distinct kinase inhibitors on human LRRK2 destabilization in the rat brain.
The contribution of GTPase activity in LRRK2 animal models has not been studied, largely owing to a lack of robust activity assays and pharmacological tools. While LRRK2 has been shown to bind GTP and exhibit a slow rate of GTP hydrolysis in vitro (13, 14, 16, 20–23), comparable activity assays in tissues are presently lacking. Putative pharmacological inhibitors of LRRK2 GTP binding have also been reported (i.e., FX2149) although their selectivity profiles and mode of action are poorly defined (56, 57). At present, mutational analysis of the Roc GTPase domain remains the only reliable method to probe its function in vivo. We have previously identified hypothesis-testing mutations in conserved motifs that regulate the GTPase activity of LRRK2 (13, 23). In our hands, the switch II motif mutation, R1398L, increases GTP hydrolysis in vitro but has no obvious effect on steady-state GTP binding (13, 23). In combination with a second P-loop mutation, R1398L/T1343V, this variant impairs GTP hydrolysis in vitro (13). Disruption of the P-loop motif via T1348N impairs GDP/GTP binding and therefore prevents GTP hydrolysis (13). Based on these in vitro studies, we are able to demonstrate that the R1398L and T1348N mutations provide robust neuroprotection against G2019S LRRK2 in the rat brain but perhaps via different mechanisms. While the G2019S/R1398L protein is stable in vivo, the G2019S/T1348N protein is highly unstable and therefore phenocopies a loss-of-function effect similar to the effects of kinase inhibitors (13). These data suggest to us instead that GTP-binding capacity is critical for maintaining the normal stability of the LRRK2 protein in vivo, although the underlying mechanism is unclear. We have previously shown that T1348N LRRK2 is unstable, interacts poorly with itself, and is enzymatically inactive in vitro and in human cells (Fig. 6) (13), potentially consistent with impaired dimerization. For the R1398L mutation that is normally dimeric in cells (13), we assume its protective effect in vivo is due to increased GTP hydrolysis activity but it could also disrupt the GTPase cycle of LRRK2 in general. Notably, G2019S/R1398L LRRK2 markedly reduces Rab10 phosphorylation compared to G2019S LRRK2 in cells (Fig. 6), suggesting that neuroprotection in vivo is most likely mediated via a kinase-dependent mechanism. This finding is consistent with the concept that increased GTP hydrolysis promotes the GDP-bound off state of LRRK2 that would lead to reduced kinase activity (15). Therefore, our data support interference with GTP hydrolysis via the switch II motif or GTP binding via the P-loop as promising neuroprotective strategies for alleviating the pathogenic effects of G2019S LRRK2. The underlying mechanism(s) involved in vivo and whether these protective effects extend to PD-linked LRRK2 mutations within the Roc-COR tandem domain (i.e., N1437H, R1441C/G/H, and Y1699C) await further study.
Collectively, our data provide a comprehensive evaluation of Ad5-LRRK2 vectors for exploring the neurodegenerative effects and mechanisms of PD-linked LRRK2 mutations in neurons of the adult rat brain. While dopaminergic neuronal loss provides a robust phenotypic readout of G2019S LRRK2 in this model, we identify axonal damage as another specific phenotype, and also define nonspecific neuropathological markers that are not informative for LRRK2 pathophysiology. As a proof of concept we demonstrate that this Ad5-LRRK2 model can be effectively used to evaluate the genetic and pharmacological contribution of enzymatic activity to LRRK2-dependent neurodegeneration. We nominate kinase inhibition, increased GTP hydrolysis, and inhibition of GTP binding as potential therapeutic strategies for attenuating the neurotoxic effects of LRRK2 mutations. The Ad5-LRRK2 rat model provides a relatively robust, flexible, and relevant animal model for rapidly exploring novel mechanisms and therapeutic strategies in PD.
Materials and Methods
Animals.
Adult female Wistar rats (180 to 200 g) were obtained from Harlan Laboratories. LRRK2 KO mice with a deletion of exon 41 (31) were kindly provided by Giorgio Rovelli and Derya Shimshek, Novartis Pharma AG, Basel Switzerland. D620N VPS35 knockin mice were reported previously (58). Time-pregnant female Sprague-Dawley outbred rats were obtained from Taconic Biosciences and P0–P1 rats were used to prepare postnatal primary cortical neuronal cultures as previously described (59). Rodents were maintained in a pathogen-free barrier facility and provided with food and water ad libitum and exposed to a 12 h light/dark cycle. Animals were treated in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals (60). All animal experiments were approved by the Van Andel Institute Institutional Animal Care and Use Committee.
Cell Culture.
Human SH-SY5Y neuroblastoma cells (ATCC) were maintained as described (61). Stable E2T-293 cells used for adenovirus production were maintained as described (32, 37). Primary cortical neurons were plated in 35-mm dishes and maintained as described (59, 61).
Adenovirus Production.
Recombinant E1, E2a, and E3-deleted (second generation) human adenovirus serotype 5 (Ad5) were produced in E2T cells and purified as previously described (32, 37, 62). Codon-optimized 3xFLAG-tagged full-length WT, G2019S, and G2019S/D1994N human LRRK2 cDNAs in pDC511 shuttle plasmid downstream of a human synapsin-1 promoter have been described (32, 37). K1906M, R1398L, and T1348N mutations were introduced into pDC511-G2019S LRRK2 by site-directed mutagenesis using the QuikChange XL kit (Stratagene), and a T1343V mutation was introduced into pDC511-G2019S/R1398L LRRK2, as described (32). Briefly, Ad5 vectors were generated in inducible E2a cells (E2T cells) by calcium phosphate transfection of human Ad5 genomic plasmid (pBHGfrtΔE1,3FLP; Microbix) and each pDC511-LRRK2 shuttle plasmid, and sequential amplification in 24-well, 6-well, and 10-cm dishes, as described (62). Cell extracts were treated with benzonase nuclease (12.5 U/mL) and subjected to two rounds of column purification using the Vivapure AdenoPACK 100RT kit (Sartorius). The concentration of viral particles was determined by spectrophotometry using OD260 measurements.
Adenoviral Infection: SH-SY5Y Cells and Primary Neurons.
SH-SY5Y cells were infected with each purified adenovirus to verify equivalent LRRK2 expression. In general, 5 × 105 cells/virus dose were plated in 35-mm dishes 24 h before infection. On the day of infection, purified viruses were mixed at the desired volumes in 0.5 mL of normal culture media. Cells were incubated with virus at 37 °C with gentle rocking every 10 min. After 30 min of incubation, 1.5 mL of fresh media was added to each dish and cells were incubated at 37 °C. At 48 h postinfection, cells were washed twice in cold TBS (50 mM Tris pH 7.5, 150 mM NaCl) and harvested in 200 μL lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 1× Complete protease inhibitor mixture [Roche], 1× phosphatase inhibitor mixtures 2 and 3 [Sigma]). Cells were rotated at 4 °C for 30 min and centrifuged at 21,000 × g for 30 min at 4 °C to prepare supernatants. Protein concentration was determined using bicinchoninic acid (BCA) assays (Pierce Biotech). Primary cortical neurons in 35-mm dishes were infected with Ad5 vectors (1.5 × 1010 viral particles) in media for 30 min on days in vitro (DIV) 3 and harvested on DIV6 in 75 μL lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 270 nM sucrose, 1% Triton X-100, 1 mM EGTA, 1× Complete protease inhibitor mixture [Roche], 0.2 mM sodium orthovanadate, 10 mM sodium fluoride, 2 mM 2-glycerophosphate, 2 mM sodium pyrophosphate, 1× phosphatase inhibitor mixture set I [524624, Millipore]). Neuronal extracts were prepared by centrifugation as above. For immunofluorescence, neurons were fixed at DIV6 in 4% PFA.
Stereotactic Rodent Surgery.
Adult female Wistar rats (Harlan Laboratories) weighing ∼180 to 200 g, or adult homozygous LRRK2 KO and wild-type littermate mice (mixed sex), were subjected to stereotactic surgery for unilateral delivery of recombinant adenoviral vectors. After the animals were deeply anesthetized with continuous isofluorane, they were placed in a stereotactic frame (model 963-A, David Kopf Instruments) and the cranium was exposed. For intranigral injection, viral vectors were delivered at a single point in the right SNpc using the following coordinates relative to bregma: anterior-posterior −5.2 mm, mediolateral −2 mm, and dorsoventral (DV) −7.8 mm relative to the skull surface. Blunt tip steel needles (34 gauge) connected through polyethylene tubing to a 10-μL Hamilton syringe were inserted slowly to the injection site and left in place for 30 s before starting the infusion. A volume of ∼2.5 μL of virus was injected at a flow rate of 0.2 μL/min with an automatic pump (CMA Microdialysis). The needle was left in place for an additional 5 min before being slowly withdrawn to allow the tissue to close over and minimize leakage of the injected virus. The skin was sealed by stapling and the animals were monitored until they recovered from anesthesia.
For intrastriatal injections, viral vectors were delivered at six points in the right dorsal striatum (two dorsoventral deposits along three needle tracks) of rats using the following coordinates relative to bregma: anterior-posterior +0.48 mm; mediolateral −2 mm, −3 mm, and −4 mm; and dorsoventral −6 mm and −4.8 mm relative to the skull surface. Volumes of ∼2.5 μL of virus were injected per site, and needles were left in place for 5 min between the first and second infusions (at DV −6 and −4.8 mm), and for an additional 5 min after the second infusion before being withdrawn. For mice, viral vectors (1 μL/site) were delivered at four points in the right dorsal striatum (two dorsoventral deposits along two needle tracks) using the coordinates: anterior-posterior +0.86 mm, mediolateral −1.2 mm and −2.2 mm, and dorsoventral −3.7 mm and −3.0 mm relative to the skull surface.
Immunohistochemistry and Immunofluorescence.
Animals were perfusion-fixed with 4% paraformaldehyde (PFA, in 0.1 M phosphate buffer, pH 7.3), and brains were removed, cryoprotected, dissected into 40-μm-thick coronal sections, and subjected to chromogenic immunohistochemistry, as previously described (63). A Zeiss Axio Imager M2 light microscope with color charge-coupled device (CCD) camera (AxioCam, Zeiss) was used to capture all images.
Immunofluorescence staining was also conducted as previously described (63). Briefly, sections were blocked in 10% normal goat serum (Invitrogen), and sequentially incubated with primary antibodies (for 48 h) and Alexa Fluor-488- or Alexa Fluor-594-conjugated secondary antibodies (Life Technologies). Sections were mounted on glass slides, coverslipped in mounting medium (Prolong containing DAPI, Invitrogen), and images were acquired on a Nikon A1plus-RSi scanning confocal microscope. For primary neurons, fixed cells were incubated with primary antibodies to FLAG-M2 (Sigma) and either pS1292-LRRK2 (MJFR-19-7-8, Abcam) or pT73-Rab10 (MJF-R21-22-5, Abcam), and anti-mouse IgG-AlexaFluor-488- and anti-rabbit IgG-AlexaFluor-546 secondary antibodies. Neurons were mounted and imaged as above.
Brain Tissue Fractionation.
Striatum and ventral midbrain from injected and noninjected hemispheres were rapidly dissected and homogenized with six volumes of Triton lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 5% glycerol, 1% Triton X-100, 1 mM EDTA, 1× EDTA-free Complete protease inhibitor mixture [Roche] and 1× phosphatase inhibitor mixture 2 and 3 [Sigma]). Tissues were disrupted using a mechanical homogenizer (IKA T10 basic, Ultra Turrax). The Triton-soluble fraction was obtained after ultracentrifugation at 100,000 × g for 30 min at 4 °C. The resulting pellets were further extracted by sonication in 3× volumes of SDS lysis buffer (50 mM Tris⋅HCl pH 7.4, 2% SDS, 1× EDTA-free Complete protease inhibitor mixture [Roche] and 1× phosphatase inhibitor mixture 2 and 3 [Sigma]). Samples were centrifuged at 21,000 × g for 30 min at 25 °C to obtain the Triton-insoluble (SDS-soluble) fraction. Protein concentration was determined using the BCA assay (Pierce Biotech).
PF-360 Dosing and Pharmacodynamic Assays.
PF-360 was synthesized as described in patent US2014/005183 at >98% purity and formulated into medicated chow at 35 or 175 mg/kg by Research Diets, as previously described (41). Animals were fed chow continuously for 7 or 35 d beginning at 7 d after stereotactic surgery. Body weight and chow consumption were monitored every 7 d. Pharmacodynamic assays to monitor LRRK2 kinase inhibition by in-diet PF-360 dosing included assessment of endogenous pSer935 levels normalized to total LRRK2 levels in brain and peripheral tissues (lung, kidney, spleen, PBMCs), or endogenous pT73-Rab10 or pT72-Rab8A levels normalized to total Rab10. To monitor Rab phosphorylation, brain and lung tissues were homogenized with six volumes of Triton lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 270 mM sucrose, 1% Triton X-100, 1 mM EGTA, 1× EDTA-free Complete protease inhibitor mixture [Roche], 0.2 mM sodium orthovanadate, 10 mM sodium fluoride, 2 mM 2-glycerophosphate, 2 mM sodium pyrophosphate, and 1× phosphatase inhibitor mixture set I [EMD Millipore, 524624]) (49). The Triton-soluble fraction was obtained after centrifugation at 21,000 × g for 30 min at 4 °C and quantitated by BCA assay for analysis. PBMCs were isolated from tail vein blood (500 µL) using SepMate-15 tubes (STEMCELL Technologies).
Western Blot Analysis.
Western blot analysis was conducted as previously described (63). Briefly, protein samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) using precast Tris-glycine gels (7.5% or 4 to 20%; Novex). Proteins were transferred to nitrocellulose membranes (0.2 μm, Amersham), blocked with 5% nonfat milk (Bio-Rad), and sequentially incubated with primary antibodies (overnight at 4 °C), and secondary antibodies conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch). Membranes were stripped between different primary antibodies using Restore Western Blot buffer (Thermo Scientific). Proteins were detected using enhanced chemiluminescence (ECL, Amersham) and digital images were acquired on a LAS-3000 image analyzer (Fujifilm). For protein quantitation, densitometry was performed on acquired digital images using Image Studio Lite, v4.0 software.
Antibodies.
Primary antibodies used include: mouse monoclonal anti-GFP (clones 7.1 and 13.1, Sigma), mouse monoclonal anti-LRRK2 (clone N241A/34, NeuroMabs), rabbit monoclonal anti-LRRK2 (clone MJFF2/c41-2, Abcam), mouse monoclonal anti-FLAG and anti-FLAG-HRP (clone M2, Sigma), rabbit monoclonal anti-pSer910-LRRK2 [clone UDD1 15 (3), Abcam], rabbit monoclonal anti-pSer935-LRRK2 [clone UDD2 10 (12), Abcam], rabbit monoclonal anti-pSer1292-LRRK2 (clone MJFR-19-7-8, Abcam), mouse monoclonal anti-β-tubulin (TUB 2.1, Sigma), rabbit polyclonal anti-dynamin-1 (PA1-660, Thermo Fisher Scientific), rabbit polyclonal anti-tyrosine hydroxylase (NB300-109, Novus Biologicals), rabbit monoclonal anti-phospho-Thr73-RAB10 (MJF-R21 or MJF-R21-22–5, Abcam), anti-phospho-Thr72-RAB8A (MJF-R20, Abcam), rabbit monoclonal anti-RAB10 (D36C4, Cell Signaling Technology), mouse monoclonal anti-human adenovirus 5, hexon capsid protein (clone 9C12, Developmental Studies Hybridoma Bank), mouse monoclonal anti-GAPDH (clone 1E6D9, Protein Tech). For bright-field microscopy, biotinylated goat anti-mouse and anti-rabbit secondary antibodies (Vector Laboratories) were employed. For Western blots, light chain-specific mouse anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP (Jackson Immunoresearch) secondary antibodies were used.
Stereological Analysis.
The unbiased stereological estimation of substantia nigra dopaminergic neurons was performed using the optical fractionator probe of the StereoInvestigator software (MicroBrightField Biosciences), as described (32, 35, 44).
Statistical Analysis.
One-way ANOVA with appropriate post hoc analysis was used for multiple comparisons, whereas unpaired, two-tailed Student’s t test was used for comparing two groups, as indicated. All data were plotted as mean ± SEM and were considered significant when P < 0.05.
Data Availability.
All relevant data are included in the paper.
Supplementary Material
Acknowledgments
This work was supported by funding from the NIH (R01 NS091719; D.J.M.), Sanofi Aventis (D.J.M.), Michael J. Fox Foundation for Parkinson’s Research (D.J.M.), Swiss National Science Foundation (Grant No. 31003A_144063; D.J.M.), and American Parkinson Disease Association (A.P.T.N). We are grateful to Drs. Philippe Bertrand and Laurent Dubois (Sanofi, Chilly-Mazarin, France) for helpful discussions and Dr. Dorian Sargent (Van Andel Research Institute) for technical assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922184117/-/DCSupplemental.
References
- 1.Obeso J. A., et al. , Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 32, 1264–1310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez D. G., Reed X., Singleton A. B., Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem. 139 (suppl. 1), 59–74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biskup S., West A. B., Zeroing in on LRRK2-linked pathogenic mechanisms in Parkinson’s disease. Biochim. Biophys. Acta 1792, 625–633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy D. G. et al.; International LRRK2 Consortium , Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 7, 583–590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paisán-Ruíz C., et al. , Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Zimprich A., et al. , Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Chang D. et al.; International Parkinson’s Disease Genomics Consortium; 23andMe Research Team , A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 49, 1511–1516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nalls M. A. et al.; International Parkinson’s Disease Genomics Consortium (IPDGC); Parkinson’s Study Group (PSG) Parkinson’s Research: The Organized GENetics Initiative (PROGENI); 23andMe; GenePD; NeuroGenetics Research Consortium (NGRC); Hussman Institute of Human Genomics (HIHG); Ashkenazi Jewish Dataset Investigator; Cohorts for Health and Aging Research in Genetic Epidemiology (CHARGE); North American Brain Expression Consortium (NABEC); United Kingdom Brain Expression Consortium (UKBEC); Greek Parkinson’s Disease Consortium; Alzheimer Genetic Analysis Group , Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biskup S., et al. , Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 60, 557–569 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Biskup S., et al. , Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 8, 102 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West A. B., et al. , Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J. Comp. Neurol. 522, 2465–2480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam M. S., Moore D. J., Mechanisms of RRK2-dependent neurodegeneration: Role of enzymatic activity and protein aggregation. Biochem. Soc. Trans. 45, 163–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biosa A., et al. , GTPase activity regulates kinase activity and cellular phenotypes of Parkinson’s disease-associated LRRK2. Hum. Mol. Genet. 22, 1140–1156 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Ito G., et al. , GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry 46, 1380–1388 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Nguyen A. P., Moore D. J., Understanding the GTPase activity of LRRK2: Regulation, function, and neurotoxicity. Adv. Neurobiol. 14, 71–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taymans J. M., et al. , LRRK2 kinase activity is dependent on LRRK2 GTP binding capacity but independent of LRRK2 GTP binding. PLoS One 6, e23207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng Z., et al. , Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci. Transl. Med. 4, 164ra161 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Steger M., et al. , Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West A. B., et al. , Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U.S.A. 102, 16842–16847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniëls V., et al. , Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J. Neurochem. 116, 304–315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis P. A., et al. , The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 357, 668–671 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., et al. , Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J. Neurochem. 103, 238–247 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Y., et al. , GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 6, e1000902 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao J., et al. , Parkinson disease-associated mutation R1441H in LRRK2 prolongs the “active state” of its GTPase domain. Proc. Natl. Acad. Sci. U.S.A. 111, 4055–4060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greggio E., et al. , Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 23, 329–341 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Skibinski G., Nakamura K., Cookson M. R., Finkbeiner S., Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies. J. Neurosci. 34, 418–433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith W. W., et al. , Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 9, 1231–1233 (2006). [DOI] [PubMed] [Google Scholar]
- 28.West A. B., et al. , Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 16, 223–232 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Lee B. D., et al. , Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 16, 998–1000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsden N., et al. , Chemoproteomics-based design of potent LRRK2-selective lead compounds that attenuate Parkinson’s disease-related toxicity in human neurons. ACS Chem. Biol. 6, 1021–1028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzig M. C., et al. , LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 20, 4209–4223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsika E., et al. , Adenoviral-mediated expression of G2019S LRRK2 induces striatal pathology in a kinase-dependent manner in a rat model of Parkinson’s disease. Neurobiol. Dis. 77, 49–61 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Daniel G., Moore D. J., Modeling LRRK2 pathobiology in Parkinson’s disease: From yeast to rodents. Curr. Top. Behav. Neurosci. 22, 331–368 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Chen C. Y., et al. , (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 19, 1623–1633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramonet D., et al. , Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One 6, e18568 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y., et al. , Robust kinase- and age-dependent dopaminergic and norepinephrine neurodegeneration in LRRK2 G2019S transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 115, 1635–1640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dusonchet J., et al. , A rat model of progressive nigral neurodegeneration induced by the Parkinson’s disease-associated G2019S mutation in LRRK2. J. Neurosci. 31, 907–912 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis T. B., Glasgow J. N., Glandon A. M., Curiel D. T., Standaert D. G., Transduction of brain dopamine neurons by adenoviral vectors is modulated by CAR expression: Rationale for tropism modified vectors in PD gene therapy. PLoS One 5, e12672 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols R. J., et al. , 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 430, 393–404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lis P., et al. , Development of phospho-specific Rab protein antibodies to monitor in vivo activity of the LRRK2 Parkinson’s disease kinase. Biochem. J. 475, 1–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly K., et al. , The G2019S mutation in LRRK2 imparts resiliency to kinase inhibition. Exp. Neurol. 309, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert K., Voutilainen M. H., Domanskyi A., Airavaara M., AAV vector-mediated gene delivery to substantia nigra dopamine neurons: Implications for gene therapy and disease models. Genes (Basel) 8, E63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman M., Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Tsika E., et al. , Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum. Mol. Genet. 23, 4621–4638 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee M. K., et al. , Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 –> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 99, 8968–8973 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo Bianco C., Ridet J. L., Schneider B. L., Deglon N., Aebischer P., Alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 99, 10813–10818 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West A. B., Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp. Neurol. 298, 236–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dzamko N., et al. , Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem. J. 430, 405–413 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mir R., et al. , The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J. 475, 1861–1883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kritzinger A., et al. , Age-related pathology after adenoviral overexpression of the leucine-rich repeat kinase 2 in the mouse striatum. Neurobiol. Aging 66, 97–111 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Mestre-Francés N., et al. , Exogenous LRRK2G2019S induces parkinsonian-like pathology in a nonhuman primate. JCI Insight 3, 98202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daher J. P., et al. , Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates α-synuclein gene-induced neurodegeneration. J. Biol. Chem. 290, 19433–19444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen M. A., et al. , Parkinson’s disease-like burst firing activity in subthalamic nucleus induced by AAV-α-synuclein is normalized by LRRK2 modulation. Neurobiol. Dis. 116, 13–27 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Andersen M. A., et al. , PFE-360-induced LRRK2 inhibition induces reversible, non-adverse renal changes in rats. Toxicology 395, 15–22 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Lobbestael E., et al. , Pharmacological LRRK2 kinase inhibition induces LRRK2 protein destabilization and proteasomal degradation. Sci. Rep. 6, 33897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T., et al. , A novel GTP-binding inhibitor, FX2149, attenuates LRRK2 toxicity in Parkinson’s disease models. PLoS One 10, e0122461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas J. M., et al. , 68 and FX2149 attenuate mutant LRRK2-R1441C-induced neural transport impairment. Front. Aging Neurosci. 8, 337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X., et al. , Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 116, 5765–5774 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stafa K., et al. , GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 8, e1002526 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 61.Williams E. T., et al. , Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum. Mol. Genet. 27, 3189–3205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou H., Beaudet A. L., A new vector system with inducible E2a cell line for production of higher titer and safer adenoviral vectors. Virology 275, 348–357 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Nguyen A. P. T., et al. , G2019S LRRK2 enhances the neuronal transmission of tau in the mouse brain. Hum. Mol. Genet. 27, 120–134 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the paper.