Abstract
A growing body of evidence demonstrates that the brain can reorganize dramatically following sensory loss. Although the existence of such neuro-plastic cross-modal changes is not in doubt, the functional significance of these changes remains unclear. The dominant belief is that reorganization is compensatory. However, results thus far do not unequivocally indicate that sensory deprivation results in enhanced abilities in other senses. Here, we consider alternative reasons besides sensory compensation that might drive the brain to reorganize after sensory loss. One such possibility is that the cortex reorganizes not to confer functional benefits, but to avoid undesirable physiological consequences of sensory deafferentation. Empirical assessment of the validity of this and other possibilities defines a rich program for future research.
Keywords: Cortical reorganization, plasticity, sensory loss, multimodal activations, sensory compensation
Investigating the teleology of cortical reorganization
The brain’s functional organization follows a prototypical schema across most individuals. This comprises a parcellation of sensory inputs from different modalities to distinct cortical regions, which in turn constitute the beginnings of cascades of processing that culminate in higher-order association areas. Of interest to our discussion here are deviations from this norm. Instances of atypical sensory development, specifically involving sensory loss in one or more modalities, can lead to dramatic changes in cortical organization. The primary sensory cortices associated with the missing modality, instead of lying ‘idle’, appear to be recruited by other sensory modalities; they come to exhibit responses to stimuli from these encroaching senses. Such reorganization is not only a remarkable demonstration of cortical plasticity, it also suggests that the nervous system follows a strategy to achieve homeostasis - loss of one modality is compensated for by providing additional neural resources to the remaining sensory streams. The accumulated evidence for cortical reorganization and its intuitively appealing explanation as a compensatory process is the starting point for our discussion. We ask whether the extant experimental results obligate us to consider additional accounts of reorganization beyond the conventional one. Given the dramatic extent of reorganization, when it occurs, investigating its teleology promises to yield insights about the basic principles of cortical function, as well as guidance regarding how best to harness or manage these changes for maximal adaptive benefit.
Cortical reorganization and its conventionally assumed ‘purpose’
There is compelling evidence for cortical reorganization in individuals with sensory loss. The data are especially striking in the domain of blindness. Several studies with human subjects using neuro-imaging techniques (such as functional magnetic resonance imaging, or fMRI) have reported activation of the occipital cortex while blind individuals perform non-visual tasks such as sound localization [1–3], tactile perception and spatial navigation [4–6], and odor perception [7]. Intriguingly, even higher level cognitive tasks such as language [8–10] and memory processing [11] have also been shown to activate occipital areas in the blind (see Figure 1 for examples). Although important cautions need to be exercised in assessing the extent of cortical reorganization via neuro-imaging [12], evidence for the existence of such changes is generally accepted to be robust. Analogous to results from humans, studies of animals that were visually-deprived soon after birth (either by lid suturing or dark rearing) also show functional reorganization in multisensory areas such as superior colliculus [13,14], the anterior ectosylvian (AE) cortex [15,16] and parietal association cortex [17]. In these areas, neurons have been found to be less responsive to visual stimuli, and more responsive to auditory or somatosensory stimuli compared to normally-sighted control animals [18,19]. The causal link between sensory deprivation and neural reorganization is strengthened by the observation that the extent and nature of the latter is modulated by the timing of the former’s onset [20,21].
Figure 1. Examples of cortical reorganization (i.e. crossmodal occipital activation) observed in early blind individuals using fMRI.
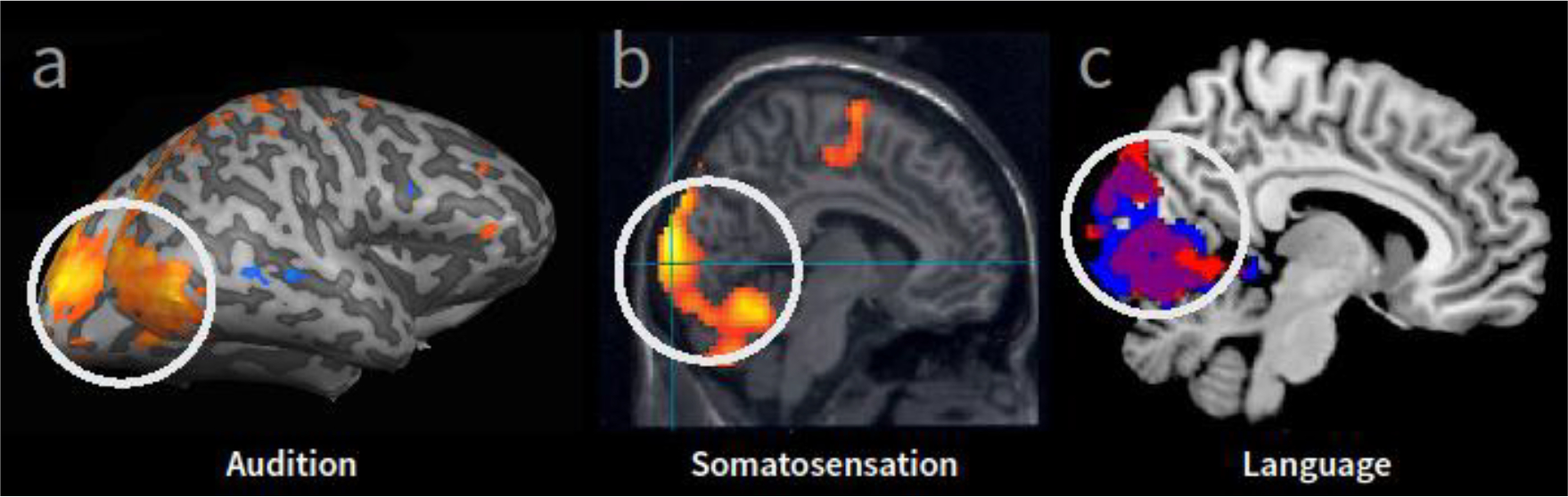
Occipital cortical areas (white circle, shown in sagittal plane) typically associated with processing visual information are found to respond to stimulation from non-visual modalities. (A) Occipital cortical regions that were activated more in early blind compared to sighted controls in response to auditory processing (sound localization task [22], modified from Renier et al., 2010). (B) Occipital cortical responses within a group of blind subjects reading Braille (compared to rest) [23] (modified from Gizewski et al., 2003). (C) Greater activity within occipital cortex in early blind compared to sighted controls in response to auditory linguistic stimuli [24] (modified from Bedny et al., 2011).
Inferences regarding the functional significance of occipital cortex activation in blind individuals during non-visual processing have come from both clinical and experimental studies. Braille reading abilities of a congenitally blind woman were found to be compromised following stroke-induced bilateral damage to her occipital cortex [25]. In a laboratory setting, transcranial magnetic stimulation (TMS) has served as a means to non-invasively and reversibly disrupt localized cortical activity and observe its impact on behavioral performance. For instance, transient and reversible disruption of occipital cortical areas of the blind has been found to causally disrupt their performance on Braille reading [26], auditory localization [27] and even verbal processing [28]. Similar causal linkages between cross-modal neural reorganization and performance have been reported using reversible cortical cooling paradigms [29,30].
Changes in non-visual task performance following disruption of occipital cortex activity in the blind suggest a compensatory account for explaining cortical reorganization. With access to the large neuronal resources of the erstwhile visual cortex, modalities such as audition and somato-sensation are expected to register significant functional gains that can help compensate, at least partially, for the loss of vision. A similar argument applies for the loss of audition; visual processing may be enhanced by coopting auditory cortex [31–33]. This notion of compensatory enhancement dates at least as far back as Darwin. In ‘Expression of Emotions in Man and Animal’ (1890, page 361) he posited that —
“When we direct our attention to any one sense, its acuteness is increased; and the continued habit of close attention, as with blind people to that of hearing, and with the blind and deaf to that of touch, appears to improve the sense in question permanently [34].”
Over the past few decades, several such results with humans have accumulated, constituting a convincing case for functional enhancement in one modality being a consequence of deprivation in another [28,35–47]. For instance, two-point tactile thresholds on the index, middle, and ring fingers of blind individuals are smaller than those in their sighted counterparts, indicative of greater tactile sensitivity [40]. Blind individuals are also reported to have a lower rate of sound localization errors relative to sighted ones [42], and improved noun-verb recall and classification performance [28]. The latter result is attributed to the linguistically evoked activations in the visual cortex of the blind. Figure 2, a–c, summarizes the data from a few representative studies. Blind individuals have also been reported to be able to use echolocation for exploring the spatial structure of their surroundings [48,49]. Interestingly, neuro-imaging studies reveal that echolocation in these individuals is associated with activation of occipital cortex [50], consistent with a compensatory account of the observed cortical functional reorganization.
Figure 2. A performance comparison of blind and sighted individuals across a variety of tasks.
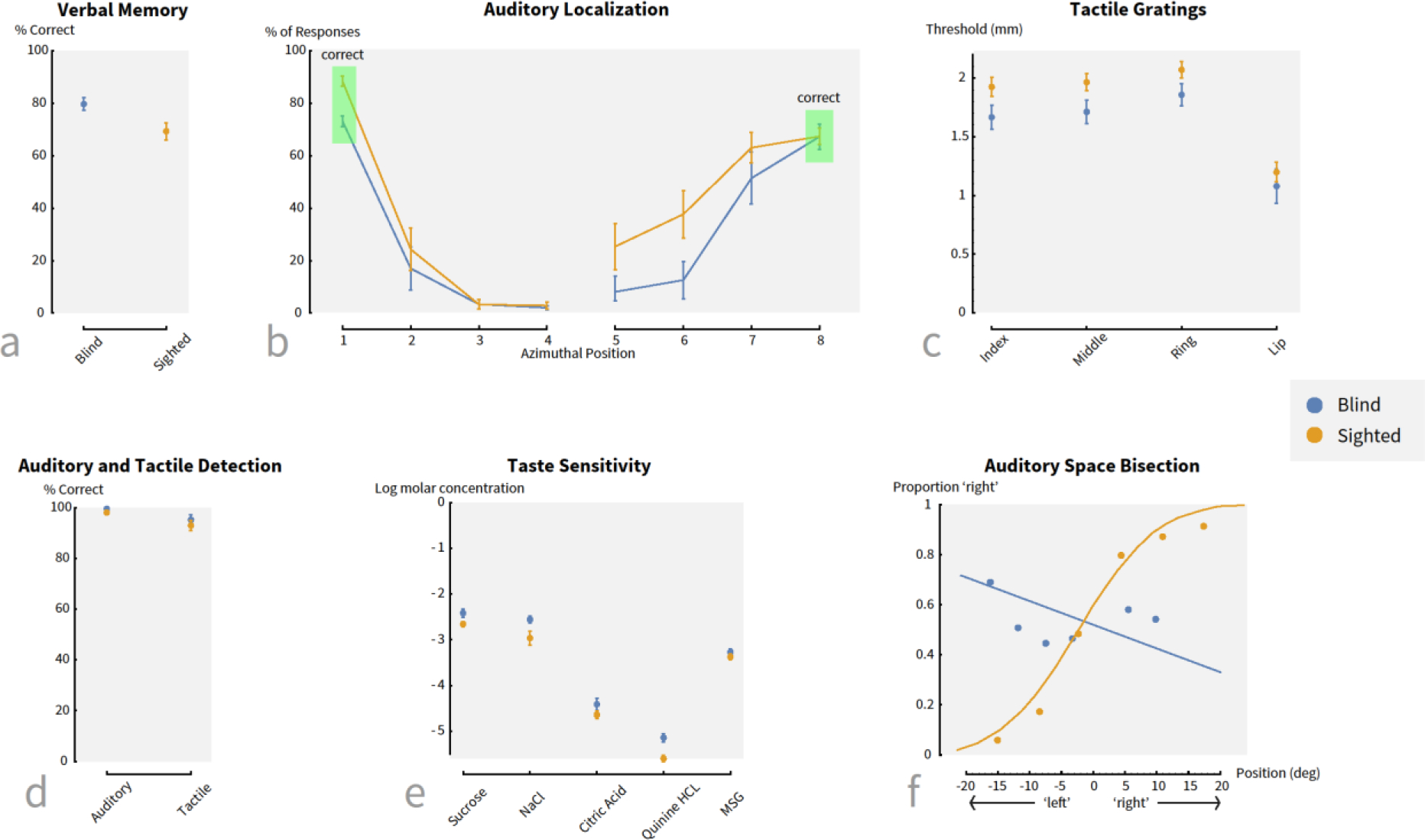
(a – c) Examples of tasks on which blind participants show an advantage relative to their sighted counterparts. (d – f) Examples of tasks on which there are either no differences between the two groups or a disadvantage for the blind. (Sources: a: [28]; b: [42]; c: [37]; d: [40]; e: [58]; f: [73]
The link between cortical reorganization and enhanced functioning of non-deprived senses is supported by several studies that have reported functional and anatomical (e.g. gray matter volume) neural covariates of compensatory behavioral adaptations in occipital cortex of blind individuals [51–53]. Similar correlations have been observed in non-human subjects. Investigation of the behavioral consequences of deprivation, specifically of auditory abilities, show that visually-deprived cats exhibit more precise sound localization abilities compared to normally-sighted cats [54]. Furthermore, visual deprivation also results in significant neural alterations after a period of several years. For example, the auditory portion of AE cortex, believed to be specialized for sound localization, is enlarged and neurons in this area exhibit sharper spatial tuning compared to neurons in normally-sighted cats [55].
The need to revisit the conventional assumption
In the face of results described above, there seems little reason to rethink the notion of cortical reorganization as a compensatory response that enhances processing of the remaining senses. It makes logical sense for changes in cortical functional organization to serve some adaptive purpose. However, not all cortical reorganization may be beneficial and can be potentially maladaptive. Reorganization of motor cortex in primates and humans correlates with poor recovery from some forms of cortical damage [56]. Sensory systems may suffer a similar fate. As an example, ferret auditory cortex shows marked reorganization as a consequence of ectopic visual input [57]. However, this reorganization subsequently inhibits the recovery of the original cortical function, suggesting that the role of sensory plasticity also may not always be for the better. Studies of chemosensation, though not very extensive so far, also point to limited, if any, compensatory effects. For instance, congenitally blind individuals have been found to have reduced taste sensitivity, and an absence of gustatory crossmodal responses in the occipital cortex [58,59]. Additionally, loss in one chemosensory modality (as in anosmia) often leads to a reduction in sensitivity in the other chemo-senses as well [60]. This reduction is especially notable given that anosmic individuals have been reported to exhibited larger responses to chemosensory stimulation in frontal and temporal cortical regions which are typically not involved in chemosensation [61]. Such evidence forces a reconsideration of the functional significance of cortical changes after sensory loss.
It is notable that the enhancement effects typically observed in remaining sensory modalities following loss of one are modest in magnitude and generally become manifest in circumscribed experimental settings. For instance, the reported two-point threshold improvements in congenitally blind subjects averaged 10% in magnitude [40]. Studies of sound localization [42] found no facilitatory effect of blindness on localization accuracy for sound sources in front of the observer (the blind actually exhibited a reduction in accuracy relative to the sighted in this condition). It was only for sound sources in the extreme periphery that the blind showed a reduction in false-alarms rates. Puzzlingly, this advantage vanished for sources placed entirely to the side. In this condition, the blind were no better than the sighted in their response accuracy, with neither approaching ceiling performance. Instead of a generalized enhancement of localization abilities in the blind, there appears to be a tradeoff; improvements in sound localization in the horizontal plane are accompanied by a worsening of this ability in the vertical plane [62]. Finally, studies of blind subjects performing a verbal memory task [28] showed about a 10% increase in performance by the blind relative to sighted controls. It is important to keep in mind the possibility that the extent of improvements exhibited by blind individuals on these tasks may be limited by performance ceilings, derived either from intrinsic biological limitations or simply the theoretical maximum obtainable in a setting. As Figure 2 shows, the level of performance of both the blind and sighted groups across many of these tasks is below the theoretical maximum. However, biologically based ceilings may well be a factor in limiting the gains exhibited by the blind.
Although small, these gains are statistically significant and do provide support for the compensatory hypothesis. But, the magnitudes of improvements are potentially pertinent from two perspectives. The first is that the extent of improvements observed is comparable to enhancements obtained through practice [63,64]. This weakens the case for invoking cross-modal cortical reorganization as a necessary causal factor underlying performance changes. The impressive skills of echolocation that have typically been associated with blindness can, in fact, be learned through practice by sighted observers as well [65]. Relatedly, comparably high levels of Braille reading proficiency have been found in early and late-blind individuals, even though early blind subjects showed more widespread reorganization, extending into temporal regions, compared to late blind subjects [66]. Although late blindness could induce different mechanisms of cortical changes than early blindness, the result argues for a dissociation between functional ability and the extent of reorganization.
The second is the mismatch between the dramatic extent of cortical reorganization observed on the one hand, and the modest functional benefit that accrues on the other. Figure 1 shows the large area of putative primary visual cortex (V1) that is activated by audition in blind individuals. A comparison of the extent of these activations and the area of typical primary auditory cortex (A1) [67,68] suggests that the primary sensory cortical area for audition has increased several-fold. And yet, the functional consequences of this marked increase are modest at best. Such seeming inefficiency of resource usage poses the question of whether functional enhancement is the primary adaptive purpose of cortical reorganization.
Several lines of evidence in the literature further motivate a re-examination of the notion of functional enhancement as being a necessary/intended consequence of sensory deprivation induced cortical reorganization. Instead of a heightening of acuity, some researchers have found either no changes in performance, or even, in some cases, a worsening (Figure 2d–f shows a few examples). For instance, some recent studies that have observed striking extents of auditory encroachment in the visual cortex (Figure 1), have found no differences in the performance of blind and sighted subjects on a range of tasks including auditory detection, identification and localization [22,69], echoing earlier findings [70]. Blind individuals were not more sensitive to a target odor, nor more proficient at choosing a correct odor label from a list of four [71]. Several studies with early blind individuals have reported impairments in auditory spatial localization performance of early blind individuals [72,73] and reduced taste sensitivity [59]. Recent reviews have catalogued several instances of non-visual performance decrement in the blind [74]. Similarly, reviews of visual abilities in profoundly deaf individuals indicate that despite evidence of cortical changes, behavioral capabilities may undergo little enhancement [75].
The extant evidence, therefore, suggests that the blind may not be ‘super-sensors’ in non-visual modalities, and may even be worse off than sighted individuals. First-person accounts provide an interesting subjective corroboration of this inference. Here’s a passage from an essay (‘You don’t look blind’) by Laura Legendary, a blind educator who specializes in disability awareness.
Nearly everywhere I go I am forced to contend with the result of widely-held beliefs about blindness. The single most often repeated myth about blindness is the belief that we have a superior sense of hearing. Let’s clear this up right now. People who are blind do not have bionic hearing. This is a myth. This myth is repeated so often, everyone tends to believe that it must be true. It is not.
Years ago, I was placed in the unfortunate position of having to complain to my apartment manager about my noisy neighbors in the building in which I lived. The apartment manager would do nothing, as he evidently believed the “heightened senses” myth. “Well, you have more sensitive hearing,” he explained, “so they just seem louder to you” (http://accessibleinsights.info/blog/2010/05/01/blogging-against-disablism-entry-you-dont-look-blind/).
A similar lack of correlation between cortical reassignment and functional outcomes is observed within single modalities. Two examples are especially notable. The first relates to the classic finding by Wiesel and Hubel (1963) of reorganization in primary visual cortex (V1) of the cat following several weeks of monocular deprivation in infancy [76]. In such cases, the normally balanced division of neuronal resources in V1 is shifted dramatically in favor of the open eye. Interestingly, the profound increase in the neural representation of the non-deprived eye leads to no improvement in its functional capabilities. As a model of human amblyopia, this is analogous to the observation that the ‘fellow eye’ not only registers no visual enhancements, but may even show compromised function [77]. Notably, the size of primary visual cortex across the population at large varies by a factor estimated to be as large as 2.5 [78], but this does not translate to correspondingly large variations in basic dimensions of visual function such as resolution acuity [79].
The second example comes from studies of somatosensory reorganization following digit amputation. The cortical area normally devoted to a particular finger is taken over by the adjacent digits following the finger’s removal/silencing [80,81]. However, this increase in cortical representation for the two adjacent digits is not accompanied by any increase in their tactile sensitivity [82].
Taken together, these findings suggest that while the conventional account of cortical reorganization as a compensatory response to heighten the remaining senses is empirically well-founded, it may be worth considering additional possibilities to arrive at a more comprehensive teleological understanding of cortical reorganization.
Possible accounts of cortical reorganization after sensory loss
Figure 3 schematizes three potential explanations for cortical reorganization. After presenting brief descriptions of each here, in the next section we consider ways in which they may be tested. It is important to note that although the three accounts emphasize conceptually distinct points, they are not mutually exclusive, and may even share some overlaps.
Figure 3. Schematic depictions of three accounts of cortical reorganization after partial sensory loss.
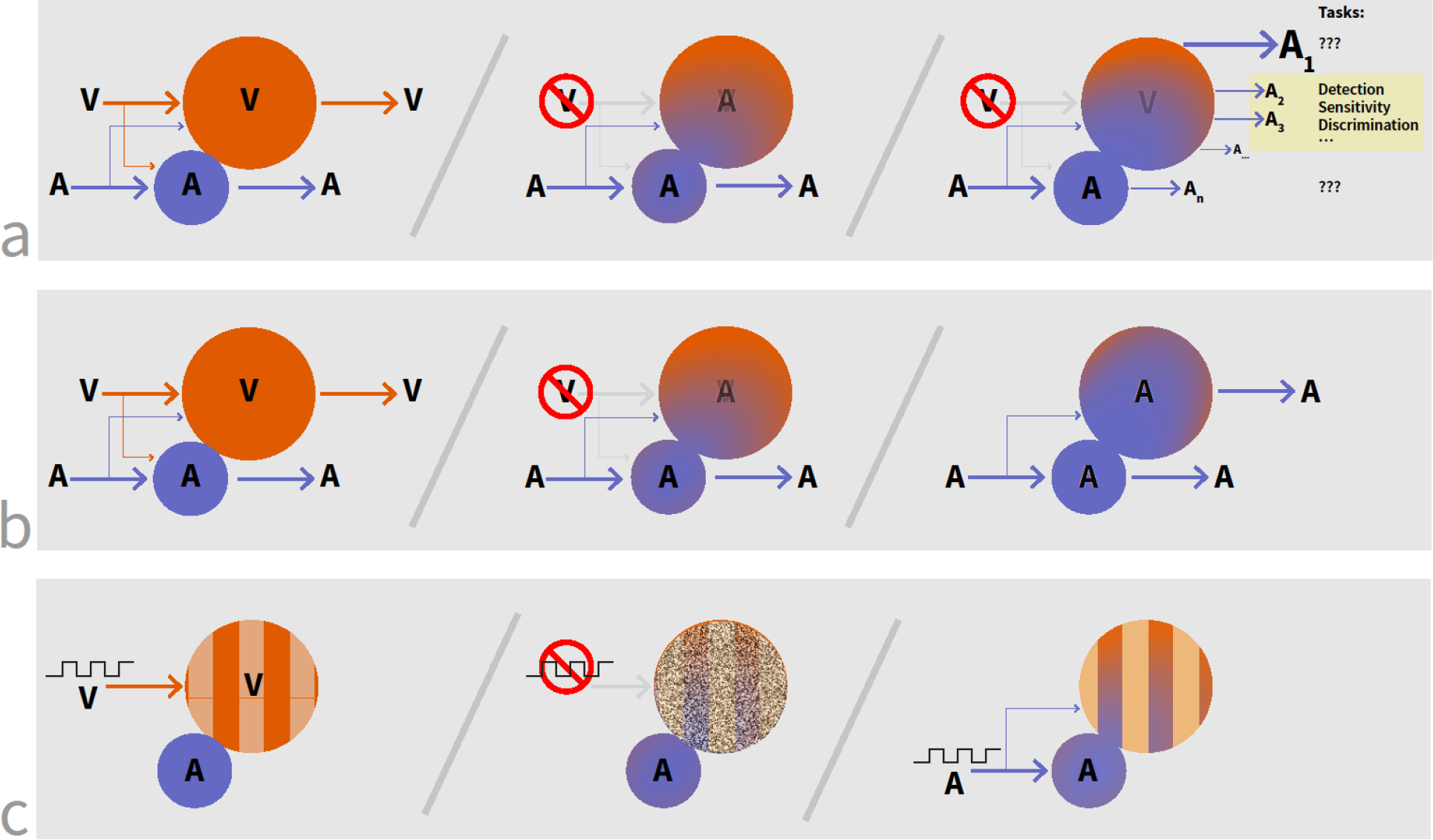
In all figures, V and A refer to ‘vision’ and ‘audition’ as example modalities. (a) Possibility 1: The cortical area devoted to the ‘lost’ sense (here, vision) is co-opted by a different modality (here, audition). This leads to changes in functional capabilities, although the dimensions along which these changes happen may be different from the conventionally assumed ones of low-level detection and discrimination thresholds. (b) Possibility 2: Loss of one modality leads to the unmasking of already present connections from a different sense. (c) Possibility 3: Structured sensory inputs (here depicted as square waves) lead to structured cortical activation. With the loss of this input, cortical activity can become paroxysmal. Structured inputs from a different modality help re-impose structured activity in the cortical area.
Possibility 1: The conventional explanation constitutes a full account but needs additional experimental evidence to demonstrate its adequacy.
Cortical reorganization may well be compensatory (Figure 3a). The reason for the limited empirical support for this possibility, as discussed in the previous section, could be that studies so far may not have focused on the ‘right’ tasks. For instance, reduced auditory detection thresholds or enhanced localization accuracy, may not be the only, nor the largest, non-visual behavioral changes in blind individuals. Instead, enhancements may be evident along hitherto unexamined aspects of auditory function that register impressive gains in the blind. It is instructive to consider an analogy. Conditions such as ‘hidden hearing loss’ [83,84] suggest that even a marked change in neural resource allocation might not necessarily influence basic detection thresholds. These empirical observations are supported by predictions from signal detection theory [85].
These enhanced abilities need not be purely perceptual but include attentional or decisional processing as well. Recent studies have shown that blind individuals exhibit enhanced connectivity between frontal and visual cortices, suggesting that activation in visual cortex from non-visual stimuli may be caused by top-down mechanisms [86,87]. Studies on sighted individuals who are extensively trained on tactile tasks also demonstrate increased connectivity between frontal and subcortical regions associated with motor and decision processes rather than in somatosensory regions [88]. Together, these results suggest that the extensive practice blind individuals have using non-visual modalities can lead to primary enhancements in executive processing, which can then, secondarily, result in the observed improvements in thresholds and accuracies when performing non-visual behavioral tasks.
Possibility 2: Reorganization is inevitable; it results from the unmasking of connections already present, and thus may not confer significant new behavioral proficiencies.
Recent evidence suggests that even in a healthy brain (i.e. one that has undergone typical sensory development), the so-called primary sensory areas are, in fact, multi-modal in nature and receive inputs from more than one nuclei in the thalamus or other primary sensory areas [89–91]. Responses of neurons in one sensory area can be modulated by connections from areas corresponding to other sensory modalities [92,93]. Under normal development, one modality becomes so dominant that the other inputs are not easily evident because they provide inputs at subthreshold levels. However, loss of the primary modality can unmask the normally dormant connections or increase their inputs to supra-threshold levels (Figure 3b). In this framework, the presence of activation from other sensory modalities in a cortical area that does not normally exhibit such responses is not so much evidence for reorganization as the unmasking of circuitry already present.
Support for this idea derives from demonstrations of multi-modal responses in the visual cortices of normally sighted individuals who undergo a period of total visual deprivation lasting merely a few days [94]. The rapid manifestation of such responses appears more consistent with the unmasking of existing inputs rather than growth of new long-range connections from other cortical or thalamic areas (while long range axonal sprouting is feasible, it typically occurs over much longer time courses [95]. Several animal studies have also yielded evidence consistent with unmasking of existing connections. For instance, there are compelling data consistent with unmasking of connections in the somato-sensory cortex following amputation of a digit [81]. Studies examining consequences of reversible sensory deactivation by pharmacological means (using lidocaine injections) in the somatosensory system of adult rats have found immediate and extensive neuronal reorganization in the cortex as well as the thalamus, implicating unmasking of connections as a mechanism [96]. There are reports of immediate enhancements in somatosensory cortical responses in adult rats after spinal-cord transection [97]. Researchers have argued for unmasking of long-range horizontal connections (i.e. pathways supporting inter-areal connections and linking cortical areas) as an account of cortical reorganization in cat V1 following retinal lesions [98,99]. Although the results reported in these studies are intra-modal, they indicate the feasibility of unmasking as a mechanism to explain at least some of the reorganization observed in adult cortex.
The consequences of unmasking of connections already present in a cortical area may include behavioral outcomes other than changes in basic sensory thresholds. For instance, if the unmasking is tantamount to changes in the weighting of connections from different sensory modalities, it may affect perceptual re-weighting, as discussed in statistical models of sensory combination. Similarly, if unmasking or reweighting occurs between top-down connections, then this hypothesis would posit changes in decisional or attentional behaviors. These different kinds of outcomes also serve to link this possibility with the previous one; the functional significance of cortical reorganization may be best assessed using tests other than those that focus on basic sensory thresholds.
Possibility 3: Reorganization is necessary to avoid maladaptive consequences such as paroxysmal activity and cortical atrophy.
Sensory inputs arising from a structured world impose structure on cortical activity. It appears that such structure is important for maintaining the stability of cortical dynamics. A growing body of evidence from non-human animal studies [100,101] shows that even partial afferent signal loss leads to paroxysmal cortical activity that can progress to electrographic seizures. Sensory signal loss, especially early in life, can also lead to other undesirable consequences such as neuronal apoptosis [102–104]. Consistent with this possibility, studies have reported significant thinning in typically centrally responsive primary visual cortex of patients with central vision loss due to macular degeneration (MD), and thickening in the peripherally responsive regions [105].
In this context, it is plausible that the redirection of other sensory modalities towards the input-deprived cortical area may be an attempt to avoid the severe physiological consequences of sensory loss (Figure 3c). The resulting reorganization is not intended to achieve compensatory enhancement, but primarily to stave off potentially harmful changes in cortical dynamics and physiology caused by the absence of normal sensory input.
How can we test these possibilities?
Testing possibility 1: Formulating a set of alternative tasks on which to assess performance.
The first possibility suggests that some of the concerns regarding the strength of the link between cross-modal reorganization and behavior, as reviewed in a preceding section, may be due to a focus on tests that do not probe the abilities where enhancements might actually be occurring. A direct way to address this issue is to design tests that explore whether blind individuals exhibit improved performance on measures besides perceptual thresholds and low-level sensory sensitivities. For example, tests of reaction times can reveal enhanced speed of processing in tactile or auditory modalities. Electro-encephalographic (EEG) studies could also be used to evaluate whether event-related potentials corresponding to tactile or auditory stimuli exhibit faster latencies in blind individuals compared to the sighted. There is some evidence that signals corresponding to the detection of incongruous words in sentences appear sooner in blind compared to sighted subjects, suggesting that blind individuals process auditory language faster than sighted people [106]. It is possible that such latency differences may exhibit a higher correlation than the conventional sensitivity measures with the extent of crossmodal cortical reorganization.
Given the neuroimaging results that blind people exhibit increased connectivity between frontal and visual cortices, another avenue to explore is whether blind individuals show enhanced executive processing skills, such as in the domains of attention or decision making. It is possible that previous results showing perceptual differences between blind and sighted individuals were an outcome of the blind being able to more effectively attend to non-visual senses. Supporting this view, it is reported that when controlling for perceptual sensitivity, the blind indeed show better spatial attention skills compared to sighted individuals [107]. Along related lines, given the evidence that behavioral improvements exhibited by blind individuals in non-visual modalities might be the result of extensive use rather than blindness itself [108], it would be important to examine whether some quantification of use correlates with extent of cortical reorganization.
According to some accounts [109,110], primary sensory cortices are fundamentally metamodal and designed to compute certain kinds of information (say, spatial locations, small scale patterns or temporal sequences) rather than a single sensory modality per se. However, they become dominated by the sensory modality that most suits a particular function. This point of view suggests that we need to consider tasks that tap into the underlying functional specialization of a given sensory cortical area. For example, the visual cortex may be specialized for spatial information processing. In normally-sighted individuals, spatial judgments of tactile stimuli activated visual cortex whereas judgments of textural properties of the same stimuli activate somatosensory cortex [111]. Therefore, auditory and tactile tasks that involve spatial judgments are more likely to show enhancements in blind individuals and may also correlate with the area of activation in visual cortex.
The field of sensory-substitution offers a potentially fruitful avenue for exploring several of the issues described above. Briefly, a visual sensory-substitution device (SSD) interprets and transforms spatial-temporal attributes of a visual scene into stimuli perceived by another sensory modality (e.g. touch or hearing) [112]. This strategy has been pursued in the design of assistive technology devices for the blind to improve functions such as object identification and obstacle avoidance (e.g the vOICe (visual to audition; Metamodal Inc.) and BrainPort (visual to tactile; Wicab Inc.). Numerous studies using SSDs have typically focused on complex, ecologically relevant perceptual tasks. These might constitute the kinds of dimensions beyond basic sensory thresholds that can reveal the consequences of cortical reorganization. Correlating behavioral performance with neuroimaging derived measures of cortical change would yield valuable data to assess potential causal dependencies between the two [113,22,10,114].
Testing possibility 2: Exploring whether cross-modal activations after sensory loss arise from unmasking of already present, but dormant inputs
If the observed cortical reorganization is a manifestation of already present inputs, the time needed to see such reorganization would be fairly short, since no new anatomical tracts need to be grown. (Interestingly, although there is little evidence of rapid long-range axonal growth, recent studies have indicated that other kinds of structural changes can indeed transpire quickly [115,116]. Indeed, a number of studies have shown functional activity changes in the brain using experimental protocols of short-term sensory deprivation [94,117,118]. A key open question in this context is whether the neuro-plastic changes observed in these studies are similar to those found in cases of prolonged sensory loss, as reviewed in section 1. Similarity between the two would strengthen the case for unmasking of dormant connections as an underlying mechanism for both conditions. Studies using TMS with normal subjects have also provided clues about tasks potentially subserved by pre-existing cross-modal connections [119]. These tasks can serve as targets to determine whether they, differentially more than others, register an enhancement following sensory loss.
Longitudinal studies using varied measures of brain activity and connectivity may help further elucidate neuro-plastic changes over time. For example, white matter tractography derived from diffusion-based imaging techniques (such as diffusion tensor imaging or high angular resolution diffusion imaging) can be used to identify underlying white matter structural connectivity profiles [120]. Combined with functional connectivity (as determined from resting state sequences) and effective connectivity (based on task specific activation) analyses, further information can be garnered regarding relative strength of connections across cortical areas [121,122]. Thus, an assessment of connectivity profiles prior to the onset of sensory loss (or specific intervention) and again after the intervention of interest, would allow for comparisons to be made regarding underlying neuroplasticity. Furthermore, associating these network connectivity changes after short and long-term deprivation with changes in behavioral performance may also help in separating the contributions of practice on the one hand and cortical reorganization per se in bringing about these changes.
Testing possibility 3: Exploring whether cortical reorganization is an adaptation to mitigate adverse physiological consequences of sensory signal loss
There are several empirical avenues for assessing the validity this idea. First, from the viewpoint that ‘encroaching’ modalities serve to impose structure on the neural activity of an area, we would expect that if the sensory loss was multi-modal, thereby precluding structured signals in any input stream, then paroxysmal activity would arise in sensory cortical areas (although, it is possible that feedback connections may play a role in imposing structured activity in the absence of sensory inputs). In other words, multi-modal sensory deprivation would be likely to result in epileptogenic activity. This is a prediction that can be tested directly by chronically recording EEG activity while an animal undergoes an extended period of sensory deprivation. The EEG traces can be analyzed for aberrant signal patterns indicative of seizures or their precursor changes in intrinsic neuronal activity using any of a host of powerful techniques that have been developed for this purpose [123,124]. Interestingly, one of the primary exclusionary criteria for use of sensory deprivation chambers is a predisposition to epilepsy. Also consistent with this idea, neonatal sensory deprivation has been found to be correlated with later petit mal seizures [125]. Intriguingly, there is some evidence that even unimodal sensory deprivation is associated with high incidence of paroxysmal EEG [126].
A second set of studies can examine how the nature of neural activity changes over time after sensory loss. For instance, we can use multi-electrode arrays or even scalp-based recordings in animals to record brain activity after blindness onset. In these recordings, it will be informative to determine whether the visual cortex displays paroxysmal activity in the short-term, and then transitions to more structured responses following the same timeline as that over which it comes to exhibit non-visual responses. Relatedly, a third kind of study can probe whether there may be a causal relationship between paroxysmal activity and cortical encroachment. Does the manifestation of the former in a cortical area serve as an attractor for the latter? One way to probe this question could involve determining whether the cessation of paroxysmal activity in an area, say by pharmacological means such as anti-epileptic drugs [127,128], also leads to a reduction in cross-modal encroachment. Although we have focused the discussion above on epileptogenic activity, the ideas also apply to other undesirable consequences such as neural apoptosis that have been seen to follow sensory deafferentation [102,129,130].
Concluding remarks
In summary, the phenomenology of cortical reorganization following sensory loss is rich and robust. To more comprehensively account for the empirical data, it is worth considering additional possibilities beyond the conventional one of cortical changes as compensatory processes. The alternative accounts we have presented here serve as experimental targets that define a program of research to understand the reasons for and functional significance of cortical reorganization. Such understanding is of significance both from a basic as well as a translational perspective. Knowing whether cortical reorganization is an inevitable epiphenomenon, serves a compensatory purpose, or is potentially maladaptive can dramatically change how we intervene in cases of sensory loss, stroke or traumatic brain injury.
Outstanding Questions.
Why does there exist such a marked discrepancy between the large extent of cortical reorganization and the modest magnitude of performance gains on tasks in the encroaching sensory modality?
Performance on which tasks, if any, undergoes the most significant enhancements after loss of one sensory modality?
Is cortical reorganization after sensory loss due primarily to unmasking of pre-existing connections from other sensory modalities? If so, then what is the purpose of these dormant connections in the normal brain?
How does cortical reorganization after short-term sensory loss compare to that arising from congenital sensory loss?
Can paroxysmal activity in a cortical area induce the growth of connections from neighboring areas to mitigate stochastic neural activity?
Highlights.
Neuroimaging studies have revealed that after loss of their primary sensory inputs, cortical areas often come to exhibit responses to inputs from other sensory modalities.
These cortical changes are sometimes, but not always, accompanied by enhancements in behavioral abilities in the ‘encroaching’ modalities, seemingly to compensate for the missing modality.
We lack a comprehensive account of why cortical reorganization happens after sensory loss. Possibilities besides compensation include unmasking of dormant inputs, and mitigation of potentially harmful physiological changes in ‘deafferented’ cortical tissue.
Acknowledgments
This work was supported by grants from the NIH/NEI (R01 EY020517 to PS and R01 EY019924 to LBM), the James McDonnell Foundation’s Scholar Award to PS, the Low Vision Research Award from Research to Prevent Blindness (RPB) to LBM, The IIE Fulbright Scholar Program to FP, the Nick Simons Foundation and the Lions Clubs International Foundation (LCIF). The authors wish to thank Sidney Diamond, Sruti Raja, Robert Ajemian, Jitendra Sharma and Wasim Malik for their feedback on this work.
Glossary
- Anosmia
Partial or total loss of the sense of smell.
- Apoptosis
cell death occurring as part of normal growth or development.
- Cortical cooling paradigms
an experimental technique (such as cryoloops and cryoplates) used to reversibly deactivate a circumscribed area of the brain for the purposes of establishing causal links between behavior and brain function.
- Ectopic visual input
Abnormal or misdirected visual input
- Electro-encephalography (EEG)
measures the aggregate of small electrical field currents generated by postsynaptic activity from neurons using electrodes placed on the surface of the scalp.
- Epileptogenic activity
abnormal neuronal firing patterns (such as in the case of a seizure).
- Functional magnetic resonance imaging (fMRI)
measures localized changes in magnetic susceptibility caused by local oxyhemaglobin and deoyhemaglobin levels (referred to as the blood oxygen level dependent, or BOLD signal). Measured changes within specific regions of the brain are associated with performing a particular task.
- Paroxysmal pattern
An episodic rather than chronic pattern; in the context of brain activity, often refers to epileptic seizures.
- Resting-state functional connectivity MRI (rsfcMRI)
measures the temporal synchronization of spontaneous fluctuations of the BOLD signal (see fMRI) in the absence of performing an explicit behavioral task. Useful for characterizing large-scale functional network circuits in the brain.
- Sensory substitution device (SSD)
A device that interprets and transforms the characteristics of one sensory modality (e.g. vision) into stimuli that can be perceived by another sensory modality (e.g. touch or hearing).
- Transcranial magnetic stimulation (TMS)
a noninvasive procedure that generates a changing magnetic field in order to induce an electrical current delivered to the brain. By placing a magnetic field generator (or “coil’) on the surface of the scalp, the induced electro-magnetic current can stimulate underlying nerve cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gougoux F et al. (2005) A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 3, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voss P et al. (2011) Relevance of spectral cues for auditory spatial processing in the occipital cortex of the blind. Front. Psychol 2, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss P and Zatorre RJ (2015) Early visual deprivation changes cortical anatomical covariance in dorsal-stream structures. NeuroImage 108, 194–202 [DOI] [PubMed] [Google Scholar]
- 4.Burton H et al. (2004) Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum. Brain Mapp 23, 210–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnon L et al. (2010) Tactile maze solving in congenitally blind individuals. Neuroreport 21, 989–992 [DOI] [PubMed] [Google Scholar]
- 6.Kupers R et al. (2010) Neural correlates of virtual route recognition in congenital blindness. Proc. Natl. Acad. Sci. U. S. A 107, 12716–12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupers R et al. (2011) Neural correlates of olfactory processing in congenital blindness. Neuropsychologia 49, 2037–2044 [DOI] [PubMed] [Google Scholar]
- 8.Burton H et al. (2002) Adaptive changes in early and late blind: a FMRI study of verb generation to heard nouns. J. Neurophysiol 88, 3359–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedny M et al. (2015) “Visual” Cortex Responds to Spoken Language in Blind Children . J. Neurosci. Off. J. Soc. Neurosci 35, 11674–11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Striem-Amit E et al. (2012) Reading with sounds: sensory substitution selectively activates the visual word form area in the blind. Neuron 76, 640–652 [DOI] [PubMed] [Google Scholar]
- 11.Raz N et al. (2007) Superior serial memory in the blind: a case of cognitive compensatory adjustment. Curr. Biol. CB 17, 1129–1133 [DOI] [PubMed] [Google Scholar]
- 12.Binda P et al. (2013) Minimizing biases in estimating the reorganization of human visual areas with BOLD retinotopic mapping. J. Vis 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidyasagar TR (1978) Possible plasticity in the rat superior colliculus. Nature 275, 140–141 [DOI] [PubMed] [Google Scholar]
- 14.Rauschecker JP and Harris LR (1983) Auditory compensation of the effects of visual deprivation in the cat’s superior colliculus. Exp. Brain Res 50, 69–83 [DOI] [PubMed] [Google Scholar]
- 15.Rauschecker JP and Korte M (1993) Auditory compensation for early blindness in cat cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci 13, 4538–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauschecker JP (1996) Substitution of visual by auditory inputs in the cat’s anterior ectosylvian cortex. Prog. Brain Res 112, 313–323 [DOI] [PubMed] [Google Scholar]
- 17.Hyvärinen J et al. (1981) Modification of parietal association cortex and functional blindness after binocular deprivation in young monkeys. Exp. Brain Res 42, 1–8 [DOI] [PubMed] [Google Scholar]
- 18.Rauschecker JP (1995) Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 18, 36–43 [DOI] [PubMed] [Google Scholar]
- 19.Bavelier D and Neville HJ (2002) Cross-modal plasticity: where and how? Nat. Rev. Neurosci 3, 443–452 [DOI] [PubMed] [Google Scholar]
- 20.Collignon O et al. (2013) Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain J. Neurol 136, 2769–2783 [DOI] [PubMed] [Google Scholar]
- 21.Voss P (2013) Sensitive and critical periods in visual sensory deprivation. Front. Psychol 4, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renier LA et al. (2010) Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron 68, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gizewski ER et al. (2003) Cross-modal plasticity for sensory and motor activation patterns in blind subjects. NeuroImage 19, 968–975 [DOI] [PubMed] [Google Scholar]
- 24.Bedny M et al. (2011) Language processing in the occipital cortex of congenitally blind adults. Proc. Natl. Acad. Sci. U. S. A 108, 4429–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton R et al. (2000) Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuroreport 11, 237–240 [DOI] [PubMed] [Google Scholar]
- 26.Cohen LG et al. (1997) Functional relevance of cross-modal plasticity in blind humans. Nature 389, 180–183 [DOI] [PubMed] [Google Scholar]
- 27.Collignon O et al. (2011) Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc. Natl. Acad. Sci. U. S. A 108, 4435–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amedi A et al. (2003) Early “visual” cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci 6, 758–766 [DOI] [PubMed] [Google Scholar]
- 29.Lomber SG et al. (2010) Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat. Neurosci 13, 1421–1427 [DOI] [PubMed] [Google Scholar]
- 30.Meredith MA et al. (2011) Crossmodal reorganization in the early deaf switches sensory, but not behavioral roles of auditory cortex. Proc. Natl. Acad. Sci. U. S. A 108, 8856–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascual-Leone A and Hamilton R (2001) The metamodal organization of the brain. Prog. Brain Res 134, 427–445 [DOI] [PubMed] [Google Scholar]
- 32.Neville H and Bavelier D (2002) Human brain plasticity: evidence from sensory deprivation and altered language experience. Prog. Brain Res 138, 177–188 [DOI] [PubMed] [Google Scholar]
- 33.Merabet LB and Pascual-Leone A (2010) Neural reorganization following sensory loss: the opportunity of change. Nat. Rev. Neurosci 11, 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darwin C (1890) The expression of the emotions in man and animals, (2nd edn) John Murray. [Google Scholar]
- 35.Craig JC (1988) The role of experience in tactual pattern perception: A preliminary report. Int. J. Rehabil. Res 11, 167–171 [Google Scholar]
- 36.Van Boven RW et al. (2000) Tactile spatial resolution in blind braille readers. Neurology 54, 2230–2236 [DOI] [PubMed] [Google Scholar]
- 37.Goldreich D and Kanics IM (2003) Tactile acuity is enhanced in blindness. J. Neurosci. Off. J. Soc. Neurosci 23, 3439–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chebat D-R et al. (2007) Tactile-’visual’ acuity of the tongue in early blind individuals. Neuroreport 18, 1901–1904 [DOI] [PubMed] [Google Scholar]
- 39.Legge GE et al. (2008) Retention of high tactile acuity throughout the life span in blindness. Percept. Psychophys 70, 1471–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong M et al. (2011) Tactile spatial acuity enhancement in blindness: evidence for experience-dependent mechanisms. J. Neurosci. Off. J. Soc. Neurosci 31, 7028–7037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alary F et al. (2009) Tactile acuity in the blind: a closer look reveals superiority over the sighted in some but not all cutaneous tasks. Neuropsychologia 47, 2037–2043 [DOI] [PubMed] [Google Scholar]
- 42.Röder B et al. (1999) Improved auditory spatial tuning in blind humans. Nature 400, 162–166 [DOI] [PubMed] [Google Scholar]
- 43.Niemeyer W and Starlinger I (1981) Do the blind hear better? Investigations on auditory processing in congenital or early acquired blindness. II. Central functions. Audiol. Off. Organ Int. Soc. Audiol 20, 510–515 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton RH et al. (2004) Absolute pitch in blind musicians. Neuroreport 15, 803–806 [DOI] [PubMed] [Google Scholar]
- 45.Gougoux F et al. (2004) Neuropsychology: pitch discrimination in the early blind. Nature 430, 309. [DOI] [PubMed] [Google Scholar]
- 46.Cuevas I et al. (2009) Odour discrimination and identification are improved in early blindness. Neuropsychologia 47, 3079–3083 [DOI] [PubMed] [Google Scholar]
- 47.Beaulieu-Lefebvre M et al. (2011) Odor perception and odor awareness in congenital blindness. Brain Res. Bull 84, 206–209 [DOI] [PubMed] [Google Scholar]
- 48.Rice CE and Feinstein SH (1965) Sonar System of the Blind: Size Discrimination. Science 148, 1107–1108 [DOI] [PubMed] [Google Scholar]
- 49.Kolarik AJ et al. (2014) A summary of research investigating echolocation abilities of blind and sighted humans. Hear. Res 310, 60–68 [DOI] [PubMed] [Google Scholar]
- 50.Thaler L et al. (2011) Neural correlates of natural human echolocation in early and late blind echolocation experts. PloS One 6, e20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Röder B et al. (2002) Speech processing activates visual cortex in congenitally blind humans. Eur. J. Neurosci 16, 930–936 [DOI] [PubMed] [Google Scholar]
- 52.Raz N et al. (2005) V1 activation in congenitally blind humans is associated with episodic retrieval. Cereb. Cortex N. Y. N 1991 15, 1459–1468 [DOI] [PubMed] [Google Scholar]
- 53.Voss P et al. (2014) Evidence for both compensatory plastic and disuse atrophy-related neuroanatomical changes in the blind. Brain J. Neurol 137, 1224–1240 [DOI] [PubMed] [Google Scholar]
- 54.Rauschecker JP and Kniepert U (1994) Auditory localization behaviour in visually deprived cats. Eur. J. Neurosci 6, 149–160 [DOI] [PubMed] [Google Scholar]
- 55.Korte M and Rauschecker JP (1993) Auditory spatial tuning of cortical neurons is sharpened in cats with early blindness. J. Neurophysiol 70, 1717–1721 [DOI] [PubMed] [Google Scholar]
- 56.Dancause N (2006) Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 12, 489–499 [DOI] [PubMed] [Google Scholar]
- 57.Mao Y (2012) The Reorganization of Primary Auditory Cortex by Invasion of Ectopic Visual Inputs.
- 58.Gagnon L et al. (2013) Reduced taste sensitivity in congenital blindness. Chem. Senses 38, 509–517 [DOI] [PubMed] [Google Scholar]
- 59.Gagnon L et al. (2015) Neural correlates of taste perception in congenital blindness. Neuropsychologia 70, 227–234 [DOI] [PubMed] [Google Scholar]
- 60.Frasnelli J et al. (2011) Crossmodal plasticity in sensory loss. Prog. Brain Res 191, 233–249 [DOI] [PubMed] [Google Scholar]
- 61.Iannilli E et al. (2007) Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res. 1139, 235–244 [DOI] [PubMed] [Google Scholar]
- 62.Voss P et al. (2015) Trade-off in the sound localization abilities of early blind individuals between the horizontal and vertical planes. J. Neurosci. Off. J. Soc. Neurosci 35, 6051–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King AJ et al. (2011) Neural circuits underlying adaptation and learning in the perception of auditory space. Neurosci. Biobehav. Rev 35, 2129–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andéol G et al. (2014) Perceptual factors contribute more than acoustical factors to sound localization abilities with virtual sources. Front. Neurosci 8, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tonelli A et al. (2016) Depth Echolocation Learnt by Novice Sighted People. PloS One 11, e0156654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burton H et al. (2002) Adaptive changes in early and late blind: a fMRI study of Braille reading. J. Neurophysiol 87, 589–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews TJ et al. (1997) Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J. Neurosci. Off. J. Soc. Neurosci 17, 2859–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Artacho-Pérula E et al. (2004) Quantitative estimation of the primary auditory cortex in human brains. Brain Res. 1008, 20–28 [DOI] [PubMed] [Google Scholar]
- 69.Renier L et al. (2013) Right occipital cortex activation correlates with superior odor processing performance in the early blind. PloS One 8, e71907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starlinger I and Niemeyer W (1981) Do the blind hear better? Investigations on auditory processing in congenital or early acquired blindness. I. Peripheral functions. Audiol. Off. Organ Int. Soc. Audiol 20, 503–509 [DOI] [PubMed] [Google Scholar]
- 71.Rosenbluth R et al. (2000) Performance of early-blind and sighted children on olfactory tasks. Perception 29, 101–110 [DOI] [PubMed] [Google Scholar]
- 72.Zwiers MP et al. (2001) A spatial hearing deficit in early-blind humans. J. Neurosci. Off. J. Soc. Neurosci 21, RC142: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gori M et al. (2014) Impairment of auditory spatial localization in congenitally blind human subjects. Brain J. Neurol 137, 288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sathian K (2014) Cross-modal plasticity in the visual system In Textbook of Neural Repair and Rehabilitation (2nd edn) (Selzer M et al. eds), pp. 140–153, Cambridge University Press [Google Scholar]
- 75.Pavani F and Bottari D (2012) Visual Abilities in Individuals with Profound Deafness A Critical Review In The Neural Bases of Multisensory Processes (Murray MM and Wallace MT, eds), CRC Press/Taylor & Francis; [PubMed] [Google Scholar]
- 76.Wiesel TN and Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol 26, 1003–1017 [DOI] [PubMed] [Google Scholar]
- 77.Chatzistefanou KI et al. (2005) Contrast sensitivity in amblyopia: the fellow eye of untreated and successfully treated amblyopes. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 9, 468–474 [DOI] [PubMed] [Google Scholar]
- 78.Dougherty RF et al. (2003) Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J. Vis 3, 586–598 [DOI] [PubMed] [Google Scholar]
- 79.Schwarzkopf DS et al. (2011) The surface area of human V1 predicts the subjective experience of object size. Nat. Neurosci 14, 28–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merzenich MM et al. (1983) Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8, 33–55 [DOI] [PubMed] [Google Scholar]
- 81.Merzenich MM et al. (1984) Somatosensory cortical map changes following digit amputation in adult monkeys. J. Comp. Neurol 224, 591–605 [DOI] [PubMed] [Google Scholar]
- 82.Vega-Bermudez F and Johnson KO (2002) Spatial acuity after digit amputation. Brain J. Neurol 125, 1256–1264 [DOI] [PubMed] [Google Scholar]
- 83.Kujawa SG and Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. Off. J. Soc. Neurosci 29, 14077–14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liberman MC and Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res 349, 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oxenham AJ (2016) Predicting the Perceptual Consequences of Hidden Hearing Loss. Trends Hear. 20, 2331216516686768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stilla R et al. (2008) Neural processing underlying tactile microspatial discrimination in the blind: a functional magnetic resonance imaging study. J. Vis 8, 13.1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Connell N and Merabet LB (2014) Uncovering the connectivity of the brain in relation to novel vision rehabilitation strategies. Neurology 83, 484–485 [DOI] [PubMed] [Google Scholar]
- 88.Sathian K et al. (2013) Neural changes with tactile learning reflect decision-level reweighting of perceptual readout. J. Neurosci. Off. J. Soc. Neurosci 33, 5387–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Falchier A et al. (2002) Anatomical evidence of multimodal integration in primate striate cortex. J. Neurosci. Off. J. Soc. Neurosci 22, 5749–5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghazanfar AA and Schroeder CE (2006) Is neocortex essentially multisensory? Trends Cogn. Sci 10, 278–285 [DOI] [PubMed] [Google Scholar]
- 91.Driver J and Noesselt T (2008) Multisensory interplay reveals crossmodal influences on “sensory-specific” brain regions, neural responses, and judgments. Neuron 57, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lakatos P et al. (2007) Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53, 279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibrahim LA et al. (2016) Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron 89, 1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merabet LB et al. (2008) Rapid and reversible recruitment of early visual cortex for touch. PloS One 3, e3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Florence SL et al. (1998) Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121 [DOI] [PubMed] [Google Scholar]
- 96.Faggin BM et al. (1997) Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc. Natl. Acad. Sci. U. S. A 94, 9428–9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humanes-Valera D et al. (2013) Reorganization of the intact somatosensory cortex immediately after spinal cord injury. PloS One 8, e69655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darian-Smith C and Gilbert CD (1994) Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368, 737–740 [DOI] [PubMed] [Google Scholar]
- 99.Das A and Gilbert CD (1995) Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature 375, 780–784 [DOI] [PubMed] [Google Scholar]
- 100.Topolnik L et al. (2003) Partial cortical deafferentation promotes development of paroxysmal activity. Cereb. Cortex N. Y. N 1991 13, 883–893 [DOI] [PubMed] [Google Scholar]
- 101.Nita DA et al. (2006) Increased propensity to seizures after chronic cortical deafferentation in vivo. J. Neurophysiol 95, 902–913 [DOI] [PubMed] [Google Scholar]
- 102.Capurso SA et al. (1997) Deafferentation causes apoptosis in cortical sensory neurons in the adult rat. J. Neurosci. Off. J. Soc. Neurosci 17, 7372–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kitajima M et al. (1997) MR changes in the calcarine area resulting from retinal degeneration. AJNR Am. J. Neuroradiol 18, 1291–1295 [PMC free article] [PubMed] [Google Scholar]
- 104.Yamaguchi M and Mori K (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. U. S. A 102, 9697–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burge WK et al. (2016) Cortical thickness in human V1 associated with central vision loss. Sci. Rep 6, 23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Röder B et al. (2000) Event-related potentials during auditory language processing in congenitally blind and sighted people. Neuropsychologia 38, 1482–1502 [DOI] [PubMed] [Google Scholar]
- 107.Collignon O et al. (2006) Improved selective and divided spatial attention in early blind subjects. Brain Res. 1075, 175–182 [DOI] [PubMed] [Google Scholar]
- 108.Sterr A et al. (1998) Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J. Neurosci. Off. J. Soc. Neurosci 18, 4417–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shimojo S and Shams L (2001) Sensory modalities are not separate modalities: plasticity and interactions. Curr. Opin. Neurobiol 11, 505–509 [DOI] [PubMed] [Google Scholar]
- 110.Ricciardi E et al. (2014) Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci. Biobehav. Rev 41, 64–77 [DOI] [PubMed] [Google Scholar]
- 111.Merabet L et al. (2004) Feeling by sight or seeing by touch? Neuron 42, 173–179 [DOI] [PubMed] [Google Scholar]
- 112.Bach-y-Rita P et al. (1969) Vision substitution by tactile image projection. Nature 221, 963–964 [DOI] [PubMed] [Google Scholar]
- 113.Amedi A et al. (2007) Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nat. Neurosci 10, 687–689 [DOI] [PubMed] [Google Scholar]
- 114.Nau AC et al. (2015) Use of sensory substitution devices as a model system for investigating cross-modal neuroplasticity in humans. Neural Regen. Res 10, 1717–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sagi Y et al. (2012) Learning in the fast lane: new insights into neuroplasticity. Neuron 73, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 116.Sampaio-Baptista C and Johansen-Berg H (2017) White Matter Plasticity in the Adult Brain. Neuron 96, 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lewald J (2007) More accurate sound localization induced by short-term light deprivation. Neuropsychologia 45, 1215–1222 [DOI] [PubMed] [Google Scholar]
- 118.Landry SP et al. (2013) Short-term visual deprivation improves the perception of harmonicity. J. Exp. Psychol. Hum. Percept. Perform 39, 1503–1507 [DOI] [PubMed] [Google Scholar]
- 119.Zangaladze A et al. (1999) Involvement of visual cortex in tactile discrimination of orientation. Nature 401, 587–590 [DOI] [PubMed] [Google Scholar]
- 120.Jones DK (2008) Studying connections in the living human brain with diffusion MRI. Cortex J. Devoted Study Nerv. Syst. Behav 44, 936–952 [DOI] [PubMed] [Google Scholar]
- 121.Friston KJ (2011) Functional and effective connectivity: a review. Brain Connect. 1, 13–36 [DOI] [PubMed] [Google Scholar]
- 122.Bauer CM et al. (2017) Multimodal MR-imaging reveals large-scale structural and functional connectivity changes in profound early blindness. PloS One 12, e0173064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramgopal S et al. (2014) Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav. EB 37, 291–307 [DOI] [PubMed] [Google Scholar]
- 124.Hawellek DJ et al. (2013) Altered intrinsic neuronal interactions in the visual cortex of the blind. J. Neurosci. Off. J. Soc. Neurosci 33, 17072–17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sitnikova E (2011) Neonatal sensory deprivation promotes development of absence seizures in adult rats with genetic predisposition to epilepsy. Brain Res. 1377, 109–118 [DOI] [PubMed] [Google Scholar]
- 126.Fishman JE et al. (1983) Congenital sensorineural deafness associated with EEG abnormalities, epilepsy and high familial incidence. Dev. Med. Child Neurol 25, 747–754 [DOI] [PubMed] [Google Scholar]
- 127.Haglund MM and Hochman DW (2005) Furosemide and mannitol suppression of epileptic activity in the human brain. J. Neurophysiol 94, 907–918 [DOI] [PubMed] [Google Scholar]
- 128.Kalappa BI et al. (2015) Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J. Neurosci. Off. J. Soc. Neurosci 35, 8829–8842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vankirk AM and Byrd CA (2003) Apoptosis following peripheral sensory deafferentation in the olfactory bulb of adult zebrafish. J. Comp. Neurol 455, 488–498 [DOI] [PubMed] [Google Scholar]
- 130.Koliatsos VE et al. (2004) Cortical interneurons become activated by deafferentation and instruct the apoptosis of pyramidal neurons. Proc. Natl. Acad. Sci. U. S. A 101, 14264–14269 [DOI] [PMC free article] [PubMed] [Google Scholar]


